Abstract
In metazoans, Polo Kinase (Plk1) controls several mitotic events including nuclear envelope breakdown, centrosome maturation and kinetochore assembly. Here we show that mitotic events regulated by Polo Like Kinase (PLK-1) in early C. elegans embryos depend on the mitochondrial-localized protein SPD-3. spd-3 mutant one-cell embryos contain abnormally positioned mitotic chromosomes and prematurely and asymmetrically disassemble the nuclear lamina. Nuclear envelope breakdown (NEBD) in C. elegans requires direct dephosphorylation of lamin by PLK-1. In spd-3 mutants PLK-1 levels are ~6X higher in comparison to control embryos and PLK-1::GFP was highly accumulated at centrosomes, the nuclear envelope, nucleoplasm, and chromosomes prior to NEBD. Partial depletion of plk-1 in spd-3 mutant embryos rescued mitotic chromosome and spindle positioning defects indicating that these phenotypes result from higher PLK-1 levels and thus activity. Our data suggests that the mitochondrial SPD-3 protein controls NEBD and chromosome positioning by regulating the endogenous levels of PLK-1 during early embryogenesis in C. elegans. This finding suggests a novel link between mitochondria and mitotic events by controlling the amount of a key mitotic regulator, PLK-1 and thus may have further implications in the context of cancers or age-related diseases and infertility as it provides a novel link between mitochondria and mitosis.
Introduction
Mitochondria are organelles in which cellular respiration occurs, as well as a variety of other biosynthetic processes, such as the regulation of Ca2+, apoptosis, and the production of reactive oxygen species (Jeong and Seol 2008; Tilokani et al. 2018; Amorim et al. 2022). Artificially disrupting mitochondrial function in oocytes and early embryos was shown to cause defects in maturation (Al-Zubaidi et al. 2021), fertilization (Pasquariello et al. 2019), errors in cell division and chromosome segregation (Wang et al. 2020; Mikwar et al. 2020), and loss of viability and defects in metabolism (Al-Zubaidi et al. 2021). These observations suggest a link between mitochondrial defects, spindle assembly abnormalities, chromosome segregation errors and aneuploidy. As mitochondria are strongly involved in metabolism and the main generators of energy in form of ATP it is possible that changes in the available energy can negatively affect spindles. In particular, the function of motor proteins, which are required for spindle assembly and chromosome segregation depends on available ATP (Sweeney and Holzbaur 2018), and changes in the ATP content could impair motor function, ultimately leading to mitotic errors (Cheng et al. 2021). However, the mechanisms of how defects in mitochondria affect spindle function in mitosis have remained elusive.
The C. elegans Spindle-defect-3 (SPD-3) protein is a mitochondria localizing protein that was isolated in an EMS-mutagenesis screen in search of cell division mutants (O’Connell et al. 1998). Previous publications have shown that defects in the SPD-3 protein in the nematode C. elegans impair homolog pairing by causing a severe reduction in the mobility of SUN-1 aggregates at the start of meiotic prophase (Labrador et al. 2013). It was suggested that this defect is due to the reduced function of cytoskeletal motors in spd-3 mutants, caused by a defect in mitochondrial function and associated changes in ATP levels (Labrador et al. 2013). This was further supported by the observation of mitotic spindle positioning defects in one-cell C. elegans spd-3 mutant embryos, which are comparable to spindle positioning defects after dynein depletion (Yoder and Han 2001; Dinkelmann et al. 2007).
Here we show that mutation of spd-3 does not only impair spindle positioning as previously reported but also chromosome positioning and the nuclear integrity during mitosis. Interestingly, we found that Polo-like kinase (PLK-1) levels are significantly increased in the spd-3 mutant. PLK-1 is a crucial mitotic kinase that functions in many aspects of cell division, such as mitotic entry (Thomas et al. 2016), centrosome maturation (Fu et al. 2015), nuclear envelope (NE) disassembly (Martino et al. 2017; Linder et al. 2017; Dawson and Wente 2017), and asymmetric cell division (Han et al. 2018) in C. elegans and mammalian cells.
PLK1 function is not limited to mitotic events and cytokinesis. Plk1 also regulates heat-shock transcription factor 1 (Kim et al. 2005), p53 (Ando et al. 2004) microtubule dynamics (Joukov and De Nicolo 2018), and mitochondrial Ca2+ homeostasis (Lee et al. 2016). The tight regulation of Plk1 during the cell cycle is essential to mitotic progression and increased levels of Plk1 have been affiliated with cancer. Increasing evidence indicates that Plk1 overexpression correlates with poor clinical outcomes (Martini et al. 2019), yet the detailed mechanisms of Plk1 regulation have remained unknown. Our data suggests that elevated PLK-1 in early C. elegans embryos leads to premature nuclear disassembly and could also account for the observed spindle defects. This finding provides a key link between mitochondria and mitosis by affecting master regulators of mitosis.
Results and Discussion
The mitochondrial protein SPD-3 is required for mitotic events
To further determine the functions of the mitochondrial protein SPD-3 during mitosis we characterized the spd-3(oj35) mutant, which was previously shown to impact spindle alignment (Dinkelmann et al. 2007). The spd-3(oj35) mutant was isolated in an ethyl methanesulfonate (EMS) mutagenesis screen in search of cell division mutants and carries a single cytosine-to-thymidine transition resulting in a leucine-to-phenylalanine change at amino acid 130 (O’Connell et al. 1998; Dinkelmann et al. 2007). The spd-3(oj35) strain is a temperature-sensitive, maternal-effect mutant, which is defective in meiosis (Labrador et al. 2013) and mitosis (O’Connell et al. 1998, Dinkelmann et al. 2007). In addition to the spd-3(oj35) mutant, 3 additional mutant strains are available (Fig. S1). Two deletion alleles, spd-3(ok1817) and spd-3(tm2969), which are null spd-3 alleles (Labrador et al. 2013). The spd-3(me85) mutant strain carries an early stop mutation and has severe meiotic defects impeding the analysis of mitosis (Labrador et al. 2013). Thus, we decided to focus on the effects of spd-3 mutation in the spd-3(oj35) strain.
To characterize the effects on mitotic spindle alignment in spd-3(oj35), we filmed embryos co-expressing mCherry::γ-tubulin and mCherry::histone H2B. Following fertilization in control (WT) embryos, the pronuclear-centrosomal complex moves to the center and rotates to align the mitotic spindle along the anterior/posterior axis (A/P axis) (Fig. S2A). This rotation is impaired in spd-3(oj35) embryos, resulting in a mitotic spindle that is misaligned to the A/P axis. Consistent with the previous report (Dinkelmann et al. 2007), we observed 54.5% of spindle misalignment at the metaphase stage in one-cell-stage spd-3(oj35) embryos (Fig. S2B). Moreover, the metaphase spindles in the spd-3(oj35) embryos are shorter in comparison to control spindles (Fig. 1A, control 14.8 ± 0.6μm (Stdev), spd-3(oj35) 12.7 ± 1.4μm). Quantification of the duration of mitosis in one-cell-stage embryos revealed that mitosis is prolonged in the spd-3(oj35) mutant embryos. While the spd-3(oj35) mutant embryos display a similar duration of the prophase stage of mitosis, which is defined as the time from pronuclear meeting to NEBD (Fig. S2C), the pro-metaphase stage, which is defined as the time from NEBD to anaphase onset, is extended (Fig. 1B). These results suggest that spd-3(oj35) does not only affect spindle positioning but also spindle length and the timing of mitotic events.
Figure 1.
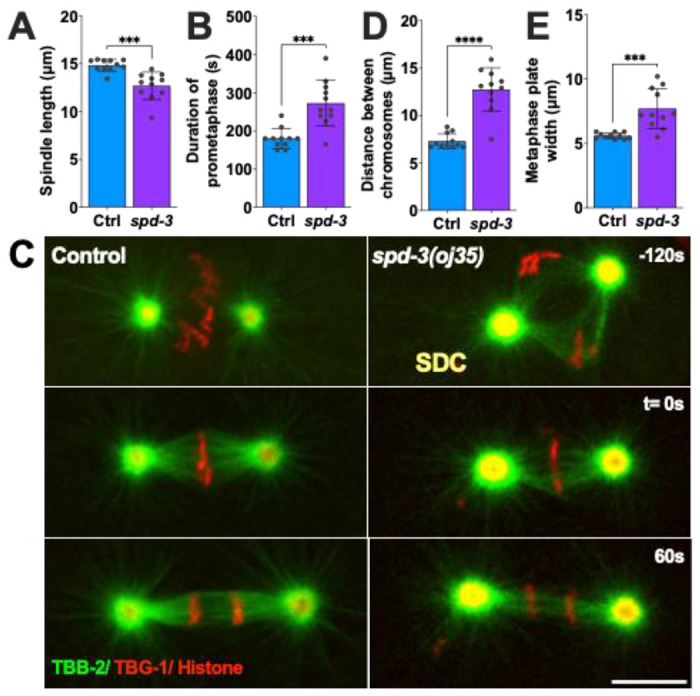
Duration of mitosis, spindle length and chromosome positioning are affected in spd-3(oj35) one-cell stage C. elegans embryos. (A) Graph plotting mitotic spindle length (pole-to-pole distance) at anaphase onset in control (Ctrl, blue bar) and spd-3(oj35) (spd-3, purple bar). (B) Plot showing prometaphase duration as defined by the time between NEBD and anaphase onset. (C) Representative fluorescence confocal images of control (left) and spd-3(oj35) (right) embryos expressing GFP::β-tubulin, mCherry::γ-tubulin, and mCherry::histone H2B. SDC indicates the socially distant chromosome stage observed in spd-3(oj35) embryos (top right). Scale bar, 10 μm. (D) Plot of the distance between chromosomes at the SDC stage. (E) Plot of the width of the metaphase plate at anaphase onset. (B, C) Times are in seconds relative to anaphase onset (t= 0s). (A, B, D, E) Error bars are SD. n = 11 embryos for control and spd-3. The significance of differences between results was determined by two-tailed Student’s t-tests, ***P<0.001, ****P<0.0001.
The SPD-3 is critical for mitotic chromosome positioning during congression
During our analysis of mitosis in the spd-3(oj35) embryos we noticed that the mutant embryos display an additional unusual phenotype during chromosome positioning and alignment. Instead of forming a normal metaphase plate in the center of the embryo, the individual sets of chromosomes (paternal and maternal) are initially positioned on the periphery of the respective nuclei, giving rise to a transient diamond shaped spindle configuration (Fig. 1C, Fig. S2D), before converging to form a metaphase plate. Inspired by the current pandemic we called this abnormal positioning stage the “socially distanced chromosome (SDC) positioning” (Fig. 1C) as the two individual sets of chromosomes, paternal and maternal, showed a large physical distance (Fig. 1D). While the chromosomes eventually form a metaphase plate in the spd-3(oj35) mutant, the resulting metaphase plates are significantly wider (Fig. 1E, control: 5.6±0.2 μm, spd-3(oj35): 7.3±1.4 μm) in comparison to control metaphase plates. This observation suggests an additional role for SPD-3 during chromosome congression.
The mitotic phenotypes of the spd-3(oj35) mutant are not caused by changes in energy metabolism
SPD-3 was identified as a mitochondria-localizing protein based on colocalization of SPD-3::GFP and mitochondria stained with MitoTracker Red CMXRos (MTR) (Fig. S1B) and immuno-EM (Dinkelmann et al. 2007, Labrador et al. 2013). We wanted to determine the mechanisms of how the mitochondrial protein SPD-3 affects chromosome positioning. As it was previously suggested that defects in the mitochondrial function in the spd-3(oj35) could lead to reduced function of cytoskeletal motors, possibly caused by variations in ATP levels, we measured the metabolic rates in spd-3(oj35) embryos by fluorescence lifetime imaging microscopy (FLIM) of NADH autofluorescence (Schneckenburger 1992; Sud et al. 2006). FLIM NADH offers a marker-free readout of the mitochondrial function of cells in their natural microenvironment and allows different pools of NADH to be distinguished within a cell (Blacker et al. 2014). NADH autofluorescence can be used as a marker of cellular redox state and indirectly also of cellular energy metabolism (Schneckenburger 1992; Sud et al. 2006). These measurements show that metabolic rates in spd-3(oj35) embryos are increased in comparison to control (Fig. S3). This agrees with previously reported increased ATP levels (Dinkelman et al 2007).
In order to test if elevated (or reduced) ATP could lead to similar mitotic phenotypes we examined the effect of depleting proteins that are part of the electron transfer chain (ETC) in C. elegans (Dancy 2015) (Table S1). CLK-1 is required for the biosynthesis of ubiquinone, a carrier in the ETC, and depletion of CLK-1 leads to elevated ATP levels (Braeckman et al. 1999; Dancy 2015). We screened embryos of the null allele clk-1(qm30) for defects in spindle and chromosome positioning but could not reproduce any of the observed phenotypes of the spd-3 (oj35) mutant in respect to spindle and chromosome positioning. We further analyzed the effects of isp-1(qm150), ucr-1(RNAi), mev-1 (RNAi), or cco-1 (RNAi), which alter the ETC function resulting in decreased ATP levels (Dillin et al. 2002; Dancy 2015). While we did observe spindle misalignment phenotypes in all examined depletions, we never detected the SDC positioning phenotype in those mitochondrial mutants (Table S1).
In summary, the spd-3(oj35) phenotype does not mimic the disruption of mitochondrial genes, suggesting that metabolic perturbations in spd-3(oj35) worms may not directly lead to the observed mitotic defects. In agreement with this, also treatment of control embryos with mitochondrial inhibitors (Dinkelman et al 2008) did not reproduce the spd-3(oj35) phenotype suggesting that the observed phenotypes are not based on misregulation of ATP levels alone.
SPD-3 is important for ER morphology
As we observed an effect on the timing from NEBD to anaphase we decided to further investigate the arrangement of ER and nucleus. The ER surrounds and encloses the pronucleus, forming a characteristic bi-membraned nuclear envelope that protects the chromatin. In order to determine the arrangement of the ER in the spd-3(oj35) mutant we generated a mutant strain that co-expressed GFP labeled SP12, an ER lumen marker, and mCherry labeled histone. Analysis of this strain revealed that the morphology of the ER is severely affected in the spd-3(oj35) mutant (Fig. 2A–C). The ER shows a significant increase in the formation of ER clusters and drastic changes in the morphology surrounding the nucleus (Fig. 2B, C). In control embryos clusters begin to form 238.1±21.9 s before anaphase onset (Fig. 2D) and become enriched around the centrosome and mitotic spindle (Fig. 2B, C). In general, the distribution of ER clusters is asymmetric (Poteryaev et al. 2005), with more clusters localizing in the anterior half of the embryo in the vicinity of the cortex (Fig. 2A). In the spd-3(oj35) mutant, excessive ER clusters begin to form earlier, at 367.7±17.5 s before anaphase onset (Fig. 2D), and are found in the cytoplasm, around the nucleus and on the cortex in both the anterior and posterior sides of the spd-3(oj35) embryo (Fig. 2A–C). In addition, gaps appear in between centrosome and pronuclei (white arrow) and in between pronuclei (yellow arrow) before anaphase onset in spd-3(oj35) embryos. These results show that ER morphology is affected in the early embryonic stage of the spd-3(oj35), suggesting a potential role for SPD-3 in these processes.
Figure 2.
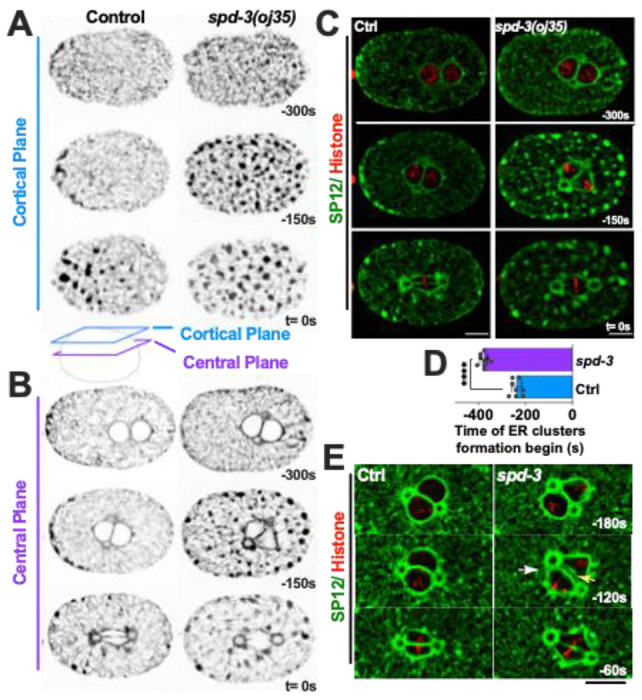
The point mutation of SPD-3 results in abnormal ER morphology. (A, B) Spinning-disk confocal images of control (left) and spd-3(oj35) (right) embryos expressing GFP::SP12 and mCherry::histone H2B. Cartoon indicates the position of a cortical plane just beneath the embryo surface (blue, A) and a central plane (purple, B) from control and spd-3(oj35) are shown. (C) Merge of the images shown in A. Control left, spd-3(oj35) right (D) Graph plotting the time mitotic ER clusters begin to form in the cortical section of embryos expressing GFP::SP12 in mitosis (n = 8 embryos for control and spd-3). Error bars are SD. Significance was determined by t-tests. ****p<0.0001. (E) Spinning-disk confocal images of the ER shape surrounding the pronucleus in control (left) and spd-3(oj35) (right) embryos coexpressing mCherry::histone H2B and GFP::SP12.. The white arrow indicates a gap appearing between the centrosome and pronuclei (t= −120s). The yellow arrow indicates a gap appearing between the pronuclei (t= −120s). (A-E) Times are in seconds relative to anaphase onset (t= 0s). Scale bars, 10μm.
In order to determine the potential effects of ER morphology on spindle and chromosome positioning, we analyzed a number of ER mutants that show drastic changes in ER morphology, in particular the ratio of clusters to tubes. The RAB-5 and YOP-1/RET-1 proteins are important for regulating the ER morphology during mitosis and controlling the kinetics of nuclear envelope disassembly (Audhya et al. 2007). The endosomal Rab-type GTPase, RAB-5, plays a role in the homotypic fusion of ER membranes (Audhya et al. 2007). The YOP-1, homologs of DP1/NogoA, localize to the ER and play a functionally redundant role in generating tubular morphology in the ER (Audhya et al. 2007). In YOP-1 and RAB-5 depleted embryos, there are fewer thick ER tubules that are poorly organized and no mitotic ER clusters form. We treated spd-3(oj35) worms with rab-5 (RNAi) (data not shown) and yop-1 (RNAi) with the aim to reduce the number of ER clusters in the cytoplasm. While we did observe fewer and smaller ER clusters in the spd-3(oj35) mutant after rab-5 and yop-1 depletion, the SDC positioning phenotype was still present (Fig. S4).
Next, as ER dynamics and morphology changes can also be induced by ER stress, we tested whether the ER stress-unfolded protein response (UPRER) is changed in the spd-3(oj35) mutant. During C. elegans UPRER two homologs of the ER-resident heat-shock protein BiP, HSP-3 and HSP-4, are essential for the formation of sheet-like ER structures (Poteryaev et al. 2005). Depletion of HSP-3 leads to upregulation of the hsp-4 gene in C. elegans (Kapulkin et al. 2005). To examine the UPRER in spd-3(oj35), we imaged worms expressing the zcIs4 transgene, a transcriptional fusion of the hsp-4 promoter to GFP, that can be used to quantify HSP-4 expression levels (Calfon et al. 2002). The zcIs4 reporter is strongly upregulated in response to treatments inducing ER stress and can thus function as a read out for UPRER activity (Calfon et al. 2002). We found an overall lower HSP-4 expression level in spd-3(oj35) mutant worms by quantification of fluorescence levels, indicating an approximately 11 fold decrease in hsp-4::gfp expression (Fig. S5A, B). This result suggested that changes in ER morphology could be induced by blocking UPRER leading to low HSP-4 expression levels in the spd-3(oj35) mutant. To further determine whether the mitotic defects could be rescued by increasing HSP-4, we treated spd-3(oj35) mutant with hsp-3 (RNAi) to increase the endogenous hsp-4 transcription (Kapulkin et al. 2005). However, the chromosome positioning and spindle defects in the spd-3(oj35) mutant are not prevented by hsp-3 (RNAi) (data not shown). In control embryos hsp-3 (RNAi) does not affect ER dynamics in the early embryo. In contrast, when HSP-4 function is reduced by RNAi, the ER accumulates in multiple foci, and the ER morphology is distinctly disrupted. However, we did not observe any chromosome positioning and spindle defects in HSP-4 depleted embryos (data not shown). Taken together, our results suggest that perturbation of the mitochondrial protein SPD-3 affects the morphology of the ER and that this might be induced by the UPRER regulatory system. However, the structural changes of the ER are most likely not responsible for the observed chromosome positioning and spindle alignment defects in the spd-3(oj35) mutant embryos.
SPD-3 is critical for nuclear envelope structure and dynamics
Our analysis of the ER morphology revealed a significant change in the shape of the pronucleus in spd-3(oj35) mutant. As the ER and the nucleus are intimately connected, we generated strains that carry either NPP-22::mNeonGreen (NPP-22::mNG) (Mauro et al. 2022), a conserved transmembrane nucleoporin, or GFP tagged lamin (Link et al. 2018), which enabled us to analyze the shape and organization of the nuclear envelope during mitosis. Analysis of these strains by light-microscopy showed that the nuclear shape is significantly altered during prometaphase in spd-3(oj35), consistent with the observed changes in ER morphology surrounding the spindle (Fig. 3A). Analysis of the lamin GFP in the spd-3(oj35) mutant background showed that in comparison to control embryos lamin is disassembled prematurely, approximately 96.5s earlier in comparison to control embryos (Fig. 3B, C). In addition, the lamin of the paternal pronucleus (164.8±52.8s) disappears earlier than the lamin on the maternal pronucleus (120.8±38.4s) (Fig. 3D). This indicates that the structural integrity of the nucleus is impaired in the spd-3(oj35) mutant and that this could potentially affect the positioning of the spindle as well as chromosomes, possibly by destabilizing nuclear membrane attachments to the centrosome, microtubules and chromosomes.
Figure 3.
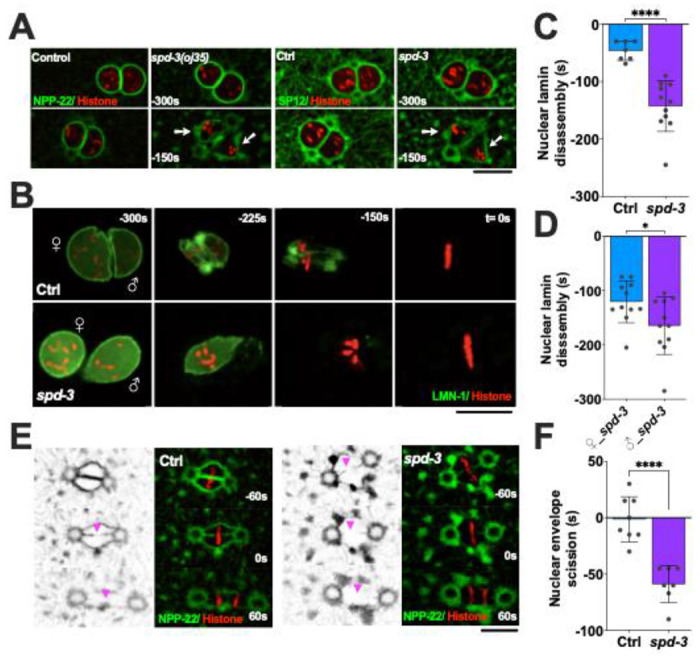
SPD-3 is involved in structural integrity and disassembly of the nuclear envelope during mitosis. (A) Spinning-disk confocal images of nuclear morphology in control (Ctrl) and spd-3(oj35) (spd-3) embryos coexpressing mCherry::histone H2B and NPP-22::mNG (left) or GFP::SP12 (right). The white arrows indicate the abnormal nuclear shape in spd-3(oj35). (B) Time-lapse sequences of control (left) and spd-3(oj35) (right) embryos expressing GFP::LMN-1 and mCherry::histone H2B. ♀ indicates female pronucleus and ♂ indicates male pronucleus. Lamin disassembles prematurely and asymmetrically in spd-3(oj35) embryos. (C) Plot of the timing of complete nuclear lamin disappearance in embryos co-expressing GFP::LMN-1 and mCherry::histone H2B in control (n=7) and spd-3(oj35) (n=11). (D) Plot of the timing of complete lamin disassembly in the female (left, n=11) and male pronucleus (right, n=11) in spd-3(oj35) embryos. (E) Fluorescence confocal images of control and spd-3(oj35) embryos expressing NPP-22::mNG and mCherry::histone H2B. Magenta arrowheads mark the site of nuclear envelope scission in control (left) and spd-3(oj35) (right). (F) Graph plotting time of nuclear envelope scission in control (n=8) and spd-3(oj35) (n=7) embryos. (A-F) Times are in seconds relative to anaphase onset (t= 0s). Scale bars, 10 μm. (C, D, F) Error bars are SD. The significance of the difference between strains was determined by t-tests. *P<0.05, ****P<0.0001.
Early nuclear envelope scission in prometaphase
The timely disassembly of the nuclear lamina is required for chromosome alignment and NE scission to allow parental chromosomes to merge on the metaphase plate in C. elegans (Rahman et al. 2015; Chase et al. 2000; Velez-Aguilera et al. 2020). We next examined whether NE scission changes between the juxtaposed pronuclei by labeling of NE (NPP-22::mNG) or ER (GFP::SP12). The NE scission event occurs less than 3 s (1.4-2.5 s) before anaphase onset in control embryos and the forming membrane gap is visible (Fig. 3E, F, Fig. S6A–C). In contrast, the NE scission event occurs early, 40-60 s (37.5-58.9 s) before anaphase onset, in spd-3(oj35) embryos.
In control embryos the reformation of new NE around the segregated chromosomes begins approximately 86.7±20.9 s after anaphase onset and the new NE is completed after 161.7±27.8 s (Fig. S6D, E). The reassembly of the NE is slightly delayed in spd-3(oj35) embryos as it is initiated 101.7±18.0 s and completed 180.0±29.0 S after anaphase onset (Fig. S6D, E). Combined these results suggest that SPD-3 is critical for the dynamics of NE disassembly during the first mitotic division in C. elegans.
Spindle and chromosome positioning defects in spd-3(oj35) are caused by PLK-1 overexpression
Nuclear disassembly is triggered by phosphorylation of lamin at multiple residues in the head and tail domain by PLK-1 in C. elegans (Chase et al. 2000; Rahman et al. 2015; Velez-Aguilera et al. 2020). Therefore, we generated a strain expressing PLK-1::GFP in spd-3(oj35) mutant to monitor the localization and expression of PLK-1. To our surprise, this strain revealed excessive amounts of PLK-1 in the spd-3(oj35) mutant localizing to centrosomes, the nuclear envelope, nucleoplasm, and chromosomes (Fig. 4A, C). We further confirmed that PLK-1 is increased by around 6-fold in the spd-3(oj35) mutant compared to N2 animals by immunoblot (Fig. 4B).
Figure 4.
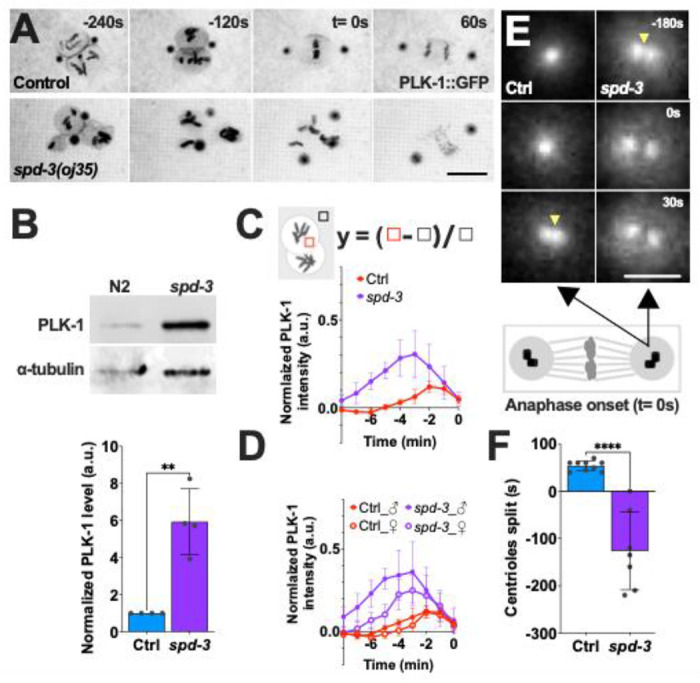
PLK-1 overexpression in the spd-3(oj35) mutant. (A) Time-lapse sequences of embryos expressing PLK-1::GFP in control (Ctrl, top) and spd-3(oj35)(spd-3, bottom). Scale bar, 10 μm. (B) Top: Western of PLK-1 protein in N2 (n=4) and spd-3(oj35) (n=4) worms, bottom: Quantification of PLK-1 expression level. (C, D) Quantification of normalized PLK-1::GFP intensity in the nucleoplasm of embryos measured from time-lapse sequences as in (A). (C) The value of PLK-1::GFP intensity in control (red, n=6) and spd-3(oj35) (purple, n=6). (D) The value of PLK-1::GFP intensity in the nucleoplasm of male and female pronuclei. Solid circles indicate the male pronucleus (n=6), empty circles indicate the female pronucleus (n=6). Cartoon showing the method used for quantification of the PLK-1::GFP fluorescence in nucleoplasm. Briefly a fixed-size box is drawn in nucleoplasm (red box) and in cytoplasm (black box) as background at each time point. The normalized PLK-1 level is calculated as [(integrated intensity in red box – integrated intensity in black box)/ integrated intensity in black box]. ♀ indicates female pronucleus and ♂ indicates male pronucleus. (E) Spinning-disk confocal images of the centrosome in control (left) and spd-3(oj35) (right) embryos expressing PLK-1::GFP. Yellow arrowheads indicate the time of centrioles splitting. Schematic showing that each centrosome contains a pair of centrioles. Black arrows indicate a centrosome. Scale bar, 2 μm. (F) Graph plotting the time of detectable centriole pair splitting in control (left, n=6 centrosomes) and spd-3(oj35) (right, n=6 centrosomes). (A, C, D, E, F) Times are in seconds relative to anaphase onset (t= 0). (B, C, D, F) Error bars are SD. The significance of the difference between strains was determined by t-tests. **P<0.01, ****P<0.0001.
In C. elegans, PLK-1 is recruited to the nuclear pore complexes (NPC) by nucleoporins NPP-1, NPP-4, and NPP-11 prior to NEBD where the importins α/β IMA-2 and IMB-1 promote the nuclear import of PLK-1, where it phosphorylates nuclear lamin (Martino et al. 2017). To determine the time point of PLK-1 import into the nucleus, we quantified the nuclear fluorescence of PLK-1::GFP in nucleoplasm during mitosis. The intensity of PLK-1::GFP reaches a peak about 2 minutes prior to anaphase onset in both pronuclei of control embryos (Figure 4C). This peak is shifted in spd-3(oj35) embryos to about 3 minutes prior to anaphase onset. In addition we detected an overall increased fluorescence of PLK-1::GFP in the paternal pronucleus relative to the maternal pronucleus (Fig. 4D). This result is consistent with the observed premature, faster lamin disassembly in the paternal pronucleus (Fig. 3D). In addition to the nucleoplasm, more PLK-1::GFP localizes to centrosomes. Centrosomes consist of a centriole pair surrounded by pericentriolar material (PCM) that nucleates and anchors microtubules (MTs) in C. elegans. In general, the centriole pair splits 53.8±10.5 s after anaphase onset during mitosis (Fig. 4E, F). Surprisingly, the centriole pair splits 126.4±82.1 s before anaphase onset in spd-3(oj35) embryos (Fig. 4E, F). This result is consistent with overexpression and inhibition of Plk1 affecting the centrioles disengagement (Lončarek et al. 2010; Schöckel et al. 2011; Fu et al. 2015). This data indicates that overexpression of PLK-1 leads to premature centrioles splitting independent of the cell cycle regulation.
To determine whether reducing PLK-1 in the nucleoplasm can avoid the socially distanced chromosomes and rescue spindle misalignment, we exposed the spd-3(oj35) worms to ima-2 (RNAi) to prevent nuclear import of PLK-1 . In control embryos ima-2 (RNAi) prevents the nuclear import of PLK-1 and this its localization to kinetochores and chromatin (Martino et al. 2017) (Fig. S7A). In addition, previous work showed that ima-2 (RNAi) is essential for spindle assembly and nuclear envelope formation in the C. elegans embryo (Askjaer et al 2002). In spd-3(oj35) mutant embryos, PLK-1::GFP still localizes to nucleoplasm and chromatin after ima-2 (RNAi) in embryos (Fig. S7A), however the amount of PLK-1 is reduced and the peak of PLK-1 fluorescence is shifted to about 2 minutes prior to anaphase onset, similar to control embryos (Fig. S7B). Interestingly, the SDC and spindle misalignment are rescued by ima-2 (RNAi) supporting the hypothesis that both phenotypes are induced by elevated amounts of PLK-1 in the nucleus (Fig. S7C, D). To test this hypothesis, we exposed the spd-3(oj35) worms to short treatments of plk-1 (RNAi) to reduce the elevated levels of PLK-1 to approximately wild-type levels (Fig 5A, B). Reduction of the PLK-1 overexpression does indeed rescue the socially distanced chromosome positioning (Fig. 5C). The spindle positioning defects seemed slightly reduced but this reduction was not significant (Fig. S7E). This supports our hypothesis that PLK-1 overexpression in the spd-3(oj35) mutant affects the nuclear integrity by inducing premature and asymmetric nuclear envelope disassembly and that this most likely affects proper chromosome and spindle positioning.
Figure 5.
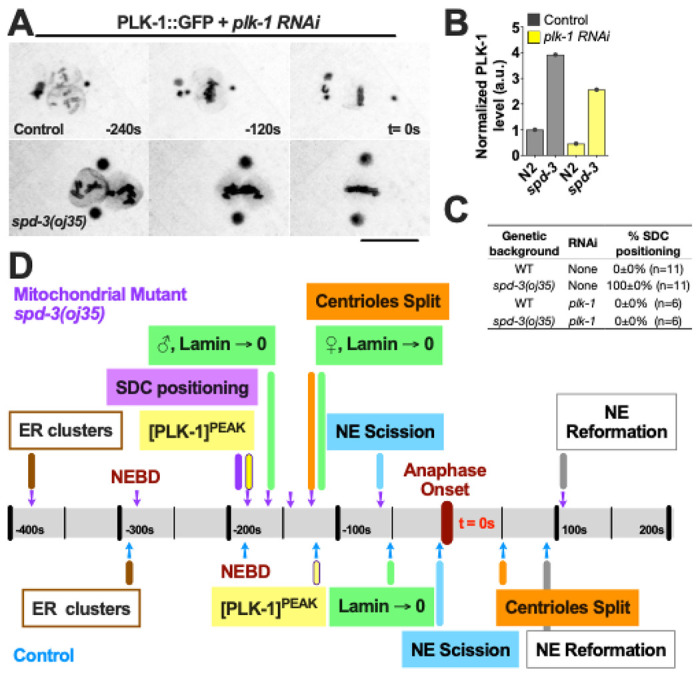
Mitotic phenotypes in the spd-3(oj35) embryos result from PLK-1 overexpression. (A) Time-lapse sequences of embryos expressing PLK-1::GFP in control (Ctrl, top) and spd-3(oj35) (spd-3, bottom) after plk-1 (RNAi) were filmed using a spinning-disk confocal. Scale bar, 10 μm. (B) Graph of the quantification of PLK-1 expression level in lysate from N2 and spd-3(oj35) without (gray bars, n=1 for each strain) and with plk-1 (RNAi) (yellow bars, n=1 for each strain) as determined by western blot. (C) Plot of the percentage of socially distanced chromosome (SDC) positioning in control and spd-3(oj35) without (gray bars, n=6 for each strain) and with plk-1 (RNAi) (yellow bars, n=6 for each strain) treatment. (D) Schematic showing the timeline of mitotic events in control and spd-3(oj35). (A, D) Times are in seconds relative to anaphase onset (t= 0).
To determine if the abnormal ER morphology is also triggered by PLK-1 overexpression we examined the ER structure after plk-1 (RNAi). We found that the ER morphology and distribution of ER clusters are not significantly different between control and plk-1 (RNAi) in spd-3(oj35) embryos, suggesting that the changes in ER morphology are not induced by elevated PLK-1 levels (Fig. S8A, B). Our finding is thus consistent with the result that the removal of ER clusters cannot rescue socially distanced chromosomes (Fig. S4). Taken together we show that defects in SPD-3 cause a spindle positioning defect and an unusual chromosome positioning, socially distanced chromosomes (SDC), phenotype during mitosis in spd-3(oj35) embryos. The fact that we did not observe comparable phenotypes in embryos defective in ATP production, suggests that mitochondrial ETC pathways are not involved in this process. The abnormal spindle and chromosome positioning are accompanied by altered ER and NE morphology, and premature and asymmetric nuclear lamin disassembly in prometaphase (Fig. 5D).
Our results revealed an increase in PLK-1 levels in the spd-3(oj35) embryos. As PLK-1 plays a direct role in lamin disassembly, by phosphorylating lamin and thus targeting it for degradation (Velez-Aguilera et al. 2020), this suggests that the elevated levels of PLK-1 are driving the premature lamin disassembly in the spd-3(oj35) mutant. In addition, our data showed that the paternal pronucleus disassembled several seconds before the maternal pronucleus. As PLK-1 localizes to centrosomes, which are initially associated with the male pronucleus this indicates that the asymmetric disassembly of the two pronuclei is also linked to excessive levels of PLK-1. We propose that the chromosome alignment defects and premature lamin disassembly, the previously described spindle positioning defect (Dinkelmann et al, 2007), and the reported reduction in mobility of SUN-1 aggregates and pairing-center binding proteins on the nuclear envelope in spd-3(me85) mutant (Labrador et al 2013) could be linked to the elevated PLK-1 levels in the spd-3(oj35) mutant.
The linker of nucleoskeleton and cytoskeleton (LINC) complex connects the nuclear lamina to cytoplasmic MTs and also contributes to centrosome attachment to the outer nuclear membrane (Cain et al. 2018). The phosphorylation of SUN1, a component of the LINC complex by Plk1 and CDK1, inhibits SUN1 interaction with the nuclear lamina (Labella et al. 2011; Patel et al. 2014). It is possible that more PLK-1 in the nucleoplasm leads to a destabilization of the outer nuclear membrane attachment to the centrosome by phosphorylation of SUN-1 and premature lamin disassembly and that this could affect spindle positioning. In addition, elevated levels of PLK-1 on the chromosomes could also affect kinetochore assembly and function and thus result in chromosome positioning defects. Lastly, we noticed that the male pronucleus expands before the pronuclear meeting (Fig. S9), the PCM increases in size and microtubule nucleation is increased in spd-3(oj35), which could all be induced by increased PLK-1 levels (Fig. S10).
Besides mitosis, PLK-1 also plays roles in mitochondrial Ca2+ homeostasis and ATP production (Lee et al. 2016). It has been shown that mitochondrial Ca2+ can positively regulate the activities of the tricarboxylic acid (TCA) cycle and electron transport chain components, stimulating ATP production (Shanmughapriya et al. 2015). The mitochondrial rho GTPase Miro controls mitochondrial Ca2+ homeostasis at the ER-mitochondria contact sites, and its activity is regulated by Polo kinase in Drosophila (Lee et al. 2016). Since spd-3(oj35) has excessive amounts of PLK-1, elevated ATP levels could be triggered by increased Miro activation affecting Ca2+ import into mitochondria. It would be interesting to further examine the interaction between ER and mitochondria and mitochondrial Ca2+ homeostasis in C. elegans.
The remaining question is how the mitochondrial protein SPD-3 affects or regulates PLK-1 levels during mitosis in c. elegans. At this point we can only speculate, but possible mechanisms include i) regulation of mRNA levels (Martin and Strebhardt 2006), ii) mRNA translation (Tanenbaum et al. 2015), iii) post-translational modifications (Macůrek et al. 2008), or iv) protein stability (Cordeiro et al. 2020). Further detailed analysis will be necessary to determine the mechanisms and pathways utilized by SPD-3 that led to increased PLK-1 levels.
In summary, our work provides a novel link between mitochondria, nuclear envelope dynamics and chromosome positioning by increasing the amount of a key mitotic regulator, PLK-1. This finding does not only provide a new role of the mitochondrial protein SPD-3 during mitosis in C. elegans but may also have further implications in the context of cancers or age-related diseases and infertility as it provides a novel link between mitochondria and mitosis.
Materials and methods
C. elegans strains and RNA-mediated interference
All C. elegans strains were cultured at 16°C before imaging and experiments (Brenner 1974). C. elegans strains and RNAi feeding clones are listed in the Supplemental Material. L4 or young adult hermaphrodites were fed with RNAi bacteria and incubated for 6-48 h at room temperature (23°C) before dissection to obtain embryos for filming (for details, see the Supplemental Material).
Live cell imaging
For live imaging of embryos, worms were dissected on glass coverslips in M9 buffer and then mounted on 2% agar pads. Imaging was conducted at 23°C (room temperature) and carried out on a on a 3i VIVO spinning-disc confocal microscope (Z1; Carl Zeiss) equipped with 488 nm and 561 nm diode laster and a Hamamatsu ORCA-Flash4.0 scientific CMOS camera (Hamamatsu) for detection. Acquisition parameters were controlled using the Sildebook 6.0 software (3i - Intelligent Imaging). For details, see the Supplemental Material.
Supplementary Material
Acknowledgments
We thank the Caenorhabditis Genetics Center, which is funded by the National Institutes of Health (NIH) National Center for Research Resources (NCRR), Jessica L. Feldman (Stanford University), Shirin Bahmanyar (Yale University), and Verena Jantsch-Plunger (University of Vienna) for strains; Orna Cohen-Fix (NIH) for RNAi feeding clones; Monica Gotta (University of Geneva) for PLK-1 antibody; Thomas Müller-Reichert (Technische Universität Dresden), Kevin F. O’Connell (NIH), and Shirin Bahmanyar (Yale University) for critical comments and discussions; Horst Wallrabe for the 2-photon FLIM microscopy training (The W.M. Keck Center for Cellular Imaging, University of Virginia). This work was supported by grant from the NIH NIGMS 1R01GM144668.
References
- Al-Zubaidi U, Adhikari D, Cinar O, Zhang Q-H, Yuen WS, Murphy MP, Rombauts L, Robker RL, Carroll J. 2021. Mitochondria-targeted therapeutics, MitoQ and BGP-15, reverse aging-associated meiotic spindle defects in mouse and human oocytes. Human Reproduction 36: 771–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amorim JA, Coppotelli G, Rolo AP, Palmeira CM, Ross JM, Sinclair DA. 2022. Mitochondrial and metabolic dysfunction in ageing and age-related diseases. Nat Rev Endocrinol 18: 243–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando K, Ozaki T, Yamamoto H, Furuya K, Hosoda M, Hayashi S, Fukuzawa M, Nakagawara A. 2004. Polo-like Kinase 1 (PLK-1) Inhibits p53 Function by Physical Interaction and Phosphorylation. Journal of Biological Chemistry 279: 25549–25561. [DOI] [PubMed] [Google Scholar]
- Askjaer P, Galy V, Hannak E, and Mattaj Iain. 2002. Ran GTPase cycle and importins and are essential for spindle formation and nuclear envelope assembly in living Caenorhabditis elegans embryos. Molecular Biology of the Cell 13, 4355–4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audhya A, Desai A, Oegema K. 2007. A role for Rab5 in structuring the endoplasmic reticulum. J Cell Biol 178: 43–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blacker TS, Mann ZF, Gale JE, Ziegler M, Bain AJ, Szabadkai G, Duchen MR. 2014. Separating NADH and NADPH fluorescence in live cells and tissues using FLIM. Nat Commun 5: 3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braeckman BP, Houthoofd K, Vreese AD, Vanfleteren JR. 1999. Apparent uncoupling of energy production and consumption in long-lived Clk mutants of Caenorhabditis elegans. Current Biology 9: 493–497. [DOI] [PubMed] [Google Scholar]
- Brenner S. 1974. The genetics of Caenorhabditis elegans. Genetics 77: 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain NE, Jahed Z, Schoenhofen A, Valdez VA, Elkin B, Hao H, Harris NJ, Herrera LA, Woolums BM, Mofrad MRK, et al. 2018. Conserved SUN-KASH Interfaces Mediate LINC Complex-Dependent Nuclear Movement and Positioning. Current Biology 28: 3086–3097.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calfon M, Zeng H, Urano F, Till JH, Hubbard SR, Harding HP, Clark SG, Ron D. 2002. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature 415: 92–96. [DOI] [PubMed] [Google Scholar]
- Cao R, Wallrabe H, Siller K, Rehman Alam S, Periasamy A. 2019. Single-cell redox states analyzed by fluorescence lifetime metrics and tryptophan FRET interaction with NAD(P)H: Single-Cell Redox States Analyzed by FLIM. Cytometry 95: 110–121. [DOI] [PubMed] [Google Scholar]
- Chase D, Serafinas C, Ashcroft N, Kosinski M, Longo D, Ferris DK, Golden A. 2000. The polo-like kinase PLK-1 is required for nuclear envelope breakdown and the completion of meiosis in Caenorhabditis elegans. genesis 26: 26–41. [DOI] [PubMed] [Google Scholar]
- Cheng A, Jiang Y, Wang T, Yu F, Ishrat I, Zhang D, Ji X, Chen M, Xiao W, Li Q, et al. 2021. Energy restriction causes metaphase delay and chromosome mis-segregation in cancer cells. Cell Cycle 20: 1195–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordeiro MH, Smith RJ, Saurin AT. 2020. Kinetochore phosphatases suppress autonomous Polo-like kinase 1 activity to control the mitotic checkpoint. Journal of Cell Biology 219: e202002020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dancy BM. 2015. Mitochondrial bioenergetics and disease in Caenorhabditis elegans. Front Biosci 20: 198–228. [DOI] [PubMed] [Google Scholar]
- Dawson TR, Wente SR. 2017. The PLK-1 Piece of the Nuclear Envelope Disassembly Puzzle. Developmental Cell 43: 115–117. [DOI] [PubMed] [Google Scholar]
- Dillin A, Hbu A-L, Arantes-Oliveira N, Lehrer-Graiwer J, Hsin H, Fraser AG, Kamath RS, Ahringer J, Kenyon C. 2002. Rates of Behavior and Aging Specified by Mitochondrial Function During Development. Science 298: 2398–2401. [DOI] [PubMed] [Google Scholar]
- Dinkelmann MV, Zhang H, Skop AR, White JG. 2007. SPD-3 Is Required for Spindle Alignment in Caenorhabditis elegans Embryos and Localizes to Mitochondria. Genetics 177: 1609–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, Hogan IM, Glover DM. 2015. The Centrosome and Its Duplication Cycle. Cold Spring Harb Perspect Biol 7: a015800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han B, Antkowiak KR, Fan X, Rutigliano M, Ryder SP, Griffin EE. 2018. Polo-like Kinase Couples Cytoplasmic Protein Gradients in the C. elegans Zygote. Current Biology 28: 60–69.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong S-Y, Seol D-W. 2008. The role of mitochondria in apoptosis. BMB Rep. 41: 011–022. [DOI] [PubMed] [Google Scholar]
- Joukov V, De Nicolo A. 2018. Aurora-PLK-1 and mitosis_2018R Science. Sci Signal 11: eaar4195. [DOI] [PubMed] [Google Scholar]
- Kapulkin V, Hiester BG, Link CD. 2005. Compensatory regulation among ER chaperones in C. elegans. FEBS Letters 579: 3063–3068. [DOI] [PubMed] [Google Scholar]
- Kim S-A, Yoon J-H, Lee S-H, Ahn S-G. 2005. Polo-like Kinase 1 Phosphorylates Heat Shock Transcription Factor 1 and Mediates Its Nuclear Translocation during Heat Stress. Journal of Biological Chemistry 280: 12653–12657. [DOI] [PubMed] [Google Scholar]
- Labella S, Woglar A, Jantsch V, Zetka M. 2011. Polo Kinases Establish Links between Meiotic Chromosomes and Cytoskeletal Forces Essential for Homolog Pairing. Developmental Cell 21: 948–958. [DOI] [PubMed] [Google Scholar]
- Labrador L, Barroso C, Lightfoot J, Müller-Reichert T, Flibotte S, Taylor J, Moerman DG, Villeneuve AM, Martinez-Perez E. 2013.Chromosome Movements Promoted by the Mitochondrial Protein SPD-3 Are Required for Homology Search during Caenorhabditis elegans Meiosis. PLOS Genetics 9: e1003497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Lee K-S, Huh S, Liu S, Lee D-Y, Hong SH, Yu K, Lu B. 2016. Mito Calcium and PLK-1_2016. Developmental Cell 37: 174–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder MI, Köhler M, Boersema P, Weberruss M, Wandke C, Marino J, Ashiono C, Picotti P, Antonin W, Kutay U. 2017. Mitotic Disassembly of Nuclear Pore Complexes Involves CDK1- and PLK-1-Mediated Phosphorylation of Key Interconnecting Nucleoporins. Developmental Cell 43: 141–156.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link J, Paouneskou D, Velkova M, Daryabeigi A, Laos T, Labella S, Barroso C, Pacheco Piñol S, Montoya A, Kramer H, et al. 2018. Transient and Partial Nuclear Lamina Disruption Promotes Chromosome Movement in Early Meiotic Prophase. Developmental Cell 45: 212–225.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lončarek J, Hergert P, Khodjakov A. 2010. Centriole Reduplication during Prolonged Interphase Requires Procentriole Maturation Governed by PLK-1. Current Biology 20: 1277–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macůrek L, Lindqvist A, Lim D, Lampson MA, Klompmaker R, Freire R, Clouin C, Taylor SS, Yaffe MB, Medema RH. 2008. Polo-like kinase-1 is activated by aurora A to promote checkpoint recovery. Nature 455: 119–123. [DOI] [PubMed] [Google Scholar]
- Martin BT, Strebhardt K. 2006. Polo-Like Kinase 1: Target and Regulator of Transcriptional Control. Cell Cycle 5: 2881–2885. [DOI] [PubMed] [Google Scholar]
- Martini A, Wang J, Brown NM, Cumarasamy S, Sfakianos JP, Rastinehad AR, Haines KG, Wiklund NP, Nair SS, Tewari AK. 2019. A transcriptomic signature of tertiary Gleason 5 predicts worse clinicopathological outcome. BJU Int 124: 155–162. [DOI] [PubMed] [Google Scholar]
- Martino L, Morchoisne-Bolhy S, Cheerambathur DK, Van Hove L, Dumont J, Joly N, Desai A, Doye V, Pintard L. 2017. Nucleoporins and plk-1_2017. Dev Cell 43: 157–171.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauro MS, Celma G, Zimyanin V, Magaj MM, Gibson KH, Redemann S, Bahmanyar S. 2022. Ndc1 drives nuclear pore complex assembly independent of membrane biogenesis to promote nuclear formation and growth. eLife 11: e75513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikwar M, MacFarlane AJ, Marchetti F. 2020. Mechanisms of oocyte aneuploidy associated with advanced maternal age. Mutation Research/Reviews in Mutation Research 785: 108320. [DOI] [PubMed] [Google Scholar]
- O’Connell KF, Leys CM, White JG. 1998. A Genetic Screen for Temperature-Sensitive Cell-Division Mutants of Caenorhabditis elegans. Genetics 149: 1303–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquariello R, Ermisch AF, Silva E, McCormick S, Logsdon D, Barfield JP, Schoolcraft WB, Krisher RL. 2019. Alterations in oocyte mitochondrial number and function are related to spindle defects and occur with maternal aging in mice and humansf. Biology of Reproduction 100: 971–981. [DOI] [PubMed] [Google Scholar]
- Patel JT, Bottrill A, Prosser SL, Jayaraman S, Straatman K, Fry AM, Shackleton S. 2014. Mitotic phosphorylation of SUN1 loosens its connection with the nuclear lamina while the LINC complex remains intact. Nucleus 5: 462–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poteryaev D, Squirrell JM, Campbell JM, White JG, Spang A. 2005. Involvement of the actin cytoskeleton and homotypic membrane fusion in ER dynamics in Caenorhabditis elegans. Mol Biol Cell 16: 2139–2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman MM, Munzig M, Kaneshiro K, Lee B, Strome S, Müller-Reichert T, Cohen-Fix O. 2015. Caenorhabditis elegans polo-like kinase PLK-1 is required for merging parental genomes into a single nucleus ed. Y. Zheng. MBoC 26: 4718–4735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneckenburger Η. 1992. Fluorescence decay kinetics and imaging of NAD(P)H and flavins as metabolic indicators. Opt Eng 31: 1447. [Google Scholar]
- Schöckel L, Möckel M, Mayer B, Boos D, Stemmann 0. 2011. Cleavage of cohesin rings coordinates the separation of centrioles and chromatids. Nat Cell Biol 13: 966–972. [DOI] [PubMed] [Google Scholar]
- Shanmughapriya S, Rajan S, Hoffman NE, Zhang X, Guo S, Kolesar JE, Hines KJ, Ragheb J, Jog NR, Caricchio R, et al. 2015. Ca2+ signals regulate mitochondrial metabolism by stimulating CREB-mediated expression of the mitochondrial Ca2+ uniporter gene MCU. Sci Signal 8: ra23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sud D, Zhong W, Beer DG, Mycek M-A. 2006. Time-resolved optical imaging provides a molecular snapshot of altered metabolic function in living human cancer cell models. Opt Express 14: 4412. [DOI] [PubMed] [Google Scholar]
- Sweeney HL, Holzbaur ELF. 2018. Motor Proteins. Cold Spring Harb Perspect Biol 10: a021931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanenbaum ME, Stern-Ginossar N, Weissman JS, Vale RD. 2015. Regulation of mRNA translation during mitosis. eLife 4: e07957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas Y, Cirillo L, Panbianco C, Martino L, Tavernier N, Schwager F, Van Hove L, Joly N, Santamaria A, Pintard L, et al. 2016. Cdk1 Phosphorylates SPAT-1/Bora to Promote PLK-1 Activation in C. elegans and Human Cells. Cell Reports 15: 510–518. [DOI] [PubMed] [Google Scholar]
- Tilokani L, Nagashima S, Paupe V, Prudent J. 2018. Mitochondrial dynamics: overview of molecular mechanisms eds. C. Garone and M. Minczuk. Essays in Biochemistry 62: 341–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velez-Aguilera G, Nkombo Nkoula S, Ossareh-Nazari B, Link J, Paouneskou D, Van Hove L, Joly N, Tavernier N, Verbavatz J-M, Jantsch V, et al. 2020. PLK-1 promotes the merger of the parental genome into a single nucleus by triggering lamina disassembly. eLife 9: e59510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallrabe H, Svindrych Z, Alam SR, Siller KH, Wang T, Kashatus D, Hu S, Linder Periasamy A. 2018. Segmented cell analyses to measure redox states of autofluorescent NAD(P)H, FAD & Trp in cancer cells by FLIM. Sci Rep 8: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X-H, Yin S, Ou X-H, Luo S-M. 2020. Increase of mitochondria surrounding spindle causes mouse oocytes arrested at metaphase I stage. Biochemical and Biophysical Research Communications 527: 1043–1049. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


