Abstract
Importance
The lack of robust neuroanatomical markers of psychosis risk has been traditionally attributed to heterogeneity. A complementary hypothesis is that variation in neuroanatomical measures in the majority of individuals at psychosis risk may be nested within the range observed in healthy individuals.
Objective
To quantify deviations from the normative range of neuroanatomical variation in individuals at clinical high-risk for psychosis (CHR-P) and evaluate their overlap with healthy variation and their association with positive symptoms, cognition, and conversion to a psychotic disorder.
Design, Setting, and Participants
Clinical, IQ and FreeSurfer-derived regional measures of cortical thickness (CT), cortical surface area (SA), and subcortical volume (SV) from 1,340 CHR-P individuals [47.09% female; mean age: 20.75 (4.74) years] and 1,237 healthy individuals [44.70% female; mean age: 22.32 (4.95) years] from 29 international sites participating in the ENIGMA Clinical High Risk for Psychosis Working Group.
Main Outcomes and Measures
For each regional morphometric measure, z-scores were computed that index the degree of deviation from the normative means of that measure in a healthy reference population (N=37,407). Average deviation scores (ADS) for CT, SA, SV, and globally across all measures (G) were generated by averaging the respective regional z-scores. Regression analyses were used to quantify the association of deviation scores with clinical severity and cognition and two-proportion z-tests to identify case-control differences in the proportion of individuals with infranormal (z<−1.96) or supranormal (z>1.96) scores.
Results
CHR-P and healthy individuals overlapped in the distributions of the observed values, regional z-scores, and all ADS vales. The proportion of CHR-P individuals with infranormal or supranormal values in any metric was low (<12%) and similar to that of healthy individuals. CHR-P individuals who converted to psychosis compared to those who did not convert had a higher percentage of infranormal values in temporal regions (5–7% vs 0.9–1.4%). In the CHR-P group, only the ADSSA showed significant but weak associations (|β|<0.09; PFDR<0.05) with positive symptoms and IQ.
Conclusions and Relevance
The study findings challenge the usefulness of macroscale neuromorphometric measures as diagnostic biomarkers of psychosis risk and suggest that such measures do not provide an adequate explanation for psychosis risk.
Keywords: clinical high-risk, positive symptoms, IQ, normative modeling, FreeSurfer, MRI
Introduction
Schizophrenia is a mental disorder characterized by psychotic and cognitive symptoms1 and significant psychosocial disability.2 Similar abnormalities are also present in individuals at clinical high-risk for psychosis (CHR-P) who typically experience attenuated or brief psychotic symptoms3 and cognitive difficulties, and an elevated risk of developing psychosis at rate of 20% at 2 years and 35% at 10 years.5 A better understanding of the neurobiology of CHR states holds the promise of improving early detection and preventive strategies.6
Multiple magnetic resonance imaging (MRI) studies have focused on identifying neuroanatomical alterations in CHR-P compared to healthy individuals (HI). Two meta-analyses of these studies have highlighted cortical thickness (CT) reductions of small effect size in the frontotemporal regions of CHR individuals7,8 while a mega-analysis of brain morphometric data from 1792 CHR-P and 1377 HI from the CHR-P Working Group of the Enhancing Neuroimaging Genetics through Meta-analysis (ENIGMA) Consortium found that such CT reductions were widespread (Cohen d range of −0.17 to −0.09).9
Recently psychiatric neuroimaging has turned to normative modeling, which quantifies individual-level deviation in brain-derived phenotypes relative to a normative reference population.10 The advantage of this approach is that it can test whether psychiatric disorders are associated with substantial deviation from healthy variation in measures of brain organization. Normative modeling has yet to be applied to CHR-P states, but there are two studies on patients with established schizophrenia that are of direct relevance.11,12 In both studies, brain morphometry measures with values below the 5th or above the 95th percentile of the normative range were respectively considered infranormal and supranormal. Lv et al. (2020)11 calculated normative models of CT from 195 HI and applied them to 322 individuals with schizophrenia; 10–15% of patients showed infranormal CT values in temporal and ventromedial frontal regions and 3% of patients had supranormal values mainly in the paracentral lobule. Wolfers and colleagues (2021)12 developed normative models from voxel-based morphometry data from three samples of HI (N1=400, N2=312, N3=256) and applied them to data from corresponding samples of patients with schizophrenia (N1=94, N2=105, N3=163); only a low percentage of voxels (<2%) had extreme values in patients across samples; voxels with infranormal values were mostly located within temporal, medial frontal, and posterior cingulate regions.
It is currently unknown whether regional deviations from healthy neuroanatomical variation in brain morphometry might be present in CHR-P individuals and whether they explain substantial variance in positive symptoms or cognition. At least one study has suggested that normative deviation is better than raw volumes in predicting psychotic symptoms.13 Addressing these questions is important for two reasons. First, vulnerabilities during brain development, as inferred from the presence of deviations from normative neuroanatomical trajectories, may set the scene for the brain changes observed in established cases of schizophrenia. Second, deviation from healthy variation in brain neuroanatomy may prove informative in identifying those CHR-P individuals that convert or experience more severe clinical presentations. To test these hypotheses, the current study derived age- and sex-specific normative models of regional morphometry from an independent dataset of HI and applied them to the ENIGMA CHR-P Working Group sample which represents the largest available dataset of individual-level morphometric measures from CHR-P individuals.9
Methods
Study Sample
The study sample was derived from the pooled dataset of CHR-P and HI held by the ENIGMA CHR-P Working Group (eMethods and eTable 1). At each site, CHR-P status was ascertained using either the Structured Interview for Prodromal Syndromes (SIPS) or the Comprehensive Assessment of At-Risk Mental States (CAARMS) (eMethods and eTable 2). Additional site-specific eligibility criteria are shown in eTable 1. At each site, whole-brain T1-weighted MRI data obtained from each participant (eMethods and eTable 3) were parcellated and segmented using standard FreeSurfer pipelines (https://surfer.nmr.mgh.harvard.edu/) to yield estimates of total intracranial volume (ICV), regional measures of CT (N=68), surface area (SA) (N=68), and subcortical volume (SV) (N=14) (eTable 4). These measures were then assessed using the ENIGMA consortium quality assessment pipeline.14–17 Ethical approval for data collection and sharing was obtained by the Institutional Review Board at each site. Participant data were shared after all identifying information was removed.
The current study sample comprises participants who had both high-quality brain morphometric data and complete SIPS or CAARMS ratings at the time of their scan (eMethods and eFigure 1). Based on these criteria we included 1,340 CHR-P individuals [47.09% female; age range: 9.5 to 39 years; mean (SD) age: 20.75 (4.74) years] and 1,237 HI [44.70% female; age range: 12 to 39.87 years; mean (SD) age: 22.32 (4.95) years] (Table 1, eTables 5 and 6). Conversion status at a mean follow-up time of 19.71 (13.97) months was available for 1,097 CHR-P individuals (Table 1 and eTable 6). Individuals that converted to a psychotic disorder (CHR-PC) (n=157) had significantly higher positive symptoms at the time of scanning (mean z-score [SD] = 0.21 [1.08]) than those who did not convert (CHR-PNC) (N=940) (mean z-score [SD] = −0.05 [1.01]; T = 2.99; P = 0.003), but the two groups did not differ in age, sex, or IQ (all P > 0.07).
Table 1.
Characteristics of the Sample at Clinical High-Risk for Psychosis (CHR-P)
| All CHR-P individuals | |
| Age, years, mean (SD) | 20.75 (4.74) |
| Female Sex, number (%) | 631 (47.09%) |
| SIPS Positive Symptoms Score, mean (SD)a | 10.93 (4.66) |
| CAARMS Positive Symptoms Scores, mean (SD)b | 10.37 (4.03) |
| IQ, mean z-score (SD)c | −0.21 (1.00) |
| Prescribed Antipsychotic Medication, number (%)d | 243 (18.63%) |
| Months of follow-up, mean (SD)e | 19.71 (13.97) |
| Converters, N (%)e | 157 (14.31%) |
| CHR-P individuals that converted to a psychotic disorder | |
| Age, years, mean (SD) | 20.09 (4.68) |
| Female Sex, number (%) | 64 (40.76%) |
| SIPS Positive Symptoms Score, mean (SD)f | 12.12 (5.06) |
| CAARMS Positive Symptoms Scores, mean (SD)f | 10.71 (4.24) |
| IQ, mean z-score (SD)g | −0.29 (1.03) |
| Prescribed Antipsychotic Medication, number (%)h | 32 (20.38%) |
CAARMS = Comprehensive Assessment of At-Risk Mental States; SIPS = Structured Interview for Psychosis-risk Syndromes; SD = standard Deviation. Positive symptom ratings at the time of scanning were available for the entire sample (N = 1340), assessed either with SIPS or CAARMS;
SIPS was used to assess CHR-P status in 806 CHR-P participants
CAARMS was used to assess CHR-P status in 534 CHR-P participants
Estimates of IQ were available in 924 CHR-P participants; z-scores were used to accommodate site differences in the instruments used (eTable 1)
Medication status at the time of scanning was available in 1304 CHR-P individuals
Conversion status was known for 1097 CHR-P participants but information about the length of the follow-up period was available for 975 CHR-P individuals
SIPS and CAARMS were respectively used to assess 115 and 42 individuals who converted
IQ estimates were available in 109 CHR-P individuals that converted
Medication status was available in 157 CHR-P individuals that converted
Clinical Data
The ratings of CAARMS and SIPS converge only for positive symptoms (eMethods and eTable 2); these ratings were converted to z-scores to enable cross-site harmonization. Similarly, IQ estimates were converted to z-scores to accommodate the different instruments used across sites (eTable 1). Information was also available on medication exposure at the time of scanning.
Normative models of brain morphometry
The normative models for each of the regional CT, SA, and SV measures (eTable 4) were generated using CentileBrain, an empirically validated framework for normative models of brain morphometry developed using data from an independent multi-site sample of 37,407 HI (53.3% female; aged 3–90 years) (https://centilebrain.org/).18 Details of the sample, procedures, model performance, and code are presented in the eMethods. Fractional polynomial regression was used to generate sex-specific models for each measure while accounting for site using ComBat-GAM harmonization.19 The ICV, mean CT, and mean SA were included in the models of the regional measures of SV, CT, and SA respectively.
Computing deviation scores of regional morphometric measures
The CentileBrain model parameters were then applied to each regional CT, SA, and SV measure of the CHR-P and HI of the ENIGMA sample. For each measure in each participant, we estimated the degree of normative deviation from the reference population mean as a z-score computed by subtracting the predicted (Ŷ) from the raw value (Yo) of that measure and then dividing the difference by the root mean square error of the model (eFigure 2).20,21 A positive/negative z-score indicates that the value of the corresponding morphometric measure is higher/lower than the normative mean. As per previous literature,15,16 we defined regional z-scores as infranormal when below z=−1.96 or supranormal when above z=1.96, corresponding to the 5th and 95th percentile respectively. Intermediate values (i.e., between z=−1.96 and z=1.96) were designated as “within normal range”.
Computation of average deviation scores
We averaged the regional z-scores in each participant to generate an “average deviation score” for CT (ADSCT), SA (ADSSA), and SV (ADSSV). ADS were not weighted for the size of the region to enhance reproducibility. Positive or negative ADS values indicate a general pattern of deviations that are above or below the normative reference values. ADS scores were further averaged to generate a “global average deviation score” (ADSG). Using the same criteria as for the z-scores, each ADS was also designated as infranormal, supranormal, or within the normal range. In supplemental analyses, we also explored alternate definitions of ADS, by averaging positive and negative z-scores separately for each neuroimaging phenotype (eMethods).
Statistical Analyses
Statistical significance across all tests performed was set at PFDR<0.05 as per the Benjamini-Hochberg false discovery rate (FDR) correction for multiple comparisons. The robustness of the results was confirmed using a leave-one-site-out approach.
The following analyses were conducted: (i) we calculated the percentage of CHR-P and HI from the ENIGMA sample that had supranormal or infra-normal z-scores in any regional measure and in any of ADSCT, ADSSA, ADSSV, and ADSG (eMethods); group differences in the proportion of individuals with supra- or infranormal z-scores were examined using the two-proportions z-test implemented in R version 4.1.2; (ii) within the CHR-P group, linear regression (implemented with the “lm” function in R version 4.1.2) was used to assess associations between positive symptoms and IQ with each regional z-score, the observed value of each regional morphometric measure, and each ADS; age was included as predictor in all aforementioned regression models due to its significant association with positive symptoms and IQ (PFDR<0.05), while sex was included only in the models with observed data as the z-scores and ADSs were derived from sex-specific models; (iii) we repeated the above analyses separately for CHR-PC and CHR-PNC individuals; and (iv) in HI, we conducted regression analyses to assess associations between IQ and the brain regional z-scores, observed values, and ADS.
Supplemental testing involved group comparisons of mean regional and ADS scores, the use of an alternate parcellation template, and repeating the aforementioned analyses in subsamples of CHR-P sub-syndromes (i.e., attenuated psychotic symptoms syndrome, brief intermittent psychotic symptoms syndrome, and genetic risk and functional deterioration syndrome); additional analyses focused on medication status and alternate ADS definitions (eMethods).
Results
Infra- and supranormal deviations in brain morphometry in CHR-P and HI
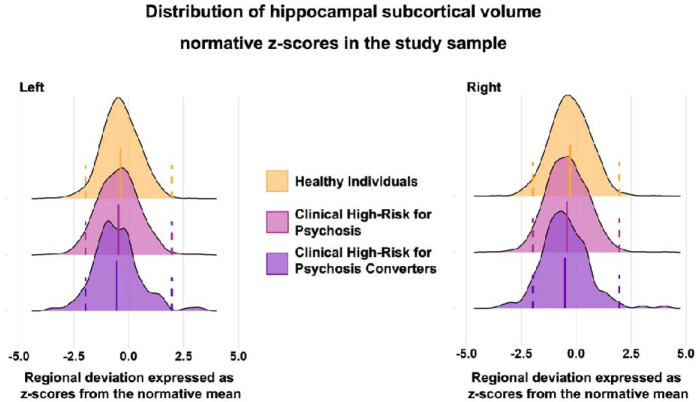
The distributions of the z-scores and observed values of all regional brain morphometric measures of CHR-P and HI showed complete overlap. The left and right hippocampus are used as exemplars in Figure 1 while the corresponding figures for all other regions are included in the eVideo. Moreover, the distribution overlap was independent of parcellation used to define subregions (eFigure 3 and 4).
Figure 1. Distribution of hippocampal subcortical volume normative z-scores in the study sample.
The figure presents the distribution of the left and right hippocampus regional normative z-scores in healthy individuals (HI), individuals at clinical high-risk for psychosis (CHR-P) and CHR-P that converted to full-blown psychosis (CHR-PC). The results for the remaining regional normative z-score are presented in the eVideo. The dotted lines represent the cutoffs for infranormal and supranormal values at z = |1.96|.
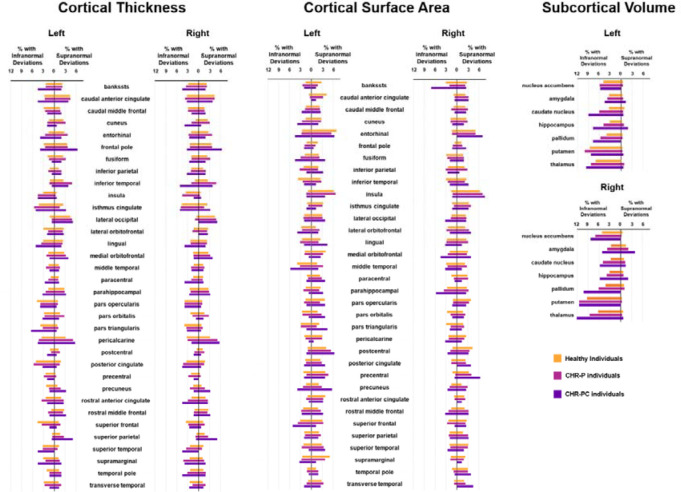
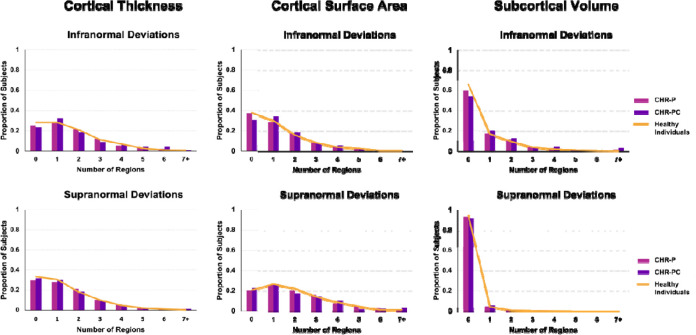
The percentage of CHR-P and HI with supra- or infranormal z-scores in each morphometric measure are shown in Figure 2. Infranormal regional CT z-scores were noted in 0.52–5.67% of CHR-P individuals and 0.49–5.01% of HI; the corresponding range for supranormal z-scores were 0.37–5.15% and 0.32–5.50%. Infranormal regional SA z-scores were noted in 0.30–3.66% of CHR-P individuals and 0.65–3.88% of HI; the corresponding range for supranormal z-scores were 1.12–7.01% and 1.21–6.95%. Infranormal regional SV z-scores were noted in 3.73–11.42% of CHR-P individuals and 2.67–9.30% of HI; the corresponding range for supranormal z-scores were 0.07–2.01% and 0.08–1.37%. Infranormal z-scores in any regional CT, SA, and SV were observed in 74.63%, 62.76%, and 40.00% across CHR-P, respectively; the corresponding infranormal z-scores of HI were 71.71%, 62.25%, and 34.93% (Figure 3). Supranormal z-scores in any regional CT, SA, and SV were observed in 70.37%, 79.70%, and 6.64% across CHR-P, respectively; the corresponding supranormal z-scores in HI is 66.61%, 79.22%, and 5.58% (Figure 3). There were no significant group differences in the percentage of individuals with supra- or infranormal regional values (PFDR>0.05; eTable 7). Compared to unmedicated CHR-individuals, those medicated had a greater proportion with supranormal regional z-scores for the surface area of the left lateral occipital lobe (χ2 = 13.92, PFDR = 0.03) but no other differences (eTable 8).
Figure 2. Percentage of subjects with infra- or supranormal regional normative z-scores.
The proportion of healthy individuals, clinical high-risk for psychosis (CHR-P), and cinical high-risk for psychosis converters with infranormal (left) and supranormal deviations (right) are presented for each hemisphere for cortical thickness, cortical surface area, and subcortical volume.
Figure 3. Distribution of the total number of regions with infra- or supranormal regional normative z-scores.
Bar plots show the distribution of the total number of regions per individual with infra-normal (top row) and supra-normal (bottom row) deviations from the normative model for A) cortical thickness, B) cortical surface area, and C) subcortical volume separately in healthy individuals, clinical high-risk for psychosis (CHR-P) individuals, and clinical high-risk for psychosis converters (CHR-PC).
Supra- and infranormal average deviation scores
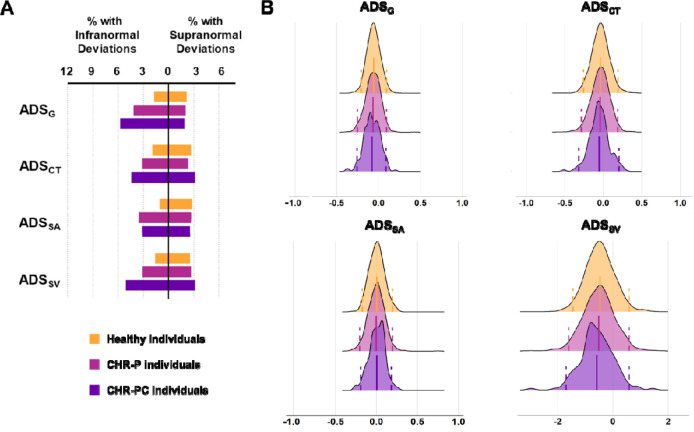
The percentage of CHR-P and HI with supra- or infranormal ADS values are shown in Figure 4A. The distributions in both groups showed a near complete overlap (Figure 4B). Infranormal ADSCT, ADSSA, ADSSV, and ADSG were respectively observed in 3.21%, 3.51%, 3.13%, and 4.18% of CHR-P individuals; the corresponding percentages in HI were 1.94%, 1.05%, 1.62%, and 1.70%. Supranormal ADSCT, ADSSA, ADSSV, and ADSG were respectively observed in 2.31%, 2.69%, 2.69%, and 2.01% of CHR-P individuals; the corresponding percentages in HI were 2.67%, 2.83%, 2.59%, and 2.18%. A significantly higher percentage of CHR-P individuals had infranormal ADSG (χ2 = 12.82, PFDR = 6.85E-4), ADSSV (χ2 = 5.68, PFDR = 0.02), and ADSSA (χ2 = 16.01, PFDR = 2.53E-4) (eTable 9). There were no differences in the percentage of CHR-P individuals with infra- or supranormal ADS depending on their medication status (PFDR>0.05; eTable 10).
Figure 4. Distributions of average deviation scores and percentage of subjects with infra- or supranormal regional normative z-scores.
A) Percentage of healthy, clinical high-risk for psychosis (CHR-P), and clinical high-risk for psychosis converters (CHR-PC) with supra- or infranormal global average deviation score (ADSG), the average deviation score for cortical thickness (ADSCT), average deviation score for cortical surface area (ADSSA), and average deviation score for subcortical volumes (ADSSV). B) The distributions of the average deviation scores in CHR-P (magenta color), CHR-PC (purple color), and healthy individuals (yellow color) for ADSG, ADSCT, ADSSA, and ADSSV. The dotted lines represent the cutoffs for infranormal and supranormal values at z = |1.96|.
Associations of regional z-scores and observed values with positive symptoms and IQ
Within the CHR-P group, positive associations were noted only between IQ and the z-scores of the left caudate volume (left: β= 0.11, PFDR = 0.05), and surface area of the left cuneus (β= 0.11, PFDR = 0.05) (eFigure 5A and B; eTable 11). When analyses were repeated using the observed regional morphometric values, there were no significant associations with IQ or positive symptoms (eTable 12). The same pattern of results for z-scores and observed values was observed when CHR-P individuals exposed to medication were excluded. In HI, no significant associations were noted either between IQ and z-scores or between IQ and the observed values (PFDR > 0.05) (eTable 13).
Associations of average deviation scores with positive symptoms and IQ
Within the CHR-P group, positive symptoms were negatively associated with ADSSA (β = −0.08, PFDR = 0.02; eFigure 5C), while IQ was positively associated with ADSSA (β = 0.09, PFDR = 0.02) and ADSG (β = 0.10, PFDR = 0.01) (eFigure 5D and E; eTable 14). This pattern of associations was robust to medication status and leave-one-out analysis (eFigure 6). In HI, positive associations were also present between IQ and ADSSA, and ADSG (eTable 14).
CHR-P individuals who converted to a psychotic disorder
The percentage of CHR-PC and CHR-PNC individuals with infranormal and supranormal regional z-scores and ADS are shown in Figure 3 and eTable 15). There was a significantly greater percentage of CHR-PC (5.10%) than HI (0.89%) with infranormal z-scores for the thickness of the right inferior temporal lobe (χ2 = 15.34, PFDR = 0.01) and a significantly greater percentage of CHR-PC (7.01%) than CHR-PNC (1.38%) with infranormal z-scores for the surface area of the right banks of the superior temporal sulcus (χ2 = 17.34, PFDR = 4.69E-3). No further differences were identified. As for the entire CHR-P sample, IQ was positively associated with ADSSA (β = 0.26, PFDR = 0.02) and ADSG (β = 0.21, PFDR = 0.05) in CHR-PC individuals. Because of the smaller sample size, associations between positive symptoms and ADSSA were no longer significant within the CHR-PC group but retained the same direction (β = −0.12, PFDR = 0.20). No other significant associations were found between regional z-scores or ADS and IQ or positive symptoms in the CHR-PC or CHR-PNC subsamples (eTable 16).
Supplemental Analyses
Group differences in regional z-scores and ADS between HI and the entire CHR-P or the CHR-PC group were of small effect sizes (Cohen’s |d| <0.26) (eTable 17). Similarly, the effect size differences in the above metrics in CHR-P exposed or not exposed to antipsychotics were also negligible (Cohen’s |d| <0.24) (eTable 17). Analyses of CHR syndromes (eResults) and alternate ADS did not provide additional insights (eTable 18).
Discussion
This study found that variation in regional neuromorphometric measures in CHR-P individuals was nested within the healthy distribution while extreme deviations were present in a minority of CHR-P individuals and at proportions similar to those observed in healthy individuals. However, a greater proportion of CHR-P individuals had infranormal ADSCT, ADSSA, and ASDSV values. Additionally, a higher percentage of CHR-PC individuals had infranormal values in temporal regions but none of the regional z-scores had meaningful associations with the severity of positive symptoms.
Prior case-control studies, including a study by Jalbrzikowski and colleagues who also used the ENIGMA CHR-P Working Group dataset, have reported subtle decrements in regional brain morphometry in CHR-P individuals.7–9 These findings are aligned with the observation of a higher proportion of CHR-P individuals had infranormal values for ADS. In the same dataset, Baldwin and colleagues,22 showed that individual-level heterogeneity was similar in CHR-P and healthy individuals and was not predictive of increased clinical severity. The current study extends our understanding of the role of brain morphometry for psychosis by showing that regional neuroanatomical variation in CHR-P individuals is nested within normative variation. A small minority of CHR-PC patients had pronounced decrements in the cortical thickness and surface area of temporal regions reinforcing the relevance of these regions for psychosis risk 7–9 and syndromal schizophrenia. 11,12,23
Regional deviation from normative patterns in the CHR-P individuals did not show meaningful associations with the severity of positive symptoms. The only exception was that higher ADSSA, indicating an overall pattern of positive regional deviations in cortical surface area, was associated with less severe positive symptoms in the CHR-P group (regardless of conversion status). The ADSSA was also positively associated with IQ both in CHR-P and healthy individuals. The strength of these associations was low (|β|<0.20). Nevertheless, these findings resonate with prior reports of higher IQ being associated with greater cortical surface area expansion24,25 and may reflect the integrity of cellular processes relating to neurite remodelling and intra-cortical myelination that play a key role in cortical surface area expansion during early adulthood. 26, 27
Limitations
The study includes the largest neuroimaging dataset of CHR-P individuals and robust normative models derived from an independent reference sample. As is common with large-scale studies, the data were collected at multiple sites using different scanners and protocols. Although we accounted for site effects using MRI data harmonization and tested the robustness of the results using leave-one-site-out analyses, residual effects cannot be fully excluded but are unlikely to have influenced the overall pattern of the results. The neuroimaging data of the CHR individuals are cross-sectional and do not capture potential longitudinal changes that may be more informative28.
Conclusions
In this study, regional variation in the neuroanatomy of CHR-P individuals was nested within the normal variation. The degree of neuroanatomical normative deviation showed minimal associations with positive symptoms and conversion status. These findings question the usefulness of neuromorphometry as a diagnostic biomarker of CHR-states.
Supplementary Material
Key points.
Question
Is the risk of psychosis associated with brain morphometric changes that deviate significantly from healthy variation?
Findings
In this study of 1340 individuals high-risk for psychosis (CHR-P) and 1237 healthy participants, individual-level variation in macroscale neuromorphometric measures of the CHR-P group was largely nested within healthy variation and was not associated with the severity of positive psychotic symptoms or conversion to a psychotic disorder.
Meaning
The findings suggest the macroscale neuromorphometric measures have limited utility as diagnostic biomarkers of psychosis risk.
Acknowledgments
This study presents independent research funded by multiple agencies. The funding sources had no role in the study design, data collection, analysis, and interpretation of the data. The views expressed in the manuscript are those of the authors and do not necessarily represent those of any of the funding agencies. SSH is supported by NIH National Institute of Mental Health, grant T32MH122394. VLC has received investigator grant 1177370 and project grant 1065742 from the National Health and Medical Research Council. CdlF-S was supported by grants 261895 and 320662 from the Consejo Nacional de Ciencia y Tecnología (CONACyT), grants from CONACyT’s Sistema Nacional de Investigadores (SNI), and grant R21 MH117434 from the National Institutes of Health. LBG was supported by the TrygFoundation; the Danish Research Council on Independent Research; the Lundbeck Foundation Center for Clinical Intervention and Neuropsychiatric Schizophrenia Research, CINS. KMH received support from NIMH grant R01MH105246. RAH has received grants from the University of Pittsburgh Medical Center. CIH was supported by grant R01 MH105246 from the National Institutes of Health. WJH was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI) funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI19C1080). KK and SK received supports from Japan Agency for Medical Research and Development (AMED) under Grant Number JP18dm0307001, JP18dm0307004, and JP19dm0207069, and JST Moonshot R&D (JPMJMS2021). MKi has received grant 2019R1C1C1002457 from the National Research Foundation of Korea and grant 21-BR-03-01 from the KBRI basic research program through Korea Brain Research Institute, funded by the Ministry of Science & ITC. TK was supported by a Brain and Behavior Research Foundation 2021 NARSAD Young Investigator Grant (ID 30112). JSK has received grant 2020M3E5D9079910 from the National Research Foundation of Korea and grant 21-BR-03-01 from the KBRI basic research program through Korea Brain Research Institute, funded by the Ministry of Science & ITC. JL received grant NMRC/TCR/003/2008 from the National Medical Research Council Translational and Clinical Research Flagship Programme. AL is supported by a National Health and Medical Research Council (NHMRC) Emerging Leadership Fellowship 2010063. RM has received grants R01MH100043 and R01MH113564 from the National Institute of Mental Health and grants from the Brain and Behavior Research Foundation and Canadian Institutes of Health Research. BN is supported by a National Health and Medical Research Council (NHMRC) Senior Research Fellowship 1137687. TN has received grants from Otsuka as well as personal fees from Astellas, Eisai, Janssen Pharmaceuticals, Meiji Seika Pharma, Sumitomo Dainippon Pharma, and Takeda. MN is supported by Mental Health Center Copenhagen, Mental Health Services in the Capital Region of Denmark, and University of Copenhagen. DN has received grants R25-A2701 and R287-2018-1485 from the Lundbeck Foundation and grants from the Mental Health Services Capital Region of Denmark. PL-O and FR-M were supported by grants from SNI. WR has received grants from the Zurich Program for Sustainable Development of Mental Health Services. DFS was supported by the US National Institutes of Health, R01MH113533. DS has received grant JP18K15509 and JP22K15745 from the JSPS KAKENHI. US has received grant 569259 from the National Health and Medical Research Council of Australia. GS is supported by the Fundació Clínic Recerca Biomèdica, the Brain and Behavior Research Foundation (NARSAD Young Investigator Award 2017, grant ID: 26731), the Alicia Koplowitz Foundation and the Spanish Ministry of Health, Instituto de Salud Carlos III “Health Research Fund” (PI15/0444; PI18/0242; PI18/00976; PI2100330). MS has received grant JP20H03598 from the Japan Society for the Promotion of Science KAKENHI and grant JP19dk0307069s0203 from the Japan Agency for Medical Research and Development. TT was supported by JSPS KAKENHI Grant Number JP18K07550. CKT is supported by the Research Council of Norway (#223273, #288083, #323951) and the South-Eastern Norway Regional Health Authority (#2019069, #2021070, #2023012, #500189). PMT and SIT are supported in part by NIMH grants R01MH123163, R01MH121246, and R01MH116147. PJU has received grant MR/L011689/1 from the UK Medical Research Council. JAW has received grant 5R01MH115031 from the National Institute of Mental Health. SJW was funded by several grants from the National Health & Medical Research Council of Australia. Core funding for ENIGMA was provided by the NIH Big Data to Knowledge (BD2K) program under consortium grant U54 EB020403 to PMT. This work was supported in part through the computational resources and staff expertise provided by Scientific Computing at the Icahn School of Medicine at Mount Sinai.
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Publishing, 2013. [Google Scholar]
- 2.GBD 2019 Mental Disorders Collaborators. Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry. 2020;9(2):137–150. doi: 10.1016/S2215-0366(21)00395-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fusar-Poli P, Borgwardt S, Bechdolf A, et al. The psychosis high-risk state: a comprehensive state-of-the-art review. JAMA Psychiatry. 2013;70(1):107–120. doi: 10.1001/jamapsychiatry.2013.269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Catalan A, Salazar de Pablo G, Aymerich C, et al. Neurocognitive Functioning in Individuals at Clinical High Risk for Psychosis: A Systematic Review and Meta-analysis. JAMA Psychiatry. 2021;78(8):859–67. doi: 10.1001/jamapsychiatry.2021.1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salazar de Pablo G, Radua J, Pereira J, et al. Probability of Transition to Psychosis in Individuals at Clinical High Risk: An Updated Meta-analysis. JAMA Psychiatry. 2021;78(9):970–978. doi: 10.1001/jamapsychiatry.2021.0830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fusar-Poli P, Salazar de Pablo G, Correll CU, et al. Prevention of Psychosis: Advances in Detection, Prognosis, and Intervention. JAMA Psychiatry. 2020;77(7):755–765. doi: 10.1001/jamapsychiatry.2019.4779 [DOI] [PubMed] [Google Scholar]
- 7.Zhao Y, Zhang Q, Shah C, Li Q, Sweeney JA, Li F, Gong Q. Cortical Thickness Abnormalities at Different Stages of the Illness Course in Schizophrenia: A Systematic Review and Meta-analysis. JAMA Psychiatry. 2022;79(6):560–570. doi: 10.1001/jamapsychiatry.2022.0799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luna LP, Radua J, Fortea L, et al. A systematic review and meta-analysis of structural and functional brain alterations in individuals with genetic and clinical high-risk for psychosis and bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2022;117:110540. doi: 10.1016/j.pnpbp.2022.110540 [DOI] [PubMed] [Google Scholar]
- 9.ENIGMA Clinical High Risk for Psychosis Working Group, Jalbrzikowski M, Hayes RA, Wood SJ, et al. Association of Structural Magnetic Resonance Imaging Measures With Psychosis Onset in Individuals at Clinical High Risk for Developing Psychosis: An ENIGMA Working Group Mega-analysis. JAMA Psychiatry. 2021;78(7):753–766. doi: 10.1001/jamapsychiatry.2021.0638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marquand AF, Wolfers T, Mennes M, Buitelaar J, Beckmann CF. Beyond Lumping and Splitting: A Review of Computational Approaches for Stratifying Psychiatric Disorders. Biol Psychiatry Cogn Neurosci Neuroimaging. 2016;1(5):433–447. doi: 10.1016/j.bpsc.2016.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lv J, Di Biase M, Cash RFH, et al. Individual deviations from normative models of brain structure in a large cross-sectional schizophrenia cohort. Mol Psychiatry 2021;26(7):3512–3523. doi: 10.1038/s41380-020-00882-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolfers T, Rokicki J, Alnaes D, Berthet P, et al. Replicating extensive brain structural heterogeneity in individuals with schizophrenia and bipolar disorder. Hum Brain Mapp 2021;42(8):2546–2555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parkes L, Moore TM, Calkins ME, et al. Transdiagnostic dimensions of psychopathology explain individuals’ unique deviations from normative neurodevelopment in brain structure. Transl Psychiatry. 2021;11(1):232. doi: 10.1038/s41398-021-01342-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Erp TGM, Hibar DP, Rasmussen JM, et al. Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA Consortium. Mol Psychiatry. 2016;21(4):547–553. doi: 10.1038/mp.2015.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Erp TGM, Walton E, Hibar DP, et al. Cortical brain abnormalities in 4474 individuals with schizophrenia and 5098 control subjects via the Enhancing Neuro Imaging Genetics Through Meta Analysis (ENIGMA) Consortium. Biol Psychiatry. 2018;84(9):644–654. doi: 10.1016/j.biopsych.2018.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ching CRK, Gutman BA, Sun D, et al. Mapping subcortical brain alterations in 22q11.2 deletion syndrome: effects of deletion size and convergence with idiopathic neuropsychiatric illness. Am J Psychiatry. 2020;177(7):589–600. doi: 10.1176/appi.ajp.2019.19060583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun D, Ching CRK, Lin A, et al. Large-scale mapping of cortical alterations in 22q11.2 deletion syndrome: convergence with idiopathic psychosis and effects of deletion size. Mol Psychiatry. 2020;25(8):1822–1834. doi: 10.1038/s41380-018-0078-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.BioRX paper coming.
- 19.Pomponio R, Erus G, Habes M, et al. Harmonization of large MRI datasets for the analysis of brain imaging patterns throughout the lifespan. Neuroimage. 2020;208:116450. doi: 10.1016/j.neuroimage.2019.116450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crawford JR, Garthwaite PH, Denham AK, Chelune GJ. Using regression equations built from summary data in the psychological assessment of the individual case: extension to multiple regression. Psychol Assess 2012;24(4):801–814. doi: 10.1037/a0027699 [DOI] [PubMed] [Google Scholar]
- 21.Potvin O, Dieumegarde L, Duchesne S, Alzheimer’s Disease Neuroimaging Initiative, CIMA-Q Group, CCNA Groups. NOMIS: Quantifying morphometric deviations from normality over the lifetime of the adult human brain. BioRxiv 2021; doi: 10.1101/2021.01.25.428063 [DOI] [Google Scholar]
- 22.Baldwin H, Radua J, Antoniades M, et al. Neuroanatomical heterogeneity and homogeneity in individuals at clinical high risk for psychosis. Transl Psychiatry. 2022;12(1):297. doi: 10.1038/s41398-022-02057-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Erp TGM, Walton E, Hibar DP, et al. Cortical Brain Abnormalities in 4474 Individuals With Schizophrenia and 5098 Control Subjects via the Enhancing Neuro Imaging Genetics Through Meta Analysis (ENIGMA) Consortium. Biol Psychiatry. 2018;84(9):644–654. doi: 10.1016/j.biopsych.2018.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fjell AM, Westlye LT, Amlien I, et al. High-expanding cortical regions in human development and evolution are related to higher intellectual abilities. Cereb Cortex. 2015;25(1):26–34. doi: 10.1093/cercor/bht201 [DOI] [PubMed] [Google Scholar]
- 25.Schnack HG, van Haren NE, Brouwer RM, Evans A, Durston S, Boomsma DI, et al. Changes in thickness and surface area of the human cortex and their relationship with intelligence. Cereb Cortex. 2015;25(6):1608–17. doi: 10.1093/cercor/bht357 [DOI] [PubMed] [Google Scholar]
- 26.Jacobs B, Driscoll L, Schall M. Life-span dendritic and spine changes in areas 10 and 18 of human cortex: a quantitative Golgi study. J Comp Neurol. 1997;386(4):661–680. [PubMed] [Google Scholar]
- 27.Miller DJ, Duka T, Stimpson CD, et al. Prolonged myelination in human neocortical evolution. Proc Natl Acad Sci U S A. 2012;109(41):16480–5. doi: 10.1073/pnas.1117943109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Collins MA, Ji JL, Chung Y, et al. Accelerated cortical thinning precedes and predicts conversion to psychosis: The NAPLS3 longitudinal study of youth at clinical high-risk. Mol Psychiatry. 2022;10.1038/s41380–022-01870–7. doi: 10.1038/s41380-022-01870-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.