Abstract
Introduction:
Multiple large clinical trauma trials have documented an increased susceptibility to infection after injury. Although neutrophils (PMNs) were historically considered a homogeneous cell type, we hypothesized that injury could alter neutrophil heterogeneity and predispose to dysfunction. To explore whether trauma modifies PMN heterogeneity, we performed an observational mass-spectrometry based cytometry (CyTOF) study on total leukocytes (TL) and low-density PMNs found in the mononuclear cell (PBMC) fraction of leukocytes from healthy controls and trauma patients.
Methods:
A total of 74 samples from 12 trauma patients, each sampled at 1 or more time points, and matched controls were fractionated and profiled by CyTOF using a panel of 44 distinct markers. After deconvolution and conservative gating on neutrophils, data were analyzed using Seurat, followed by clustering of principal components.
Results:
11 distinct neutrophil populations were resolved in control and trauma neutrophils based on differential protein surface marker expression. Trauma markedly altered the basal heterogeneity of neutrophil subgroups seen in the control samples, with loss of a dominant population of resting neutrophils marked by high expression of C3AR and low levels of CD63, CD64 and CD177 (cluster 1), and expansion of 2 alternative neutrophil populations, one of which is marked by high expression of CD177 with suppression of CD10, CD16, C3AR, CD63, CD64 (cluster 6). Remarkably, following trauma a substantially larger percentage of neutrophils sediment in the monocyte fraction. These low-density neutrophils (LDNs) bear markers of functional exhaustion and form a unique trauma-induced population (cluster 9) with markedly upregulated expression of active surface adhesion molecules (activated CD11b/CD18), with suppression of nearly all other surface markers, including receptors for formyl peptides, leukotrienes, chemokines, and complement.
Conclusions:
Circulating neutrophils demonstrate considerable evidence of functional heterogeneity that is markedly altered by trauma. Trauma induces evolution of a novel, exhausted low-density neutrophil population with immunosuppressive features.
Keywords: neutrophil heterogeneity, signal transduction, trauma, mass cytometry, CD11b/CD18
Introduction
Trauma triggers a rapid innate immune response to protect against infection and aid clearance of devitalized tissues, followed by a refractory immune state with suppressed neutrophil, monocyte/macrophage and T cell function (1). Neutrophils make up about 60% of total leukocytes in normal human blood and are essential for antimicrobial defense and coordination of pro- and anti-inflammatory responses (2). Once released from the bone marrow, mature post-mitotic neutrophils contain granules housing proteases and anti-microbial proteins. Neutrophil functions including phagocytosis, reactive oxygen species (ROS) production, and NETosis are essential for fighting infection early after trauma, but may also result in auto-inflammatory tissue damage contributing to organ failure (3). For example, after trauma neutrophils are primed for increased extracellular ROS production and protease release by cytokines and products of coagulation, leading to lung and liver injury (4, 5). Understanding how neutrophil populations change in response to injury may help in clarifying the pathophysiology underlying complications that arise during or after trauma.
Neutrophils have historically been considered a homogenous population. Neutrophil heterogeneity, however, has been proposed in several different fields of research, particularly oncology (6–10) but functional and phenotypic definition of these subsets remains an area of confusion and ongoing debate (11). For example, Marini et. al. described a population of CD10+ mature neutrophils that suppressed T cell activation via arginase 1 activity, while a CD10- neutrophil population was immunostimulatory in granulocyte colony-stimulating factor-treated donors, and in patients with solid tumors or lymphoma (6). In contrast, Pillay et al. showed that in the setting of severe injury, CD62L-dim CD16+ neutrophils were hypersegmented and suppressed T cell activation via H2O2 production (12).
Besides flow cytometry, atypical neutrophil populations can be identified by changes in cell density using density gradient centrifugation. Neutrophils in normal healthy donors typically exhibit high density, while those isolated from inflammatory settings can sediment at low density similar to that of mononuclear cells (13, 14). Bryk et al. showed that low-density neutrophils (LDNs) from trauma patients expressed high levels of arginase 1 and had increased CD11b and CD66b expression (15), suggesting neutrophil activation and degranulation, while a recent study from Munder and colleagues showed that human granulocyte arginase activity could suppress T cell activation (16). A common limitation in these and other studies is that neutrophil populations were defined by only a few markers or a specific physical characteristic. More detailed characterization of low- and high-density neutrophils in the circulation after trauma has not been performed. In this study, we hypothesized that trauma would alter neutrophil heterogeneity, and set out to determine phenotypical differences between neutrophils of different densities in the circulation of trauma patients and healthy controls using highly-multiplexed mass cytometry (CyTOF).
Methods
Patient Selection
This study included trauma patients enrolled in the HALO protocol at Beth Israel Deaconess Medical Center (BIDMC) for whom fresh samples were concurrently available with laboratory personnel required for CyTOF sample preparation. The HALO protocol enrolled trauma patients from BIDMC between April 2017 and March 2019 who were older than 18, not pregnant, and without immunosuppressive conditions, and who were within 24 hours of a traumatic injury with hypotension, tachycardia, clinical evidence of hypoperfusion, or required blood transfusion or mechanical ventilation (Table S1, Supplemental Digital Content 1). To specify features of neutrophil heterogeneity associated with trauma, and avoid confounders arising from neutrophil changes in non-trauma conditions requiring hospitalization, control samples were obtained from six healthy volunteers, some of whom donated more than once. A fresh volunteer blood sample was processed alongside fresh trauma samples every time samples were collected. A total of 20 trauma and 17 control blood samples were used. Our study was approved by the BIDMC Institutional Review Board (2016-P-000144), the Committee on Use of Humans as Experimental Subjects at MIT, and the USAMRDC Office of Research Protections Human Research Protection Office (A-19664). In addition, pre-operative and intra-operative venous blood samples were collected from one patient undergoing a major oncologic liver resection under an NIH institutional review board-approved protocol (NCT01915225).
Blood Sample Processing
Peripheral blood was drawn into Vacutainer ethylenediaminetetraacetic acid (EDTA) tubes (Becton Dickinson, Franklin Lakes, NJ) at 0, 1, 2–3, 4–6, and/or 7–14 days after injury, and simultaneously from healthy volunteer controls, per protocol. For total leukocyte isolation, blood (6mL) was aliquoted and red blood cell lysed with 30 mL of ammonium chloride-based lysis buffer (17). Cells were resuspended in CryoStor CS10 (BioLife Solutions, Bothell, WA) freezing medium, aliquoted (106 cells per vial), and stored in liquid nitrogen until CyTOF staining. Neutrophils present within these total leukocyte fractions are referred to as total neutrophils (TNs).
For the low-density layer collection, blood (4 mL) diluted 1:1 with PBS was overlaid onto SepMate tubes (STEM CELL Technologies, Vancouver, Canada) containing Ficoll-Plus (4.5mL) (GE Healthcare, Chicago, IL) and centrifuged at 1200g for 10 minutes. The lower-density layer containing PBMCs and LDNs was resuspended in culture medium (RPMI 1640 supplemented with 5% heat-inactivated fetal calf serum, 1 mM glutamine, 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 2 mM nonessential amino acids, penicillin/streptomycin/amphotericin B, and 2.5×10−5 M 2-mercaptoethanol (all products from Life Technologies, Carlsbad, CA)) and centrifuged at 200g for 5 minutes. 106 cells per vial were frozen in CryoStor CS10 as described above.
CyTOF Staining and Analysis
Cells were thawed for 3 minutes at 37°C in culture medium. 20 U/mL heparin sodium salt and 25 U/mL benzonase nuclease were added to prevent cell clumping (Sigma-Aldrich, St. Louis, MO). CyTOF staining was performed at room temperature. Cells were stained with Cell-ID cisplatin (Fluidigm, San Francisco, CA) for viability, washed, and blocked with TruStain FcX (BioLegend, San Diego, CA) for 10 minutes. Cells were then stained with CyTOF antibodies labeled with rare earth metal isotopes using MaxPar reagent kits from Fluidigm (Table S2, Supplemental Digital Content 1) for 30 minutes. After fixation and permeabilization of the cells using the FoxP3 Staining Buffer Set (eBioscience, San Diego, CA), samples were barcoded using combinations of palladium isotopes as described (18), allowing the entire mixture of samples to be analyzed in a small number of CyTOF runs over 2 days. 18.75 μM iridium intercalator solution (Fluidigm, San Francisco, CA) was added to the cells, and cells were subsequently washed and reconstituted in Milli-Q filtered distilled water with EQ four element calibration beads (Fluidigm, San Francisco, CA). Cells were analyzed on a Helios CyTOF Mass Cytometer (Fluidigm, San Francisco, CA) (19).
Seurat analysis of CyTOF data
FCS files (74 samples) were read into R1 (v 4.2.0) using flowCore2 (v 2.8.0), and loaded into Seurat3 (v 4.1.1), a package generally used for the analysis of single-cell RNA sequencing and multi-omic data (20). Events were excluded from further analysis if they had a bead channel value ≥ 500, a DNA1 channel value ≥ 2500, a DNA2 channel value ≥ 4000, or a viability channel value ≥ 50, indicating beads, dead cells, debris, or doublets (2.8% of each sample on average).
5000 cells per patient sample were randomly selected. Marker intensities for these 370k cells were transformed by Seurat’s LogNormalize function, and a principal components-transformed version of the data used with Seurat’s IntegrateData function to combine the samples into a single dataset using as a reference two samples each of control total leukocytes, control PBMCs, trauma total leukocytes, and trauma PBMCs.
All cells were jointly clustered by the Leiden algorithm4, and clusters annotated as likely B cells (CD20+), T cells (CD3+), NK cells (CD56+), and monocytes (CD14+) were discarded. Remaining cells were subjected to a second round of clustering and discarding of non-neutrophils. The remaining stringently selected CD15+/CD66b+ neutrophils were clustered a final time by the Leiden algorithm with a resolution of 0.5The FindAllMarkers function of Seurat was used on log-transformed marker intensity data to identify surface expression markers of each cluster (Table S3, Supplemental Digital Content 1).
Statistical analysis of cluster proportions by sample type
A Kruskal-Wallis test with Benjamini-Hochberg false discovery rate correction was used to assess the difference between the fraction of neutrophils from different sample types in each cluster (Figure S1, Supplemental Digital Content 2). For those with a corrected false discovery rate < 0.1, Benjamini-Hochberg-corrected post-hoc Wilcoxon tests were used to determine which pairs of sample types differed significantly. Reporting conformed to STROBE guidelines (Checklist, Supplemental Digital Content 3).
Flow Cytometry
Venous blood was collected from a patient undergoing oncologic liver resection preoperatively and 5 hours into the operation. Total leukocytes and PBMCs were processed as described above. Cells were stained with fluorophore-conjugated antibodies against CD15 SSEA-1, CD66b, and total and activated CD11b and CD18, for 30 minutes on ice. Flow cytometry was performed on a BD LSRFortessa, LSR II (Becton Dickinson, Franklin Lakes, NJ) and data analyzed using FlowJo (Tree Star, San Francisco, CA).
Results
A subset of morphologically normal neutrophils sediment in the PBMC layer of whole blood and increase after trauma.
To investigate the effects of trauma on neutrophil heterogeneity, peripheral blood samples were collected from trauma patients (Table S1, Supplemental Digital Content 1) and normal controls for CyTOF analysis. Each blood sample was used to provide both a total leukocyte (TL) fraction, and a separate low-density ‘mononuclear cell fraction’ based on cell sedimentation in a Ficoll-Hypaque gradient (21) (Figure 1A), since neutrophils aberrantly sedimenting in this low-density layer have been previously described, particularly in the oncology literature (22). Indeed, morphologically fully mature-appearing neutrophils were observed in the low-density layer in both the trauma patients and the normal controls, and were quite prominent in samples from the trauma patients (Figure 1B). We did not observe immature band forms of neutrophils in the low-density layer, and if anything, the LDNs from the trauma patients appeared to be somewhat hypersegmented (Figure 1B).
Figure 1. Sample preparation and CyTOF identification of major leukocyte subsets including CD15+ /CD66b+ neutrophils.
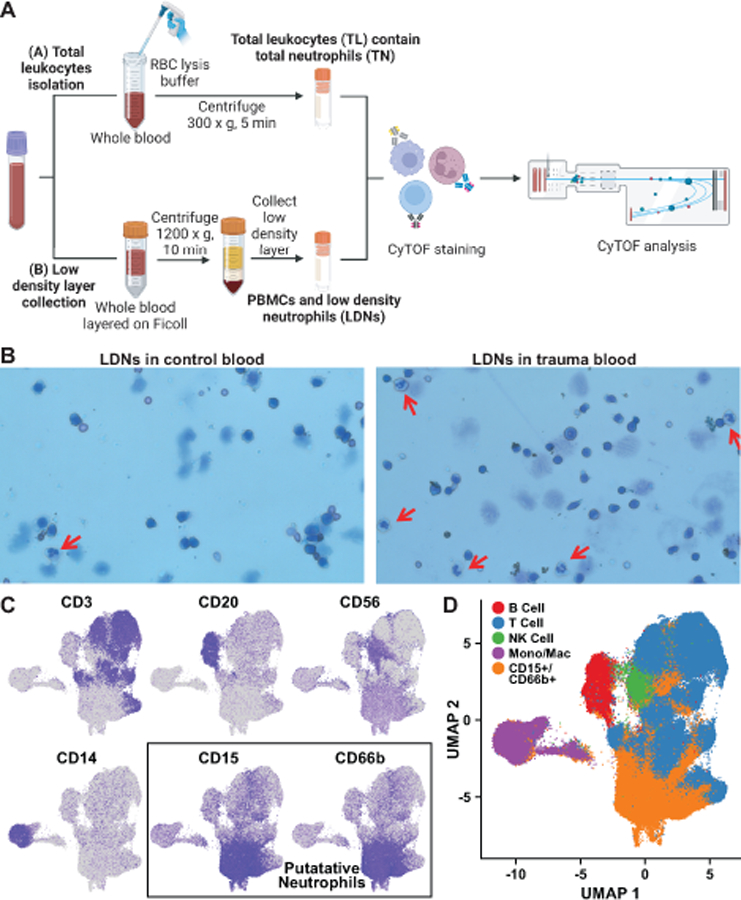
A. Separation procedure for purification of total leukocytes (TL) including total neutrophils (TN), and the PBMC layer including low-density neutrophils (LDN). B. Cells in the PBMC layer were visualized by Wright’s stain. Mature neutrophils are indicated by the red arrows. C, D. Markers including CD3, CD20, CD14, CD15, and CD66b (C) were used to identify clusters of cells as B cells, T cells, monocytes/macrophages, or neutrophils (D). Blue color intensity in individual marker plots indicates level of surface marker detection by CyTOF, log-normalized as described in methods, with the bluest color corresponding to the highest expression of that marker.
Both TLs, and cells from the mononuclear cell layer were then labeled using heavy metal-derivatized antibodies directed at a panel of 44 markers as described previously (Table S2, Supplemental Digital Content 1) (19). These markers were selected based on their ability to distinguish between different classes of leukocytes (T cells, B cells, monocytes, etc.) while also being enriched for surface markers relevant to neutrophil function. Following labeling, cells were subjected to mass cytometry and the levels of each of the 44 markers were quantified in all samples on a per-cell basis (Figure 1A).
CyTOF analysis identifies major leukocyte subsets including CD15+ /CD66b+ neutrophils.
To gain insight into this complex high-dimensional data, 5,000 cells from each of the total leukocyte fractions and from the mononuclear fractions of each sample were randomly subsampled, and all of the subsamples then combined together into a single large dataset and analyzed using Seurat, an R package designed for single-cell studies that clusters cells using weighted nearest-neighbors data (20). The data were then visualized using the Uniform Manifold Approximation and Projection (UMAP) (23) to create a 2-dimensional representation in which close proximity of adjacent points within the map, corresponding to individual cells, reflects strong similarity in surface marker expression (Figure S2, Supplemental Digital Content 2). As shown in Figure 1C-D, distinct clusters corresponding to putative B cells (CD20+ cells), T cells (CD3+ cells), NK cells (CD56+ cells), monocytes/macrophages (CD14+ cells) and neutrophils (CD15+/CD66b+ cells) were easily identified using these traditional cell-type markers.
Neutrophils can be categorized into eleven clusters based on surface marker expression, of which two are trauma-specific.
The CD15/CD66b double-positive cells, corresponding to neutrophils (Figures 1C-D), were then re-clustered for surface marker expression using Seurat, subjected to a second round of annotation to more stringently remove any non-neutrophil cell types, and the remaining data visualized with UMAP so that neutrophils that most similarly express the same set of surface markers appear near each other in the 2D plot (Figure 2A). Notably, as shown in the bottom of Figure 2A, a large region of the surface marker expression UMAP space, the upper left half, is populated by both control and trauma neutrophils. In contrast the lower right of the plot, as indicated by the blue outline, is disproportionately occupied by trauma neutrophils. This indicates that the space of neutrophil surface expression phenotypes displayed by control neutrophils is shared with trauma neutrophils, but there are unique surface marker phenotypes that are specific to the trauma neutrophil population. Moreover, a portion of the expression landscape that is occupied only by the trauma neutrophils is particularly highly enriched in low-density trauma neutrophils, a phenomenon not shared to nearly the same degree in control neutrophils (Figure 2A, compare dark red and dark black symbols).
Figure 2. Clustering identifies eleven varieties of neutrophil, two of which are trauma-specific.
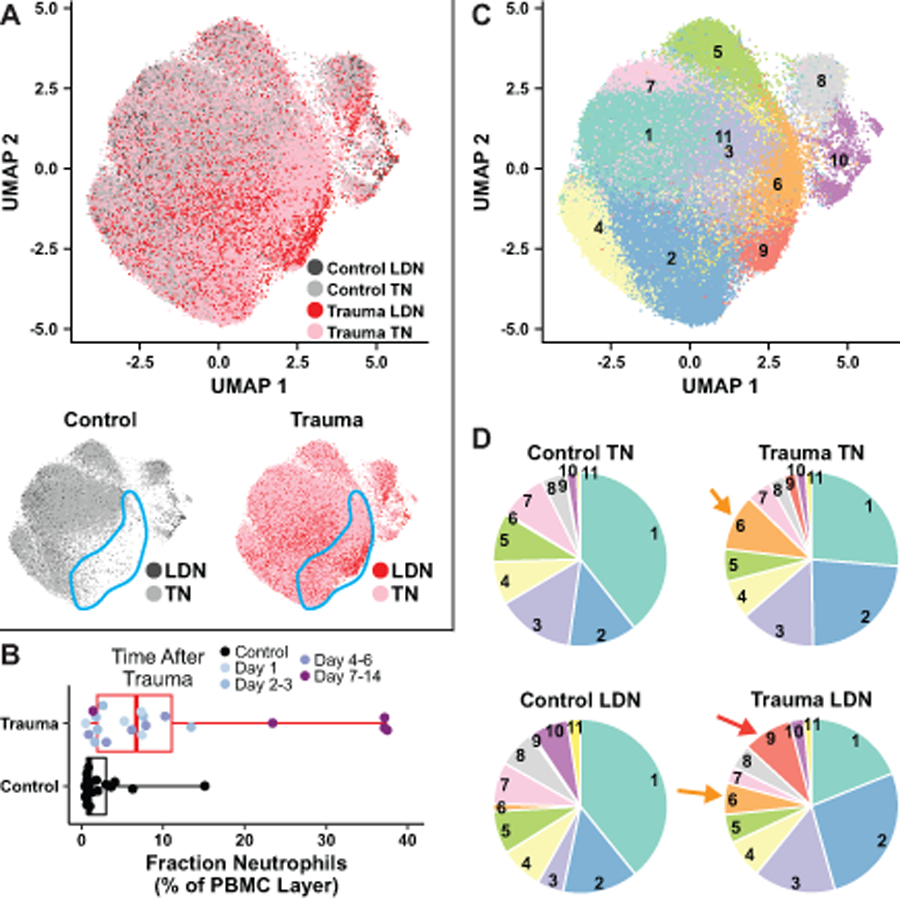
A. Above, CD15+, CD66b+ cells (neutrophils) from the entire collection of trauma patients and controls analyzed by Seurat and displayed in two dimensions with UMAP, with similar cells near each other, colored by sample type. Below, control neutrophils (left) and trauma neutrophils (right) in the same projection as in A are colored by their presence in total leukocytes (TN) or their co-sedimentation in the PBMC layer (LDN). The blue outlined area indicates a region of UMAP space occupied by trauma neutrophils but severely depleted of control neutrophils. B. Box-and-whisker plot showing percentage of cells sedimenting in the PBMC layer that were CD15+/CD66b+ in the control and trauma samples. Boxes cover the interquartile range (25–75th percentile) with a line marking the median, and whiskers extend through the full range of the data. C. UMAP projection from A, colored for each Seurat-defined neutrophil cluster. D. Pie charts indicate the total proportion of neutrophils of each sample type that belong to each of the 11 clusters. Orange arrows indicate cluster 6, present only in trauma neutrophils, and red arrow indicates cluster 9, present only in low-density trauma neutrophils.
Interestingly, in the control samples, very few of the cells sedimenting in the low-density layer were neutrophils, with a median value of ~1.0% percent of all cell types present (Figure 2B). Following trauma, however, the median percentage of neutrophils sedimenting in the mononuclear cell layer increased over 6-fold, and this enhanced percentage of LDNs was particularly noticeable at late times after trauma (Figure 2B). Taken together, these data indicate that trauma creates unique patterns of surface marker expression on peripheral blood neutrophils, and increases the population of neutrophils that co-sediment with mononuclear cells in the low-density layer. Seurat-based analysis of all CD15+/CD66b+ cells in the CyTOF dataset separated the entire population of cells from control and trauma patients into 11 distinct clusters of neutrophils (Figure 2C).
Notably, the proportion of cells in each cluster, however, varied with the type of sample from which the cells arose (Figure 2D). In the two largest clusters, cluster 1 and cluster 2, there is an evident shift of the total neutrophil population out of cluster 1, and into cluster 2, in the trauma samples relative to the controls, although this did not rise to the level of statistical significance (Figure S1, Supplemental Digital Content 2). There were other statistically significant differences between sample types, for example in clusters 5, 8, and 10. The most striking findings, however, were statistically significant differences in clusters 6 and 9 depending on whether the samples were controls or trauma patients. Almost none of the TNs or the LDNs from normal control samples were present in cluster 6, whereas both TNs and LDNs from trauma patients were found in this cluster. By contrast, cluster 9 was uniquely populated by low-density neutrophils from trauma patients, and was essentially absent in both total and low-density neutrophils from healthy controls. Analysis of the distribution of neutrophil clusters as a function of time after trauma is suggestive of an increase in clusters 6 and 9 at early times after trauma that then resolves, while the increase in cluster 2 appears to arise later (Figure S3, Supplemental Digital Content 2) although the limited number of samples in this study precludes a definitive statistical statement.
A population of CD10-low, CD177-high neutrophils (cluster 6) that is rare in control total neutrophils is enriched in low-density neutrophils and trauma neutrophils.
Analysis of individual markers for their prevalence in specific clusters (Figure S4, Supplemental Digital Content 2) revealed that neutrophils within cluster 6 expressed, on average, over 7.5-fold lower levels of CD10, a generally accepted marker of neutrophil maturity (6), than neutrophils in the other clusters (Figures 3A-C). The only upregulated marker present on cluster 6 neutrophils is CD177, which was expressed about 2.5-fold higher in cluster 6 relative to other clusters. Notably, all other surface markers significantly differentially expressed in cluster 6 neutrophils were depleted relative to their levels in other clusters (Figure 3C). While only 0.3% of control total neutrophils occupy cluster 6, control low-density neutrophils, trauma total neutrophils, and trauma low-density neutrophils belong to cluster 6 at rates of 1.4%, 10.4%, and 5.9%, respectively. On a per-sample basis, the relative absence of cluster 6 neutrophils in control total neutrophil samples is evident, and statistically significant (Figure 3D). As discussed below, we believe cluster 6 represents neutrophils recently mobilized from the bone marrow.
Figure 3. Cluster 6, highly enriched in trauma neutrophils, represents CD177-high, CD10-low immature neutrophils.
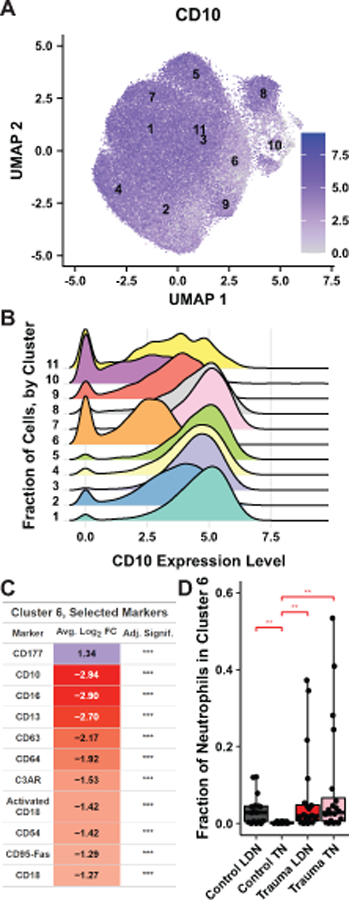
A. A UMAP projection as in Figure 3A colored by log-normalized surface expression of CD10, with cluster centroids labeled. B. A ridge plot indicates the distribution of CD10 expression level as shown in A, for each cluster. C. Average log2 fold-changes in surface expression of selected markers in cluster 6 neutrophils vs. neutrophils in all other clusters is shown, colored by sign and magnitude. Bonferonni-adjusted significance is given (*p<0.05, **p < 0.01, ***p < 0.001). D. Fraction of neutrophils of each sample that belong to cluster 6, grouped by sample type. Boxplots as described in Figure 3C. Stars indicate the significance of a post-hoc Benjamini-Hochberg corrected Wilcoxon test (*p<0.05, **p<0.01, ***p<0.001, ****p<10−4).
A cluster high in activated CD11b/CD18 integrin (cluster 9) is enriched among trauma low-density neutrophils.
Cluster 9 is a second neutrophil subpopulation uniquely found in trauma patients. Remarkably, neutrophils in cluster 9 express, on average, 5-fold as much activated CD11b as other neutrophils (Figure 4A), and 4.4-fold as much activated CD18 (Figure 4B). These two proteins are the components of integrin αMβ2, also known as the complement receptor CR3, which binds to a wide variety of ligands present at sites of injury, including fibrinogen, complement components (immobilized C3b, Factor H), and platelet factor 4, among others (24).
Figure 4. Low-density trauma neutrophils are enriched for a population with high levels of activated CD11b and CD18.
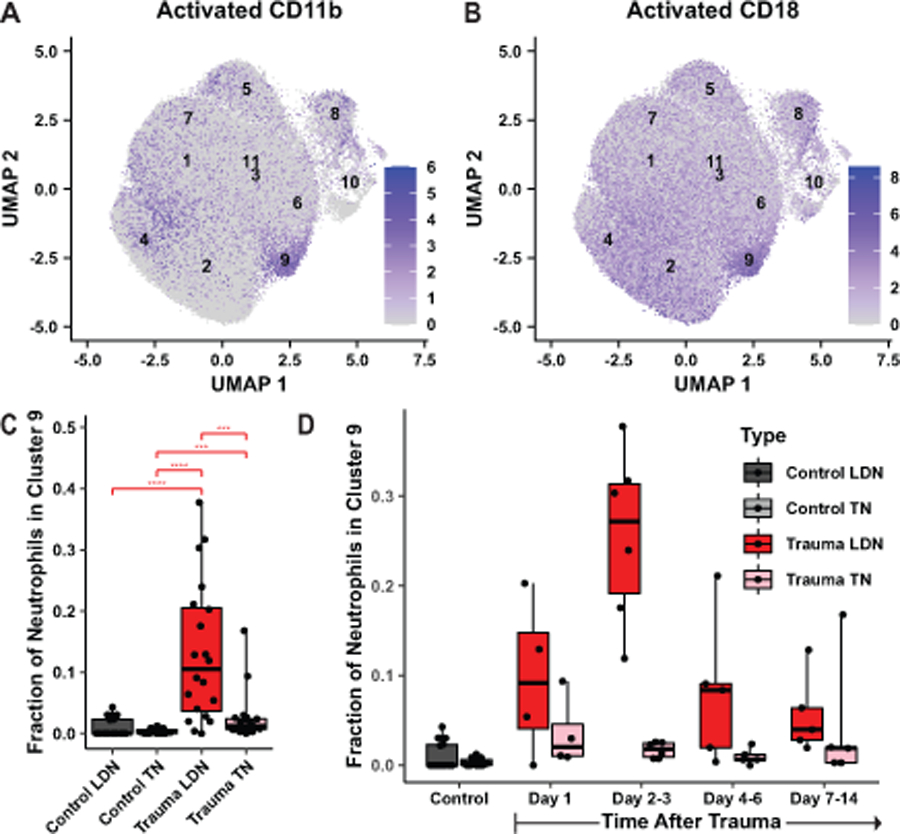
A, B. Log-normalized expression of activated CD11b (A) or activated CD18 (B) in all neutrophils is shown by intensity of blue color, with cluster centroids labeled. C, D. The fraction of neutrophils of each sample that belong to cluster 9 is shown, grouped by sample type (C) or time after trauma (D). Boxplots are as described in Figure 3C. Stars indicate the significance of a post-hoc Benjamini-Hochberg corrected Wilcoxon test (*p<0.05, **p<0.01, ***p<0.001, ****p<10−4).
Importantly, cluster 9 neutrophils are significantly and specifically enriched among trauma low-density neutrophils (9.2%) relative to trauma total neutrophils (2.3%), control total neutrophils (0.3%), and control low-density neutrophils (0.5%). All three of these differences are statistically significant on a per-sample basis (Figure 4C). The emergence of cluster 9 in the low-density neutrophil population in trauma samples appears to peak 2–3 days after trauma (Figure 4D).
In contrast to their activated forms, the total surface expression levels of CD11b and CD18 did not differ markedly from other clusters (Figure 5A). Other than activated CD11b/CD18, essentially every other surface receptor that recognizes neutrophil-activating ligands, including the receptors for IL-8 (CXCR1), IgG-Fc (CD64), leukotrienes (BLT1), the complement C3a fragment (C3AR), Fas ligand (CD95-Fas) and N-formyl peptides (FPR1, FPR2), had their surface expression significantly downregulated in the neutrophils in cluster 9 (Figure 5B).
Figure 5. Cluster 9 neutrophils are not highly enriched for total CD11b or CD18 and have reduced expression of neutrophil activating ligands.
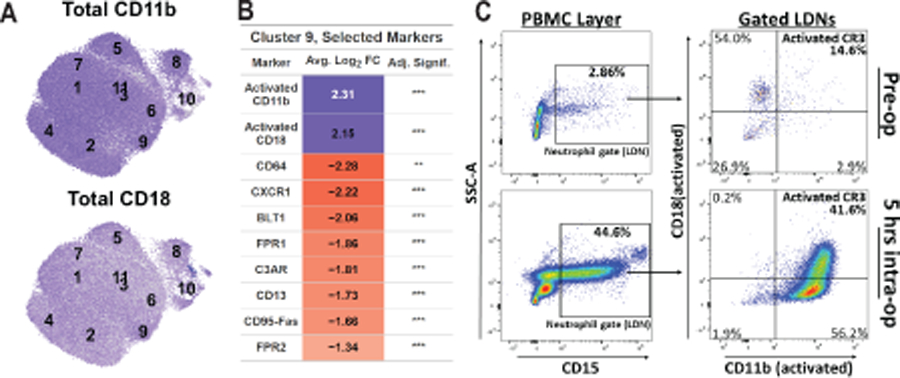
A. Log-normalized expression of total CD11b (top panel) or total CD18 (bottom panel) in all neutrophils is shown by intensity of blue color, with cluster centroids labeled. B. For a selection of markers, the average log2 fold-change of markers in cluster 9 neutrophils vs. all other neutrophils is given, colored by sign and magnitude. Bonferonni-adjusted significance is given (*p<0.05, **p < 0.01, ***p < 0.001). C. Flow cytometric analysis of cell surface expression of activated CD11b and CD18 in CD15+ LDNs within the PBMC layer of peripheral blood before (top panels) and during (bottom panels) a scheduled surgical hepatic resection.
This finding of a novel population of trauma-specific CD11b/CD18-activated neutrophils sedimenting in the PBMC layer was somewhat unexpected, and prompted us to ask whether the existence of this unique neutrophil subpopulation was specific to trauma patients, or might instead reflect an alteration in circulating neutrophils as part of a general physiological response to significant tissue damage. We therefore performed an experiment to further explore and preliminarily validate this surprising finding using cell fractionation followed by traditional flow cytometry with specific markers for activated CD11b/CD18 in a single patient during the process of an extensive hepatic resection for cancer. As shown in Figure 5C, prior to surgery the PBMC layer contained fewer than 3% LDNs, of which over 85% did not contain an active CD11b/CD18 heterodimer. Strikingly, 5 hours into the hepatic resection, LDNs comprised nearly 45% of cells in the PBMC layer, with over 40% of these LDNs displaying both activated CD11b and activated CD18 on their surface. Taken together, we interpret these findings as evidence that significant tissue injury, either from accidental trauma or from surgery itself, results in the appearance in the peripheral circulation of previously activated neutrophils that co-sediment with PBMCs in a low-density layer upon density gradient centrifugation, consistent with neutrophil degranulation (15). In addition, the loss of cell surface expression of receptors capable of further activating these cells is in good agreement with the designation of low-density neutrophils as a potentially immunosuppressive cell type.
Enhanced expression of single cell surface markers appears to define other neutrophil subtypes.
Finally, we noted that, although the majority of the 44 surface markers showed relatively broad expression in both trauma and control neutrophils (Figure S4, Supplemental Digital Content 2), when individual surface markers were analyzed on a per-cluster basis, a number of clusters appear to be defined by high cell surface expression of a single cell surface marker, often corresponding to a single specific receptor sub-type (Figure 6A). For example, neutrophils in cluster 4, 5, 7, and 8 have uniquely high expression of C5L2, TLR4, CD20, and CXCR2, respectively. These findings suggest that neutrophil heterogeneity may result, in part, as a consequence of functional specialization of neutrophils for activation by specific types of ligands, although this remains to experimentally tested.
Figure 6. A model for changes in neutrophil heterogeneity in response to trauma.
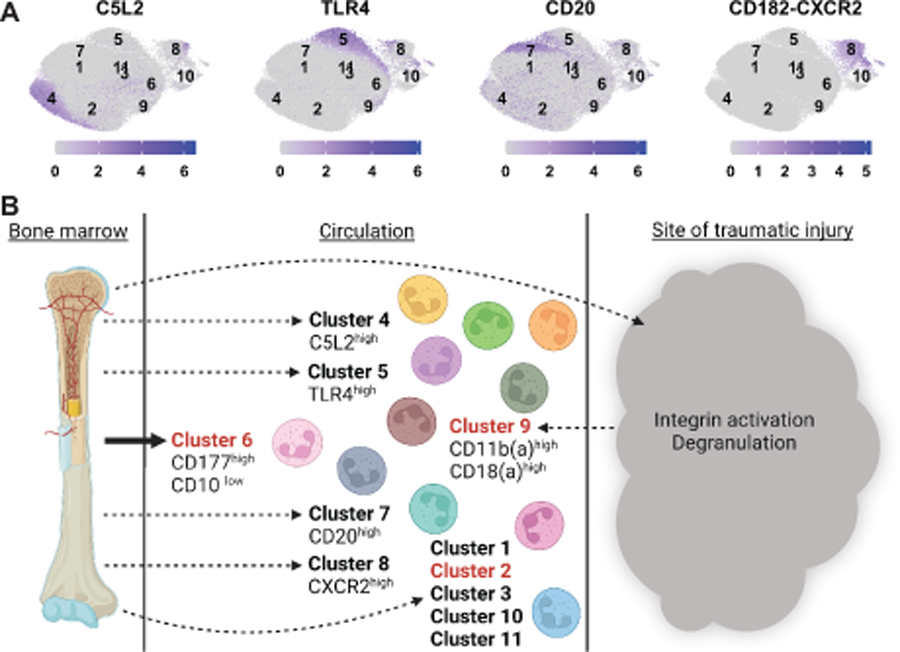
A. Log-normalized expression of indicated markers, chosen to exemplify heterogenous distribution of expression, is shown by intensity of blue color, with cluster centroids labeled. B. Eleven neutrophil subpopulations are observed in trauma patients and healthy controls. Several clusters (4, 5, 7, and 8) each show enhanced expression of one particular type of surface receptor relative to all other neutrophil subtypes. Two clusters (6 and 9), are specific to trauma. Cluster 6 neutrophils appear to be newly released from the bone marrow. Cluster 9 contains low-density neutrophils, which display signs of having been activated and degranulated as a consequence of signaling from the site of injury.
Discussion
Neutrophils have long been considered a homogeneous population of phagocytic cells that function at early times in the innate immune response. In this work we demonstrate significant heterogeneity in neutrophils at the level of surface marker expression in normal humans, show that this baseline heterogeneity is markedly altered by trauma and tissue damage, and identify 2 unique trauma-specific neutrophil subpopulations (Figure 6B).
One of these populations was distinguished by high levels of CD177 and low levels of CD10. The function of CD177, a GPI-anchored surface molecule lacking an intracellular domain, and absent in 3–5% of normal individuals, remains enigmatic. Artificial activation of CD177 with antibodies has been shown to enhance signaling through β2 integrins and impair neutrophil migration (25), but the natural ligand for CD177 is unknown. CD177 is best appreciated as a marker of bone marrow neutrophils and on circulating neutrophils mobilized from the bone marrow in response to G-CSF treatment (26, 27). In contrast, CD10, a transmembrane glycoprotein with neutral endopeptidase activity, is specifically expressed by mature neutrophils during their terminal stage of differentiation (28), and was previously reported to have decreased surface expression on circulating neutrophils from patients after thermal injury (29). Our finding of a specific population of neutrophils that display both elevated CD177 and decreased CD10 expression suggests that this population is composed of relatively immature neutrophils that have recently been mobilized from the bone marrow. Indeed, Monneret and colleagues identified CD177 mRNA as the single most upregulated gene product in neutrophils from patients with septic shock, which was similarly negatively correlated with the levels of CD10 mRNA (30), and proposed that this population was a marker of sepsis. Our findings suggest instead that this neutrophil subpopulation arises from a pathophysiological need for emergency granulopoiesis regardless of cause.
A second trauma-specific neutrophil population was distinguished by low-density, high levels of the conformationally activated forms of CD11b/CD18, and reduced expression of essentially every other inflammatory ligand receptor on their surface. These neutrophils almost certainly represent neutrophils that have been previously activated by either inside-out or outside-in integrin signaling (31), and have released their granule contents. Both of these features are entirely consistent with an immunosuppressive neutrophil phenotype described by Ochoa and colleagues in humans after trauma (15), and by Schmielau and Finn in humans with advanced cancer (32), although the immunomodulatory effect of these neutrophils on T-cell proliferation was not directly explored in the current work. Whether these neutrophils remained fully within the circulation throughout their activation process, or represent marginated or previously extravasated neutrophils that have then returned to the circulation from the site of injury is not known, but merits future investigation. Our finding that this circulating neutrophil subpopulation can be created in response to both accidental trauma and following elective surgical liver resection in a highly controlled setting suggests that these cells may contribute to the functional immunosuppressive state that occurs after significant tissue damage regardless of etiology. In principle, serially monitoring this cell population over time might assist in evaluating the overall immune status of patients during the convalescent phase after injury/surgery.
Finally, among the 11 distinct classes of neutrophils identified in both normal controls and trauma patients, several classes had similar levels of expression of most common surface markers but higher levels of one specific marker. This finding suggests an element of functional specialization whereby specific subsets of neutrophils are specifically wired to respond preferentially to a particular signal such as LPS-binding to TLR4 in cluster 5 neutrophils (33), or C5a-binding to C5L2 in cluster 4 neutrophils (34). An integrative model of our findings on changes in neutrophil heterogeneity in response to trauma is shown in Figure 6B.
There are several limitations to our study. First, our study consisted of phenotypic neutrophil characterization of 12 trauma patients, some sampled at multiple times, along with matched controls. It will be important in future studies to further validate the 11 distinct neutrophil clusters using a larger patient cohort. Secondly, we did not experimentally validate that some or all of the low-density neutrophils that observed after tissue trauma are functionally immunosuppressive, although similar studies performed by others in the setting of both trauma and cancer have shown this (15, 35, 36). Finally, the concept of functional neutrophil specialization, with specific subpopulations of neutrophils predisposed to preferentially respond to a particular activating signal based on selective surface expression of high levels of a particular receptor merits additional experimental investigation. In particular, it will be important in future experiments to directly compare the immunosuppressive properties of cluster 9 neutrophils, and further delineate the mechanisms underlying the putative functions associated with this phenotype.
Supplementary Material
Supplemental Digital Content 1. Supplemental Tables S1-S3. xlsx
Supplemental Digital Content 2. Supplemental Figures S1-S4. pdf
Supplemental Digital Content 3. STROBE Checklist. docx
Acknowledgements
We gratefully acknowledge intellectual contributions from Drs. Charles H. Cook, Xueyang Yu, Stephanie Gregory, and other members of the Yaffe laboratory and HALO consortium. Some figures were created using BioRender.
Funding Disclosure
The work was funded by DoD Peer Reviewed Medical Research Program, Contract Number W81XWH-16-1-0464, NIH grants ES028374, P30-ES002109, AI148232, AI138318, and NIH intramural funds, and grants from the Charles and Marjorie Holloway Foundation and the MIT Center for Precision Cancer Medicine.
Footnotes
Conflict of Interest Statement
The authors have no conflicts of interest related to the current work.
This study was presented at the 81st Annual Meeting of the American Association for the Surgery of Trauma, September 22, 2022, in Chicago, IL.
References
- 1.Huber-Lang M, Lambris JD, Ward PA. Innate immune responses to trauma. Nat Immunol 2018;19(4):327–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burn GL, Foti A, Marsman G, Patel DF, Zychlinsky A. The Neutrophil. Immunity 2021;54(7):1377–91. [DOI] [PubMed] [Google Scholar]
- 3.Finlay LD, Conway Morris A, Deane AM, Wood AJ. Neutrophil kinetics and function after major trauma: A systematic review. World J Crit Care Med 2021;10(5):260–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Condliffe AM, Kitchen E, Chilvers ER. Neutrophil priming: pathophysiological consequences and underlying mechanisms. Clin Sci (Lond) 1998;94(5):461–71. [DOI] [PubMed] [Google Scholar]
- 5.Barrett CD, Hsu AT, Ellson CD, B YM, Kong YW, Greenwood JD, et al. Blood clotting and traumatic injury with shock mediates complement-dependent neutrophil priming for extracellular ROS, ROS-dependent organ injury and coagulopathy. Clin Exp Immunol 2018;194(1):103–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grieshaber-Bouyer R, Nigrovic PA. Neutrophil Heterogeneity as Therapeutic Opportunity in Immune-Mediated Disease. Front Immunol 2019;10:346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silvestre-Roig C, Hidalgo A, Soehnlein O. Neutrophil heterogeneity: implications for homeostasis and pathogenesis. Blood 2016;127(18):2173–81. [DOI] [PubMed] [Google Scholar]
- 8.Hedrick CC, Malanchi I. Neutrophils in cancer: heterogeneous and multifaceted. Nat Rev Immunol 2022;22(3):173–87. [DOI] [PubMed] [Google Scholar]
- 9.Shaul ME, Eyal O, Guglietta S, Aloni P, Zlotnik A, Forkosh E, et al. Circulating neutrophil subsets in advanced lung cancer patients exhibit unique immune signature and relate to prognosis. FASEB J 2020;34(3):4204–18. [DOI] [PubMed] [Google Scholar]
- 10.Zhu YP, Eggert T, Araujo DJ, Vijayanand P, Ottensmeier CH, Hedrick CC. CyTOF mass cytometry reveals phenotypically distinct human blood neutrophil populations differentially correlated with melanoma stage. J Immunother Cancer 2020;8(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quail DF, Amulic B, Aziz M, Barnes BJ, Eruslanov E, Fridlender ZG, et al. Neutrophil phenotypes and functions in cancer: A consensus statement. J Exp Med 2022;219(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pillay J, Kamp VM, van Hoffen E, Visser T, Tak T, Lammers JW, et al. A subset of neutrophils in human systemic inflammation inhibits T cell responses through Mac-1. J Clin Invest 2012;122(1):327–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blanco-Camarillo C, Aleman OR, Rosales C. Low-Density Neutrophils in Healthy Individuals Display a Mature Primed Phenotype. Front Immunol 2021;12:672520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hardisty GR, Llanwarne F, Minns D, Gillan JL, Davidson DJ, Gwyer Findlay E, et al. High Purity Isolation of Low Density Neutrophils Casts Doubt on Their Exceptionality in Health and Disease. Front Immunol 2021;12:625922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bryk JA, Popovic PJ, Zenati MS, Munera V, Pribis JP, Ochoa JB. Nature of myeloid cells expressing arginase 1 in peripheral blood after trauma. J Trauma 2010;68(4):843–52. [DOI] [PubMed] [Google Scholar]
- 16.Vonwirth V, Bulbul Y, Werner A, Echchannaoui H, Windschmitt J, Habermeier A, et al. Inhibition of Arginase 1 Liberates Potent T Cell Immunostimulatory Activity of Human Neutrophil Granulocytes. Front Immunol 2020;11:617699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seshadri A, Brat GA, Yorkgitis BK, Giangola M, Keegan J, Nguyen JP, et al. Altered monocyte and NK cell phenotypes correlate with posttrauma infection. J Trauma Acute Care Surg 2019;87(2):337–41. [DOI] [PubMed] [Google Scholar]
- 18.Zunder ER, Finck R, Behbehani GK, Amir el AD, Krishnaswamy S, Gonzalez VD, et al. Palladium-based mass tag cell barcoding with a doublet-filtering scheme and single-cell deconvolution algorithm. Nat Protoc 2015;10(2):316–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Emmons TR, Giridharan T, Singel KL, Khan ANH, Ricciuti J, Howard K, et al. Mechanisms Driving Neutrophil-Induced T-cell Immunoparalysis in Ovarian Cancer. Cancer Immunol Res 2021;9(7):790–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hao Y, Hao S, Andersen-Nissen E, Mauck WM, 3rd, Zheng S, Butler A, et al. Integrated analysis of multimodal single-cell data. Cell 2021;184(13):3573–87 e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cui C, Schoenfelt KQ, Becker KM, Becker L. Isolation of polymorphonuclear neutrophils and monocytes from a single sample of human peripheral blood. STAR Protoc 2021;2(4):100845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scapini P, Marini O, Tecchio C, Cassatella MA. Human neutrophils in the saga of cellular heterogeneity: insights and open questions. Immunol Rev 2016;273(1):48–60. [DOI] [PubMed] [Google Scholar]
- 23.McInnes L, Healy J, Melville J. Umap: Uniform manifold approximation and projection for dimension reduction. arXiv preprint arXiv:180203426 2018.
- 24.Lamers C, Pluss CJ, Ricklin D. The Promiscuous Profile of Complement Receptor 3 in Ligand Binding, Immune Modulation, and Pathophysiology. Front Immunol 2021;12:662164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bai M, Grieshaber-Bouyer R, Wang J, Schmider AB, Wilson ZS, Zeng L, et al. CD177 modulates human neutrophil migration through activation-mediated integrin and chemoreceptor regulation. Blood 2017;130(19):2092–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Kleijn S, Kox M, Sama IE, Pillay J, van Diepen A, Huijnen MA, et al. Transcriptome kinetics of circulating neutrophils during human experimental endotoxemia. PLoS One 2012;7(6):e38255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Passamonti F, Pietra D, Malabarba L, Rumi E, Della Porta MG, Malcovati L, et al. Clinical significance of neutrophil CD177 mRNA expression in Ph-negative chronic myeloproliferative disorders. Br J Haematol 2004;126(5):650–6. [DOI] [PubMed] [Google Scholar]
- 28.Elghetany MT. Surface antigen changes during normal neutrophilic development: a critical review. Blood Cells Mol Dis 2002;28(2):260–74. [DOI] [PubMed] [Google Scholar]
- 29.McCormack RT, Nelson RD, Solem LD, LeBien TW. Decreased expression of the common acute lymphoblastic leukaemia antigen (CALLA/CD10) on neutrophils from patients with thermal injury. Br J Haematol 1988;69(2):189–95. [DOI] [PubMed] [Google Scholar]
- 30.Demaret J, Venet F, Plassais J, Cazalis MA, Vallin H, Friggeri A, et al. Identification of CD177 as the most dysregulated parameter in a microarray study of purified neutrophils from septic shock patients. Immunol Lett 2016;178:122–30. [DOI] [PubMed] [Google Scholar]
- 31.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell 2002;110(6):673–87. [DOI] [PubMed] [Google Scholar]
- 32.Schmielau J, Finn OJ. Activated granulocytes and granulocyte-derived hydrogen peroxide are the underlying mechanism of suppression of t-cell function in advanced cancer patients. Cancer Res 2001;61(12):4756–60. [PubMed] [Google Scholar]
- 33.Park BS, Lee JO. Recognition of lipopolysaccharide pattern by TLR4 complexes. Exp Mol Med 2013;45:e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cain SA, Monk PN. The orphan receptor C5L2 has high affinity binding sites for complement fragments C5a and C5a des-Arg(74). J Biol Chem 2002;277(9):7165–9. [DOI] [PubMed] [Google Scholar]
- 35.Ochoa JB, Strange J, Kearney P, Gellin G, Endean E, Fitzpatrick E. Effects of L-arginine on the proliferation of T lymphocyte subpopulations. JPEN J Parenter Enteral Nutr 2001;25(1):23–9. [DOI] [PubMed] [Google Scholar]
- 36.Saraiva DP, Correia BF, Salvador R, de Sousa N, Jacinto A, Braga S, et al. Circulating low density neutrophils of breast cancer patients are associated with their worse prognosis due to the impairment of T cell responses. Oncotarget 2021;12(24):2388–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content 1. Supplemental Tables S1-S3. xlsx
Supplemental Digital Content 2. Supplemental Figures S1-S4. pdf
Supplemental Digital Content 3. STROBE Checklist. docx


