Abstract
We describe a case of neoehrlichiosis in an immunocompetent child with acute febrile illness in South Africa. Neoehrlichiosis was diagnosed by PCR on 16S rDNA from bone marrow aspirate. Phylogenetic analysis indicated an organism closely related to Candidatus Neoehrlichia. Clinicians should be aware of possible ehrlichiosis even in immunocompetent patients.
Keywords: neoehrlichiosis, zoonoses, vector-borne infections, bacteria, Candidatus Neoehrlichia, ehrlichiosis, tickborne infections, South Africa
Ehrlichia species and related organisms are obligate intracellular membrane-bound bacteria transmitted via ticks between a range of mammalian animal hosts, including humans. In recent years, taxonomy and phylogeny of these bacterial species has been revised. The family Anaplasmataceae comprises 5 genera: Ehrlichia, Anaplasma, Neorickettsia, Neoehrlichia, and Wolbachia. However, the number of recognized species and candidate species has substantially increased in recent years. We describe a case of neoehrlichiosis in an immunocompetent child in South Africa.
The Study
A previously healthy 9-year-old boy from Eastern Cape Province, South Africa, was admitted to the hospital with a 3-week history of fever, back pain, and myalgia. Symptoms failed to respond to a course of azithromycin prescribed by his general practitioner for suspected tickbite fever. The child lived on a farm and was exposed to a variety of domestic companion and farm animals, but he had no history of recent tick bites or travel.
At admission, results of a physical examination were unremarkable, except for a temperature of >40°C. The patient had no rash, skin lesions, lymphadenopathy, or clinically detectable hepatosplenomegaly. Laboratory investigation results were unremarkable (Table). The patient received empiric treatment with ceftriaxone for 5 days, but symptoms did not improve.
Table. Laboratory and imaging investigations in a case of neoehrlichiosis in a symptomatic immunocompetent child, South Africa.
| Investigation | Patient result | Reference value or range |
|---|---|---|
| C-reactive protein, mg/L | 17.2 | <5 |
| Leukocytes, × 109 cells/L | 15.8 | 4.5–13.5 |
| Neutrophils, × 109 cells/L | 10.98 | 1.50–8.5 |
| Lymphocytes, × 109 cells/L | 2.99 | 1.0–6.5 |
| Monocytes, × 109 cells/L | 1.50 | 0–0.8 |
| Platelets, × 109 cells /L | 219 | 140–420 |
| Hemoglobin, g/dL |
12.7 |
11.5–15.5 |
| Blood cultures | No growth | NA |
| Urine culture | No growth | NA |
| Rickettsia conorii/R. africae serology and PCR on blood | Negative | NA |
| SARS-CoV-2 PCR, nasopharyngeal swab and serology | Negative | NA |
| Cytomegalovirus PCR and serology | Negative | NA |
| Epstein-Barr serology | Negative | NA |
| Widal test | Negative | NA |
| Mastazyme Brucella ELISA | IgM and IgG equivocal | NA |
| Multiplex PCR for atypical and viral respiratory pathogens* | Negative | NA |
| QuantiFERON Gold test for tuberculosis† |
Negative |
NA |
| Imaging | ||
| Ultrasound of the abdomen | 15 cm spleen | 10–11 cm at this age |
| Chest radiograph | No abnormalities detected | NA |
| Cervical and thoracic spine radiograph | No abnormalities detected | NA |
| Transthoracic echocardiography | No abnormalities detected | NA |
| Magnetic resonance imaging spine | No abnormalities detected | NA |
*FilmArray RP2.1plus (BioFire Diagnostics, https://www.biofiredx.com). †QuantiFERON, https://www.quantiferon.com.
Clinicians considered brucellosis because of initial equivocal Brucella serologic test results, despite absence of definite exposure history, and began treatment with doxycycline, rifampin, and gentamicin. The patient gradually experienced partial improvement over the next week.
Clinicians performed bone marrow aspirate and trephine to exclude malignancy and investigate possible brucellosis; results showed a reactive inflammatory background. PCR for Brucella spp. was negative, and cultures yielded no growth. However, PCR and sequencing of a 598-bp section of the hypervariable 5 to 8 regions of the 16S rRNA gene (1), revealed probable Ehrlichia species.
Clinicians discontinued rifampin and gentamicin but continued doxycycline for a total of 14 days, and the symptoms completely resolved. The child remained well at follow-up 12 months later. The child’s mother gave written informed consent for publication, and approval was granted by the Human Research Ethics Committee of the University of Cape Town (reference no. 551/2022).
We subjected the bone marrow samples to a reverse line blot hybridization assay for simultaneous detection and differentiation of the most common Ehrlichia and Anaplasma spp. by using a PCR targeting the 16S rRNA gene (2,3). PCR product hybridized with the Ehrlichia/Anaplasma genus-specific probe but not with any species-specific probes, suggesting a novel species or variant of a known species.
We PCR amplified the near full-length 16S rRNA gene (1,467 bp) using primers fD1 and rP2 (4), cloned and sequenced the resultant amplicons. We assembled and edited the generated sequences using the Staden Package (https://staden.sourceforge.net). The consensus sequence was deposited in Genbank (accession no. OP208838). BLASTn (https://blast.ncbi.nlm.nih.gov) homology search results revealed no identical sequences in the public databases. The most closely related sequences, at 96%–98% identity, were various Candidatus Neoehrlichia 16S rDNA sequences, including Candidatus N. mikurensis (strain TK4456R), Candidatus N. lotoris (strain RAC413R), and Candidatus N. arcana (strain HT94R) (Figure).
Figure.
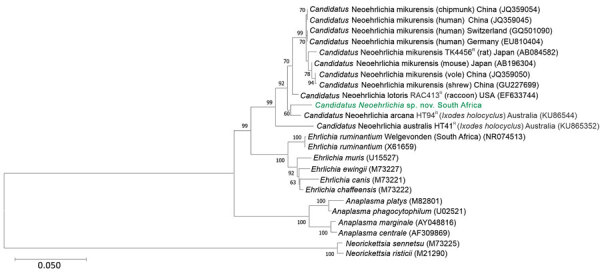
Phylogenetic tree of Candidatus Neoehrlichia species detected in a case of neoehrlichiosis in a symptomatic immunocompetent child, South Africa. We used Kimura 2-parameter plus gamma plus invariable site substitution model in MEGA X (https://www.megasoftware.net) to infer a maximum-likelihood phylogenetic tree in combination with the bootstrap method using 1,000 replicates. Green bold text indicates Candidatus Neoehrlichia species detected from the patient in this case-study; we compared this isolate to other Anaplasmataceae species detected from ticks, humans, and mammals available in GenBank (indicated by species name and GenBank accession number; host species and country are given for other Candidatus Neoehrlichia species). Scale bar indicates nucleotide substitutions per site.
We aligned the consensus sequence with closely related 16S rDNA sequences from Genbank by using ClustalX (http://www.clustal.org). We manually examined the alignments and truncated to smallest sequence size, a 1,264-bp Candidatus N. australis sequence, by using BioEdit 7.2.5 (https://bioedit.software.informer.com). We used MEGA X (https://www.megasoftware.net) to determine Kimura 2-parameter plus gamma plus invariable site substitution as the best-fit model. We used it in combination with the bootstrap method using 1,000 replicates to infer a maximum-likelihood phylogenetic tree, which indicated that our obtained sequence clustered with Candidatus N. arcana, although with low bootstrap support (60%) (Figure). The sequence was closely related to, but distinct from, Candidatus N. lotoris and Candidatus N. mikurensis. As previously shown (5,6), Candidatus Neoehrlichia mikurensis, Candidatus N. lotoris, and Candidatus N. arcana are distinct from other genera in the Anaplasmataceae family and form a well-supported sister clade to the genus Ehrlichia. On the basis of whole-genome sequencing, Candidatus N. mikurensis appears to be more closely related to Ehrlichia chaffeensis than to E. ruminantium, suggesting that establishing the genus Neoehrlichia might have been premature (7).
We calculated estimated evolutionary divergence by determining the number of nucleotide differences between similar sequences. We removed all ambiguous positions for each sequence pair (pairwise deletion option), which left a total of 1,264 positions in the final dataset. Our obtained sequence differed from GenBank reference strains by 23 bp for Candidatus N. lotoris, 24 bp for Candidatus N. arcana, and 34 bp for Candidatus N. mikurensis. These reference species differ by 46–50 bp from each other. Our findings confirmed that the ehrlichial organism we identified is a member of the family Anaplasmataceae and most closely related to, but distinct from, Candidatus N. lotoris and Candidatus N. arcana. Until appropriate type-material can be deposited and the species is formally described, we will refer to this novel organism as Candidatus Neoehrlichia sp. South Africa (OP208838).
Conclusions
We report a novel ehrlichial organism, most closely related to the genus Candidatus Neoehrlichia, a sister genus to Anaplasma and Ehrlichia, detected in the bone marrow aspirate of an acutely febrile immunocompetent child from South Africa. Candidatus N. lotoris has been described from raccoons in Georgia, USA, and an associated tick species (7), but it has not been detected in humans or any other wild vertebrate species. Candidatus N. arcana has been described from Ixodes holocyclus ticks in Australia (8), but its pathogenic significance has not yet been determined (6). Candidatus N. mikurensis was described in 2004 (5), and a human case was reported in 2010 (9). Candidatus N. mikurensis appears to be widely distributed in Europe (10), especially in central Europe and Scandinavia, as well as in Asia (11). Data from Africa are limited, but Candidatus N. mikurensis has been isolated from dog ticks in Nigeria (12). The host reservoir of Candidatus N. mikurensis is small mammals, and its vectors include Ixodes and Haemaphysalis tick species (10).
Most human neoehrlichia infections described to date have been caused by Candidatus N. mikurensis, and clinical findings are usually nonspecific, including fever, headache, and myalgia; however, vasculitis and thrombo-embolic events can occur (13). Infections are more commonly recognized in immunocompromised patients; infections in immunocompetent patients can be mild or asymptomatic (14).
Another probable clinical neoehrlichia case from Africa was reported in 2013 in a traveler who returned to Denmark from Tanzania (15). The researchers described a potentially novel neoehrlichia species isolated from the patient’s blood. On the basis of analysis of short, 300–345-bp fragments, the species was closely related to both Candidatus N. lotoris and Candidatus N. mikurensis (15).
Ehrlichiae and related organisms are usually detected via molecular methods, including PCR of 16S rRNA, or groEL/groESL and gltA genes. Application of new molecular methods is leading to an explosion in discovery of related novel species or variants, as noted in Australia (6) and elsewhere. Recognition of associated human disease is increasing, especially in immunocompetent patients. Further elucidation of the taxonomic status of the novel ehrlichial organism identified in this case study would require whole-genome sequencing or multilocus sequence analysis targeting genes for which Neoehrlichia gene sequences are available, including 16S rRNA or ftsZ, gatB, groEL, and lipA.
Although tickborne rickettsial infections are common in South Africa, Anaplasmataceae infections likely are undiagnosed because of lack of awareness among clinicians and unavailability of laboratory tests. Of note, Ehrlichia spp. are not susceptible to macrolides and our case would not have been recognized if the patient had initially been treated with doxycycline.
In conclusion, our case represents an expansion of the known range of neoehrlichiosis. Clinicians should be aware of neoehrlichia as a possible tickborne cause of acute febrile illness in Africa.
Acknowledgments
We thank Emma Hooijberg for reviewing some of the microscopic images of the bone marrow aspirate preparation.
Biography
Dr. Bamford is a clinical microbiologist with Pathcare and the University of Cape Town, East London, South Africa. Her primary research interests include antimicrobial resistance and stewardship as well as the appropriate use of laboratory diagnostic tests.
Footnotes
Suggested citation for this article: Bamford C, Blumberg LH, Bosman M, Frean J, Hoek KGP, Miles J, et al. Neoehrlichiosis in symptomatic immunocompetent child, South Africa. Emerg Infect Dis. 2023 Feb [date cited]. https://doi.org/10.3201/eid2902.221451
These authors contributed equally to this article.
References
- 1.Xu J, Smyth CL, Buchanan JA, Dolan A, Rooney PJ, Millar BC, et al. Employment of 16 S rDNA gene sequencing techniques to identify culturable environmental eubacteria in a tertiary referral hospital. J Hosp Infect. 2004;57:52–8. 10.1016/j.jhin.2004.01.011 [DOI] [PubMed] [Google Scholar]
- 2.Bekker CP, de Vos S, Taoufik A, Sparagano OA, Jongejan F. Simultaneous detection of Anaplasma and Ehrlichia species in ruminants and detection of Ehrlichia ruminantium in Amblyomma variegatum ticks by reverse line blot hybridization. Vet Microbiol. 2002;89:223–38. 10.1016/S0378-1135(02)00179-7 [DOI] [PubMed] [Google Scholar]
- 3.Schouls LM, Van De Pol I, Rijpkema SG, Schot CS. Detection and identification of Ehrlichia, Borrelia burgdorferi sensu lato, and Bartonella species in Dutch Ixodes ricinus ticks. J Clin Microbiol. 1999;37:2215–22. 10.1128/JCM.37.7.2215-2222.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weisburg WG, Barns SM, Pelletier DA, Lane DJ. 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol. 1991;173:697–703. 10.1128/jb.173.2.697-703.1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawahara M, Rikihisa Y, Isogai E, Takahashi M, Misumi H, Suto C, et al. Ultrastructure and phylogenetic analysis of ‘Candidatus Neoehrlichia mikurensis’ in the family Anaplasmataceae, isolated from wild rats and found in Ixodes ovatus ticks. Int J Syst Evol Microbiol. 2004;54:1837–43. 10.1099/ijs.0.63260-0 [DOI] [PubMed] [Google Scholar]
- 6.Gofton AW, Doggett S, Ratchford A, Ryan U, Irwin P. Phylogenetic characterisation of two novel Anaplasmataceae from Australian Ixodes holocyclus ticks: ‘Candidatus Neoehrlichia australis’ and ‘Candidatus Neoehrlichia arcana’. Int J Syst Evol Microbiol. 2016;66:4256–61. 10.1099/ijsem.0.001344 [DOI] [PubMed] [Google Scholar]
- 7.Grankvist A, Jaén-Luchoro D, Wass L, Sikora P, Wennerås C. Comparative genomics of clinical isolates of the emerging tick-borne pathogen Neoehrlichia mikurensis. Microorganisms. 2021;9:1488. 10.3390/microorganisms9071488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gofton AW, Doggett S, Ratchford A, Oskam CL, Paparini A, Ryan U, et al. Bacterial profiling reveals novel “Ca. Neoehrlichia”, Ehrlichia, and Anaplasma species in Australian human-biting ticks. PLoS One. 2015;10:e0145449. 10.1371/journal.pone.0145449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Welinder-Olsson C, Kjellin E, Vaht K, Jacobsson S, Wennerås C. First case of human “Candidatus Neoehrlichia mikurensis” infection in a febrile patient with chronic lymphocytic leukemia. J Clin Microbiol. 2010;48:1956–9. 10.1128/JCM.02423-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Portillo A, Santibáñez P, Palomar AM, Santibáñez S, Oteo JA. ‘Candidatus Neoehrlichia mikurensis’ in Europe. New Microbes New Infect. 2018;22:30–6. 10.1016/j.nmni.2017.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jha P, Kim CM, Kim DM, Yoon NR, Jha B, Park JW, et al. First detection and identification of Candidatus Neoehrlichia mikurensis in South Korea. PLoS One. 2018;13:e0209685. 10.1371/journal.pone.0209685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamani J, Baneth G, Mumcuoglu KY, Waziri NE, Eyal O, Guthmann Y, et al. Molecular detection and characterization of tick-borne pathogens in dogs and ticks from Nigeria. PLoS Negl Trop Dis. 2013;7:e2108. 10.1371/journal.pntd.0002108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Höper L, Skoog E, Stenson M, Grankvist A, Wass L, Olsen B, et al. Vasculitis due to Candidatus Neoehrlichia mikurensis: a cohort study of 40 Swedish patients. Clin Infect Dis. 2021;73:e2372–8. 10.1093/cid/ciaa1217 [DOI] [PubMed] [Google Scholar]
- 14.Welc-Falęciak R, Siński E, Kowalec M, Zajkowska J, Pancewicz SA. Asymptomatic “Candidatus Neoehrlichia mikurensis” infections in immunocompetent humans. J Clin Microbiol. 2014;52:3072–4. 10.1128/JCM.00741-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwameis M, Auer J, Mitteregger D, Simonitsch-Klupp I, Ramharter M, Burgmann H, et al. Anaplasmataceae-specific PCR for diagnosis and therapeutic guidance for symptomatic neoehrlichiosis in immunocompetent host. Emerg Infect Dis. 2016;22:281–4. 10.3201/eid2202.141762 [DOI] [PMC free article] [PubMed] [Google Scholar]


