Abstract
Much attention has focused on the Mycobacterium tuberculosis molecular chaperone chaperonin (Cpn) 60.2 (Hsp 65) in the pathology of tuberculosis because of its immunogenicity and ability to directly activate human monocytes and vascular endothelial cells. However, M. tuberculosis is one of a small group of bacteria that contain multiple genes encoding Cpn 60 proteins. We have now cloned and expressed both M. tuberculosis proteins and report that the novel chaperonin 60, Cpn 60.1, is a more potent inducer of cytokine synthesis than is Cpn 60.2. This is in spite of 76% amino acid sequence similarity between the two mycobacterial chaperonins. The M. tuberculosis Cpn 60.2 protein activates human peripheral blood mononuclear cells by a CD14-independent mechanism, whereas Cpn 60.1 is partially CD14 dependent and contains a peptide sequence whose actions are blocked by anti-CD14 monoclonal antibodies. The cytokine-inducing activity of both chaperonins is extremely resistant to heat. Cpn 60.1 may be an important virulence factor in tuberculosis, able to activate cells by diverse receptor-driven mechanisms.
The current global epidemic of tuberculosis is responsible for 3 to 4 million deaths each year (8). Chronic inflammation is the hallmark of tuberculosis, and substantial efforts have been made to identify the bacterial components responsible. The molecular chaperone chaperonin (Cpn) 60.2 of Mycobacterium tuberculosis, also known as Hsp 65, has attracted significant attention because of its immunogenicity (5, 6) and possible role in autoimmunity (23). It was recently shown that, in addition to acting as an immunogen, mycobacterial Cpn 60.2 can stimulate myeloid cells to synthesize proinflammatory cytokines (9, 18, 20) and that this activity is CD14 independent (27). Other bacterial Cpn 60 proteins have also been reported to induce cytokine synthesis (11, 13, 21). These findings have led us to propose that the chaperonin 60 proteins of bacteria function as virulence determinants and may be important in directly inducing host inflammatory responses (15).
Most bacteria encode only one chaperonin 60 protein. However, M. tuberculosis expresses a second cpnL gene (14), designated cpnL1, with the original cpnL (Hsp 65) gene now being designated cpnL2 (4). The gene encoding cpnL2 is not adjacent to the gene encoding the cochaperone, Cpn 10, which is the normal arrangement in bacteria (10). In contrast, the cpnL1 gene and the gene encoding cpnS1 are separated by only 98 bp (14) and may represent an operon. The proteins encoded by these two genes share 76% amino acid sequence similarity (14).
Surprisingly, nothing is known about the role of Cpn 60.1 in the normal functioning of M. tuberculosis or in the pathology induced by this organism. In this study, we have cloned the M. tuberculosis cpnL1 and cpnL2 genes, expressed and purified both proteins, and compared their capacities to stimulate human peripheral blood mononuclear cells (PBMC) to produce pro- and anti-inflammatory cytokines. We have also tested a number of synthetic Cpn 60 peptides for cytokine-inducing activity. These peptides were predicted to be T-cell epitopes (3). We find that, depending on the cytokine measured, M. tuberculosis Cpn 60.1 is between 10- and 100-fold more active in inducing cytokine synthesis than is the Cpn 60.2 protein. We have confirmed that cell activation induced by Cpn 60.2 is CD14 independent but have found that Cpn 60.1 is partially CD14 dependent and have identified a peptide within Cpn 60.1 that stimulated cytokine production and was blocked by anti-CD14. These results suggest that Cpn 60.1, and its derived peptides, may represent an important M. tuberculosis proinflammatory signal.
MATERIALS AND METHODS
Cloning and expression of M. tuberculosis Cpn 60.1 and 60.2.
The gene coding for Cpn 60.1 was amplified and cloned in the expression vector pET22b (Novagen, Nottingham, United Kingdom). Production and purification of the recombinant Cpn 60.1 from Escherichia coli were performed by metal chelate affinity chromatography. The purified protein was then dialyzed against 10 mM ammonium bicarbonate. The recombinant Cpn 60.2 was purified from E. coli by standard ion-exchange chromatography followed by dialysis against 10 mM ammonium bicarbonate (22). Protein purity was demonstrated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and mass spectrometry.
Cloning and expression of Actinobacillus actinomycetemcomitans autolysin.
The gene encoding the autolysin (N-acetylmuramyl-l-alanine amidase) of A. actinomycetemcomitans was PCR amplified from chromosomal DNA and cloned in the expression vector pET21a (Novagen). The protein was purified by metal chelate chromatography and dialyzed against phosphate-buffered saline. Protein purity was demonstrated by SDS-PAGE.
Peptide synthesis.
A number of M. tuberculosis Cpn 60.1 and Cpn 60.2 peptides and one GroEL peptide (Table 1) were prepared by solid-phase synthesis and purified to 95% purity by reverse-phase high-pressure liquid chromatography. Purity was demonstrated by SDS-PAGE and mass spectrometry.
TABLE 1.
Chaperonin 60 peptides used in this study
| Protein | Peptide no. | Peptide sequence |
|---|---|---|
| GroEL | 197–221 | GYLSPYFINKPETGAVELESPFILL |
| M. tuberculosis Cpn 60.1 | 84–95 | AGDGTTTATILA |
| 195–219a | KGFLSAYFVTDFDNQQAVLEDALI | |
| 219–233 | LLHQDKISSLPDLLP | |
| 220–242 | LHQDKISSLPDLLPLLEKVAGTG | |
| 272–281 | VAVKGPYFGD | |
| 403–419 | AAVEEGIV PGGGASLIH | |
| M. tuberculosis Cpn 60.2 | 195–219 | LGYISGYFVTDPERQEAVLEDPYILL |
| 256–270 | ALSTLVVNKIRGTFK | |
| 260–271 | LVVNKIRGTFKS | |
| 262–273 | VKNIRGTFKSVA | |
| 403–419 | AAVEEGIVAGGGVTLL |
This is the only peptide with cytokine-inducing activity.
LAL assay.
The LPS content of the recombinant chaperonins and peptides was determined using the Limulus amoebocyte lysate (LAL) test. All reagents were purchased from Associates of Cape Cod (Liverpool, United Kingdom), and the assay was carried out according to the manufacturer's instructions.
Preparation of human PBMC.
Human PBMC were prepared from buffy coat blood from healthy donors by density gradient centrifugation and differential adherence and cultured as described elsewhere (21). Cell viability was assessed by measurement of trypan blue uptake. In some experiments, the PMBC were further purified by T-cell depletion using T-cell rosetting with the RosetteSep reagent (StemCell Technologies, Vancouver, Canada) used according to the manufacturer's instructions. Depletion was monitored by flow cytometry using a FACScan instrument (Becton Dickinson) with the data being analyzed using WinMDI version 2.8.
Determination of cytokine production.
PPBMC (2 × 106 cells/ml) were exposed to a range of concentrations of recombinant chaperonins or peptides. Polymyxin B was added at a concentration of 20 μg/ml to neutralize any contaminating lipopolysaccharide (LPS). In some assays, the polymyxin B was omitted and cells were pretreated with one of three anti-CD14 monoclonal antibodies at 15 μg/ml: MY4 (Beckman Coulter, High Wycombe, United Kingdom) and 60bca and 26ic (American Type Culture Collection, Manassas, Va.). As a control for the blocking effects of these antibodies, irrelevant isotype-matched antibodies (Sigma) were added to cells at the same concentrations. After 16 h in the presence of activators, medium was collected and cytokine levels were determined by two-site enzyme-linked immunosorbent assay (ELISA). Data were analyzed using Student's t test.
Cytokine assays.
Interleukin 1β (IL-1β), tumor necrosis factor alpha (TNF-α), IL-6, and IL-8 antibodies and all cytokine standards were provided by the National Institute for Biological Standards and Control. IL-1β, TNF-α, and IL-6 ELISA methods were as described previously (21). Paired antibodies for assay of IL-10, gamma interferon (IFN-γ), and granulocyte-macrophage colony-stimulating factor (GM-CSF) were from Pharmingen (Oxford, United Kingdom), and IL-4 and IL-12 antibodies were from BioSource (Watford, United Kingdom). The ELISA protocols for IL-4, IL-8, IL-10, IL-12, GM-CSF, and IFN-γ were similar to that for IL-6 (21).
Controls for LPS contamination.
In addition to the use of polymyxin B and anti-CD14 monoclonal antibodies, the Cpn 60 proteins were subjected to other tests for LPS contamination. Both recombinant Cpn 60 proteins (at a concentration of 200 μg/ml) and E. coli LPS (Sigma) (at a concentration of 100 ng/ml) were subjected to the following conditions: (i) boiling for 20 min, (ii) autoclaving twice for 20 min, and (iii) exposure to 200 ng of proteinase K/ml for 2 h at 50°C (Sigma). To inactivate the proteinase K, the solutions containing this protein and the Cpn 60 proteins or LPS were boiled for 20 min. The samples were then diluted to 1 and 5 μg/ml for Cpn 60.1 and 60.2, respectively, and 1 ng/ml for LPS and tested for their ability to activate PBMC cytokine synthesis. To determine what effect these treatments were having on the Cpn 60 proteins, they were separated on commercially available 4 to 20% gradient gels (Invitrogen, Groningen, The Netherlands) and stained with Coomassie blue.
Secondary structure predictions of peptides.
The consensus method, Jpred (7), was used to predict the secondary structures of the peptides via the server at http://jpred.ebi.ac.uk/.
RESULTS
Physicochemical characteristics of recombinant Cpn 60 proteins and LPS content.
The purity of the recombinant chaperonin 60 proteins was analyzed by SDS-PAGE and mass spectrometry, and no major contaminating species were evident using either technique (data not shown). The endotoxin content of the two recombinant proteins was low and within the range of 0.012 to 0.12 ng of endotoxin/μg of protein.
Comparison of the cytokine-inducing activities of M. tuberculosis Cpn 60.1 and Cpn 60.2.
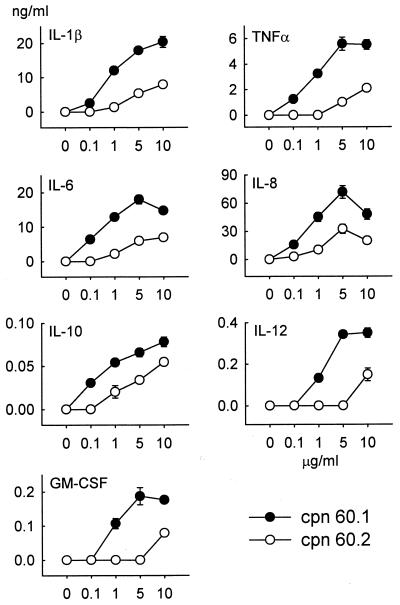
Blood obtained from 10 healthy individuals has been used to determine the relative responsiveness of human PBMC to the mycobacterial Cpn 60 proteins. All assays were done in the presence of polymyxin B to block any stimulation by contaminating LPS (see below). Both chaperonins stimulated PBMC to produce a range of proinflammatory cytokines including IL-1β, IL-6, IL-8, IL-12, TNF-α, and GM-CSF and the monocyte-deactivating cytokine IL-10. Neither Cpn 60 protein elicited the production of IFN-γ or IL-4 from the PBMC of any individual. A typical response is shown in Fig. 1. This shows the general finding with these 10 samples of PBMC that they responded to lower concentrations of Cpn 60.1 than of Cpn 60.2. Thus, Cpn 60.1 concentrations as low as 100 ng/ml (1.8 nM) could stimulate cytokine production. In contrast, PBMC generally required 10 μg of Cpn 60.2/ml (180 nM) to trigger the production of cytokines. The PBMC from all blood samples responded to Cpn 60.1 in terms of the production of the cytokines shown in Fig. 1. However, three individuals failed to produce IL-12 in response to M. tuberculosis Cpn 60.2. The addition of the mycobacterial chaperonin 60 proteins to cultured cells had no effect on cell viability.
FIG. 1.
Comparison of the PBMC cytokine-inducing activities of M. tuberculosis Cpn 60.1 and Cpn 60.2 over the dose range of 100 ng/ml to 10 μg/ml (1.8 to 180 nM). Cytokine concentrations were measured by two-site ELISA. Both chaperonins failed to induce the synthesis of IL-4 or IFN-γ. Each data point represents the mean ± standard error of triplicate cultures from a representative experiment.
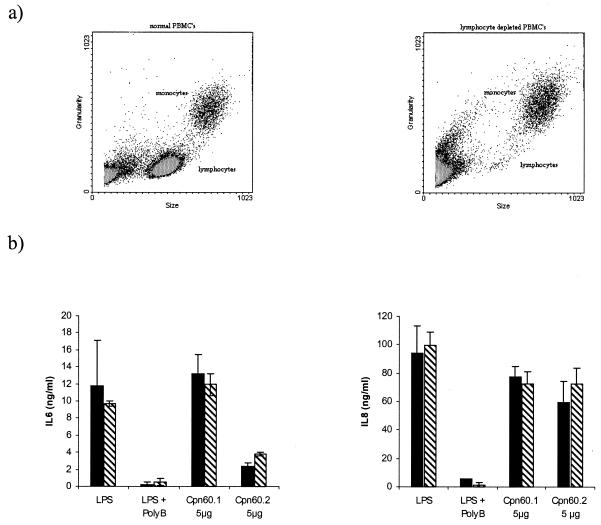
To determine if the induction of cytokine synthesis was due to a direct effect on the monocytes or to an indirect stimulation via the T-cell population in the PBMC, the latter population was selectively removed by rosetting. Such treatment removed the majority of the CD3-bearing lymphocytes (Fig. 2a) but had no significant effect on the production of IL-6 or IL-8 in response to LPS or the two mycobacterial chaperonins (Fig. 2b).
FIG. 2.
Typical experiment showing the effect of T-cell depletion on the cytokine-stimulating activities of the mycobacterial chaperonins as assessed by assay of IL-6 and IL-8 synthesis. PBMC were depleted of CD3 cells by rosetting with the RosetteSep reagent from StemCell Technologies. The depletion was assessed by flow cytometry (a), and the effect of depletion on IL-6 and IL-8 production by the remaining cell population was measured (b). Results are expressed as the means ± standard errors of triplicate cultures. ▧, nondepleted cells; ▪, depleted cells. PolyB, polymyxin B.
Controls for LPS contamination.
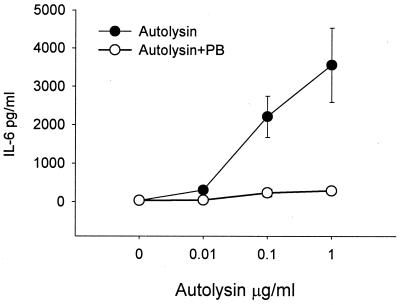
While polymyxin B is the agent most often used to control for LPS contamination, it is held by some workers to be less effective at blocking protein-bound LPS than free LPS. This could explain the negative effects found with polymyxin B when it was incubated with the mycobacterial chaperonins. However, when polymyxin B was added to recombinant bacterial autolysin, purified, like the chaperonins, by metal chelation chromatography, it proved possible to inhibit completely the cytokine-inducing activity, showing that this protein was not a cytokine-inducing molecule and that its activity was due solely to contaminating LPS (Fig. 3).
FIG. 3.
Effect of adding polymyxin B (PB) on the IL-6-inducing activity of the autolysin of A. actinomycetemcomitans. Results are expressed as the means ± standard errors of triplicate cultures from a representative experiment.
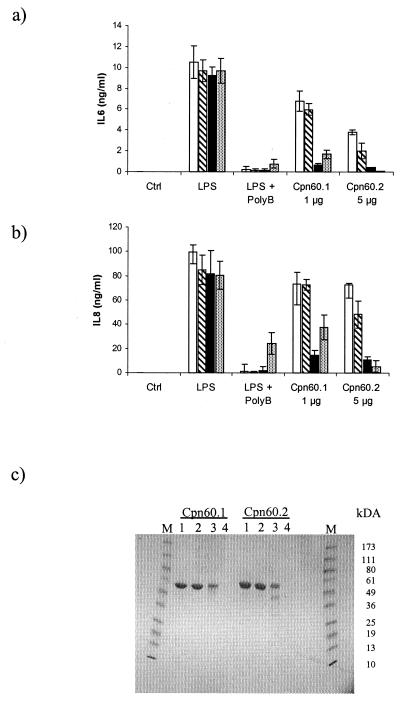
Heat denaturation is a good method of discriminating biologically active proteins from LPS. Heating the mycobacterial chaperonins to 100°C for 20 min failed to inhibit the cytokine-inducing activity of these proteins or of LPS. To significantly inhibit the cytokine-inducing activity of the chaperonins required autoclaving for 40 min. Autoclaving also appeared to cause some breakdown of the chaperonin proteins (Fig. 4c). In contrast, this treatment had no effect on the cytokine-inducing activity of the LPS. Similarly, proteolysis of the chaperonins with proteinase K significantly inhibited their biological activity while having, as expected, no effect on the biological activity of the LPS (Fig. 4).
FIG. 4.
Effects of boiling, autoclaving, and exposure to proteinase K on the IL-6 (a)- and IL-8 (b)-stimulating activities of the M. tuberculosis Cpn 60 proteins and E. coli LPS. Cpn 60.1 and Cpn 60.2 were analyzed at 1 and 5 μg/ml, respectively. LPS was tested at 1 ng/ml after exposure to the various treatments. The effects of the various treatments on protein integrity were analyzed by SDS-PAGE (c): no treatment (lanes 1), boiling (lanes 2), autoclaving (lanes 3), and proteinase K treatment (lanes 4). Results are expressed as the means ± standard errors of triplicate cultures from a representative experiment. □, no treatment; ▧, boiling; ▪, autoclaving; ░⃞, proteinase K. PolyB, polymyxin B.
Inhibitory effect of anti-CD14 monoclonal antibodies.
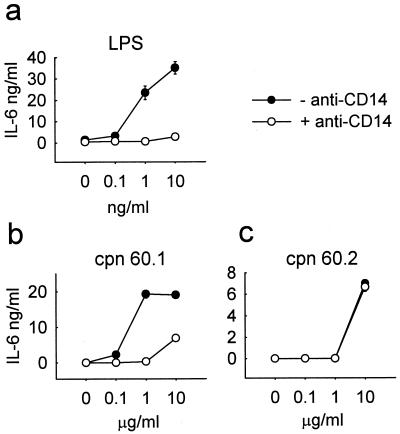
The CD14-binding and -neutralizing antibody 60bca inhibited the LPS-stimulated induction of the synthesis of all cytokines measured in this study. The effect of this antibody was assessed with all the human PBMC samples used in this study, but for clarity, only the results of one representative experiment showing the response of IL-6 production are presented (Fig. 5a). In contrast, 60bca failed to inhibit the cytokine-inducing activity of M. tuberculosis Cpn 60.2 (Fig. 5c), confirming previous reports of the CD14 independency of this mycobacterial protein (27). However, all samples of PBMC exposed to M. tuberculosis Cpn 60.1 showed some degree of inhibition when the monoclonal antibody 60bca was present in the medium (Fig. 5b). At 1 μg of Cpn 60.1/ml, the antibody completely blocked cytokine induction. However, with 10 μg of Cpn 60.1/ml, this total inhibition was overcome and significant cytokine synthesis occurred. An isotype-matched irrelevant antibody used at the same concentration as 60bca had no inhibitory effect on cytokine induction induced by LPS or the two chaperonin proteins. In some experiments, another neutralizing anti-CD14 monoclonal antibody, MY4, was also shown to block the cytokine-inducing activity of LPS and M. tuberculosis Cpn 60.1 but not that of M. tuberculosis Cpn 60.2.
FIG. 5.
Effect of anti-CD14 monoclonal antibody 60bca on IL-6 production by PBMC stimulated with LPS or M. tuberculosis Cpn 60 proteins. (a) LPS-stimulated IL-6 production by PBMC is inhibited by pretreatment with 15 μg of anti-CD14 monoclonal antibody 60bca per ml. (b) M. tuberculosis Cpn 60.1-stimulated IL-6 production is partially inhibited by anti-CD14 pretreatment. (c) In contrast, M. tuberculosis Cpn 60.2-stimulated IL-6 production is unaffected by anti-CD14 pretreatment. Each data point represents the mean ± standard error for triplicate cultures from a representative experiment.
Activity of synthetic peptides.
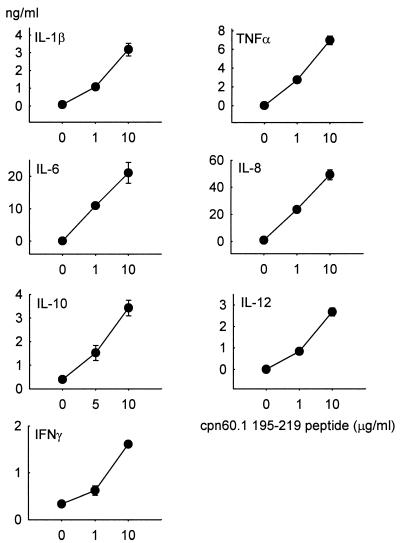
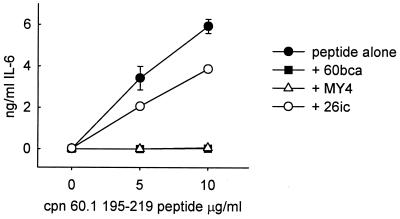
A number of M. tuberculosis chaperonin 60 peptides were synthesized (Table 1) and were analyzed by SDS-PAGE and mass spectrometry, which failed to demonstrate the presence of contaminants. The levels of LPS in these peptides were below the limit of LAL assay detection. Of these mycobacterial peptides, only the peptide homologue of residues 195 to 219 of M. tuberculosis Cpn 60.1 stimulated human PBMC to synthesize the same range of cytokines as did the parent molecule, albeit at about a 20-fold-higher molar concentration. In addition, and in contrast to the parent protein, this peptide stimulated the synthesis of IFN-γ (Fig. 6). The cytokine-inducing activity of this peptide was completely inhibited by anti-CD14 monoclonal antibodies MY4 and 60bca but not by the CD14-binding, but nonneutralizing, antibody 26ic (Fig. 7). An isotype-matched monoclonal antibody for 60bca also failed to have any effect on the cytokine-inducing activity of this peptide. In contrast to the activity of the M. tuberculosis Cpn 60.1 peptide, the corresponding peptides of M. tuberculosis Cpn 60.2 and GroEL were not able to induce cytokine synthesis.
FIG. 6.
Dose-dependent stimulation of PBMC cytokine synthesis by the M. tuberculosis Cpn 60.1 peptide 195–219. This peptide induces the synthesis of a range of cytokines including IFN-γ. Each point represents the mean ± standard error for triplicate cultures from a representative experiment.
FIG. 7.
Inhibition of IL-6-inducing activity of M. tuberculosis Cpn 60.1 peptide 195–219 by neutralizing anti-CD14 antibodies MY4 and 60bca but not by nonneutralizing anti-CD14 antibody 26ic. Each point represents the mean ± standard error for triplicate cultures from a representative experiment.
Secondary structure predictions of peptides.
Comparison of the three peptides revealed that the Cpn 60.1 peptide has the most regular structure with a significant span of α-helix at the C terminus. In Cpn 60.2 and GroEL, there are proline substitutions which tend to break up regular hydrogen-bonded structures. This is apparent in the Cpn 60.2 peptide, which has a lower α-helical content, and in the GroEL peptide, which has very little regular structure (Table 2).
TABLE 2.
Secondary structure predictions of chaperonin peptidesa
| Protein | Position and sequence |
|---|---|
| Cpn 60.1 | 195 KGFLSAYFVTDFDNQQAVLEDALIL 219 |
| EEEEEE HHHHHHHHHH | |
| Cpn 60.2 | 195 KGYISGYFVTDPERQEAVLEDPYIL 219 |
| EEEEEE HHHHHHH | |
| GroEL | 197 RGYLSPYFINKPETGAVELESPFIL 221 |
| E EEEE | |
| IIBIBISBXXXXXSBXBXBXXBXBB |
E, β-sheet; H, α-helix; I, exposed to internal cavity; B, buried; S, intersubunit contact; X, exterior exposure. The table shows an alignment of the peptide sequences tested for the simulation of cytokine secretion. The secondary structures were predicted using the consensus method Jpred (7) via the server at http://jpred.ebi.ac.uk/ and are listed under each sequence. The environments of each amino acid side chain (I, B, S, and X) in the GroEL structure (25) are listed in the bottom row.
DISCUSSION
The worldwide resurgence of tuberculosis requires that we understand how the causative organism, M. tuberculosis, produces tissue pathology. The chronic inflammatory pathology of tuberculosis obviously suggests that overproduction of proinflammatory cytokines lies at the heart of this infection. What components of M. tuberculosis are responsible for cytokine synthesis? Much attention has focused on the Cpn 60.2 protein (Hsp 65) of M. tuberculosis because of its striking immunogenicity (5, 6, 23) and because in recent years a number of reports have appeared supporting the hypothesis that bacterial Cpn 60 proteins can stimulate human monocytes to secrete proinflammatory cytokines (9, 11, 13, 18, 20, 21). The content of chaperonin 60 protein in M. tuberculosis can increase from 1 to 10% or more under conditions of stress (26), such as are likely to occur during infection. This suggests that chaperonin 60 may play a major role in bacterial virulence by acting, like cytokines, as an intercellular signal (15). The sequence conservation of chaperonin 60 proteins suggests that these molecules will have very similar intercellular signaling functions, irrespective of their source. This concept was challenged, however, when it was found that the Cpn 60.2 proteins of M. tuberculosis and Mycobacterium leprae, in contrast to GroEL, failed to stimulate the breakdown of murine bone in culture (11, 17). In the present study, we have compared the two cpnL gene products of M. tuberculosis for their ability to stimulate human PBMC to produce a range of pro- and anti-inflammatory cytokines. While the Cpn 60.2 protein of M. tuberculosis has been studied extensively, nothing was known about the activity of the product of the second cpnL gene (cpnL1) of this bacterium.
M. tuberculosis Cpn 60.2 stimulated human PBMC to synthesize and secrete a range of proinflammatory cytokines and the anti-inflammatory cytokine IL-10 but only at the highest concentration used (5 to 10 μg/ml, or 90 to 180 nM). This confirms previous studies of the potency of M. tuberculosis Cpn 60.2 as a cytokine-inducing mediator (18, 20, 24). In contrast, recombinant M. tuberculosis Cpn 60.1 was active at concentrations as low as 100 ng/ml (1.8 nM) and always produced a greater maximum response than did the Cpn 60.2 protein, or even LPS. Cytokines produced included the potent proinflammatory cytokines IL-1β, TNF-α, IL-6, IL-8, and IL-12. However, production of the antimycobacterial cytokine IFN-γ, or the Th2 cytokine IL-4, was not observed. This was in spite of the capacity of both mycobacterial chaperonins to induce IL-12 synthesis. Both chaperonins also induced the production of the anti-inflammatory cytokine IL-10. The conclusion from the 10 individual human blood samples tested in this study is that chaperonin 60.1 is up to 2 log orders more potent as a cytokine-stimulating agonist than is Cpn 60.2 and has a substantially greater efficacy. This substantial difference in potency is surprising given the sequence homology of these two proteins.
Depletion of T cells from the PBMC had no significant effect on the production of IL-6 and IL-8 induced by both chaperonins. The supports the hypothesis that these chaperonin proteins are directly stimulating the monocyte population in peripheral blood.
Both mycobacterial chaperonin 60 proteins had been expressed in E. coli, and it was possible that the cytokine-inducing activity was due to LPS contamination. Addition of polymyxin B to PBMC stimulated with these chaperonins had no inhibitory effect. However, it is claimed by various workers that protein-associated LPS is not inhibited, or not inhibited as effectively, by polymyxin B. In our experience, the LPS contaminating recombinant proteins expressed in E. coli can always be blocked by polymyxin B. An example of a recombinant protein with no cytokine-inducing activity in the presence of polymyxin B but significant activity in its absence is the autolysin of the oral bacterium A. actinomycetemcomitans (Fig. 3). One of the simple controls for LPS contamination of proteins is to expose the protein to heat. If the bioactivity is due to the protein, then heating will destroy it. If the activity is due to the LPS, then heating will have no effect. In this study, we have boiled both LPS and the chaperonins for 20 min without affecting their cytokine-inducing activities. However, when the LPS and the chaperonins were autoclaved, the activity of the former was, again, unaffected while that of the latter was significantly reduced. In addition, proteinase K caused significant inhibition of the activity of the chaperonins without influencing that of LPS. These results clearly show that the chaperonins are extremely heat-stable proteins. They also reveal that the cytokine-inducing activity of the chaperonins is not due to contaminating LPS.
Addition of anti-CD14 monoclonal antibodies, at concentrations that totally inhibited nanogram-per-milliliter concentrations of LPS, failed to inhibit the cytokine-inducing activity of the mycobacterial chaperonin 60.2 protein, confirming a previous report (27). However, the situation with Cpn 60.1 was not so clear-cut. In eight individuals tested, cytokine-inducing activity was reduced, but not totally blocked, by anti-CD14 monoclonal antibodies, suggesting that CD14 is at least partially involved in the activation of human leukocytes by this molecular chaperone.
In unpublished studies (A. R. M. Coates and P. Mascagni) of the T-cell reactivity of Cpn 60.1, a number of peptides were synthesized on the basis that they contained predicted T-cell epitopes (5). These peptides were tested for cytokine-inducing activity, and only one (M. tuberculosis Cpn 60.1 peptide, residues 195 to 219) was active. This peptide stimulated human PBMC to secrete the same panoply of cytokines as that induced by the intact recombinant Cpn 60.1. In addition, it was found that this peptide, in contrast to the parent molecule, also stimulated the synthesis of IFN-γ. The amount of endotoxin in this synthetic peptide was below the detection limit of the LAL assay, but it was found that its activity was inhibited by neutralizing anti-CD14 monoclonal antibodies but not by a nonneutralizing anti-CD14 antibody. The same peptides in M. tuberculosis Cpn 60.2 (residues 195 to 219) and in GroEL (residues 197 to 221) were completely inactive. Is this Cpn 60.1 peptide (KGFLSAYFVTDFDNQQAVLEDALI) responsible for conferring some or all of the cytokine-inducing activity of the molecular chaperone and for the inhibitory effect of anti-CD14 monoclonal antibodies? The answer to this question is complicated by the fact that peptide 195–219 stimulates IFN-γ synthesis, while the parent molecule does not. This would suggest that this peptide is normally hidden in the intact Cpn 60.1 protein. Indeed, analysis of the homologous sequence in the GroEL crystal structure (25) indicates that, while the predicted α-helix of the Cpn 60.1 peptide would be on the outside of the Cpn 60.1 structure, if it were to exist as a tetradecameric assembly similar to the GroEL structure, the rest of the peptide would be buried within the wall of the assembly or protrude into the interior of the complex. We do not know which residues confer biological activity on this peptide but conclude that, whatever they are, they are inaccessible to the receptor on the target cell. This suggests that some other region or regions of Cpn 60.1 are responsible for the cytokine-inducing activity of this protein.
The reasons for the differences in the biological activities of the three peptides are not clear. The most stringent analysis would be a comparison of peptides 195–219 from Cpn 60.1 and Cpn 60.2, where the former has an α-helix that extends more towards the C terminus (Table 2). In Cpn 60.2 and GroEL, there are proline substitutions that tend to break up regular hydrogen-bonded structures, and this may contribute to the lack of bioactivity of these peptides.
In previous studies, we reported that the E. coli Cpn 60 (GroEL) is a potent stimulator of cytokine-driven murine bone resorption (11) and human PBMC cytokine synthesis (21). In the former study (11), we established that bone from the C3H/HeJ, LPS-unresponsive mouse would respond to GroEL but, as expected, not to LPS. The defect in the C3H/HeJ mouse is a single nucleotide mutation which renders the cellular LPS sensor, TLR4, unresponsive (2). In studies of GroEL-induced cytokine synthesis, we found that the activity of this chaperonin is not inhibited by anti-CD14 monoclonal antibodies (21). Therefore, GroEL does not activate cells by binding to the CD14-TLR4 complex. In this study, we show that the mycobacterial Cpn 60 proteins are also cytokine inducing but vary in their dependence on CD14. The involvement of TLR4 in M. tuberculosis Cpn 60.1- and Cpn 60.2-induced cell activation has yet to be determined, as CD14 is not necessarily required for interaction of TLR4 with other ligands (16).
It has recently been reported that the monocyte-activating capacity of human and chlamydial Cpn 60 proteins is inhibited by anti-CD14 monoclonal antibodies. CD14 negative cells also fail to respond to these chaperonins. However, after transfection with CD14, cells become responsive to these two Cpn 60 proteins (12). Another molecular chaperone, HSP70, has also been recently reported to induce cytokine synthesis by interacting with CD14 (1). Our own findings in this and other studies (21) suggest that the cellular receptors for chaperonin 60 proteins are diverse and may include CD14-TLR4 and other, as yet undefined, receptors. Further work is necessary to determine (i) the relative contributions of the two Cpn 60 proteins in M. tuberculosis virulence (by using knockout mutants), (ii) the full range of cell surface receptors that bind Cpn 60 proteins and produce cell activation, and (iii) the structure-activity relationships of this fascinating group of proteins.
M. tuberculosis contains two chaperonin 60 proteins with 70% amino acid sequence similarity. Both proteins have the capacity to stimulate human PBMC to synthesize and secrete proinflammatory cytokines. In spite of the sequence conservation of these proteins, there are substantial differences in their cytokine-inducing potency and efficacy, with Cpn 60.1 being substantially more active than Cpn 60.2. It is unclear if such differences in activity are due to the fact that the chaperonin 60 proteins differ in their CD14 dependency and may bind to different receptors. Alternatively, they could be due to differences in the C-terminal sequences or to differences in the oligomeric structures of these proteins resulting in some form of partial agonism (19). Whatever the mechanism, these studies show that M. tuberculosis Cpn 60.1 is a powerful stimulator of proinflammatory cytokine production and may play a role in the inflammatory pathology of tuberculosis.
ACKNOWLEDGMENTS
This work was supported by the Sir Jules Thorn Charitable Trust and the Arthritis Research Campaign (Programme Grant HO600).
We acknowledge the help of M. Stevens in the flow cytometry studies.
REFERENCES
- 1.Asea A, Kraeft S K, Kurt-Jones E A, Stevenson M A, Chen L B, Finberg R W, Koo G C, Calderwood S K. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med. 2000;6:435–442. doi: 10.1038/74697. [DOI] [PubMed] [Google Scholar]
- 2.Beutler B. Endotoxin, toll-like receptor 4, and the afferent limb of innate immunity. Curr Opin Microbiol. 2000;3:23–28. doi: 10.1016/s1369-5274(99)00046-6. [DOI] [PubMed] [Google Scholar]
- 3.Coates A R, Cookson J, Barton G J, Zvelebil M J, Sternberg M J. AIDS vaccine predictions. Nature. 1987;326:549–550. doi: 10.1038/326549c0. [DOI] [PubMed] [Google Scholar]
- 4.Coates A R, Shinnick T M, Ellis R J. Chaperonin nomenclature. Mol Microbiol. 1993;8:787. doi: 10.1111/j.1365-2958.1993.tb01624.x. [DOI] [PubMed] [Google Scholar]
- 5.Coates A R M. Immunological aspects of chaperonins. In: Ellis R A, editor. The chaperonins. London, United Kingdom: Academic Press; 1996. pp. 267–296. [Google Scholar]
- 6.Cohen I R, Young D B. Autoimmunity, microbial immunity and the immunological homunculus. Immunol Today. 1991;12:105–110. doi: 10.1016/0167-5699(91)90093-9. [DOI] [PubMed] [Google Scholar]
- 7.Cuff J A, Clamp M E, Siddiqui A S, Finlay M, Barton G J. JPred: a consensus secondary structure prediction server. Bioinformatics. 1998;14:892–893. doi: 10.1093/bioinformatics/14.10.892. [DOI] [PubMed] [Google Scholar]
- 8.Dye C, Scheele S, Dolin P, Pathania V, Raviglione M C. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. W. H. O. Global Surveillance and Monitoring Project. JAMA. 1999;282:677–686. doi: 10.1001/jama.282.7.677. [DOI] [PubMed] [Google Scholar]
- 9.Friedland J S, Shattock R, Remick D G, Griffin G E. Mycobacterial 65-kD heat shock protein induces release of proinflammatory cytokines from human monocytic cells. Clin Exp Immunol. 1993;91:58–62. doi: 10.1111/j.1365-2249.1993.tb03354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hemmingsen S M, Woolford C S, van der Vies M, Tilly K, Dennis D T, Georgopoulos C P, Hendrix R W, Ellis R J. Homologous plant and bacterial proteins chaperone oligomeric protein assembly. Nature. 1988;333:330–334. doi: 10.1038/333330a0. [DOI] [PubMed] [Google Scholar]
- 11.Kirby A C, Meghji S, Nair S P, White P, Reddi K, Nishihara T, Nakashima K, Willis A C, Sim R, Wilson M, Henderson B. The potent bone-resorbing mediator of Actinobacillus actinomycetemcomitansis homologous to the molecular chaperone GroEL. J Clin Investig. 1995;96:1185–1194. doi: 10.1172/JCI118150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kol A, Lichtman A H, Finberg R W, Libby P, Kurt-Jones E A. Cutting edge: heat shock protein (HSP) 60 activates the innate immune response: CD14 is an essential receptor for HSP60 activation of mononuclear cells. J Immunol. 2000;164:13–17. doi: 10.4049/jimmunol.164.1.13. [DOI] [PubMed] [Google Scholar]
- 13.Kol A, Bourcier T, Lichtman A H, Libby P. Chlamydial and human heat shock protein 60s activate human vascular endothelium, smooth muscle cells, and macrophages. J Clin Investig. 1999;103:571–577. doi: 10.1172/JCI5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kong T H, Coates A R, Butcher P D, Hickman C J, Shinnick T M. Mycobacterium tuberculosisexpresses two chaperonin-60 homologs. Proc Natl Acad Sci USA. 1993;90:2608–2612. doi: 10.1073/pnas.90.7.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lewthwaite J, Skinner A, Henderson B. Are molecular chaperones microbial virulence factors? Trends Microbiol. 1998;6:426–428. doi: 10.1016/s0966-842x(98)01362-6. [DOI] [PubMed] [Google Scholar]
- 16.Means T K, Wang S, Lien E, Yoshimura A, Golenbock D T, Fenton M J. Human toll-like receptors mediate cellular activation by Mycobacterium tuberculosis. J Immunol. 1999;163:3920–3927. [PubMed] [Google Scholar]
- 17.Meghji S, White P A, Nair S P, Reddi K, Heron K, Henderson B, Zaliani A, Fossati G, Mascagni P, Hunt J F, Roberts M M, Coates A R. Mycobacterium tuberculosischaperonin 10 stimulates bone resorption: a potential contributory factor in Pott's disease. J Exp Med. 1997;186:1241–1246. doi: 10.1084/jem.186.8.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peetermans W E, Langermans J A M, van der Hulst E, van Embden J D, van Furth R. Murine peritoneal macrophages activated by the mycobacterial 65-kilodalton heat shock protein express enhanced microbicidal activity in vitro. Infect Immun. 1993;61:868–875. doi: 10.1128/iai.61.3.868-875.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pliska V. Partial agonism: mechanisms based on ligand-receptor interactions and on stimulus-response coupling. J Recept Signal Transduct Res. 1999;19:597–629. doi: 10.3109/10799899909036675. [DOI] [PubMed] [Google Scholar]
- 20.Retzlaff C, Yamamoto Y, Hoffman P S, Friedman H, Klein T W. Bacterial heat shock proteins directly induce cytokine mRNA and interleukin-1 secretion in macrophage cultures. Infect Immun. 1994;62:5689–5693. doi: 10.1128/iai.62.12.5689-5693.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tabona P, Reddi K, Khan S, Nair S P, Crean S J, Meghji S, Wilson M, Preuss M, Miller A D, Poole S, Carne S, Henderson B. Homogeneous Escherichia colichaperonin 60 induces IL-1 beta and IL-6 gene expression in human monocytes by a mechanism independent of protein conformation. J Immunol. 1998;161:1414–1421. [PubMed] [Google Scholar]
- 22.Thole J E, Keulen W J, De Bruyn J, Kolk A H, Groothuis D G, Berwald L G, Tiesjema R H, van Embden J D. Characterization, sequence determination, and immunogenicity of a 64-kilodalton protein of Mycobacterium bovis BCG expressed in Escherichia coliK-12. Infect Immun. 1987;55:1466–1475. doi: 10.1128/iai.55.6.1466-1475.1987. . (Erratum, 55:1949.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Eden W, van der Zee R, Paul A G, Prakken B J, Wendling U, Anderton S M, Wauben M H. Do heat shock proteins control the balance of T-cell regulation in inflammatory diseases? Immunol Today. 1998;19:303–307. doi: 10.1016/s0167-5699(98)01283-3. [DOI] [PubMed] [Google Scholar]
- 24.Verdegaal M E, Zegveld S T, van Furth R. Heat shock protein 65 induces CD62e, CD106, and CD54 on cultured human endothelial cells and increases their adhesiveness for monocytes and granulocytes. J Immunol. 1996;157:369–376. [PubMed] [Google Scholar]
- 25.Xu Z, Horwich A L, Sigler P B. The crystal structure of the asymmetric GroEL-GroES-(ADP)7 chaperonin complex. Nature. 1997;388:741–750. doi: 10.1038/41944. [DOI] [PubMed] [Google Scholar]
- 26.Young D B, Garbe T R. Heat shock proteins and antigens of Mycobacterium tuberculosis. Infect Immun. 1991;59:3086–3093. doi: 10.1128/iai.59.9.3086-3093.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y, Doerfler M, Lee T C, Guillemin B, Rom W N. Mechanisms of stimulation of interleukin-1 beta and tumor necrosis factor-alpha by Mycobacterium tuberculosiscomponents. J Clin Investig. 1993;91:2076–2083. doi: 10.1172/JCI116430. [DOI] [PMC free article] [PubMed] [Google Scholar]