Abstract
The Plasmodium berghei-infected mouse model is a well-recognized model for human cerebral malaria. Mice infected with P. berghei exhibit (i) metabolic acidosis (pH < 7.3) associated with elevated plasma lactate concentrations, (ii) significant (P < 0.05) vascular leakage in their lungs, hearts, kidneys, and brains, (ii) significantly (P < 0.05) higher cell and serum glutamate concentrations, and (iv) significantly (P < 0.05) lower mean arterial blood pressures. Because these complications are similar to those of septic shock, the simplest interpretation of these findings is that the mice develop shock brought on by the P. berghei infection. To determine whether the immune system and specifically CD8+ T cells mediate the key features of shock during P. berghei malaria, we depleted CD8+ T cells by monoclonal antibody (mAb) treatment and assessed the complications of malarial shock. P. berghei-infected mice depleted of CD8+ T cells by mAb treatment had significantly reduced vascular leakage in their hearts, brains, lungs, and kidneys compared with infected controls treated with rat immunoglobulin G. CD8-depleted mice were significantly (P < 0.05) protected from lactic acidosis, glutamate buildup, and diminished HCO3− levels. Although the blood pressure decreased in anti-CD8 mAb-treated mice infected with P. berghei, the cardiac output, as assessed by echocardiography, was similar to that of uninfected control mice. Collectively, our results indicate that (i) pathogenesis similar to septic shock occurs during experimental P. berghei malaria, (ii) respiratory distress with lactic acidosis occurs during P. berghei malaria, and (iii) most components of circulatory shock are ameliorated by depletion of CD8+ T cells.
Circulatory shock is defined as an inadequacy of blood flow in multiple organ systems that leads to inadequate delivery of nutrients to tissues and inadequate removal of waste products (reviewed in reference 14). The most common causes of circulatory shock are cardiac and circulatory abnormalities, such as myocardial infarction, and hemorrhage. Less common but no less deadly is the development of circulatory shock caused by an infectious agent, also called septic shock (45). Bacteria or bacterial products in septic shock initiate an inflammatory response that feeds on itself, becomes uncontrolled, and ultimately destroys the host (45). Leukocytes, including T cells, secrete cytokines (such as tumor necrosis factor alpha [TNF-α], interleukin 1 [IL-1], and gamma interferon [IFN-γ]) that further enhance the inflammatory response, leading to endothelial dysfunction. The endothelial dysfunction leads to increased vascular permeability, which in turn decreases blood volume, diminishes perfusion of tissues, and results in interstitial edema. In the absence of adequate blood flow, cells must rely on glycolysis for energy production and consequently produce lactic acid. While a variety of reflexes and compensatory mechanisms are activated in response to shock, these efforts to restore normal tissue perfusion can fail, which leads to a further reduction in cardiac output, more lactic acidosis, and ultimately tissue necrosis. Unless this cascade of immune destruction and tissue necrosis is interrupted, death results.
Malaria is a leading cause of morbidity and mortality. Patients with severe Plasmodium falciparum malaria develop the following complications: coma or cerebral malaria, respiratory distress with lactic acidosis, anemia, and occasionally renal failure. The mechanism of cerebral malaria pathogenesis is being intensely debated, and there are two major hypotheses, the mechanical hypothesis and the inflammatory hypothesis (reviewed in references 8 and 31). In the mechanical hypothesis, parasitized red blood cells bind to the endothelium, causing minithrombi, which in turn lead to the petechial hemorrhaging that is observed on autopsy, tissue hypoxia, and ultimately death. The inflammatory hypothesis states that the immune response to parasites leads to vascular damage in the brain, coma, and ultimately death. Clark et al. have proposed that the inflammatory response leads to breakdown of the blood-brain barrier and that nitric oxide is a key mediator of pathology (6). Infection with P. falciparum increases the levels of inflammatory cytokines (TNF-α, IL-1β, and IFN-γ) in serum. Individuals with a single nucleotide polymorphism in the OCT-1 site of the TNF-α promoter region have a fourfold-greater risk of developing cerebral malaria and respiratory distress (30). The inflammatory cytokines are believed to upregulate expression of several adhesion molecules, such as ICAM-1, VCAM-1, and CD36. CD36 and ICAM-1 are used by the parasite for cytoadherence to capillary endothelium (1), but these molecules are also known to be important for leukocyte endothelial adhesion (43). The precise pathologic mechanisms in humans are difficult to identify for obvious ethical reasons.
There are two well-characterized models of cerebral malaria (10, 28, 36, 38). The advantages and disadvantages of these models have been reviewed elsewhere (8). It has been proposed that the Plasmodium yoelii model is better than the Plasmodium berghei model because P. yoelii-parasitized erythrocytes, like P. falciparum-parasitized erythrocytes, bind to brain microvasculature. However, Hearn et al. reported that P. berghei-parasitized erythrocytes adhere to brain microvasculature, indicating that both P. yoelii and P. berghei mimic P. falciparum in this regard (15, 19, 20). We selected the P. berghei model for this study because P. berghei-infected mice develop impaired consciousness, whereas P. yoelii-infected mice do not (10, 28, 38). In the respects pertinent to this study, the P. berghei-infected mouse model remarkably mimics P. falciparum infection in humans. Virtually all mice with a susceptible background (C57BL/6 mice) that are infected with P. berghei develop cerebral malaria on day 6 of infection and die between days 6 and 12, which is the time window for the development of cerebral malaria (36). In contrast, only 20% of resistant mice (BALB/c and A/J mice) succumb to cerebral malaria. Mice that succumb after day 12 die of hyperparasitemia.
The immune response is vital for pathogenesis of P. berghei malaria. Elevated levels of inflammatory cytokines are detected in sera of P. berghei-infected mice, and endothelial cell adhesion molecules are also upregulated (7, 12). If anti-LFA1 monoclonal antibodies (mAbs) are administered or ICAM-1-deficient mice are used, cerebral malaria does not develop (9, 13). Petechial hemorrhaging is observed in brains of P. berghei-infected mice, and there is a breakdown of the blood-brain barrier, as determined by Monastal blue and Evans blue dye leakage, prior to the onset of cerebral symptoms (33). In contrast to immunologically intact mice, mice lacking T and B cells or CD8+ T cells do not develop cerebral malaria, indicating that the immune response is required for pathogenesis (11, 16, 44). Type 1 cytokines (IL-2, IFN-γ, and TNF-α) are also required for pathogenesis of experimental cerebral malaria (7, 27, 44). The role of the immune system in P. yoelii pathogenesis and the reason for death remain to be determined. We selected CD8+ T cells to test whether the immune system contributes to circulatory shock during malaria because these cells are required for malarial pathogenesis and depletion is easily verified (44).
Based on the findings described above and similar manifestations in humans with severe P. falciparum malaria, we hypothesized that P. berghei-infected mice develop complications of circulatory shock with multiple organ dysfunction and that the immune response mediates the damage. To test this hypothesis, we first assessed whether P. berghei-infected mice develop key features of circulatory shock, including (i) metabolic acidosis, (ii) increased vascular permeability and tissue edema, (iii) respiratory distress, and (iv) decreased mean arterial blood pressure. All of these key features of circulatory shock, which are anticipated in septic shock, were observed in P. berghei-infected mice. To determine which of these features are immune mediated, we examined the development of these complications during P. berghei malaria in mice depleted of CD8+ T cells by mAb treatment. We found that P. berghei-infected mice depleted of CD8+ T cells were markedly protected from most of the features of circulatory shock described above, whereas rat immunoglobulin G (IgG)-treated control mice developed malarial shock.
MATERIALS AND METHODS
Parasite, infection, and treatment of mice.
The malarial parasite used in this study, P. bergehi ANKA, a gift from William Weidanz, was maintained and used as described previously (17). This strain kills susceptible C57BL/6 mice on about day 6 after infection. Frozen parasite stabilate was therefore injected intraperitoneally (i.p.) into a resistant BALB/c source mouse. Blood was obtained from the source BALB/c mouse to generate the inoculum used for the experimental C57BL/6 mice. All experimental mice (both test mice and control mice) were identically infected, so the use of BALB/c mice should not have affected the development of malarial pathogenesis. The experimental mice were injected i.p. with 106 erythrocytes parasitized with P. berghei, and parasitemia was assessed by examining between 200 and 1,000 erythrocytes in Giemsa-stained thin blood films.
Female C57BL/6 experimental mice and BALB/c source mice were purchased from Jackson Laboratories (Bar Harbor, Maine) when they were 4 to 5 weeks old, and they were provided food and water ad libitum. In each experiment, age- and sex-matched groups consisting of between four and eight C57BL/6 mice were used, and the animals were between 6 and 12 weeks old. The animals were housed at the Louisiana State University Health Sciences Center Animal Care Facility, an American Association of Laboratory Animal Care-approved facility. All procedures were approved by the Animal Resources Advisory Committee of the Louisiana State University Health Sciences Center.
To deplete CD8+ T cells, we injected i.p. 1 mg of anti-CD8 mAb (rat IgG mAb, clone 53-6.72), which is a depleting mAb (24), on day 1 of P. berghei infection into mice. Control mice were injected i.p. with 1 mg of rat IgG (Accurate Scientific). The extent of depletion was assessed on day 4 of infection by obtaining about 50 μl of blood and analyzing 30 μl of this blood for the presence of CD3+ CD8+ cells by flow cytometry.
Flow cytometry.
Flow cytometry was performed as described previously (42). Briefly, erythrocytes from 30 μl of mouse blood were lysed by hypotonic shock. The cells were washed to remove erythrocyte debris, and Fc block was added to the cell suspension to minimize nonspecific binding of mAbs. After 10 min of incubation, biotinylated anti-CD8 mAb (Pharmingen, San Diego, Calif.) was added, and the cell suspension was incubated with the antibody for 30 min. After the cells were washed, CD3-fluorescein isothiocyanate, CD4-phycoerythrin, and streptavidin-allophycocyanin (Pharmingen) were added to the suspension, and the mixture was incubated for 30 min. The cells were washed and resuspended in 0.5 ml of phosphate-buffered saline (PBS), and propidium iodide (Sigma Chemical Co., St. Louis, Mo.) was added 5 min before data acquisition in order to exclude dead cells. Flow cytometry data for the cell suspension were acquired with a FACSCalibur (Becton Dickinson) by using the CellQuest program and were analyzed by using the Attractors program (Becton Dickinson).
Assessment of vascular leakage and tissue edema.
Vascular leakage into selected organs during P. berghei malaria was assessed as described by Tateishi et al. (39). Briefly, 0.2 ml of 2% Evans Blue dye in saline was injected intravenously into each mouse. The dye was allowed to circulate for 10 min, and then the mouse was anesthetized with xylazine (7.5 mg/kg) and ketamine (150 mg/kg) injected i.p. for 6 min. The right atrium was snipped, and then 25 ml of PBS was injected into the left ventricle and 20 ml of PBS was injected into the right ventricle to remove dye from the vasculature. Selected organs were removed from the animal, weighed, and placed in a test tube containing 1 ml of N,N-dimethyl formamide. The amount of Evans Blue dye in each organ was assessed by incubating the organ in 1 ml of N,N-dimethyl formamide (Sigma) for 48 h to extract the Evans Blue dye. The A630 of the Evans Blue dye solution was measured with a spectrophotometer. To ensure that measurements were in the linear range of the spectrophotometer, the solution was diluted with N,N-dimethyl formamide until the A630 was less than 0.7. The absorbance value was divided by the weight of the tissue to normalize for the amount of tissue. The ratio of wet weight to dry weight was calculated by dividing the weight of the organ immediately after dissection (wet weight) by the weight of the organ after it was dried overnight at 80°C.
Lactate, bicarbonate, and amino acid assessment.
To precipitate proteins, serum samples were promptly treated with an equal volume of ice-cold 5% trichloroacetic acid, left on ice for 10 min, and then centrifuged at 10,000 × g for 10 min. Aliquots of the protein-free supernatant containing free amino acids were then treated with o-phthaladehyde (Fluka, Buchs, Switzerland) for precolumn derivatization and injected into a C18 column (4.6 by 250 mm; Microsorb; Varian, Walnut Creek, Calif.) for separation of the derivatized amino acids. The column effluent was passed through a fluorescence detector along with major amino acid standards that formed peaks at the following retention times: aspartate, 7.85 min; glutamate and glutamine, 16.4 min; homoserine (internal standard), 17.5 min; and alanine, 20.5 min. The plasma concentrations of the major amino acids were obtained by dividing the sample peak areas by the peak areas for the corresponding standards. The coefficients of variation for replicate determinations of aspartate and glutamate in plasma were 1.6 and 2.3%, respectively (n = 3), and for whole blood the coefficients of variation were 2.8 and 5.2%, respectively; the levels of recovery of the glutamate and aspartate standards (100 nmol) added to plasma were 103% ± 4% and 106% ± 5%, respectively.
The plasma lactate concentration was determined enzymatically with protein-free trichloroacetic acid extracts by using lactate dehydrogenase-catalyzed oxidation of lactate to pyruvate coupled to NADH formation from NAD monitored spectrophotometrically at 340 nm (Sigma); the concentration of lactate was calculated by dividing the absorbance by the absorbance for the lactate standard (4.4 mM). The coefficient of variation for replicate determinations of control plasma lactate concentrations was 3% (n = 5). The plasma bicarbonate concentration was measured by determining the total CO2 content manometrically with a microgasometer (32); under the conditions used the serum bicarbonate represented virtually all of the CO2.
Echocardiographic assessment of cardiac output.
Mean arterial blood pressure was determined in anesthetized mice (mice given sodium pentobarbital [50 mg/kg] and ketamine [50 mg/kg] i.p.) by using a common carotid artery catheter and a BP-1 pressure monitor (World Precision Instruments). In vivo echocardiography of mouse hearts and aortas was performed with a 15-MHz linear array transducer interfaced with a Sequoia C256 (Acuson) as previously described (18). In accordance with the American Society of Echocardiography recommendations (37), parameters were measured by using the leading-edge technique. Two-dimension-guided, M-mode echocardiograms were captured from long- and short-axis views of an aorta to obtain the diameter (d). From the diameter, the cross-sectional area (CSA) of the aorta was calculated as follows: CSA = π × (d/2)2. Using pulse wave doppler analysis, we determined the velocity-time integral (VTI) . The stroke volume (SV) was then calculated with the following equation: SV = CSA × VTI. The heart rate (HR) was directly measured from the M-mode tracings of the aorta. The cardiac output (CO) was then calculated with the following equation: CO = HR × SV. All echocardiogram evaluations were performed for at least 10 cardiac cycles per mouse and in a blind fashion by one observer.
Statistical analysis.
Analysis of variance with the Statview program (SAS Institute) was performed to statistically compare all measurements with a P value cutoff of 0.05. Means and standard deviations are reported below.
RESULTS
On day 4 of P. berghei infection, mice appear to be healthy and to have no obvious signs of disease. Virtually all C57BL/6 mice successfully infected (by i.p. or intravenous injection of 106 P. berghei-parasitized erythrocytes) develop signs and symptoms of cerebral malaria on day 6 (rarely day 7) of infection; the mice become lethargic, lose their righting and gripping reflexes, and die hours later. The majority of the mice on day 6 of P. berghei infection have cracks in their skulls, suggesting that severe brain swelling occurs. On average, about one mouse per experiment is excluded because of inadequate parasitemia. Most investigators believe that inadequate infection of mice is due to injection of the parasite inoculum into an area outside the peritoneal cavity, such as the bladder. Inadequately infected mice are mice with levels of parasitemia of <0.5% on day 4 of infection and are excluded from analysis; these mice generally succumb on day 8 or 9 of infection. Mice fail to gain weight during the infection and exhibit significant (P < 0.05) weight loss by day 6 of infection.
The P. berghei model is therefore highly reproducible. However, there are subtle differences in disease presentation from experiment to experiment. In all cases, we observed significant damage in both brains and lungs, but in some experiments damage appeared to be greater in the lungs than in the brains, whereas in other experiments the converse was true.
Clinical and pathological signs of circulatory shock occur during P. berghei infection.
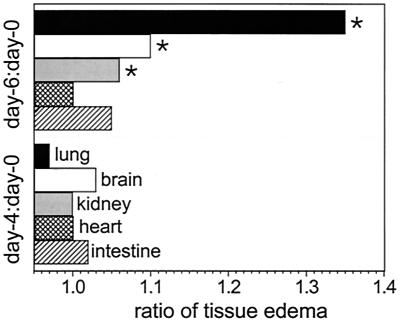
To verify that the observed increase in vascular permeability led to tissue edema (which also occurs during septic shock), we determined the ratios of wet weight to dry weight for selected organs during P. berghei infection and evaluated other parameters, such as histology and whether the skull was intact. Brains, lungs, and kidneys, but not hearts and intestines, contained significantly (P < 0.05) greater amounts of fluid on day 6 in P. berghei-infected mice than in uninfected controls (Fig. 1). The P values were 0.001, 0.0001, and 0.04 for brains, lungs, and kidneys, respectively, and 0.95 and 0.49 for hearts and intestines, respectively. About 50% of the mice on day 6 of P. berghei infection had cracked skulls, and the extents of the cracks were related to the degrees of brain edema and neurological complications (loss of righting and gripping reflexes). Microscopic evaluation of hematoxylin- and eosin-stained sections of lungs obtained from P. berghei-infected mice on day 6 of infection revealed marked infiltration of mononuclear cells and granulocytes into alveolar space and walls and thickening of alveolar walls. Severe hemorrhaging and moderate congestion were observed in lungs of infected animals. In addition, there were modest amounts of pink-stained alveolar spaces, which is characteristic of lung edema.
FIG. 1.
Tissue edema during P. berghei malaria. The ratios of wet weight to dry weight for selected tissues were determined at zero time and on days 4 and 6 of infection for groups of five mice. The ratios for days 4 and 6 were divided by ratios for zero time. An asterisk indicates statistical significance (P < 0.05) for a comparison of a group of infected mice and uninfected controls.
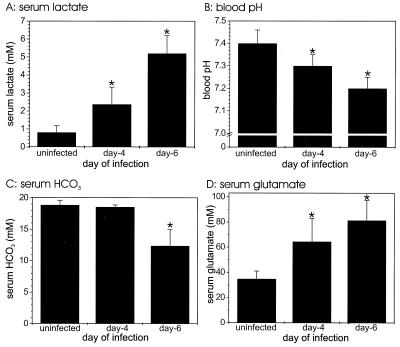
To determine whether P. berghei-infected mice develop acidosis, a clinical sign of circulatory shock, we assessed serum lactate contents and pH values for groups of C57BL/6 mice during P. berghei infection. The levels of serum lactate in infected mice were significantly (P < 0.05) elevated on day 4 of infection compared with the levels in uninfected controls, and the levels increased even more by day 6 (P = 0.04 and P < 0.0001, respectively) (Fig. 2A). The levels of serum lactate in mice on day 6 of infection were about five times higher than the levels in uninfected controls. The pH of serum declined markedly during P. berghei malaria, and mice were acidotic (pH < 7.3) on days 4 and 6 of infection (P = 0.03 and P < 0.0001, respectively) (Fig. 2B). The levels of serum HCO3−, which buffers the blood at pH 7.4, declined significantly (P < 0.0001) by day 6 of infection compared with the levels in uninfected controls (Fig. 2C).
FIG. 2.
Acidosis of blood during P. berghei malaria. Serum lactate (A), HCO3− (C), and glutamate (D) levels were measured for groups of five mice at zero time and on days 4 and 6 of P. berghei malaria. The blood pH (B) was measured in a separate set of experimental animals. Similar results were obtained in duplicate experiments. In addition, the results for controls used in the experiments whose results are shown in Fig. 5 were similar. An asterisk indicates statistical significance (P < 0.05) for a comparison of a group of infected mice and uninfected controls.
To determine whether glutamate levels in serum were high and possibly inhibited production of HCO3− by the kidneys, we determined the levels of selected amino acids during P. berghei malaria. Significantly elevated levels of glutamate were detected in plasma on days 4 and 6 of infection compared with the levels in uninfected controls (P = 0.008 and P = 0.0006, respectively) (Fig. 2D). No significant changes in the serum levels of glycine, aspartate, and alanine were detected during P. berghei malaria. However, the levels of glutamate and aspartate in the erythrocytes were significantly (P < 0.05) higher during P. berghei malaria; the glutamate concentrations at zero time and on days 4 and 6 were 97 ± 20, 256 ± 46, and 239 ± 57 mM, respectively, and the aspartate concentrations at these times were 79 ± 17, 201 ± 65, and 238 ± 3 mM, respectively. These findings suggested that there was active transport into the infected erythrocytes.
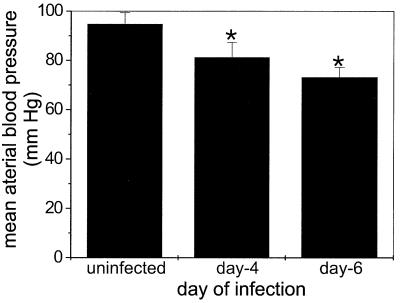
To investigate whether increased vascular permeability and tissue edema caused the blood pressure to fall during P. berghei malaria (which also occurs during septic shock), we measured the blood pressure of infected C57BL/6 mice. The mean arterial blood pressure decreased significantly (P < 0.05) on day 4 of P. berghei infection compared with the mean arterial blood pressure of uninfected controls and fell further by day 6 (Fig. 3).
FIG. 3.
Mean arterial blood pressure during P. berghei malaria. Mean arterial blood pressure was measured for groups of five mice at zero time and on days 4 and 6 of P. berghei malaria. Similar results were obtained a duplicate experiment. An asterisk indicates statistical significance (P < 0.05) for a comparison of a group of infected mice and uninfected controls.
Clinical symptoms of circulatory shock during P. berghei malaria are markedly reduced after depletion of CD8+ T cells.
CD8-depleted mice did not exhibit any neurological signs of cerebral malaria (such as loss of the righting reflex or the ability to grip) and were not lethargic, whereas rat IgG-treated controls showed neurological signs of cerebral malaria and were lethargic. The CD8-depleted mice were adequately infected because the parasitemia in anti-CD8 mAb-treated mice was similar than that in rat IgG-treated controls. Therefore, the two groups of mice had similar clinical symptoms of uncomplicated malaria (i.e., fever and anemia). In each of the experiments with CD8-depleted mice, we determined the percentage of CD3+ CD8+ T cells in the peripheral blood by flow cytometry on day 4 of infection and performed an experimental analysis on day 6. Few, if any, CD3+ CD8+ cells (0.0 ± 0.0%) were detected by flow cytometry in the anti-CD8 mAb-treated mice, whereas more than 8% of the peripheral blood leukocytes in rat IgG-treated mice were labeled with anti-CD3 and anti-CD8 mAbs. The percentages of CD8+ T cells in peripheral blood were similar for infected rat IgG-treated mice and uninfected controls.
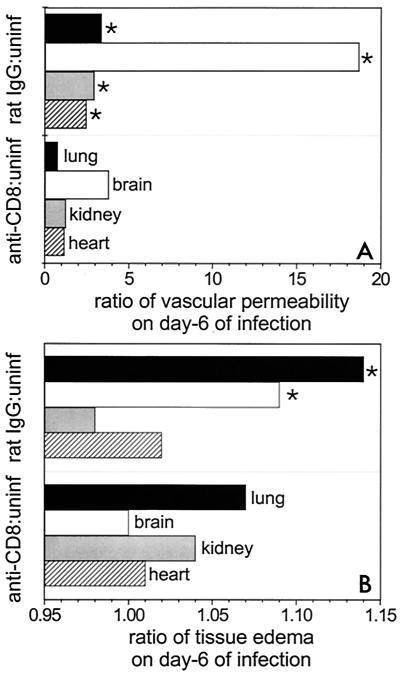
To determine whether CD8+ T cells in the tissues actually contribute to the increased vascular permeability during experimental malaria, we measured vascular permeability by the Evans Blue dye leakage technique on day 6 of P. berghei infection of CD8-depleted mice. There was a marked, significant (P < 0.05) reduction in vascular permeability in the brains, lungs, kidneys, and hearts of anti-CD8 mAb-treated mice with P. berghei malaria compared with the vascular permeability in infected rat IgG-treated controls (Fig. 4A). The P values were 0.0001, <0.0001, 0.002, and 0.0009 for the brains, lungs, hearts, and kidneys, respectively. There was marked tissue edema in lungs and brains of rat IgG-treated controls on day 6 of P. berghei infection (Fig. 4B). However, CD8 depletion significantly (P < 0.05) protected against edema in brains, lungs, and kidneys during P. berghei malaria compared with the results obtained for rat IgG-treated and infected controls; the P values were <0.0001 and 0.01 for brains and lungs, respectively, and 0.07, 0.8, and 0.6 for kidneys, hearts, and intestines, respectively. None of the anti-CD8 mAb-treated mice infected with P. berghei showed signs of cracked skulls, whereas two of five infected rat IgG-treated mice had cracked skulls. In some experiments vascular permeability appeared to predominate in lungs, whereas in other experiments changes in brains predominated (Fig. 1 and 4).
FIG. 4.
Ratios of vascular permeability (A) and tissue edema (B) in CD8+ T-cell-depleted mice (anti-CD8) infected with P. berghei malaria to vascular permeability and tissue edema in uninfected controls (uninf). These ratios are compared with the ratios of vascular permeability (A) and tissue edema (B) in rat IgG-treated and infected mice (rat IgG) to vascular permeability and tissue edema in uninfected controls. Vascular permeability was determined by the Evans Blue technique for selected tissues from groups of eight mice. The ratios of wet weight to dry weight for selected tissues were determined on day 6 of infection for groups of five mice. The vascular permeability or ratio of wet weight to dry weight for infected groups of animals (anti-CD8 mAb and rat IgG mAb treated) were divided by values for uninfected animals. Similar results were obtained in replicate experiments for vascular permeability and in duplicate experiments for tissue edema. An asterisk indicates statistical significance (P < 0.05) for a comparison of a group of infected CD8-depleted mice and infected rat IgG-treated controls.
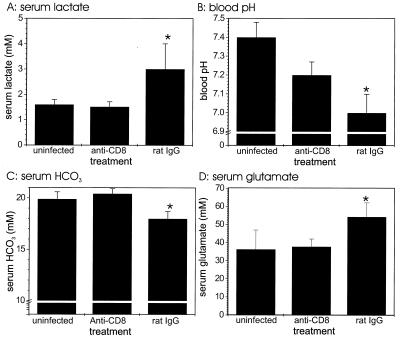
To determine whether CD8+ T cells actually contribute to pathogenesis of circulatory shock during experimental malaria, we assessed acidosis in P. berghei-infected mice depleted of CD8+ T cells by mAb treatment. The serum lactate, HCO3−, and glutamate contents of infected CD8-depleted mice were significantly (P < 0.05) decreased compared with the contents of infected rat IgG-injected controls (Fig. 5A, C, and D); the P values were 0.005, 0.0002, and 0.01 for lactate, HCO3−, and glutamate levels, respectively. The blood pH (Fig. 5B) was significantly (P = 0.01) higher in CD8-depleted mice infected with P. berghei than in infected rat IgG-treated controls.
FIG. 5.
Acidosis of blood in CD8+ T-cell-depleted mice infected with P. berghei malaria. Serum lactate (A), HCO3− (C), and glutamate (D) levels were measured in groups of four mice at zero time and on day 6 of P. berghei malaria. The groups of infected mice were treated with either anti-CD8 mAb or control rat IgG. The blood pH (B) was measured with a separate set of experimental animals. Two of five mice in the control rat IgG-injected group died on day 6 of infection before blood was obtained for pH measurement, so the pH values are averages based on the data for three mice. Similar results were obtained in a duplicate experiment. An asterisk indicates statistical significance (P < 0.05) for a comparison of a group of infected mice and uninfected controls.
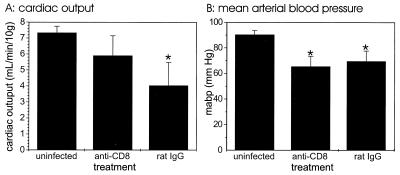
To determine whether the observed changes in mean arterial blood pressure were also dependent on CD8+ T cells, we assessed blood pressure on day 6 of infection in C57BL/6 mice depleted of CD8+ T cells by mAb treatment. CD8-depleted mice infected with P. berghei had a mean arterial blood pressure similar to that of infected rat IgG-treated mice, and both of the values were lower than the value obtained for the uninfected controls (Fig. 6). However, cardiac output was significantly (P < 0.05) lower in infected rat IgG-treated mice than in either infected anti-CD8 mAb-treated mice (P = 0.02) or uninfected controls (P = 0.0005). Cardiac output was slightly lower in infected CD8-depleted mice than in uninfected mice, but the difference was not statistically significant.
FIG. 6.
Cardiac output (A) and mean arterial blood pressure (B) in CD8+ T-cell-depleted mice infected with P. berghei. The mean arterial blood pressure was measured for groups of five mice at zero time and on day 6 of P. berghei malaria. The groups of infected mice were treated with either anti-CD8 mAb or control rat IgG. This experiment was performed once. An asterisk indicates statistical significance (P < 0.05) for a comparison of a group of infected mice and uninfected controls.
DISCUSSION
Our studies were designed to examine the hypothesis that complications that occur during P. berghei malaria in mice are consistent with circulatory shock. Unlike most studies of P. berghei malaria, which focus exclusively on changes in the brain, in our study we examined tissues that are damaged in humans with P. falciparum malaria to develop a complete picture of malarial pathogenesis. Our observation that vascular leakage occurs in the brains of mice with P. berghei malaria prior to the onset of symptoms confirms the results of similar studies (5, 40). The finding that breakdown of the blood-brain barrier occurs prior to the onset of symptoms is important because it shows that vascular leakage occurs not merely because an animal is moribund and tissue integrity is failing. Rather, the breakdown of the blood-brain barrier may contribute to the animal's demise. Increased vascular leakage was also observed in lungs, hearts, and kidneys. This result confirmed our previous findings obtained by using Evans Blue dye leakage and a radiolabeled mAb technique (41). It also agrees in large part with the results of semiquantitative Monastral Blue studies in which increased vascular permeability was observed in lungs, brains, hearts, and kidneys (33); however, Neill and Hunt also observed increased permeability in the liver and spleen, which are blood filtration organs with little, if any, permeability barrier. Endothelial cells actively transport Monastral Blue across the endothelium, and there is no relationship between hemorrhage and extrusion of Monastral Blue into tissue, suggesting that Monastral Blue extrusion does not always reflect vascular leakage. Collectively, the results indicate that vascular permeability increases markedly during P. berghei malaria in several organs besides the brain.
One consequence of increased vascular permeability is tissue edema. An increase in the ratio of wet weight to dry weight is a well-recognized indication of tissue edema. We observed significant (P < 0.05) increases in the ratios of wet weight to dry weight in the lungs and brains of P. berghei-infected mice compared with the ratios for uninfected controls; this finding indicates that tissue edema occurs during experimental malaria. Even in our genetically controlled (both parasite and mouse) model of malaria, we observed striking increases in brain permeability in some experiments (Fig. 1), striking increases in lung permeability in other experiments (Fig. 4), and equal increases in brain permeability and in lung permeability in still other experiments. This reflects the situation in humans; some patients with P. falciparum develop mainly cerebral malaria, other patients develop respiratory distress, and most patients develop a combination of cerebral malaria and respiratory distress (29).
Increased vascular permeability and tissue edema contribute to poor tissue perfusion, resulting in buildup of waste products in the blood. The increased lung vascular permeability and lung edema interfere with gas exchange in P. berghei-infected mice compared with gas exchange in uninfected controls. When tissue oxygenation is poor, a switch to glycolysis occurs, and this results in lactic acid production, tissue acidosis, and a drop in the extracellular pH. The malarial parasite also uses glycolysis, and its production of lactic acid may exacerbate the acidosis. Indeed, the lactic acid concentration in serum increases markedly during P. berghei malaria compared with the serum lactic acid concentration in uninfected controls. Acidosis (pH < 7.3), which occurs on day 4 of P. berghei infection, when the animals appear healthy, is usually prevented by increasing the amount of serum HCO3−. Our data indicate that malaria results in increased serum glutamate levels. The levels of glutamate observed during malaria should inhibit HCO3− production by the kidneys (4), resulting in the observed decline in serum HCO3− levels on day 6 of infection. The high levels of glutamate may also contribute to neurological complications of malaria because glutamate has profound effects on the central nervous system. Collectively, the changes indicate that significant (P < 0.05) metabolic derangements occur with experimental malaria, possibly as a result of initial changes in vascular permeability.
The complications of shock observed in P. berghei-infected mice are similar to those of septic shock. In both diseases, there is multiple organ failure (particularly lung, brain, kidney, and heart failure) and respiratory distress with lactic acidosis. Our data showing (i) increased vascular leakage and edema in the lungs and (ii) histological changes with infiltrating cells, alveolar wall thickening, congestion, and fluid in alveolar space support the contention that respiratory distress occurs during P. berghei malaria in mice. This respiratory distress exacerbates metabolic complications and contributes to the vicious cycle during malarial pathogenesis and septic shock. As in septic shock, hemodynamic changes also occur during P. berghei malaria. The observed decreases in mean arterial blood pressure and cardiac output on day 6 of P. berghei infection probably exacerbate the metabolic complications, thereby creating positive feedback and leading ultimately to death of the animal. These findings collectively indicate that mice infected with P. berghei are in circulatory shock, which resembles septic shock.
There are reports of shock occurring in patients with severe P. falciparum malaria, and the World Health Organization lists shock as a poor prognostic indicator for malaria (3, 23). There are also numerous parallels between complications in patients with severe malaria and complications in patients with septic shock, including a prominent role of TNF in pathogenesis (21). While some workers report no breakdown in the blood-brain barrier in humans with cerebral malaria (2, 26), other workers have observed increased blood-brain permeability which was associated with poor outcome (34, 35). In addition, retinal hemorrhages are poor prognostic factors and are believed to reflect petechial hemorrhaging into the brain (5, 25). Lactic acidosis and shock are other poor prognostic factors for severe P. falciparum malaria (22). Thus, we propose that circulatory shock mediates damage in the brains and lungs of some patients with severe P. falciparum malaria. Consequently, P. berghei infection of mice is a model for severe P. falciparum malaria and should allow researchers to study malarial shock.
The concept that malarial pathogenesis is circulatory shock actually unifies the mechanical hypothesis and the inflammatory hypothesis. For example, minithrombi occur in the late stages of septic shock. These minithrombi may be analogous to erythrocyte rosetting during P. falciparum malaria and congestion in cerebral blood vessels. The immune response directed at the parasite may cause the vascular leakage and edema that are observed and contribute to the circulatory complications of malaria, which is the basis for the inflammatory hypothesis.
To test whether the immune response contributes to the circulatory complications of malaria, we assessed these complications in CD8 T-cell-depleted mice. We selected CD8+ T lymphocytes because this T-cell subset is required for death due to experimental cerebral malaria (16, 44). Mice depleted of CD8+ T cells by mAb during P. berghei malaria exhibit (i) attenuated vascular permeability and edema in their lungs and brains, (ii) diminished lactic acidosis, and (iii) no increase in serum glutamate levels compared with the levels in rat IgG-treated and infected controls. CD8-depleted mice infected with P. berghei also exhibit significantly (P < 0.05) lower cardiac output than rat IgG-treated and infected controls. Collectively, these findings indicate that CD8-depleted mice exhibit markedly ameliorated circulatory shock compared with the circulatory shock of rat IgG-treated controls. The pathogenic mechanism(s) mediating cerebral malaria and respiratory distress may in fact be similar because depletion of CD8+ T cells protects against vascular permeability changes and edema in both brains and lungs.
The reemergence of P. falciparum, which causes severe malaria, and the development of drug resistance by the parasite highlight the need to develop new ways of treating malaria. To develop new treatments for patients with severe malaria, we need to better understand its pathogenesis. Our observations indicate that P. berghei-infected mice develop circulatory shock, which may be the underlying mechanism for both cerebral malaria and respiratory distress with acidosis. The immune system (specifically CD8+ T cells) contributes to the development of these complications. If our concept of circulatory shock underlying malarial pathogenesis is validated after analysis of patients with severe P. falciparum malaria, then novel treatment modalities may be developed due to an improved understanding of malarial pathogenesis. Thus, we predict that treatments for septic shock should also be effective in blunting the severe complications of malaria.
ACKNOWLEDGMENTS
This research was supported by NIH grants KO8 AI01438(to W.-L. Chang), RO1 HL60849 (to D. J. Lefer), PO1 DK43785 (to D. N. Granger and D. J. Lefer), and RO1 AI40667 (to H. C. van der Heyde).
We thank Philippe Bauer for his assistance with lactic acid and bicarbonate measurements.
REFERENCES
- 1.Aikawa M, Iseki M, Barnwell J W, Taylor D, Oo M M, Howard R J. The pathology of human cerebral malaria. Am J Trop Med Hyg. 1990;43:30–37. doi: 10.4269/ajtmh.1990.43.30. [DOI] [PubMed] [Google Scholar]
- 2.Brown H C, Chau T T, Mai N T, Day N P, Sinh D X, White N J, Hien T T, Farrar J, Turner G D. Blood-brain barrier function in cerebral malaria and CNS infections in Vietnam. Neurology. 2000;55:104–111. doi: 10.1212/wnl.55.1.104. [DOI] [PubMed] [Google Scholar]
- 3.Bruneel F, Gachot B, Timsit J F, Wolff M, Bedos J P, Regnier B, Vachon F. Shock complicating severe falciparum malaria in European adults. Intensive Care Med. 1997;23:698–701. doi: 10.1007/s001340050396. [DOI] [PubMed] [Google Scholar]
- 4.Carter P, Welbourne T. Glutamate transport regulation of renal glutaminase flux in vivo. Am J Physiol. 1997;273:E521–E527. doi: 10.1152/ajpendo.1997.273.3.E521. [DOI] [PubMed] [Google Scholar]
- 5.Chang-Ling T, Neill A L, Hunt N H. Early microvascular changes in murine cerebral malaria detected in retinal whole mounts. Am J Pathol. 1992;140:1121–1130. [PMC free article] [PubMed] [Google Scholar]
- 6.Clark I A, al Yaman F M, Jacobson L S. The biological basis of malarial disease. Int J Parasitol. 1997;27:1237–1249. doi: 10.1016/s0020-7519(97)00121-5. [DOI] [PubMed] [Google Scholar]
- 7.de Kossodo S, Grau G E. Role of cytokines and adhesion molecules in malaria immunopathology. Stem Cells. 1993;11:41–48. doi: 10.1002/stem.5530110108. [DOI] [PubMed] [Google Scholar]
- 8.Eling W M, Kremsner P G. Cytokines in malaria, pathology and protection. Biotherapy. 1994;7:211–221. doi: 10.1007/BF01878487. [DOI] [PubMed] [Google Scholar]
- 9.Favre N, Da Laperousaz C, Ryffel B, Weiss N A, Imhof B A, Rudin W, Lucas R, Piguet P F. Role of ICAM-1 (CD54) in the development of murine cerebral malaria. Microbes Infect. 1999;1:961–968. doi: 10.1016/s1286-4579(99)80513-9. [DOI] [PubMed] [Google Scholar]
- 10.Finley R W, Mackey L J, Lambert P H. Virulent P. berghei malaria: prolonged survival and decreased cerebral pathology in cell-dependent nude mice. J Immunol. 1982;129:2213–2218. [PubMed] [Google Scholar]
- 11.Grau G E, Piguet P F, Engers H D, Louis J A, Vassalli P, Lambert P H. L3T4+ T lymphocytes play a major role in the pathogenesis of murine cerebral malaria. J Immunol. 1986;137:2348–2354. [PubMed] [Google Scholar]
- 12.Grau G E, Piguet P F, Vassalli P, Lambert P H. Involvement of tumour necrosis factor and other cytokines in immune-mediated vascular pathology. Int Arch Allergy Appl Immunol. 1989;88:34–39. doi: 10.1159/000234744. [DOI] [PubMed] [Google Scholar]
- 13.Grau G E, Pointaire P, Piguet P F, Vesin C, Rosen H, Stamenkovic I, Takei F, Vassalli P. Late administration of monoclonal antibody to leukocyte function-antigen 1 abrogates incipient murine cerebral malaria. Eur J Immunol. 1991;21:2265–2267. doi: 10.1002/eji.1830210939. [DOI] [PubMed] [Google Scholar]
- 14.Guyton A C, Hall J E. Circulatory shock and physiology of its treatment. In: Guyton A C, Hall J E, editors. Textbook of medical physiology. W. B. Philadelphia, Pa: Saunders Company; 1996. pp. 285–293. [Google Scholar]
- 15.Hearn J, Rayment N, Landon D N, Katz D R, de Souza J B. Immunopathology of cerebral malaria: morphological evidence of parasite sequestration in murine brain microvasculature. Infect Immun. 2000;68:5364–5376. doi: 10.1128/iai.68.9.5364-5376.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hermsen C, van de Wiel T, Mommers E, Sauerwein R, Eling W. Depletion of CD4+ or CD8+ T-cells prevents Plasmodium berghei induced cerebral malaria in end-stage disease. Parasitology. 1997;114:7–12. doi: 10.1017/s0031182096008293. [DOI] [PubMed] [Google Scholar]
- 17.Hoffmann E J, Weidanz W P, Long C A. Susceptibility of CXB recombinant inbred mice to murine plasmodia. Infect Immun. 1984;43:981–985. doi: 10.1128/iai.43.3.981-985.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoffmeyer M R, Jones S P, Ross C R, Sharp B, Grisham M B, Laroux F S, Stalker T J, Scalia R, Lefer D J. Myocardial ischemia/reperfusion injury in NADPH oxidase-deficient mice. Circ Res. 2000;87:812–817. doi: 10.1161/01.res.87.9.812. [DOI] [PubMed] [Google Scholar]
- 19.Kaul D K, Liu X D, Nagel R L, Shear H L. Microvascular hemodynamics and in vivo evidence for the role of intercellular adhesion molecule-1 in the sequestration of infected red blood cells in a mouse model of lethal malaria. Am J Trop Med Hyg. 1998;58:240–247. doi: 10.4269/ajtmh.1998.58.240. [DOI] [PubMed] [Google Scholar]
- 20.Kaul D K, Nagel R L, Llena J F, Shear H L. Cerebral malaria in mice: demonstration of cytoadherence of infected red blood cells and microrheologic correlates. Am J Trop Med Hyg. 1994;50:512–521. doi: 10.4269/ajtmh.1994.50.512. [DOI] [PubMed] [Google Scholar]
- 21.Knight J C, Udalova I, Hill A V, Greenwood B M, Peshu N, Marsh K, Kwiatkowski D. A polymorphism that affects OCT-1 binding to the TNF promoter region is associated with severe malaria. Nat Genet. 1999;22:145–150. doi: 10.1038/9649. [DOI] [PubMed] [Google Scholar]
- 22.Krishna S, Waller D W, ter Kuile F, Kwiatkowski D, Crawley J, Craddock C F, Nosten F, Chapman D, Brewster D, Holloway P A. Lactic acidosis and hypoglycaemia in children with severe malaria: pathophysiological and prognostic significance. Trans R Soc Trop Med Hyg. 1994;88:67–73. doi: 10.1016/0035-9203(94)90504-5. [DOI] [PubMed] [Google Scholar]
- 23.Lagudis S, Camargo L F, Meyer E C, Fernandes C J, Akamine N, Knobel E. Hyperdynamic shock in falciparum malaria. Intensive Care Med. 2000;26:142. doi: 10.1007/s001340050029. [DOI] [PubMed] [Google Scholar]
- 24.Ledbetter J A, Rouse R V, Micklem H S, Herzenberg L A. T cell subsets defined by expression of Lyt-1:2,3 and Thy-1 antigens. Two-parameter immunofluorescence and cytotoxicity analysis with monoclonal antibodies modifies current views. J Exp Med. 1980;152:280–295. doi: 10.1084/jem.152.2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Looareesuwan S, Warrell D A, White N J, Chanthavanich P, Warrell M J, Chantaratherakitti S, Changswek S, Chongmankongcheep L, Kanchanaranya C. Retinal hemorrhage, a common sign of prognostic significance in cerebral malaria. Am J Trop Med Hyg. 1983;32:911–915. doi: 10.4269/ajtmh.1983.32.911. [DOI] [PubMed] [Google Scholar]
- 26.Looareesuwan S, Warrell D A, White N J, Sutharasamai P, Chanthavanich P, Sundaravej K, Juel-Jensen B E, Bunnag D, Harinasuta T. Do patients with cerebral malaria have cerebral oedema? A computed tomography study. Lancet. 1983;i:434–437. doi: 10.1016/s0140-6736(83)91437-x. [DOI] [PubMed] [Google Scholar]
- 27.Lucas R, Lou J N, Juillard P, Moore M, Bluethmann H, Grau G E. Respective role of TNF receptors in the development of experimental cerebral malaria. J Neuroimmunol. 1997;72:143–148. doi: 10.1016/s0165-5728(96)00185-3. [DOI] [PubMed] [Google Scholar]
- 28.Mackey L J, Hochmann A, June C H, Contreras C E, Lambert P H. Immunopathological aspects of Plasmodium berghei infection in five strains of mice. II. Immunopathology of cerebral and other tissue lesions during the infection. Clin Exp Immunol. 1980;42:412–420. [PMC free article] [PubMed] [Google Scholar]
- 29.Marsh K, Forster D, Waruiru C, Mwangi I, Winstanley M, Marsh V, Newton C, Winstanley P, Warn P, Peshu N. Indicators of life-threatening malaria in African children. N Engl J Med. 1995;332:1399–1404. doi: 10.1056/NEJM199505253322102. [DOI] [PubMed] [Google Scholar]
- 30.McGuire W, Hill A V, Allsopp C E, Greenwood B M, Kwiatkowski D. Variation in the TNF-alpha promoter region associated with susceptibility to cerebral malaria. Nature. 1994;371:508–510. doi: 10.1038/371508a0. [DOI] [PubMed] [Google Scholar]
- 31.Miller L H, Good M F, Milon G. Malaria pathogenesis. Science. 1994;264:1878–1883. doi: 10.1126/science.8009217. [DOI] [PubMed] [Google Scholar]
- 32.Natelson S. Routine use of ultramicro methods in the clinical laboratory. Am J Pathol. 1951;21:1153–1158. [PubMed] [Google Scholar]
- 33.Neill A L, Hunt N H. Pathology of fatal and resolving Plasmodium berghei cerebral malaria in mice. Parasitology. 1992;105:165–175. doi: 10.1017/s0031182000074072. [DOI] [PubMed] [Google Scholar]
- 34.Newton C R, Crawley J, Sowumni A, Waruiru C, Mwangi I, English M, Murphy S, Winstanley P A, Marsh K, Kirkham F J. Intracranial hypertension in Africans with cerebral malaria. Arch Dis Child. 1997;76:219–226. doi: 10.1136/adc.76.3.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Newton C R, Peshu N, Kendall B, Kirkham F J, Sowunmi A, Waruiru C, Mwangi I, Murphy S A, Marsh K. Brain swelling and ischaemia in Kenyans with cerebral malaria. Arch Dis Child. 1994;70:281–287. doi: 10.1136/adc.70.4.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rest J R. Cerebral malaria in inbred mice. I. A new model and its pathology. Trans R Soc Trop Med Hyg. 1982;76:410–415. doi: 10.1016/0035-9203(82)90203-6. [DOI] [PubMed] [Google Scholar]
- 37.Schiller N B, Shah P M, Crawford M, DeMaria A, Devereux R, Feigenbaum H, Gutgesell H, Reichek N, Sahn D, Schnittger I. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2:358–367. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 38.Shear H L, Marino M W, Wanidworanun C, Berman J W, Nagel R L. Correlation of increased expression of intercellular adhesion molecule-1, but not high levels of tumor necrosis factor-alpha, with lethality of Plasmodium yoelii 17XL, a rodent model of cerebral malaria. Am J Trop Med Hyg. 1998;59:852–858. doi: 10.4269/ajtmh.1998.59.852. [DOI] [PubMed] [Google Scholar]
- 39.Tateishi H, Mitsuyama K, Toyonaga A, Tomoyose M, Tanikawa K. Role of cytokines in experimental colitis: relation to intestinal permeability. Digestion. 1997;58:271–281. doi: 10.1159/000201454. [DOI] [PubMed] [Google Scholar]
- 40.Thumwood C M, Hunt N H, Clark I A, Cowden W B. Breakdown of the blood-brain barrier in murine cerebral malaria. Parasitology. 1988;96:579–589. doi: 10.1017/s0031182000080203. [DOI] [PubMed] [Google Scholar]
- 41.van der Heyde H C, Bauer P, Sun G, Chang W L, Yin L, Fuseler J W, Granger D N. Assessing vascular permeability during experimental cerebral malaria by a radiolabeled monoclonal antibody technique. Infect Immun. 2001;69:3460–3465. doi: 10.1128/IAI.69.5.3460-3465.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van der Heyde H C, Manning D D, Weidanz W P. Role of CD4+ T cells in the expansion of the CD4-, CD8- gamma delta T cell subset in the spleens of mice during blood-stage malaria. J Immunol. 1993;151:6311–6317. [PubMed] [Google Scholar]
- 43.von Andrian U H, Mackay C R. T-cell function and migration. Two sides of the same coin. N Engl J Med. 2000;343:1020–1034. doi: 10.1056/NEJM200010053431407. [DOI] [PubMed] [Google Scholar]
- 44.Yanez D M, Manning D D, Cooley A J, Weidanz W P, van der Heyde H C. Participation of lymphocyte subpopulations in the pathogenesis of experimental murine cerebral malaria. J Immunol. 1996;157:1620–1624. [PubMed] [Google Scholar]
- 45.Young L S. Sepsis syndrome. In: Mandel G L, Bennett J E, Dolin R, editors. Principles and practice of infectious diseases. Philadelphia, Pa: Churchill Livingstone; 2000. pp. 806–820. [Google Scholar]