Abstract
Objective:
Despite improved access to antiretroviral therapy (ART) for people living with HIV (PLHIV), HIV continues to contribute considerably to morbidity and mortality. Increasingly, advanced HIV disease (AHD) is found among PLHIV who are ART-experienced.
Design:
Using a multi-state model we examined associations between engagement with care and AHD on ART in South Africa.
Methods:
Using data from IeDEA Southern Africa, we included PLHIV from South Africa, initiating ART from 2004–2017 aged >5 years with a CD4 cell count at ART start and ≥1 subsequent measure. We defined a gap as no visit for ≥18 months. Five states were defined: “AHD on ART” (CD4<200 cells/mm3), “Clinically Stable on ART” (CD4 ≥200 or if no CD4 count, viral load <1000 copies/ml), “Early Gap” (commencing ≤18 months from ART start), “Late Gap” (commencing >18 months from ART start), and “Death”.
Results:
Among 32452 PLHIV, men and those aged 15–25 were more likely to progress to unfavourable states. Later year of ART start was associated with a lower probability of transitioning from AHD to clinically stable, increasing the risk of death following AHD. In stratified analyses, those starting ART with AHD in later years were more likely to re-engage in care with AHD following a gap, and to die following AHD on ART.
Conclusions:
In more recent years those with AHD on ART were more likely to die, and AHD at re-engagement in care increased. To further reduce HIV-related mortality, efforts to address the challenges facing these more vulnerable patients are needed.
Keywords: advanced HIV disease, engagement in care, people living with HIV, antiretroviral therapy, Southern Africa
Introduction
Despite the successful scale-up in antiretroviral therapy (ART) programmes in the last decade, many patients continue to experience advanced HIV disease (AHD). A large proportion of people living with HIV (PLHIV) continue to present to care with low CD4 cell counts, despite implementation of the treat all policy.[1–3] Reductions in HIV-related mortality have plateaued in more recent years,[4] and HIV continues to contribute considerably to hospital admissions and mortality in high burden settings.[5]
In South Africa, a growing proportion of adult HIV-related mortality is among ART-experienced PLHIV,[6] and recent studies found that AHD is increasingly diagnosed among ART-experienced patients who are returning to care after treatment interruption, or are poorly adherent, or infected with a virus that is resistant to ART.[5, 7]
Few studies have examined longitudinal trends in patient engagement in care and AHD on ART as treatment programmes have expanded and matured in southern Africa. We aimed to estimate the transition probabilities to and from an AHD on ART state using a multi-state model, and to describe associations with transitions.
Methods
Study population
We used routinely collected data contributed to the International epidemiology Databases to Evaluate AIDS Southern Africa (IeDEA-SA) collaboration from the Khayelitsha ART programme.[8] Khayelitsha is a peri-urban township in Cape Town, South Africa, with an estimated population of 500,000, and one of the highest HIV burdens with a prevalence among pregnant women of 34%. By 2017 approximately 40,000 patients were receiving ART from public sector clinics.[9, 10] During the study period ART initiation guidelines were revised several times to expand from ART eligibility based on CD4 cell count and other co-morbidities, to all PLHIV becoming eligible for ART irrespective of CD4 cell count by 2016.[9, 11–14] Ethical approval to contribute anonymized, individual data to IeDEA-SA was obtained from the University of Cape Town.
Eligibility criteria
We included all patients initiating ART from 2004–2017 aged >5 years at ART initiation, with a CD4 cell count at ART initiation and at least one subsequent measure, ensuring ≥18 months between ART initiation and database closure (October 2018).
States of care / Measures
We defined states of ART care from the time of each patients first CD4 cell count after ART initiation. We defined a gap in care as having no recorded visit for ≥18 months, ensuring patients were truly out of care and without ART during that time. Early and late gaps were defined according to when they occurred during each patient’s follow-up. We defined five states : “AHD on ART” (CD4 <200 cells/mm3), “Stable on ART” (CD4 ≥200 cells/mm3 or if no CD4 count available in 12 month period, having a suppressed viral load (VL) (VL <1000 copies/ml)), “Early Gap” (gap commencing ≤18 months from ART initiation), “Late Gap” (gap commencing >18 months from ART initiation), and “Death”. For periods with ongoing visits but no recorded CD4 cell count and no VL for >12 months, time on ART was assigned to a censored state. Time on ART was also assigned to the censored state for periods with a recorded VL >1000 copies/ml, and no recorded CD4 cell count for >12 months. The model assumed periods spent in this censored state were in either of the two “on ART” states.
The World Health Organization (WHO) defines AHD among adults and children >5 years as having a CD4 <200 cells/mm3 or WHO Stage 3 or 4 disease.[15] Clinical staging data was not available throughout patient follow-up, so we used CD4 cell count only to indicate AHD. We defined the stable state using both CD4 and VL measures, since from 2013 CD4 monitoring on ART was recommended only at 1 year after ART initiation, with VL monitoring recommended at months 6, 12, and annually thereafter.[13]
Vital status was ascertained through linkage to the national population registry for patients with a recorded South African national identity number. Follow-up time was measured until death, transfer or database closure.
Model description
A parametric, continuous-time multi-state Markov model was used to identify associations with transitioning between states. We modelled 12 transitions among 5 states, with reverse transitions allowed except to early gap and from death (Figure 1), with transitions to and from AHD on ART being the primary interest. The early gap state was the initial state for any patient with a gap commencing ≤18 months on ART. This ensured that transitions to a gap state from either of the “on ART” states were to the late gap state, removing any potential time dependence. We estimated the five-year probability of transition between states. We included the following fixed covariates to adjust for differences in transition by sex (male or female), year of ART initiation (2004–2006, 2007–2009, 2010–2012, 2013–2017), CD4 cell count at ART initiation (0–50, 51–200, 201–500 and >500 cells/mm3), and age at ART initiation (5-≤15, >15-≤25, >25-≤40 and >40 years). We also included the time-updated covariates of any recorded prior tuberculosis diagnosis and any recorded pregnancy. For the pregnancy covariate, to adjust for any potential increase in risk of a gap post-partum,[16] we defined the post-partum period as between 9 months and 2 years after the first antenatal date.
Figure 1:
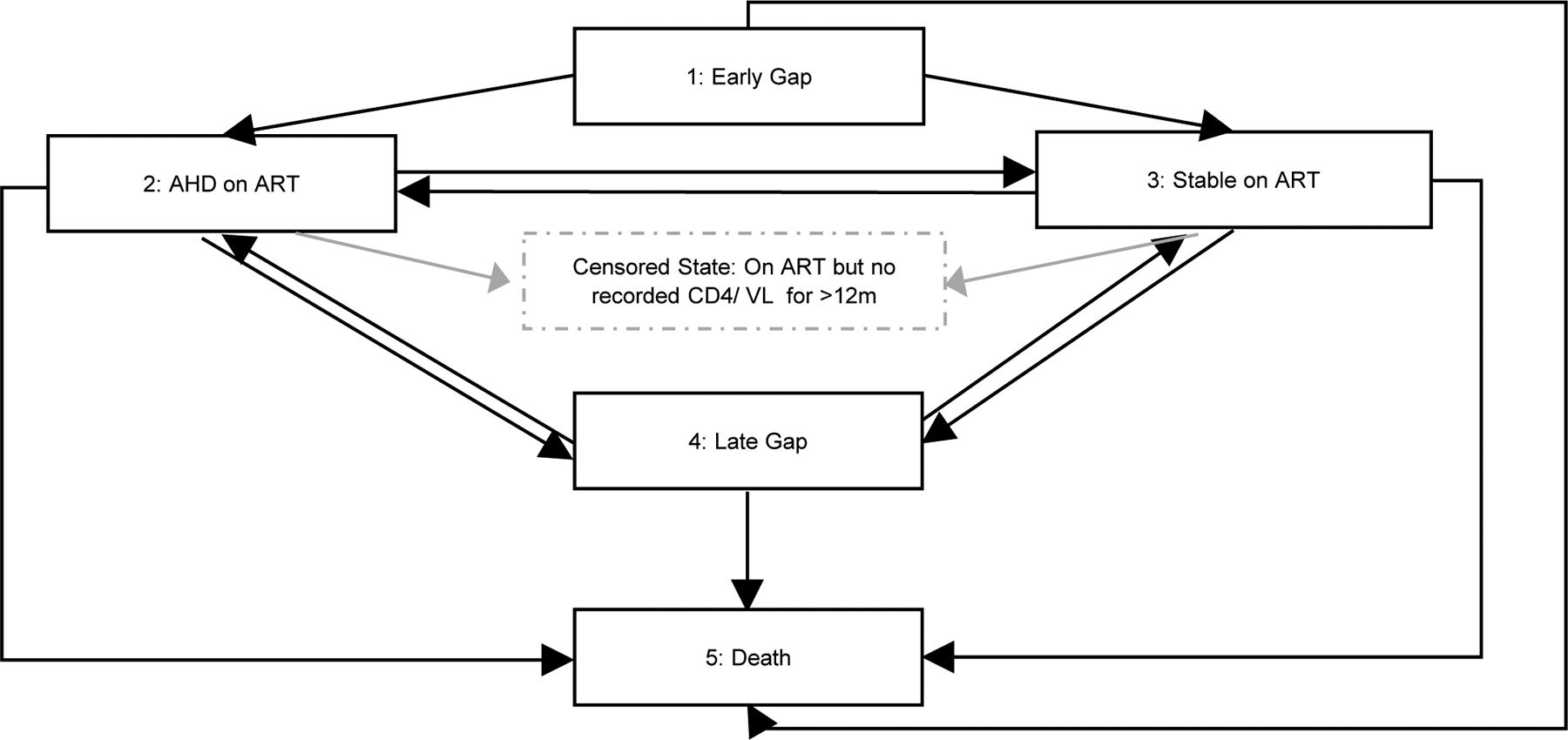
Diagram of states and transitions of the multi-state model
ART: Antiretroviral treatment
Gap in care: no recorded visit for ≥18 months
Early Gap: gap commencing ≤18 months from ART start
Late Gap: gap commencing >18 months from ART start
AHD on ART: Advanced HIV disease on ART
Stable on ART: CD4 ≥200 cells/mm3 or if no CD4 count available in 12 monthperiod, having viral load (VL) (VL <1000 copies/ml))
Censored state: In care with no recorded CD4 cell count or viral load for >12 months, or no CD4 cell count and VL ≥1000 copies/ml
Multi-state models assume time spent in each state follow an exponential distribution and transition probabilities are independent of previous states. Covariate effects are assumed constant over time and multiplicative, similar to Cox proportional hazard models. The model allowed for unobserved transitions between the AHD and Stable on ART states, while the exact time of transitions to and from gap states and to death were specified.
Sensitivity analyses were performed to address potential biases. Since ART eligibility changed during the study period and was also based on clinical criteria which we were unable to assess, we performed a stratified analysis by CD4 cell count at ART initiation to explore potential differences in the effect of year of ART initiation on transition probabilities. Those who started ART in the most recent years have shorter follow-up, with late gaps occurring at a shorter duration on ART. We repeated our analysis using a restricted dataset which included each patient’s first 5 years on ART only. To explore the effect of missing CD4 cell count data during follow-up we modelled a best and worst case scenario where all time in the censored state was assigned to Stable or to AHD on ART respectively. To understand the potential effects of differences in mortality ascertainment, we repeated our analysis using a restricted dataset including only those with South African identity numbers.
We used Stata version 15 for descriptive statistics, and the msm package in R for the multi-state model.[17]
Results
Table S1 (Supplemental Digital Content (SDC) 1) summarizes characteristics of patients included and excluded from our study. Our analysis included 32452 patients, of whom 63% were linked to the national population registry. Among those excluded, 6718 (81%) initiated ART after 2013 and had no CD4 cell count after ART initiation. At ART initiation median age was 32.9 years (interquartile range (IQR): 27.9–39.2), median CD4 cell count was 175 (IQR: 94–267), 70% were female of whom 26% experienced pregnancy during follow-up, and 33% had evidence of TB prior to or during follow-up. (Table 1)
Table 1:
Characteristics of patients >5 years initiating ART between 2004–2017 in Khayelitsha, South Africa (n=32452)
| Characteristic | Total | AHD on ART | AHD after ART start | After Early Gap | After Late Gap | After Stable | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | 32452 | 11222 | 35% | 6510 | 58% | 997 | 9% | 1053 | 9% | 3416 | 30% | ||
| Sex | Males | 9877 | 30% | 4314 | 38% | 2682 | 41% | 611 | 61% | 358 | 34% | 1203 | 35% |
| Females | 22575 | 70% | 6908 | 62% | 3828 | 59% | 386 | 39% | 695 | 66% | 2213 | 65% | |
| Age at ART start (years) | Median (IQR) | 32.9 (27.9–39.2) | 33.3 (28.3–39.4) | 34.4 (29.4–40.4) | 31.1 (26.5–37.1) | 30.5 (26.6–35.5) | 33.0 (27.9–39.3) | ||||||
| 5-≤15 | 404 | 1% | 97 | 1% | 35 | 1% | 8 | 1% | 15 | 1% | 41 | 1% | |
| >15-≤25 | 3618 | 11% | 1133 | 10% | 492 | 8% | 155 | 16% | 172 | 16% | 391 | 11% | |
| >25-≤40 | 21048 | 65% | 7383 | 66% | 4269 | 66% | 679 | 68% | 733 | 70% | 2192 | 64% | |
| >40 | 7382 | 23% | 2609 | 23% | 1714 | 26% | 155 | 16% | 133 | 13% | 792 | 23% | |
| CD4 cell count at ART start | Median (IQR) | 175 (94–267) | 97 (46–163) | 69 (31–125) | 147 (89–217) | 129 (69–188) | 112 (60–176) | ||||||
| 0–50 | 4192 | 13% | 3028 | 27% | 2438 | 37% | 125 | 13% | 179 | 17% | 685 | 20% | |
| 51–200 | 14793 | 46% | 6561 | 58% | 3621 | 56% | 577 | 58% | 652 | 62% | 2171 | 64% | |
| 201–500 | 12035 | 37% | 1576 | 14% | 434 | 7% | 288 | 29% | 213 | 20% | 538 | 16% | |
| >500 | 1432 | 4% | 57 | 1% | 17 | 0% | 7 | 1% | 9 | 1% | 22 | 1% | |
| Year of ART start | 2004–2006 | 4513 | 14% | 2296 | 20% | 1339 | 21% | 90 | 9% | 240 | 23% | 1103 | 32% |
| 2007–2009 | 6956 | 21% | 3160 | 28% | 1862 | 29% | 201 | 20% | 357 | 34% | 1140 | 33% | |
| 2010–2012 | 9949 | 31% | 3248 | 29% | 1745 | 27% | 398 | 40% | 365 | 35% | 837 | 25% | |
| 2013–2017 | 11034 | 34% | 2518 | 22% | 1564 | 24% | 308 | 31% | 91 | 9% | 336 | 10% | |
| Post-partum during follow-up | 5804 | 18% | 1683 | 15% | 795 | 12% | 190 | 19% | 233 | 22% | 612 | 18% | |
| TB at ART start or after | 10657 | 33% | 5434 | 48% | 3315 | 51% | 387 | 39% | 491 | 47% | 1761 | 52% | |
| TB at ART start | 7251 | 22% | 3563 | 32% | 2233 | 34% | 268 | 27% | 317 | 30% | 1087 | 32% | |
TB: Tuberculosis
AHD: Advanced HIV disease
IQR: Interquartile range
Early Gap: gap in care (no recorded visit for ≥18 months) starting ≤18 months of ART start
Late Gap: gap in care (no recorded visit for ≥18 months) starting >18 months since ART start
Stable: CD4 ≥200 cells/mm3 or if no CD4 count available in 12 month period, having viral load (VL) (VL <1000 copies/ml))
Post-partum period defined as between 9 months and 2 years after the first antenatal date
Over a median follow-up of 6.1 years (IQR 3.4–8.9), 2859 (9%) died, 28226 (87%) experienced the stable state, 11222 (35%) had AHD on ART, 4518 (14%) had an early gap, and 6814 (21%) a late gap in care. Of those with AHD on ART, 3239 (29%) had 2 or more episodes of AHD on ART, separated by either a gap or a stable state. Most patients with AHD on ART had AHD as their starting state (6510, 58%), almost a third (3416, 30%) had AHD directly after being stable, 9% (997) after an early gap and 9% (1053) after a late gap.
While prevalence of AHD on ART declined between 2004–2017, (40% to 8%), the number of PLHIV with AHD on ART has remained stable since 2009. (Figure 2) Prevalence of late gap has steadily increased, while prevalence of stable state has remained steady since 2013.
Figure 2:
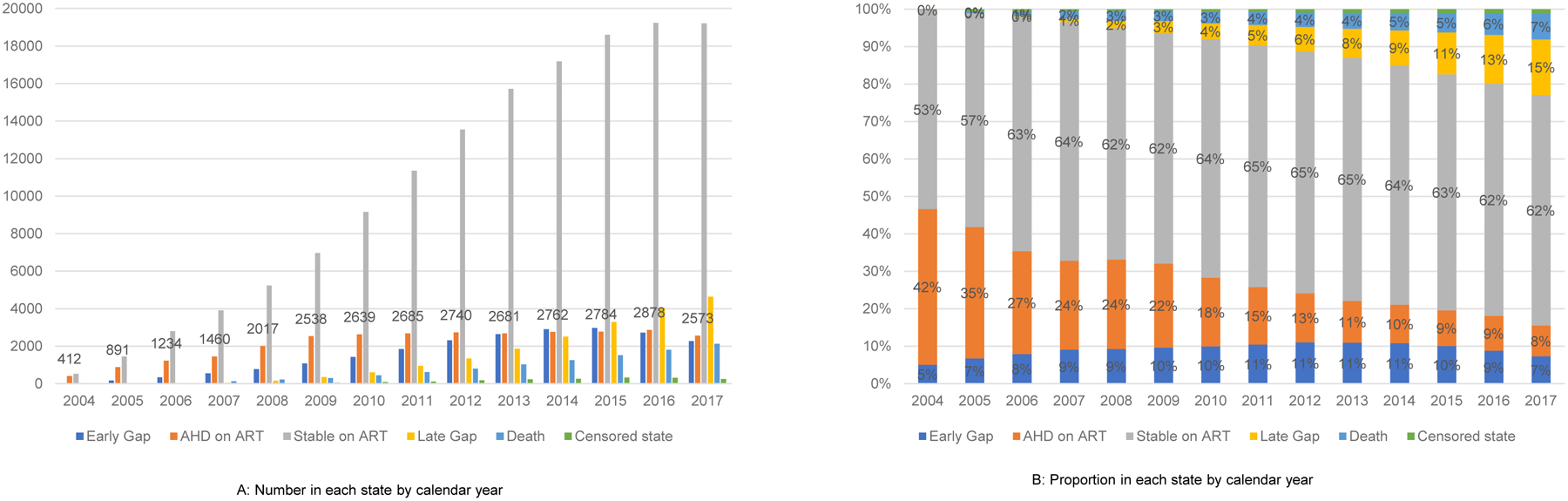
Number (A) and proportion (B) in each state by calendar year
Figure 3 summarizes the key results of our multi-state model by sex, age and year of ART start. We found men were more likely to transition to unfavourable states (AHD on ART, late gap and death), and less likely to transition to the stable state compared to women.(Figure 3A) Those aged 15–25 years were more likely to transition from stable to AHD on ART and late gap, and from AHD on ART to late gap. Those aged 15–25 had a similar risk of death to those aged >40 years.(Figure 3B) Later year of ART initiation was associated with reduced transition from stable to AHD on ART, increased transition from stable to late gap and increased re-engagement in care in either the AHD on ART or stable states. Later year was also associated with reduced transition from AHD on ART to stable, and increased transition from AHD on ART to death. (Figure 3C)
Figure 3:
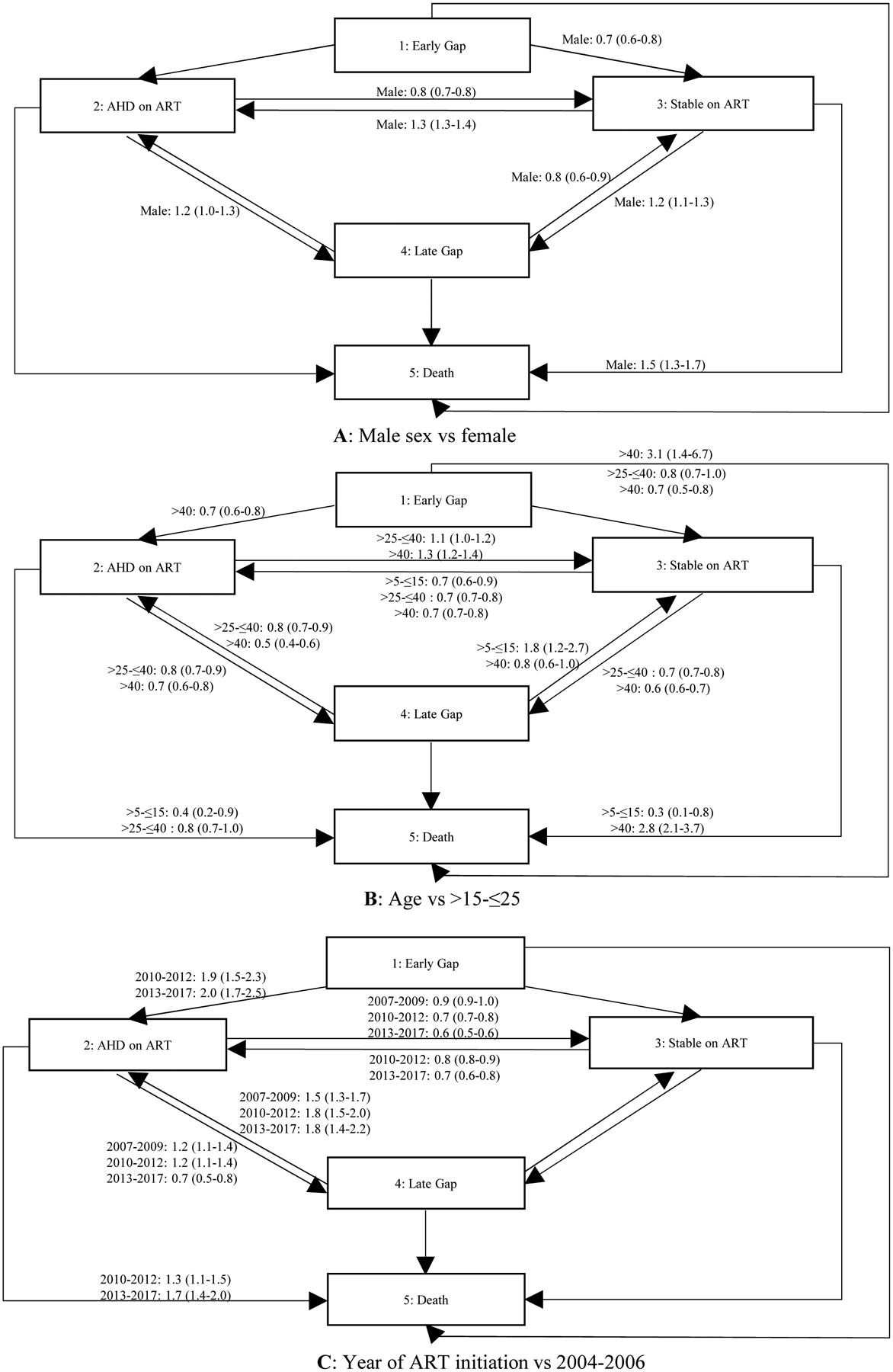
Estimated hazard ratios from a multi-state model, adjusted hazard ratios (95% CI)
Lower CD4 cell count at ART initiation was associated with worse progression, with increased transition to AHD on ART, and reduced transition to stable. Those with higher CD4 cell count at ART initiation were more likely to transition to late gap. Having tuberculosis and being post-partum was associated with increased risk of progression to AHD on ART from stable.(Table S2, SDC 2)
Sensitivity Analyses
In stratified analysis by CD4 cell count at ART initiation, there were too few transitions to model associations for those with CD4 >500 cells/mm3. For the other strata, aHRs differed by year of ART initiation (Table S3, SDC 3). For those starting ART with low CD4 cell count (CD4 0–50 and 50–200), later year of ART initiation was associated with increased risk of re-engaging in care with AHD. Transitioning from AHD to stable was less likely, while mortality increased with later year. For those starting ART with higher CD4 count (CD4 of 200–500), transitions from stable to AHD on ART were less likely, while transitions from stable to late gap increased with later year.
When restricting the dataset to include each patient’s first 5 years on ART, results were similar, although for transitions from late gap many of the confidence intervals were no longer significant when compared with the full dataset. (Table S4, SDC 4)
We did not see significant differences when exploring the effects of missing CD4 cell count data (Tables S5 and S6, SDC 5 and 6), except for transitions from Stable to AHD on ART in the worst case scenario. This suggests that we may be overestimating the association between initiating ART with AHD and experiencing AHD on ART following a Stable state. However, the worst case scenario is somewhat unrealistic since stable patients may be more likely to have longer periods without CD4 monitoring.
When we restricted our analysis to only those linked to the national population registry (Table S7, SDC 7), we found associations with transitions from AHD on ART did not change substantially, although some were removed by widening of confidence intervals due to smaller study size. Similarly there was little change in the results for transitions to AHD on ART, but notably there was no longer an association between year of ART start and transition from late gap to AHD on ART.
Re-engaging in care
Figures 4 and S1 (SDC 8) show the five-year probabilities of resuming ART after either gap state, and the proportions re-engaging with AHD.
Figure 4:
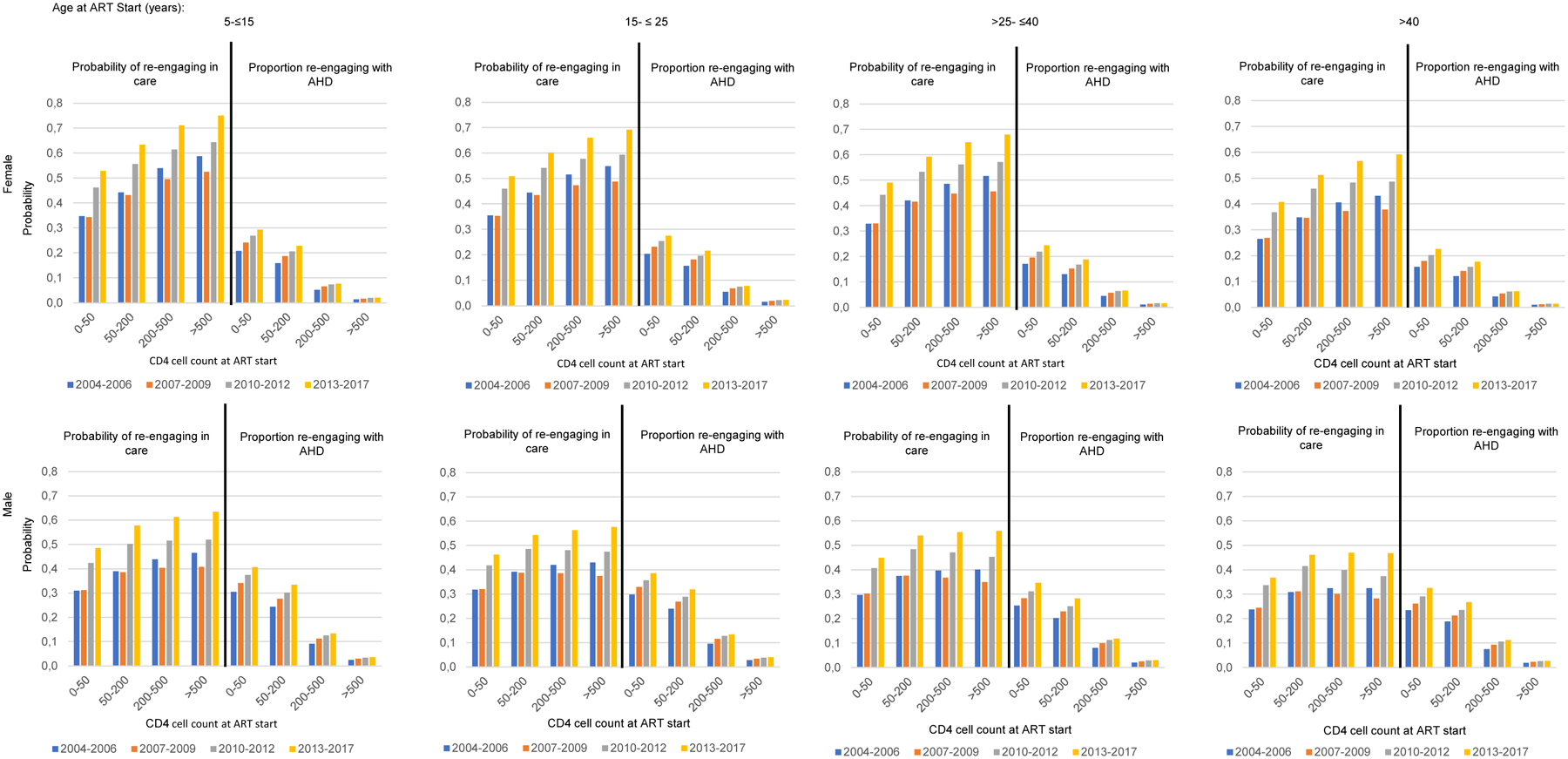
Five-year probability of re-engaging in care following an early gap, and proportion re-engaging with AHD
Across all covariates the probability of re-engaging after a gap was higher after an early compared to a late gap. Women, those with higher CD4 cell count at ART initiation and younger PLHIV had the highest probability of re-engaging in care.
Across all covariates re-engaging in care with AHD was higher following a late gap compared to an early gap. Among those re-engaging with AHD, a higher proportion were men. There were smaller differences between age groups, with those aged 15–25 years being more likely to re-engage with AHD. Higher CD4 cell count at ART initiation dramatically reduced the proportion re-engaging with AHD. In later years a higher proportion re-engaged with AHD especially among those with low CD4 cell count at ART initiation.
Validation
Comparing model projections with the observed prevalence of different states, our model overpredicted AHD on ART and underpredicted the stable state. (Figure S2, SDC 9) However, the observed prevalence assumed that where no CD4 cell count is recorded, the patient remained in their previous state, unlike our model which allowed for unobserved transitions. With stable patients less likely to have their CD4 cell count measured and longer times between measures, the observed data may overestimate the prevalence of the stable state and underestimate AHD on ART.
Discussion
While the prevalence of AHD has substantially decreased, the number of PLHIV experiencing AHD on ART remained stable since 2009. We found that in more recent years those with AHD on ART were increasingly more likely to die, and less likely to become stable. Over time, progression to AHD from stable became less likely, but re-engaging in care with AHD following a gap became increasingly more likely. The proportion of patients in a stable state has not improved since 2013, and an increasing proportion are in the late gap state. We found that male sex, age 15–25, and lower CD4 cell count at ART initiation were associated with progression to unfavourable states. Those aged 15–25 at ART initiation were as likely to die following AHD compared to older age groups, and more likely to transition to AHD from any state than any other age group.
Those initiating ART with low CD4 cell counts in more recent years appear to be a particularly vulnerable group. In our stratified analysis we found that among those with low CD4 count at ART initiation, those initiating ART in later year were less likely to transition from AHD to stable, more likely to die following AHD, and more likely to have AHD when re-engaging in care. Re-engaging in care with AHD was far more likely among those who initiate ART with low compared to higher CD4 cell counts. While those with higher CD4 cell count at ART initiation were less likely to progress to AHD, and more likely to transition to late gap, higher CD4 cell count dramatically reduced the proportion with AHD among those re-engaging. Even so, for adults and adolescents initiating ART more recently with CD4 cell count of 200–500, among those re-engaging in care >9% of women and >15% of men were estimated to do so with AHD, suggesting that AHD will continue to be a significant burden to the health system.
According to recent hospital-based studies among PLHIV in Southern Africa, relatively few admitted patients are ART naïve.[18–20] This marks a change from earlier years where most AHD was seen among ART-naïve patients, to an emerging challenge of patients with AHD, aware of their HIV status and ART-exposed, but with very high mortality. Several studies have employed multi-state models to examine engagement in care and HIV disease progression.[21–23] A Zambian study exploring patient stability found transitions to an unstable state and gaps in care were common. They found similar results to our study, with lower rates of transition from a stable state to instability, and higher rates of gaps among those enrolling more recently. Men had lower rates of transition from an unstable to a clinically stable state. They did not report transitions to death or include an adolescent age group.[23]
Other Southern African studies simultaneously examining engagement in care and mortality among PLHIV include a Zambian group-based multi-trajectory analysis.[24] In broad agreement with our findings, they found higher risk of mortality with worse engagement trajectory. They identified trajectory groups that distinguished between early and late LTFU, supporting the two gap states employed in our model. Trajectory group strongly predicted mortality, independent of baseline characteristics. They did not explore trends over time. Our findings are consistent with a number of studies which show that those with lower CD4 cell count at ART initiation, men and adolescents have poorer outcomes.[25, 26] Our finding that higher CD4 cell count was associated with increased probability of late gap is also consistent with other studies.[27, 28]
While several studies have demonstrated declining AHD at ART initiation, few have examined outcomes of patients with AHD once on treatment. To our knowledge this is the only study examining engagement in care and AHD on ART in Southern Africa. With many PLHIV already ART-exposed, and an increasing number experiencing gaps in care, our study used methods allowing for repeated disengagement and re-engagement in care, instead of the more traditional linear ‘HIV cascade of care’ approach. Our study follows a large public sector cohort over many years, providing insights into the outcomes of patients experiencing AHD on ART and how these have changed as ART programmes have grown and policies shifted to more inclusive ART eligibility criteria. Our findings are likely generalizable to other urban cohorts in the region where similar expansion in ART programmes occurred.
Our study has several limitations. Our results regarding trends over time are subject to some bias, since those initiating ART more recently had shorter follow-up, and their “late gaps” thus occurred at shorter duration on ART. Our multi-state model assumes transition probabilities are independent of time, whereas, adherence and engagement in care may vary with duration on ART. Some potential bias was removed by including early and late gap states and our sensitivity analysis found little bias when restricting data to each patient’s first 5 years on ART. There may be some bias in our estimates due to differences in mortality ascertainment with calendar year of ART initiation, and further research is needed to quantify re-engagement in care with AHD. In the earliest years those with clinical disease were eligible to start treatment regardless of CD4 cell count. While we included prior tuberculosis diagnosis as a covariate, we were unable to include other clinical data. Those initiating ART with higher CD4 cell counts during the earlier years of ART were likely to have clinical illness, which would worsen their prognosis compared to patients initiating ART with higher CD4 cell counts in later years. Our stratified analysis attempted to remove this bias, but without knowing how the prevalence of clinical illness at ART initiation has changed over time, we remain cautious in interpreting the hazard ratios especially for those with higher CD4 count at ART initiation. It is possible that AHD is underestimated in more recent years due to reduced CD4 monitoring. We made use of VL data, to include periods without CD4 cell count measurement but with suppressed VL in the stable state. We may, therefore, underestimate AHD since low CD4 cell count can persist even with viral suppression.[29] With limited CD4 cell count data in later years we did not model CD4 cell count trajectory which may more accurately predict transitions to and from AHD. Our model could not account for trajectory through previous states, potentially an important predictor of outcomes.
Our finding that death following AHD is increasingly more likely suggests a change in patient profile. Those initiating ART with a low CD4 cell count in more recent years did not access treatment earlier in their disease progression despite it being available. Their low CD4 cell count not only makes them more vulnerable to morbidity and mortality, but they are likely a population facing extra barriers in accessing care and may have more complicated clinical and psychosocial needs as a result. While CD4 cell count at ART initiation has increased, there are concerns that starting ART without disease may increase disengagement from care, ultimately leading to increased AHD after ART initiation. Our study found that low CD4 cell count at ART initiation is strongly predictive of AHD on ART, and while those with higher CD4 cell count were indeed more likely to experience gaps care, higher CD4 cell count was also protective against having AHD when re-engaging. This strongly supports universal ART combined with measures that reduce barriers to re-engaging in care, and normalise interruptions, as a means of reducing AHD. While only a small proportion of those with high CD4 cell count at ART initiation re-engage with AHD, with the increasing proportion of patients who experience gaps in care, AHD is likely to grow, and models of care such as welcome back services[30–32] will be important in identifying those who require more urgent care. As ART programmes shift to a differentiated care approach, VL monitoring has been emphasized as an important tool in identifying stable patients.[33] Our study emphasizes the importance of CD4 cell count measurement, at least at ART initiation, in identifying more vulnerable patients. More data from the treat all era is needed to understand how AHD trends are likely to change.
In conclusion, while AHD is declining in the modern ART era, those with AHD are more likely to die, and AHD at re-engagement in care is increasing. To further reduce HIV-related mortality, efforts to address the challenges facing these more vulnerable patients are needed, along with programmes to ensure long-term retention in care.
Supplementary Material
Acknowledgements
Computations were performed using facilities provided by the University of Cape Town’s ICTS High Performance Computing team. The authors are grateful to all patients and staff at the Khayelitsha HIV care programme included in this analysis and to the data centre staff.
Support for this study was provided by the US National Institute of Allergy and Infectious Diseases (NIAID) through the International epidemiological Databases to Evaluate AIDS, Southern Africa (IeDEA-SA), Grant no 5U01AI069924-04
Footnotes
Conflicts of Interest:
The authors declare no conflicts of interest.
References
- 1.Leeme TB, Mine M, Lechiile K, Mulenga F, Mosepele M, Mphoyakgosi T, et al. Utility of CD4 count measurement in the era of universal antiretroviral therapy: an analysis of routine laboratory data in Botswana. HIV medicine 2021; 22(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brennan AT, Maskew M, Larson BA, Tsikhutsu I, Bii M, Vezi L, et al. Who is seeking antiretroviral treatment for HIV now? Characteristics of patients presenting in Kenya and South Africa in 2017–2018. Journal of the International AIDS Society 2019; 22(9):e25358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lilian RR, Rees K, Mabitsi M, McIntyre JA, Struthers HE, Peters RPH. Baseline CD4 and mortality trends in the South African human immunodeficiency virus programme: Analysis of routine data. South Afr J HIV Med 2019; 20(1):963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.UNAIDS. UNAIDS Fact Sheet - World AIDS Day 2020. In; 2020.
- 5.Meintjes G, Kerkhoff AD, Burton R, Schutz C, Boulle A, Van Wyk G, et al. HIV-Related Medical Admissions to a South African District Hospital Remain Frequent Despite Effective Antiretroviral Therapy Scale-Up. Medicine (Baltimore) 2015; 94(50):e2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson LF, May M, Dorrington RE, Cornell M, Boulle A, Egger M, et al. Estimating the impact of antiretroviral treatment on adult mortality trends in South Africa: A mathematical modelling study. PLoS medicine in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Osler M, Hilderbrand K, Goemaere E, Ford N, Smith M, Meintjes G, et al. Community CD4 count as a proxy for ongoing morbidity and mortality risk over ten years of increasing antiretroviral therapy coverage in South Africa. in press. [DOI] [PMC free article] [PubMed]
- 8.Egger M, Ekouevi DK, Williams C, Lyamuya RE, Mukumbi H, Braitstein P, et al. Cohort Profile: the international epidemiological databases to evaluate AIDS (IeDEA) in sub-Saharan Africa. Int J Epidemiol 2012; 41(5):1256–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stinson K, Goemaere E, Coetzee D, van Cutsem G, Hilderbrand K, Osler M, et al. Cohort Profile: The Khayelitsha antiretroviral programme, Cape Town, South Africa. Int J Epidemiol 2017; 46(2):e21. [DOI] [PubMed] [Google Scholar]
- 10.Boulle A, Van Cutsem G, Hilderbrand K, Cragg C, Abrahams M, Mathee S, et al. Seven-year experience of a primary care antiretroviral treatment programme in Khayelitsha, South Africa. Aids 2010; 24(4):563–572. [DOI] [PubMed] [Google Scholar]
- 11.National Department of Health SA. Implementation of the universal Test and Treat strategy for HIV positive patients and differentiated care for stable patients. In; 2016.
- 12.National Department of Health SA. National consolidated guidelines for the prevention of mother-to-child transmission of HIV (PMTCT) and the management of HIV in children, adolescents and adults. In; 2014.
- 13.National Department of Health SA. The South African Antiretroviral Treatment Guidelines. 2013.
- 14.National Department of Health SA. Clinical guidelines for the management of HIV and AIDS in Adults and Adolescents. In; 2010.
- 15.Guidelines for managing advanced HIV disease and rapid initiation of antiretroviral therapy. In. Geneva: World Health Organization; 2017. [PubMed] [Google Scholar]
- 16.Phillips TK, Clouse K, Zerbe A, Orrell C, Abrams EJ, Myer L. Linkage to care, mobility and retention of HIV-positive postpartum women in antiretroviral therapy services in South Africa. Journal of the International AIDS Society 2018; 21 Suppl 4:e25114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jackson C Multi-State Models for Panel Data: The msm Package for R. 2011 2011; 38(8):28. [Google Scholar]
- 18.Quan V, Toro-Silva S, Sriruttan C, Chetty V, Chihota V, Candfield S, et al. Pathways to care and outcomes among hospitalised HIV-seropositive persons with cryptococcal meningitis in South Africa. PloS one 2019; 14(12):e0225742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barak T, Neo DT, Tapela N, Mophuthegi P, Zash R, Kalenga K, et al. HIV-associated morbidity and mortality in a setting of high ART coverage: prospective surveillance results from a district hospital in Botswana. Journal of the International AIDS Society 2019; 22(12):e25428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laher AE, Venter WDF, Richards GA, Paruk F. Profile of presentation of HIV-positive patients to an emergency department in Johannesburg, South Africa. South Afr J HIV Med 2021; 22(1):1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang L, Krebs E, Min JE, Mathews WC, Nijhawan A, Somboonwit C, et al. Combined estimation of disease progression and retention on antiretroviral therapy among treated individuals with HIV in the USA: a modelling study. Lancet HIV 2019; 6(8):e531–e539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gillis J, Loutfy M, Bayoumi AM, Antoniou T, Burchell AN, Walmsley S, et al. A Multi-State Model Examining Patterns of Transitioning Among States of Engagement in Care in HIV-Positive Individuals Initiating Combination Antiretroviral Therapy. Journal of acquired immune deficiency syndromes 2016; 73(5):531–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roy M, Holmes C, Sikazwe I, Savory T, Mwanza MW, Bolton Moore C, et al. Application of a Multistate Model to Evaluate Visit Burden and Patient Stability to Improve Sustainability of Human Immunodeficiency Virus Treatment in Zambia. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2018; 67(8):1269–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mody A, Eshun-Wilson I, Sikombe K, Schwartz SR, Beres LK, Simbeza S, et al. Longitudinal engagement trajectories and risk of death among new ART starters in Zambia: A group-based multi-trajectory analysis. PLoS medicine 2019; 16(10):e1002959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cornell M, Schomaker M, Garone DB, Giddy J, Hoffmann CJ, Lessells R, et al. Gender differences in survival among adult patients starting antiretroviral therapy in South Africa: a multicentre cohort study. PLoS medicine 2012; 9(9):e1001304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsondai PR, Braithwaite K, Fatti G, Bolton Moore C, Chimbetete C, Rabie H, et al. Characteristics and outcomes of adolescents living with perinatally acquired HIV within Southern Africa. Aids 2020; 34(15):2275–2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grimsrud A, Cornell M, Schomaker M, Fox MP, Orrell C, Prozesky H, et al. CD4 count at antiretroviral therapy initiation and the risk of loss to follow-up: results from a multicentre cohort study. J Epidemiol Community Health 2016; 70(6):549–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaplan SR, Oosthuizen C, Stinson K, Little F, Euvrard J, Schomaker M, et al. Contemporary disengagement from antiretroviral therapy in Khayelitsha, South Africa: A cohort study. PLoS medicine 2017; 14(11):e1002407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Balachandra S, Rogers JH, Ruangtragool L, Radin E, Musuka G, Oboho I, et al. Concurrent advanced HIV disease and viral load suppression in a high-burden setting: Findings from the 2015–6 ZIMPHIA survey. PloS one 2020; 15(6):e0230205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ford N, Geng E, Ellman T, Orrell C, Ehrenkranz P, Sikazwe I, et al. Emerging priorities for HIV service delivery. PLoS medicine 2020; 17(2):e1003028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nabaggala MS, Parkes-Ratanshi R, Kasirye R, Kiragga A, Castlenuovo B, Ochaka I, et al. Re-engagement in HIV care following a missed visit in rural Uganda. BMC research notes 2018; 11(1):762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Layer EH, Brahmbhatt H, Beckham SW, Ntogwisangu J, Mwampashi A, Davis WW, et al. “I pray that they accept me without scolding:” experiences with disengagement and re-engagement in HIV care and treatment services in Tanzania. AIDS Patient Care STDS 2014; 28(9):483–488. [DOI] [PubMed] [Google Scholar]
- 33.Working Group on Modelling of Antiretroviral Therapy Monitoring Strategies in Sub-Saharan A, Phillips A, Shroufi A, Vojnov L, Cohn J, Roberts T, et al. Sustainable HIV treatment in Africa through viral-load-informed differentiated care. Nature 2015; 528(7580):S68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


