ABSTRACT
Purpose:
COVID-19 continues to be an urgent World issue. Receptors of angiotensin converting enzyme 2 (ACE2), gateway of SARS-CoV-2, are present in the lungs, bladder, prostate, and testicles. Therefore, these organs face high risk of damage caused by the virus and this mechanism may explain non-respiratory symptoms of the disease.
Materials and Methods:
This systematic review, guided by the PRIMSA statement, was proposed to elucidate possible urological complications of COVID-19. Searches were carried out in Medline (PubMed), Cochrane (CENTRAL), Embase, MedRxiv and LILACS. Bias analysis was made using the specific Newcastle-Ottawa Scale for each study design.
Results:
Search was carried out until April 2022, and 8,477 articles were identified. Forty-nine of them were included in this systematic review. There is evidence that lower urinary tract symptoms and acute scrotum may be signs of COVID-19 in men, although in a small proportion. Also, the disease may have a transitory impact on male fertility, evidenced by several alterations in sperm counts. However, it must be clarified whether this impact is transitory, or may last for longer periods. Several patients showed reduction of total value of testosterone. Two authors linked low levels of testosterone with worse outcomes of COVID-19, suggesting that the hormone may be used as an early biomarker of the severity of the disease. Moreover, it is extremely unlikely that SARS-CoV-2 is transmitted by semen.
Conclusion:
This systematic review identified possible repercussions of COVID-19 in the urinary as well as in the male reproductive system.
Keywords: COVID-19, SARS-CoV-2, Infertility, Testosterone
INTRODUCTION
In December 2019 began, in the Chinese province of Wuhan, the outbreak of COVID-19 caused by a new coronavirus, the SARS-CoV-2, and on March 11th, 2020, the World Health Organization officially declared it a pandemic. Until August 2022, more than two years after the beginning of the outbreak, the virus reached all continents, affecting approximately 586 million and killed 6,4 million people (1). Although several countries have controlled the disease and have high vaccination rates, there are still some countries where immunization has not reached levels high enough to reduce virus circulation. Also, there are concerns regarding new variants and population groups that refuse the vaccine (2-4). Therefore, elucidation of the effects of SARS-CoV-2 is still very important and relevant.
Receptors of angiotensin converting enzyme 2 (ACE2) are the gateway for the entrance of the virus into the cells. The virus uses the ACE2 receptors for entrance and serin-protease TMPRSS2 receptors for priming of spike protein, similarly to what is observed in SARS-CoV (5, 6). Besides pneumocytes type II, RNA sequencing showed that these receptors are also expressed at myocardial, esophageal, kidney proximal contorted tubules, and urothelium bladder cells (7), and at testicles (spermatogonia, Leydig and Sertoli cells) (8), cholangiocytes (9), ileum and colon enterocytes (10), suggesting that these organs are potentially damaged by SARS-CoV-2, and that this mechanism may explain non-respiratory symptoms caused by the virus. Furthermore, in 2002, during the outbreak of severe acute respiratory syndrome (SARS) it was observed that orchitis is one of the complications of SARS (11). This may be one complication of COVID-19 since SARS-CoV, and SARS-CoV-2 have 79.5% genetic similarity (12) and bind similarly to ACE2 receptors (5, 6).
Several authors have emphasized the need of urological monitoring of COVID-19 patients, not only during the disease, but also to long term complications (13–15). Therefore, a systematic review would be crucial to synthetize the major urologic aspects of SARS-CoV-2. New symptoms of the disease may be detected, expanding alert signs, helping doctors to diagnose better COVID-19, and predicting patients at risk to develop the most aggressive forms of the disease. Once the consequences to urinary and urologic systems are identified, medical decisions may be based on stronger evidence than on the ones currently used.
OBJECTIVE
The objective of this systematic review was to identify possible urological consequences or complications of patients that were infected by SARS-CoV-2.
METHODOLOGY
This systematic review was conducted according to the PRISMA statement (16), a recommendation that consists of a checklist and flow diagram to help researchers to improve the report of their systematic reviews and was registered at PROSPERO (17) under the register CRD42020206155. Systematic review of the literature was done using the database Medline (PubMed), Cochrane (CENTRAL), Embase and LILACS. The following search terms were used: (COVID-19 OR COVID19 OR Coronavirus OR 2019-nCOV OR SARS-CoV-2) AND (Urology OR Urological OR Urologic OR Urogenital OR Genitourinary OR Epididymis OR Penis OR Penile OR Prostate OR Scrotum OR Testis OR Testes OR Testicles OR Testicular OR Orchi* OR Leydig OR Sertoli OR Sperm OR Seminal OR Semen).
Two authors evaluated independently the titles and abstracts of the studies, and those meeting the inclusion criteria were selected for this review. In case of disagreements, a third author was consulted.
Articles were selected according to the following eligibility criteria: (I) COVID-19 effects on the urological system; (II) full articles, without language restrictions; (III) articles with relevant outcomes for this review.
Two authors have done the bias analysis using the Newcastle-Ottawa Scale specific to each study design.
RESULTS
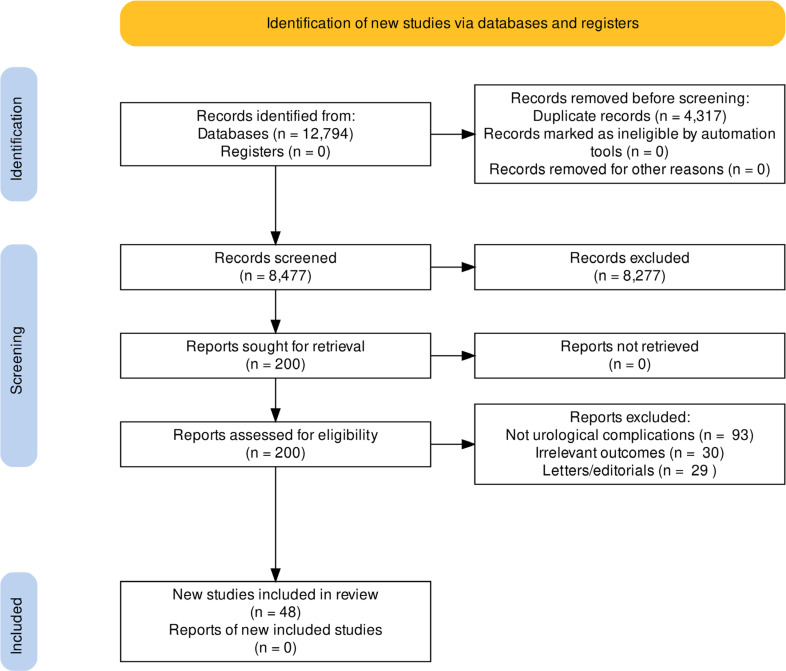
Search was carried out until April 2022 and retrieved a total of 12,794 articles from the scientific databases (Medline: 3,957; Embase: 4,896; LILACS: 356; CENTRAL Cochrane: 3,585). After removal of duplicates, 8,477 titles and abstracts were evaluated and 201 were selected to full reading. According to eligibility criteria, 49 articles (19-68) involving 3,008 infected patients with SARS-CoV-2 were included in this systematic review (Figure-1). Study characteristics are summarized in Table-1.
Figure 1. Identification of new studies via databases and registers.
Table 1. Included articles characteristics.
| Study | Study design | Location | N | Age (years) | Severity | Study | Study design | Location | N | Age (years) | Severity |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Achua et al. (2021) (19) | Cohort | Miami, FL, USA | 6 | 56 (20–87) | Autopsy | La Marca et al. (2020) (43) | Case report | Modena, Italy | 1 | 43 | Death |
| Addar et al. (2020) (20) | Case report | Riad, Saudi Arabia | 1 | 62 | Severe | Lam et al. (2020) (44) | Case report | Pembrokeshire, United Kingdom | 1 | 67 | Death |
| Alkhatatbeh et al. (2020) (21) | Cohort | Zarqa, Jordan | 253 | 43 (1–78) | Critical: 12; Severe: 48; Mild: 152; Asymptomatic: 53 | Lamamri et al. (2021) (45) | Case report | Le Chesnay, France | 1 | 62 | Severe |
| Best et al. (2021) (22) | Cohort | Miami, FL, USA | 30 | 40 | Hospitalized: 8 Non-hospitalized: 22 | Lamb et al. (2020) (46) | Cohort | Royal Oak, MI, USA | 4 | 68 (51–85) | – |
| Bridwell et al. (2020) (23) | Case report | San Antonio, TX, USA | 1 | 37 | Mild | Li et al. (2020) (47) | Cohort | Beijing, China | 38 | – | Acute phase: 23 Recovered: 15 |
| Burke et al. (2021) (24) | Cohort | Orlando, FL, USA | 18 | 32 (24–57) | Moderate: 16; Mild: 2 | Li H et al. (2020) (48) | Case series Cross sectional | Wuhan, China | 23 | 40,8 ± 8,5 | Ordinary: 14; Mild: 9 |
| Can et al. (2021) (25) | Cohort | Istanbul, Turkey | 94 | 57.5±16.6 | > 3 weeks hospitalized | Ma et al. (2020) (49) | Cohort | Wuhan, China | 119 | 39 (35–44) | Critical: 2; Severe: 14; Moderate: 100; Mild: 3 |
| Carreño et al. (2021) (26) | Case report | Bogota, Colombia | 1 | 39 | Death | Machado et al. (2021) (50) | Cross sectional | Arkansas, USA | 15 | 23,36 (19–43) | – |
| Çayan et al. (2020) (27) | Cohort | Mersin, Turkey | 46 | 45,07 ± 18,28 | ICU | Mannur et al. (2020) (51) | Case report | Telangana, India | 1 | 36 | Mild |
| 129 | Hospital ward | Mumm et al. (2020) (52) | Case series | Munich, Germany | 57 | 62 (59–78) | – | ||||
| 46 | Asymptomatic | Nie et al. (2021) (53) | Case series | Wuhan, China | 5 | – | – | ||||
| Chen et al. (2020) (28) | Cohort | Wuhan, China | 83 | 54,2 (38–69) | Non-Severe | Ning et al. (2020) (54) | Case series | Wuhan, China | 112 | 55,5 (23–83) | Severe: 72; Mild: 40 |
| 59 | 64 (47–78) | Severe | Pan et al. (2020) (55) | Cross sectional | Wuhan, China | 34 | 37 | – | |||
| Dhar et al. (2020) (29) | Case series | Detroit, MI, USA | 39 | 63,5 (56 - 68,75) | – | Paoli et al. (2020) (56) | Case report | Rome, Italy | 1 | 31 | Moderate |
| Duarte–Neto et al. (2020) (30) | Case series | Sao Paulo, SP, Brazil | 5 | 69 (33-83) | – | Pavone et al. (2020) (57) | Case series | Palermo, Italy | 9 | 42 (31-60) | Mild |
| Ediz et al. (2020) (31) | Cohort | Istanbul, Turkey | 91 | 38 | – | Rastrelli et al. (2020) (58) | Case series | Florence, Italy | 4 | 74,5 (59,5–85,0) | ICU/death |
| Erbay et al. (2020) (32) | Cohort | Karaman, Turkey | 43 | 31.06 ± 4.2 | Moderate | 6 | 72 (33,0–83,5) | Respiratory ICU | |||
| 26 | 30.4 ± 4.8 | Mild | 21 | 63 (55,0–66,5) | Hospital ward | ||||||
| Flaifel et al. (2020) (33) | Case series | New York, NY, USA | 10 | 49,5 (22-83) | Death | Rawlings et al. (2020) (59) | Cohort | San Diego, CA, USA | 6 | (28-45) | – |
| Fraietta et al. (2021) (34) | Case series | Sao Paulo, SP, Brazil | 22 | 29,5 (23–33) | Mild: 20 Moderate: 2 | Ruan et al. (2020) (60) | Case series | Wuhan, China | 55 | 31,15 ± 5,32 | All recovered – Severe: 32; Moderate: 31; Mild: 11 |
| Gacci et al. (2021) (35) | Cross sectional | Florence, Italy | 43 | (18-65) | ICU: 5; Hospital ward: 26; non-Hospitalized: 12 | Salonia et al. (2021) (61) | Case-control | Milan, Italy | 34 | 67,0 (59,0–72,0) | Deaths |
| Gagliardi et al. (2020) (36) | Case report | Forte dei Marmi, Italy | 1 | 14 | Mild | 51 | 60,0 (53,0–66,0) | Severe | |||
| Guo et al. (2020) (37) | Prospective Cohort | Jinan, China | 23 | 41,04 ± 11,56 | Moderate: 5; Mild: 18 | 174 | 57,0 (49–65,5) | Moderate | |||
| Holtmann et al. (2020) (38) | Cohort | Dusseldorf, Germany | 4 | 40,8 ± 8,7 | Moderate | 27 | 49,0 (45,0–55,0) | Mild | |||
| 14 | 42,7 ± 10,4 | Mild | Silverman et al. (2021) (62) | Case report | Dayton, OH, USA | 1 | 68 | Severe | |||
| Kadihasanoglu et al. (2020) (39) | Cohort | Istanbul, Turkey | 12 | 49,9 ± 12,5 | Severe | Song et al. (2020) (63) | Case series | Nanjing, China | 13 | (22–67) | – |
| 30 | Moderate | Temiz et al. (2020) (64) | Cross sectional | Istanbul, Turkey | 30 | 37,21 ± 8,59 | – | ||||
| 47 | Mild | Xu et al. (2020) (65) | Cohort | Wuhan, China | 39 | 60 (46,5–65,5) | Severe: 19; Moderate: 20 | ||||
| Kaya et al. (2020) (40) | Cohort | Eskisehir, Turkey | 19 | 38,9 ± 13 | Hospitalized | Yang et al. (2020) (66) | Case series | Wuhan, China | 12 | 65 (42-87) | Severe: 3; Moderate: 5; Mild: 2 |
| Kayaaslan et al. (2020) (41) | Cohort | Ankara, Turkey | 16 | 33,5 (18–54) | Moderate: 5; Mild: 11 | Zhu et al. (2020) (67) | Case report | Tianmen, China | 1 | 30 | Severe |
| Kim et al. (2020) (42) | Case report | Boston, MA, USA | 1 | 42 | Mild | ||||||
Lower urinary tract symptoms
Lower urinary tract symptoms (LUTS) were reported in 5 studies (25, 29, 40, 46, 52). Mumm et al. (52) in a series of 57 cases, reported that 7 patients showed increase of urinary frequency, with a medium of 13.7 urinations at the day of admittance and 11.6 at the 5th day. Other two series, Dhar et al. (29) and Lamb et al. (46), also reported increased frequency in 39 and 4 patients, respectively. Both also verified that patients reported nocturia. Dhar et al. (29) related that 85% of patients presented 13 or more urinations per day and 87% at least 4 urinations at night. Also, Lamb et al. (46) reported urgency and urinary incontinence in 4 patients.
International Prostate Symptom Score (IPSS) was applied in 113 patients in two studies. Kaya et al. (40) did not find significant score differences among previous, during and at hospitalization due to COVID-19. This result is similar to that of Can et al. (25) in patients under 50 years of age (n=32). However, in patients with more than 50 years old (n=62) it was verified an increase of IPSS during hospitalization. Value before COVID-19 was 5.1±4.1 and during infection 9.0±6.4 (p<0. Covid 0001).
Acute scrotum
Testicle involvement was reported in some of the articles reviewed. Chen et al. (28) studying 142 patients, reported 6 with orchitis, 7 with epididymitis, 19 orchitis-epididymitis and 28 scrotal infections, being the two last more common in patients severely ill (non-severe 3 vs. severe 4; non-severe: 5 vs. severe 10, p<0.05). Ediz et al. (31) (N=91) reported 10 patients with orchitis-epididymitis or testicular pain, and 9 with testicular edema. Pan et al. (55) (N=34) and Holtmann et al. (38) (N=18) reported respectively 6 and 1 case of testicular discomfort. Ning et al. (54) (N=112) did not report any patient with testicular edema, and Alkhatatbeh et al. (21) (N=253) did not report any patient with orchitis. Two case reports reported testicular pain (42,43), one bilateral orchitis (23) and one orchitis-epididymitis in a 14-year-old teenager (36).
La Marca et al. (43) patient presented initial bilateral testicular pain, that evolved 3 days later to dyspnea and death after one week of hospitalization. All other patients presented manifestations of acute scrotum as their initial presentation.
Autopsies
In this review, it was included 6 autopsies of patients that died due to complications of SARS-CoV-2 infection (19, 30, 33, 48, 53, 66). Yang et al. (66) reported that in 12 patients the medium of quantity of Leydig cells was inferior to control patients (p<0.01). In 9 patients, tubular lesion was described (4 with severe lesion, 5 moderate and 2 mild). Moreover, alterations of spermatogenesis were found in 8 patients. Nie et al. (53) performed autopsies in 5 patients, finding alterations which suggested a disfunction or reduction of Leydig cells and impaired spermatogenesis and alteration of motility of sperms. Li et al. (48) compared 6 patients with COVID-19 with 6 controls and found more apoptotic testicular cells in those infected (p=0.018). In addition, interstitial edema and congestion of testicles and epididymis were reported. Duarte-Neto et al. (30) described two patients with orchitis, Flaifel et al. (33) multifocal testicular microthrombus in 2 patients and Achua et al. (19) reported that 3 patients had spermatogenesis alterations.
Spermatic parameters
Several alterations such as azoospermia, oligozoospermia, criptozoospermia and teratozoospermia were found (35, 48, 51, 67). Gacci et al. (35) reported that 25.6% of patients developed some of these alterations and that concentration of sperm was lower in more severely will patients. All 9 patients (9/23) reported by Li et al. (48) with oligospermia had fathered children previously to COVID-19 through natural pregnancies. What's more, when compared to controls, sperm concentration of patients with Covid-10 were significantly lower (40.6x106/mL in controls (2, 5, 27, 61), 13.8x106/mL (2, 5, 8, 36) in patients with mild COVID-19 and 10.9x106/mL in ordinary cases). Best et al. (22) observed that patients with the disease had sperm concentrations (11.5 vs. 21.5 x 106/mL; p=0.0048) and total sperm count (12.5 vs. 59.2x106/ejaculation; p=0.0024) lower than control group. Holtmann et al. (38) observed that patients with moderate COVID-19 had reduced total sperm count (11.9±13.4x106/ejaculate) and motility (total amount with progressive motility: 2.4±2.7x106/mL; total amount with full motility: 4.7±5.5x106) while patients with mild disease had not significant alterations (total sperm count: 243.7±140.4 x 106/ejaculate; total amount with progressive motility: 125.3±96.4x106; total amount with full motility: 157.1±120.8x106) when compared to controls (total sperm count: 233.1±234.4x106/ejaculate; total amount with full motility: 102.1±102.3x106; total amount with full motility: 124.0±124.9x106). Finally, Erbay et al. (32) found reduction of progressive motility (28.81%±9.7 vs. 20.92%±9.1, p=0.002) and total (48.69%±12.1 vs. 33.41%±12;3, p<0.001) and reduced vitality (62%±7.0 vs. 58.1%±7.1, p=0.03) when compared to sperm analysis before and after the disease in patients with mild COVID-19, however without alteration of sperm concentration. In patients with moderate COVID-19 it was found reduction in all sperm parameters when compared to controls (total volume: 3.34 mL±1.1 vs. 2.74 mL±0.9, p<0.001; concentration: 35.01x106/mL±14.1 vs. 30.63x106/mL vs. 17.2; p=0.008; total count: 114.53x106±93.66 vs. 90.38x106±83.66; p=0.001; progressive motility: 30.16%±12.1 vs. 21.40%±10.1, p<0.001; total motility : 50.74%±13.4 vs. 31,42%±13.3; p<0.001; vitality 64.6±5.6 vs. 57;4±6.8, p=0.001).
Presence of SARS-CoV-2 in Semen
The semen of 428 patients of 19 articles was analyzed for possible presence of SARS-CoV-2 (22, 24, 34, 35, 37, 38, 41, 47-50, 54–57, 59, 60-62). Patients included had different severity levels of COVID-19 (from asymptomatic to ICU patients) and semen samples were collected in different periods of the disease. Some patients collected at day 1 and others after 109 days of first symptoms. Of all 428 analyzed samples, only 8 presented the virus in the semen (35, 47, 50).
Priapism
Five authors reported (N=5) patients with COVID-19 and priapism (20, 26, 44, 45, 62). All had severe forms of the disease (shortness of breath and dyspnea (N=5); intubation and mechanical ventilation (N=3), oxygen support via nasal catheter (N=1), and via CPAP (N=1)) and 2 died. Also, all had a risk factor (overweight/obesity, hypertension, dyslipidemia, diabetes) and 4 were older (62-68 years old). In 5, priapism was ischemic. Interventions in 4 patients included aspiration of blood (n=2), and intracavernous administration of phenylephrine (n=2), etilephrine (n=1) or adrenaline (n=1). Of these, one died and the other three reverted priapism recovering from COVID-19. Lam's et al. (44) patient died before any treatment for priapism.
Hormones
Total Testosterone
Salonia et al. (61) reported that 89.8% of patients with COVID-19 had total testosterone lower than normal (<9.2 nmol/L) while only 14.9% in controls (2.5 (1.0-4.7) vs. 10.4 nmL/L (8.1-13.4); p<0.0001). Furthermore, lower levels of total testosterone in more severely ill patients were observed. Using multiple variated analysis made by the author, total testosterone was inversely associated with admission to ICU (OR=0.54; p<0.0001 (IC95%=0.43-0.67)) and death (OR=0.68; p=0.002 (IC95%=0.53-0.86)). Kadihasanoglu et al. (39) also showed that patients with COVID-19 presented lower values of total testosterone than controls (182.52 vs. 332 ng/dL; p<0.0001). Rastrelli et al. (58) showed in 31 patients admitted to respiratory ICU (5.0 nmol/L (1.8-7.6)) or ICU/deaths (1;0 nmol/L (0.2-1.9)) lower levels than normal (considered by the author - <8.6 nmol/L), while hospital ward patients presented normal values (medium) (8.8 (4.1-16.2)). Ma et al. (49) (COVID-19: 3.97 (3.14-5,74)) vs. control: 4.43 ng/mL (3;53-5.24); p=0.1886) and Xu et al. (65) (COVID-19: 3.3932±1.081 vs. control: 3.838 ng/mL±0.96; p<0.05) did not find significant differences among patients with COVID-19 and controls. Lastly, Çayan et al. (27) reported that patients showed a medium of normal values (308 ng/dL (18-931); normality: ≥ 300 ng/mL), but hypogonadism was observed in 113 patients (51.1%). Additionally, in 24 patients that presented the previous values of testosterone (before SARS-CoV-2 infection) a reduction of total values was observed, from 458±198 ng/dL to 315±120 ng/dL (p=0.003).
FSH
Xu et al. (65) was the only author that found significant differences of values between sick patients and health controls (although at normal range) (1.27-19.26 mLU/mL), with lower levels in patients with COVID-19 (8.763±4.952 vs. 14.407±12.918; p<0.05). Ma et al. (49) and Kadihasanoglu et al. (39) did not find differences between groups. Salonia et al. (61), showed that more severely ill patients had lower levels of FSH, although did not also find any significant differences between patients with or without COVID-19 (mild: 7.0 mU/mL (3.9-8.3); moderate: 6.9 (4.5-9.9); severe: 3.9 (2.6-5.8); deaths: 4.6 (3.8-6.4); p<0.0001). However, Çayan, et al. (27) reported that patients at ICU had higher levels than asymptomatic patients (8.41±4.38 mIU/mL vs. 5.26±2.68; p=0.02).
LH
A significant difference was found between COVID-19 patients and controls in four articles. In three of them, the patients showed higher values of LH (Ma, et al. (49)): 6.36 mIU/mL (4.63-8.37) vs. 3.38 (2.48-4.52), p<0.0001; Salonia et al. (61): 4.7 mU/mL (3.0-6.7) vs. 4.1 (3.0-5.4), p=0.005; Kadihasanoglu et al. (39): 5.67±4.52 U/L vs. 4.1±2.62, p=0.0001). On the other side, Xu et al. (65) reported that patients with COVID-19 showed lower levels than controls (5.519 mIU/mL±2.705 vs. 8.051±6.048, p<0.05). Rastrelli et al. (58) reported that patients with COVID-19 at ICU or that died presented higher levels than those admitted to hospital wards (11.2 U/L (9.0-19.3) vs. 6.6 (4.6-9.6); p=0.037) and above normality (LH: 1.7-8.6 U/L). On the other side, Çayan et al. (27) did not find any statistically significant differences among asymptomatic patients (5.31±2.38 mIU/mL) in hospital wards (5;73±2.22) and those at ICU (5.97-3.17).
Prolactin
Kadihasanoglu et al. (39) showed that patients with COVID-19 had higher levels than controls (9.6±5.59 ug/L vs. 7.5±1.86; p=0.0007). But Xu et al. (65) did not find any difference between patients with or without COVID-19 and Çayan et al. (27) did not find significant differences among different levels of severity of the disease.
Estradiol
Xu et al. (65) reported significant differences between groups (COVID-19: 50.9±18.8 vs. controls: 34.9±18.5; p<0.05) with values above normal (≤47 pg/mL) in patients with COVID-19. Salonia et al. (61) found higher levels in patients with COVID-19 than in controls (35.0 pg/mL (22.4-44.2) vs. 23;3 (19.0-27.9); p<0.05) and, finally, Çayan et al. (27) did not find significant differences among different severities of the disease.
DISCUSSION
SARS-CoV-2 infection may cause several impacts on male urinary and genital systems. Many important changes in semen parameters in patients (azoospermia, oligozoospermia and criptozoospermia) comparing before and after infection were observed. The presence of the virus in semen was rare and reported in only 8 of the 428 samples analyzed. Patients with COVID-19 showed reduced values of total testosterone, with lower values in more critically ill patients. Furthermore, urinary frequency increase, LUTS, nocturia, urgency and incontinence were reported in several patients. Orchitis, epididymitis, edema, pain, and tenderness of testicles were also reported by several authors, demonstrating a possible testicular impact of COVID-19. Five cases of priapism were also reported. Other findings, such as IPSS alteration and changes in levels of hormones were heterogeneous.
Since the beginning of COVID-19 pandemics, there is great concern about the effects of SARS-CoV-2 on urological system, especially on the male reproductive system, due to the presence of ACE2 and TMPRSS2 receptors and the great similarity of SARS-CoV-2 and SARS-CoV. The presence of these receptors in male genital organs and in urinary bladder may explain a possible direct mechanism of action of this virus in these organs, which may explain several findings such as LUTS, IPSS increase, orchitis, epididymitis and alterations of sperm count.
Another possible explanation for the urological involvement that may occur concurrently with the direct attack of the virus is the damage caused by the inflammatory activity. COVID-19 is considered an inflammatory disease, evidenced by the cytokines storm (68). It is possible that the production of oxygen reactive species may stimulate pathways for cytokine release with exaggerated inflammatory response (69) with several cellular damage. Endothelitis caused by the virus may be one of the multiple mechanisms that cause LUTS and increase of urinary frequency (70). Duarte-Neto et al. (71) suggests that the clearance of viral antigens in the testis take longer than expected and that this can induce severe cellular changes, such as loss of Leydig cells.
In the included articles, it was observed several alterations in patients’ sperm parameters, such as azoospermia, oligozoospermia and criptozoospermia. One important finding was reported by Li et al. (48), that showed that all patients with oligospermia had already fathered children by natural conception, suggesting that the disease may impair, even if transitorily, male fertility, and that the alterations may or may not be present before infection. The physiopathology of COVID-19, in particular the inflammatory response, may damage testicular cells and compromise the quality of sperm. It is also possible that fertility may be affected by several drugs used during treatment of COVID-19, such as antibiotics, corticosteroids, chloroquine, among others (72). Carneiro et al. (73) points out that there are asymptomatic epididymal injuries since they found color Doppler ultrasound changes in 42.5% of patients without symptoms of epididymitis. They hypothesize that these injuries can have deleterious impact on seminal parameters. More studies comparing spermatic parameters before and after the disease are needed to define if infertility may be a complication of COVID-19. It is also important to follow up patients over time to verify if the impact is transitory or if it can be long-lasting.
Evidence synthesis showed that the disease may affect testosterone levels. Three articles showed that patients with COVID-19 had lower values compared to controls, particularly those more severely affected. Rastrelli et al. (58) demonstrated that the values were lower the more severe the disease. Patients with COVID-19 showed values below normal according to authors. This result is extremely important since Salonia et al. (61) univariate analysis showed that total testosterone level was inversely associated with admission to ICU, suggesting that the hormone may be an early biomarker of severity of COVID-19. Such association was also suggested by Rastrelli et al. (58). It is possible that the reduction of testosterone may last until after the recovery of COVID-19, impacting men's sex lives. Teixeira et al. (74) suggest that a decreased testosterone/luteinizing hormone ratio correlated with high levels of C-reactive protein and white blood cell count, reported by some authors, can mean a transient stage of hypogonadism. In an experimental study, Carrasco et al. (75) inoculated in rats nucleocapsid protein, that have high IgG antibodies against it in COVID-19 patients and found low serum levels of testosterone and free testosterone in these rats compared with a control group. They suggest that this hormone imbalance can be linked with a post-COVID-19 syndrome hypogonadism. However, prospective studies are needed to clarify all this hypothesis.
Regarding other hormones, the findings were heterogeneous. The great variation of findings may be due to previous diseases and conditions (before infection by SARS-CoV-2) that could have affected hormonal levels of patients. However, since these studies were observational and without previous knowledge of hormonal levels before the disease, it is not possible to conclude with accuracy the reasons of the findings.
Aside from the possible pathological effects that the disease can cause, sexual transmission of the virus was uncertain at the beginning of the pandemic. This review concluded that transmission through semen is highly unlikely. In only 8 of 428 samples analyzed the virus was found. Paoli et al. (76) suggested that virus detection could have occurred due to contamination of sample during masturbation, a non-sterile way of collecting, different from the nasal swab or venipuncture. There is also the possibility of cough contamination. Massarotti et al. (77) believes that contamination may have occurred due to residual virus from the respiratory tract. There is evidence of the presence of SARS-CoV-2 in urine (78), although in a few patients. Moreover, even if RT-PCR in semen is positive, the result only implies the presence of viral RNA in the sample, and that there may be no viable virus to be transmitted through semen ((Paoli et al. (76)). New studies are needed to determine if it is possible, even in a small proportion of patients, the transmission of SARS-CoV-2 through semen.
The COVID-19 pandemic strongly impacted all medical specialties and urology was no different. Initially, the biggest concern was about the risk of patients undergoing surgery to contract SARS-CoV-2, especially cancer patients. Anjos-Silva et al. (79) reported that the longer the hospital stay after urological surgeries, the greater the risk of contracting COVID-19 and being a fatal case. It was considered that elective urological surgeries can be safe but that urgent cases need special care to avoid contamination. Zampolli et al. (80) demonstrated that robotic and laparoscopic surgeries are safe regarding the risk of infection by SARS-CoV-2 and that the fact that they lead to a shorter hospital stay is a benefit in this situation. Several authors have been suggesting protocols to reduce the risk of infection by the virus. It is agreed that all staff members should wear Protective Personal Equipment, such as protective eyewear and N95 or PFF2 (81) masks. Furthermore, all patients should be considered suspects until proven otherwise and that all healthcare professionals should be tested in case of suspicion (82). Regarding the postponement of surgeries, especially those involving cancer patients, it is extremely important that each case is analyzed individually, considering the patient's condition and preferences and hospital conditions for big surgeries (81, 83). Cancellations and postponements of elective surgeries, medical appointments, diagnostic procedures, and non-emergency surgeries were very common in this period, strongly harming the training of residents in urology. Prezotti et al. (84) analyzed the impact of the pandemic on urology medical residencies through questionnaires. Residents estimate that the median damage to the urological training was 6.0 [3.4 -7.7] in a scale from 0-10. In addition to the impairment in training, there was an important impact on health and quality of life, with several residents reporting weight gain, reduced physical activity, development of depressive symptoms, in addition to increased alcohol consumption and smoking. Faced with this impact on urological practice, one way to work around some of these problems is the implementation of telemedicine. Despite the impossibility of carrying out a physical examination, online consultations were of great importance in this period, reducing the chance of infection by SARS-CoV-2, promoting self-care, and enabling the training of residents (85).
Evidence of the urological involvement in patients infected by SARS-CoV-2 is limited. Bias analysis showed that only 5 articles presented low risk of bias and all others presented moderate or high risk of bias (Table-2). Most studies had low methodological quality, with only a limited number of patients, with heterogeneous characteristics regarding severity of the disease, age, comorbidities and received treatment. Lack of follow-up after COVID-19 is also another limiting factor since most studies were not longitudinal and due to the short period since the beginning of the pandemics. There are no studies that report exam results before and after the disease, limiting the extension of the conclusions of this review. It was not possible to perform a metanalysis due to impossibility to compare studies with different methodologies (study designs) and different measures used. Prospective studies with good methodologic quality, and longer follow-up are needed to determine the real impact of the disease on the male genital and urinary systems. This systematic review summarizes in a single article the main changes that COVID-19 can cause in the urological system. We describe several points that should be further investigated, such as changes in sperm parameters, since it has a potential impact on the reproductive life of men, and pathological findings of the virus attack on the testes. In the discussion, we were able to discuss the findings with several authors, providing urologists with an overview of the involvement of the urological system.
Table 2. Bias analysis.
| Author | Best et al. (2021) (22) | Burke et al. (2021) (24) | Can et al. (2021) (25) | Çayan et al. (2020) (27) | Erbay et al. (2021) (32) | Guo et al. (2021) (37) | Holtmann et al. (2020) (38) | Kadihasanoglu et al. (2021) (39) | Kaya et al. (2021) (40) | Ma et al. (2021) (49) |
|---|---|---|---|---|---|---|---|---|---|---|
| Selection | ||||||||||
| 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 2 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 |
| 3 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 4 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| Comparability | ||||||||||
| 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 |
| Outcome | ||||||||||
| 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 |
| 2 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 |
| 3 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Total | 7 | 2 | 5 | 4 | 8 | 4 | 6 | 6 | 5 | 6 |
Cohort – Newcastle-Ottawa Scale
More information see APPENDIX 1
CONCLUSION
Although further studies are needed, this systematic review identified possible urological consequences or complications of COVID-19 such as changes of micturition pattern, urological urgencies, autopsies findings, sperm alterations, hormonal changes, and that the sexual transmission is highly unlikely.
APPENDIX 1:
Cross-sectional – Newcastle-Ottawa Scale.
| Author | Achua et al. (2021) (19) | Alkhatatbeh et al. (2020) (21) | Chen et al. (2021) (28) | Ediz et al. (2021) (31) | Gacci et al. (2021) (35) | Kayaaslan et al. (2020) (41) | Lamb et al. (2020) (46) | Li et al. (2020) (47) | |
|---|---|---|---|---|---|---|---|---|---|
| Selection | |||||||||
| 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | |
| 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 3 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | |
| Confounder | |||||||||
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Outcome | |||||||||
| 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | |
| Total | 3 | 3 | 3 | 2 | 3 | 3 | 1 | 2 | |
| Li et al. (2020) (48) | Machado et al. (2021) (50) | Pan et al. (2020) (55) | Rastrelli et al. (2021) (58) | Rawlings et al. (2020) (59) | Ruan et al. (2020) (61) | Temiz et al. (2021) (64) | Xu et al. (2021) (65) | ||
|---|---|---|---|---|---|---|---|---|---|
| Selection | |||||||||
| 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | |
| 2 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | |
| 3 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| Confounder | |||||||||
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | |
| Outcome | |||||||||
| 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| Total | 3 | 3 | 3 | 4 | 2 | 3 | 6 | 5 | |
Case report – Newcastle-Ottawa Scale.
| Author | Addar et al. (2020) (21) | Bridwell et al. (2021) (23) | Carreño et al. (2021) (26) | Gagliardi et al. (2020) (36) | Kim et al. (2020) (42) | La Marca et al. (2020) (43) | Lamamri et al. (2021) (45) | Mannur et al. (2020) (51) | Paoli et al. (2020) (56) | Silverman et al. (2021) 62) | Zhu et al. (2021)(67) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Selection | ||||||||||||
| 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Confounder | ||||||||||||
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Exposure | ||||||||||||
| 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Total | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | |
Case series – Newcastle-Ottawa Scale.
| Author | Dhar et al. (2020) (29) | Flaifel et al. (2021) (33) | Fraietta et al. (2022) (34) | Li et al. (2020) (41) | Mumm et al. (2020) (52) | Nie et al. (2021) (53) | Ning et al. (2020) (54) | Pavone et al. (2020) (57) | Song et al. (2020) (63) | Yang et al. (2020) (66) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Selection | |||||||||||
| 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| 2 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | |
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Confounder | |||||||||||
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Exposure | |||||||||||
| 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | |
| 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Total | 2 | 3 | 3 | 3 | 3 | 3 | 3 | 4 | 3 | 3 | |
Case-control – Newcastle-Ottawa Scale.
| Author | Salonia et al. (2021) (61) | |
|---|---|---|
| Selection | ||
| 1 | 1 | |
| 2 | 0 | |
| 3 | 1 | |
| 4 | 1 | |
| Comparability | ||
| 1 | 1 | |
| Exposure | ||
| 1 | 1 | |
| 2 | 1 | |
| 3 | 1 | |
| Total | 7 | |
REFERENCES
- 1.Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20:533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harvey WT, Carabelli AM, Jackson B, Gupta RK, Thomson EC, Harrison EM, et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat Rev Microbiol. 2021;19:409–424. doi: 10.1038/s41579-021-00573-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khateeb J, Li Y, Zhang H. Emerging SARS-CoV-2 variants of concern and potential intervention approaches. Crit Care. 2021;25:244–244. doi: 10.1186/s13054-021-03662-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prieto Curiel R, González Ramírez H. Vaccination strategies against COVID-19 and the diffusion of anti-vaccination views. Sci Rep. 2021;11:6626–6626. doi: 10.1038/s41598-021-85555-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Q, Zhang Y, Wu L, Niu S, Song C, Zhang Z, et al. Structural and Functional Basis of SARS-CoV-2 Entry by Using Human ACE2. Cell. 2020;181:894.e9–904.e9. doi: 10.1016/j.cell.2020.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271.e8–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zou X, Chen K, Zou J, Han P, Hao J, Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 2020;14:185–192. doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galsgaard KD, Jepsen SL, Kjeldsen SAS, Pedersen J, Wewer Albrechtsen NJ, Holst JJ. Alanine, arginine, cysteine, and proline, but not glutamine, are substrates for, and acute mediators of, the liver-α-cell axis in female mice. Am J Physiol Endocrinol Metab. 2020;318:E920–E929. doi: 10.1152/ajpendo.00459.2019. [DOI] [PubMed] [Google Scholar]
- 9.Chai X, Hu L, Zhang Y, Han W, Lu Z, Ke A, et al. Specific ACE2 Expression in Cholangiocytes May Cause Liver Damage After 2019-nCoV Infection. Biorxiv. 2020;02:04–04. [Internet]. Avalilable at. < https://www.biorxiv.org/content/10.1101/2020.02.03.931766v1>. [Google Scholar]
- 10.Zhang H, Kang Z, Gong H, Xu D, Wang J, Li Z, et al. Digestive system is a potential route of COVID-19: an analysis of single-cell coexpression pattern of key proteins in viral entry process. Gut. 2020;69:1010–1018. [Internet]. Available at. < https://gut.bmj.com/content/69/6/1010>. [Google Scholar]
- 11.Xu J, Qi L, Chi X, Yang J, Wei X, Gong E, et al. Orchitis: a complication of severe acute respiratory syndrome (SARS) Biol Reprod. 2006;74:410–416. doi: 10.1095/biolreprod.105.044776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo YR, Cao QD, Hong ZS, Tan YY, Chen SD, Jin HJ, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak - an update on the status. Mil Med Res. 2020;7:11–11. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang S, Zhou X, Zhang T, Wang Z. The need for urogenital tract monitoring in COVID-19. Nat Rev Urol. 2020;17:314–315. doi: 10.1038/s41585-020-0319-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ronco C, Reis T. Kidney involvement in COVID-19 and rationale for extracorporeal therapies. Nat Rev Nephrol. 2020;16:308–310. doi: 10.1038/s41581-020-0284-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tamás F, Tibor S, Anita C, Boris H, Péter N. COVID-19 research: promising tracks leading to uro-oncology. Int Urol Nephrol. 2020;52:995–997. doi: 10.1007/s11255-020-02490-2. Erratum in: Int Urol Nephrol. 2021;53:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535–b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.University of York . PROSPERO: international prospective register of systematic reviews. National Institute for Health Research; [Accessed June, 2021]. Centre for Reviews and Dissemination. [Internet]. Available at. < https://www.crd.york.ac.uk/prospero/>. [Google Scholar]
- 18.CEBM Oxford . Oxford Centre for Evidence-based Medicine. Levels of Evidence; 2009. [Accessed June. 2020]. [Internet]. Available at. < https://www.cebm.net/2009/06/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/>. [Google Scholar]
- 19.Achua JK, Chu KY, Ibrahim E, Khodamoradi K, Delma KS, Iakymenko OA, et al. Histopathology and Ultrastructural Findings of Fatal COVID-19 Infections on Testis. World J Mens Health. 2021;39:65–74. doi: 10.5534/wjmh.200170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Addar A, Al Fraidi O, Nazer A, Althonayan N, Ghazwani Y. Priapism for 10 days in a patient with SARS-CoV-2 pneumonia: a case report. J Surg Case Rep. 2021;2021 doi: 10.1093/jscr/rjab020. rjab020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alkhatatbeh H, Alzaghari D, Alkhashman A, Azab M, Edwan GMA, Abufaraj M. Does severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) cause orchitis in patients with coronavirus disease 2019 (COVID-19)? Arab J Urol. 2020;18:129–133. doi: 10.1080/2090598X.2020.1798862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Best JC, Kuchakulla M, Khodamoradi K, Lima TFN, Frech FS, Achua J, et al. Evaluation of SARS-CoV-2 in Human Semen and Effect on Total Sperm Number: A Prospective Observational Study. World J Mens Health. 2021;39:489–495. doi: 10.5534/wjmh.200192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bridwell RE, Merrill DR, Griffith SA, Wray J, Oliver JJ. A coronavirus disease 2019 (COVID-19) patient with bilateral orchitis. Am J Emerg Med. 2021;42:260.e3–260.e5. doi: 10.1016/j.ajem.2020.08.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burke CA, Skytte AB, Kasiri S, Howell D, Patel ZP, Trolice MP, et al. A cohort study of men infected with COVID-19 for presence of SARS-CoV-2 virus in their semen. J Assist Reprod Genet. 2021;38:785–789. doi: 10.1007/s10815-021-02119-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Can O, Erkoç M, Ozer M, Karakanli MU, Otunctemur A. The effect of COVID-19 on lower urinary tract symptoms in elderly men. Int J Clin Pract. 2021;75:e14110. doi: 10.1111/ijcp.14110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carreño B DV, Perez CP, Vasquez D, Oyola JA, Suarez O, Bedoya C. Veno-Occlusive Priapism in COVID-19 Disease. Urol Int. 2021;105(9-10):916–919. doi: 10.1159/000514421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Çayan S, Uğuz M, Saylam B, Akbay E. Effect of serum total testosterone and its relationship with other laboratory parameters on the prognosis of coronavirus disease 2019 (COVID-19) in SARS-CoV-2 infected male patients: a cohort study. Aging Male. 2020;23:1493–1503. doi: 10.1080/13685538.2020.1807930. [DOI] [PubMed] [Google Scholar]
- 28.Chen L, Huang X, Yi Z, Deng Q, Jiang N, Feng C, et al. Ultrasound Imaging Findings of Acute Testicular Infection in Patients With Coronavirus Disease 2019: A Single-Center-Based Study in Wuhan, China. J Ultrasound Med. 2021;40:1787–1794. doi: 10.1002/jum.15558. [DOI] [PubMed] [Google Scholar]
- 29.Dhar N, Dhar S, Timar R, Lucas S, Lamb LE, Chancellor MB. De Novo Urinary Symptoms Associated With COVID-19: COVID-19-Associated Cystitis. J Clin Med Res. 2020;12:681–682. doi: 10.14740/jocmr4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duarte AN, Neto, Monteiro RAA, da Silva LFF, Malheiros DMAC, de Oliveira EP, Theodoro J, Filho, et al. Pulmonary and systemic involvement in COVID-19 patients assessed with ultrasound-guided minimally invasive autopsy. Histopathology. 2020;77:186–197. doi: 10.1111/his.14160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ediz C, Tavukcu HH, Akan S, Kizilkan YE, Alcin A, Oz K, et al. Is there any association of COVID-19 with testicular pain and epididymo-orchitis? Int J Clin Pract. 2021;75:e13753. doi: 10.1111/ijcp.13753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Erbay G, Sanli A, Turel H, Yavuz U, Erdogan A, Karabakan M, et al. Short-term effects of COVID-19 on semen parameters: A multicenter study of 69 cases. Andrology. 2021;9:1060–1065. doi: 10.1111/andr.13019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Flaifel A, Guzzetta M, Occidental M, Najari BB, Melamed J, Thomas KM, et al. Testicular Changes Associated With Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Arch Pathol Lab Med. 2021;145:8–9. doi: 10.5858/arpa.2020-0487-LE. [DOI] [PubMed] [Google Scholar]
- 34.Fraietta R, de Carvalho RC, Camillo J, Groner MF, Truzzi JCCI, Petkov CN, et al. SARS-CoV-2 is not found in human semen during mild COVID-19 acute stage. Andrologia. 2022;54:e14286. doi: 10.1111/and.14286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gacci M, Coppi M, Baldi E, Sebastianelli A, Zaccaro C, Morselli S, et al. Semen impairment and occurrence of SARS-CoV-2 virus in semen after recovery from COVID-19. Hum Reprod. 2021;36:1520–1529. doi: 10.1093/humrep/deab026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gagliardi L, Bertacca C, Centenari C, Merusi I, Parolo E, Ragazzo V, et al. Orchiepididymitis in a Boy With COVID-19. Pediatr Infect Dis J. 2020;39:e200–e202. doi: 10.1097/INF.0000000000002769. [DOI] [PubMed] [Google Scholar]
- 37.Guo L, Zhao S, Li W, Wang Y, Li L, Jiang S, et al. Absence of SARS-CoV-2 in semen of a COVID-19 patient cohort. Andrology. 2021;9:42–47. doi: 10.1111/andr.12848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holtmann N, Edimiris P, Andree M, Doehmen C, Baston-Buest D, Adams O, et al. Assessment of SARS-CoV-2 in human semen-a cohort study. Fertil Steril. 2020;114:233–238. doi: 10.1016/j.fertnstert.2020.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kadihasanoglu M, Aktas S, Yardimci E, Aral H, Kadioglu A. SARS-CoV-2 Pneumonia Affects Male Reproductive Hormone Levels: A Prospective, Cohort Study. J Sex Med. 2021;18:256–264. doi: 10.1016/j.jsxm.2020.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaya Y, Kaya C, Kartal T, Tahta T, Tokgöz VY. Could LUTS be early symptoms of COVID-19. Int J Clin Pract. 2021;75:e13850. doi: 10.1111/ijcp.13850. [DOI] [PubMed] [Google Scholar]
- 41.Kayaaslan B, Korukluoglu G, Hasanoglu I, Kalem AK, Eser F, Akinci E, et al. Investigation of SARS-CoV-2 in Semen of Patients in the Acute Stage of COVID-19 Infection. Urol Int. 2020;104(9-10):678–683. doi: 10.1159/000510531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim J, Thomsen T, Sell N, Goldsmith AJ. Abdominal and testicular pain: An atypical presentation of COVID-19. Am J Emerg Med. 2020;38:1542.e1–1542.e3. doi: 10.1016/j.ajem.2020.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.La Marca A, Busani S, Donno V, Guaraldi G, Ligabue G, Girardis M. Testicular pain as an unusual presentation of COVID-19: a brief review of SARS-CoV-2 and the testis. Reprod Biomed Online. 2020;41:903–906. doi: 10.1016/j.rbmo.2020.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lam G, McCarthy R, Haider R. A Peculiar Case of Priapism: The Hypercoagulable State in Patients with Severe COVID-19 Infection. Eur J Case Rep Intern Med. 2020;7:001779–001779. doi: 10.12890/2020_001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lamamri M, Chebbi A, Mamane J, Abbad S, Munuzzolini M, Sarfati F, et al. Priapism in a patient with coronavirus disease 2019 (COVID-19) Am J Emerg Med. 2021;39:251.e5–251.e7. doi: 10.1016/j.ajem.2020.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lamb LE, Dhar N, Timar R, Wills M, Dhar S, Chancellor MB. COVID-19 inflammation results in urine cytokine elevation and causes COVID-19 associated cystitis (CAC) Med Hypotheses. 2020;145:110375–110375. doi: 10.1016/j.mehy.2020.110375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li D, Jin M, Bao P, Zhao W, Zhang S. Clinical Characteristics and Results of Semen Tests Among Men With Coronavirus Disease 2019. JAMA Netw Open. 2020;3:e208292. doi: 10.1001/jamanetworkopen.2020.8292. Erratum in: JAMA Netw Open. 2020;3:e2010845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li H, Xiao X, Zhang J, Zafar MI, Wu C, Long Y, Lu W, Pan F, Meng T, Zhao K, Zhou L, Shen S, Liu L, Liu Q, Xiong C. Impaired spermatogenesis in COVID-19 patients. EClinicalMedicine. 2020 Nov;28:100604–100604. doi: 10.1016/j.eclinm.2020.100604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ma L, Xie W, Li D, Shi L, Ye G, Mao Y, et al. Evaluation of sex-related hormones and semen characteristics in reproductive-aged male COVID-19 patients. J Med Virol. 2021;93:456–462. doi: 10.1002/jmv.26259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Machado B, Barcelos Barra G, Scherzer N, Massey J, Dos Santos Luz H, Henrique Jacomo R, et al. Presence of SARS-CoV-2 RNA in Semen-Cohort Study in the United States COVID-19 Positive Patients. Infect Dis Rep. 2021;13:96–101. doi: 10.3390/idr13010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mannur S, Jabeen T, Khader MA, Rao LSS. Post-COVID-19-associated decline in long-term male fertility and embryo quality during assisted reproductive technology. QJM. 2021;114:328–330. doi: 10.1093/qjmed/hcab019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mumm JN, Osterman A, Ruzicka M, Stihl C, Vilsmaier T, Munker D, et al. Urinary Frequency as a Possibly Overlooked Symptom in COVID-19 Patients: Does SARS-CoV-2 Cause Viral Cystitis? Eur Urol. 2020;78:624–628. doi: 10.1016/j.eururo.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nie X, Qian L, Sun R, Huang B, Dong X, Xiao Q, et al. Multi-organ proteomic landscape of COVID-19 autopsies. Cell. 2021;184:775.e14–791.e14. doi: 10.1016/j.cell.2021.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ning J, Li W, Ruan Y, Xia Y, Wu X, Hu K, et al. Effects of 2019 Novel Coronavirus on Male Reproductive System: A Retrospective Study. Preprints. 2020 2020040280. [Internet]. Available at. < https://www.preprints.org/manuscript/202004.0280/v1>. [Google Scholar]
- 55.Pan F, Xiao X, Guo J, Song Y, Li H, Patel DP, et al. No evidence of severe acute respiratory syndrome-coronavirus 2 in semen of males recovering from coronavirus disease 2019. Fertil Steril. 2020;113:1135–1139. doi: 10.1016/j.fertnstert.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Paoli D, Pallotti F, Colangelo S, Basilico F, Mazzuti L, Turriziani O, et al. Study of SARS-CoV-2 in semen and urine samples of a volunteer with positive naso-pharyngeal swab. J Endocrinol Invest. 2020;43:1819–1822. doi: 10.1007/s40618-020-01261-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pavone C, Giammanco GM, Baiamonte D, Pinelli M, Bonura C, Montalbano M, et al. Italian males recovering from mild COVID-19 show no evidence of SARS-CoV-2 in semen despite prolonged nasopharyngeal swab positivity. Int J Impot Res. 2020;32:560–562. doi: 10.1038/s41443-020-00344-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rastrelli G, Di Stasi V, Inglese F, Beccaria M, Garuti M, Di Costanzo D, et al. Low testosterone levels predict clinical adverse outcomes in SARS-CoV-2 pneumonia patients. Andrology. 2021;9:88–98. doi: 10.1111/andr.12821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rawlings SA, Ignacio C, Porrachia M, Du P, Smith DM, Chaillon A. No Evidence of SARS-CoV-2 Seminal Shedding Despite SARS-CoV-2 Persistence in the Upper Respiratory Tract. Open Forum Infect Dis. 2020;7:ofaa325–ofaa325. doi: 10.1093/ofid/ofaa325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ruan Y, Hu B, Liu Z, Liu K, Jiang H, Li H, et al. No detection of SARS-CoV-2 from urine, expressed prostatic secretions, and semen in 74 recovered COVID-19 male patients: A perspective and urogenital evaluation. Andrology. 2021;9:99–106. doi: 10.1111/andr.12939. [DOI] [PubMed] [Google Scholar]
- 61.Salonia A, Pontillo M, Capogrosso P, Gregori S, Tassara M, Boeri L, et al. Severely low testosterone in males with COVID-19: A case-control study. Andrology. 2021;9:1043–1052. doi: 10.1111/andr.12993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Silverman ML, VanDerVeer SJ, Donnelly TJ. Priapism in COVID-19: A thromboembolic complication. Am J Emerg Med. 2021 Jul;45:686.e5–686.e6. doi: 10.1016/j.ajem.2020.12.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Song C, Wang Y, Li W, Hu B, Chen G, Xia P, et al. Absence of 2019 novel coronavirus in semen and testes of COVID-19 patients†. Biol Reprod. 2020;103:4–6. doi: 10.1093/biolre/ioaa050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Temiz MZ, Dincer MM, Hacibey I, Yazar RO, Celik C, Kucuk SH, et al. Investigation of SARS-CoV-2 in semen samples and the effects of COVID-19 on male sexual health by using semen analysis and serum male hormone profile: A cross-sectional, pilot study. Andrologia. 2021;53:e13912. doi: 10.1111/and.13912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xu H, Wang Z, Feng C, Yu W, Chen Y, Zeng X, et al. Effects of SARS-CoV-2 infection on male sex-related hormones in recovering patients. Andrology. 2021;9:107–114. doi: 10.1111/andr.12942. [DOI] [PubMed] [Google Scholar]
- 66.Yang M, Chen S, Huang B, Zhong JM, Su H, Chen YJ, et al. Pathological Findings in the Testes of COVID-19 Patients: Clinical Implications. Eur Urol Focus. 2020;6:1124–1129. doi: 10.1016/j.euf.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhu M, Chen D, Zhu Y, Xiong X, Ding Y, Guo F, et al. Long-term sero-positivity for IgG, sequelae of respiratory symptoms, and abundance of malformed sperms in a patient recovered from severe COVID-19. Eur J Clin Microbiol Infect Dis. 2021;40:1559–1567. doi: 10.1007/s10096-021-04178-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hu B, Huang S, Yin L. The cytokine storm and COVID-19. J Med Virol. 2021;93:250–256. doi: 10.1002/jmv.26232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Delgado-Roche L, Mesta F. Oxidative Stress as Key Player in Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV) Infection. Arch Med Res. 2020;51:384–387. doi: 10.1016/j.arcmed.2020.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Duarte AN, Neto, Teixeira TA, Caldini EG, Kanamura CT, Gomes-Gouvêa MS, Dos Santos ABG, et al. Testicular pathology in fatal COVID-19: A descriptive autopsy study. Andrology. 2022;10:13–23. doi: 10.1111/andr.13073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Semet M, Paci M, Saïas-Magnan J, Metzler-Guillemain C, Boissier R, Lejeune H, et al. The impact of drugs on male fertility: a review. Andrology. 2017;5:640–663. doi: 10.1111/andr.12366. [DOI] [PubMed] [Google Scholar]
- 73.Carneiro F, Teixeira TA, Bernardes FS, Pereira MS, Milani G, Duarte AN, Neto, et al. Radiological patterns of incidental epididymitis in mild-to-moderate COVID-19 patients revealed by colour Doppler ultrasound. Andrologia. 2021;53:e13973. doi: 10.1111/and.13973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Teixeira TA, Bernardes FS, Oliveira YC, Hsieh MK, Esteves SC, Duarte AN, Neto, et al. SARS-CoV-2 and Multi-Organ damage - What men's health specialists should know about the COVID-19 pathophysiology. Int Braz J Urol. 2021;47:637–646. doi: 10.1590/S1677-5538.IBJU.2020.0872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lucio Carrasco CH, Noda P, Barbosa AP, Vieira Borges da Silva EK, Gasque Bomfim C, Ventura Fernandes BH, et al. SARS-CoV-2 Nucleocapsid Protein is Associated With Lower Testosterone Levels: An Experimental Study. Front Physiol. 2022;13:867444–867444. doi: 10.3389/fphys.2022.867444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Paoli D, Pallotti F, Turriziani O, Mazzuti L, Antonelli G, Lenzi A, et al. SARS-CoV-2 presence in seminal fluid: Myth or reality. Andrology. 2021;9:23–26. doi: 10.1111/andr.12825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Massarotti C, Garolla A, Maccarini E, Scaruffi P, Stigliani S, Anserini P, et al. SARS-CoV-2 in the semen: Where does it come from? Andrology. 2021;9:39–41. doi: 10.1111/andr.12839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kashi AH, De la Rosette J, Amini E, Abdi H, Fallah-Karkan M, Vaezjalali M. Urinary Viral Shedding of COVID-19 and its Clinical Associations: A Systematic Review and Meta-analysis of Observational Studies. Urol J. 2020;17:433–441. doi: 10.22037/uj.v16i7.6248. [DOI] [PubMed] [Google Scholar]
- 79.Silva GCDA, Abe DK, Pedrenho R, Neto, Vilares RN, Cordeiro MD, Coelho RF, et al. Evaluation of uro-oncological surgical treatment during the Sars-CoV-2 pandemic in a Brazilian tertiary oncology institution, the new world epicenter. Int Braz J Urol. 2021;47:378–385. doi: 10.1590/S1677-5538.IBJU.2020.0479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zampolli HC, Rodriguez AR. Laparoscopic and Robotic Urology Surgery during Global Pandemic COVID19. Int Braz J Urol. 2020;46(suppl.1):215–221. doi: 10.1590/S1677-5538.IBJU.2020.S113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mazzucchi E, Torricelli FCM, Vicentini FC, Marchini GS, Danilovic A, Batagello CA, et al. The impact of COVID-19 in medical practice. A review focused on Urology. Int Braz J Urol. 2021;47:251–262. doi: 10.1590/S1677-5538.IBJU.2020.99.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Carneiro A, Wroclawski ML, Nahar B, Soares A, Cardoso AP, Kim NJ, et al. Impact of the COVID-19 Pandemic on the Urologist's clinical practice in Brazil: a management guideline proposal for low- and middle-income countries during the crisis period. Int Braz J Urol. 2020;46:501–510. doi: 10.1590/S1677-5538.IBJU.2020.04.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ficarra V, Novara G, Abrate A, Bartoletti R, Crestani A, De Nunzio C, et al. Urology practice during the COVID-19 pandemic. Minerva Urol Nefrol. 2020;72:369–375. doi: 10.23736/S0393-2249.20.03846-1. [DOI] [PubMed] [Google Scholar]
- 84.Prezotti JA, Henriques JVT, Favorito LA, Canalini AF, Machado MG, Brandão TBV, et al. Impact of COVID-19 on education, health and lifestyle behaviour of Brazilian urology residents. Int Braz J Urol. 2021;47:753–776. doi: 10.1590/S1677-5538.IBJU.2021.99.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Esperto F, Prata F, Civitella A, Pang KH, Marchioni M, Tuzzolo P, et al. Implementation and strategies to ensure adequate coordination within a Urology Department during the COVID-19 pandemic. Int Braz J Urol. 2020;46(suppl.1):170–180. doi: 10.1590/S1677-5538.IBJU.2020.S122. [DOI] [PMC free article] [PubMed] [Google Scholar]