Abstract
Locomotor movements cause visual images to be displaced across the eye, a retinal slip that is counteracted by stabilizing reflexes in many animals. In insects, optomotor turning causes the animal to turn in the direction of rotating visual stimuli, thereby reducing retinal slip and stabilizing trajectories through the world. This behavior has formed the basis for extensive dissections of motion vision. Here, we report that under certain stimulus conditions, two Drosophila species, including the widely studied D. melanogaster, can suppress and even reverse the optomotor turning response over several seconds. Such ‘anti-directional turning’ is most strongly evoked by long-lasting, high-contrast, slow-moving visual stimuli that are distinct from those that promote syn-directional optomotor turning. Anti-directional turning, like the syn-directional optomotor response, requires the local motion detecting neurons T4 and T5; a subset of lobula plate tangential cells, CH cells, show involvement in these responses. Imaging from a variety of direction-selective cells in the lobula plate shows no evidence of dynamics that match the behavior, suggesting that the observed inversion in turning direction emerges downstream of the lobula plate. Further, anti-directional turning declines with age and exposure to light. These results show that Drosophila optomotor turning behaviors contain rich, stimulus-dependent dynamics that are inconsistent with simple reflexive stabilization responses.
Intro
Visual navigation requires active mechanisms to stabilize trajectories through the world. Insects exhibit an optomotor turning response, a behavior in which they rotate their bodies in the direction of visual patterns that rotate about them (Buchner, 1976; Götz and Wenking, 1973; Hassenstein and Reichardt, 1956). This behavior is analogous to optomotor turning responses in fish (Clark, 1981) and the optokinetic response in mammals (Koerner and Schiller, 1972). In insects, this response is thought to be a course-stabilization mechanism that minimizes retinal slip, allowing animals to maintain their trajectory in the face of external or unexpected rotational forces (Götz and Wenking, 1973; Götz, 1975). For instance, if an insect attempts to walk in a straight line, it may slip and turn to the right. From the point of view of the insect, this turn is observed as optic flow rotating to the left. By responding to this leftward optic flow with a leftward turn, the insect can recover its original trajectory. The optokinetic response, similarly, acts to stabilize eye position relative to the visual scene, even as the head rotates (Schweigart et al., 1997).
In fruit flies, the optomotor response relies on well-characterized circuitry (Yang and Clandinin, 2018). Photoreceptor signals are split into parallel ON and OFF pathways in the lamina and medulla (Behnia et al., 2014; Clark et al., 2011; Joesch et al., 2010; Strother et al., 2014), that are not direction-selective. These signals provide input to T4 and T5 cells, which compute direction-selective responses along four directions for every point in the fly visual field (Bausenwein et al., 1992; Henning et al., 2022; Maisak et al., 2013; Shinomiya et al., 2019; Takemura et al., 2013). The outputs of T4 and T5 cells are then summed across visual space by lobula plate tangential cells (LPTCs) (Barnhart et al., 2018; Joesch et al., 2008; Maisak et al., 2013; Mauss et al., 2015; Schnell et al., 2012). Different LPTCs provide distinct signals about the overall pattern of motion surrounding the fly, and have been linked to head and body movements (Haikala et al., 2013; Kim et al., 2017; Krapp and Hengstenberg, 1996).
Interestingly, there have been several reports of flies turning in the direction opposite to what would be predicted from the optomotor turning response. In some cases, these counter-intuitive behaviors were observed using periodic stimuli with spatial wavelengths smaller than the receptive field of individual ommatidia, and thus can be accounted for by aliasing (Buchner, 1976; Götz, 1964; Götz, 1970). Work in a tethered flight simulator showed that when a moving pattern is presented in front of the fly, the animal turned in the direction of the stimulus motion (Tammero et al., 2004), as expected (Goetz, 1968). However, if the moving pattern was presented behind the fly, it attempted to turn in the direction opposite to stimulus motion (Tammero et al., 2004). In a different experimental preparation, rotational patterns were presented on a dome around freely-walking flies (Williamson et al., 2018). Under these conditions, flies generally turned in the direction of motion of the stimulus, but these rotations were often punctuated by brief, large-magnitude saccades in the opposite direction. Similarly, experiments using flight simulators have reported spikes in the torque in the direction opposite the stimulus rotation (Wolf and Heisenberg, 1990).
Here we show that rotational stimuli can elicit strong, consistent anti-directional behavior in two drosophilid species, D. melanogaster and D. yakuba. We report that flies respond to high contrast, high luminance rotational motion stimuli by first turning in the direction of stimulus motion, and then reversing their trajectory after approximately one second, depending on the species. In Drosophila melanogaster, we characterize the dynamics of this behavior and the stimuli that drive it. The behavior depends critically on adaptation to back-to-front motion. We use the genetic tools available in Drosophila melanogaster to show that this behavior relies on the motion detecting neurons T4 and T5. Silencing HS and CH, two widefield neurons downstream of T4 and T5, resulted in small changes in this complex turning behavior. However, the visually evoked responses of these direction-selective neurons could not account for these behaviors. Thus, behavioral reversal must be mediated by more downstream circuitry. Overall, these results show that flies generate behavioral signals that oppose the direction of visual motion, showing that Drosophila turning responses to wide-field visual motion stimuli are more complex than a simple stabilizing reflex.
Results
Anti-directional turning responses to high contrast stimuli
Optomotor turning responses are central for gaze stabilization, so we sought to examine the stability of this response across different conditions. Many studies have investigated this behavior using low contrast and low light intensity stimuli or both (Bahl et al., 2013; Bosch et al., 2015; Buchner, 1976; Götz and Wenking, 1973; Rister et al., 2007; Seelig et al., 2010), at a variety of different speeds. However, natural scenes can have relatively high contrast and luminance, conditions have been poorly explored in the laboratory. In this experiment, we presented flies with rotational stimuli using high contrast and relatively high luminance.
We tethered individual female D. melanogaster above a freely rotating ball to characterize the optomotor response (Buchner, 1976; Creamer et al., 2019) (Fig. 1a). As expected, low contrast, slow-moving sinusoidal gratings caused flies to turn in the same direction as the moving gratings via the classical optomotor turning response (Fig. 1b) (Bahl et al., 2013; Bahl et al., 2015; Buchner, 1976; Clark et al., 2011; Clark et al., 2014; Creamer et al., 2018; Götz, 1964; Hassenstein and Reichardt, 1956; Leonhardt et al., 2016; Salazar-Gatzimas et al., 2016; Seelig et al., 2010; Silies et al., 2013; Strother et al., 2018; Strother et al., 2017; Tammero et al., 2004). However, when we changed the stimulus to high contrast sinusoidal gratings (nominal 100% Weber contrast), flies turned in the stimulus direction for approximately 1 second, but then reversed course, and turned in the direction opposite to the stimulus motion for the duration of the stimulus presentation. Because this turning response is in the opposite direction of stimulus and the syn-directional optomotor turning response, we refer to it as anti-directional turning.
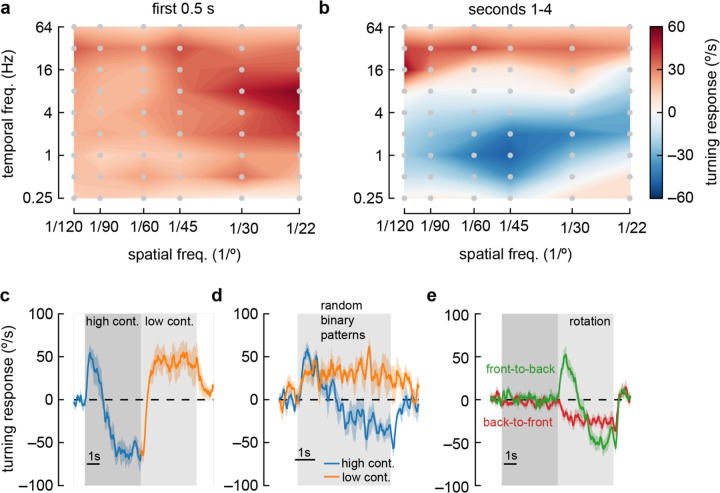
Figure 1. Flies turn opposite to the stimulus direction in high contrast conditions.
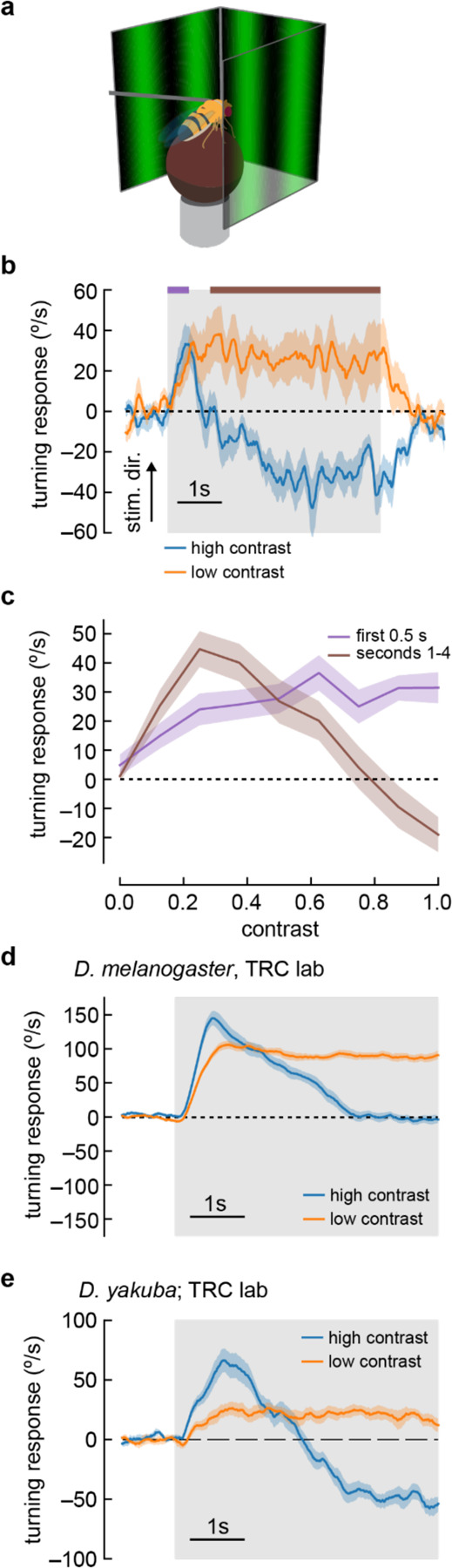
a) We measured fly turning behavior as they walked on an air-suspended ball. Stimuli were presented over 270 degrees around the fly.
b) We presented drifting sinusoidal gratings for 5 seconds (shaded region) with either high contrast (c = 1.0) or low contrast (c = 0.25). When high contrast sinusoidal gratings were presented, flies initially turned in the same direction as the stimulus, then started turning in the opposite direction after ~1 second of stimulation. Under low contrast conditions, flies turned continuously in the same direction as the stimulus. In these experiments, the sine waves had a wavelength of 60° and a temporal frequency of 1 Hz. Shaded patches represent ±1 SEM. N= 10 flies.
c) We swept contrast between 0 and 1 and measured the mean turning response during the first 0.5 seconds (purple, purple bar in b) and during the last 4 seconds of the stimulus (brown, brown line in b). The response in the first 0.5 seconds increased with increasing contrast, while the response in the last four seconds increased from c = 0 to c = 0.25, and then decreased with increasing contrast, until flies turned in the direction opposite the stimulus direction at the highest contrasts. N = 20 flies.
d) We repeated the presentation of drifting sinusoidal gratings, this time in the lab of author TRC, using a similar behavioral apparatus. Stimulus parameters were as described in (b). In these experiments, the population average shows that flies proceeded to zero net turning at high contrasts, but some individual flies exhibited anti-directional turning responses. N = 20 flies.
e) We repeated the experiments with D. yakuba, also in the lab of TRC, and observed that this species exhibited a robust anti-directional turning response to high contrast gratings and a classical syn-directional turning response to low contrast gratings. N = 11 flies.
We swept a range of contrasts and compared the fly turning in the first 500 milliseconds to the turning after one second (Fig. 1c). As contrast increased, the flies turned faster during the first half second of stimulus presentation, reaching a plateau at around 0.5 contrast, consistent with previous results (Bahl et al., 2015; Buchner, 1976; Duistermars et al., 2007; Heisenberg and Buchner, 1977; McCann and MacGinitie, 1965; Strother et al., 2017). Fly behavior after the first second of stimulation was more complex. As contrast increased from 0 to 0.25, flies turned in the same direction as the stimulus, with faster turning as the contrast increased. When the contrast was greater than 0.25, turning decreased, lowering to no net sustained turning at around 0.8 contrast. Above a contrast of 0.8, flies began to turn in the direction opposite the stimulus.
These initial experiments took place in the lab of author DAC. To confirm that these unexpected responses did not reflect some idiosyncrasy of one specific behavioral apparatus or environment, we repeated these experiments in a second lab, that of author TRC. Under similar conditions, using the same strain of Drosophila melanogaster, we reproduced the rapid deceleration after an initial, transient syn-directional response (Fig. 1d), with some individual flies exhibiting significant anti-directional turning, as in the experiments in the first lab (Supp. Fig. S1). This demonstrates that the key features of this behavioral response are stable across experimental systems and laboratories, though the magnitude of reverse-turning behavior in D. melanogaster is sensitive to some unknown experimental parameter differences between the laboratories.
Individual strains of D. melanogaster, and other drosophilid species, display significant variation in their locomotor patterns during walking (York et al., 2022). Indeed, when we tested a Canton-S D. melanogaster strain, we observed mild but significant anti-directional turning at long timescales (Supp. Fig. S2b). We reasoned that a strong test of the generality of anti-directional turning would be to examine turning behavior in another species, and selected D. yakuba. Strikingly, D. yakuba also displayed anti-directional turning behavior under similar conditions (Fig. 1e). Thus, this behavior is not an idiosyncratic feature of a single laboratory strain.
Conditions for anti-directional turning behaviors
While anti-directional turning behaviors have been reported before, other groups have presented similar stimuli without observing anti-directional behavior (Bahl et al., 2013; Bosch et al., 2015; Buchner, 1976; Götz and Wenking, 1973; Seelig et al., 2010). We wondered what aspects of our experimental setup could lead to these behavioral differences. In our experiments, anti-directional turning was strongly linked to display brightness (Supp. Fig. S2a). When the mean brightness of the screens was reduced from 100 cd/m2 to 1 cd/m2, we saw no anti-directional turning in 5 second trials (though average optomotor behavior did decrease over the course of the stimulus presentation). When we further reduced the mean brightness to 0.1 cd/m2, flies persisted in their optomotor behavior throughout the stimulus presentation. We note, however, that at low luminance, low levels of ambient light in the nominally dark experimental rig could also reduce the effective contrast of the stimulus.
We tested a variety of other factors that might affect anti-directional turning. Anti-directional turning occurred when experiments were run both at hot temperatures and at room temperature (Supp. Fig. S2b). We also observed anti-directional behavior when flies were reared in the dark and on different media. We also tested a number of other experiment conditions (Supp. Fig. S2c). Flies responded with anti-directional turning to high contrast stimuli presented at both blue and green wavelengths. We glued fly heads to their thorax to ensure stimuli could not be affected by head movements (Haikala et al., 2013; Kim et al., 2017), but found no difference between head-fixed and head-free flies. There were, however, a few factors that did modulate anti-directional turning behavior. In particular, rearing D. melanogaster at 25°C instead of 20°C or testing flies that were two weeks old instead of 12–60 hours old both reduced overall turning behavior and eliminated anti-directional turning. In these cases, optomotor turning still decreased over the course of the 5 second, high contrast trials, but did not reverse. As details of rearing temperature and the age at which behavior tests are run often vary across labs, it is likely that these factors account for the differences between our observations and the previous literature.
Distinct spatiotemporal tuning of the anti-directional behavioral response
To further characterize the anti-directional response, we swept the spatial and temporal frequency of the sinusoidal grating stimulus. Using only Weber contrasts of 1, we compared the early response (first quarter second, Fig. 2a) to the late response (after one second, Fig. 2b). Drosophila melanogaster always turned in the optomotor direction during the early stimulus response. In this early response, flies turned most vigorously to stimuli with short spatial frequencies (~20° wavelength) and fast temporal frequencies (~8 Hz), in agreement with earlier studies (Creamer et al., 2018; Strother et al., 2018; Tammero et al., 2004). However, during the long-term response to high-contrast stimuli, flies only turned in the optomotor direction at very high temporal frequencies (> ~16 Hz) and at very low temporal frequencies (<0.5 Hz). At intermediate temporal frequencies, flies showed a sustained anti-directional response. The maximal anti-directional response was achieved at 1 Hz and 45° wavelength, distinct from the conditions for peak classical turning responses. Interestingly, the stimuli that elicit the strongest anti-directional response appear similar to those that maximally activate T4 and T5 neurons when those neurons are measured in head-fixed flies (Arenz et al., 2017; Creamer et al., 2018; Leong et al., 2016; Maisak et al., 2013; Strother et al., 2018; Wienecke et al., 2018).
Figure 2. Anti-directional turning behavior has distinct tuning and is driven by adaptation.
a) Heatmap of fly turning velocity during the first 0.5 seconds of sinusoidal grating stimulation under high contrast conditions and variable temporal and spatial frequencies. The flies turned in the direction of the stimulus across all conditions and responded most to 8 Hz, 22-degree stimuli. N = 16,21,17,21,7, and 22 flies for spatial frequencies 1/120, 1/90, 1/60, 1/45, 1/30 and 1/22 degrees respectively.
b) Heatmap as in (a), measured during the last four seconds of stimulation. Flies turned in the same direction as the stimulus at high and low temporal frequencies, but in the opposite direction of the stimulus at intermediate temporal frequencies, with a maximal anti-directional response at wavelengths between 30° and 60°.
c) Switching stimulus contrast from high to low after 5 seconds caused flies to revert to syn-directional behavior after the anti-directional response. N = 7 flies.
d) Presenting rotating random binary patterns (5-degree vertical strips rotating at 150 degrees/second) induced anti-directional turning similar to that elicited by rotating sine wave gratings. N = 7 flies.
e) We presented flies with five seconds of “translational” stimuli (dark shaded region), with high contrast sinusoidal gratings moving either front-to-back or back-to-front, bilaterally, for five seconds. After that, we presented high contrast rotational sinusoidal grating stimuli (60° wavelength, 1 Hz). Front-to-back stimulation did not affect the subsequent response to rotational stimuli, but back-to-front stimuli caused flies to turn immediately in the opposite direction of the stimulus. N = 18 flies.
Anti-directional turning results from adaptation effects
We were intrigued by the switch from syn-directional to anti-directional turning behavior. To investigate the dynamics of these changes, we presented a rotating sinusoidal stimulus at contrast 1 for five seconds, and then changed the contrast to 0.25 (Fig. 2c). After the switch to low contrast, the flies quickly reverted classical, syn-directional optomotor behavior, demonstrating that no long-term switch in directional turning occurs during high contrast stimulus presentation. This effect did not depend on the periodic nature of these stimuli; a rotating stimulus consisting of 5°-wide vertical bars with randomly-chosen, binary contrasts (Clark et al., 2014) yielded similar behavioral responses (Fig. 2d).
To further isolate the causes of this switch in behavior, we developed a stimulus to adapt the fly to different stimuli before presenting high-contrast rotational sinusoidal gratings to elicit the anti-directional turning response. This adapting stimulus consisted of five seconds of high contrast ‘translational’ stimuli, which was then followed by a rotational stimulus (Fig. 2e). The translational stimuli consisted of both left and right hemifields moving either front-to-back or back-to-front across the fly’s two eyes (Creamer et al., 2018). These stimuli resulted in no net turning by the flies (Creamer et al., 2018; Silies et al., 2013). Adapting the fly with front-to-back stimuli did not have a strong effect on the subsequent response to rotational stimuli. However, adapting with back-to-front stimuli generated responses that no longer showed an initial syn-directional turning response, but instead exhibited anti-directional turning immediately after the rotational stimulus began. This result indicates that the anti-directional turning results from slow-timescale changes that depend on strong back-to-front motion stimulation.
Anti-directional turning is elicited when stimuli are presented in front of the fly
A previous report of anti-directional turning behavior in flying tethered flies showed that flies turn in the opposite direction to stimuli that are presented behind their midline (Tammero et al., 2004). To test whether our results were caused by this effect, we split our stimulus into three regions: 90 degrees in front of the fly, 45 degrees in front of the midline on either side of the fly, and 45 degrees behind the midline on either side of the fly (Fig. 3a). We found that flies displayed anti-directional turning when presented with stimuli only in the front region or only just in front of the midline (Fig. 3bc). They did not display anti-directional turning when moving stimuli were presented behind the midline (Fig. 3bc). This suggests a different mechanism from the behaviors that depend on posterior spatial location to elicit reverse-turning (Tammero et al., 2004).
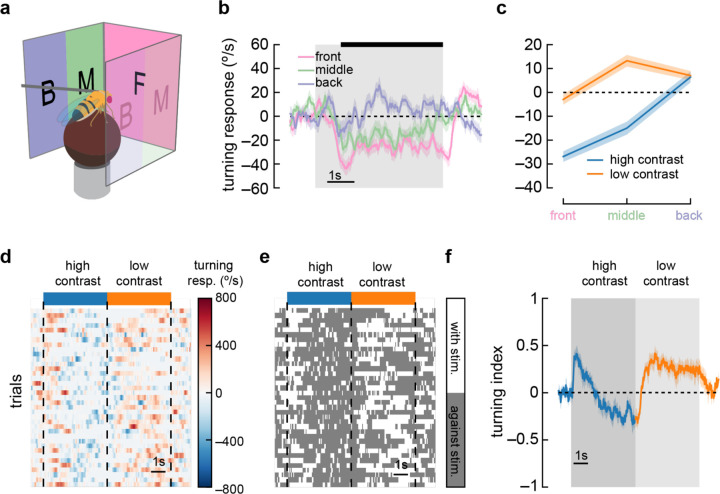
Figure 3. Anti-directional turning is driven by stimuli in the forward-facing visual field and is not driven by saccades.
a) We divided our panoramic display into three sections — the front 90°, the 45° behind the fly on either side, and a middle 45°.
b) High contrast sinusoidal gratings were presented on each of these three display sections, with the remaining sections blank. Flies turned syn-directionally when stimuli were presented behind the fly, and turned anti-directionally when stimuli were presented in front of the fly. Shaded patches represent ±1 SEM. N = 55 flies.
c) Average turning in the last 4 seconds of the stimulus (black bar in b), in low contrast and high contrast conditions. Shaded patches in the time trace plots represent ±1 SEM. N = 55 flies.
d) A single fly responds to many trials of sinusoidal grating stimuli at high contrast (blue bar) and low contrast (orange bar). We show a heatmap of the fly’s responses over time (horizontal axis) and across trials (vertical axis).
e) We can ignore the magnitude of the turning and instead only quantify whether the fly was turning in the same direction as the stimulus (white area) or in the opposite direction (dark gray area). This shows sustained anti-directional turning, not brief saccades.
f) Averaging the direction (but not magnitude) of turning across trials and across flies yields a turning index for each point in time. Shaded patches in the time trace plots represent ±1 SEM. N = 7 flies.
Anti-directional responses do not depend on saccades
Anti-directional saccades have been reported in walking and flying flies (Williamson et al., 2018; Wolf and Heisenberg, 1990). In walking flies (Williamson et al., 2018), flies largely turned in syn-directionally, but these turns were sometimes interrupted by brief, high-amplitude saccades in the opposite direction, against the stimulus direction. If such saccades were frequent or high amplitude, the net effect could shift the average turning we measured, creating apparent anti-directional turning. To investigate this possibility, we plotted the turning response on a per-trial basis (Fig. 3d). We then discarded information about the magnitude of the turns and considered only the direction of the turning at each point in time (Fig. 3e). Strikingly, in many trials, flies continued to turn opposite to the stimulus for several seconds, a behavior unlike brief saccades. We then calculated a turning index for each response timepoint (sampled at 60 Hz). This turning index represented the fraction of trials where the fly turned in the direction of the stimulus at each timepoint minus the fraction of trials where the fly turned in the opposite direction (Fig. 3f). Since this turning index does not include the magnitude of turning, it is strongly affected by sustained low-amplitude turns and discounts any brief high-amplitude saccades. When presented with high contrast stimuli, flies maintained a negative turning index, indicating that sustained turns, and not high velocity saccades, underlie this anti-directional turning behavior.
Anti-directional turning requires elementary motion detectors
What neurons are involved in this anti-directional turning behavior? Previous work demonstrated that T4 and T5 are required for directional neural responses (Schnell et al., 2012), as well as for optomotor turning (Maisak et al., 2013; Salazar-Gatzimas et al., 2018; Salazar-Gatzimas et al., 2016), for walking speed regulation (Creamer et al., 2018), and for responses to visual looming stimuli (Schilling and Borst, 2015). We silenced the neurons T4 and T5 using shibirets (Kitamoto, 2001) and measured responses to sinusoidal stimuli that switched from high to low contrast (Fig. 4a). Flies in which T4 and T5 had been silenced displayed only minimal responses to motion stimuli, with anti-directional turning suppressed along with classical syn-directional turning. Thus, we conclude that, like optomotor turning behaviors, this anti-directional behavior depends critically on signals from T4 and T5.
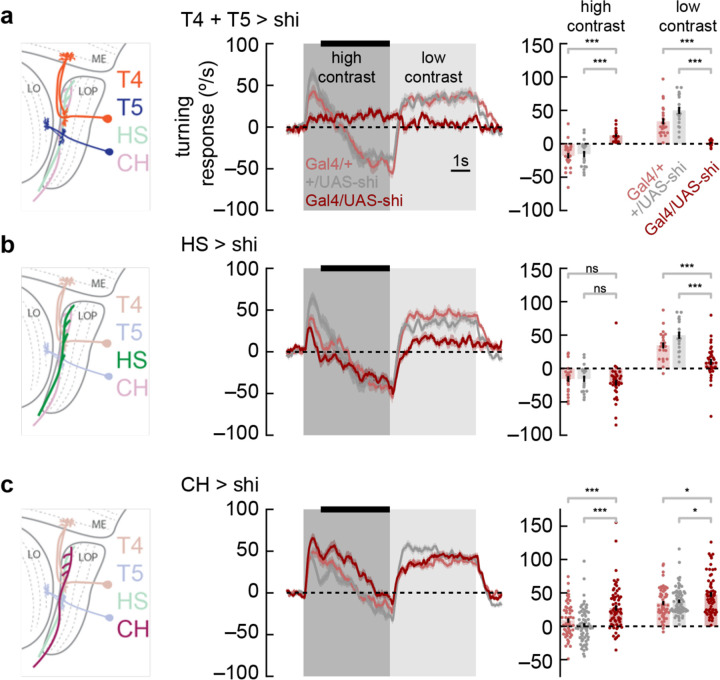
Figure 4. Syn-directional and anti-directional turning share common circuitry.
a) We silenced T4 and T5 neurons by expressing shibirets selectively in those neurons. We measured turning behavior during a contrast-switching stimulus (as in Fig. 2c). Results from flies with T4 and T5 silenced shown in dark red, while controls are in light red and gray. Average fly behavior during the last four seconds of the first contrast (black bar on left) shown as bars on the right, with individual fly behavior shown as dots. Note that the data labeled “low contrast” are from experiments in which the low-contrast stimulus was shown before the high contrast stimulus. Shaded patches in the time trace plots represent ±1 SEM, as do vertical lines on bar plots. *** indicates experimental results are significantly different from results, P < 0.001 via a two-sample Student t-test. * indicates P < 0.05. N = 17, 24, 19 flies with genotypes T4T5/Shibirets, T4T5/+, +/Shibirets.
b) Results from HS silencing as in a. Silencing HS reduced syn-directional turning behavior ( P < 0.001) but did not have a strong effect on anti-directional turning. N = 34, 21, 19 flies with genotypes HS/Shibirets, HS/+, +/Shibirets.
c) Results from CH silencing as in a. CH silencing reduced the degree of anti-directional turning (P < 0.001). N = 63, 57, 70 flies with genotypes CH/Shibirets, CH/+, +/Shibirets.
Anti-directional turning requires the CH lobula plate tangential cell
Since the switch from optomotor to anti-directional behavior seems to be dependent on the direction of motion adaptation (Fig. 2e), we reasoned that neurons involved in this behavior were likely to be downstream from T4 and T5. Horizontal System (HS) cells are well-studied postsynaptic partners of T4 and T5 (Joesch et al., 2008; Joesch et al., 2010). These lobula plate tangential cells integrate information from front-to-back and back-to-front selective T4 and T5 cells across the fly’s visual field (Mauss et al., 2015). HS cells have been implicated in visually-evoked head turns (Kim et al., 2017) and body rotations in flight (Haikala et al., 2013), as well as in maintenance of walking direction (Fujiwara et al., 2022). When we silenced HS neurons, we found small deficits in syn-directional turning behavior, but not in anti-directional turning behavior (Fig. 4b), indicating that HS cells synaptic output is not required specifically for anti-directional turning behavior.
Next, we turned to the CH lobula plate tangential cells. These cells are GABAergic and are both pre-synaptic and post-synaptic in the lobula plate (Wei et al., 2020). In blowflies, these neurons play an inhibitory role in an interconnected LPTC circuit that shapes behavior (Borst and Weber, 2011). When we silenced these neurons, we found a small increase in syn-directional turning and a decrease in anti-directional turning (Fig. 4c). Overall, silencing this neuron type caused the flies to turn more in the direction of motion. This result suggests that CH activity contributes to the anti-directional turning response. However, since adapting to back-to-front translational stimuli significantly affected the dynamics of anti-directional turning, it seems likely that other neurons beyond HS and CH are involved, since these two neurons both respond selectively to front-to-back motion (Eckert and Dvorak, 1983; Joesch et al., 2008).
Early direction-selective cells do not adapt to the stimulus
The anti-directional turning response is preceded by an initial syn-directional response. This change in behavior must be the result of changes in neural activity, but this change could happen at any point along the neural pathway between photoreceptors and motor neurons. In order to constrain possible mechanisms for generating the anti-directional turning behaviors, we used calcium imaging to interrogate the activity of direction selective neurons during high and low contrast stimulation (Fig. 5a). However, as calcium imaging experiments using two photon microscopy require additional spectral filtering of the projector, we first confirmed that these spectral differences did not alter anti-directional turning responses. To do this, we re-measuring the anti-directional turning behavior using optical filtering matched to the conditions needed for imaging. Using this spectrally distinct illuminant, we observed both syn-directional and anti-directional turning behaviors, following the previously observed dynamics (Supp. Fig. S3).
Figure 5. Responses in early direction-selective cells do not show a reduction or reversal of response on the timescale of the behavior.
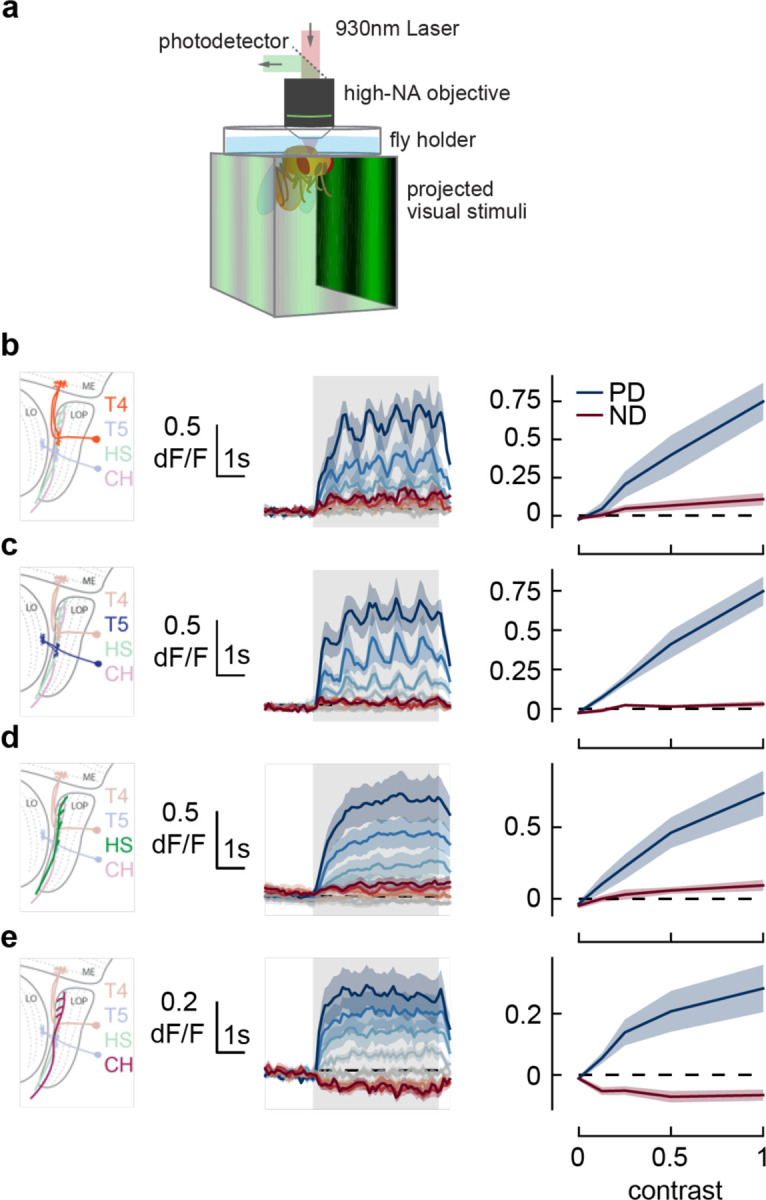
a) We used two-photon microscopy to measure calcium activity in lobula plate neurons while presenting sinusoidal gratings at a range of contrasts.
b) T4 cells, marked in orange (left), responded to drifting sinusoidal gratings with increased calcium activity (middle). Darker colors indicate higher contrast, preferred direction in blue, null direction in red. When integrated across the stimulus presentation (right), calcium activity increased with stimulus contrast. N = 8 flies.
c-e) As in b) measuring calcium activity in T5, HS, and CH cells. N = 8, 10, 15 flies.
As T4 and T5 neurons play a critical role in both the syn- and anti-directional turning responses, we first measured the calcium activity of these neurons as they responded to sine wave gratings at a range of contrasts in their preferred and null directions. The T4 and T5 neurons responded to sine wave gratings in their preferred direction by increasing their calcium activity for the full duration of the stimulus presentation, reaching a plateau after approximately 1 second (Fig. 5bc, middle). As we increased the contrast of the preferred direction stimuli, we found that both T4 and T5 cells had increased calcium activity throughout the contrast range (Fig. 5bc, right), consistent with prior measurements (Maisak et al., 2013). Thus, the responses of T4 and T5 cells do not capture the transition from syn-directional to anti-directional turning behavior.
Next we examined two LPTCs downstream of T4 and T5 cells. Calcium activity in HS cells followed similar trends to T4 and T5. Calcium signals increased at the start of preferred direction stimuli presentation and stayed high until the end of the presentation (Fig. 5d, middle). Increasing contrast caused stronger calcium responses with a mild saturation effect at high contrast (Fig. 5d, right), consistent with prior voltage measurements (Joesch et al., 2008). These results indicate that the changes in the time course of optomotor behavior at high contrast are not related to changes in HS activity. Finally, we measured calcium activity in CH cells. CH cells responded to visual stimuli more quickly than HS cells (Fig. 5e, middle), and showed decreased calcium signals in response to null direction stimuli (Fig. 5e, right). However, they showed sustained responses to high contrast stimuli, as in T4, T5, and HS. These measurements suggest that the switch from syn- to anti-directional turning behavior is driven by cells downstream of or parallel to T4, T5, HS, and CH.
Adult plasticity in anti-directional turning behavior
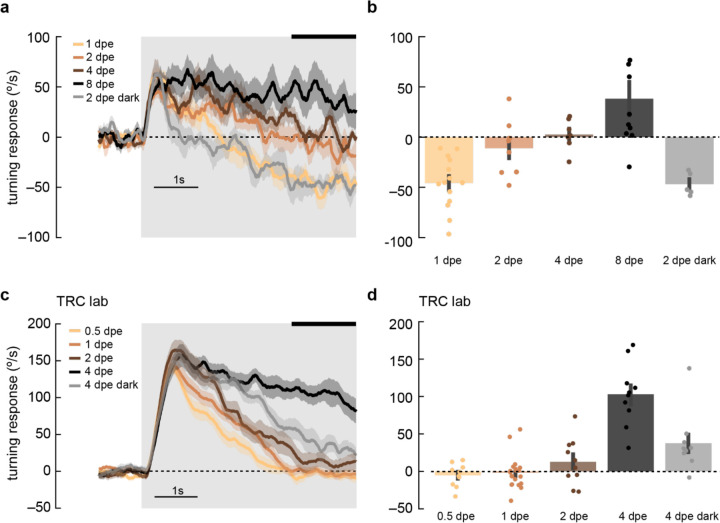
In behaving flies, the strength of anti-directional turning was dependent both on rearing temperature, which alters the rate of growth, and on age (Supp. Fig. S2). This raises the possibility that syn- and anti-directional turning responses might be plastic during the early adult stages of development. To probe this possibility, we presented 1 Hz, high-contrast, rotating sinusoidal grating at various stages during early adulthood (Fig. 6). Strikingly, as flies aged from 0.5 to 4 days post eclosion (dpe), the initial syn-directional turning became less transient and more sustained, indicative of a weaker anti-directional turning drive. We then wondered whether this plasticity was intrinsically programmed, or dependent on visual input. To explore this possibility, we reared flies in darkness to 2 or 4 dpe and measured their turning responses (Fig. 6, gray). Dark-reared flies exhibited a stronger deceleration away from syn-directional turning, similar to that found in more juvenile flies, arguing that visual input may sculpt the balance of syn- and anti-directional turning. Finally, we examined whether optomotor response plasticity could be detected in D. yakuba. However, in this species, anti-directional responses were stable across the first four days of adulthood, arguing that the role of visual experience in shaping these responses is itself evolutionarily tuned in drosophilids (Supp. Fig. 4).
Figure 6. Maturation of optomotor response in early adulthood.
a) Adult flies at various ages post eclosion were presented with 5-second, high-contrast, rotating sinusoidal gratings as in Fig 2b. As the flies aged from 1 day post eclosion (dpe) to 2, 4, and 8 dpe, the initial anti-directional turning response transitioned into syn-directional turning. Dark-rearing flies at 2 dpe reduced this maturation effect. Shaded patches represent ±1 SEM. N = 5–14 flies.
b) The last 1.5 seconds of the mean turning velocity of each fly was averaged, and the population response was plotted.
c) As in (a) but in the TRC lab, using 0.5, 1, 2, and 4 dpe, with dark rearing for 4 dpe. With maturation, the syn-directional turning became less transient. N = 9–15 flies.
d) As in (b) but for data in (c).
Discussion
In this study, we found we could elicit robust turning in the opposite direction of high contrast motion stimuli (Fig. 1). This behavior is qualitatively different from other turning behaviors reported in the literature (Figs. 2 and 3), but shares elements with the circuitry necessary for optomotor behavior (Fig. 4). However, the switch from optomotor behavior to anti-directional turning behavior is not a reflection of changes in the activity of known direction-selective neuron types in the early visual system (Fig. 5). Moreover, this anti-directional turning behavior exhibits a degree of experience-dependent plasticity (Fig. 6).
Anti-directional turning is distinct from other against-stimuli behaviors
The anti-directional turning behavior we have characterized is distinct from previous reports of flies turning in the direction opposite to the stimulus motion. First, some opposite-direction turning behaviors can be explained by stimulus aliasing (Buchner, 1976). Aliasing cannot explain our results because the stimulus that maximally activates anti-directional behavior has a spatial frequency of 1/60 cycles per degree, well below the Nyquist frequency of the fly eye (~1/10 cycles per degree) (Buchner, 1976; Götz, 1970) and below reports of higher acuity vision in flies (Juusola et al., 2017). Aliasing would also not explain the dependence on stimulus contrast.
Second, our observations also cannot be explained by stimuli to the rear of the fly driving it in the opposite direction (Tammero et al., 2004), since we observe anti-directional turning even when stimuli are only presented in only the 90 degrees in front of the fly (Fig. 3).
Third, it is also distinct from previous reports of reverse body saccades (Williamson et al., 2018) since it manifests in persistent turns in the opposite direction of the stimulus and can be measured even when the magnitude of the turns is discarded (Fig. 3).
Fourth, the behavior observed here also appears to be distinct from previously-observed stimulus-density dependent behavioral reversals (Katsov and Clandinin, 2008). Those previously reported behaviors showed immediate reversals, but it took ~1 second for flies in our paradigm to switch between optomotor and anti-directional behaviors.
Anti-directional turning is unlikely to be due to adaptation to contrast alone
In mammalian retina, the direction preference of cells can switch because of upstream circuit adaptation (Rivlin-Etzion et al., 2012; Vlasits et al., 2014). However, we do not believe the anti-directional turning we observe has similar causes. In the mammalian retina, direction switching occurs when non-direction-selective neurons adapt to high contrast stimuli, which distorts the downstream direction-selective computation. Since the adaptation in those experiments occurs in non-direction-selective neurons, it cannot be affected by the direction of the adapter stimulus. However, we see differences in turning behavior depending on whether we adapt with front-to-back or back-to-front stimuli (Fig. 2e). This observation rules out a mechanism based solely on contrast, since the contrast content of front-to-back and back-to-front stimuli are identical.
The fly’s visual system, however, adapts its gain to stimulus contrast (Drews et al., 2020; Matulis et al., 2020). Importantly, the phenomenology of the anti-directional turning also argues that the contrast adaptation is incomplete or heterogeneous among neurons, since contrast 1 and contrast 0.25 stimuli result in such different behaviors. Contrast adaptation reported in the fly is also faster than the 1–2 seconds preceding the shift to anti-directional turning in these experiments.
Anti-directional turning behavior may require specific experimental and rearing conditions
Despite these previous reports of anti-directional turning under certain conditions, other labs have measured sustained optomotor turning in response to high contrast stimuli (Bosch et al., 2015; Götz and Wenking, 1973; Seelig et al., 2010; Strother et al., 2017). We suspect that the two major causes of this difference are display brightness and rearing temperature. Some experiments employ displays with mean luminances less than 5 cd/m2 (Rister et al., 2007; Seelig et al., 2010; Strother et al., 2017). Our screens, with a mean luminance of 100 cd/m2, are substantially brighter, but not especially bright when compared to natural scenes. In daytime natural scenes, foliage and the ground have average luminances of 200–500 cd/m2 and the sky has an average luminance of around 4000 cd/m2 (Frazor and Geisler, 2006). We therefore suspect that as researchers move to using displays that can more accurately depict natural scene luminances, anti-directional turning behaviors will be encountered more frequently.
Rearing conditions also had a significant influence on anti-directional turning behavior. Flies reared at 25°C showed less anti-directional behavior than those reared at 20°C. We also found differences based on fly age and fly strain. Our rearing conditions and choice of fly strain have all been optimized during previous experiments to yield strong optomotor responses. Since anti-directional behavior at high contrast usually occurs under conditions that yield strong optomotor turning at low contrast, these optimized conditions may be required to observe anti-directional behavior. Temperature, in particular, has developmental effects on neural connectivity (Kiral et al., 2021). Notably, all three of these parameters vary significantly across the field, with prior studies varying rearing temperatures from 18 to 20 to 25°C (see for instance (Creamer et al., 2018; Juusola et al., 2017; Ketkar et al., 2020; Mongeau and Frye, 2017; Strother et al., 2017)), ages from 1 day to 10 days (see for instance (Bahl et al., 2013; Silies et al., 2013; Tammero et al., 2004)), and strain between CantonS or OregonR (see for instance (Clark et al., 2011; Rister et al., 2007)). Thus, we believe that these factors likely account for the fact that this phenomenon has not previously been reported.
Tuning of anti-directional turning matches tuning of direction selective neurons
The study of anti-directional turning behavior may yield clues about the temporal tuning of fly motion detectors. Optomotor behavior is tuned to visual stimuli in the range of 8–22 Hz (Creamer et al., 2018; Strother et al., 2018; Tammero et al., 2004; Tuthill et al., 2013), while anti-directional behavior is tuned to stimuli in the 0.5–4 Hz range (Fig. 1). Intriguingly, this slower tuning matches the tuning of T4, T5, and HS neurons, as measured via calcium imaging or electrophysiology (Chiappe et al., 2010; Creamer et al., 2018; Joesch et al., 2008; Maisak et al., 2013). Previous studies have suggested that the difference in tuning between behavior and imaging are due to octopamine released during behavior, and not necessarily released during imaging (Arenz et al., 2017; Chiappe et al., 2010; Strother et al., 2018). In this work, we demonstrate a motion-related behavior tuned to low frequencies, comparable to those in neural measurements, during behavior that requires T4 and T5 neurons. Overall, this suggests that T4 and T5 are required for behaviors with very different temporal tuning, which in turn suggests that the temporal tuning of behavior is not determined solely by T4 and T5 tuning, but by other, parallel pathways as well.
Anti-directional turning is unlikely to occur in nature
In a natural environment, flies are unlikely to encounter a situation where they see continuous motion in the same direction for more than 1 second. Measurements of free walking behavior have shown that the time constant of the autocorrelation of fly turning is around 100 ms (DeAngelis et al., 2019; Katsov et al., 2017). This means that the anti-directional turning studied here has likely not been directly subject to evolutionary pressures. However, the fact that we observed strong anti-directional turning in D. yakuba indicates that anti-directional turning is not an idiosyncratic behavior of D. melanogaster. It seems likely that the behavior reflects some other requirement of fly behavior, whose circuits are engaged by this stimulus. In this context, the anti-directional turning response represents a promising avenue to further constrain the underlying mechanisms of motion detection and integration. Indeed, just as illusory motion stimuli have placed key constraints on the circuits and algorithms for visual motion detection in flies and vertebrates (Agrochao et al., 2020; Clark et al., 2011; Clark et al., 2014; Eichner et al., 2011; Leonhardt et al., 2016; Salazar-Gatzimas et al., 2018; Salazar-Gatzimas et al., 2016; Theobald et al., 2008; Tuthill et al., 2011) (Adelson and Bergen, 1985; Anstis and Rogers, 1975; Conway et al., 2005; Hassenstein and Reichardt, 1956; Hu and Victor, 2010; Livingstone et al., 2001; Livingstone and Conway, 2003; Mo and Koch, 2003; Orger et al., 2000), we believe that the anti-directional turning we describe here will provide additional insights.
In summary, we have presented evidence of a transition from syn-directional turning to no turning or to anti-directional turning when high contrast stimuli are presented to the fly. This persists across laboratory environments and across Drosophila species and shows plasticity with age. This behavior suggests than turning in response to rotational stimuli is not a simple reflex. Instead, the turning likely represents a superposition of behaviors driven by distinct circuits and elicited by different characteristics of the stimulus and different states of the fly. This complexity makes the optomotor response a model for studying the interactions of circuits as they control the low-dimensional behaviors that change an animal’s orientation.
Methods
Fly strains
Strains used in these experiments are listed in the tables below:
Genotypes of files used in imaging experiments: +; +; HS-Gal4/UAS-jGCaMP7b, +; UAS-C6f/+; T4T5-Gal4/UAS-mtdTomato, w/+; +; CH-Gal4/UAS-jGCaMP7b.
Fly rearing (DAC lab)
Unless otherwise noted, flies were reared at 20 degrees Celsius in Panasonic MIR-154-PA incubators (Panasonic/PHC, Tokyo, Japan). The flies were circadian entrained on 12-hour light-dark cycles. Flies were raised on Archon Scientific glucose food (recipe D20102, Archon Scientific, Durham, NC). We used CO2 to anesthetize flies more than 12 hours before the behavioral experiments.
Flies were tested for behavior in rigs built in the labs of DAC and TRC. Behavior shown in Figs. 1d, 1e, 6c, 6d, S1, and S4 was acquired in the lab of TRC, while the rest was obtained in the lab of DAC.
Fly rearing (TRC lab)
Flies were reared at 25°C, on molasses-based food, and circadian entrained on 12-hour light-dark cycles. Flies were collected within three hours of eclosion using brief CO2 anesthetization. D. melanogaster and D. yakuba were raised under identical conditions. Dark-reared flies were put in a dark chamber within 3 hours of eclosion. Flies tested at 0.5 days post eclosion were collected during the first two hours of the light cycle and were exposed to light until they were tested.
Stimulus generation and behavioral turning assays (DAC lab)
Stimuli were presented using DLP Lightcrafter (Texas Instruments, Dallas, TX) projectors (Creamer et al., 2019). Mirrors were used to bounce the projected light onto three screens made of back-projection material, surrounding the fly. The screens covered the front 270 degrees around the fly, and ~45 degrees in elevation above and below the fly. The projectors were set to monochrome mode (green unless otherwise noted), updating at 180 Hz. Stimulus video was generated through a custom MATLAB (Mathworks, Natick, MA) application using PsychToolbox (Kleiner et al., 2007). Stimuli were mapped onto a virtual cylinder around the fly and the MATLAB application generated a viewpoint-corrected video signal.
Behavioral experiments were performed 12–60 hours after staging. For behavioral experiments, we selected female flies, and co-housed them with males after staging. Flies were cold-anesthetized and fixed to needles using UV-cured epoxy (Norland optical adhesive #63, Norland Products, Cranbury, NJ). Flies were then placed above air-suspended polypropylene balls. These balls were 6 mm in diameter and weighed ~120 mg. The balls were painted with two layers of marker coatings- a base silver layer and a red top layer. The motion of balls was detected by either a Parallax mouse sensor board (Parallax, Rocklin, CA) with an MCS-12086 sensor (Unity Opto Technology, Taipei, Taiwan), or a custom board with an ADNS 2080 sensor (Avago Technologies / Broadcom Inc, San Jose, TX). The data from these sensors were transferred to a custom MATLAB application via an Arduino Uno board.
Stimulus generation and behavioral turning assays (TRC lab)
Stimuli were presented using a DLP Lightcrafter (Texas Instruments, Dallas, TX) projector. Three coherent optic fibers were used to direct the projected light onto three screens made of back-projection material, surrounding the fly (Clark et al., 2011; Clark et al., 2014). The screens covered the front 270 degrees around the fly, and ~45 degrees in elevation above and below the fly. The projectors were set to monochrome mode, updating at 120 Hz. Stimulus video was generated through Flystim (https://github.com/ClandininLab/flystim), a custom Python application developed in the Clandinin Lab (Turner et al., 2022). Stimuli were mapped onto a virtual cylinder around the fly and Flystim generated a viewpoint-corrected video signal.
Behavioral experiments were performed 12–48 hours after eclosion, as described in the figures. Flies were cold-anesthetized and fixed to needles using UV-cured adhesive (Bondic, Niagara Falls, NY). Flies were then placed above air-suspended balls made with LAST-A-FOAM FR-4615 polyurethane foam (General Plastics, Tacoma, WA). These balls were 9 mm in diameter and weighed ~91.7 mg. The motion of balls was detected by a Flea3 FL3-U3-13Y3M camera (Teledyne Flir, Wilsonville, OR) and Fictrac software (Moore et al., 2014).
Imaging procedures
Two photon imaging (Fig. 5) was performed as previously described (Tanaka and Clark, 2022). Briefly, two-photon images were acquired with a Scientifica microscope at between 6 and 13 Hz using a 930 nm femtosecond laser (SpectraPhysics, Santa Clara, USA) using ScanImage (Pologruto et al., 2003). Visual stimuli were presented on three screens occupying 270° of azimuthal angle about the fly using projectors (Creamer et al., 2019). Optical filters on the projector and emission filters prevented the visual stimulus light from leaking into the two-photon images.
Regions of interest (ROIs) were extracted from image timeseries using a watershed algorithm. Responsive ROIs were included in the analyses. For T4 and T5 neurons, each ROI was identified as a T4-dominant or T5-dominant ROI by its response to light vs. dark edges, following prior procedures (Agrochao et al., 2020). For all neuron types, responses were averaged over ROIs and over trials of each stimulus type to obtain a measurement for each fly; these fly measurements acted as the independent measurements to compute means and standard error bars for the figure.
Statistical tests
Throughout the paper, each fly was considered an independent sample for statistical purposes. Means and standard errors were computed over flies. For imaging experiments, regions of interest from a specific neuron type were first averaged within each fly, creating a value for each fly’s response. These values were used to calculate means and standard errors over the tested flies. In the silencing experiments, a 2-sample Student t-test was used to test for significant differences between the experimental genotype and parental controls.
Supplementary Material
Table 1:
Parental stock genotypes
| Name | Genotype | Source | Stock # |
|---|---|---|---|
| Wildtype | +; +; + (IsoD1) | (Gohl et al., 2011) | N/A |
| T4T5-Gal4 | +; +; R42F06-Gal4 (IsoD1 background) | BDSC | BDSC 41253 |
| HS-Gal4 | +; +; R27B03-Gal4 (IsoD1 bg) | (Seelig et al., 2010) | BDSC 49211 |
| CH-Gal4 | w; +; R35A10-Gal4 (Janelia bg) | BDSC | BDSC 49897 |
| UAS-Shibirets | +; +; UAS-Shibirets (IsoD1 bg) | (Silies et al., 2013) | N/A |
| Empty Gal4 | w; +; pBDPGAL4.1Uw (Janelia bg) | BDSC | BDSC 68384 |
| GCaMP6f | w; UAS-GCaMP6f; + | BDSC | BDSC 42747 |
| jGCaMP7b | w; +; UAS-jGCaMP7b | BDSC | BDSC 79029 |
| mtdTomato | w; +; UAS-mtdTomato | BDSC | BDSC 30124 |
Table 2:
Genotypes of flies used in behavior experiments
| Experimental | Gal4 Control | UAS Control | Background Control |
|---|---|---|---|
| T4T5-Gal4 x UAS-Shibirets: +; +; R42F06-Gal4/UAS-Shibirets | T4T5-Gal4 x IsoD1: +;+;R42F06-Gal4/+ | IsoD1 x UAS-Shibirets: +; +; +/UAS-Shibirets | IsoD1: +; +; + |
| HS-Gal4 x UAS-Shibirets: +; +; R27B03-Gal4/UAS-Shibirets | HS-Gal4 x IsoD1: +; +; R27B03-Gal4/+ | IsoD1 x UAS-Shibirets: +; +; +/UAS-Shibirets | IsoD1: +; +; + |
| CH-Gal4 x UAS-Shibirets: w/+; +; R35A10-Gal4/UAS-Shibirets | CH-Gal4 x IsoD1: w/+; +; R35A10-Gal4/+ | Empty Gal4 x UAS-Shibirets: +/w; +; pBDPGAL4.1Uw /UAS-Shibirets | Empty Gal4 X IsoD1: +/w; +; +/ pBDPGAL4.1Uw |
Acknowledgements
This work was supported by NIH R01EY026555 (DAC), R01EY022638 (TRC), and by a Chan-Zuckerberg Investigator Award (TRC). MC was supported by an NDSEG Fellowship; RT was supported by the Takenaka Foundation; MSC was supported by an NSF GRFP; NCBM was supported by a CAPES fellowship; JS was supported by a Ford Foundation Fellowship.
Citations
- Adelson E., and Bergen J. (1985). Spatiotemporal energy models for the perception of motion. JOSA A 2, 284–299. [DOI] [PubMed] [Google Scholar]
- Agrochao M., Tanaka R., Salazar-Gatzimas E., and Clark D.A. (2020). Mechanism for analogous illusory motion perception in flies and humans. Proc. Natl. Acad. Sci. 117, 23044–23053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anstis S.M., and Rogers B.J. (1975). Illusory reversal of visual depth and movement during changes of contrast. Vision Res. 15, 957–IN956. [DOI] [PubMed] [Google Scholar]
- Arenz A., Drews M.S., Richter F.G., Ammer G., and Borst A. (2017). The temporal tuning of the Drosophila motion detectors is determined by the dynamics of their input elements. Curr. Biol. 27, 929–944. [DOI] [PubMed] [Google Scholar]
- Bahl A., Ammer G., Schilling T., and Borst A. (2013). Object tracking in motion-blind flies. Nat. Neurosci. 16, 730–738. [DOI] [PubMed] [Google Scholar]
- Bahl A., Serbe E., Meier M., Ammer G., and Borst A. (2015). Neural mechanisms for Drosophila contrast vision. Neuron 88, 1240–1252. [DOI] [PubMed] [Google Scholar]
- Barnhart E.L., Wang I.E., Wei H., Desplan C., and Clandinin T.R. (2018). Sequential nonlinear filtering of local motion cues by global motion circuits. Neuron 100, 229–243. e223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bausenwein B., Dittrich A., and Fischbach K.-F. (1992). The optic lobe of Drosophila melanogaster. Cell Tissue Res. 267, 17–28. [DOI] [PubMed] [Google Scholar]
- Behnia R., Clark D.A., Carter A.G., Clandinin T.R., and Desplan C. (2014). Processing properties of ON and OFF pathways for Drosophila motion detection. Nature 512, 427–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst A., and Weber F. (2011). Neural Action Fields for Optic Flow Based Navigation: A Simulation Study of the Fly Lobula Plate Network. PLOS ONE 6, e16303. 10.1371/journal.pone.0016303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch D.S., van Swinderen B., and Millard S.S. (2015). Dscam2 affects visual perception in Drosophila melanogaster. Frontiers in behavioral neuroscience 9, 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchner E. (1976). Elementary movement detectors in an insect visual system. Biol. Cybern. 24, 85–101. [Google Scholar]
- Chiappe M.E., Seelig J.D., Reiser M.B., and Jayaraman V. (2010). Walking modulates speed sensitivity in Drosophila motion vision. Curr. Biol. 20, 1470–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D. (1981). Visual responses in developing zebrafish. Brachydanio rerio. [Google Scholar]
- Clark D.A., Bursztyn L., Horowitz M.A., Schnitzer M.J., and Clandinin T.R. (2011). Defining the computational structure of the motion detector in Drosophila. Neuron 70, 1165–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D.A., Fitzgerald J.E., Ales J.M., Gohl D.M., Silies M., Norcia A.M., and Clandinin T.R. (2014). Flies and humans share a motion estimation strategy that exploits natural scene statistics. Nat. Neurosci. 17, 296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway B.R., Kitaoka A., Yazdanbakhsh A., Pack C.C., and Livingstone M.S. (2005). Neural basis for a powerful static motion illusion. J. Neurosci. 25, 5651–5656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creamer M.S., Mano O., and Clark D.A. (2018). Visual Control of Walking Speed in Drosophila. Neuron 100, 1460–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creamer M.S., Mano O., Tanaka R., and Clark D.A. (2019). A flexible geometry for panoramic visual and optogenetic stimulation during behavior and physiology. J. Neurosci. Methods 323, 48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeAngelis B.D., Zavatone-Veth J.A., and Clark D.A. (2019). The manifold structure of limb coordination in walking Drosophila. eLife 8, e46409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drews M.S., Leonhardt A., Pirogova N., Richter F.G., Schuetzenberger A., Braun L., Serbe E., and Borst A. (2020). Dynamic Signal Compression for Robust Motion Vision in Flies. Curr. Biol. [DOI] [PubMed] [Google Scholar]
- Duistermars B., Chow D., Condro M., and Frye M. (2007). The spatial, temporal and contrast properties of expansion and rotation flight optomotor responses in Drosophila. J. Exp. Biol. 210, 3218. [DOI] [PubMed] [Google Scholar]
- Eckert H., and Dvorak D.R. (1983). The centrifugal horizontal cells in the lobula plate of the blowfly, Phaenicia sericata. J. Insect Physiol. 29, 547–560. [Google Scholar]
- Eichner H., Joesch M., Schnell B., Reiff D.F., and Borst A. (2011). Internal structure of the fly elementary motion detector. Neuron 70, 1155–1164. [DOI] [PubMed] [Google Scholar]
- Frazor R.A., and Geisler W.S. (2006). Local luminance and contrast in natural images. Vision Res. 46, 1585–1598. [DOI] [PubMed] [Google Scholar]
- Fujiwara T., Brotas M., and Chiappe M.E. (2022). Walking strides direct rapid and flexible recruitment of visual circuits for course control in Drosophila. Neuron. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz K.G. (1968). Flight control in Drosophila by visual perception of motion. Biol. Cybern. 4, 199–208. [DOI] [PubMed] [Google Scholar]
- Gohl D.M., Silies M.A., Gao X.J., Bhalerao S., Luongo F.J., Lin C.C., Potter C.J., and Clandinin T.R. (2011). A versatile in vivo system for directed dissection of gene expression patterns. Nat. Methods 8, 231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Götz K. (1964). Optomotorische untersuchung des visuellen systems einiger augenmutanten der fruchtfliege Drosophila. Biol. Cybern. 2, 77–92. [DOI] [PubMed] [Google Scholar]
- Götz K., and Wenking H. (1973). Visual control of locomotion in the walking fruitfly Drosophila. J. Comp. Physiol. A 85, 235–266. [Google Scholar]
- Götz K.G. (1970). Fractionation of Drosophila Populations According to Optomotor Traits. Journal of Experimental Biology 52, 419–436. [DOI] [PubMed] [Google Scholar]
- Götz K.G. (1975). The optomotor equilibrium of theDrosophila navigation system. Journal of comparative physiology 99, 187–210. 10.1007/BF00613835. [DOI] [Google Scholar]
- Haikala V., Joesch M., Borst A., and Mauss A.S. (2013). Optogenetic control of fly optomotor responses. J. Neurosci. 33, 13927–13934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassenstein B., and Reichardt W. (1956). Systemtheoretische Analyse der Zeit-, Reihenfolgen-und Vorzeichenauswertung bei der Bewegungsperzeption des Rüsselkäfers Chlorophanus. Zeits. Naturforsch. 11, 513–524. [Google Scholar]
- Heisenberg M., and Buchner E. (1977). The role of retinula cell types in visual behavior ofDrosophila melanogaster. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology 117, 127–162. [Google Scholar]
- Henning M., Ramos-Traslosheros G., Gür B., and Silies M. (2022). Populations of local direction–selective cells encode global motion patterns generated by self-motion. Science advances 8, eabi7112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Q., and Victor J.D. (2010). A set of high-order spatiotemporal stimuli that elicit motion and reverse-phi percepts. J. Vis. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joesch M., Plett J., Borst A., and Reiff D. (2008). Response properties of motion-sensitive visual interneurons in the lobula plate of Drosophila melanogaster. Curr. Biol. 18, 368–374. [DOI] [PubMed] [Google Scholar]
- Joesch M., Schnell B., Raghu S., Reiff D., and Borst A. (2010). ON and OFF pathways in Drosophila motion vision. Nature 468, 300–304. [DOI] [PubMed] [Google Scholar]
- Juusola M., Dau A., Song Z., Solanki N., Rien D., Jaciuch D., Dongre S.A., Blanchard F., de Polavieja G.G., and Hardie R.C. (2017). Microsaccadic sampling of moving image information provides Drosophila hyperacute vision. Elife 6, e26117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsov A., and Clandinin T. (2008). Motion processing streams in Drosophila are behaviorally specialized. Neuron 59, 322–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsov A.Y., Freifeld L., Horowitz M.A., Kuehn S., and Clandinin T.R. (2017). Dynamic structure of locomotor behavior in walking fruit flies. eLife 6, e26410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketkar M.D., Sporar K., Gür B., Ramos-Traslosheros G., Seifert M., and Silies M. (2020). Luminance information is required for the accurate estimation of contrast in rapidly changing visual contexts. Curr. Biol. 30, 657–669. e654. [DOI] [PubMed] [Google Scholar]
- Kim A.J., Fenk L.M., Lyu C., and Maimon G. (2017). Quantitative predictions orchestrate visual signaling in Drosophila. Cell 168, 280–294. e212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiral F.R., Dutta S.B., Linneweber G.A., Hilgert S., Poppa C., Duch C., von Kleist M., Hassan B.A., and Hiesinger P.R. (2021). Brain connectivity inversely scales with developmental temperature in Drosophila. Cell Rep. 37, 110145. [DOI] [PubMed] [Google Scholar]
- Kitamoto T. (2001). Conditional modification of behavior in Drosophila by targeted expression of a temperature-sensitive shibire allele in defined neurons. J. Neurobiol. 47, 81–92. [DOI] [PubMed] [Google Scholar]
- Kleiner M., Brainard D., Pelli D., Ingling A., Murray R., and Broussard C. (2007). What’s new in Psychtoolbox-3. Perception 36, 1. [Google Scholar]
- Koerner F., and Schiller P.H. (1972). The optokinetic response under open and closed loop conditions in the monkey. Exp. Brain Res. 14, 318–330. [DOI] [PubMed] [Google Scholar]
- Krapp H.G., and Hengstenberg R. (1996). Estimation of self-motion by optic flow processing in single visual interneurons. Nature 384, 463–466. [DOI] [PubMed] [Google Scholar]
- Leong J.C.S., Esch J.J., Poole B., Ganguli S., and Clandinin T.R. (2016). Direction selectivity in Drosophila emerges from preferred-direction enhancement and null-direction suppression. J. Neurosci. 36, 8078–8092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonhardt A., Ammer G., Meier M., Serbe E., Bahl A., and Borst A. (2016). Asymmetry of Drosophila ON and OFF motion detectors enhances real-world velocity estimation. Nat. Neurosci. 19, 706–715. [DOI] [PubMed] [Google Scholar]
- Livingstone M., Pack C., and Born R. (2001). Two-dimensional substructure of MT receptive fields. Neuron 30, 781–793. [DOI] [PubMed] [Google Scholar]
- Livingstone M.S., and Conway B.R. (2003). Substructure of direction-selective receptive fields in macaque V1. J. Neurophysiol. 89, 2743–2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisak M.S., Haag J., Ammer G., Serbe E., Meier M., Leonhardt A., Schilling T., Bahl A., Rubin G.M., Nern A., et al. (2013). A directional tuning map of Drosophila elementary motion detectors. Nature 500, 212–216. [DOI] [PubMed] [Google Scholar]
- Matulis C.A., Chen J., Gonzalez-Suarez A., Behnia R., and Clark D.A. (2020). Heterogeneous temporal contrast adaptation in Drosophila direction-selective circuits. Curr. Biol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauss A.S., Pankova K., Arenz A., Nern A., Rubin G.M., and Borst A. (2015). Neural circuit to integrate opposing motions in the visual field. Cell 162, 351–362. [DOI] [PubMed] [Google Scholar]
- McCann G.D., and MacGinitie G. (1965). Optomotor response studies of insect vision. Proceedings of the Royal Society of London. Series B. Biological Sciences 163, 369–401. [DOI] [PubMed] [Google Scholar]
- Mo C.-H., and Koch C. (2003). Modeling reverse-phi motion-selective neurons in cortex: double synaptic-veto mechanism. Neural Comput. 15, 735–759. [DOI] [PubMed] [Google Scholar]
- Mongeau J.-M., and Frye M.A. (2017). Drosophila spatiotemporally integrates visual signals to control saccades. Curr. Biol. 27, 2901–2914. e2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore R.J., Taylor G.J., Paulk A.C., Pearson T., van Swinderen B., and Srinivasan M.V. (2014). FicTrac: a visual method for tracking spherical motion and generating fictive animal paths. J. Neurosci. Methods 225, 106–119. [DOI] [PubMed] [Google Scholar]
- Orger M.B., Smear M.C., Anstis S.M., and Baier H. (2000). Perception of Fourier and non-Fourier motion by larval zebrafish. Nat. Neurosci. 3, 1128–1133. [DOI] [PubMed] [Google Scholar]
- Pologruto T.A., Sabatini B.L., and Svoboda K. (2003). ScanImage: flexible software for operating laser scanning microscopes. Biomed. Eng. Online 2, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rister J., Pauls D., Schnell B., Ting C., Lee C., Sinakevitch I., Morante J., Strausfeld N., Ito K., and Heisenberg M. (2007). Dissection of the peripheral motion channel in the visual system of Drosophila melanogaster. Neuron 56, 155–170. [DOI] [PubMed] [Google Scholar]
- Rivlin-Etzion M., Wei W., and Feller M.B. (2012). Visual stimulation reverses the directional preference of direction-selective retinal ganglion cells. Neuron 76, 518–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar-Gatzimas E., Agrochao M., Fitzgerald J.E., and Clark D.A. (2018). The Neuronal Basis of an Illusory Motion Percept Is Explained by Decorrelation of Parallel Motion Pathways. Curr. Biol. 28, 3748–3762. e3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar-Gatzimas E., Chen J., Creamer M.S., Mano O., Mandel H.B., Matulis C.A., Pottackal J., and Clark D.A. (2016). Direct measurement of correlation responses in Drosophila elementary motion detectors reveals fast timescale tuning. Neuron 92, 227–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling T., and Borst A. (2015). Local motion detectors are required for the computation of expansion flow-fields. Biology open, bio. 012690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell B., Raghu S.V., Nern A., and Borst A. (2012). Columnar cells necessary for motion responses of wide-field visual interneurons in Drosophila. J. Comp. Physiol. A 198, 389–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweigart G., Mergner T., Evdokimidis I., Morand S., and Becker W. (1997). Gaze stabilization by optokinetic reflex (OKR) and vestibulo-ocular reflex (VOR) during active head rotation in man. Vision Res. 37, 1643–1652. [DOI] [PubMed] [Google Scholar]
- Seelig J., Chiappe M., Lott G., Dutta A., Osborne J., Reiser M., and Jayaraman V. (2010). Two-photon calcium imaging from head-fixed Drosophila during optomotor walking behavior. Nat. Methods. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinomiya K., Huang G., Lu Z., Parag T., Xu C.S., Aniceto R., Ansari N., Cheatham N., Lauchie S., Neace E., et al. (2019). Comparisons between the ON-and OFF-edge motion pathways in the Drosophila brain. eLife 8, e40025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silies M., Gohl D.M., Fisher Y.E., Freifeld L., Clark D.A., and Clandinin T.R. (2013). Modular use of peripheral input channels tunes motion-detecting circuitry. Neuron 79, 111–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strother J.A., Nern A., and Reiser M.B. (2014). Direct observation of ON and OFF pathways in the Drosophila visual system. Curr. Biol. 24, 976–983. [DOI] [PubMed] [Google Scholar]
- Strother J.A., Wu S.-T., Rogers E.M., Eliason J.L., Wong A.M., Nern A., and Reiser M.B. (2018). Behavioral state modulates the ON visual motion pathway of Drosophila. Proc. Natl. Acad. Sci. USA 115, E102–E111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strother J.A., Wu S.-T., Wong A.M., Nern A., Rogers E.M., Le J.Q., Rubin G.M., and Reiser M.B. (2017). The emergence of directional selectivity in the visual motion pathway of Drosophila. Neuron 94, 168–182. e110. [DOI] [PubMed] [Google Scholar]
- Takemura S. y., Bharioke A., Lu Z., Nern A., Vitaladevuni S., Rivlin P.K., Katz W.T., Olbris D.J., Plaza S.M., Winston P., et al. (2013). A visual motion detection circuit suggested by Drosophila connectomics. Nature 500, 175–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tammero L., Frye M., and Dickinson M. (2004). Spatial organization of visuomotor reflexes in Drosophila. J. Exp. Biol. 207, 113–122. [DOI] [PubMed] [Google Scholar]
- Tanaka R., and Clark D.A. (2022). Neural mechanisms to exploit positional geometry for collision avoidance. Curr. Biol. 32, 2357–2374.e2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theobald J.C., Duistermars B.J., Ringach D.L., and Frye M.A. (2008). Flies see second-order motion. Curr. Biol. 18, R464–R465. [DOI] [PubMed] [Google Scholar]
- Turner M.H., Krieger A., Pang M.M., and Clandinin T.R. (2022). Visual and motor signatures of locomotion dynamically shape a population code for visual features in Drosophila. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuthill J.C., Chiappe M.E., and Reiser M.B. (2011). Neural correlates of illusory motion perception in Drosophila. Proc. Natl. Acad. Sci. USA 108, 9685–9690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuthill J.C., Nern A., Holtz S.L., Rubin G.M., and Reiser M.B. (2013). Contributions of the 12 neuron classes in the fly lamina to motion vision. Neuron 79, 128–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlasits A.L., Bos R., Morrie R.D., Fortuny C., Flannery J.G., Feller M.B., and Rivlin-Etzion M. (2014). Visual stimulation switches the polarity of excitatory input to starburst amacrine cells. Neuron 83, 1172–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei H., Kyung H.Y., Kim P.J., and Desplan C. (2020). The diversity of lobula plate tangential cells (LPTCs) in the Drosophila motion vision system. J. Comp. Physiol. A 206, 139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wienecke C.F., Leong J.C., and Clandinin T.R. (2018). Linear Summation Underlies Direction Selectivity in Drosophila. Neuron. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson W.R., Peek M.Y., Breads P., Coop B., and Card G.M. (2018). Tools for Rapid High-Resolution Behavioral Phenotyping of Automatically Isolated Drosophila. Cell Rep. 25, 1636–1649. e1635. [DOI] [PubMed] [Google Scholar]
- Wolf R., and Heisenberg M. (1990). Visual control of straight flight in Drosophila melanogaster. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology 167, 269–283. [DOI] [PubMed] [Google Scholar]
- Yang H.H., and Clandinin T.R. (2018). Elementary motion detection in Drosophila: algorithms and mechanisms. Ann. Rev. Vis. Sci. 4, 143–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- York R.A., Brezovec L.E., Coughlan J., Herbst S., Krieger A., Lee S.-Y., Pratt B., Smart A.D., Song E., and Suvorov A. (2022). The evolutionary trajectory of drosophilid walking. Curr. Biol. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.