Abstract
Objective:
We tested the hypothesis that treatment with the GLP-1/glucagon receptor agonist SAR425899 would lead to a smaller decrease in sleeping metabolic rate (SMR; kcal/day) than expected from the loss of lean and fat mass (metabolic adaptation).
Methods:
This Phase 1b, double-blind, randomized, placebo-controlled study was conducted at two centers in inpatient metabolic wards. Thirty-five healthy males and females with overweight and obesity (36.5±7.1 years) were randomized to a calorie-reduced diet (−1000 kcal/day) and escalating doses (0.06–0.2 mg/day) of SAR425899 (N=17) or placebo (N=18) for 19 days. SMR was measured by whole-room calorimetry.
Results:
Both groups lost weight (−3.68±1.37 kg placebo; −4.83±1.44 kg SAR425899). Those treated with SAR425899 lost more weight, fat mass, and fat free mass (p<0.05) due to a greater achieved energy deficit than planned. The SAR425899 group had a smaller reduction in body composition-adjusted SMR (p=0.002) as compared to placebo, but not 24-hour energy expenditure. Fat oxidation and ketogenesis increased in both groups, with significantly greater increases with SAR425899 (p<0.05).
Conclusions:
SAR425899 led to reduced selective metabolic adaptation and increased lipid oxidation which are believed to be beneficial for weight loss and weight loss maintenance.
Keywords: Metabolic adaptation, fat oxidation, glucagon-like peptide-1/glucagon receptor dual agonist, weight loss
Introduction
The incretin hormones glucagon-like peptide-1 (GLP-1) and glucagon have shown promise as weight loss therapies. GLP-1 regulates nutrient metabolism and decreases food intake. Significant weight loss has been observed with administration of the long acting GLP-1 receptor (GLP-1R) agonists liraglutide (1) and semaglutide (2), primarily by affecting satiety and food intake. Glucagon is another satiety hormone that also regulates nutrient metabolism (3). Glucagon receptor (GCGR) activation seems to promote weight loss via increased energy expenditure (EE) in preclinical models while the impact in humans is unclear (4–6). Glucagon’s action through the GCGR to promote weight loss may be though the inhibition of appetite or reduced food intake (7). Given the actions of GLP-1 and glucagon on energy intake and expenditure, dual agonists of GLP-1R and GCGR have been shown to produce weight loss while avoiding the hyperglycemic effects of pure glucagon agonism (6).
Loss of body energy stores is primarily driven by: a reduction of energy intake (EI) (8,9) and/or an increase in EE, and/or a reduction EE that is smaller than expected based on changes in body composition (reduced metabolic adaptation) (8–11). It is now recommended that next generation weight loss agents should target not only weight loss, but also a decrease in metabolic adaptation and an increase in fat oxidation thus preventing the loss of fat free mass (FFM) (12). The contribution of these metabolic parameters to the weight loss induced by the dual activation of GLP-1R and GCGR is unknown.
We therefore evaluated effects of a novel dual GLP-1R/GCGR agonist with higher potency for the GLP1-R, SAR425899, on energy metabolism such as EE and fat oxidation (13,14). This short duration study implemented the appropriate experimental paradigm to quantify the effect of SAR425899 on 24-hour sleep metabolic rate (SMR normalized to 24-hours) and substrate oxidation following multiple escalating subcutaneous doses over 19 days. We achieved this by using caloric restriction with the goal similar changes in weight and body composition in the two groups so that we could adequately evaluate metabolic adaptation. To address metabolic adaptation, we elected to use SMR because it has the lowest interindividual variability since it is not affected by factors such as physical activity, stress, and food consumption (15,16). We hypothesized that a program of caloric restriction plus SAR425899, compared to caloric restriction alone, would attenuate of the expected fall in SMR (reduced metabolic adaptation) and stimulate fat oxidation.
Methods
The details of the study design were previously reported. Below, we briefly describe these components and provide details for aspects of the study not reported elsewhere (17).
Design:
This was a Phase Ib, two-center, double-blind, randomized, placebo-controlled study that enrolled the first participant 4/17/2018 and completed the last participant on 12/14/2018. Treatment duration was 19 days of SAR425899 with once daily dosing in the morning. Dose escalations occurred after 4, 8 and 12 days of treatment after a 7-day run-in period (Figure S1). This trial (NCT03376802) was approved by the Institutional Review Boards of the two study sites: AdventHealth and Pennington Biomedical Research Center. All potential participants provided written informed consent.
Participants:
Volunteers were generally healthy, weight stable, males and females, BMI 28–40 kg/m2 and 18–50 years of age. Supplementary Appendix 1 provides inclusion and exclusion criteria. Thirty-five participants were randomized. Eighteen participants were randomly allocated to placebo and 17 to SAR425899. Study treatment discontinuation due to adverse events (AEs) or personal reasons occurred for 7 participants. This left 17 in the placebo group and 11 in the SAR425899 group for the pharmacodynamic assessments presented herein (CONSORT Diagram; Figure S2). Demographic, anthropometric, and site enrollment was similar between study completers and the people who were randomized but did not complete the study (Table S1)
Randomization and Dosing:
Participants were randomized 1:1 centrally by the sponsor to the treatment groups (SAR425899/placebo) using site and sex as stratification factors. The following SAR425899 doses were administered subcutaneously: 0.06 mg from Day 1–4, 0.12 mg from Day 5–8, 0.16 mg from Day 9–12, and 0.2 mg from Day 13–19. A placebo solution was administered at equivalent volumes. The pen-type injector (TactiPen) was provided for each participant separately from the cartridge kits. A double-blind design was implemented with cartridges (SAR425899 or placebo) and pen devices being indistinguishable. Table S2 provides pharmacokinetic data (secondary endpoint).
Safety and Tolerability:
To address our secondary objectives of safety and tolerability, adverse events were monitored throughout the study based on prior studies (18,19) via medical history, vital signs, physical examination, and standard laboratory assessments (hematology, biochemistry, coagulation, urinalysis). Safety assessments are detailed in Supplementary Appendix 2.
Nutrition Intervention:
Caloric restriction of the placebo group was necessary due to the confounding effects of metabolic adaptation, which is defined as a decrease in EE greater than predicted by the change in weight and body composition (20–23). To ensure the achievement of a 1000-kcal deficit and similar weight loss, participants were housed in a metabolic ward during the treatment period (13).
Participants consumed a weight-maintaining outpatient diet until randomization (17). After baseline measures and randomization, both groups were placed a 1000-kcal deficit per day (17). Such a caloric deficit was a priori estimated to result in a reduction of body weight of ~3 kg, fat mass of ~1.6 kg and a decrease in 24-hour EE of ~350 kcal/day within 3 weeks of treatment in both arms. This amount of weight loss is similar to what has been observed in other studies with SAR425899 (13). A fixed calorie deficit was selected over other methods (fixed body weight reduction or % calorie deficit) based on data from a validated mathematical model of human metabolism (24,25), suggesting more consistent change of body weight, FFM and FM in people with disparate body weight. The diet consisted of 15% protein, 30% fat, and 55% carbohydrates. 100% diet consumption was required. Overall adherence (kcals presented versus weighed back unconsumed food) was 94% (Table S3). Table S4 shows a list of allowed and disallowed beverages and seasonings. A sample menu is provided in Table S5 with extended methods in Supplementary Appendix 2.
Energy Requirements:
Run-In Period:
Energy needs for the outpatient days (Days -7 through -4) were estimated using the following equation (rounded to the nearest 100 kcals) derived from doubly labeled water studies: 24-hour energy expenditure (24-hour EE; kcal/day)=1279 + 18.3 (weight, kg) + 2.3 (age, y) - 338 (sex: 1=female, 0=male) (17,26). Energy requirements for the first calorimetry day (Day -3) were estimated using the following equation derived from calorimeter studies: 24EE (kcal/day)=26.2([LM(kg)]) + 5.2([FM(kg)]) − 2.32([age(y)]) − 96(African American) + 546 (27). Within-day adjustments, if needed, were made based on software predictions that were input into published calorimetry equations (27). Between-day adjustments were based on the prior day.
Treatment Period:
Energy needs for the treatment period (Days 1–19) were estimated using the following equation, assuming a 20% increased EE on the metabolic ward compared to that in the calorimeters: EI=[EE in calorimeter (average Days -3,-2,-1) × 1.2 ] – 1000kcal (17).
Whole-body Room Indirect Calorimetry:
Standards for calorimetry operations utilized in our study are described in Chen et al. (16) and calorimetry methods described in Allerton et al. (17). Briefly, 24-hour EE and related components [sleeping metabolic rate (SMR)- primary endpoint; secondary endpoints- resting metabolic rate (RMR); basal metabolic rate (BMR); thermic effect of food (TEF)], and substrate oxidation [respiratory exchange ratio (RER); during sleep (RERsleep); over a period of 24-hours (RER24-hour); during rest (RERrest), basal (RERbasal); oxidation rates (protein, carbohydrate and fat)] were measured by indirect calorimetry on 3 consecutive days during the run-in period (Days -3, -2 and -1: baseline) and at the end of the treatment period (Days 17, 18 and 19: post-treatment). Calorimeters at both sites were previously cross-validated and the same calorimeter was used for each participant at baseline and post-treatment (28). Participants followed the activity schedule shown in Table S6. Supplementary Appendix 2 includes descriptions of all components of EE and substrate oxidation measured. 24-hour urine was collected, urine volume and collection duration were determined, and a sample analyzed for nitrogen and ketone bodies.
Physical Activity:
Physical activity level (PAL) while in the calorimeter was calculated as follows: PAL=24-hour EE/RMR.
Body Composition:
Dual-energy X-ray absorptiometry scanning (Lunar iDXA; GE Healthcare) was carried out in the fasted state according to standardized procedures (secondary endpoint).
Clinical Labs and Biomarkers:
The following pharmacodynamic circulating biomarkers were measured (secondary endpoints): fasting plasma glucose, hemoglobin A1c, lipid biomarkers (free fatty acids, triglycerides, total cholesterol, HDL/LDL-cholesterol); ketone bodies (blood and urine); and leptin.
Statistical Analyses:
Sample size assessment was based on the primary endpoint of the study to compare the change from baseline to end of treatment in SMR (kcal/day) between SAR425899 and placebo. The calculation was performed for a one-sided test with Type 1 error level alpha=0.05. A value of approximately 50 kcal/day was expected for the standard deviation (SD) for change from baseline in SMR. For power calculation, SD between 45 and 55 kcal/day and effect sizes between 36 and 84 kcal/day were used based on in-silico trial simulations using existing validated models (24,29,30) using SAR425899 phase 1 trial data as input (13). With 12 evaluable participants per treatment arm, if the true SD was as much as 50 kcal/day and the effect size 60 kcal/day, the power was expected to be 88.5%.
Multivariate analysis of variance (MANOVA) of repeated measures was used to compare differences between calorimetry days at baseline with a group interaction factor for EE components, substrate oxidation rates including RER variables and EI. The between-day coefficient of variation (CV) was calculated for each individual variable: (N=number of participants; K, number of days measured (3); M, measured value each day) and relative CV (%CV, (CV/mean)) (31).
The primary analysis of SMR change from baseline was based on a linear mixed model with treatment and stratification factors (site and gender) as class effects, SMR at baseline and change in body weight as covariates, subject as random effect and study day as a repeating factor. Treatment difference for change from baseline was based on corresponding contrasts and reported with point estimate, two-sided 90% confidence interval (CI), and corresponding one-sided p-value. Similar analyses were conducted for the rest of EE components and substrate oxidation variables. Changes in weight and body composition from baseline were calculated and adjusted to baseline values. Percent differences were calculated for all components of EE and independent sample t-tests were performed to compare differences between groups.
Metabolic adaptation was also evaluated by generating regression equations to predict EE from baseline body composition variables (LM and FM) and used using equations to predict baseline and post treatment 24-hour EE and SMR. Metabolic adaptation was calculated with the equation: [(Estimated EEpost-treatment−Estimated EEBaseline) − (Measured EEpost-treatment −Measured EEBaseline)] (32).
We calculated EE balance (EEbal) using daily EI and the change of body energy storage pools (kg) via the following equation: EEbal = EI − ρFFM ΔLM − ρFM ΔFM (33) where ρLM=1,100 kcal/kg is the energy density of FFM and ρFM=9,300 kcal/kg is the energy density of body FM (34).
Results
Population Characteristics
The study population consisted of females and males aged 36.1±7.3 years with a higher percentage of males enrolled in the study (67.9%). The population was diverse with 40.7% white, 44.4% black, 14.8% Asian, unknown, multiple or other, and 32.1% Hispanic or Latino ethnicity. The baseline clinical characteristics indicate that we enrolled a population with overweight and obesity without evidence of diabetes or dyslipidemia, other than slightly elevated triglycerides. There were no differences in baseline characteristics between groups (Table 1).
Table 1:
Demographics and Baseline Characteristics
| Placebo | SAR425899 | All | p-value | |
|---|---|---|---|---|
| (N = 17) | (N = 11) | |||
| Age [years; mean (SD)] | 36.1 (7.3) | 35.7 (8.6) | 36.0 (7.7) | 0.896 |
| Sex [n (%)] | ||||
| Female | 6 (35.3) | 3 (27.3) | 9 (32.1) | 0.655 |
| Male | 11 (64.7) | 8 (72.7) | 19 (67.9) | |
| Race [n (%)] 1 | ||||
| White | 8 (50.0) | 3 (27.3) | 11 (40.7) | 0.490 |
| Black or African American | 6 (37.5) | 6 (54.5) | 12 (44.4) | |
| Other2 | 2 (12.5) | 2 (18.2) | 4 (14.8) | |
| Ethnicity | ||||
| Hispanic or Latino | 7 (41.2) | 2 (18.2) | 9 (32.1) | 0.327 |
| Not Hispanic or Latino | 9 (52.9) | 7 (63.6) | 16 (57.1) | |
| Not Reported | 1 (5.9) | 2 (18.2) | 3 (10.7) | |
| Weight [kg; mean (SD)] | 91.54 (12.15) | 93.76 (10.10) | 92.41(11.24) | 0.604 |
| BMI [kg/m2; mean (SD)] | 30.33 (2.23) | 30.95 (2.55) | 30.57 (2.34) | 0.521 |
| Fat Mass [kg; mean (SD)] | 33.6 (6.1) | 35.4 (6.7) | 34.3 (6.3) | 0.473 |
| Fat Free Mass [kg; mean (SD)] | 57.9 (11.6) | 58.3 (9.3) | 58.0 (10.5) | 0.931 |
| HbA1C [%; mean (SD)] | 5.477 (0.286) | 5.236 (0.505) | 5.380 (0.396) | 0.173 |
| Glucose [mmol/L; mean (SD)] | 5.261 (0.500) | 5.126 (0.437) | 5.208 (0.473) | 0.458 |
| Total Cholesterol [mmol/L; mean (SD)] | 4.986 (1.098) | 4.759 (0.838) | 4.897 (0.993) | 0.542 |
| HDL [mmol/L; mean (SD)] | 1.167 (0.306) | 1.246 (0.313) | 1.198 (0.305) | 0.515 |
| LDL [mmol/L; mean (SD)] | 3.00 (0.90) | 2.55 (0.84) | 2.89 (0.79) | 0.277 |
| Triglycerides [mmol/L; mean (SD)] | 1.783 (0.920) | 1.947 (2.000) | 1.848 (1.411) | 0.802 |
Data presented for participants that completed baseline and post-treatment calorimetry visits (pharmacodynamic population). Variables are shown as mean (± standard deviation- SD) or n (%). Placebo vs. SAR425899 at baseline. Comparison by unpaired t-test.
n = 16 for placebo group race variable.
Other = unknown, multiple or Asian.
Calorimetry data was collected over 3 days at baseline and post-treatment (Figure S1). Baseline EE, respiratory exchange ratio (RER), EI and substrate oxidation parameters are shown in Table S7. As expected, EE components and RERs were not significantly different between groups over the three measurements at baseline (pre-treatment). At baseline, 24-hour RER reflected the provided diet (food quotient=0.885). There was a between-day difference in RER24-hour and a trend in RERbasal (p<0.0001 and 0.047, respectively), but the absolute change was quite small, had minimal variation between days, remained within the expected substrate oxidation based on food quotient, and was not biologically meaningful (35). EI, substrate oxidation (carbohydrate and fat), and urinary ketones differed slightly between days in both groups (p=0.008, <0.0001, <0.0001, and <0.05, respectively), but there was no significant difference between treatment groups.
SAR425899 Safety and Tolerability in the Safety Population
No serious adverse events or severe treatment emergent AEs (TEAEs) were reported during the study. TEAEs leading to permanent treatment discontinuation (Table S8) were reported in both the placebo group and the SAR425899 group. Gastrointestinal disorders were observed in 9/18 (50.0%) participants in the placebo group and 15/17 (88.2%) participants in the SAR425899 group. Particularly, the frequency of vomiting was high for the SAR425899 group (58.8% of all participants exposed to SAR425899), with no such events in the placebo group. The most frequently reported TEAEs, reported by ≥3 participants in either the placebo group or the SAR425899 group (for the safety population), can be found in Table S8 and adverse events by primary organ class can be found in Table S9. Supplementary Appendix 2 contains additional details on adverse event assessments.
Weight Loss is Greater, and Body Composition Profile is More Favorable with SAR425899 due to a Greater Deficit in Energy Intake
Under conditions of calorie restriction, the mean change from baseline for body weight (adjusted for baseline weight) on Days 5, 9, 16, and 20 was −2.11±1.11, −2.51±1.35, −3.10±1.39, and −3.68±1.37 kg for placebo, and −1.85±1.17, −2.56±1.41, −3.99±1.46, and −4.83±1.44 kg for SAR425899 (p≤0.0001 and p=0.002 for time and interaction time × group effects, respectively) (Figure 1A). The mean change from baseline (±SD) for FM and FFM (kg) at Days 16 and 20 was −1.5±0.5 kg and −2.4±1.2 kg for SAR425899, and −1.0±0.5 kg and −1.8±0.8 kg for placebo, respectively (p≤0.0001 and p=0.01 for time and interaction time × group effects, for fat mass; p≤0.0001 and p=0.02 for time and interaction time × group effects, for FFM) (Figure 1B, 1C). Multiple comparisons between groups and timepoints revealed significant between group differences for weight and FM only (Figure 1A, 1B).
Figure 1: SAR425899 Leads to Greater Weight and Fat Mass Reductions.
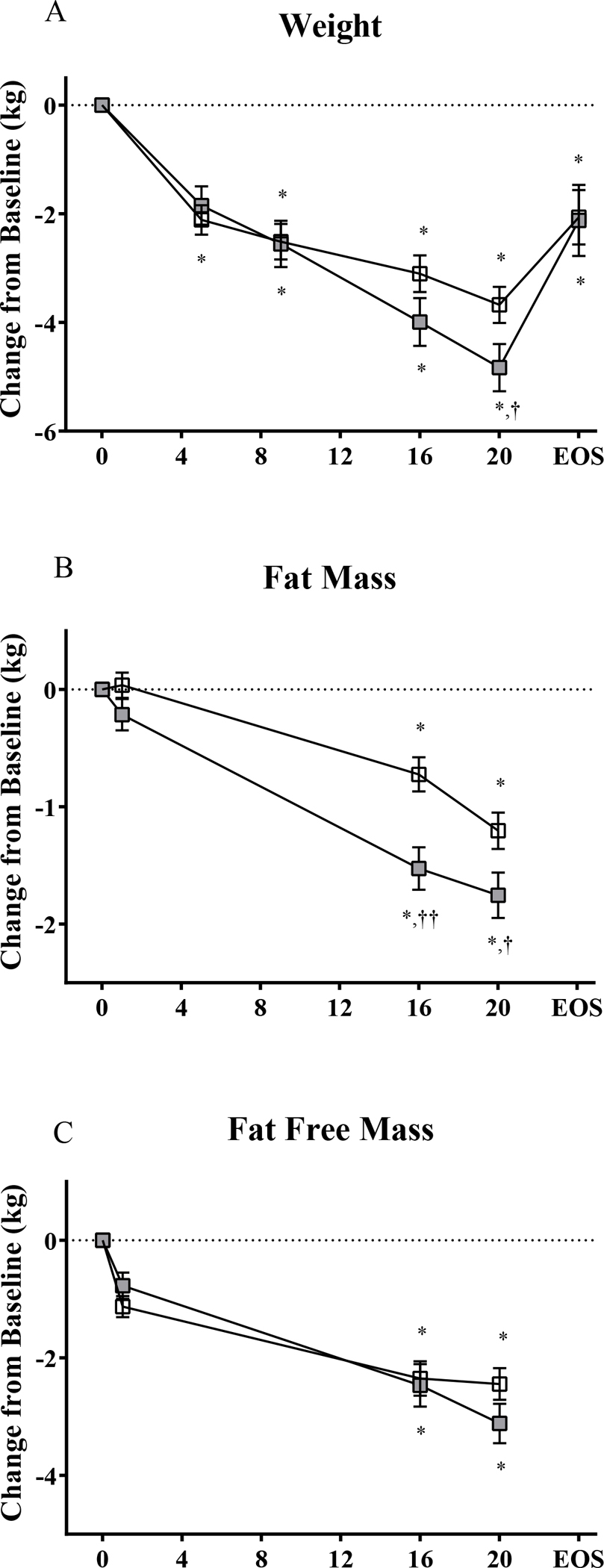
(A) Weight was evaluated at baseline and Days 5, 9, 16, 20 and end of study (EOS; 6–8 days after last dose and end of calorie restricted diet). (B, C) Body composition (by dual energy X-ray absorptiometry) was evaluated at baseline and Days 1, 16 and 20 (B- fat mass; C- fat free mass). Comparisons done by repeated measures multivariate analysis of variance (N = 17 Placebo; N = 11 SAR425899). *, significant difference from baseline at p < 0.05. †, group difference at p < 0.05. ††, group difference at p < 0.01. Placebo, open box; SAR425899, grey box.
As expected with caloric restriction and weight loss, there were significant improvements in several metabolic parameters between Day 1 and Day 20 including glycemic control, plasma lipids, and leptin that were similar in both groups (Table 2). Free fatty acids were increased in both groups (p=0.005) with a significantly greater increase in the SAR425899 group (p=0.02, Table 2).
Table 2:
Circulating Metabolic Parameters at Baseline and Post-Intervention
| Variables | Placebo (n=17) | SAR425898 (n=11) | p-value | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BASELINE | POST | BASELINE | POST | ||||||||||||
|
|
|
|
|
|
|||||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Time | Time × Group | ||||||
|
|
|
|
|
|
|
||||||||||
| HbA1C | (%) | 5.48 | ± | 0.29 | 5.39 | ± | 0.28 | 5.24 | ± | 0.50 | 5.07 | ± | 0.35 | 0.004 | 0.357 |
|
|
|
||||||||||||||
| Glucose | (mmol/L) | 5.26 | ± | 0.50 | 5.14 | ± | 0.35 | 5.13 | ± | 0.44 | 4.79 | ± | 0.37 | 0.012 | 0.222 |
|
|
|
||||||||||||||
| Total Cholesterol | (mmol/L) | 4.99 | ± | 1.10 | 4.48 | ± | 1.09 | 4.76 | ± | 0.84 | 4.07 | ± | 0.99 | <0.0001 | 0.397 |
|
|
|
||||||||||||||
| HDL | (mmol/L) | 1.17 | ± | 0.31 | 1.08 | ± | 0.28 | 1.25 | ± | 0.31 | 1.04 | ± | 0.21 | <0.0001 | 0.074 |
|
|
|
||||||||||||||
| LDL 1 | (mmol/L) | 57.7 | ± | 65.4 | 53.9 | ± | 62.4 | 65.4 | ± | 56.6 | 61.2 | ± | 57.4 | 0.115 | 0.929 |
|
|
|
||||||||||||||
| Triglycerides | (mmol/L) | 1.78 | ± | 0.92 | 1.27 | ± | 0.64 | 1.95 | ± | 2.00 | 1.03 | ± | 0.75 | 0.0002 | 0.232 |
| Free Fatty Acids 2 | (mmol/L) | 0.439 | ± | 0.127 | 0.464 | ± | 0.160 | 0.398 | ± | 0.164 | 0.612 | ± | 0.161 | 0.005 | 0.020 |
|
|
|
||||||||||||||
| Leptin | (μg/L) | 44.7 | ± | 35.9 | 31.4 | ± | 25.5 | 46.0 | ± | 29.3 | 28.3 | ± | 17.2 | <0.0001 | 0.495 |
Variables are shown for baseline (Day 1) and post treatment (Day 20; POST) as mean +/− standard deviation (SD).
n (Placebo) =17 and n (SAR425899) = 10
n (Placebo) =16 and n (SAR425899) = 9
n (Placebo) =13 and n (SAR425899) = 9.
Time and time X group comparisons done by repeated measures multivariate analysis of variance.
We next evaluated the potential impact of unintended factors on the changes in body weight and body composition. We planned an energy deficit between baseline and the treatment period of 1000 kcal/day for both groups. As expected, there was significant daily reduction in EI from baseline approximating 1000 kcal in each group (p<0.05; Figure S3A). There were no statistically significant differences in EI between groups over the 19-day intervention period (Figure S3A, Table 3). However, there was a trend toward a higher daily change in EI in the SAR425899 group than planned (Placebo −989±489 kcal/day; SAR425899: −1442±667 kcal/day; p=0.07). We found no significant differences in physical activity level between placebo and SAR425899 (Figure S3B).
Table 3.
Evaluation of Factors Related to Changes in Body Energy Stores
| Energy Intake Variables | Placebo | SAR425899 | Difference | P-value |
|---|---|---|---|---|
| El Intervention (kcal/19 days) | 29197 ± 7198 | 23609 ± 8408 | 5588 ± 2974 | 0.09 |
| Change in Energy Stores (kcal) | −13927 ± 5884 | −19678 ± 6568 | 5752 ± 2382 | 0.03 |
| EE Balance (kcal) | 43123 ± 2145 | 43287 ± 2666 | −164 ± 3422 | 0.96 |
Energy intake (EI) intervention is the total energy intake over the 19-day study period. The change in energy stores represents the change of the body energy storage pools (in kg) based on measured fat mass and fat free mass (9,300 (kcal/kg) × FM change (kg)) − (1,100 (kcal/kg) × lean change (kg)). Energy expenditure (EE) balance represents the difference between EI and change in energy stores (EI - Change in ES). Data shown as mean ± standard deviation. Comparison between Placebo and SAR425899 done by an independent sample t-test.
Because differences in EI were quantitatively different between groups, we evaluated the change in body energy stores (BES) in relationship to EI by calculating EE balance (EEbal) (33). This approach utilizes the principle of energy balance under conditions of weight loss (EI=EE + change in BES), and re-arranges the equation to solve for EE to determine whether EI was a factor in the differences in BED (36). Similar to changes in weight and body composition, the change in BES was significantly higher in the SAR425899 group (p=0.03). However, we found that EEbal was quantitatively similar between the groups (p=0.96; Table 3).
Reduced Metabolic Adaptation with SAR425899
As expected from body weight loss, SMR declined in both treatment groups from baseline until end of treatment (p<0.001; Figure S4A). While participants in the SAR425899 group lost more weight than the placebo group, the decrease from baseline for SMR (on-treatment calorimeter days, ±SD) was larger in the placebo group compared to SAR425899 (p=0.002; Figure 2A) after adjusting for changes in body composition (lean and fat mass). The SMR percent decrease from baseline for SAR4256899 adjusted for change in body composition was statistically significantly smaller than for placebo (p=0.001; Figure 2F). Similar results were obtained when adjusting for baseline body weight and change in body weight (Figure S5A and B). There were significant reductions in other EE components over time (Figure S4 B–D), but there were no differences between groups or after adjustment for body composition (Figure 2 B–E).
Figure 2: Baseline and Post-Intervention Values and Relative Changes in Energy Expenditure Components Adjusted for Body Composition.
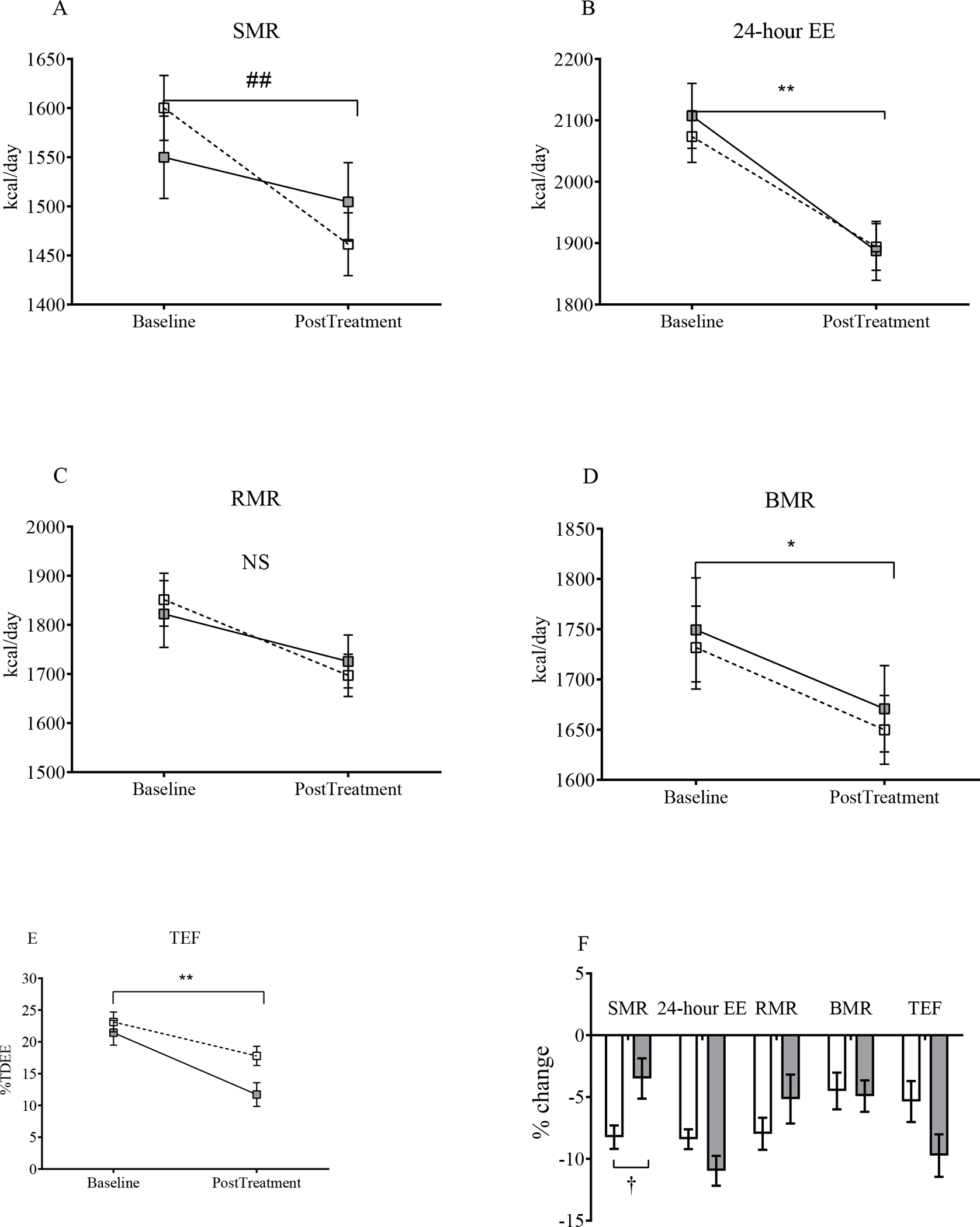
(A-E) Sleeping metabolic rate (SMR), 24-hour energy expenditure (24-hour EE), resting metabolic rate (RMR), basal metabolic rate (BMR) and thermic effect of feeding (TEF), respectively, at baseline and post-treatment. The reduction in SMR was lower in the SAR425899 group. (F) Percent changes in the same components. There was smaller percent change in SEE in the SAR425899 group. Grey and white squares and bars represent means and standard error of mean for the SAR425899 and placebo groups, respectively. Comparisons of least square means absolute change done by repeated measures multivariate analysis of variance and percent change by independent sample t-tests. ##, p<0.01 for time × group effect. **, p<0.01 for time effect in both groups. †, p<0.05 for group effect. Placebo, open box/bar; SAR425899, grey box/bar.
To further evaluate differences in metabolic adaptation, we used regression equations to predict EE from baseline body composition variables (Figure 3A and 3B) and found that SAR425899 treatment led to an equivalent metabolic adaptation for 24-hour EE (Figure 3C) but a blunting of metabolic adaptation during sleep (Figure 3D).
Figure 3: Sleeping and 24-Hour Metabolic Adaptation.

Scatterplots are measured vs. predicted 24-hour energy expenditure (24-hour EE) (A) and sleeping metabolic rate (SMR) (C) at baseline to generate the regression equations utilized to estimate 24-hour EE and SMR at baseline and post-treatment (average of Days 17–19). Metabolic adaptation for 24-hour EE and SMR was calculated by: [(Estimated EEpost-treatment−Estimated EEBaseline) − (Measured EEpost-treatment −Measured EEBaseline)] (B, D). **, p<0.01 for time effect in both groups. #, p<0.05 for time × group effect. Placebo, open box; SAR425899, grey box.
Increased Fat Oxidation and Ketogenesis with SAR425899
We found that RER24-hour and all subcomponents decreased in both groups toward end of treatment (Figure 4A–D). These changes were significantly greater with SAR425899 than with placebo. For example, while both groups started with an RER24-hour of 0.89, the placebo group decreased to 0.85±0.02 and the SAR425899 decreased to 0.80±0.03 (p<0.05; Figure 4B).
Figure 4: Baseline and Post-Intervention Values for Respiratory Exchange Ratio Components.

(A-D) Sleep respiratory exchange ratio (RQsleep), 24-hour RER (RER24-h), rest RER (RERrest) and basal RER (RERbasal), respectively, at baseline and post-intervention. The absolute reductions were significantly greater in the SAR425899 group. Grey and white squares and bars represent means and standard error of mean for the SAR425899 and placebo groups, respectively. Comparisons of least square means absolute change done by repeated measures multivariate analysis of variance and percent change by independent sample t-tests. # and ###, p<0.05 and p<0.001 respectively, for time × group effect. Placebo, open box; SAR425899, grey box.
To further define the role of substrate oxidation with SAR425899 treatment, we evaluated fat and carbohydrate oxidation (FatOx and CarbOx, respectively) across all EE parameters. The SAR425899 group had significantly higher FatOx during sleep, total daily FatOx (24-hour), and FatOx during the basal and resting EE periods (p=0.01, 0.001, 0.002, 0.003, respectively; Figure 5A–D). The only difference in CarbOx was over 24-hour, where placebo had higher CarbOx than SAR425899, likely due to the shift to fat oxidation in the SAR425899 group (p=0.006; Figure 5E–H).
Figure 5: SAR425899 Leads to an Increase in Fat Oxidation and Circulating Ketone Bodies.
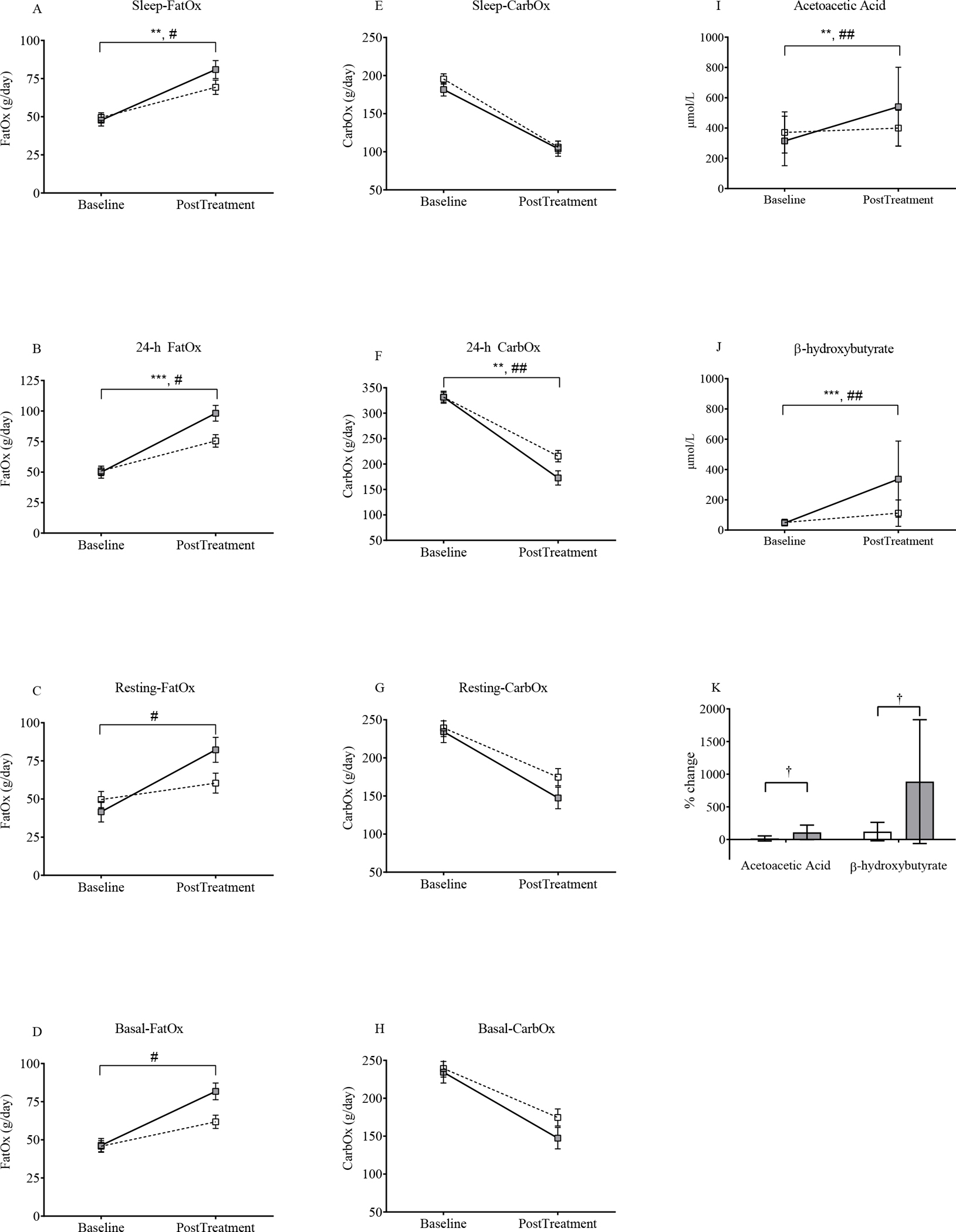
Fat (FatOx) and carbohydrate (CarbOx) oxidation rates were calculated for all energy expenditure (EE) components: sleep metabolic rate (A, E), total daily EE (24-h) (B, F), resting (C, G) and basal (D, H). FatOx was higher in the SAR425899 group for sleep, 24-h, resting and basal. 24-h CarbOx was higher in the placebo group. Absolute values of acetoacetic acid (I) and β-hydroxybutyrate (J) at baseline and post-intervention. There was an increase in both groups with a greater increase in SAR425899 group. (K) There was also a greater percent change in the SAR425899 group in both ketones. Grey and white squares represent means and standard error of mean for the SAR425899 and placebo groups, respectively. Comparisons of least square means absolute change done by repeated measures multivariate analysis of variance and percent change by independent sample t-tests. # and ##, p<0.05 and p<0.01 respectively, for time × group effect. ** and ***, p<0.01 and p<0.001 for time effect in both groups; †, p<0.05 for group effect (for panel K). Placebo, open box/bar; SAR425899, grey box/bar.
Ketone bodies (acetoacetic acid and β hydroxybutyrate) were increased in both groups (time effect; p< 0.01 and <0.001 for acetoacetic acid and β hydroxybutyrate; Figure 5I and J). The increase in both ketone bodies was significantly higher in the SAR425899 group (p<0.01, respectively for interaction of time × group; Figure 5I and J). The percent change in both ketone bodies was significantly greater for SAR425899 (p<0.05; Figure 5K).
The differences observed in FatOx and ketogenesis could have been due to a larger energy imbalance in the SAR425899 group. After adjusting for change in energy stores, these differences between groups remained significant (46.0±4.8 vs 26.0±3.8 g/day, p=0.005; 280.0±60.8 vs 75.4±49.7 μmol/L, p=0.02 and 226.8±58.5 vs 27.8±46.1 μmol/L, p=0.02 for SAR425899 vs Placebo and 24-h FatOx, β-hydroxybutyrate and acetoacetic acid, respectively; Figure S6).
Discussion
To address the EE effects of dual GLP-1/GCGR agonism, we conducted a metabolic ward study under rigorous environmental controls that were intended to clamp EI. We observed a greater weight loss and reduction in fat mass with SAR425899 plus caloric restriction versus caloric restriction alone. This greater change in body energy stores was at least partly due to our inability to clamp EI equivalently between groups. Despite this limitation, we found intriguing metabolic effects of SAR425899 that could be important drivers for weight loss. Specifically, we found a statistically significantly smaller reduction in SMR, but not 24h-EE, with SAR425899 (before and after adjustment for change in body weight and body composition). The SMR results are an indication of reduced metabolic adaptation with SAR425899. We also found greater fat oxidation and ketogenesis that was not due to the greater changes in weight and body composition. These metabolic effects of SAR425899 are important concepts for the further development of weight loss therapies since both low EE and low fat oxidation are risk factors for weight gain that impair loss of fat mass (12,37).
Both preclinical and short clinical studies of the dual GLP-1R/GCGR agonist oxyntomodulin have shown increases in total, resting and/or activity related EE (10,38). Neither of those studies utilized weight loss through calorie restriction to quantify changes in EE without confounding from greater metabolic adaptation in the treated group. The absolute difference in SMR over an 8-hour sleep period was ~34 kcal/day. The absolute sleep metabolic adaptation for the placebo group was 61±60 kcal/day whereas in the SAR425899 group it was −31±79 kcal/day. This suggests that ramping up of EE during sleep may account for some of the metabolic benefits with SAR425899 treatment. Because of the small effect on SMR, we conclude that the clinical relevance of this result remains an open question. This conclusion is supported by a study showing that SAR425899 is a weak GCGR agonist, suggesting that the GLP-1/GCGR ratio in SAR425899 may require additional optimization (39). We found that the greater change in body energy stores in the SAR425899 group was at least partially explained by differences in EI based on a calculation of EEbal. This implies that the observed body energy store differences were likely attributable to the EI differences. Therefore, our hypothesis of a role of glucagon agonism on increased EE is not entirely supported by our data, in line with our previous findings (4,5).
Despite the limited impact of SAR425899 on EE, the blunted metabolic adaptation observed in the SAR425899 group may be important for weight loss maintenance (12). Our findings are supported by our recent study where we observed reduced metabolic adaptation to be a distinguishing phenotype in people who meet their predicted weight loss targets vs. those that do not (40).
We postulated that the reduced metabolic adaptation we saw with GLP-1R/GCGR agonism under conditions of caloric restriction might be coupled with substrate switching. RER24-hour and other subcomponents decreased in both groups toward the end of treatment, demonstrating a shift towards lipid oxidation. The change from baseline was roughly 2-fold higher under treatment with SAR425899 compared to placebo (22.8 vs 50.9 g/day). This difference represents an improved negative fat energy balance of 196 grams of fat per week. In line with increased flux through the lipolytic and fat oxidation machinery, there was an 886% increase in β-hydroxybutyrate in the SAR425899 group (vs. a 121% increase in placebo) and a 109% increase in the SAR425899 group in acetoacetic acid (vs. a 16% increase in placebo). Impaired fat oxidation is part of the obese phenotype (41–43) and a key barrier to weight loss maintenance (44,45). Importantly, this increased fat oxidation and ketogenic metabolic profile was independent of the greater changes in weight and body composition in the SAR425899 group. Therefore, our results suggest that unlike caloric restriction (41), SAR425899 improves impairments in fat oxidation, and this might contribute to weight loss success under certain conditions (12).
There were several limitations of our study. There were more dropouts in the SAR425899 group, which could have biased our results. This is unlikely given that that baseline characteristics of the people who were randomized but did not complete the study were similar to those who completed the study. We also only found metabolic adaptation effects to the study drug on SMR and not 24-hour EE. Whether this selective adaptation response is meaningful in long-term weight loss maintenance using this drug cannot be addressed in this study. We did not have a GLP-1R arm in the study, making it difficult to know if the observed changes are due to the presence of GCGR.
Conclusion
In conclusion, we uncovered effects of SAR425899 on metabolic pathways that are relevant for weight loss and weight loss maintenance: a selective reduction in metabolic adaptation and a quantitatively relevant increase in fat oxidation with a parallel increase in ketogenesis. We propose that an optimal combination of GLP-1 and GCGR agonism may translate to greater weight loss success in conditions where EI is not clamped. Continued development of incretin-based weight loss therapeutics would, therefore, represent a fruitful area for continued research.
Supplementary Material
Study Importance.
What is already known?
Preclinical studies demonstrate that glucagon alone or in combination with GLP-1 increases energy expenditure (EE) and weight loss.
Short-term studies in humans are equivocal regarding the EE effects of glucagon.
What does this study add?
We tested the hypothesis that, during weight loss, a novel dual agonist of GLP-1 and glucagon receptors (SAR425899) would lead to a smaller reduction in SMR (kcal/day) than predicted by changes in body composition during weight loss (metabolic adaptation).
We found that SAR425899 led to reduced metabolic adaptation, increased fat oxidation and enhanced ketogenesis independent of the changes in body energy stores.
How might these results change the direction of research?
After accounting for changes in energy stores, SAR425899 leads to metabolic benefits (lower selective metabolic adaptation and increased fat oxidation) that may be important for weight loss and weight loss maintenance.
Pending the development of an ideal combination of agonists that is well tolerated, these metabolic benefits could lead to improved weight loss and longer-term weight loss maintenance.
Acknowledgments:
We thank our study participants without whom this work would not be possible and the expert research kitchen, metabolic ward and indirect calorimetry teams at the Translational Research Institute and Pennington Biomedical Research Center for the excellent execution of this trial. Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Funding:
This trial was funded by Sanofi, the study Sponsor. Study drug was provided by Sanofi. Sanofi was involved in study design in collaboration with site investigators, data analysis, and manuscript review. Sanofi was not involved in data collection. The manuscript was prepared by Dr. Corbin and other members of the research team at the study sites. Sanofi was permitted to review the manuscript and suggest changes, but the final decision on content was exclusively retained by the principal investigators.
Abbreviations:
- 24-hour EE
24-hour energy expenditure
- AE
adverse event
- BMR
Basal metabolic rate
- EE
energy expenditure
- EI
energy intake
- FFM
fat free mass
- FM
fat mass
- GCGR
Glucagon receptor
- GLP-1
Glucagon-like peptide-1
- MANOVA
multivariate analysis of variance
- PAL
physical activity level
- RER
respiratory exchange ratio
- RMR
resting metabolic rate
- SMR
sleeping metabolic rate
- TEAE
treatment emergent adverse event
- TEF
thermic effect of feeding
Footnotes
Disclosure Summary:
SRS has received research grants or contracts from Bayer Pharma; serves as a consultant to Novartis, Eli Lilly, Novo Nordisk, and Zealand Pharma.
ER has received research grants, contracts or unrestricted gifts from Eli Lilly, ICON, Novartis and Sanofi-Aventis; receives consulting fees from Energesis Pharmaceuticals, Eli Lilly USA, Merck, Amway, Nutrilite Health Institute- Amway, Kintai Therapeutics, YSOPIA (LNC Therapeutics), Big Sky Health, Generian; has received payment or honoraria for lectures, presentations, speakers bureau, manuscript writing or educational events from Open Academi- Venice, National Institutes of Health; has received support for attending meetings or travel from SSIB, Grand Rounds- Oregon, Florida State University, Copenhagen Bioscience Conference, National Institutes of Health, New York University, Open Academy, Aegean Conference on Precision Nutrition, Tulane Personalized Health Institute, Societe Francaise du Diabete, European Association for the Study of Obesity, American Association of Clinical Endocrinology; is the Editor in Chief of Obesity.
KDH received support for the present manuscript from the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases; received support for attending meetings and/or travel from Pennington Biomedical Research Center and American College of Sports Medicine.
PPL, BG and JT are employees of Sanofi. JT owns Sanofi stock.
SRS and ER received a research grant from Sanofi-Aventis which allowed the conduct of this study.
All other authors have nothing to disclose.
Clinical Trial Registration: This trial is registered on ClinicalTrials.gov- NCT03376802
References
- 1.Vilsbøll T, Christensen M, Junker AE, Knop FK, Gluud LL. Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analyses of randomised controlled trials. BMJ (Clinical research ed). 2012;344:d7771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilding JPH, Batterham RL, Calanna S, Davies M, Van Gaal LF, Lingvay I, McGowan BM, Rosenstock J, Tran MTD, Wadden TA, Wharton S, Yokote K, Zeuthen N, Kushner RF. Once-Weekly Semaglutide in Adults with Overweight or Obesity. New England Journal of Medicine. 2021;384(11):989–1002. [DOI] [PubMed] [Google Scholar]
- 3.Habegger KM, Heppner KM, Geary N, Bartness TJ, DiMarchi R, Tschöp MH. The metabolic actions of glucagon revisited. Nature reviews Endocrinology. 2010;6(12):689–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whytock KL, Carnero EA, Vega RB, Tillner J, Bock C, Chivukula K, Yi F, Meyer C, Smith SR, Sparks LM. Prolonged Glucagon Infusion Does Not Affect Energy Expenditure in Individuals with Overweight/Obesity: A Randomized Trial. Obesity (Silver Spring, Md). 2021;29(6):1003–1013. [DOI] [PubMed] [Google Scholar]
- 5.Chakravarthy M, Parsons S, Lassman ME, Butterfield K, Lee AY, Chen Y, Previs S, Spond J, Yang S, Bock C, Yi F, Moon J, Wohlers-Kariesch E, Smith SR, Meyer C. Effects of 13-Hour Hyperglucagonemia on Energy Expenditure and Hepatic Glucose Production in Humans. Diabetes. 2017;66(1):36–44. [DOI] [PubMed] [Google Scholar]
- 6.Tan TM, Field BC, McCullough KA, Troke RC, Chambers ES, Salem V, Gonzalez Maffe J, Baynes KC, De Silva A, Viardot A, Alsafi A, Frost GS, Ghatei MA, Bloom SR. Coadministration of glucagon-like peptide-1 during glucagon infusion in humans results in increased energy expenditure and amelioration of hyperglycemia. Diabetes. 2013;62(4):1131–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geary N, Kissileff HR, Pi-Sunyer FX, Hinton V. Individual, but not simultaneous, glucagon and cholecystokinin infusions inhibit feeding in men. The American journal of physiology. 1992;262(6 Pt 2):R975–980. [DOI] [PubMed] [Google Scholar]
- 8.Cohen MA, Ellis SM, Le Roux CW, Batterham RL, Park A, Patterson M, Frost GS, Ghatei MA, Bloom SR. Oxyntomodulin suppresses appetite and reduces food intake in humans. The Journal of clinical endocrinology and metabolism. 2003;88(10):4696–4701. [DOI] [PubMed] [Google Scholar]
- 9.Wynne K, Park AJ, Small CJ, Patterson M, Ellis SM, Murphy KG, Wren AM, Frost GS, Meeran K, Ghatei MA, Bloom SR. Subcutaneous oxyntomodulin reduces body weight in overweight and obese subjects: a double-blind, randomized, controlled trial. Diabetes. 2005;54(8):2390–2395. [DOI] [PubMed] [Google Scholar]
- 10.Wynne K, Park AJ, Small CJ, Meeran K, Ghatei MA, Frost GS, Bloom SR. Oxyntomodulin increases energy expenditure in addition to decreasing energy intake in overweight and obese humans: a randomised controlled trial. International journal of obesity (2005). 2006;30(12):1729–1736. [DOI] [PubMed] [Google Scholar]
- 11.Bagger J, Holst J, Vilsbøll T, Knop FK. Glucagon and the gut hormones GLP-1 and oxyntomodulin increase resting energy expenditure in man. Regulatory Peptides. 2012;177:S15–S16. [Google Scholar]
- 12.Christoffersen BØ, Sanchez-Delgado G, John LM, Ryan DH, Raun K, Ravussin E. Beyond appetite regulation: Targeting energy expenditure, fat oxidation, and lean mass preservation for sustainable weight loss. Obesity. 2022;30(4):841–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tillner J, Posch MG, Wagner F, Teichert L, Hijazi Y, Einig C, Keil S, Haack T, Wagner M, Bossart M, Larsen PJ. A novel dual glucagon-like peptide and glucagon receptor agonist SAR425899: Results of randomized, placebo-controlled first-in-human and first-in-patient trials. Diabetes, obesity & metabolism. 2019;21(1):120–128. [DOI] [PubMed] [Google Scholar]
- 14.Visentin R, Schiavon M, Göbel B, Riz M, Cobelli C, Klabunde T, Dalla Man C. Dual glucagon-like peptide-1 receptor/glucagon receptor agonist SAR425899 improves beta-cell function in type 2 diabetes. Diabetes, obesity & metabolism. 2020;22(4):640–647. [DOI] [PubMed] [Google Scholar]
- 15.Schoffelen PF, Westerterp KR. Intra-individual variability and adaptation of overnight- and sleeping metabolic rate. Physiol Behav. 2008;94(2):158–163. [DOI] [PubMed] [Google Scholar]
- 16.Chen KY, Smith S, Ravussin E, Krakoff J, Plasqui G, Tanaka S, Murgatroyd P, Brychta R, Bock C, Carnero E, Schoffelen P, Hatamoto Y, Rynders C, Melanson EL. Room Indirect Calorimetry Operating and Reporting Standards (RICORS 1.0): A Guide to Conducting and Reporting Human Whole-Room Calorimeter Studies. Obesity (Silver Spring, Md). 2020;28(9):1613–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allerton TD, Carnero EA, Bock C, Corbin KD, Luyet P-P, Smith SR, Ravussin E. Reliability of measurements of energy expenditure and substrate oxidation using whole-room indirect calorimetry. Obesity. 2021;29(9):1508–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bettge K, Kahle M, Abd El Aziz MS, Meier JJ, Nauck MA. Occurrence of nausea, vomiting and diarrhoea reported as adverse events in clinical trials studying glucagon-like peptide-1 receptor agonists: A systematic analysis of published clinical trials. Diabetes, obesity & metabolism. 2017;19(3):336–347. [DOI] [PubMed] [Google Scholar]
- 19.Robinson LE, Holt TA, Rees K, Randeva HS, O’Hare JP. Effects of exenatide and liraglutide on heart rate, blood pressure and body weight: systematic review and meta-analysis. BMJ open. 2013;3(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Redman LM, Smith SR, Burton JH, Martin CK, Il’yasova D, Ravussin E. Metabolic Slowing and Reduced Oxidative Damage with Sustained Caloric Restriction Support the Rate of Living and Oxidative Damage Theories of Aging. Cell metabolism. 2018;27(4):805–815.e804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin CK, Heilbronn LK, de Jonge L, DeLany JP, Volaufova J, Anton SD, Redman LM, Smith SR, Ravussin E. Effect of calorie restriction on resting metabolic rate and spontaneous physical activity. Obesity (Silver Spring, Md). 2007;15(12):2964–2973. [DOI] [PubMed] [Google Scholar]
- 22.Heilbronn LK, de Jonge L, Frisard MI, DeLany JP, Larson-Meyer DE, Rood J, Nguyen T, Martin CK, Volaufova J, Most MM, Greenway FL, Smith SR, Deutsch WA, Williamson DA, Ravussin E. Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. Jama. 2006;295(13):1539–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ravussin E, Smith SR, Ferrante AW Jr. Physiology of Energy Expenditure in the Weight-Reduced State. Obesity. 2021;29(S1):S31–S38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hall KD, Chow CC. Estimating changes in free-living energy intake and its confidence interval. The American journal of clinical nutrition. 2011;94(1):66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hall KD, Sacks G, Chandramohan D, Chow CC, Wang YC, Gortmaker SL, Swinburn BA. Quantification of the effect of energy imbalance on bodyweight. Lancet (London, England). 2011;378(9793):826–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Redman LM, Kraus WE, Bhapkar M, Das SK, Racette SB, Martin CK, Fontana L, Wong WW, Roberts SB, Ravussin E. Energy requirements in nonobese men and women: results from CALERIE. The American journal of clinical nutrition. 2014;99(1):71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lam YY, Redman LM, Smith SR, Bray GA, Greenway FL, Johannsen D, Ravussin E. Determinants of sedentary 24-h energy expenditure: equations for energy prescription and adjustment in a respiratory chamber. The American journal of clinical nutrition. 2014;99(4):834–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hall KD, Chen KY, Guo J, Lam YY, Leibel RL, Mayer LE, Reitman ML, Rosenbaum M, Smith SR, Walsh BT, Ravussin E. Energy expenditure and body composition changes after an isocaloric ketogenic diet in overweight and obese men. The American journal of clinical nutrition. 2016;104(2):324–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Göbel B, Sanghvi A, Hall KD. Quantifying energy intake changes during obesity pharmacotherapy. Obesity (Silver Spring, Md). 2014;22(10):2105–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanghvi A, Redman LM, Martin CK, Ravussin E, Hall KD. Validation of an inexpensive and accurate mathematical method to measure long-term changes in free-living energy intake. The American journal of clinical nutrition. 2015;102(2):353–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ulijaszek SJ, Kerr DA. Anthropometric measurement error and the assessment of nutritional status. The British journal of nutrition. 1999;82(3):165–177. [DOI] [PubMed] [Google Scholar]
- 32.Doucet E, St-Pierre S, Alméras N, Després JP, Bouchard C, Tremblay A. Evidence for the existence of adaptive thermogenesis during weight loss. The British journal of nutrition. 2001;85(6):715–723. [DOI] [PubMed] [Google Scholar]
- 33.Hall KD, Guo J, Chen KY, Leibel RL, Reitman ML, Rosenbaum M, Smith SR, Ravussin E. Methodologic considerations for measuring energy expenditure differences between diets varying in carbohydrate using the doubly labeled water method. The American journal of clinical nutrition. 2019;109(5):1328–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heymsfield SB, Waki M, Kehayias J, Lichtman S, Dilmanian FA, Kamen Y, Wang J, Pierson RN, Jr. Chemical and elemental analysis of humans in vivo using improved body composition models. The American journal of physiology. 1991;261(2 Pt 1):E190–198. [DOI] [PubMed] [Google Scholar]
- 35.Westerterp KR. Food quotient, respiratory quotient, and energy balance. The American journal of clinical nutrition. 1993;57(5):759S–765S. [DOI] [PubMed] [Google Scholar]
- 36.Ravelli MN, Schoeller DA. An objective measure of energy intake using the principle of energy balance. International journal of obesity (2005). 2021;45(4):725–732. [DOI] [PubMed] [Google Scholar]
- 37.Ravussin E, Lillioja S, Knowler WC, Christin L, Freymond D, Abbott WGH, Boyce V, Howard BV, Bogardus C. Reduced Rate of Energy Expenditure as a Risk Factor for Body-Weight Gain. New England Journal of Medicine. 1988;318(8):467–472. [DOI] [PubMed] [Google Scholar]
- 38.Scott R, Minnion J, Tan T, Bloom SR. Oxyntomodulin analogue increases energy expenditure via the glucagon receptor. Peptides. 2018;104:70–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eriksson O, Haack T, Hijazi Y, Teichert L, Tavernier V, Laitinen I, Berglund JE, Antoni G, Velikyan I, Johansson L, Pierrou S, Wagner M, Tillner J. Receptor occupancy of dual glucagon-like peptide 1/glucagon receptor agonist SAR425899 in individuals with type 2 diabetes. Scientific Reports. 2020;10(1):16758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whytock KL, Corbin KD, Parsons SA, Pachori A, Bock CP, Jones KP, Smith JS, Yi F, Xie H, Petucci CJ, Gardell SJ, Smith SR. Metabolic adaptation characterizes short-term resistance to weight loss induced by a low-calorie diet in overweight/obese individuals. The American journal of clinical nutrition. 2021;114(1):267–280. [DOI] [PubMed] [Google Scholar]
- 41.Simoneau JA, Veerkamp JH, Turcotte LP, Kelley DE. Markers of capacity to utilize fatty acids in human skeletal muscle: relation to insulin resistance and obesity and effects of weight loss. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 1999;13(14):2051–2060. [DOI] [PubMed] [Google Scholar]
- 42.Kelley DE, Goodpaster B, Wing RR, Simoneau J-A. Skeletal muscle fatty acid metabolism in association with insulin resistance, obesity, and weight loss. American Journal of Physiology-Endocrinology and Metabolism. 1999;277(6):E1130–E1141. [DOI] [PubMed] [Google Scholar]
- 43.Weyer C, Pratley RE, Salbe AD, Bogardus C, Ravussin E, Tataranni PA. Energy Expenditure, Fat Oxidation, and Body Weight Regulation: A Study of Metabolic Adaptation to Long- Term Weight Change. The Journal of Clinical Endocrinology & Metabolism. 2000;85(3):1087–1094. [DOI] [PubMed] [Google Scholar]
- 44.Seidell JC, Muller DC, Sorkin JD, Andres R. Fasting respiratory exchange ratio and resting metabolic rate as predictors of weight gain: the Baltimore Longitudinal Study on Aging. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 1992;16(9):667–674. [PubMed] [Google Scholar]
- 45.Dandanell S, Husted K, Amdisen S, Vigelsø A, Dela F, Larsen S, Helge JW. Influence of maximal fat oxidation on long-term weight loss maintenance in humans. Journal of Applied Physiology. 2017;123(1):267–274. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


