Abstract
Streptococcus dysgalactiae increasingly is recognized as a pathogen of concern for human health. However, longitudinal surveillance data describing temporal trends of S. dysgalactiae are scarce. We retrospectively identified all β-hemolytic streptococcal bloodstream infections reported in Bergen, in western Norway, during 1999–2021. To explore S. dysgalactiae disease burden in a broader context, we mapped the incidence of all microbial species causing bloodstream infections during 2012–2021. We found S. dysgalactiae incidence rates substantially increased during the study period; by 2021, S. dysgalactiae was the fifth most common pathogen causing bloodstream infections in our region. We noted genotypic shifts and found that the rising trend was related in part to the introduction and expansion of the stG62647 emm-type. S. dysgalactiae is among the most common causes of bloodstream infections in western Norway, and increased surveillance and unambiguous species identification are needed to monitor the disease burden attributable to this pathogen.
Keywords: Streptococcus dysgalactiae, bacteria, streptococci, beta-hemolytic streptococcus, bloodstream infections, epidemiology, incidence rates, Norway
Streptococcus dysgalactiae is a potent pathogen that has similar disease manifestations as S. pyogenes, including cellulitis, necrotizing soft tissue infections, and streptococcal toxic shock syndrome (1–3). Nevertheless, in contrast to the well-established surveillance networks for S. pyogenes and S. agalactiae infections in many countries, epidemiologic reports on S. dysgalactiae are scarce (4–6).
The S. dysgalactiae epithet and delineation of its 2 subspecies, dysgalactiae and equisimilis, was proposed as late as 1996, and until recently identification of this pathogen has relied predominantly on phenotypic β-hemolysis and a Lancefield agglutination reaction (7). However, the promiscuous repertoire of Lancefield antigens potentially displayed by S. dysgalactiae (mostly C or G, occasionally A or L) has caused considerable taxonomic confusion (8). In addition, S. equi harbors the C antigen and S. canis harbors the G antigen, which adds further ambiguity to speciation. Although S. equi and S. canis rarely cause human disease, their added ambiguity further illustrates that the Lancefield classification lacks taxonomic resolution to the species level for the group C and G Streptococcus (GCGS).
Clinical and epidemiologic features of S. dysgalactiae have often been fragmented and concealed in separate scientific reports on group C and group G Streptococcus, a practice that continues to some extent (9–12). This practice has likely underestimated S. dysgalactiae incidence rates and clouded its clinical significance. Moreover, most recent publications involving precise identification of S. dysgalactiae comprise insufficient sample sizes or observation periods to evaluate temporal trends (3,13–15).
Drawing power from the enhanced taxonomic precision of sequencing technology and mass spectrometry, we revisited S. dysgalactiae bloodstream infection epidemiology in a health region in western Norway during the past 23 years. Moreover, to explore S. dysgalactiae disease burden in a broader context, we compared S. dysgalactiae incidence against all other pathogens that cause bloodstream infections.
Methods
Study Setting and Definitions
Health Region Bergen (Bergen, Norway) had a catchment area of ≈460,000 inhabitants in 2021 and encompasses the tertiary care institution Haukeland University Hospital and 2 local hospitals, Haraldsplass Deaconess Hospital and Voss Hospital. The region is served by a single microbiology laboratory located at Haukeland University Hospital.
We reviewed the electronic records of the microbiology laboratory and retrospectively identified all bloodstream infections caused by S. pyogenes, S. agalactiae, and S. dysgalactiae in Health Region Bergen during 1999–2021. We also extracted information on specimen type, sample date and species identification, as well as patient age, sex, and residential postal code.
We defined bloodstream infection as microbial growth in blood cultures collected from patients in a hospital setting. We considered cases from which repeated cultures were positive for the same identified organism within 30 days of initial isolation as a single bloodstream infection. To avoid referral bias, we excluded patients with a residential postal code outside the catchment areas of the 3 hospitals at the time of specimen sampling.
To explore the incidence of S. dysgalactiae bloodstream infections in a wider context, we also retrospectively identified bloodstream infections caused by other pathogens during 2012–2021 in our region. We confined the study period to a decade that had relatively stable standards and protocols for pathogen identification in the routine microbiology laboratory. The main tool for species identification during this era was matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry and the MALDI Biotyper database (Bruker Daltonik, https://www.bruker.com), which received annual or biannual updates from the manufacturer.
Bacterial Identification of β-hemolytic Streptococci
Primary identification in the routine microbiology laboratory was based on β-hemolytic reaction on 5% sheep-blood agar, colony size of >0.5 mm after 24 h incubation, and Lancefield serogroup specificity (Oxoid, http://www.oxoid.com). Identification of β-hemolytic streptococci was supplemented with MALDI-TOF mass spectrometry beginning in 2012. For this study, we retrieved all β-hemolytic streptococcal isolates identified in bloodstream infections during 1999–2021 from the freezer and confirmed species identity by MALDI-TOF mass spectrometry. Moreover, we taxonomically verified all strains identified as S. dysgalactiae, S. canis, S. equi, or S. pyogenes by using either emm typing (16) or whole genome sequencing (17), as previously described. A subset of these strains had already been characterized by emm typing in previous studies (1,17).
Statistical Analyses
We calculated incidence rates by using contemporary population data extracted from Statistics Norway (https://www.ssb.no). We tested temporal trends for statistical significance by using negative binomial regression analysis and evaluated nonparametric data by using the Mann Whitney U-test. We considered a 2-sided p value <0.05 statistically significant. We used SPSS Statistics 26.0 (IBM, https://www.ibm.com) for statistical analyses, Excel 2016 (Microsoft, https://www.crosoft.com) to create graphs, and R version 4.1.2 (The R Foundation for Statistical Computing, https://www.r-project.org) to construct violin plots. Regional Ethics Committee West, Norway, conducted institutional ethics review and approved this study (approval no. 2021/350196).
Results
A total of 1,179 cases of β-hemolytic streptococcal bloodstream infections were detected during 1999–2021, comprising 309 cases of S. pyogenes, 513 cases of S. agalactiae, and 354 cases of S. dysgalactiae. We excluded 3 Lancefield group C isolates identified as S. equi from the analysis. No cases of S. canis bloodstream infection were detected. Whole-genome sequencing identified one S. dysgalactiae isolate as S. dysgalactiae subspecies dysgalactiae (18), whereas the remaining 353 isolates belonged to the subspecies equisimilis.
We plotted temporal trends for β-hemolytic streptococcal bloodstream infections (Figure 1). The incidence rate of S. dysgalactiae increased significantly from 1.4 to 7.6 (incidence rate ratio [IRR] 1.064, 95% CI 1.045–1.083; p<0.0001) per 100,000 inhabitants, during 1999–2021; by the end of the study period S. dysgalactiae had become the leading cause of β-hemolytic streptococcal bloodstream infections. Conversely, S. pyogenes displayed decreasing incidence rates (IRR 0.969, 95% CI 0.949–0.988; p = 0.002), whereas we detected no significant temporal change for S. agalactiae bloodstream infections (IRR 1.006, 95% CI 0.989–1.024; p = 0.46).
Figure 1.
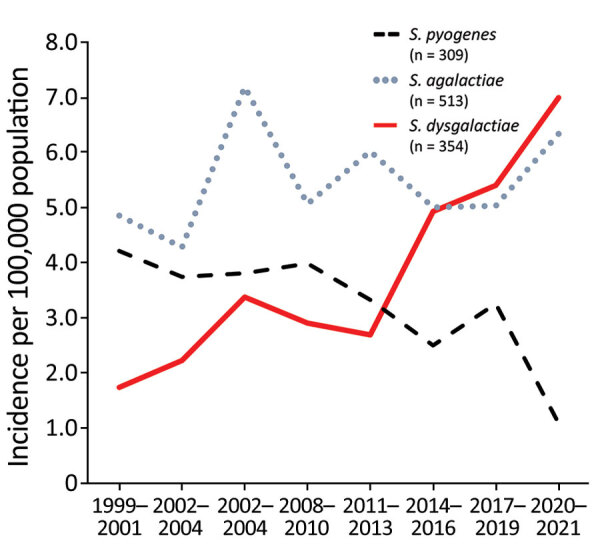
β-hemolytic streptococcal bloodstream infections caused by Streptococcus pyogenes, S. agalactiae, and S. dysgalactiae, western Norway, 1999–2021. We calculated incidence rates for β-hemolytic streptococcal bloodstream infections in Health Region Bergen, Bergen, Norway, in 3-year periods, except 2020–2021. We calculated incidence rates as the total number of cases during each time period, divided by the number of years in the period.
Reviewing all bloodstream infections in Health Region Bergen during 2012–2021, we identified 12,346 cases caused by 292 different bacterial species (Appendix Table 1). We excluded 1,678 cases of coagulase-negative staphylococci from our study because we considered these species to be likely skin contaminants. Thus, we included 10,668 cases of bloodstream infection for further analysis. We ranked the most frequent pathogens detected per year and calculated the relative proportion of each pathogen (Figure 2).
Figure 2.
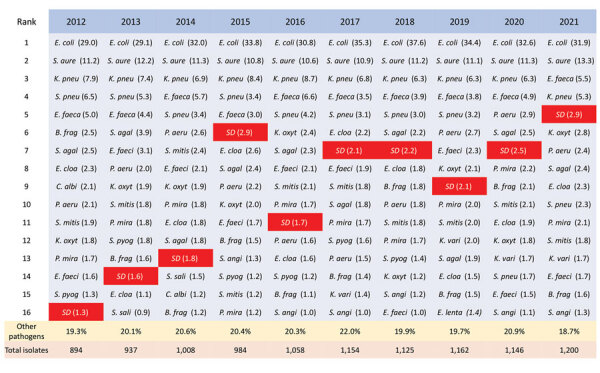
Leading causes of bloodstream infections, western Norway, 2012–2021. We investigated bloodstream infections in Health Region Bergen, Bergen, Norway, and ranked the most common pathogens detected in each year by frequency. Red shading indicates Streptococcus dysgalactiae (abbreviated as SD). Numbers in parentheses represent the percentage of all identified cases of bloodstream infections for each pathogen. Percentages of other pathogens detected and the total number of bloodstream infections per year are shown across the bottom. B. frag, Bacteroides fragilis; C. albi, Candida albicans; E. cloa, Enterobacter cloacae; E.coli, Escherichia coli; E. faeca, Enterococcus faecalis; E. faeci, Enterococcus faecium; E. lenta, Eggerthella lenta; K. oxyt, Klebsiella oxytoca; K. pneu, Klebsiella pneumoniae; K. vari, Klebsiella variicola; P. aeru, Pseudomonas aeruginosa; P. mira, Proteus mirabilis; S. agal, Streptococcus agalactiae; S. angi, Streptococcus anginosus; S. aure, Staphylococcus aureus; S. mitis, Streptococcus mitis; S. pneu, Streptococcus pneumoniae; S. pyog, Streptococcus pyogenes; S. sali, Streptococcus salivarius.
The proportion of bloodstream infections caused by S. dysgalactiae gradually expanded from 1.3% to 2.9% during the study period, and S. dysgalactiae climbed from being the 16th most frequent common in 2012 to the 5th most common in 2021. We noted significantly increasing incidence rates for 3 pathogens: S. dysgalactiae (IRR 1.086, 95% CI 1.037–1.137; p<0.0001), Escherichia coli (IRR 1.038, 95% CI 1.018–1.058; p = 0.0002), and Staphylococcus aureus (IRR 1.031, 95% CI 1.011–1.052; p = 0.002). We found that rates of S. pneumoniae bloodstream infections decreased significantly during 2012–2021 (IRR 0.915, 95% CI 0.878–0.953; p<0.0001).
The 3 β-hemolytic streptococcal species were associated with different profiles of age distribution (Figure 3). S. dysgalactiae was intimately linked to higher age (median 75 years, range 4–100 years), and only 2 patients with S. dysgalactiae bloodstream infections were <18 years of age. The S. dysgalactiae cases were among much older patients than were S. pyogenes (median age 53 years, range 1–95 years; p<0.0001) and S. agalactiae (median age 64 years, range 0–99 years; p<0.0001) cases. In fact, the median age in the S. dysgalactiae group was significantly higher than that for any other major streptococcal pathogen (p<0.0001), including S. pneumoniae (median age 67 years), S. mitis/oralis (median age 65 years), S. anginosus (median age 62 years), S. salivarius (median age 61 years), and S. sanguinis (median age 71 years) (Figure 4).
Figure 3.
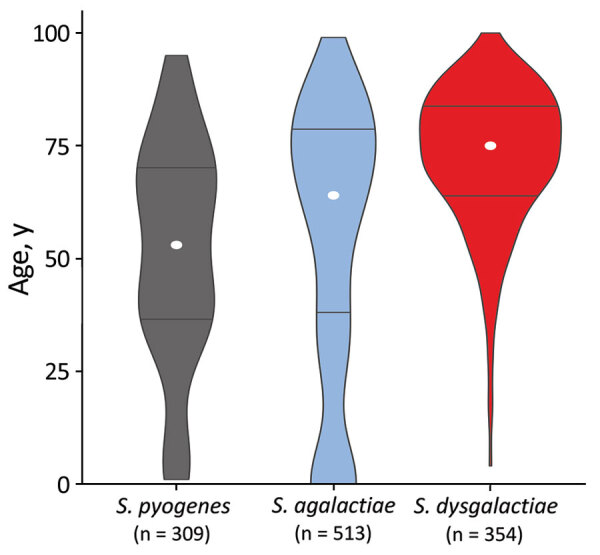
Age distribution for patients with β-hemolytic streptococcal bloodstream infections, western Norway, 1999–2021. The violin plot is based on 1,176 cases of β-hemolytic streptococcal bloodstream infection in Health Region Bergen, Bergen, Norway. The total number of cases is indicated for each species. The width of each figure corresponds to the proportion of patients in that age group. White circles represent the median age and horizontal bars indicate interquartile range.
Figure 4.
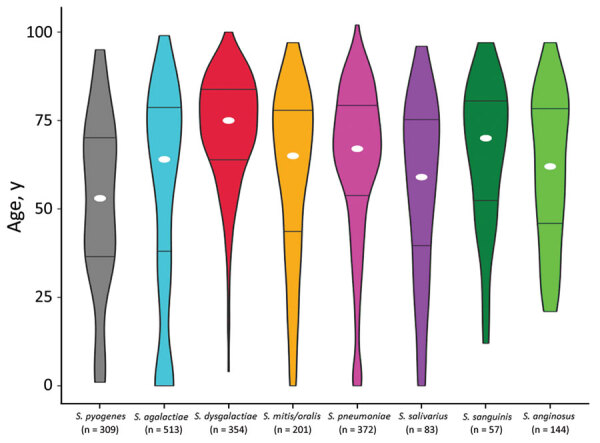
Age distribution for patients with streptococcal bloodstream infections, western Norway, 1999–2021. The violin plot is based on 2,033 cases of bloodstream infection caused by the 8 most common streptococcal species in Health Region Bergen, Bergen, Norway. The total number of cases is indicated for each species. The width of each figure corresponds to the proportion of patients in that age group. White circles indicate the median age and horizontal bars indicate interquartile range.
We also assessed emm-type distribution among the S. dysgalactiae bloodstream infection isolates (Appendix Table 2). We detected high genotypic diversity, comprising 30 different emm-types. Genotypic shifts during the study period were evident, and emm-type stG62647 gradually became dominant beginning in 2014 (Figure 5). Inversely, 1 major emm-type from early in the study, stG643, was infrequently detected after 2015. The remaining emm-types displayed annual fluctuations, but we observed no temporal trend. Two isolates were not available for emm sequencing.
Figure 5.
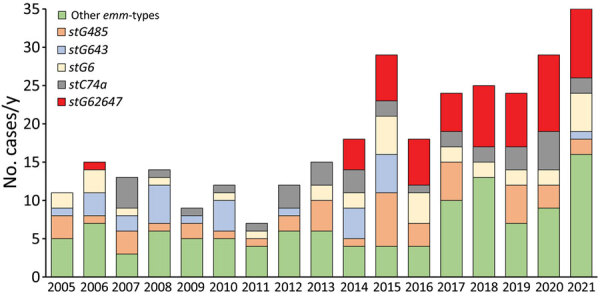
Distribution of the most common emm-types among Streptococcus dysgalactiae bloodstream infections, western Norway, 2005–2021. We identified most common emm-types among 351 S. dysgalactiae bloodstream infections in Health Region Bergen, Bergen, Norway. Two isolates were not available for typing, and the S. dysgalactiae subspecies dysgalactiae isolate could not be typed.
Discussion
In this large epidemiologic study of invasive S. dysgalactiae bloodstream infections in western Norway, we found that S. dysgalactiae is rapidly emerging as a potent pathogen and currently is the fifth most common cause of bloodstream infections in the Bergen health region. S. dysgalactiae also appears to have surpassed S. pyogenes and S. agalactiae as the leading cause of invasive β-hemolytic streptococcal disease in Japan and Finland (2,3). Moreover, studies from several geographic regions, including Canada, Hungary, Denmark, and Australia, have noted substantial increases in incidence rates for invasive GCGS infections during the past decade (19–22).
Unfortunately, many larger epidemiologic studies on β-hemolytic streptococcal infections have been hampered by taxonomic imprecision. Several studies report separate incidence curves for group C and group G Streptococcus, and some have even exclusively included group G Streptococcus (10,11,23,24). The lack of species identification is also reflected in the scarce published national surveillance data.
National surveillance on antimicrobial resistance in Norway reports combined annual incidence rates of GCGS bloodstream infections (25). In line with our findings, GCGS has been the leading β-hemolytic streptococcal pathogen in Norway since 2017 (Figure 6, panel A). Although not necessarily equivalent to S. dysgalactiae, GCGS is a fair estimate of incidence, as demonstrated by the data retrieved from our health region in this study. Nevertheless, the GCGS epithet represents an aggregate of several bacterial species and might easily shroud the magnitude of S. dysgalactiae infections from clinicians.
Figure 6.
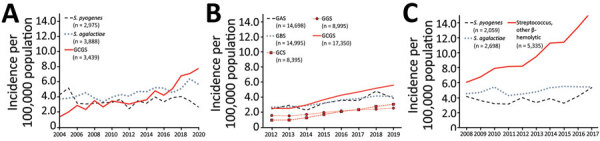
National surveillance data for β-hemolytic streptococcal bloodstream infections in 3 countries: A) Norway, 2004–2020; B) United Kingdom, 2012–2019; C) Finland, 2008–2017. We compiled data from annual surveillance reports published during the given time periods (6,25–27). The 3 countries use different surveillance methods. UK surveillance is based on voluntary reporting from the microbiology laboratories, whereas in Norway and Finland, surveillance data are collected electronically from the laboratories’ information systems. We calculated incidence rates by acquiring contemporary population data from Norway (https://www.ssb.no), the United Kingdom (https://www.ons.gov.uk), and Finland (https://www.stat.fi). We used taxonomic labels that appeared in the original publications, except GCGS, which we constructed for the purpose of this study by combining incidence data for GCS and GGS. GAS, group A Streptococcus; GBS, group B Streptococcus; GCS, group C Streptococcus; GGS, group G Streptococcus; GCGS, group C and G Streptococcus.
The United Kingdom has monitored β-hemolytic streptococcal bloodstream infections for several decades but tracks group C and group G Streptococcus infections by separate incidence curves (Figure 6, panel B) (12). However, that practice can make group C and group G Streptococcus appear to be relatively uncommon causes of bloodstream infections. Combining those incidence curves could reveal a completely different picture.
In Finland, the annual incidence rates of invasive S. pyogenes and S. agalactiae disease are reported separately, whereas S. dysgalactiae infections are included in the other β-hemolytic streptococci category (6). Of note, the category of other β-hemolytic streptococci greatly expanded during 2008–2017 to become the 4th most common cause of bloodstream infections in Finland (Figure 6, panel C).
Such taxonomic imprecision is detrimental to the surveillance of S. dysgalactiae infections. The Lancefield agglutination reaction is a cheap and pragmatic method for sorting β-hemolytic streptococcal isolates but lacks sufficient phylogenetic resolution. To report reliable and comprehensible epidemiologic data, identification to the species level is necessary. MALDI-TOF mass spectrometry is a relatively cheap and efficient alternative, but concerns have been raised about its ability to discern S. dysgalactiae from S. canis and S. pyogenes (28). However, modifications and updates to the MALDI-TOF database has largely resolved this issue (29). Thus, we propose that future epidemiologic surveillance and publications report the identity of S. dysgalactiae to the species level.
The increasing burden of S. dysgalactiae disease is likely multifactorial. In line with findings from previous reports, our reports showed this pathogen exhibited a predilection for older persons (3,22). This preference could be a consequence of immunosenescence but likely also reflects the burden of underlying conditions. Some reports have identified predisposing factors in >90% of the invasive S. dysgalactiae cases (13,30). One report explored risk factors for invasive β-hemolytic streptococcal disease and found the highest average Charlson Comorbidity Index score among patients with GCGS bloodstream infections, including a strong association with diabetes (22). In Norway, the estimated life expectancy increased by >5 years during the study period (https://www.ssb.no), and the prevalence of diabetes in the population of Norway has doubled in the past decade (https://www.fhi.no). These developments are projected to continue; thus, the epidemiologic trend for invasive S. dysgalactiae disease is unlikely to revert.
Shorter term abrupt changes in incidence rates could be related in part to epidemiologic shifts in circulating emm-types. We previously documented the introduction of the stG62647 emm-type into our health region in 2013 (17). This emm-type appears to have continued to expand and constituted a major contribution to the increasing rates of S. dysgalactiae bloodstream infections.
In line with our findings, the stG62647 genotype appears to have gained a strong foothold in several geographic regions, accounting for 47% of the S. dysgalactiae isolates in Germany during 2012–2016 (15), 31% in Canada during 2012–2014 (13), and 26% in Switzerland during 2006–2015 (14). The rapid expansion of this genotype could be related to a lack of herd immunity in the population, but the emergence of a clone with enhanced virulence potential cannot be ruled out. We previously documented a disruption of the streptococcal invasive locus in this genotype, a feature previously associated with increased virulence (17,31). However, the exact pathogenetic implications of this genetic event have yet to be experimentally elucidated.
Historically, S. dysgalactiae has been considered an opportunistic microbe with low pathogenicity, but its potential for severe disease manifestations should not be disregarded. In a large, prospective, multicenter study (32), S. dysgalactiae was the second most common cause of monomicrobial necrotizing soft tissue infections, and mortality rates for S. dysgalactiae cases were even higher than for S. pyogenes cases. Moreover, >40% of case-patients experienced septic shock. Recent data from the Swedish Registry of Infective Endocarditis indicated that S. dysgalactiae endocarditis was associated with an aggressive clinical course, and rates of embolic events and surgical intervention comparable to endocarditis caused by Staphylococcus aureus (33). Of note, the stG62647 genotype was reported as the predominant emm-type in both these studies.
Our study is limited by a retrospective design, increasing the risk for taxonomic misclassification. We cannot rule out the possibility that improved taxonomic resolution during the study period could account for some of the increase in the S. dysgalactiae disease burden. However, during a thorough review and reconfirmation of all registered cases of β-hemolytic streptococcal infections, including S. canis and S. equi, we did not identify evidence for such misclassification of the streptococcal isolates.
Prevention measures taken against the SARS-CoV-2 pandemic had a substantial effect on incidence rates of bacterial diseases, particularly for those caused by airway pathogens (34). In line with those effects, epidemiology of bloodstream infections observed in our study might have been influenced to some extent by restrictions during the early pandemic phases, for instance, the rapid decline of S. pneumoniae bloodstream infections observed in 2020. Nevertheless, the rising trend of S. dysgalactiae predates the pandemic on both a national and international level; this pathogen became the 6th most common cause of bloodstream infections in our region by 2015.
In conclusion, our findings indicate that S. dysgalactiae is a potent and common pathogen that should not be overlooked. Enhanced taxonomic precision of this streptococcal species is needed to ensure that epidemiologic data are unambiguous and comprehensible. Moreover, increased attention to S. dysgalactiae disease is warranted, and S. dysgalactiae should be included in national surveillance programs on equal terms with S. pyogenes and S. agalactiae.
Additional information and bacterial strains for Streptococcus dysgalactiae and other bloodstream infections, Norway, 2019–2021.
Acknowledgments
We thank the staff at the microbiological department at Haukeland University Hospital for excellent technical assistance and for providing access to their laboratory facilities. We particularly acknowledge Haima Mylvaganam for her significant contribution to streptococcal research at Haukeland University Hospital during the past 2 decades and for inspiration to continued scientific exploration.
This study received financial contributions from the Western Norway Regional Health Authority (grant no. 912231).
Biography
Dr. Oppegaard is an infectious disease specialist at the Department of Infectious Diseases, Haukeland University Hospital, Bergen, Norway. He is also an associate professor at the University of Bergen, Norway. His research interests focuses on beta-hemolytic streptococcal infections, spanning from clinical features and epidemiology to dissection of microbial genetics and host responses.
Footnotes
Suggested citation for this article: Oppegaard O, Glambek M, Skutlaberg DH, Skrede S, Sivertsen N, Kittang BR. Streptococcus dysgalactiae bloodstream infections, Norway, 1999–2021. Emerg Infect Dis. 2023 Feb [date cited]. https://doi.org/10.3201/eid2902.221218
References
- 1.Oppegaard O, Mylvaganam H, Kittang BR. Beta-haemolytic group A, C and G streptococcal infections in Western Norway: a 15-year retrospective survey. Clin Microbiol Infect. 2015;21:171–8. 10.1016/j.cmi.2014.08.019 [DOI] [PubMed] [Google Scholar]
- 2.Rantala S, Vuopio-Varkila J, Vuento R, Huhtala H, Syrjänen J. Clinical presentations and epidemiology of β-haemolytic streptococcal bacteraemia: a population-based study. Clin Microbiol Infect. 2009;15:286–8. 10.1111/j.1469-0691.2008.02672.x [DOI] [PubMed] [Google Scholar]
- 3.Wajima T, Morozumi M, Hanada S, Sunaoshi K, Chiba N, Iwata S, et al. Molecular Characterization of Invasive Streptococcus dysgalactiae subsp. equisimilis, Japan. Emerg Infect Dis. 2016;22:247–54. 10.3201/eid2202.141732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.US Centers for Disease Control and Prevention. Active bacterial core surveillance [cited 2022 Nov 16]. www.cdc.gov/abcs/reports-findings/surv-reports.html
- 5.Australian Government, Department of Health and Aged Care. Australian national notifiable diseases case definition [cited 2022 Nov 16]. https://www.health.gov.au/resources/publications/invasive-group-a-streptococcal-disease-igas-surveillance-case-definition
- 6.Finnish Institute for Health and Welfare. Infectious diseases in Finland 2017. Helsinki: The Institute; 2017. [Google Scholar]
- 7.Vandamme P, Pot B, Falsen E, Kersters K, Devriese LA. Taxonomic study of lancefield streptococcal groups C, G, and L (Streptococcus dysgalactiae) and proposal of S. dysgalactiae subsp. equisimilis subsp. nov. Int J Syst Bacteriol. 1996;46:774–81. 10.1099/00207713-46-3-774 [DOI] [PubMed] [Google Scholar]
- 8.Facklam R. What happened to the streptococci: overview of taxonomic and nomenclature changes. Clin Microbiol Rev. 2002;15:613–30. 10.1128/CMR.15.4.613-630.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bradley SF, Gordon JJ, Baumgartner DD, Marasco WA, Kauffman CA. Group C streptococcal bacteremia: analysis of 88 cases. Rev Infect Dis. 1991;13:270–80. 10.1093/clinids/13.2.270 [DOI] [PubMed] [Google Scholar]
- 10.Leitner E, Zollner-Schwetz I, Zarfel G, Masoud-Landgraf L, Gehrer M, Wagner-Eibel U, et al. Prevalence of emm types and antimicrobial susceptibility of Streptococcus dysgalactiae subsp. equisimilis in Austria. Int J Med Microbiol. 2015;305:918–24. 10.1016/j.ijmm.2015.10.001 [DOI] [PubMed] [Google Scholar]
- 11.Lo HH, Cheng WS. Distribution of virulence factors and association with emm polymorphism or isolation site among beta-hemolytic group G Streptococcus dysgalactiae subspecies equisimilis. APMIS. 2015;123:45–52. 10.1111/apm.12305 [DOI] [PubMed] [Google Scholar]
- 12.UK Health Security Agency. Laboratory surveillance of pyogenic and non-pyogenic streptococcal bacteraemia in England: 2020 update. Health Protection Report, vol. 15, no. 19. London: The Agency; 2021. [Google Scholar]
- 13.Lother SA, Demczuk W, Martin I, Mulvey M, Dufault B, Lagacé-Wiens P, et al. Clonal clusters and virulence factors of group C and G Streptococcus causing severe infections, Manitoba, Canada, 2012–2014. Emerg Infect Dis. 2017;23:1079–88. 10.3201/eid2307.161259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruppen C, Rasmussen M, Casanova C, Sendi P. A 10-year observational study of Streptococcus dysgalactiae bacteraemia in adults: frequent occurrence among female intravenous drug users. Swiss Med Wkly. 2017;147:w14469. [DOI] [PubMed] [Google Scholar]
- 15.Rößler S, Berner R, Jacobs E, Toepfner N. Prevalence and molecular diversity of invasive Streptococcus dysgalactiae and Streptococcus pyogenes in a German tertiary care medical centre. Eur J Clin Microbiol Infect Dis. 2018;37:1325–32. 10.1007/s10096-018-3254-2 [DOI] [PubMed] [Google Scholar]
- 16.Kittang BR, Langeland N, Mylvaganam H. Distribution of emm types and subtypes among noninvasive group A, C and G streptococcal isolates in western Norway. APMIS. 2008;116:457–64. 10.1111/j.1600-0463.2008.00976.x [DOI] [PubMed] [Google Scholar]
- 17.Oppegaard O, Mylvaganam H, Skrede S, Lindemann PC, Kittang BR. Emergence of a Streptococcus dysgalactiae subspecies equisimilis stG62647-lineage associated with severe clinical manifestations. Sci Rep. 2017;7:7589. 10.1038/s41598-017-08162-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jordal S, Glambek M, Oppegaard O, Kittang BR. New tricks from an old cow: infective endocarditis caused by Streptococcus dysgalactiae subsp. dysgalactiae. J Clin Microbiol. 2015;53:731–4. 10.1128/JCM.02437-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gajdács M, Ábrók M, Lázár A, Burián K. Beta-haemolytic group A, C and G streptococcal infections in Southern Hungary: a 10-year population-based retrospective survey (2008–2017) and a review of the literature. Infect Drug Resist. 2020;13:4739–49. 10.2147/IDR.S279157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lambertsen LM, Ingels H, Schønheyder HC, Hoffmann S; Danish Streptococcal Surveillance Collaboration Group 2011. Nationwide laboratory-based surveillance of invasive beta-haemolytic streptococci in Denmark from 2005 to 2011. Clin Microbiol Infect. 2014;20:O216–23. 10.1111/1469-0691.12378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harris P, Siew DA, Proud M, Buettner P, Norton R. Bacteraemia caused by beta-haemolytic streptococci in North Queensland: changing trends over a 14-year period. Clin Microbiol Infect. 2011;17:1216–22. 10.1111/j.1469-0691.2010.03427.x [DOI] [PubMed] [Google Scholar]
- 22.Couture-Cossette A, Carignan A, Mercier A, Desruisseaux C, Valiquette L, Pépin J. Secular trends in incidence of invasive beta-hemolytic streptococci and efficacy of adjunctive therapy in Quebec, Canada, 1996-2016. PLoS One. 2018;13:e0206289. 10.1371/journal.pone.0206289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trell K, Nilson B, Rasmussen M. Species and emm-type distribution of group C and G streptococci from different sites of isolation. Diagn Microbiol Infect Dis. 2016;86:467–9. 10.1016/j.diagmicrobio.2016.09.008 [DOI] [PubMed] [Google Scholar]
- 24.Schwartz IS, Keynan Y, Gilmour MW, Dufault B, Lagacé-Wiens P. Changing trends in β-hemolytic streptococcal bacteremia in Manitoba, Canada: 2007-2012. Int J Infect Dis. 2014;28:211–3. 10.1016/j.ijid.2014.03.1376 [DOI] [PubMed] [Google Scholar]
- 25.NORM and NORM-VET. Usage of antimicrobial agents and occurrence of antimicrobial resistance in Norway, 2004–2020 [cited 2022 Nov 16]. https://unn.no/fag-og-forskning/norm-norsk-overvakingssystem-for-antibiotikaresistens-hos-mikrober#rapporter
- 26.Public Health England. Laboratory surveillance of pyogenic and non-pyogenic streptococcal bacteraemia in England: 2019 update. Health protection report; vol. 14, no. 24. London: The Agency; 2020. [Google Scholar]
- 27.Public Health England. Voluntary surveillance of pyogenic and non-pyogenic streptococcal bacteraemia 2016: appendix data for England only. Health protection report; vol. 11, no. 41. London: The Agency; 2017. [Google Scholar]
- 28.Jensen CS, Dam-Nielsen C, Arpi M. Matrix-assisted laser desorption/ionization-time of flight mass spectrometry identification of large colony beta-hemolytic streptococci containing Lancefield groups A, C, and G. Infect Dis (Lond). 2015;47:575–9. 10.3109/23744235.2015.1043940 [DOI] [PubMed] [Google Scholar]
- 29.Nybakken EJ, Oppegaard O, Gilhuus M, Jensen CS, Mylvaganam H. Identification of Streptococcus dysgalactiae using matrix-assisted laser desorption/ionization-time of flight mass spectrometry; refining the database for improved identification. Diagn Microbiol Infect Dis. 2021;99:115207. 10.1016/j.diagmicrobio.2020.115207 [DOI] [PubMed] [Google Scholar]
- 30.Oppegaard O, Skrede S, Mylvaganam H, Kittang BR. Temporal trends of β-haemolytic streptococcal osteoarticular infections in western Norway. BMC Infect Dis. 2016;16:535. 10.1186/s12879-016-1874-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Michael-Gayego A, Dan-Goor M, Jaffe J, Hidalgo-Grass C, Moses AE. Characterization of sil in invasive group A and G streptococci: antibodies against bacterial pheromone peptide SilCR result in severe infection. Infect Immun. 2013;81:4121–7. 10.1128/IAI.00359-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bruun T, Rath E, Madsen MB, Oppegaard O, Nekludov M, Arnell P, et al. ; INFECT Study Group. Risk factors and predictors of mortality in streptococcal necrotizing soft-tissue infections: a multicenter prospective study. Clin Infect Dis. 2021;72:293–300. 10.1093/cid/ciaa027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bläckberg A, Nilson B, Özenci V, Olaison L, Rasmussen M. Infective endocarditis due to Streptococcus dysgalactiae: clinical presentation and microbiological features. Eur J Clin Microbiol Infect Dis. 2018;37:2261–72. 10.1007/s10096-018-3367-7 [DOI] [PubMed] [Google Scholar]
- 34.Steens A, Knol MJ, Freudenburg-de Graaf W, de Melker HE, van der Ende A, van Sorge NM. Pathogen- and type-specific changes in invasive bacterial disease epidemiology during the first year of the COVID-19 pandemic in the Netherlands. Microorganisms. 2022;10:972. 10.3390/microorganisms10050972 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional information and bacterial strains for Streptococcus dysgalactiae and other bloodstream infections, Norway, 2019–2021.


