Abstract
Purpose
Preoperative breast MRI is used to evaluate for additional cancer and extent of disease for newly diagnosed breast cancer, yet benefits and harms of preoperative MRI are not well documented. We examined whether preoperative MRI yields additional biopsy and cancer detection by extent of breast density.
Methods
We followed women in the Breast Cancer Surveillance Consortium with an incident breast cancer diagnosed from 2005–2017. We quantified breast biopsies and cancers detected within 6 months of diagnosis by preoperative breast MRI receipt, overall and by breast density, accounting for MRI selection bias using inverse probability weighted logistic regression.
Results
Among 19,324 women with newly diagnosed breast cancer, 28% had preoperative MRI, 11% additional biopsy, and 5% additional cancer detected. Four times as many women with preoperative MRI underwent additional biopsy compared to women without MRI (22.6% v. 5.1%). Additional biopsy rates with preoperative MRI increased with increasing breast density (27.4% for extremely dense compared to 16.2% for almost entirely fatty breasts). Rates of additional cancer detection were almost four times higher for women with v. without MRI (9.9% v. 2.6%). Conditional on additional biopsy, age-adjusted rates of additional cancer detection were lowest among women with extremely dense breasts, regardless of imaging modality (with MRI: 35.0%; 95%CI=27.0%−43.0%; without MRI: 45.1%; 95%CI=32.6%−57.5%).
Conclusion
For women with dense breasts, preoperative MRI was associated with much higher biopsy rates, without concomitant higher cancer detection. Preoperative MRI may be considered for some women, but selecting women based on breast density is not supported by evidence.
Keywords: preoperative MRI, breast density, breast biopsy, occult cancer, Breast Cancer Surveillance Consortium, cancer detection rate
INTRODUCTION
Breast density may impact women newly diagnosed with breast cancer by underestimating extent of disease for the affected breast because of masking on mammography1 and increased risk of mammographically occult cancer in the contralateral breast.2 Breast MRI relies on differential contrast enhancement to better characterize cancers relative to surrounding breast tissue and is not influenced by breast density. Breast MRI use after a cancer diagnosis (i.e., ‘preoperative’ breast MRI, before first surgery) in the U.S. has increased in the past two decades, even though the evidence is not clear about the relative harms and benefits, particularly for subgroups of women.3 There is some evidence breast surgeons use density as a criterion for ordering preoperative MRI based on the suggested higher cancer yield for women with dense breasts.4
The primary rationale for preoperative MRI is to better define extent of newly diagnosed breast cancer and to evaluate whether there is a mammographically occult tumor in the contralateral breast.5 Better characterization of extent of the tumor(s) upon diagnosis can change surgical choice, lead to improved surgical options (fewer repeat surgeries, better identification of women for successful breast conserving surgery, wider excision when indicated, mastectomy for women with multifocal/multicentric disease) and improved patient-centered and clinical long-term outcomes. While there is evidence for additional cancer detection with MRI, its high sensitivity results in detecting benign or possibly indolent lesions, which may change women’s clinical treatment choices towards more aggressive care without providing recurrence or survival benefit.6–9 A review of more than 8 small and single-institution studies determined that preoperative MRI detected additional cancer in 10–34% of ipsilateral breasts and 3–23% of contralateral breasts.10,11 While notable subgroup differences in occult malignancy detection were not reported, several single institution studies have found that women with high breast density or lobular histology benefited most from preoperative MRI.11–13 No randomized controlled trials or population-based observational studies of preoperative MRI to date have studied outcomes by extent of breast density and clinical guidelines for its use are not well substantiated by breast density.14,15
We sought to provide population-based, generalizable evidence to inform evidence gaps in understanding the comparative effectiveness of preoperative MRI by breast density categories. We estimated rates of additional biopsies and detected cancers among women undergoing preoperative breast MRI vs. not, overall, and by breast density.
PATIENTS AND METHODS
Data Sources
Data from six Breast Cancer Surveillance Consortium (BCSC)16 breast imaging registries (Carolina Mammography Registry, New Hampshire Mammography Network, Vermont Breast Cancer Surveillance System, San Francisco Mammography Registry, Metropolitan Chicago Breast Cancer Registry, and Kaiser Permanente Washington) prospectively collected information related to women’s breast imaging use and assessments, benign and malignant breast pathology, breast cancer outcomes, and other clinical and sociodemographic characteristics. Registries collect data through a combination of women’s self-report (socio-demographics, first-degree family history), radiology imaging systems, pathology records, electronic health records, and North American Association of Central Cancer Registries (NAACCR)-affiliated cancer registries. Data from the registries were pooled and analyzed at the Statistical Coordinating Center.
The institutional review boards of the participating BCSC16 registries and Statistical Coordinating Center approved all study activities through passive consent (three registries) or waiver of written consent (two registries and the Statistical Coordinating Center). This study was Health Insurance Portability and Accountability Act compliant. Registries and the Statistical Coordinating Center received a federal Certificate of Confidentiality and other protections for the identities of women, physicians, and facilities. Our study was registered on ClinicalTrials.gov (NCT02980848) and followed the Good Research Practices guidelines for comparative effectiveness research.17
Study Population
We studied women ages 18–89 years with a biopsy-determined incident invasive breast cancer or ductal carcinoma in situ (DCIS) (index biopsy) diagnosed between 2005 and 2017, identified in the BCSC pathology records. Women were required to have evidence of surgery (lumpectomy or uni- or bilateral mastectomy) within 6 months of the index biopsy in either the pathology or cancer registry records. Women were excluded if the index biopsy was missing laterality, if they had no mammogram within one month before the index biopsy, and if there was no measure of breast density within 10 years before the index biopsy. For 98.2% of the study population, the breast density measure was determined within the 18 months prior to the index biopsy
Study Variables
Main Exposure
We compared women who received preoperative MRI (completed between index biopsy and surgery) (Figure 1) to women who did not receive preoperative MRI. Breast MRI with contrast and dedicated breast coils was performed for women in the study population based on clinical recommendation of the treating physicians. We evaluated breast density as a potential effect modifier of preoperative breast MRI associated with additional biopsies/biopsy yield. Breast density was classified based on mammographic assessment using BI-RADS density categories: a=almost entirely fatty, b=scattered fibroglandular densities, c=heterogeneously dense, and d=extremely dense.18 Non-dense breasts includes categories a and b; Dense breasts includes categories c and d.
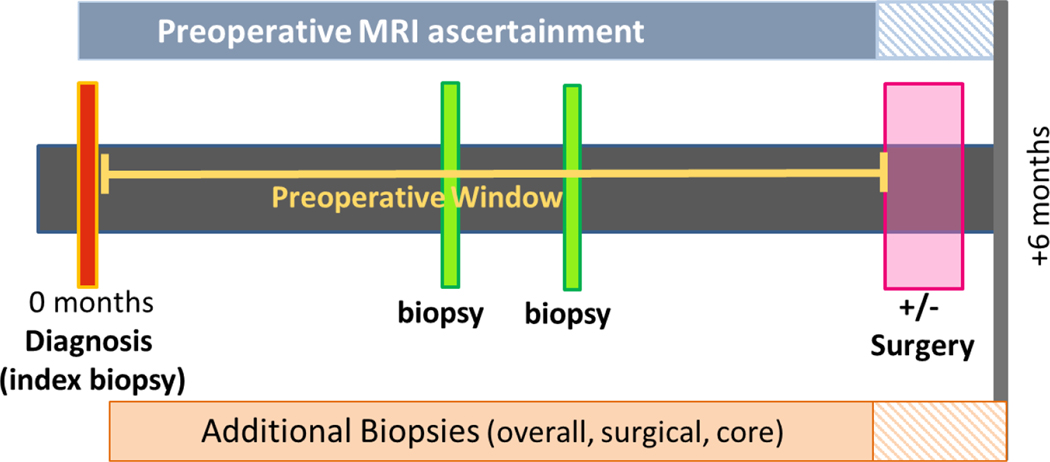
Figure 1.
Preoperative MRI and additional biopsy and cancer outcome windows
Definitions: 1) Preoperative window = index biopsy (the biopsy associated with the diagnosis) date to surgery; 2) Preoperative MRI = from index biopsy to surgery) 3) Index biopsy = biopsy associated with the diagnosis; 4) Additional biopsies = biopsy after index biopsy and before surgery; 5) Positive biopsy = invasive or DCIS = additional cancer detected; 6) Surgery type = lumpectomy or mastectomy.
Main Outcomes
The primary outcomes were rates of additional biopsies and additional cancer detected. Additional biopsy rate was defined as the number of individuals with at least one additional biopsy performed after the date of diagnosis and before surgery over the total number of women in that subgroup. Rates of additional cancers detected were defined as the number of individuals with an additional breast cancer diagnosed (i.e., biopsy positive for DCIS or invasive carcinoma after index biopsy before surgery) over the total number of women in that subgroup, calculated separately for ipsilateral and contralateral cancers. Secondary outcomes included definitive surgery type (mastectomy or lumpectomy, with laterality noted) and rate by biopsy type (core vs. surgical). Pathology information was collected on all breast biopsies and breast surgeries performed on women with a breast cancer diagnosis. These data include type of biopsy (fine needle aspiration, core biopsy, surgical biopsy) or surgery (lumpectomy, mastectomy including laterality), up to five Systematized Nomenclature of Medicine-Clinical Terms codes describing biopsy results, and cancer characteristics (e.g., stage, tumor size).
Other Key Variables
We included covariates measured prior to diagnosis: women’s age, race/ethnicity, educational attainment for ZIP code of residence based on the 2010 Census percentage of individuals with a high school degree (in quartiles), first-degree family history of breast cancer, initial cancer laterality, previous biopsies/aspirations, menopausal status, index biopsy year, DCIS v. invasive index biopsy result, mode of detection (screen-detected, interval detected following a negative screening mammogram, clinically detected with no mammogram in prior 27 months)19, and invasive cancer histology (lobular, ductal including mixed ductal/lobular, DCIS, other).
Statistical Analysis
We computed frequency distributions overall and subdivided by breast density and compared them for individuals with and without preoperative MRI. Cross-tabulations were performed by breast density to examine the relation of MRI use to additional biopsies, overall and by biopsy type, and additional cancer detected. We calculated propensity scores using generalized boosted regression modeling to obtain the probability of MRI receipt given age, race/ethnicity, diagnosis year, density, BCSC registry site, biopsy history, education, menopausal status, index cancer type (invasive v. DCIS), and mode of detection. Stabilized inverse probability weights were calculated from the estimated propensity scores and used as weights and applied to the logistic regression models for each outcome by preoperative breast MRI receipt, overall and by breast density categories. We estimated the standardized predictive margins for each outcome by averaging the predicted rates over the age distribution, and estimated 95% Wald confidence intervals using the delta method.20 If negative CI limits were observed, we alternatively computed confidence intervals by applying inverse link transform to the confidence limits on the logit scale. Analyses were conducted on the full denominator of the study population to estimate rates for all individuals with a breast cancer diagnosis, and separately in sub-analyses using the denominator of individuals who had an additional biopsy. We used Stata Statistical Software, Release 15 (College Station, TX: StataCorp LP.), R version 3.6.2 for all analyses.
RESULTS
Among 19,324 women diagnosed with breast cancer during the study period, 5,455 (28%) underwent preoperative MRI. Women with MRI were younger, with 28% <50 years of age, compared to 18% of women without MRI (Table 1). We found no differences in race/ethnicity or first-degree family history of breast cancer by preoperative breast MRI. However, women with MRI lived in a ZIP code with higher level of educational attainment, were more likely to have a previous breast biopsy, more likely to have an interval rather than screen-detected index breast cancer, and more likely to have invasive lobular histology (Table 1). A higher proportion of women with MRI compared to no MRI had heterogeneously dense (48% v. 39%) or extremely dense breasts (15% v. 8%). When stratifying by breast density, we found that for women with extremely dense breasts, those with an MRI had a slightly higher percentage of invasive cancer (MRI: 78%, without MRI: 72%) (Appendix 1).
Table 1.
| No MRI (N, %) | MRI (N, %) | |||
|---|---|---|---|---|
| Overall | Overall | |||
| Total N = 19,324 | 13,869 (72.0 %) | 5,455 (28.0%) | ||
| Age At Exam (years) | ||||
| <50 | 2529 | 18.2 | 1521 | 27.9 |
| 50–59 | 3401 | 24.5 | 1599 | 29.3 |
| 60–69 | 3930 | 28.3 | 1472 | 27.0 |
| 70–79 | 2771 | 20.0 | 714 | 13.1 |
| 80–89 | 1238 | 8.9 | 149 | 2.7 |
| Race (NCI Reporting Standard) | ||||
| White, non Hispanic | 10366 | 76.1 | 4057 | 75.5 |
| Black, non Hispanic | 1382 | 10.1 | 594 | 11.1 |
| Asian | 1260 | 9.2 | 404 | 7.5 |
| Non-Hispanic Pacific Islander, Alaska Native, American Indian |
68 | 0.5 | 27 | 0.5 |
| Hispanic | 550 | 4.0 | 292 | 5.4 |
| Other, Mixed, Unknown | 243 | 1.8 | 81 | 1.5 |
| First Degree Family History of Breast Cancer | ||||
| No | 9974 | 76.5 | 3890 | 75.8 |
| Yes | 3062 | 23.5 | 1242 | 24.2 |
| Unknown | 833 | 6.0 | 323 | 5.9 |
| Previous biopsy/aspiration | ||||
| None | 8463 | 65.9 | 2925 | 61.9 |
| Biopsy Only | 2856 | 22.2 | 1320 | 27.9 |
| Aspiration Only | 615 | 4.8 | 224 | 4.7 |
| Biopsy and Aspiration | 904 | 7.0 | 259 | 5.5 |
| Unknown | 1031 | 7.4 | 727 | 13.3 |
| Menopause | ||||
| Pre | 2847 | 23.1 | 1660 | 35.3 |
| Post | 9460 | 76.9 | 3047 | 64.7 |
| Unknown | 1562 | 11.3 | 748 | 13.7 |
| Current hormone therapy use | ||||
| No | 9146 | 95.4 | 3566 | 96.2 |
| Yes | 436 | 4.6 | 141 | 3.8 |
| Unknown | 4287 | 30.9 | 1748 | 32.0 |
| Laterality of index biopsy2 | ||||
| Left | 6770 | 48.8 | 2683 | 49.2 |
| Right | 6936 | 50.0 | 2724 | 49.9 |
| Bilateral | 163 | 1.2 | 48 | 0.9 |
| Index biopsy2 year | ||||
| 2005–2008 | 4532 | 32.7 | 591 | 10.8 |
| 2009–2011 | 3395 | 24.5 | 1425 | 26.1 |
| 2012–2014 | 3447 | 24.9 | 1812 | 33.2 |
| 2015–2017 | 2495 | 18.0 | 1627 | 29.8 |
| Geo coding education | ||||
| <=Q1 | 3324 | 26.1 | 1288 | 25.8 |
| Q1 – Q2 | 3515 | 27.6 | 1228 | 24.6 |
| Q2 – Q3 | 3296 | 25.9 | 1393 | 27.9 |
| > Q3 | 2593 | 20.4 | 1075 | 21.6 |
| Unknown | 1141 | 8.2 | 471 | 8.6 |
| Index biopsy2 | ||||
| DCIS | 3103 | 22.4 | 1049 | 19.2 |
| Invasive3 | 10766 | 77.6 | 4406 | 80.8 |
| Mode of Detection4 | ||||
| Screen Detected | 8754 | 64.3 | 3178 | 59.1 |
| Interval Detected | 2759 | 20.3 | 1303 | 24.3 |
| Clinically Detected | 2102 | 15.4 | 892 | 16.6 |
| Unknown Mode | 254 | 1.8 | 82 | 1.5 |
| Breast Density | ||||
| Almost entirely fat | 1442 | 10.4 | 284 | 5.2 |
| Scattered fibroglandular | 5996 | 43.2 | 1745 | 32.0 |
| Heterogeneously dense | 5347 | 38.6 | 2622 | 48.1 |
| Extremely dense | 1084 | 7.8 | 804 | 14.7 |
| Histology Type | ||||
| DCIS | 3103 | 28.8 | 1049 | 23.5 |
| Invasive, Ductal | 6741 | 62.5 | 2813 | 63.1 |
| Invasive, Lobular | 733 | 6.8 | 521 | 11.7 |
| Invasive, Ductal & Lobular | 210 | 1.9 | 73 | 1.6 |
| Unknown | 3082 | 22.2 | 999 | 18.3 |
| Surgery Type | ||||
| Lumpectomy | 9787 | 70.6 | 3250 | 59.6 |
| Mastectomy | 4082 | 29.4 | 2205 | 40.4 |
| All | 13869 | 5455 | ||
Preoperative MRI was defined as any MRI completed between index biopsy and the definitive surgery
Index biopsy was defined as the biopsy associated with the pathologically-determined breast cancer diagnosis of invasive breast cancer or ductal carcinoma in situ.
Invasive cancer includes lobular invasive, ductal invasive, and other invasive.
Mode of detection is as follows: Screen detected: cancer detected within 12 months of a positive screening mammogram; Interval detected: cancer detected cancer within 27 months of a prior negative screening exam; Clinically detected: Cancer detected on a diagnostic exam with no mammogram in prior 27 months.
Four times as many women with pre-operative MRI underwent ≥1 additional biopsy compared to women without MRI (22.6% v. 5.1%) (Table 2). Crude biopsy rates increased with increasing breast density, with 27.4/100 of women with extremely dense breasts having additional biopsy compared to 16.2/100 of women with almost entirely fatty breasts. Crude additional cancer detection rates for the entire study population were approximately three times higher for women with MRI (9.9/100 v. 2.6/100 without MRI). There were no notable differences in additional cancer detection by density for women with or without MRI, including by laterality. Additional ipsilateral cancers were detected at about a 3-fold higher rate than contralateral (7.6/100 v. 2.5/100), with most being detected by MRI.
Table 2.
Summary of additional biopsies and biopsy outcomes in relation to pre-operative imaging modality overall and by breast density among a cohort of women with a recent breast cancer diagnosis (N=19,324).
| BIRADS Density | |||||
|---|---|---|---|---|---|
|
| |||||
| Overall | a: Almost entirely fatty | b: Scattered fibroglandular | c: Hetero-genously dense | d: Extremely dense | |
| No MRI | 13869 | 1442 | 5996 | 5347 | 1084 |
| MRI | 5455 | 284 | 1745 | 2622 | 804 |
|
| |||||
| Additional Biopsy (N, %) | |||||
|
| |||||
| Any laterality | |||||
|
| |||||
| No MRI | 711 (5.1) | 44 (3.1) | 286 (4.8) | 310 (5.8) | 71 (6.5) |
| MRI | 1232 (22.6) | 46 (16.2) | 318 (18.2) | 648 (24.7) | 220 (27.4) |
|
| |||||
| Additional Cancer (N, %) | |||||
|
| |||||
| Any laterality | |||||
|
| |||||
| No MRI | 356 (2.6) | 22 (1.5) | 158 (2.6) | 141 (2.6) | 35 (3.2) |
| MRI | 540 (9.9) | 25 (8.8) | 158 (9.1) | 274 (10.5) | 83 (10.3) |
|
| |||||
| Ipsilateral | |||||
|
| |||||
| No MRI | 303 (2.2) | 19 (1.3) | 140 (2.3) | 114 (2.1) | 30 (2.8) |
| MRI | 414 (7.6) | 13 (4.6) | 119 (6.8) | 214 (8.2) | 68 (8.5) |
|
| |||||
| Contralateral | |||||
|
| |||||
| No MRI | 51 (0.4) | 3 (0.2) | 15 (0.3) | 28 (0.5) | 5 (0.5) |
| MRI | 136 (2.5) | 13 (4.6) | 45 (2.6) | 66 (2.5) | 12 (1.5) |
|
| |||||
| Surgery (N, %) | |||||
|
| |||||
| Mastectomy as definitive surgery | |||||
|
| |||||
| No MRI | 4082 (29.4) | 350 (24.3) | 1486 (24.8) | 1784 (33.4) | 462 (42.6) |
| MRI | 2205 (40.4) | 104 (36.6) | 555 (31.8) | 1137 (43.4) | 409 (50.9) |
Weighted logistic regression models showed rates (per 100 women) for additional biopsy were several-fold higher across all density categories in women with MRI v. without (p<0.05 for across all density groups), with the highest rates among women with extremely dense breasts (with MRI: 25.6; 95% CI:21.7–29.6; without MRI: 5.2 (3.9, 6.6)) and the lowest in women with almost entirely fatty breasts (with MRI: 16.8% CI:11.3–22.2; without MRI: 3.3 (2.3–4.3) (Table 3). Both core biopsy and surgical biopsy rates mirrored the results for overall additional biopsy rates; they were very low and did not notably differ between the MRI and no MRI groups, but did increase with increasing density (Appendix Table 2). Additional contralateral cancer on MRI decreased with breast density, while the ipsilateral pattern not monotone. We also observed higher mastectomy rates with MRI across all density groups (almost entirely fatty: with MRI: 39.7 (95% CI:32.1–47.3); without MRI: 26.9 (95% CI: 24.5–29.4); extremely dense: with MRI:47.1 (95% CI:42.7–51.5); without MRI: 42.3 (95%CI: 38.9–45.7p-value = 0.087. Mastectomy rates were higher among women with MRI and across all density groups, and higher among women with denser breasts, regardless of preoperative MRI. (Table 3, p<0.05 for all comparisons except for extremely dense group)
Table 3.
Adjusted rates*(per 100 women) for additional biopsies and biopsy outcomes (per 100 women) in relation to pre-operative imaging modality using inverse weighted probability regression methods among a cohort of women with breast cancer (N=19,324) stratified by BIRADS breast density categories.
| BIRADS Density | ||||
|---|---|---|---|---|
| a: Almost entirely fatty | b: Scattered fibroglandular | c: Heterogenously dense | d: Extremely dense | |
| Adjusted Rate (95% Confidence Interval) | ||||
|
| ||||
| Additional Biopsies | ||||
|
| ||||
| No MRI | 3.3 (2.3,4.3) | 4.9 (4.3,5.5) | 5.4 (4.8,6) | 5.2 (3.9,6.6) |
| MRI | 16.8 (11.3,22.2) | 20.6 (17.9,23.3) | 23.6 (21.4,25.9) | 25.6 (21.7,29.6) |
|
| ||||
| Additional Cancer Detected | ||||
|
| ||||
| No MRI | 1.5 (0.9,2.2) | 2.6 (2.2,3.0) | 2.4 (2.0,2.9) | 2.4 (1.6,3.3) |
| MRI | 8.4 (4.4,12.4) | 9.5 (7.7,11.4) | 10.5 (8.9,12.2) | 8.8 (6.5,11.1) |
|
| ||||
| Additional Cancer (Ipsilateral) | ||||
|
| ||||
| No MRI | 1.4 (0.8,2.0) | 2.4 (2.0,2.8) | 1.9 (1.5,2.2) | 2.0 (1.3,2.8) |
| MRI | 4.7 (1.5,8.0) | 6.8 (5.4,8.3) | 8.1 (6.6,9.6) | 6.7 (4.7,8.6) |
|
| ||||
| Additional Cancer (Contralateral) | ||||
|
| ||||
| No MRI | 0.2 (0,0.5) | 0.2 (0.1,0.3) | 0.6 (0.4,0.8) | 0.4 (0,0.8) |
| MRI | 3.9 (1.5,6.3) | 2.5 (1.5,3.4) | 2.7 (1.9,3.5) | 1.4 (0.6,2.2) |
|
| ||||
| Mastectomy | ||||
|
| ||||
| No MRI | 26.9 (24.5,29.4) | 26.6 (25.4,27.9) | 33.7 (32.3,35.1) | 42.3 (38.9,45.7) |
| MRI | 39.7 (32.1,47.3) | 33.1 (29.9,36.3) | 41.7 (39.1,44.3) | 47.1 (42.7,51.5) |
|
| ||||
| Core Biopsy | ||||
|
| ||||
| No MRI | 2.5 (1.6,3.4) | 3.7 (3.2,4.3) | 4.8 (4.2,5.4) | 4.3 (3.1,5.6) |
| MRI | 16.8 (11.3,22.2) | 19.6 (16.9,22.3) | 22.3 (20.1,24.4) | 23.1 (19.5,26.7) |
|
| ||||
| Surgical Biopsy | ||||
|
| ||||
| No MRI | 0.3 (0,0.6) | 0.6 (0.4,0.8) | 0.4 (0.3,0.6) | 1.1 (0.3,2.0) |
| MRI | 0.2 (0,1.6) | 0.6 (0.1,1.0) | 0.6 (0.2,1.0) | 1.6 (0.3,6.8) |
Rates are from weighted logistic regression model adjusting by age and propensity score model weights that include registry, age, race/ethnicity, education, previous breast biopsy, breast density, menopausal status, index cancer type, mode of detection of index cancer and year of diagnosis.
In sub-analyses including only the women with additional biopsy (N=1,943), we calculated adjusted rate of additional cancer detection overall and by laterality. For women with additional biopsy(ies), the lowest additional cancer detection rates were among women with extremely dense breasts, regardless of imaging modality (with MRI: 35.0 (95% CI: 27.0–43.0); without MRI: 45.1 (95%CI: 32.6–57.5) (Table 4). For all density categories, women with MRI consistently had lower additional ipsilateral cancer rates than women without MRI who presumably had alternative breast imaging biopsy guidance. The trend was the opposite for contralateral cancers for women without dense breasts; MRI was associated with higher rates of contralateral cancers for the two lowest density categories (Table 4).
Table 4.
Adjusted rates*(per 100 women) for biopsy outcomes in relation to pre-operative imaging modality using inverse weighted probability regression methods among women who had an additional biopsy (N=1,943) stratified by BIRADS breast density categories.
| BIRADS Density | ||||
|---|---|---|---|---|
|
| ||||
| a: Almost entirely fatty | b: Scattered fibroglandular | c: Hetero-genously dense | d: Extremely dense | |
| Adjusted Rate (95% Confidence Interval) | ||||
|
| ||||
| Additional Cancer Detected | ||||
|
| ||||
| No MRI | 45.0 (30.1,59.8) | 53.7 (47.4,59.9) | 45.3 (39.4,51.3) | 45.1 (32.6,57.5) |
| MRI | 50.6 (32.8,68.4) | 46.3 (39,53.6) | 44.2 (39.1,49.3) | 35.0 (27.0,43.0) |
|
| ||||
| Additional Cancer (Ipsilateral) | ||||
|
| ||||
| No MRI | 42.0 (26.9,57.1) | 48.6 (42.3,54.9) | 34.8 (29.3,40.4) | 38.1 (26.1,50.0) |
| MRI | 28.2 (11.5,44.9) | 33.3 (26.8,39.8) | 34.3 (29.3,39.4) | 26.2 (19.1,33.4) |
|
| ||||
| Additional Cancer (Contralateral) | ||||
|
| ||||
| No MRI | 3.9 (1.2,12.2) | 4.6 (2.3,6.9) | 11.2 (7.1,15.4) | 6.9 (1,12.9) |
| MRI | 20.7 (8.1,33.3) | 11.6 (7.6,15.6) | 10.8 (7.6,13.9) | 5.3 (2.2,8.4) |
Rates are from weighted logistic regression model adjusting by age and propensity score model weights that include registry, age, race/ethnicity, education, previous breast biopsy, breast density, menopausal status, index cancer type, mode of detection of index cancer and year of diagnosis.
DISCUSSION
This study provides the largest, generalizable study to date of the effects of preoperative MRI on additional biopsies and additional cancer detection for women with breast cancer when explicitly considering extent of breast density. Overall, we found more than a 4-fold increase in additional breast biopsies in women who received preoperative MRI compared to women without MRI. Among individuals receiving preoperative MRI, those with dense breasts had twice the rates of additional biopsies compared to those with non-dense breasts. Finding additional lesions that lead to biopsy must be placed in the context of biopsy yield. Additional cancer detection was higher following preoperative MRI compared to without MRI, but density did not play a notable role; this was shown for both ipsilateral and contralateral biopsies. Thus, for women with heterogeneously or extremely dense breasts (categories c and d), preoperative MRI was associated with much higher biopsy rates, without concomitant higher cancer detection, compared to women with non-dense breasts.
Our study helps to address the clinical issue of whether breast density is an appropriate clinical factor to consider when determining who may benefit from preoperative MRI among individuals with newly diagnosed breast cancer. As in the recent study of preoperative MRI in 1,396 women from a regional breast imaging center,3 breast density did not result in significant benefit. Similarly, in two other single-institution studies, density was found to be unrelated to detecting occult cancer with preoperative MRI.21,22 Our findings mirror those results in a large national sample including all four BI-RADS breast density categories and evaluated biopsy type and yield. Thus, our results provide substantive evidence that MRI’s ability to detect additional occult cancer is not modified preferentially by breast density for any of the four density categories. However, density does impact likelihood of undergoing additional biopsies for women with preoperative MRI, with higher rates for women with dense breasts and thus higher benign (false negative) biopsy rates in women with dense breasts.
Among women with preoperative MRI, we found additional biopsy rates were twice as high for women with dense vs. non-dense breasts. Taking the two-fold higher biopsy rates together with similar rates of additional cancer detection across density categories, our analysis demonstrated a lower biopsy yield for women with dense breasts. This shift in the benefit-to-harm ratio may be salient to women’s workup and management choices. Systematic reviews have identified the diagnostic work-up period following an initial breast cancer diagnosis as one of heightened psychological distress for women, with short- and long-term implications for mental health, treatment decision-making, and future screening participation.23,24 Decreasing cancer worry and satisfaction with treatment decisions may be of particular concern to women with dense breasts and for those whose cancers were not identified or detected by mammography. However, since breast MRI has a high negative predictive value, women may feel reassured by a negative MRI examination, potentially reducing cancer worry, and unnecessary surgery. At the same time, undergoing additional biopsy delays treatment, which may produce anxiety for women, or may identify risk-associated epithelial hyperplasia that may inappropriately bias surgical treatment decisions.
Although our study was the largest to date in the U.S. to examine intermediate outcomes of preoperative MRI overall and by breast density categories, there were limitations. First, we were not able to quantify the exact sequences of additional imaging and biopsy within the preoperative window, so cannot definitively attribute an additional biopsy to the preoperative MRI. However, this presumption of a preoperative MRI contributing to an additional biopsy in that same preoperative window corresponds to typical clinical practice. Secondly, we were not able to report on the effect of MRI on additional cancer detection by breast density in conjunction with other clinical characteristics, such as histology and subtype due to small numbers. Further, we were not able to assess whether the cancer was upgraded based on additional biopsies. As with most prior studies, we did not have information on whether women and their doctors modified treatment plans following preoperative MRI findings and did not examine re-operation rates. Other outcomes should also be considered, such as high negative predictive value that may be useful for decisions regarding surgical management. Despite these limitations, the results when considered in conjunction with clinician and individual perspectives, could inform decisions for women newly diagnosed with breast cancer.
This study contributes new, generalizable evidence on preoperative breast MRI effectiveness compared to mammography alone by extent of breast density. Specifically, women with dense breasts are suspected to benefit through additional cancer yield from preoperative breast MRI similar to women without dense breasts; however, we observed they were more likely to experience additional biopsy without an increase in cancer detection. Thus, while preoperative MRI may be important for some women for additional cancer detection, selecting women based on breast density is not supported by evidence.
ACKNOWLEDGEMENTS
Research reported in this work was funded through a Patient-Centered Outcomes Research Institute (PCORI) Program Award (PCS-1504-30370). Data collection for this research was additionally supported by the Breast Cancer Surveillance Consortium with funding from the National Cancer Institute (P01CA154292, U54CA163303, R01CA149365) and the Agency for Health Research and Quality (R01 HS018366-01A1). The collection of cancer and vital status data used in this study was supported in part by several state public health departments and cancer registries throughout the U.S. For a full description of these sources, please see: https://www.bcsc-research.org/about/work-acknowledgement. All statements in this report, including its findings and conclusions, are solely those of the authors and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute (PCORI), its Board of Governors or Methodology Committee, nor those of the National Cancer Institute or the National Institutes of Health. We thank the participating women, mammography facilities, and radiologists for the data they have provided for this study. You can learn more about the BCSC at: http://www.bcsc-research.org/.
The collection of cancer incidence and vital status data used in this study was supported, in part, by:
• The California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute’s Surveillance, Epidemiology and End Results Program under contract HHSN261201000140C awarded to the Cancer Prevention Institute of California, contract HHSN261201000035C awarded to the University of Southern California, and contract HHSN261201000034C awarded to the Public Health Institute; and the Centers for Disease Control and Prevention’s National Program of Cancer Registries, under agreement U58-DP003862-01 awarded to the California Department of Public Health;
• The Vermont Cancer Registry, supported in part by Cooperative Agreement NU58DP006322 from the Centers for Disease Control and Prevention, awarded to the Vermont State Agency of Human Services.
• The Cancer Surveillance System of the Fred Hutchinson Cancer Research Center, which is funded by contracts N01-CN-005230, N01-CN-67009, N01-PC-35142, HHSN261201000029C, and HHSN261201300012I from the Surveillance, Epidemiology and End Results (SEER) Program of the National Cancer Institute with additional support from the Fred Hutchinson Cancer Research Center and the State of Washington;
• The New Hampshire State Cancer Registry supported in part by cooperative agreement U55/CCU-121912 awarded to the New Hampshire Department of Health and Human Services, Division of Public Health Services, Bureau of Disease Control and Health Statistics, Health Statistics and Data Management Section;
• The North Carolina Central Cancer Registry, which is partially supported by the Centers for Disease Control and Prevention under cooperative agreement DP12-120503CONT14;
• Manuscripts including data from the Metro Chicago Breast Cancer Registry were supported in part by the Illinois Department of Public Health, Illinois State Cancer Registry which is partially supported by the Centers for Disease Control and Prevention under cooperative agreement DP12-120504CONT15.
The ideas and opinions expressed herein are those of the authors and endorsement by the State of California, the California Department of Public Health; Illinois Department of Public Health; New Hampshire Department of Health and Human Services; the National Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors is not intended nor should be inferred.
We thank the participating women, mammography facilities, and radiologists for the data they have provided for this study.
Funding:
Primary funding source: PCORI CS-1504-30370
Availability of data and material:
Publicly-available data and instructions for requesting additional data are available at: Breast Cancer Surveillance Consortium (BCSC) Data :: BCSC (bcsc-research.org)
APPENDIX
Appendix
Appendix Table 1.a.
Study population characteristics for women with breast cancer (13,869) without preoperative MRI1,2 overall and by breast density from 2005–2017.
| a: Almost entirely fatty | b: Scattered fibroglandular | c: Heterogeneously dense | d: Extremely dense | |||||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | |
| Total N = 13,869 | 1442 | 10.4 | 5996 | 43.2 | 5347 | 38.6 | 1084 | 7.8 |
| Age At Exam | ||||||||
| <50 | 54 | 3.8 | 555 | 9.2 | 1401 | 26.2 | 519 | 47.9 |
| 50–59 | 182 | 12.6 | 1467 | 24.5 | 1437 | 26.9 | 315 | 29.1 |
| 60–69 | 541 | 37.5 | 1879 | 31.3 | 1362 | 25.5 | 148 | 13.7 |
| 70–79 | 440 | 30.5 | 1445 | 24.1 | 810 | 15.2 | 76 | 7.0 |
| 80–89 | 225 | 15.6 | 650 | 10.8 | 337 | 6.3 | 26 | 2.4 |
| Race (NCI Reporting Standard) | ||||||||
| White, non Hispanic | 1112 | 78.6 | 4597 | 77.9 | 3914 | 74.6 | 743 | 69.9 |
| Black, non Hispanic | 164 | 11.6 | 639 | 10.8 | 530 | 10.1 | 49 | 4.6 |
| Asian | 63 | 4.5 | 423 | 7.2 | 554 | 10.6 | 220 | 20.7 |
| Non-Hispanic Pacific Islander, Alaska Native, American Indian | 10 | 0.7 | 24 | 0.4 | 29 | 0.6 | 5 | 0.5 |
| Hispanic | 66 | 4.7 | 221 | 3.7 | 217 | 4.1 | 46 | 4.3 |
| Other, Mixed, Unknown | 27 | 1.9 | 92 | 1.5 | 103 | 1.9 | 21 | 1.9 |
| Family History of Breast Cancer | ||||||||
| No | 1053 | 77.1 | 4273 | 76.2 | 3869 | 76.9 | 779 | 75.5 |
| Yes | 313 | 22.9 | 1332 | 23.8 | 1164 | 23.1 | 253 | 24.5 |
| Unknown | 76 | 5.3 | 391 | 6.5 | 314 | 5.9 | 52 | 4.8 |
| Previous biopsy/aspiration | ||||||||
| None | 1002 | 74.0 | 3683 | 66.1 | 3169 | 64.5 | 609 | 61.3 |
| Biopsy Only | 240 | 17.7 | 1238 | 22.2 | 1151 | 23.4 | 227 | 22.8 |
| Aspiration Only | 37 | 2.7 | 264 | 4.7 | 248 | 5.0 | 66 | 6.6 |
| Biopsy and Aspiration | 75 | 5.5 | 391 | 7.0 | 346 | 7.0 | 92 | 9.3 |
| Unknown | 88 | 6.1 | 420 | 7.0 | 433 | 8.1 | 90 | 8.3 |
| Menopause | ||||||||
| Pre | 60 | 4.4 | 745 | 13.8 | 1490 | 32.2 | 552 | 59.3 |
| Post | 1302 | 95.6 | 4645 | 86.2 | 3134 | 67.8 | 379 | 40.7 |
| Unknown | 80 | 5.6 | 606 | 10.1 | 723 | 13.5 | 153 | 14.1 |
| Current HRT Use | ||||||||
| No | 980 | 97.5 | 3762 | 95.3 | 3590 | 95.0 | 814 | 95.7 |
| Yes | 25 | 2.5 | 185 | 4.7 | 189 | 5.0 | 37 | 4.3 |
| Unknown | 437 | 30.3 | 2049 | 34.2 | 1568 | 29.3 | 233 | 21.5 |
| Laterality | ||||||||
| Left | 707 | 49.0 | 2912 | 48.6 | 2611 | 48.8 | 540 | 49.8 |
| Right | 722 | 50.1 | 3004 | 50.1 | 2676 | 50.1 | 534 | 49.3 |
| Both | 13 | 0.9 | 80 | 1.3 | 60 | 1.1 | 10 | 0.9 |
| Index biopsy year | ||||||||
| 2005–2008 | 423 | 29.3 | 1829 | 30.5 | 1906 | 35.7 | 374 | 34.5 |
| 2009–2011 | 372 | 25.8 | 1399 | 23.3 | 1331 | 24.9 | 293 | 27.0 |
| 2012–2014 | 380 | 26.3 | 1609 | 26.8 | 1213 | 22.7 | 245 | 22.6 |
| 2015–2017 | 267 | 18.5 | 1159 | 19.3 | 897 | 16.8 | 172 | 15.9 |
| Geo coding education | ||||||||
| <=Q1 | 404 | 29.9 | 1452 | 26.6 | 1241 | 25.3 | 227 | 22.1 |
| Q1 – Q2 | 392 | 29.1 | 1551 | 28.5 | 1301 | 26.5 | 271 | 26.4 |
| Q2 – Q3 | 291 | 21.6 | 1429 | 26.2 | 1305 | 26.6 | 271 | 26.4 |
| > Q3 | 262 | 19.4 | 1017 | 18.7 | 1058 | 21.6 | 256 | 25.0 |
| Unknown | 93 | 6.5 | 547 | 9.1 | 442 | 8.3 | 59 | 5.4 |
| Index biopsy2 | ||||||||
| DCIS | 219 | 15.2 | 1311 | 21.9 | 1267 | 23.7 | 306 | 28.2 |
| Invasive3 | 1223 | 84.8 | 4685 | 78.1 | 4080 | 76.3 | 778 | 71.8 |
| Mode of Detection4 | ||||||||
| Screen Detected | 851 | 60.3 | 4151 | 70.3 | 3248 | 61.9 | 504 | 48.1 |
| Interval Detected | 213 | 15.1 | 1000 | 16.9 | 1216 | 23.2 | 330 | 31.5 |
| Clinically Detected | 348 | 24.6 | 754 | 12.8 | 787 | 15.0 | 213 | 20.3 |
| Unknown Mode | 30 | 2.1 | 91 | 1.5 | 96 | 1.8 | 37 | 3.4 |
| Histology Type | ||||||||
| DCIS | 219 | 18.6 | 1311 | 28.3 | 1267 | 30.4 | 306 | 37.5 |
| Invasive, Ductal | 847 | 72.1 | 2934 | 63.4 | 2516 | 60.4 | 444 | 54.4 |
| Invasive, Lobular | 89 | 7.6 | 302 | 6.5 | 297 | 7.1 | 45 | 5.5 |
| Invasive, Ductal & Lobular | 20 | 1.7 | 83 | 1.8 | 86 | 2.1 | 21 | 2.6 |
| Unknown | 267 | 18.5 | 1366 | 22.8 | 1181 | 22.1 | 268 | 24.7 |
| Surgery type | ||||||||
| Lumpectomy | 1092 | 75.7 | 4510 | 75.2 | 3563 | 66.6 | 622 | 57.4 |
| Mastectomy | 350 | 24.3 | 1486 | 24.8 | 1784 | 33.4 | 462 | 42.6 |
| All | 1442 | 5996 | 5347 | 1084 | ||||
Appendix Table 1.b.
Study population characteristics for women with breast cancer (13,869) with preoperative MRI1,2 overall and by breast density from 2005–2017.
| a: Almost entirely fatty | b: Scattered fibroglandular | c: Heterogeneously dense | d: Extremely dense | |||||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | |
| Total N = 5,455 | 284 | 5.2 | 1745 | 32.0 | 2622 | 48.1 | 804 | 14.7 |
| Age At Exam | ||||||||
| <50 | 14 | 4.9 | 227 | 13.0 | 847 | 32.3 | 433 | 53.9 |
| 50–59 | 34 | 12.0 | 566 | 32.4 | 775 | 29.6 | 224 | 27.9 |
| 60–69 | 147 | 51.8 | 589 | 33.8 | 635 | 24.2 | 101 | 12.6 |
| 70–79 | 75 | 26.4 | 297 | 17.0 | 302 | 11.5 | 40 | 5.0 |
| 80–89 | 14 | 4.9 | 66 | 3.8 | 63 | 2.4 | 6 | 0.8 |
| Race (NCI Reporting Standard) | ||||||||
| White, non Hispanic | 219 | 78.5 | 1313 | 76.1 | 1949 | 75.7 | 576 | 72.6 |
| Black, non Hispanic | 41 | 14.7 | 239 | 13.8 | 264 | 10.2 | 50 | 6.3 |
| Asian | 6 | 2.2 | 77 | 4.5 | 213 | 8.3 | 108 | 13.6 |
| Non-Hispanic Pacific Islander, Alaska Native, American Indian | . | . | 11 | 0.6 | 14 | 0.5 | 2 | 0.3 |
| Hispanic | 13 | 4.7 | 86 | 5.0 | 136 | 5.3 | 57 | 7.2 |
| Other, Mixed, Unknown | 5 | 1.8 | 19 | 1.1 | 46 | 1.8 | 11 | 1.4 |
| Family History of Breast Cancer | ||||||||
| No | 207 | 76.7 | 1225 | 73.9 | 1878 | 76.4 | 580 | 77.7 |
| Yes | 63 | 23.3 | 433 | 26.1 | 580 | 23.6 | 166 | 22.3 |
| Unknown | 14 | 4.9 | 87 | 5.0 | 164 | 6.3 | 58 | 7.2 |
| Previous biopsy/aspiration | ||||||||
| None | 181 | 72.4 | 948 | 63.3 | 1386 | 60.3 | 410 | 60.1 |
| Biopsy Only | 62 | 24.8 | 413 | 27.6 | 659 | 28.7 | 186 | 27.3 |
| Aspiration Only | 4 | 1.6 | 53 | 3.5 | 124 | 5.4 | 43 | 6.3 |
| Biopsy and Aspiration | 3 | 1.2 | 83 | 5.5 | 130 | 5.7 | 43 | 6.3 |
| Unknown | 34 | 12.0 | 248 | 14.2 | 323 | 12.3 | 122 | 15.2 |
| Menopause | ||||||||
| Pre | 17 | 6.4 | 291 | 19.3 | 914 | 40.5 | 438 | 65.1 |
| Post | 250 | 93.6 | 1219 | 80.7 | 1343 | 59.5 | 235 | 34.9 |
| Unknown | 17 | 6.0 | 235 | 13.5 | 365 | 13.9 | 131 | 16.3 |
| Current HRT Use | ||||||||
| No | 197 | 99.0 | 1043 | 96.0 | 1727 | 95.9 | 599 | 96.6 |
| Yes | 2 | 1.0 | 44 | 4.0 | 74 | 4.1 | 21 | 3.4 |
| Unknown | 85 | 29.9 | 658 | 37.7 | 821 | 31.3 | 184 | 22.9 |
| Laterality | ||||||||
| Left | 135 | 47.5 | 858 | 49.2 | 1278 | 48.7 | 412 | 51.2 |
| Right | 144 | 50.7 | 869 | 49.8 | 1323 | 50.5 | 388 | 48.3 |
| Both | 5 | 1.8 | 18 | 1.0 | 21 | 0.8 | 4 | 0.5 |
| Index biopsy year | ||||||||
| 2005–2008 | 23 | 8.1 | 177 | 10.1 | 317 | 12.1 | 74 | 9.2 |
| 2009–2011 | 66 | 23.2 | 473 | 27.1 | 658 | 25.1 | 228 | 28.4 |
| 2012–2014 | 104 | 36.6 | 587 | 33.6 | 844 | 32.2 | 277 | 34.5 |
| 2015–2017 | 91 | 32.0 | 508 | 29.1 | 803 | 30.6 | 225 | 28.0 |
| Geo coding education | ||||||||
| <=Q1 | 93 | 35.0 | 437 | 27.6 | 584 | 24.4 | 174 | 23.5 |
| Q1 – Q2 | 69 | 25.9 | 390 | 24.7 | 583 | 24.3 | 186 | 25.2 |
| Q2 – Q3 | 49 | 18.4 | 445 | 28.1 | 709 | 29.6 | 190 | 25.7 |
| > Q3 | 55 | 20.7 | 309 | 19.5 | 522 | 21.8 | 189 | 25.6 |
| Unknown | 18 | 6.3 | 164 | 9.4 | 224 | 8.5 | 65 | 8.1 |
| Index biopsy2 | ||||||||
| DCIS | 38 | 13.4 | 317 | 18.2 | 516 | 19.7 | 178 | 22.1 |
| Invasive3 | 246 | 86.6 | 1428 | 81.8 | 2106 | 80.3 | 626 | 77.9 |
| Mode of Detection4 | ||||||||
| Screen Detected | 141 | 51.3 | 1197 | 69.5 | 1512 | 58.5 | 328 | 41.5 |
| Interval Detected | 58 | 21.1 | 291 | 16.9 | 687 | 26.6 | 267 | 33.8 |
| Clinically Detected | 76 | 27.6 | 235 | 13.6 | 385 | 14.9 | 196 | 24.8 |
| Unknown Mode | 9 | 3.2 | 22 | 1.3 | 38 | 1.5 | 13 | 1.6 |
| Histology Type | ||||||||
| DCIS | 38 | 14.3 | 317 | 22.0 | 516 | 24.3 | 178 | 28.5 |
| Invasive, Ductal | 195 | 73.3 | 935 | 64.8 | 1299 | 61.2 | 384 | 61.4 |
| Invasive, Lobular | 29 | 10.9 | 168 | 11.6 | 269 | 12.7 | 55 | 8.8 |
| Invasive, Ductal & Lobular | 4 | 1.5 | 23 | 1.6 | 38 | 1.8 | 8 | 1.3 |
| Unknown | 18 | 6.3 | 302 | 17.3 | 500 | 19.1 | 179 | 22.3 |
| Surgery type | ||||||||
| Lumpectomy | 180 | 63.4 | 1190 | 68.2 | 1485 | 56.6 | 395 | 49.1 |
| Mastectomy | 104 | 36.6 | 555 | 31.8 | 1137 | 43.4 | 409 | 50.9 |
| All | 284 | 1745 | 2622 | 804 | ||||
Appendix Table 2.
Adjusted rates*(per 100 women) of for biopsy type in relation to pre-operative imaging modality using inverse weighted probability regression methods among women who had an additional biopsy (N=1,943) stratified by BIRADS breast density categories.
| BIRADS Density | ||||
|---|---|---|---|---|
|
| ||||
| Outcome | a: Almost entirely fatty | b: Scattered fibroglandular | c: Hetero-genously dense | d: Extremely dense |
| Additional | Adjusted Rate (95% Confidence Interval) | |||
|
| ||||
| Core Biopsy | ||||
|
| ||||
| No MRI | 75.7 (62.6,88.9) | 75.6 (70.3,80.9) | 88.5 (84.9,92.1) | 82.5 (73.7,91.4) |
| MRI | 100 | 95.4 (92.7,98.0) | 94.2 (91.8,96.5) | 90.1 (81.8,98.4) |
|
| ||||
| Surgical Biopsy | ||||
|
| ||||
| No MRI | 9.6 (1.1,18.1) | 12.2 (8.4,16) | 7.9 (4.8,10.9) | 19.7 (8.1,31.4) |
| MRI | 1.3 (0.2,8.7) | 2.6 (0.3,4.9) | 2.4 (0.8,4.0) | 5.9 (1.3,23.9) |
Rates are from weighted logistic regression model adjusting by age and propensity score model weights that include registry, age, race/ethnicity, education, previous breast biopsy, breast density, menopausal status, index cancer type, mode of detection of index cancer and year of diagnosis.
Footnotes
The National Cancer Institute had no role in the study’s design; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication. Likewise, the content in this manuscript is solely the responsibility of the authors and does not necessarily represent the views of PCORI, its Board of Governors or Methodology Committee.
Reproducible Research Statement
Study protocol and statistical code: Available on request, please contact kpwa.scc@kp.org with specific queries. Data set: Available after study aims of funded grants are addressed and with appropriate contracts.
ClinicalTrials.gov: NCT02980848; registered 2017
DECLARATIONS
Conflicts of interest/Competing interests Authors report no conflicts of interest or competing interests
Code availability: Not applicable
Ethics approval and Consent to Participate
The institutional review boards of the participating Breast Cancer Surveillance Consortium (BCSC) registries and Statistical Coordinating Center approved all study activities through passive consent (three registries) or waiver of written consent (two registries and the Statistical Coordinating Center). This study was Health Insurance Portability and Accountability Act compliant. Registries and the Statistical Coordinating Center received a federal Certificate of Confidentiality and other protections for the identities of women, physicians, and facilities. Our study was registered on ClinicalTrials.gov (NCT02980848) and followed the Good Research Practices guidelines for comparative effectiveness research.
Consent for publication All authors consent to publication of this manuscript
REFERENCES
- 1.Martin LJ, Boyd NF. Mammographic density. Potential mechanisms of breast cancer risk associated with mammographic density: hypotheses based on epidemiological evidence. Breast cancer research : BCR. 2008;10(1):201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hwang ES, Miglioretti DL, Ballard-Barbash R, Weaver DL, Kerlikowske K, National Cancer Institute Breast Cancer Surveillance C. Association between breast density and subsequent breast cancer following treatment for ductal carcinoma in situ. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2007;16(12):2587–93. Epub 2007/12/19. [DOI] [PubMed] [Google Scholar]
- 3.Rahbar H, Hippe DS, Alaa A, Cheeney SH, van der Schaar M, Partridge SC, Lee CI. The value of patient and tumor factors in predicting preoperative breast MRI outcomes. Radiology: Imaging Cancer. 2020:2(4):e1900999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elmi A, Conant EF, Kozlov A, Young AJ, Long Q, Doot RK, McDonald ES. Preoperative breast MR imaging in newly diagnosed breast cancer: Comparison of outcomes based on mammographic modality, breast density and breast parenchymal enhancement. Clin Imaging. 2020. Oct 13;70:18–24. doi: 10.1016/j.clinimag.2020.10.021. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. https://www.nejm.org/doi/full/10.1056/nejmoa065447 .
- 6.Liberman L. Breast MR imaging in assessing extent of disease. Magnetic resonance imaging clinics of North America. 2006;14(3):339–49, vi. [DOI] [PubMed] [Google Scholar]
- 7.Houssami N, Ciatto S, Macaskill P, Lord SJ, Warren RM, Dixon JM, Irwig L. Accuracy and surgical impact of magnetic resonance imaging in breast cancer staging: systematic review and meta-analysis in detection of multifocal and multicentric cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26(19):3248–58. Epub 2008/05/14. [DOI] [PubMed] [Google Scholar]
- 8.Brennan ME, Houssami N, Lord S, Macaskill P, Irwig L, Dixon JM, Warren RM, Ciatto S. Magnetic resonance imaging screening of the contralateral breast in women with newly diagnosed breast cancer: systematic review and meta-analysis of incremental cancer detection and impact on surgical management. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27(33):5640–9. Epub 2009/10/07. [DOI] [PubMed] [Google Scholar]
- 9.Anaout A, Catley C, Booth CM, McInnes M, Graham I, Kumar V, Simos D, Van Walraven C, Clemons M. Use of preoperative magnetic resonance imaging for breast cancer. A Canadian population-based study. JAMA Oncology. 2015;1(9):1238–1250. [DOI] [PubMed] [Google Scholar]
- 10.Deurloo EE, Peterse JL, Rutgers EJT, Besnard APE, Muller SH, Gilhuijs KA. Additional breast lesions in patients eligible for breast-conserving therapy by MRI: impact of preoperative management and potential benefit of computerised analysis. European Journal of Cancer. 2005;41(10):1393–1401. [DOI] [PubMed] [Google Scholar]
- 11.DeMartini W, Lehman C. A review of current evidence-based clinical applications for breast magnetic resonance imaging. Topics in magnetic resonance imaging. 2008;19(3):143–50. [DOI] [PubMed] [Google Scholar]
- 12.Debald M, Abramian A, Nemes L, et al. : Who may benefit from preoperative breast MRI? A single-center analysis of 1102 consecutive patients with primary breast cancer. Breast Cancer Research and Treatment 2015;153:531–537. [DOI] [PubMed] [Google Scholar]
- 13.Gutierrez RL, DeMartini WB, Silbergeld JJ, Eby PR, Peacock S, Javid SH, Lehman CD. High cancer yield and positive predictive value: outcomes at a center routinely using preoperative breast MRI for staging. Am J Roent. 2010;196: W93–W99. [DOI] [PubMed] [Google Scholar]
- 14.National Comprehensive Cancer Network. NCCN Guidelines for Breast Cancer (Version 2.2015). 2015. Contract No.: October 15, 2014. [Google Scholar]
- 15.Houssami N, Hayes DF. Review of preoperative magnetic resonance imaging (MRI) in breast cancer: should MRI be performed on all women with newly diagnosed, early stage breast cancer? CA: a cancer journal for clinicians. 2009;59(5):290–302. Epub 2009/08/15. [DOI] [PubMed] [Google Scholar]
- 16.Breast Cancer Surveillance Consortium (BCSC). https://bcsc-research.org/ Last accessed 11/10/20.
- 17.National Institute for Health Research. Good Research Practice guidelines. https://www.nihr.ac.uk/health-and-care-professionals/learning-and-support/good-clinical-practice.htm Last accessed 11/10/20.
- 18.American College of Radiology. Breast Imaging Reporting and Data System® (BI-RADS®) Reston, VA: American College of Radiology; 2013. [Google Scholar]
- 19.Breast Cancer Surveillance Consortium. https://www.bcsc-research.org/application/files/4516/0096/5722/BCSC_Data_Definitions_v3__2020.09.23.pdf. Last accessed: 12/12/20
- 20.Oehlert GW: A note on the delta method. American Statistician. 1992, 46: 27–29. [Google Scholar]
- 21.Lehman CD, Gatsonis C, Kuhl CK, Hendrick RE, Pisano ED, Hanna L, Peacock S, Smazal SF, Maki DD, Julian TB, DePeri ER, Bluemke DA, Schnall MD. ACRIN Trial 6667 Investigators Group. MRI evaluation of the contralateral breast in women with recently diagnosed breast cancer. New England J Medicine. 2007;356(13):1295–303. [DOI] [PubMed] [Google Scholar]
- 22.Gutierrez RL, DeMartini WB, Silbergeld JJ, Eby PR, Peacock S, Javid SH, Lehman CD. High cancer yield and positive predictive value: outcomes at a center routinely using preoperative breast MRI for staging. Am J Roent. 2010;196: W93–W99. [DOI] [PubMed] [Google Scholar]
- 23.Montgomery M, McCrone SH. Psychological distress associated with the diagnostic phase for suspected breast cancer: systematic review. J Adv Nurs. 2010;66(11):2372–90. [DOI] [PubMed] [Google Scholar]
- 24.Bond M, Pavey T, Welch K, Cooper C, Garside R, Dean S, Hyde C. Systematic review of the psychological consequences of false-positive screening mammograms. Health Technol Assess. 2013;17(13):1–170, v-v. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Publicly-available data and instructions for requesting additional data are available at: Breast Cancer Surveillance Consortium (BCSC) Data :: BCSC (bcsc-research.org)