Abstract
Even after decades of research, the mechanism of neurodegeneration remains understudied, hindering the discovery of effective treatments for neurodegenerative diseases. Recent reports suggest that ferroptosis could be a novel therapeutic target for neurodegenerative diseases. While polyunsaturated fatty acid (PUFA) plays an important role in neurodegeneration and ferroptosis, how PUFAs may trigger these processes remains largely unknown. PUFA metabolites from cytochrome P450 and epoxide hydrolase metabolic pathways may modulate neurodegeneration. Here, we test the hypothesis that specific PUFAs regulate neurodegeneration through the action of their downstream metabolites by affecting ferroptosis. We find that the PUFA, dihomo gamma linolenic acid (DGLA), specifically induces ferroptosis-mediated neurodegeneration in dopaminergic neurons. Using synthetic chemical probes, targeted metabolomics, and genetic mutants, we show that DGLA triggers neurodegeneration upon conversion to dihydroxyeicosadienoic acid through the action of CYP-EH, representing a new class of lipid metabolite that induces neurodegeneration via ferroptosis.
Introduction
By 2050, the projected population older than age 65 is expected to be more than double, reaching over 1.5 billion, and the projected population older than 80 is predicted to triple to 426 million.1 As aging is a risk factor for neurodegeneration, it is expected that the population with dementia will significantly increase in the near future.2 However, the mechanisms of neurodegeneration remain unclear, and effective preventative measures and treatment are currently lacking.3 Therefore, identifying molecular mechanisms underlying neurodegeneration is an unmet medical need. While tauopathy, neuroinflammation, and excitotoxicity may play key roles in neurodegeneration, recent studies provide compelling evidence that ferroptosis could be a new mechanism underlying neurodegeneration.3–5 Ferroptosis is a non-apoptotic form of regulated cell death that is driven by an increase of iron-dependent lipid peroxidation in the cellular membrane.4,6 Epidemiological studies showed that patients with Parkinson’s disease (PD) or Alzheimer’s disease (AD) have elevated iron and lipid peroxide levels in the brain as compared to healthy controls, which is consistent with ferroptosis.5,7–12 The regulatory mechanism of ferroptosis in brain cells are understudied, although polyunsaturated fatty acids (PUFAs) play a critical role in this process.13–17
PUFAs are key structural components of plasma membranes and play a critical role in neuronal functions.18 Generally, ω-3 and ω-6 PUFAs are two of the major classes of PUFAs present in human diet.19 Human studies have demonstrated that an increase in plasma ω-3/ω-6 PUFA ratio decreases the risk of neurodegenerative diseases, including AD and PD.20–23 Nonetheless, even after decades of epidemiological studies in mammalian and cell-based models, how PUFAs affect neurodegeneration is poorly understood, with reported results that are contradictory.21,24,25 While most efforts in research have investigated the neuroprotective effects of ω-3 PUFA supplementation, few studies have examined the role of ω-6 PUFAs in neurodegeneration.26–28 This is surprising since the modern western diet has dramatically increased our consumption of ω-6 PUFAs.29,30 While the exact role of ω-6 PUFAs in neurodegenerative diseases is not understood, it is known that supplementing mammalian cells with ω-6 PUFAs sensitizes cells to ferroptosis.13–15,31 In addition, ω-6 dihomo-gamma-linolenic acid (20:3n-6, DGLA) induces ferroptosis in the C. elegans germline, while earlier studies have suggested that an epoxide metabolite of DGLA may mediate germ cell death.17,32
Although the mechanisms by which ω-6 PUFAs mediate biological effects remain undefined, recent studies have demonstrated that ω-6 PUFAs metabolites resulting from cytochrome P450 (CYP) enzymes and epoxide hydrolases (EHs) action are key signaling molecules for human physiology.18,33,34 As such, this study was initiated to test whether specific ω-6 PUFAs modulate neurodegeneration via their downstream CYP metabolites and to investigate if ferroptosis plays a role in the observed biology. Because the CYP enzymes and EHs are differentially expressed in tissues and cell types (Table S1),35–39 and the expression of both enzymes are significantly affected by cell passages,40–42 it is difficult to pinpoint a specific cell line suitable for our study. Therefore, a whole animal study is necessary to uncover this novel mechanism without worrying metabolites not being generated locally or overlooking critical cell-cell communications facilitated by these lipid metabolites.
To facilitate our study, we took an interdisciplinary approach by combining a simple genetic animal model, an inhibitor of a metabolic enzyme, synthesized lipid metabolites, and targeted metabolomics to systematically investigate the crosstalk between lipid metabolism, neurodegeneration, and ferroptosis. With this approach, we first demonstrate that among five tested PUFAs, only DGLA induces neurodegeneration in select neurons in C. elegans, with more pronounced effects in dopaminergic neurons, and to a lesser extent in glutaminergic neurons, with no observable effects in cholinergic and GABAergic neurons. Furthermore, we demonstrate that the DGLA-induced neurodegeneration is mediated through its downstream CYP-EHs metabolite, dihydroxyeicosadienoic acid (DHED), and ferroptosis is likely the mechanism involved in DHED-induced neurodegeneration.
Results
DGLA, but neither ω-3 nor other ω-6 PUFAs, induces degeneration specifically in dopaminergic neurons
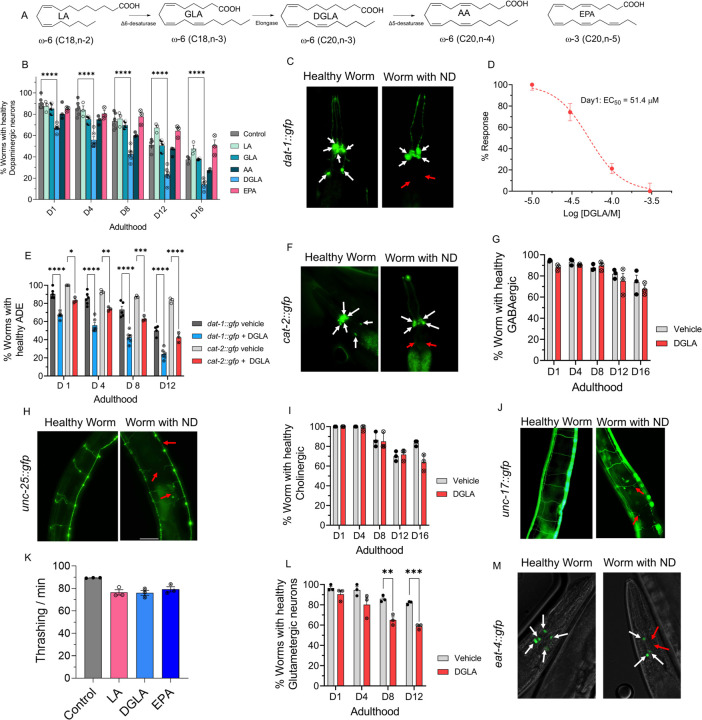
Our prior lipidomic analysis shows that C. elegans absorbs exogenous PUFAs.43,44 To study the effect of dietary PUFAs on neuronal health span, we supplemented Pdat-1::gfp worms, in which the dopaminergic neurons are labeled by green fluorescent protein (GFP), with different ω-6 PUFAs and eicosapentaenoic acid (20:5n-5, EPA), the most abundant ω-3 PUFA in C. elegans,45 and tracked the dopaminergic neurons throughout the worm lifespan using fluorescent imaging (Figure 1A–C). Supplementation was done at the larvae stage 4 (L4) when C. elegans has a fully developed neuronal system thus enabling the investigation of neurodegeneration independent of neurodevelopment.46 Among the tested PUFAs, only DGLA induced significant degeneration in the dopaminergic neurons (Figure 1B). Furthermore, DGLA triggered degeneration in dopaminergic neurons in a dose-dependent manner with an EC50= 51.4 μM and 31.2 μM at day 1 and day 8 adulthood, respectively (Figure 1D, and S1). We also showed that the vehicle control, ethanol, did not change dopaminergic neurons’ health span as compared to the control (Figure S2). In addition, we found that different types of dopaminergic neurons in the hermaphrodite had varying sensitivities to treatment with DGLA, with the ADE neurons (ADE>>>CEP>PDE) being the most impacted (Figure S3). Moreover, loss of the GFP signal did not appear to result from transcriptional repression of the Pdat-1∷GFP transgene induced by treatment with DGLA, since a similar trend was observed with the Pcat-2∷GFP transgenic line upon treatment with DGLA (Figure 1E and F)). We then examined whether DGLA can induce degeneration in other major types of neurons that play key roles in neurodegenerative diseases including GABAergic, glutaminergic, and cholinergic neurons. Significant neurodegeneration was not observed in GABAergic (Punc-25::gfp) and cholinergic neurons (Punc-17::gfp) worms supplemented with DGLA (Figure 1G–J). These findings were further confirmed with a lack of significant changes in thrashing assays in C. elegans treated with DGLA (Figure 1K), which requires cholinergic and GABAergic neurons activity.47,48 In the case of glutamatergic neurons (Peat-4::gfp), treatment with DGLA caused cell loss in glutamatergic neurons later in the C. elegans lifespan compared to the dopaminergic neurons (Figure 1L and M). Altogether, our results suggest that the effect of PUFAs on neurodegeneration is structurally specific. In addition, in contrast to conclusions reported previously,13,14,49 our data showed that treatment of the more peroxidizable arachidonic acid and EPA did not trigger neurodegeneration. Furthermore, our results indicate that the effect of DGLA on neurodegeneration is neuron-type selective, warranting future studies that may shine light on the molecular mechanism(s).
Figure 1. DGLA, but not other ω-3 and ω-6 PUFAs, induces degeneration, specifically in dopaminergic neurons.
(A) Structure of different ω-6 and ω-3 PUFAs examined in this study; (B) Percentage (%) of worms with healthy dopaminergic neurons for Pdat-1::gfp with and without supplementation with 100 μM of different ω-6 and ω-3 PUFAs. (C) Fluorescent images of Pdat-1::gfp worms with healthy and degenerated dopaminergic neurons (White arrows represent healthy neurons, and red arrows show degenerated/disappeared neurons); (D) Dose response curve: the effect of different DGLA concentrations on degeneration of ADE neurons on Day 1 adulthood; (E) Comparing the ADE neurons degeneration in Pdat-1::gfp and Pcat-2::gfp supplemented with 100 μM DGLA; (F) Fluorescent images of Pcat-2::gfp worms with healthy and degenerated dopaminergic neurons (White arrows represents healthy neurons, and red arrow shows degeneration/disappearance neurons); (G) Percentage (%) of worms with healthy GABAergic neurons for Punc-25::gfp with and without supplementation with 100 μM DGLA; (H) Fluorescence images of Punc-25::gfp worm with healthy and degenerated GABAergic neurons (Red arrows show different signs of neurodegeneration including ventral cord break, commissure break and branches); (I) Percentage (%) of worms with healthy cholinergic neurons for Punc-17::gfp with and without supplementation with 100 μM DGLA; (J) Fluorescence Images of Punc-17::gfp worms with healthy and degenerated cholinergic neurons (Red arrows show different signs of neurodegeneration including ventral cord break, commissure break and branches); (K)Thrashing on Day 8 adulthood of wild-type raised on 100 μM of LA, DGLA, and EPA; (L) Percentage (%) of worms with healthy glutamatergic neurons with Peat-4::gfp with and without supplementation with 100 μM DGLA; (M) Fluorescent images of Peat-4::gfp worms with healthy and degenerated glutamatergic neurons (White arrows represent healthy neurons, and red arrows show degenerated/disappeared neurons). All supplementations were done at the L4 stage. For all experiments N=3, and about 20 worms were tested on each trial. Two-way analysis of variance (ANOVA), Tukey’s multiple comparison test for (B) and (D); and t test for K: *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P < 0.0001, non-significant is not shown.
The remaining studies focused on the degeneration of dopaminergic neurons, as was found they are most sensitive to DGLA treatment. Because more robust data were obtained with transgenic C. elegans Pdat1::gfp, the rest of our studies were conducted using this strain. Most of the experiments were performed using day 1 and day 8 adults, enabling the determination of acute and chronic effects of DGLA treatment on neurodegeneration. Day 8 worms resemble a middle-aged population of C. elegans, thus the effect of DGLA treatment on age-associated neurodegeneration can also be investigated without a significant loss (death) of the tested population, facilitating the throughput of our studies.
DGLA induces neurodegeneration in dopaminergic neurons through ferroptosis
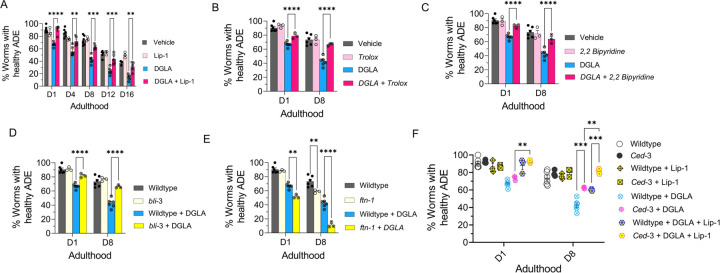
Recent studies show that treatment with DGLA can induce ferroptosis in germ cells and cause sterility in C. elegans.17 To test whether treatment with DGLA induces degeneration in dopaminergic neurons through ferroptosis, Pdat-1::gfp expressing worms were co-treated with DGLA and liproxstatin-1 (Lip-1), a radical-trapping antioxidant and ferroptosis inhibitor.50 While C. elegans treated with Lip-1 alone showed no significant effect on age-associated degeneration of dopaminergic neurons as compared to the vehicle control, co-treatment of DGLA with Lip-1 fully rescued the neurodegeneration triggered by DGLA in day 1 adults and largely rescued DGLA-induced neurodegeneration in day 8 adults (Figure 2A). Encouraged by these results, we examined neurodegeneration caused by ferroptosis in DGLA-treated worms using pharmacological and genetic approaches. An increase in the labile iron (II) pool and membrane lipid peroxidation are molecular hallmarks of ferroptosis.6,51 Therefore, we tested whether treatment with Trolox, a water-soluble form of vitamin E and lipid peroxidation inhibitor, and 2,2’-bipyridine, an iron (II) chelator, alleviates DGLA-induced neurodegeneration. Co-treatment with either Trolox or 2,2’-bipyridine rescued DGLA-induced neurodegeneration, suggesting that both the labile iron (II) pool and membrane lipid peroxidation are involved in DGLA-induced neurodegeneration (Figure 2B and C). To specifically investigate whether ferroptosis is involved in DGLA-induced neurodegeneration, was also pursued a genetic approach.
Figure 2. DGLA Induces neurodegeneration in dopaminergic neurons via ferroptosis.
(A) Percentage (%) of worms with healthy ADE neurons of worms exposed to 100 μM DGLA ± 250 μM Liproxstatin-1; (B) Percentage (%) of worms with healthy ADE neurons in wild-type C. elegans treated with 100 μM DGLA ± 500 μM Trolox (vitamin E); (C) Percentage (%) of worms with healthy ADE neurons for Pdat-1::gfp worms treated with 100 μM DGLA± 100 μM 2,2’-bipyridine; (D) Percentage (%) of worms with healthy ADE neurons in Pdat-1::gfp and Pdat-1::gfp;bli-3 worms treated with 100 μM DGLA; (E) Percentage (%) of worms with healthy ADE neurons for Pdat-1::gfp and Pdat-1::gfp; ftn-1 worms treated with 100 μM DGLA; (F)Percentage (%) of worms with healthy ADE neurons with Pdat-1::gfp and Pdat-1::gfp; ced-3 worms treated with 100 μM DGLA ± 250 μM Liproxstatin-1. All supplementations were done at the L4 stage. A two-way analysis of variance (ANOVA), Tukey’s multiple comparison test. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P < 0.0001, NS, not significant. DGLA: Dihomo-γ-linolenic acid, LA: Linoleic acid, EPA: eicosapentaenoic acid, Lip-1: Liproxstatin-1.
Previous studies have shown that nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX) family of superoxide-producing enzymes (NOX/DUOX) plays a critical role in ferroptosis in mammals and can exacerbate dopaminergic neurotoxicity triggered by ferroptosis inducers.4,52 In addition, ferritin (FTN) is also a key ferroptosis regulatory protein, and genetic knockout of FTN has been shown to sensitize C. elegans to ferroptosis.17,53 To further test whether ferroptosis is involved in DGLA-induced neurodegeneration, two new transgenic C. elegans strains were created by crossing the Pdat-1::gfp with transgenic strains that carry either a loss of function of bli-3 (C. elegans homolog of NOX) mutant or genetic knockout of ftn-1 (Figure 2D, 2E, and S4). Our results indicated that the loss of function bli-3 mutant reduced the degeneration of dopaminergic neurons triggered by DGLA (Figure 2D). Worms with loss of function mutations of BLI-3 attenuated ability to generate reactive oxygen species, thus minimizing lipid peroxidation, and as a result, reduced ferroptosis.17,54 This result further confirms the pharmacological observation after supplementing worms with the lipophilic antioxidant vitamin E (Trolox), which led to the suppression of neurodegeneration in DGLA-treated worms (Figure 2B). Furthermore, genetic knockout of ftn-1 enhanced DGLA-induced neurodegeneration, suggesting that DGLA requires the labile iron (II) pool to exert its effect on dopaminergic neurons (Figure 2E). Our data strongly suggest that DGLA causes degeneration of dopaminergic neurons at least partly through ferroptosis. As illustrated in Figure 2A, while Lip-1 fully rescued DGLA-induced neurodegeneration for day 1 adults, such rescuing effect diminished as C. elegans aged. Furthermore, the EC50 of DGLA in triggering neurodegeneration for day 1 and day 8 adults is different. Therefore, we hypothesize that chronic treatment of DGLA induces other programmed cell-death pathways, like apoptosis, leading to neurodegeneration.
To test whether DGLA also induces neurodegeneration through apoptosis,55 an additional transgenic strain was developed. The CED-3 protein, a key enzyme involved in apoptosis, was genetically knocked out in worms in which the dopaminergic neurons were labeled by GFP17,56 to create transgenic line, Pdat-1::gfp;ced-3(n717). Interestingly, while no significant difference was observed between Pdat-1::gfp;ced-3(n717) and Pdat-1::gfp worms treated with DGLA at day 1 adulthood, worms that had the ced-3 genetic knockout demonstrated partial rescue from dopaminergic neuron degeneration induced by DGLA (Figure 2F) at day 8 of adulthood. Furthermore, the ced-3 knockout worms at day 8 adulthood that were co-treated with Lip-1 were fully rescued from dopaminergic neurodegeneration induced by DGLA (Figure 2F). These results could explain the differences observed for day 1 and day 8 worms treated with Lip-1 and DGLA (Figure 2A), as well as the differences in the EC50 of DGLA-induced neurodegeneration between day 1 adults (EC50 = 51.4 μM) and day 8 adults (EC50 = 31.2 μM) (Figure 1D). The lower EC50 for DGLA-induced neurodegeneration for day 8 adults suggests that other neurodegenerative mechanisms (i.e., apoptosis, autophagy) are involved and could either synergize or provide an additive effect with DGLA-induced neurodegeneration. Together, these results suggest that dietary DGLA induces neurodegeneration via ferroptosis in early adulthood and both ferroptosis and additional mechanism(s), such as apoptosis, are induced by DGLA in dopaminergic neurodegeneration in middle-aged C. elegans.
Downstream metabolites of DGLA are key players in neurodegeneration induced by DGLA treatment.
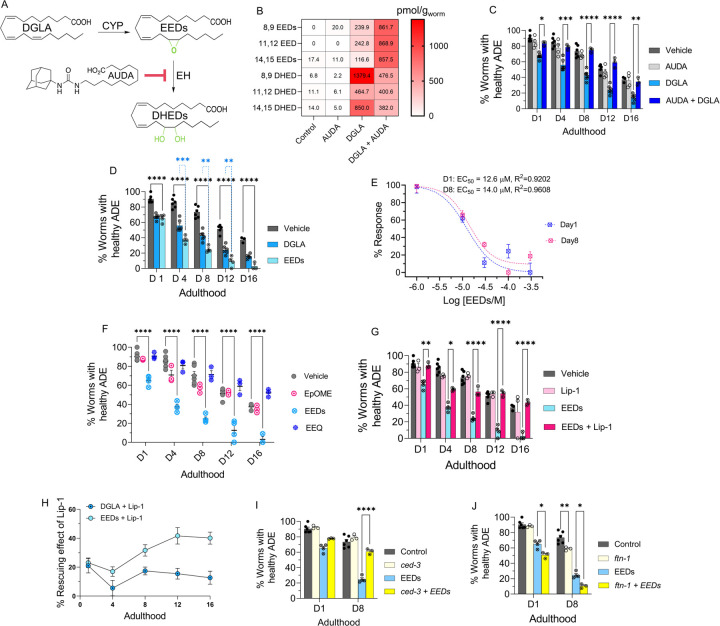
In mammals, DGLA and other PUFAs are mono-oxygenated by cytochrome P450 enzymes (CYPs) to hydroxy- and epoxy-PUFAs. Epoxy-PUFAs are further hydrolyzed by epoxide hydrolases (EHs) to the dihydroxy-PUFAs (Figure 3A).18 Numerous animal and human studies have demonstrated that endogenous levels of CYP and EH metabolites produced from various PUFAs are highly correlated to dietary intake of the corresponding PUFAs,57–59 in stark contrast to metabolites generated by cyclooxygenases and lipoxygenases, which are less correlated.60–62 Both epoxy- and dihydroxy-PUFAs are key signaling molecules for mammalian physiology including, but not limited to, neuroprotection.18,63,64 Therefore, we hypothesized that DGLA primarily induces ferroptosis-mediated neurodegeneration via its CYP and/or EH metabolites, a previously unexplored area. To test this hypothesis, we first investigated whether the CYP/EH metabolism is involved in DGLA-induced ferroptosis-mediated neurodegeneration by investigating how treatment with DGLA impacts CYP/EH metabolism. Our results indicated that treatment with 100 μM DGLA increased the whole animal endogenous levels of the corresponding epoxyeicosadienoic acid (EED) and dihydroxyeicosadienoic acid (DHED) to ~200 pmol/g and ~800 pmol/g, respectively (regioisomer-dependent Figure S5), using our oxylipin analysis (Pourmand et. al, unpublished, see experimental methods in SI). These results were similar to the endogenous levels of EPA CYP/EH metabolites, epoxyeicosatetraenoic acid (EpETEs) and dihydroxyeicosatetraenoic acids (DHETE) which are 50–919 pmol/g and 0–458 pmol/g, respectively (regioisomer-dependent) in intact C. elegans, suggesting that the increased level of EED and DHED is physiologically relevant (Figures 3B and S5). Therefore, we sought to determine whether these downstream metabolites (EED and DHED) are key mediators for neurodegeneration induced by treatment with DGLA. Transgenic C. elegans (Pdat-1::gfp) were co-treated with DGLA and 12-(1-adamantane-1-yl-ureido-) dodecanoic acid (AUDA, 100 μM), an EH inhibitor with selective action to inhibit the function of CEEH1 and CEEH2 (C. elegans EH1 and EH2 isoforms).65 AUDA treatment increased the level of EED and decreased the DHED in vivo concentration, and fully rescued dopaminergic neurodegeneration induced by DGLA, suggesting that the CYP/EH-derived downstream metabolites of DGLA play a critical role in DGLA-induced neurodegeneration (Figures 3B, C).
Figure 3. EED, epoxy metabolites downstream of DGLA, induce neurodegeneration by ferroptosis.
(A) DGLA is metabolized to EED and DHED through the CYP and epoxide hydrolase enzymes, respectively; and AUDA inhibits epoxide hydrolase; (B) Oxylipin profile representing the pmol/g of EED and DHED regioisomers in worms treated with 100 μM of DGLA ± 100 μM AUDA compared to control (C) Percentage (%) of worms with healthy ADE neurons for Pdat-1::gfp treated with 100 μM of DGLA ± 100 μM AUDA; (D) Percentage (%) of worms with healthy ADE neurons for Pdat-1::gfp worms treated with 100 μM DGLA and 100 μM EED; (E) Dose response curve: the effect of different concentration of EED on degeneration of ADE neurons on Day 1 and Day 8 adulthood; (F) Percentage (%) of worms with healthy ADE neurons in Pdat-1::gfp worms treated with 100 μM of different Ep-PUFAs, EpOME, and EEQ; (G) Percentage (%) of worms with healthy ADE neurons of worms treated with 100 μM DGLA ± 100 μM Liproxstatin-1;(H) Comparison the effect of 250 μM Liproxstatin-1 on Pdat-1::gfp worms treated with 100 μM of DGLA compared to 100 μM EED;(I) Percentage (%) of worms with healthy ADE neurons with Pdat-1::gfp and Pdat-1::gfp;ced-3 worms treated with 100 μM; (J) Percentage (%) of worms with healthy ADE neurons for Pdat-1::gfp and Pdat-1::gfp; ftn-1 worms treated with 100 μM DGLA; All supplementations were done at the L4 stage. Two-way analysis of variance (ANOVA), Tukey’s multiple comparison test. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P < 0.0001, without * not significant. DGLA: Dihomo-γ-linolenic acid, EED: epoxyeicosadienoic acids, DHED: dihydroxyeicosadienoic acids, CYP: Cytochrome P450, EH: epoxide hydrolase, AUDA: 12-(1-adamantane-1-yl-ureido-) dodecanoic acid, Lip-1: Liproxstatin-1, EpOME: epoxyoctadecenoic acids; EEQ: epoxyeicosatetraenoic acid.
Nonetheless, these results do not discriminate between AUDA’s ability to stabilize the level of EED in vivo for the observed rescue, or to block the production of DHED metabolites that result from inhibiting C. elegans EHs (Figures 3B and S6). To distinguish between the latter two possibilities, we synthesized both EED and DHED and tested their effects in C. elegans following the procedures in previous reports.66–69 Treatment with 100 μM EED at the L4 stage induced a more severe neurodegenerative phenotype than treatment with DGLA at the same concentration in the dopaminergic neurons in all tested ages (Figure 3D), with a much lower EC50 (12.6 μM vs 51.4 μM) as compared to DGLA on day 1 adult (Figures 3E and 1D). The same trend was observed in glutamatergic neurons when comparing treatments of EED and DGLA, and similar to DGLA treatment, no significant neurodegeneration was observed in GABAergic and cholinergic neurons after treatment with EED (Figure S7).
To test whether the effect of EED is structurally specific, C18:1 epoxyoctadecenoic acid (EpOME), an epoxy-metabolite of LA, and a more peroxidizable C20:4 epoxyeicosatetraenoic acid (EEQ), an epoxy-metabolite of EPA, were examined (Figure 3F) and had no effects on neurodegeneration. These results indicate that the effect of epoxy-PUFAs on neurodegeneration is specific to EED, but not other epoxy-PUFAs. Similar to the neurodegeneration induced by DGLA, co-treatment with Lip-1 rescued neurodegeneration caused by EED, and Lip-1 was more effective in alleviating EED induced neurodegeneration compared to DGLA-induced neurodegeneration (Figures 3G, H). Furthermore, like DGLA, neuronal degeneration induced by EED was not rescued by a genetic knockout of ced-3. Genetic knockout of ftn-1 escalates the effect of EED in both day 1 and day 8 adult, again suggesting that ferroptosis plays a critical role in EED-induced neurodegeneration (Figures 3I and J).
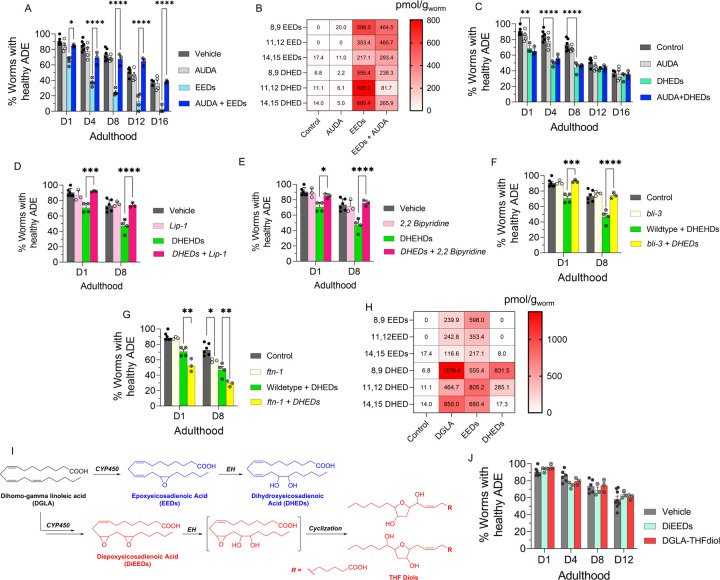
Our results further suggested that DGLA metabolites are lipid mediators responsible for the effect of DGLA on neurodegeneration. Inhibition of EED hydrolysis using an EH inhibitor (AUDA) resulted in rescue of EED-induced neurodegeneration in C. elegans (Figure 4A). The oxylipin profile of worms co-treated with EED and AUDA at 100 μM shows that blocking the metabolism of epoxy-PUFAs to dihydroxy-PUFAs, specifically EED to DHED with AUDA, stabilizes endogenous levels of epoxy-PUFAs including EED, and decreases the in vivo levels of dihydroxy-PUFAs and DHED (Figures 4B and S8). Altogether, our data further suggest that specific DGLA downstream metabolites, either EED or DHED, are responsible for DGLA-mediated neurodegeneration.
Figure 4. DHEDs, dihydroxy fatty acids downstream of DGLA/EED, are key candidates for neurodegeneration induced by DGLA in dopaminergic neurons.
(A) Percentage (%) of worms with healthy ADE neurons in Pdat-1::gfp worms treated with 100 μM of EED ± 100 μM AUDA; (B) Oxylipin profile representing pmol/g of EED and DHED regioisomers in worms treated with 100 μM of EED ± 100 μM AUDA compared to control. (C) Percentage (%) of worms with healthy ADE neurons for Pdat-1::gfp treated with 100 μM of DHED ± 100 μM AUDA; (D) Percentage (%) of worms with healthy ADE neurons of worms exposed to 100 μM DHED ± 250 μM Liproxstatin-1; (E) Percentage (%) of worms with healthy ADE neurons for Pdat-1::gfp worms treated with 100 μM DHEDs ± 100 μM 2,2’-bipyridine; (F) Percentage (%) of worms with healthy ADE neurons for Pdat-1::gfp and Pdat-1::gfp;ftn-1 worms treated with 100 μM DHED; (G) Percentage (%) of worms with healthy ADE neurons in Pdat-1::gfp and Pdat-1::gfp;bli-3 worms treated with 100 μM DEHDs; (H) Oxylipin profile representing the pmol/g of EED and DHED regioisomers in worms treated with 100 μM of DGLA, EED, and DHED compared to control. (I) two possible metabolisms of DGLA through the CYP/EH pathways; the alternative metabolism is that CYP can do two consecutive oxidations (or under oxidative stress) to yield diepoxies EED, which then EH will open one epoxide which under physiological conditions can cyclize to THF diols. (J) Percentage (%) of worms with healthy ADE neurons for Pdat-1::gfp treated with 100 μM of DiEED and 100 μM DGLA-THFdiol. All supplementations were done at the L4 stage. Two-way analysis of variance (ANOVA), Tukey’s multiple comparison test. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P < 0.0001, without * not significant. DGLA: Dihomo-γ-linolenic acid, EED: epoxyeicosadienoic acid, DHED: dihydroxyeicosadienoic acid, CYP: Cytochrome P450, EH: epoxide hydrolase, AUDA: 12-(1-adamantane-1-yl-ureido-) dodecanoic acid, DiEED: diepoxyeicosadienoic acid.
We further corroborated our hypothesis by supplementing Pdat-1::gfp worms with 100 μM of DHED, which showed significant neurodegeneration compared to the vehicle control (Figure 4C). Intriguingly, co-treatment with AUDA and DHED did not alleviate neurodegeneration induced by DHED, further confirming that DHED is likely the main driver of dopaminergic neurodegeneration in our model (Figure 4C). Co-treatment with AUDA alleviated DGLA-induced neurodegeneration likely by blocking the formation of DHED. In addition, co-treatment with Lip-1 and 2,2-bipyridine rescued the neurodegeneration caused by DHED (Figures 4D, and E). Furthermore, the loss of function bli-3 mutant also reduced the degeneration of dopaminergic neurons triggered by DHED, and genetic knockout of ftn-1 augments DHED-induced neurodegeneration (Figures 4F, and G). Together, these results suggest that the labile iron (II) pool and subsequently ferroptosis are involved in the effect of DHED on dopaminergic neurons. It is noteworthy that we did not observe more severe neurodegeneration induced by DHED as compared to EED supplementation. This effect may be due to the difference in lipid transport mechanism between dihydroxy-PUFAs, PUFAs and Ep-PUFAs, as suggested by a previous study.70 DHED is not absorbed as well as EED, and thus are not as potent at the same concentration. This hypothesis was confirmed by oxylipin profiling, which showed significantly lower DHED levels (especially for 11,12 and 14,15 DHED) in worms treated with 100 μM DHED as compared to those treated with 100 μM EED or 100 μM DGLA (Figures 4D and S9). While DGLA and EED exhibit a continuous increase in dopaminergic neurodegeneration over their lifespan, DHED exhibited a plateau after day 8, which suggests the presence of a possible mechanism for removal of the offending agent, either by inducing downstream metabolism or activating lipid transport of DHED after chronic treatment. Our data strongly suggest that DGLA/EED induces neurodegeneration through their downstream metabolites. Beyond DHED, there have been a few reports of other downstream CYP-mediated metabolites, namely epoxy-hydroxy-PUFA and diepoxy-PUFAs, which ultimately undergo spontaneous intramolecular cyclization in physiological conditions to form an understudied class of metabolites, the tetrahydrofuran-diols (THF-diols). These THF-diols could be a new class of lipid mediators in mammals.71,72 To examine these potential mediators of biological activity, isomeric vicinal diepoxyeicosenoic acid (DiEEe) and its corresponding THF-diol (DGLA-THF-diol) were synthesized and incubated with the worms (see the experimental section in SI and Figure 4E). However, no significant loss was observed in dopaminergic neurons in worms treated with 100 μM DiEEe and its corresponding THF-diol compared to the vehicle control (Figure 4F). These results strongly suggest that DHED constitutes a novel class of lipid mediators that induces neurodegeneration largely mediated by ferroptosis. In addition, EpETE and EpOME produced from EPA and LA, respectively, showed no effect on neurodegeneration, which further corroborates the effect of DHED on ferroptosis-mediated neurodegeneration is structurally selective. Furthermore, the results obtained from the treatment with more peroxidizable EPA and EpETE indicate that the effect of DHED is not due to an increase in peroxidation of the cell membrane, a known mechanism that sensitizes ferroptosis.15,73–75 As a whole, the results summarized above are contrast with reports on the effect of PUFAs76–78 or their metabolites, such as lipoxygenase’s metabolites,50,73,77 ether lipids,79 etc. on ferroptosis.
Discussion
Our studies revealed that DHED, CYP-EH metabolite of DGLA, is a novel class of lipid molecules that triggers ferroptosis-mediated degeneration in select neuron types in C. elegans. Our study addresses critical gaps in knowledge in the field of lipid pharmacology, neurodegeneration, and ferroptosis, including how ω-6 PUFAs may trigger neurodegeneration and the identity of endogenous signaling molecules that induces ferroptosis-mediated neuronal cell death. Most research investigates the beneficial effects of ω-3 PUFA supplementation in neurodegenerative diseases, with contradictory findings.26,27,30 Few studies have tested the effect of ω-6 PUFAs on neurodegeneration.28 This is of high interest since ω-6 PUFA levels are typically high in western diets. Our findings in C. elegans demonstrate that, unlike other PUFAs, the ω-6 DGLA induces ferroptosis-mediated degeneration specifically in dopaminergic and to a lesser extent in glutaminergic neurons. Recent reports suggest PUFAs play a critical role in ferroptosis and treating cells with PUFAs, their ether-lipid metabolites, and lipoxygenase metabolites, hydroperoxyeicotetraenoic acids, sensitize cells to ferroptosis, but not induce ferroptosis themselves.50,79 However, specific endogenous mediators that regulate the upstream pathway of ferroptosis remain unknown, although synthetic compounds such as erastin, RSL-3, and natural products like α-eleostearic acid have been identified as agents that can induce ferroptosis.13–15,31 Our results indicate that DGLA induces ferroptosis-mediated neuronal death likely through its downstream endogenous CYP-EH metabolites, DHED, and EH plays a critical role in modulating DGLA-mediated ferroptosis. Our study complements the elegant work showing that DGLA induces ferroptosis in germline and cancer cells.17 The identification of potential lipid signaling molecules represents a critical first step to investigating the molecular mechanism behind the effects of PUFAs on ferroptosis-mediated neurodegeneration.
Recent reports demonstrated that the expression of soluble EH, an human ortholog of CEEH 1/2, is upregulated in patients with neurodegenerative diseases including Parkinson’s disease and Alzheimer’s disease and, inhibition of soluble EH is beneficial for neurodegeneration in multiple neurodegenerative diseases animal models.18,80–83 While the specific role of soluble EH in neurodegeneration is largely unknown, these studies suggested that the epoxy-fatty acids, the substrates of soluble EH, are neuroprotective, and the corresponding downstream EH metabolites dihydroxy-fatty acids have no effect, although a few studies in cell and animal models have showed that these dihydroxy-fatty acids can have detrimental or toxic effects on cells.84,85 Our results provide an alternate perspective of how neurodegeneration could be regulated endogenously by modulating EH activity to increase ferroptotic metabolites, namely DHED, which has seldom been studied.
In this study, we employed an approach, which comprised of a simple animal model, an inhibitor of a metabolic enzyme, synthesized lipid metabolites, and targeted metabolomics to systematically investigate the crosstalk between lipid metabolism, neurodegeneration, and ferroptosis in a highly efficient way. We have not only identified the key mediator for ferroptosis-mediated neurodegeneration but have also revealed that DGLA and its metabolites have more pronounced effects on dopaminergic neurons, mild effects on glutaminergic neurons, and no effects on cholinergic and GABAergic neurons in C. elegans. Our results complement previous studies by Zille. et al., which showed that different cell types could have distinct regulatory pathways for ferroptosis.86 While the specific mechanism behind why DGLA and its metabolites, DHED, are more detrimental to dopaminergic neurons remains unknown, Solano Fonseca et al. reported a similar vulnerability of different neuron-types in response to biomechanical injury and suggested that such observation could be due to different physiological regulatory mechanisms between different neuron types.87 Such neuron type-specific effects triggered by DGLA and DHED warrant future investigation to uncover potential new neurodegeneration mechanisms. Investigating ferroptosis at the systems level to understand differential ferroptosis mechanisms between tissues is challenging, owing to the lack of appropriate genetic and imaging tools. The genetic malleability of C. elegans provides a suitable platform for the study of ferroptosis in a tissue-specific manner. Furthermore, as illustrated in Table S1, most cell lines do not express soluble EH, a human ortholog of CEEHs and studies have demonstrated that different tissues express CYP enzymes and soluble epoxide hydrolase differently.35–38 Therefore, a whole animal approach is more appropriate for us to explore this novel mechanism and C. elegans provides a simple animal model. In addition, the adaptability to high-throughput studies of C. elegans allows us quickly to dissect complicated pathways. As such, it is possible to explore how ferroptosis can be regulated differentially by endogenous signaling molecules, such as DHED, between cell-types in an intact organism. Furthermore, the chemical tools developed and utilized for this study lead to the exploration of novel hypotheses that aim to unravel PUFAs effects on organismal physiology, an area that is not only understudied, but also is challenging to execute in mammalian models and humans.
Conclusion
Oxidized lipid metabolites are key mediators for organismal physiology. Ferroptosis, characterized by an increase of iron-dependent lipid peroxidation, could be a novel mechanism for neurodegeneration. In this study, we reported that exogenous DGLA triggers neurodegeneration predominantly in dopaminergic neurons via its downstream cytochrome P450-epoxide hydrolase (CYP-EH) metabolite, dihydroxyeicosadienoic acid (DHED). The observed neurodegeneration induced by DGLA/DHED is likely mediated by ferroptosis at the early stages and a combination of ferroptosis and apoptosis after chronic treatment with DGLA/DHED. This study revealed that CYP-EH polyunsaturated fatty acid (PUFA) metabolism is one of the key intrinsic regulatory mechanisms of ferroptosis-mediated neurodegeneration, and EH could be a novel target for ferroptosis-mediated diseases.
Supplementary Material
Acknowledgments
Some nematode strains were provided by the Caenorhabditis Genetics Center, funded by the NIH Office of Research Infrastructure Programs (P40 OD010440). The EM641 Pcat-2::gfp strain was a gift from Dr. Scott Emmons. The GA912 ftn-1(ok3625) strain was a gift from Dr. David Gems (University College London, London, UK). Funding to K.S.S.L. was provided by the NIGMS R35 GM146983, and Pearl Aldrich Endowment for aging research. Funding to J.A. was provided by the NIH (R03 AG075465). J.L.W. was supported by NIH R01 GM133883. K.S.S.L. and J. A. were partially supported by startup funding from Michigan State University. M.S. was partially supported by Pearl Aldrich Endowment for aging research. Funding to T.R was graciously provided by Integrative Pharmacological Sciences Training Grant NIH T32GM142521. We acknowledge the MSU RTSF Mass Spectrometry and Metabolomics Core Facilities for support with oxylipin and lipidomic analysis. We would like to thank Dr. Brian Ackley (University of Kansas) for his advice on the neuronal studies; Dr. Scott Dixon for his comments for this study, as well as Mr. Devon Dattmore, Ms. Leslie Ramirez, and Ms. Heather deFeijter-Rupp (Michigan State University) for their help and assistance.
Footnotes
Supporting Information
The Supporting Information including experimental methods and materials, supplemental figures and table, synthesis and characterization of DGLA metabolites.
Reference
- (1).United Nations Department of Economic and Social Affairs. References. In World Population Ageing 2019; UN, 2020; pp 37–38. 10.18356/7f55a58e-en. [DOI] [Google Scholar]
- (2).Hou Y.; Dan X.; Babbar M.; Wei Y.; Hasselbalch S. G.; Croteau D. L.; Bohr V. A. Ageing as a Risk Factor for Neurodegenerative Disease. Nat. Rev. Neurol. 2019, 15 (10), 565–581. 10.1038/s41582-019-0244-7. [DOI] [PubMed] [Google Scholar]
- (3).Gan L.; Cookson M. R.; Petrucelli L.; La Spada A. R. Converging Pathways in Neurodegeneration, from Genetics to Mechanisms. Nat. Neurosci. 2018, 21 (10), 1300–1309. 10.1038/s41593-018-0237-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Dixon S. J.; Lemberg K. M.; Lamprecht M. R.; Skouta R.; Zaitsev E. M.; Gleason C. E.; Patel D. N.; Bauer A. J.; Cantley A. M.; Yang W. S.; Morrison B. 3rd; Stockwell B. R. Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell 2012, 149 (5), 1060–1072. 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Ren J.-X.; Sun X.; Yan X.-L.; Guo Z.-N.; Yang Y. Ferroptosis in Neurological Diseases. Front. Cell. Neurosci. 2020, 14, 218. 10.3389/fncel.2020.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Dixon S. J.; Stockwell B. R. The Hallmarks of Ferroptosis. Annu. Rev. Cancer Biol. 2019, 3 (1), 35–54. 10.1146/annurev-cancerbio-030518-055844. [DOI] [Google Scholar]
- (7).Plascencia-Villa G.; Perry G. Implication of Ferroptosis Iron‐dependent Programmed Cell Death Mechanism in Neurodegeneration. Alzheimers. Dement. 2020, 16 (S3). 10.1002/alz.043978. [DOI] [Google Scholar]
- (8).Do Van B.; Gouel F.; Jonneaux A.; Timmerman K.; Gelé P.; Pétrault M.; Bastide M.; Laloux C.; Moreau C.; Bordet R.; Devos D.; Devedjian J.-C. Ferroptosis, a Newly Characterized Form of Cell Death in Parkinson’s Disease That Is Regulated by PKC. Neurobiol. Dis. 2016, 94, 169–178. 10.1016/j.nbd.2016.05.011. [DOI] [PubMed] [Google Scholar]
- (9).Nikseresht S.; Bush A. I.; Ayton S. Treating Alzheimer’s Disease by Targeting Iron. Br. J. Pharmacol. 2019, 176 (18), 3622–3635. 10.1111/bph.14567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Du L.; Zhao Z.; Cui A.; Zhu Y.; Zhang L.; Liu J.; Shi S.; Fu C.; Han X.; Gao W.; Song T.; Xie L.; Wang L.; Sun S.; Guo R.; Ma G. Increased Iron Deposition on Brain Quantitative Susceptibility Mapping Correlates with Decreased Cognitive Function in Alzheimer’s Disease. ACS Chem. Neurosci. 2018, 9 (7), 1849–1857. 10.1021/acschemneuro.8b00194. [DOI] [PubMed] [Google Scholar]
- (11).Martin-Bastida A.; Ward R. J.; Newbould R.; Piccini P.; Sharp D.; Kabba C.; Patel M. C.; Spino M.; Connelly J.; Tricta F.; Crichton R. R.; Dexter D. T. Brain Iron Chelation by Deferiprone in a Phase 2 Randomised Double-Blinded Placebo Controlled Clinical Trial in Parkinson’s Disease. Sci. Rep. 2017, 7 (1). 10.1038/s41598-017-01402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Devos D.; Moreau C.; Devedjian J. C.; Kluza J.; Petrault M.; Laloux C.; Jonneaux A.; Ryckewaert G.; Garçon G.; Rouaix N.; Duhamel A.; Jissendi P.; Dujardin K.; Auger F.; Ravasi L.; Hopes L.; Grolez G.; Firdaus W.; Sablonnière B.; Strubi-Vuillaume I.; Zahr N.; Destée A.; Corvol J.-C.; Pöltl D.; Leist M.; Rose C.; Defebvre L.; Marchetti P.; Cabantchik Z. I.; Bordet R. Targeting Chelatable Iron as a Therapeutic Modality in Parkinson’s Disease. Antioxid. Redox Signal. 2014, 21 (2), 195–210. 10.1089/ars.2013.5593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Feng H.; Stockwell B. R. Unsolved Mysteries: How Does Lipid Peroxidation Cause Ferroptosis? PLoS Biol. 2018, 16 (5), e2006203. 10.1371/journal.pbio.2006203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Das U. N. Saturated Fatty Acids, MUFAs and PUFAs Regulate Ferroptosis. Cell Chem. Biol. 2019, 26 (3), 309–311. 10.1016/j.chembiol.2019.03.001. [DOI] [PubMed] [Google Scholar]
- (15).Yamada N.; Karasawa T.; Kimura H.; Watanabe S.; Komada T.; Kamata R.; Sampilvanjil A.; Ito J.; Nakagawa K.; Kuwata H.; Hara S.; Mizuta K.; Sakuma Y.; Sata N.; Takahashi M. Ferroptosis Driven by Radical Oxidation of N-6 Polyunsaturated Fatty Acids Mediates Acetaminophen-Induced Acute Liver Failure. Cell Death Dis. 2020, 11 (2), 144. 10.1038/s41419-020-2334-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Agmon E.; Solon J.; Bassereau P.; Stockwell B. R. Modeling the Effects of Lipid Peroxidation during Ferroptosis on Membrane Properties. Sci. Rep. 2018, 8 (1). 10.1038/s41598-018-23408-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Perez M. A.; Magtanong L.; Dixon S. J.; Watts J. L. Dietary Lipids Induce Ferroptosis in Caenorhabditiselegans and Human Cancer Cells. Dev. Cell 2020, 54 (4), 447–454.e4. 10.1016/j.devcel.2020.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Sarparast M.; Dattmore D.; Alan J.; Lee K. S. S. Cytochrome P450 Metabolism of Polyunsaturated Fatty Acids and Neurodegeneration. Nutrients 2020, 12 (11), 3523. 10.3390/nu12113523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Shahidi F.; Ambigaipalan P. Omega-3 Polyunsaturated Fatty Acids and Their Health Benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 345–381. 10.1146/annurev-food-111317-095850. [DOI] [PubMed] [Google Scholar]
- (20).Eriksdotter M.; Vedin I.; Falahati F.; Freund-Levi Y.; Hjorth E.; Faxen-Irving G.; Wahlund L.-O.; Schultzberg M.; Basun H.; Cederholm T.; Palmblad J. Plasma Fatty Acid Profiles in Relation to Cognition and Gender in Alzheimer’s Disease Patients during Oral Omega-3 Fatty Acid Supplementation: The OmegAD Study. J. Alzheimers. Dis. 2015, 48 (3), 805–812. 10.3233/JAD-150102. [DOI] [PubMed] [Google Scholar]
- (21).Jernerén F.; Cederholm T.; Refsum H.; Smith A. D.; Turner C.; Palmblad J.; Eriksdotter M.; Hjorth E.; Faxen-Irving G.; Wahlund L.-O.; Schultzberg M.; Basun H.; Freund-Levi Y. Homocysteine Status Modifies the Treatment Effect of Omega-3 Fatty Acids on Cognition in a Randomized Clinical Trial in Mild to Moderate Alzheimer’s Disease: The OmegAD Study. J. Alzheimers. Dis. 2019, 69 (1), 189–197. 10.3233/JAD-181148. [DOI] [PubMed] [Google Scholar]
- (22).Kalmijn S.; Launer L. J.; Ott A.; Witteman J. C.; Hofman A.; Breteler M. M. Dietary Fat Intake and the Risk of Incident Dementia in the Rotterdam Study. Ann. Neurol. 1997, 42 (5), 776–782. 10.1002/ana.410420514. [DOI] [PubMed] [Google Scholar]
- (23).Simopoulos A. P. Importance of the Ratio of Omega-6/Omega-3 Essential Fatty Acids: Evolutionary Aspects. World Rev. Nutr. Diet. 2003, 92, 1–22. 10.1159/000073788. [DOI] [PubMed] [Google Scholar]
- (24).Ballenger J. C. Dietary Patterns and Risk of Dementia: The Three-City Cohort Study. Year b. psychiatry appl. ment. health 2009, 2009, 278–279. 10.1016/s0084-3970(08)79239-9. [DOI] [Google Scholar]
- (25).Phillips M. A.; Childs C. E.; Calder P. C.; Rogers P. J. No Effect of Omega-3 Fatty Acid Supplementation on Cognition and Mood in Individuals with Cognitive Impairment and Probable Alzheimer’s Disease: A Randomised Controlled Trial. Int. J. Mol. Sci. 2015, 16 (10), 24600–24613. 10.3390/ijms161024600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Lebbadi M.; Julien C.; Phivilay A.; Tremblay C.; Emond V.; Kang J. X.; Calon F. Endogenous Conversion of Omega-6 into Omega-3 Fatty Acids Improves Neuropathology in an Animal Model of Alzheimer’s Disease. J. Alzheimers. Dis. 2011, 27 (4), 853–869. 10.3233/JAD-2011-111010. [DOI] [PubMed] [Google Scholar]
- (27).Ajith T. A. A Recent Update on the Effects of Omega-3 Fatty Acids in Alzheimer’s Disease. Curr. Clin. Pharmacol. 2018, 13 (4), 252–260. 10.2174/1574884713666180807145648. [DOI] [PubMed] [Google Scholar]
- (28).Ma Q.-M.; Zhu C.; Caoili K.; Frautschy S. A.; Cole G. M. The Novel Omega‐6 Fatty Acid Docosapentaenoic Acid Positively Modulates Brain Innate Immune Response for Resolving Neuroinflammation at Early and Late Stages of Humanized APOE‐based Alzheimer’s Disease Models. Alzheimers. Dement. 2020, 16 (S9). 10.1002/alz.044178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Simopoulos A. P. Evolutionary Aspects of Diet and Essential Fatty Acids. World Rev. Nutr. Diet. 2001, 88, 18–27. 10.1159/000059742. [DOI] [PubMed] [Google Scholar]
- (30).Blasbalg T. L.; Hibbeln J. R.; Ramsden C. E.; Majchrzak S. F.; Rawlings R. R. Changes in Consumption of Omega-3 and Omega-6 Fatty Acids in the United States during the 20th Century. Am. J. Clin. Nutr. 2011, 93 (5), 950–962. 10.3945/ajcn.110.006643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Doll S.; Proneth B.; Tyurina Y. Y.; Panzilius E.; Kobayashi S.; Ingold I.; Irmler M.; Beckers J.; Aichler M.; Walch A.; Prokisch H.; Trümbach D.; Mao G.; Qu F.; Bayir H.; Füllekrug J.; Scheel C. H.; Wurst W.; Schick J. A.; Kagan V. E.; Angeli J. P. F.; Conrad M. ACSL4 Dictates Ferroptosis Sensitivity by Shaping Cellular Lipid Composition. Nat. Chem. Biol. 2017, 13 (1), 91–98. 10.1038/nchembio.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Deline M.; Keller J.; Rothe M.; Schunck W.-H.; Menzel R.; Watts J. L. Epoxides Derived from Dietary Dihomo-Gamma-Linolenic Acid Induce Germ Cell Death in C. Elegans. Sci. Rep. 2015, 5, 15417. 10.1038/srep15417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Santos D.; Laila R. B.; Fleming I. Role of Cytochrome P450-Derived, Polyunsaturated Fatty Acid Mediators in Diabetes and the Metabolic Syndrome. Prostaglandins & Other Lipid Mediators 2020, 148. [DOI] [PubMed] [Google Scholar]
- (34).Shoieb S. M.; El-Ghiaty M. A.; Alqahtani M. A.; El-Kadi A. O. S. Cytochrome P450-Derived Eicosanoids and Inflammation in Liver Diseases. Prostaglandins Other Lipid Mediat. 2020, 147, 106400. 10.1016/j.prostaglandins.2019.106400. [DOI] [PubMed] [Google Scholar]
- (35).Enayetallah A. E.; French R. A.; Thibodeau M. S.; Grant D. F. Distribution of Soluble Epoxide Hydrolase and of Cytochrome P450 2C8, 2C9, and 2J2 in Human Tissues. J. Histochem. Cytochem. 2004, 52 (4), 447–454. 10.1177/002215540405200403. [DOI] [PubMed] [Google Scholar]
- (36).Sura P.; Sura R.; Enayetallah A. E.; Grant D. F. Distribution and Expression of Soluble Epoxide Hydrolase in Human Brain. J. Histochem. Cytochem. 2008, 56 (6), 551–559. 10.1369/jhc.2008.950659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Harris T. R.; Hammock B. D. Soluble Epoxide Hydrolase: Gene Structure, Expression and Deletion. Gene 2013, 526 (2), 61–74. 10.1016/j.gene.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Booth Depaz I. M.; Toselli F.; Wilce P. A.; Gillam E. M. J. Differential Expression of Cytochrome P450 Enzymes from the CYP2C Subfamily in the Human Brain. Drug Metab. Dispos. 2015, 43 (3), 353–357. 10.1124/dmd.114.061242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Karlsson M.; Zhang C.; Méar L.; Zhong W.; Digre A.; Katona B.; Sjöstedt E.; Butler L.; Odeberg J.; Dusart P.; Edfors F.; Oksvold P.; von Feilitzen K.; Zwahlen M.; Arif M.; Altay O.; Li X.; Ozcan M.; Mardinoglu A.; Fagerberg L.; Mulder J.; Luo Y.; Ponten F.; Uhlén M.; Lindskog C. A Single–Cell Type Transcriptomics Map of Human Tissues. Sci. Adv. 2021, 7 (31). 10.1126/sciadv.abh2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Vierck J. L.; Dodson M. V. Interpretation of Cell Culture Phenomena. Methods Cell Sci. 2000, 22 (1), 79–81. [DOI] [PubMed] [Google Scholar]
- (41).Fleming I. Cytochrome P450 Epoxygenases as EDHF Synthase(s). Pharmacol. Res. 2004, 49 (6), 525–533. 10.1016/j.phrs.2003.11.016. [DOI] [PubMed] [Google Scholar]
- (42).Pozzi A.; Macias-Perez I.; Abair T.; Wei S.; Su Y.; Zent R.; Falck J. R.; Capdevila J. H. Characterization of 5,6- and 8,9-Epoxyeicosatrienoic Acids (5,6- and 8,9-EET) as Potent in Vivo Angiogenic Lipids. J. Biol. Chem. 2005, 280 (29), 27138–27146. 10.1074/jbc.M501730200. [DOI] [PubMed] [Google Scholar]
- (43).Perez M. A.; Clostio A. J.; Houston I. R.; Ruiz J.; Magtanong L.; Dixon S. J.; Watts J. L. Ether Lipid Deficiency Disrupts Lipid Homeostasis Leading to Ferroptosis Sensitivity. PLoS Genet. 2022, 18 (9), e1010436. 10.1371/journal.pgen.1010436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Watts J. L.; Browse J. Genetic Dissection of Polyunsaturated Fatty Acid Synthesis in Caenorhabditis Elegans. Proc. Natl. Acad. Sci. U. S. A. 2002, 99 (9), 5854–5859. 10.1073/pnas.092064799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Watts J. L. Using Caenorhabditis Elegans to Uncover Conserved Functions of Omega-3 and Omega-6 Fatty Acids. J. Clin. Med. Res. 2016, 5 (2). 10.3390/jcm5020019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Pathak A.; Chatterjee N.; Sinha S. Developmental Trajectory of Caenorhabditis Elegans Nervous System Governs Its Structural Organization. PLoS Comput. Biol. 2020, 16 (1), e1007602. 10.1371/journal.pcbi.1007602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Gjorgjieva J.; Biron D.; Haspel G. Neurobiology of Caenorhabditis Elegans Locomotion: Where Do We Stand? Bioscience 2014, 64 (6), 476–486. 10.1093/biosci/biu058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Altun Z. F.; Hall D. H. WormAtlas Hermaphrodite Handbook - Nervous System - General Description. WormAtlas 2005. 10.3908/wormatlas.1.18. [DOI] [Google Scholar]
- (49).Muhoberac B. B.; Vidal R. Iron, Ferritin, Hereditary Ferritinopathy, and Neurodegeneration. Front. Neurosci. 2019, 13, 1195. 10.3389/fnins.2019.01195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Friedmann Angeli J. P.; Schneider M.; Proneth B.; Tyurina Y. Y.; Tyurin V. A.; Hammond V. J.; Herbach N.; Aichler M.; Walch A.; Eggenhofer E.; Basavarajappa D.; Rådmark O.; Kobayashi S.; Seibt T.; Beck H.; Neff F.; Esposito I.; Wanke R.; Förster H.; Yefremova O.; Heinrichmeyer M.; Bornkamm G. W.; Geissler E. K.; Thomas S. B.; Stockwell B. R.; O’Donnell V. B.; Kagan V. E.; Schick J. A.; Conrad M. Inactivation of the Ferroptosis Regulator Gpx4 Triggers Acute Renal Failure in Mice. Nat. Cell Biol. 2014, 16 (12), 1180–1191. 10.1038/ncb3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Li J.; Cao F.; Yin H.-L.; Huang Z.-J.; Lin Z.-T.; Mao N.; Sun B.; Wang G. Ferroptosis: Past, Present and Future. Cell Death Dis. 2020, 11 (2), 88. 10.1038/s41419-020-2298-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Hou L.; Huang R.; Sun F.; Zhang L.; Wang Q. NADPH Oxidase Regulates Paraquat and Maneb-Induced Dopaminergic Neurodegeneration through Ferroptosis. Toxicology 2019, 417, 64–73. 10.1016/j.tox.2019.02.011. [DOI] [PubMed] [Google Scholar]
- (53).Chen X.; Yu C.; Kang R.; Tang D. Iron Metabolism in Ferroptosis. Front Cell Dev Biol 2020, 8, 590226. 10.3389/fcell.2020.590226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Chávez V.; Mohri-Shiomi A.; Garsin D. A. Ce-Duox1/BLI-3 Generates Reactive Oxygen Species as a Protective Innate Immune Mechanism in Caenorhabditis Elegans. Infect. Immun. 2009, 77 (11), 4983–4989. 10.1128/iai.00627-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Erekat N. S. Apoptosis and Its Therapeutic Implications in Neurodegenerative Diseases. Clin. Anat. 2022, 35 (1), 65–78. 10.1002/ca.23792. [DOI] [PubMed] [Google Scholar]
- (56).Webster C. M.; Deline M. L.; Watts J. L. Stress Response Pathways Protect Germ Cells from Omega-6 Polyunsaturated Fatty Acid-Mediated Toxicity in Caenorhabditis Elegans. Dev. Biol. 2013, 373 (1), 14–25. 10.1016/j.ydbio.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Naoe S.; Tsugawa H.; Takahashi M.; Ikeda K.; Arita M. Characterization of Lipid Profiles after Dietary Intake of Polyunsaturated Fatty Acids Using Integrated Untargeted and Targeted Lipidomics. Metabolites 2019, 9 (10), 241. 10.3390/metabo9100241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Keenan A. H.; Pedersen T. L.; Fillaus K.; Larson M. K.; Shearer G. C.; Newman J. W. Basal Omega-3 Fatty Acid Status Affects Fatty Acid and Oxylipin Responses to High-Dose N3-HUFA in Healthy Volunteers. J. Lipid Res. 2012, 53 (8), 1662–1669. 10.1194/jlr.P025577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Wang W.; Yang J.; Nimiya Y.; Lee K. S. S.; Sanidad K.; Qi W.; Sukamtoh E.; Park Y.; Liu Z.; Zhang G. ω-3 Polyunsaturated Fatty Acids and Their Cytochrome P450-Derived Metabolites Suppress Colorectal Tumor Development in Mice. J. Nutr. Biochem. 2017, 48, 29–35. 10.1016/j.jnutbio.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Fischer R.; Konkel A.; Mehling H.; Blossey K.; Gapelyuk A.; Wessel N.; von Schacky C.; Dechend R.; Muller D. N.; Rothe M.; Luft F. C.; Weylandt K.; Schunck W.-H. Dietary Omega-3 Fatty Acids Modulate the Eicosanoid Profile in Man Primarily via the CYP-Epoxygenase Pathway. J. Lipid Res. 2014, 55 (6), 1150–1164. 10.1194/jlr.M047357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Schebb N. H.; Ostermann A. I.; Yang J.; Hammock B. D.; Hahn A.; Schuchardt J. P. Comparison of the Effects of Long-Chain Omega-3 Fatty Acid Supplementation on Plasma Levels of Free and Esterified Oxylipins. Prostaglandins Other Lipid Mediat. 2014, 113–115, 21–29. 10.1016/j.prostaglandins.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Schmöcker C.; Zhang I.; Kiesler S.; Kassner U.; Ostermann A.; Steinhagen-Thiessen E.; Schebb N.; Weylandt K.-H. Effect of Omega-3 Fatty Acid Supplementation on Oxylipins in a Routine Clinical Setting. Int. J. Mol. Sci. 2018, 19 (1), 180. 10.3390/ijms19010180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Morisseau C.; Hammock B. D. Impact of Soluble Epoxide Hydrolase and Epoxyeicosanoids on Human Health. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 37–58. 10.1146/annurev-pharmtox-011112-140244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Deng Y.; Theken K. N.; Lee C. R. Cytochrome P450 Epoxygenases, Soluble Epoxide Hydrolase, and the Regulation of Cardiovascular Inflammation. J. Mol. Cell. Cardiol. 2010, 48 (2), 331–341. 10.1016/j.yjmcc.2009.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Harris T. R.; Aronov P. A.; Jones P. D.; Tanaka H.; Arand M.; Hammock B. D. Identification of Two Epoxide Hydrolases in Caenorhabditis Elegans That Metabolize Mammalian Lipid Signaling Molecules. Arch. Biochem. Biophys. 2008, 472 (2), 139–149. 10.1016/j.abb.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Lee K. S. S.; Henriksen N. M.; Ng C. J.; Yang J.; Jia W.; Morisseau C.; Andaya A.; Gilson M. K.; Hammock B. D. Probing the Orientation of Inhibitor and Epoxy-Eicosatrienoic Acid Binding in the Active Site of Soluble Epoxide Hydrolase. Arch. Biochem. Biophys. 2017, 613, 1–11. 10.1016/j.abb.2016.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Wagner K.; Lee K. S. S.; Yang J.; Hammock B. D. Epoxy Fatty Acids Mediate Analgesia in Murine Diabetic Neuropathy. Eur. J. Pain 2017, 21 (3), 456–465. 10.1002/ejp.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Cinelli M. A.; Yang J.; Scharmen A.; Woodman J.; Karchalla L. M.; Lee K. S. S. Enzymatic Synthesis and Chemical Inversion Provide Both Enantiomers of Bioactive Epoxydocosapentaenoic Acids. J. Lipid Res. 2018, 59 (11), 2237–2252. 10.1194/jlr.D089136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Cinelli M. A.; Lee K. S. S. Asymmetric Total Synthesis of 19,20-Epoxydocosapentaenoic Acid, a Bioactive Metabolite of Docosahexaenoic Acid. J. Org. Chem. 2019, 84 (23), 15362–15372. 10.1021/acs.joc.9b02378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Fang X.; Weintraub N. L.; McCaw R. B.; Hu S.; Harmon S. D.; Rice J. B.; Hammock B. D.; Spector A. A. Effect of Soluble Epoxide Hydrolase Inhibition on Epoxyeicosatrienoic Acid Metabolism in Human Blood Vessels. Am. J. Physiol. Heart Circ. Physiol. 2004, 287 (6), H2412–20. 10.1152/ajpheart.00527.2004. [DOI] [PubMed] [Google Scholar]
- (71).Halarnkar P. P.; Nourooz-Zadeh J.; Kuwano E.; Jones A. D.; Hammock B. D. Formation of Cyclic Products from the Diepoxide of Long-Chain Fatty Esters by Cytosolic Epoxide Hydrolase. Arch. Biochem. Biophys. 1992, 294 (2), 586–593. 10.1016/0003-9861(92)90729-g. [DOI] [PubMed] [Google Scholar]
- (72).Moghaddam M. Novel Metabolic Pathways for Linoleic and Arachidonic Acid Metabolism. Biochim. Biophys. Acta Gen. Subj. 1996, 1290 (3), 327–339. 10.1016/0304-4165(96)00037-2. [DOI] [PubMed] [Google Scholar]
- (73).Yang W. S.; Kim K. J.; Gaschler M. M.; Patel M.; Shchepinov M. S.; Stockwell B. R. Peroxidation of Polyunsaturated Fatty Acids by Lipoxygenases Drives Ferroptosis. Proc. Natl. Acad. Sci. U. S. A. 2016, 113 (34), E4966–75. 10.1073/pnas.1603244113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (74).Magtanong L.; Ko P.-J.; To M.; Cao J. Y.; Forcina G. C.; Tarangelo A.; Ward C. C.; Cho K.; Patti G. J.; Nomura D. K.; Olzmann J. A.; Dixon S. J. Exogenous Monounsaturated Fatty Acids Promote a Ferroptosis-Resistant Cell State. Cell Chem. Biol. 2019, 26 (3), 420–432.e9. 10.1016/j.chembiol.2018.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (75).Conrad M.; Pratt D. A. The Chemical Basis of Ferroptosis. Nat. Chem. Biol. 2019, 15 (12), 1137–1147. 10.1038/s41589-019-0408-1. [DOI] [PubMed] [Google Scholar]
- (76).Dierge E.; Debock E.; Guilbaud C.; Corbet C.; Mignolet E.; Mignard L.; Bastien E.; Dessy C.; Larondelle Y.; Feron O. Peroxidation of N-3 and n-6 Polyunsaturated Fatty Acids in the Acidic Tumor Environment Leads to Ferroptosis-Mediated Anticancer Effects. Cell Metab. 2021, 33 (8), 1701–1715.e5. 10.1016/j.cmet.2021.05.016. [DOI] [PubMed] [Google Scholar]
- (77).Kagan V. E.; Mao G.; Qu F.; Angeli J. P. F.; Doll S.; Croix C. S.; Dar H. H.; Liu B.; Tyurin V. A.; Ritov V. B.; Kapralov A. A.; Amoscato A. A.; Jiang J.; Anthonymuthu T.; Mohammadyani D.; Yang Q.; Proneth B.; Klein-Seetharaman J.; Watkins S.; Bahar I.; Greenberger J.; Mallampalli R. K.; Stockwell B. R.; Tyurina Y. Y.; Conrad M.; Bayır H. Oxidized Arachidonic and Adrenic PEs Navigate Cells to Ferroptosis. Nat. Chem. Biol. 2017, 13 (1), 81–90. 10.1038/nchembio.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (78).Lee J.-Y.; Nam M.; Son H. Y.; Hyun K.; Jang S. Y.; Kim J. W.; Kim M. W.; Jung Y.; Jang E.; Yoon S.-J.; Kim J.; Kim J.; Seo J.; Min J.-K.; Oh K.-J.; Han B.-S.; Kim W. K.; Bae K.-H.; Song J.; Kim J.; Huh Y.-M.; Hwang G.-S.; Lee E.-W.; Lee S. C. Polyunsaturated Fatty Acid Biosynthesis Pathway Determines Ferroptosis Sensitivity in Gastric Cancer. Proc. Natl. Acad. Sci. U. S. A. 2020, 117 (51), 32433–32442. 10.1073/pnas.2006828117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (79).Zou Y.; Henry W. S.; Ricq E. L.; Graham E. T.; Phadnis V. V.; Maretich P.; Paradkar S.; Boehnke N.; Deik A. A.; Reinhardt F.; Eaton J. K.; Ferguson B.; Wang W.; Fairman J.; Keys H. R.; Dančík V.; Clish C. B.; Clemons P. A.; Hammond P. T.; Boyer L. A.; Weinberg R. A.; Schreiber S. L. Plasticity of Ether Lipids Promotes Ferroptosis Susceptibility and Evasion. Nature 2020, 585 (7826), 603–608. 10.1038/s41586-020-2732-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (80).Ren Q.; Ma M.; Yang J.; Nonaka R.; Yamaguchi A.; Ishikawa K.-I.; Kobayashi K.; Murayama S.; Hwang S. H.; Saiki S.; Akamatsu W.; Hattori N.; Hammock B. D.; Hashimoto K. Soluble Epoxide Hydrolase Plays a Key Role in the Pathogenesis of Parkinson’s Disease. Proc. Natl. Acad. Sci. U. S. A. 2018, 115 (25), E5815–E5823. 10.1073/pnas.1802179115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (81).Lee H.-T.; Lee K.-I.; Chen C.-H.; Lee T.-S. Genetic Deletion of Soluble Epoxide Hydrolase Delays the Progression of Alzheimer’s Disease. J. Neuroinflammation 2019, 16 (1), 267. 10.1186/s12974-019-1635-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (82).Ghosh A.; Comerota M. M.; Wan D.; Chen F.; Propson N. E.; Hwang S. H.; Hammock B. D.; Zheng H. An Epoxide Hydrolase Inhibitor Reduces Neuroinflammation in a Mouse Model of Alzheimer’s Disease. Sci. Transl. Med. 2020, 12 (573). 10.1126/scitranslmed.abb1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (83).Griñán-Ferré C.; Codony S.; Pujol E.; Yang J.; Leiva R.; Escolano C.; Puigoriol-Illamola D.; Companys-Alemany J.; Corpas R.; Sanfeliu C.; Pérez B.; Loza M. I.; Brea J.; Morisseau C.; Hammock B. D.; Vázquez S.; Pallàs M.; Galdeano C. Pharmacological Inhibition of Soluble Epoxide Hydrolase as a New Therapy for Alzheimer’s Disease. Neurotherapeutics 2020. 10.1007/s13311-020-00854-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (84).Kundu S.; Roome T.; Bhattacharjee A.; Carnevale K. A.; Yakubenko V. P.; Zhang R.; Hwang S. H.; Hammock B. D.; Cathcart M. K. Metabolic Products of Soluble Epoxide Hydrolase Are Essential for Monocyte Chemotaxis to MCP-1 in Vitro and in Vivo. J. Lipid Res. 2013, 54 (2), 436–447. 10.1194/jlr.M031914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (85).Hildreth K.; Kodani S. D.; Hammock B. D.; Zhao L. Cytochrome P450-Derived Linoleic Acid Metabolites EpOMEs and DiHOMEs: A Review of Recent Studies. J. Nutr. Biochem. 2020, 86 (108484), 108484. 10.1016/j.jnutbio.2020.108484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (86).Zille M.; Kumar A.; Kundu N.; Bourassa M. W.; Wong V. S. C.; Willis D.; Karuppagounder S. S.; Ratan R. R. Ferroptosis in Neurons and Cancer Cells Is Similar but Differentially Regulated by Histone Deacetylase Inhibitors. eNeuro 2019, 6 (1), ENEURO.0263–18.2019. 10.1523/ENEURO.0263-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (87).Solano Fonseca R.; Metang P.; Egge N.; Liu Y.; Zuurbier K. R.; Sivaprakasam K.; Shirazi S.; Chuah A.; Arneaud S. L.; Konopka G.; Qian D.; Douglas P. M. Glycolytic Preconditioning in Astrocytes Mitigates Trauma-Induced Neurodegeneration. Elife 2021, 10. 10.7554/eLife.69438. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.