Abstract
Fungi biosynthesize a diversity of secondary metabolites, small organic bioactive molecules that play diverse roles in fungal ecology. Fungal secondary metabolites are often encoded by physically clustered sets of genes known as biosynthetic gene clusters (BGCs). Fungi in the genus Penicillium produce diverse secondary metabolites that have been both useful (e.g., the antibiotic penicillin and the cholesterol-lowering drug mevastatin) and harmful (e.g., the mycotoxin patulin and the immunosuppressant gliotoxin) to human affairs. BGCs often also encode resistance genes that confer self-protection to the secondary metabolite-producing fungus. Some Penicillium species, such as Penicillium lilacinoechinulatum and Penicillium decumbens, are known to produce gliotoxin, a secondary metabolite with known immunosuppressant activity; however, an evolutionary characterization of the BGC responsible for gliotoxin biosynthesis among Penicillium species is lacking. Here, we examine the conservation of genes involved in gliotoxin biosynthesis and resistance in 35 Penicillium genomes from 23 species. We found homologous, less fragmented gliotoxin BGCs in 12 genomes, mostly fragmented remnants of the gliotoxin BGC in 21 genomes, whereas the remaining two Penicillium genomes lacked the gliotoxin BGC altogether. In contrast, we observed broad conservation of homologs of resistance genes that reside outside the BGC across Penicillium genomes. Evolutionary rate analysis revealed that BGCs with higher numbers of genes evolve slower than BGCs with few genes. Even though the gliotoxin BGC is fragmented to varying degrees in nearly all genomes examined, ancestral state reconstruction suggests that the ancestor of Penicillium species possessed the gliotoxin BGC. Our analyses suggest that genes that are part of BGCs can be retained in genomes long after the loss of secondary metabolite biosynthesis.
Keywords: comparative genomics, evolutionary biology, secondary metabolic gene clusters, duplication and loss, plant pathogen, secondary metabolism, specialized metabolism
Introduction
Gliotoxin is a secondary metabolite produced by certain fungi, including the major opportunistic human pathogen Aspergillus fumigatus (Raffa and Keller 2019). Secondary metabolites are bioactive molecules of low molecular weight that are not required for the organism’s growth but aid survival in harsh environments (Raffa and Keller 2019). Genes that participate in the biosynthesis of secondary metabolites, including gliotoxin, typically reside next to each other in fungal genomes and form biosynthetic gene clusters (BGCs) (Rokas et al. 2020). The gliotoxin BGC is implicated in human pathogenicity because gliotoxin suppresses the immune response of the mammalian host through diverse mechanisms, including by inhibiting protein complexes necessary for the generation of antimicrobial reactive oxygen species, decreasing cytotoxic activities of T lymphocytes, and preventing integrin activation (Dolan et al. 2015; Raffa and Keller 2019). Gliotoxin’s role in modulating host biology suggests that it is a virulence factor (Raffa and Keller 2019). For example, virulence is attenuated in certain animal models of disease when gliP, the non-ribosomal peptide synthetase gene involved in gliotoxin biosynthesis, is deleted (Sugui et al. 2007).
Fungi that produce gliotoxin need to be resistant to the toxin. Several genes contribute to resistance, such as the thioredoxin reductase gene gliT, located within the gliotoxin BGC (Schrettl et al. 2010). gliT deletion strains of A. fumigatus exhibit resistance to gliotoxin oxidation and unchecked methylation (Owens et al. 2015). As a result, gliT-deficient A. fumigatus are hypersensitive to gliotoxin (Owens et al. 2015). Other resistance genes encoding transcription factors, transporters, and oxidoreductases, reside outside the BGC and – like gliT – are found in both gliotoxin-producing and non-producing species (Castro et al. 2022). For example, the transcription factor RglT is the primary regulator of gliT (Ries et al. 2020). Seven other genes are known to be regulated by rglT and contribute to gliotoxin resistance: gtmA (encodes a bis-thiomethyltransferase, AFUA_2G11120), kojR (transcription factor, AFUA_5G06800), abcC1 (ABC-transporter, AN7879/AFUA_1G10390), mtrA (methyltransferase, AN3717/AFUA_6G12780), AN9051 (oxidoreductase, AFUA_7G00700), AN1472 (MFS transporter, AFUA_8G04630), and AN9531 (NmrA-transcription factor, AFUA_7G06920) (Castro et al. 2022).
Though progress has been made in understanding the mechanisms and functions of the gliotoxin biosynthetic pathway, several questions remain, especially concerning the evolutionary and ecological significance of this BGC in lineages that contain a mix of biotechnologically and medically relevant fungi, such as Penicillium (Steenwyk et al. 2019). For example, Penicillium camemberti and Penicillium roqueforti contribute to cheese production (Nelson 1970; Lessard et al. 2012), whereas Penicillium expansum, Penicillium digitatum, and Penicillium italicum are postharvest pathogens of citrus fruits, stored grains, and other cereal crops (Marcet-Houben et al. 2012; Ballester et al. 2015; Li et al. 2015). Examination of the gliotoxin BGC in the genomes of Penicillium species will shed light on the evolution of the gliotoxin BGC within Aspergillaceae, the family encompassing both Aspergillus and Penicillium species.
Considering the close relatedness of Penicillium and Aspergillus, it is interesting that evidence of gliotoxin production is scant within the former. To fill this gap, we employed a genome-scale approach to infer the evolutionary history of the gliotoxin BGC among 35 strains of 23 Penicillium species. We found that most Penicillium genomes examined contained fragmented gliotoxin BGCs and two lacked a BGC. However, some P. expansum strains had two homologous gliotoxin BGCs. Codon optimization analysis reveals that genes in Penicillium BGCs are lowly optimized, whereas genes in Aspergillus gliotoxin BGCs are highly optimized.
In contrast, gliotoxin resistance genes in Penicillium and Aspergillus fungi have similar degrees of codon optimization, suggesting that Penicillium species encounter exogenous gliotoxin in their environments. Examination of evolutionary rates revealed that genes from highly fragmented gliotoxin BGCs evolved at significantly higher rates than genes from less fragmented BGCs, suggesting that less fragmented BGCs have been experiencing relaxation of selective constraints for longer. Ancestral state reconstructions indicate that the Penicillium ancestor possessed a less fragmented gliotoxin BGC, followed by distinct trajectories of duplication and loss, highlighting the diverse evolutionary pathways of the gliotoxin BGC in Penicillium species.
Materials and Methods
I. Data collection and quality assessment
We retrieved the genomes and gene annotations of 35 Penicillium strains from 23 species as well as of two outgroups (Aspergillus fumigatus and Aspergillus fischeri) from NCBI (https://www.ncbi.nlm.nih.gov/) (Table S1).
Genome assembly and annotation quality were examined to evaluate whether the dataset is sufficient for comparative genomics. The quality and characteristics of the genomes (N50, L50, assembly size, number of scaffolds, and gene count) were evaluated using BioKIT (v0.1.0) (Steenwyk et al. 2022) (Figure S1). The average N50 value was 1,850,972.1 bases, where 46% of proteomes consisted of N50 values greater than 1 Mb, and the lowest N50 value was 31,119 bases for P. expansum CMP 1. Gene annotation completeness was assessed using BUSCO (v5.0.0) (Waterhouse et al. 2018) (Figure S2). BUSCO uses a predetermined set of near-universally conserved single-copy genes (or BUSCO genes) to identify their presence in a query proteome (characterized as single-copy, duplicated, or fragmented) or absence. We used the 4,181 BUSCO genes from the Eurotiales OrthoDB dataset (Manni et al. 2021; Zdobnov et al. 2021). Nearly all the genomes have high BUSCO gene coverage (average: 95.9% ± 3.1%), with the lowest percentages being for P. coprophilum (87.9%) and P. decumbens (85.3%).
II. Identification and characterization of gliotoxin BGC and resistance genes
a. Identification of gliotoxin GBC and resistance genes
The representative gliotoxin BGC (BGC0000361, Download date: April 2022) from the Aspergillus fumigatus Af293 reference strain was downloaded from the Minimum Information about a Biosynthetic Gene Cluster (MiBIG) database (Kautsar et al. 2019). Command-line NCBI BLASTP (Camacho et al. 2009) searches for the Af293 gliotoxin BGC against the proteome of each species were executed. Highly similar sequences were identified using an expectation value threshold of 1e-4 and a query coverage of 50%. The resulting BLAST outputs were then cross-referenced with the NCBI feature table file, which contains genome location information for each gene, and parsed to identify clusters of homologs. Less fragmented BGCs are defined as having at least 7 / 13 genes from the query gliotoxin BGC present, including gliP, encoding the core nonribosomal peptide synthetase (Castro et al. 2022); mostly fragmented clusters are defined as having at least 3 /13 genes from the gliotoxin BGC without a requirement for this cluster to include gliP. When identifying BGCs, we allowed up to four genes between each pair of adjacent homologs using the A. fumigatus Af293 BGC from the MiBIG database (Kautsar et al. 2019) as reference (Castro et al. 2022).
To rule out gene annotation errors in cases where we infer genes to be absent, we conducted command-line NCBI tBLASTn searches for the Af293 gliotoxin BGC against the genome sequences. Highly similar sequences were identified using an expectation value threshold of 1e-10. The resulting outputs were analyzed, and no new presence/absence information was found.
Sequence similarity searches were also conducted for eight gliotoxin resistance genes (abcC1/AN7879, mtrA/AN3717, AN9051, AN1472, AN9531, rglT, gtmA/AFU2G11120, kojR/AFUA_5G06800), three of which were transcription factors (AN9531, rglT, kojR). We used an expectation value threshold of 1e-3 and a query coverage threshold of 50%; we used a lower query coverage threshold of 40% for the three transcription factors.
b. Codon bias
To estimate the potential functional significance of the partial gliotoxin BGCs present in Penicillium genomes, mean gene-wise relative synonymous codon usage (gRSCU) was determined for each clustered gli gene across all proteomes using BioKIT (Steenwyk et al. 2022). This provides insight into how codon usage bias influences the expression level of a particular gene. The percentile rankings of each of the present and clustered gli genes were calculated using the R package dplyr (v1.0.9) (Wickham et al. 2022), and these values, for each species, were then plotted using the R package ggplot2 (Wickham 2016).
c. Synteny Analysis
Alignments of representative Penicillium genomes with less and more fragmented gliotoxin BGCs were generated using a GenomeDiagram in Biopython (Cock et al. 2009). Five genomes (A. fumigatus Af293, P. flavigenum IBT 14082, P. roqueforti FM164, P. nordicum DAOMC 185683, and P. expansum CMP1) with the largest number of different, homologous gli cluster genes above seven, and including gliP, were chosen to visualize the conservation of synteny of less fragmented gliotoxin BGCs across the phylogeny. Similarly, the five genomes (P. steckii IBT 24891, P. vulpinum IBT 29486, P. rubens 43M1, P. camemberti FM 013, and P. italicum PHI 1) with the greatest number of different, homologous gli cluster genes above three and below seven, and not needing to include gliP, were chosen to visualize synteny of mostly fragmented BGCs across the phylogeny.
III. Phylogenetic Analysis
a. Species Tree Inference
The evolutionary relationships of Penicillium species were obtained from a previous study (Steenwyk et al. 2019) using treehouse (Steenwyk and Rokas 2019). For three species with population-level data, within-species relationships were inferred using phylogenomics. To do so, protein sequences of BUSCO genes were first aligned using MAFFT (v7.490) with the --auto parameter (Katoh and Standley 2013). Codon-based alignments were generated by threading the corresponding DNA sequences onto the protein alignment with the thread_dna function in PhyKIT (v1.11.2) (Steenwyk et al. 2021). The resulting nucleotide alignments were trimmed using ClipKIT (v1.3.0) (Steenwyk et al. 2020) with default parameters. The resulting aligned and trimmed sequences were concatenated into a supermatrix with 8,124,861 sites using the create_concat function in PhyKIT. We then inputted the concatenated matrix into IQ-TREE 2 (v2.0.6), a software that implements a maximum likelihood framework for inferring phylogenies. All other evolutionary relationships between species were constrained following the relationships inferred in a previously published study (Steenwyk and Rokas 2019). The best-fitting substitution model (GTR+F+I+G4) was determined using ModelFinder (Kalyaanamoorthy et al. 2017).
b. Single-gene tree inference
To infer the evolutionary history of genes in the gliotoxin BGCs, individual gli genes were compiled and aligned with MAFFT (v7.490) using the --auto parameter (Katoh and Standley 2013). The corresponding nucleotide sequences for each file were obtained from the CDS files for each species, using the faidx function of BioKIT (v0.1.0) (Steenwyk et al. 2022). These nucleotide sequences were then threaded onto the protein alignments using the thread_dna function of PhyKIT (Steenwyk et al. 2021), resulting in a codon-based alignment. All individual codon-based gene alignments were trimmed with ClipKIT (Steenwyk et al. 2020) with default parameters. The trimmed alignments were used to construct a phylogeny using IQ-TREE 2 (Minh et al. 2020). The best-fitting substitution model was chosen for each gli gene using Bayesian information criteria (BIC) implemented in ModelFinder (Kalyaanamoorthy et al. 2017) from IQ-TREE 2. Branch support in each phylogenetic tree was assessed by 1000 bootstraps using ultrafast bootstrapping approximation (Hoang et al. 2018). Tree visualization was carried out using the R packages ape (v5.6.2) (Paradis and Schliep 2019) and phytools (v1.0.3) (Revell 2012).
To characterize variation in the evolution of individual genes of the gliotoxin BGC, the trimmed alignments and maximum-likelihood trees from IQ-TREE 2 were used as input into the evolutionary_rate, total_tree_length, and pairwise_identity functions of PhyKIT to estimate two tree-based measures of evolutionary rate and one sequence-based measure. Evolutionary rate is defined as the total tree length divided by the number of terminals (Telford et al. 2014; Steenwyk et al. 2021). The total tree length is the sum of all branches (Steenwyk et al. 2021).
c. Ancestral state reconstructions
Ancestral state reconstruction for each gene of the gliotoxin BGC across three discrete characters (“Presence clustered,” “Presence unclustered,” and “Absence”) was estimated using phytools (v1.0.3) (Revell 2012). Presence generally indicates that a homolog of the particular gene was identified. “Presence clustered” identifies an existing homolog of the specific gene within a maximum distance of four genes from other homologs of the gliotoxin BGC. “Presence unclustered” identifies an existing homolog of the particular gene without clustering. “Absence” indicates that no homolog of the specific gene was identified. Estimation of ancestral character states was done using the Dollo parsimony method. This method assumes that a complex character lost during the evolution of a particular lineage cannot be regained (Rogozin et al. 2006). Count, a software package for the evolutionary analysis of homolog family sizes, was used to generate these ancestral state reconstructions (Csűös 2010).
d. Tree Topology Testing
Tree topology testing was used to determine whether the duplication event resulting in the two less fragmented, homologous gliotoxin BGCs in P. expansum strains MD 8 and d1 occurred solely in the lineage of P. expansum or deeper in the tree. IQTREE 2 (Minh et al. 2020) was used to compute log-likelihoods of a constrained tree (monophyly of P. expansum gliP homologs) and the observed tree in which a polyphyly of gliP in both clusters is seen (inconsistent with the known species tree). 1000 RELL replicates (Kishino et al. 1990) were performed. The AU test results (Shimodaira 2002) was used for comparison.
Results and Discussion
I. The gliotoxin BGC is fragmented in Penicillium species
Presence / absence data of the 13 genes in the gliotoxin BGC among the 23 Penicillium species analyzed reveals that the cluster is largely fragmented in the genus Penicillium (Figure 1). The proteomes of 12 strains from 5 Penicillium species (P. arizonense, P. flavigenum, P. roqueforti, P. nordicum, P. expansum), possessed less fragmented BGCs, and the proteomes of 23 strains from 18 Penicillium species had mostly fragmented BGCs (Figure S3-S15). Two less fragmented BGCs, which contained 10 / 13 genes and 7 / 13 genes, were identified in P. expansum strains d1 and MD 8, respectively. Regardless of the number of less fragmented BGCs found, to our knowledge, none of the Penicillium species in question are known to produce gliotoxin, except P. decumbens (Feng et al. 2018), suggesting that the absence of clustering in this species may be due to strain heterogeneity and requires further exploration.
Fig. 1. Phylogeny of Penicillium genomes.
Different genera are depicted using different-colored boxes. Aspergillus is shown in red and Penicillium in blue. Shaded circles next to species / strain names indicate gliotoxin production information from the literature, or lack thereof (Fischer et al. 2000; Spikes et al. 2008; Knowles et al. 2020; Redrado et al. 2022). Shaded squares in the second column depict number of clusters identified. Remaining color strips depict gene presence clustered (black), presence unclustered (gray), and absence (white) according to the requirements outlined in the Methods section. Ancestral state reconstructions of each gene of the gliotoxin BGC (for the ancestor of Penicillium species) are presented in pie charts below the phylogeny.
II. A complete gliotoxin BGC was present in the ancestor of Penicillium species
Ancestral state reconstruction revealed the presence of all 13 genes in the gliotoxin BGC in the ancestor of the Penicillium species used in our study (Figure 1). We infer that the first gene lost was gliH, which is absent from 25 of the 35 Penicillium strains examined. The gliH gene encodes an acetyltransferase that, when deleted, results in a loss of gliotoxin production in A. fumigatus (Schrettl et al. 2010; Castro et al. 2022). Thus, the early loss of gliH in the genus Penicillium may have been the key determinant of a lack of gliotoxin production. Further, the synteny of genes in the BGC is mostly conserved and similar to the arrangement of the A. fumigatus Af293 gliotoxin BGC across representative, less fragmented BGCs, such as P. flavigenum IBT 14082 and P. expansum CMP 1 (Figure 2). In contrast, there is extensive divergence in synteny conservation among mostly fragmented BGCs (Figure 2). To our knowledge, none of the Penicillium species examined are known to produce gliotoxin, except P. decumbens (Feng et al. 2018), suggesting that the absence of clustering in this species may be due to strain heterogeneity and requires further exploration.
Fig. 2. Conservation of gliotoxin BGC synteny for representative Penicillium species.
Synteny analysis of representative genomes with less fragmented (A) and mostly fragmented (B) gliotoxin BGCs. Each interval along the track represents 2 kb.
III. Resistance genes are broadly conserved
The presence/absence results of the eight resistance genes, portrayed in Figure 1, suggest that their origins predate the Aspergillus and Penicillium genera (Figure S16-S23). All species possessed abcC1, AN1472, AN9051, AN9531, and kojR homologs. In addition, only Penicillium species with mostly fragmented gliotoxin BGCs lacked at least one resistance gene, such as gtmA, mtrA, and rglT. Penicillium chrysogenum lacked both rglT and gliT, an observation consistent with the transcriptional dependency of gliT to rglT (Ries et al. 2020).
IV. Penicillium species have experienced changes in gliotoxin BGC synteny over time
All genes of the gliotoxin BGC were broadly found within the genus Penicillium, except for gliH, yet most were sparsely clustered (Figure 1). More specifically, 12 out of 35 Penicillium species/strains were found to have a less fragmented, homologous BGC. Two strains of Penicillium expansum (d1 and MD 8) were found to have two BGCs. Evidence of variation in gene presence / absence is also evident within species. For example, Penicillium roqueforti shows population variation in the presence of gliZ, a major transcriptional regulator of gliotoxin biosynthesis (Bok et al. 2006); five strains of P. roqueforti lack gliZ whereas one strain has the gene. As a result, we can conclude that the ancestor of P. roqueforti had a gliZ homolog, but the gene was lost over time in most of the strains, highlighting the importance of population-level sampling. Overall, we can see that the gliotoxin BGC has experienced relocations and duplications of its genes, specifically in Penicillium expansum strains d1 and MD 8, as is expected in the formation of most secondary metabolite-producing BGCs (Rokas et al. 2018).
V. Few Penicillium species contain codon-optimized gliotoxin BGCs
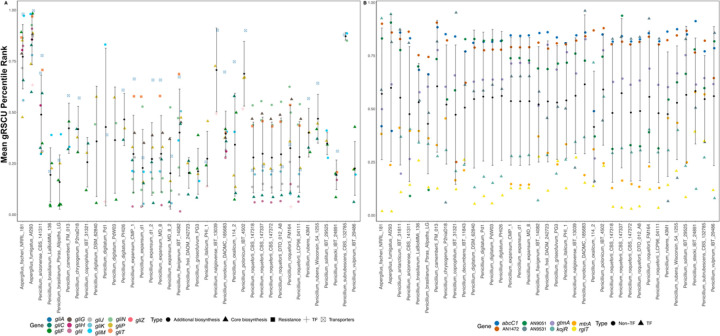
Compared to the two outgroup Aspergillus species, A. fumigatus and A. fischeri, Penicillium species have much lower gRSCU value rankings (Figure 3). Specifically, the mean gRSCU percentile rank of gliotoxin BGC genes among the Aspergillus outgroups is 0.81, while that among the Penicillium species is 0.35; these scores suggest that gli genes from Aspergillus are more codon-optimized than gli genes from Penicillium. Regardless of mean gRSCU values, gliT and gliA homologs, when present, are ranked consistently in the top three to four clustered genes. However, when considering resistance genes, the spread and range of their gRSCU values are similar across all species. The mean gRSCU percentile rank of gliotoxin resistance genes among the Aspergillus outgroups is 0.58, while that among the Penicillium species is 0.53. This allows us to infer that these Penicillium species may ecologically encounter exogenous gliotoxin, making gliT, encoding a gliotoxin-neutralizing enzyme, gliA, encoding a transporter that exports gliotoxin, and non-TF resistance genes such as abcC1, encoding an ABC-transporter, rank in the top percentiles among each of the species’ gene sets.
Fig. 3. Gene-wise relative synonymous codon usage (gRSCU) for gliotoxin BGC and resistance genes.
(A) Percentile rankings of gene-wise relative synonymous codon usage (gRSCU) among gliotoxin BGC genes, in comparison to all other genes. Types / functionality of each gene of the gliotoxin BGC is depicted by shape in the categories of “Core biosynthesis”, “Additional biosynthesis”, “Resistance”, “Transcription Factor”, “Transporter” (B) Percentile ranking of gene-wise relative synonymous codon usage (gRSCU) among gliotoxin resistance genes, in comparison to all other genes. Types / functionality of each resistance gene is depicted by shape in the categories of “Non-Transcription Factor and Transcription Factor”.
VI. gli genes in less fragmented clusters are evolving at a slower rate than mostly fragmented clusters
In the comparison of tree-based and sequence-based measures of evolutionary rate, gli genes from less fragmented clusters are evolving at a significantly slower pace (p<0.0001) than those from mostly fragmented clusters across all three metrics, as seen by a two-way ANOVA with an additive model (Figure 4, Figure S3-S15). This difference highlights a notable feature of many BGCs, the fact that they are rapidly evolving, hinted at by their high variability and narrow taxonomic range (Rokas et al. 2020).
Fig. 4. Evolutionary rate comparison across gliotoxin BGCs.
Multi-method comparison of evolutionary rates between less fragmented and mostly fragmented gliotoxin BGCs. Less fragmented clusters were required to contain a gliP ortholog and at least 7 different genes of the cluster. Mostly fragmented clusters had no requirement to contain a gliP ortholog and only needed to contain at least 3 different genes of the cluster. (A) Comparison of evolutionary rates, as a function of total tree length divided by the number of taxa, between less fragmented and mostly fragmented gliotoxin BGCs. (B) Comparison of total tree length between less fragmented and mostly fragmented gliotoxin BGCs. (C) Comparison of pairwise identity between less fragmented and mostly fragmented gliotoxin BGCs.
VII. A duplication of the gliotoxin BGC may have occurred before the divergence between P. flavigenum and P. roqueforti
We conducted a tree topology test to infer when the gliotoxin BGCs found in P. flavigenum occurred. The maximum likelihood phylogeny suggests that this duplication occurred before the divergence between P. flavigenum and P. roqueforti. An alternative hypothesis is that duplication occurred within P. expansum. This alternative hypothesis would be supported by monophyly of P. expansum homologs of BGC genes. After conducting a tree topology test comparing log likelihood values between the maximum likelihood phylogeny and an alternative tree wherein P. expansum gliP homologs were constrained to be monophyletic, we found that the constrained topology was significantly rejected (Approximately Unbiased test, p = 7.34e-110) (Figure S24). In other words, it is unlikely duplication occurred within P. expansum lineage; instead, duplication likely occurred more anciently, prior to the diversification of P. expansum.
Conclusions
The ancestor of Penicillium species likely possessed a complete gliotoxin BGC. A duplication event of the BGC occurred in one lineage, likely prior to the divergence of P. flavigenum and P. roqueforti. Also, the presence/absence results of the eight resistance genes suggest that their origins predate the Aspergillus and Penicillium genera suggesting that resistance has long been important among these species. The genes in Penicillium gliotoxin BGCs are less codon optimized (gRSCU percentile rank mean: 0.35) compared to their Aspergillus counterparts (gRSCU percentile rank mean: 0.81) suggesting that gli genes are much more often expressed in Aspergillus species than in Penicillium. However, less fragmented BGCs within Penicillium species are evolving at a slower rate than mostly fragmented clusters, suggestive of potential functionality.
Although informative, this work only utilizes publicly available protein annotations of biotechnologically and medically relevant Penicillium fungi, making it important to expand upon the species/strains studied. Moreover, this same targeted gliotoxin analysis within a larger phylogeny of Aspergillus species, for which there is greater evidence of the production of this secondary metabolite, may be helpful. An analysis of gliotoxin BGCs encoded in all fungi would also provide us with more insight into the evolutionary mechanisms that give rise to BGC diversity. In addition, expanding on the causes of conservation of less fragmented gliotoxin BGCs within a variety of Penicillium strains may be important, especially because evidence of production is lacking. As a result, this exciting reality encourages further understanding of the motivating hypothesis that individual secondary metabolites are “cards” of virulence in a larger “hand” that fungi possess.
Acknowledgements
We thank members of the Rokas Laboratory at Vanderbilt University for support and feedback on this work. We also thank the Vanderbilt Data Science Institute for their undergraduate enrichment opportunities. This work was performed in part using resources contained within the Advanced Computing Center for research and Education at Vanderbilt University in Nashville, TN.
Funding
C.B. was supported by the Vanderbilt University Data Science Institute—Summer Research Program. J.L.S. and A.R. were funded by the Howard Hughes Medical Institute through the James H. Gilliam Fellowships for Advanced Study program. Research in A.R.’s lab is supported by grants from the National Science Foundation (DEB-2110404), the National Institutes of Health/National Institute of Allergy and Infectious Diseases (R01 AI153356), and the Burroughs Wellcome Fund.
Footnotes
Conflicts of interest
J.L.S. is a scientific consultant for Latch AI Inc. J.L.S. is a scientific advisor for WittGen Biotechnologies. A.R. is a scientific consultant for LifeMine Therapeutics, Inc.
Data availability
The authors affirm that all data necessary for confirming the conclusions of the article are present within the article, figures, tables, and supplemental material.
Works Cited
- Ballester A.-R., Marcet-Houben M., Levin E., Sela N., Selma-Lázaro C. et al. , 2015. Genome, Transcriptome, and Functional Analyses of Penicillium expansum Provide New Insights Into Secondary Metabolism and Pathogenicity. MPMI 28: 232–248. [DOI] [PubMed] [Google Scholar]
- Bok J. W., Chung D., Balajee S. A., Marr K. A., Andes D. et al. , 2006. GliZ, a transcriptional regulator of gliotoxin biosynthesis, contributes to Aspergillus fumigatus virulence. Infect Immun 74: 6761–6768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho C., Coulouris G., Avagyan V., Ma N., Papadopoulos J. et al. , 2009. BLAST+: architecture and applications. BMC Bioinformatics 10: 421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro P. A. de, Colabardini A. C., Moraes M., Horta M. A. C., Knowles S. L. et al. , 2022. Regulation of gliotoxin biosynthesis and protection in Aspergillus species. PLOS Genetics 18: e1009965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cock P. J. A., Antao T., Chang J. T., Chapman B. A., Cox C. J. et al. , 2009. Biopython: freely available Python tools for computational molecular biology and bioinformatics. Bioinformatics 25: 1422–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csűös M., 2010. Count: evolutionary analysis of phylogenetic profiles with parsimony and likelihood. Bioinformatics 26: 1910–1912. [DOI] [PubMed] [Google Scholar]
- Dolan S. K., O’Keeffe G., Jones G. W., and Doyle S., 2015. Resistance is not futile: gliotoxin biosynthesis, functionality and utility. Trends in Microbiology 23: 419–428. [DOI] [PubMed] [Google Scholar]
- Feng H., Liu S., Su M., Kim E. L., Hong J. et al. , 2018. Gliotoxin is Antibacterial to Drug-resistant Piscine Pathogens. Nat Prod Sci 24: 225–228. [Google Scholar]
- Fischer G., Müller T., Schwalbe R., Ostrowski R., and Dott W., 2000. Species-specific profiles of mycotoxins produced in cultures and associated with conidia of airborne fungi derived from biowaste. International Journal of Hygiene and Environmental Health 203: 105–116. [DOI] [PubMed] [Google Scholar]
- Hoang D. T., Chernomor O., von Haeseler A., Minh B. Q., and Vinh L. S., 2018. UFBoot2: Improving the Ultrafast Bootstrap Approximation. Mol Biol Evol 35: 518–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyaanamoorthy S., Minh B. Q., Wong T. K. F., von Haeseler A., and Jermiin L. S., 2017. ModelFinder: fast model selection for accurate phylogenetic estimates. Nat Methods 14: 587–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K., and Standley D. M., 2013. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol Biol Evol 30: 772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kautsar S. A., Blin K., Shaw S., Navarro-Muñoz J. C., Terlouw B. R. et al. , 2019. MIBiG 2.0: a repository for biosynthetic gene clusters of known function. Nucleic Acids Research gkz882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishino H., Miyata T., and Hasegawa M., 1990. Maximum likelihood inference of protein phylogeny and the origin of chloroplasts. J Mol Evol 31: 151–160. [Google Scholar]
- Knowles S. L., Mead M. E., Silva L. P., Raja H. A., Steenwyk J. L. et al. , 2020. Gliotoxin, a Known Virulence Factor in the Major Human Pathogen Aspergillus fumigatus, Is Also Biosynthesized by Its Nonpathogenic Relative Aspergillus fischeri. mBio 11: e03361–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessard M.-H., Bélanger G., St-Gelais D., and Labrie S., 2012. The Composition of Camembert Cheese-Ripening Cultures Modulates both Mycelial Growth and Appearance. Applied and Environmental Microbiology 78: 1813–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B., Zong Y., Du Z., Chen Y., Zhang Z. et al. , 2015. Genomic Characterization Reveals Insights Into Patulin Biosynthesis and Pathogenicity in Penicillium Species. MPMI 28: 635–647. [DOI] [PubMed] [Google Scholar]
- Manni M., Berkeley M. R., Seppey M., and Zdobnov E. M., 2021. BUSCO: Assessing Genomic Data Quality and Beyond. Current Protocols 1: e323. [DOI] [PubMed] [Google Scholar]
- Marcet-Houben M., Ballester A.-R., de la Fuente B., Harries E., Marcos J. F. et al. , 2012. Genome sequence of the necrotrophic fungus Penicillium digitatum, the main postharvest pathogen of citrus. BMC Genomics 13: 646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minh B. Q., Schmidt H. A., Chernomor O., Schrempf D., Woodhams M. D. et al. , 2020. IQ-TREE 2: New Models and Efficient Methods for Phylogenetic Inference in the Genomic Era. Mol Biol Evol 37: 1530–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson J. Howard., 1970. Production of Blue cheese flavor via submerged fermentation by Penicillium roqueforti. J. Agric. Food Chem. 18: 567–569. [Google Scholar]
- Owens R. A., O’Keeffe G., Smith E. B., Dolan S. K., Hammel S. et al. , 2015. Interplay between Gliotoxin Resistance, Secretion, and the Methyl/Methionine Cycle in Aspergillus fumigatus. Eukaryotic Cell 14: 941–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradis E., and Schliep K., 2019. ape 5.0: an environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 35: 526–528. [DOI] [PubMed] [Google Scholar]
- Raffa N., and Keller N. P., 2019. A call to arms: Mustering secondary metabolites for success and survival of an opportunistic pathogen. PLOS Pathogens 15: e1007606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redrado S., Esteban P., Domingo M. P., Lopez C., Rezusta A. et al. , 2022. Integration of In Silico and In Vitro Analysis of Gliotoxin Production Reveals a Narrow Range of Producing Fungal Species. Journal of Fungi 8: 361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revell L. J., 2012. phytools: an R package for phylogenetic comparative biology (and other things). Methods in Ecology and Evolution 3: 217–223. [Google Scholar]
- Ries L. N. A., Pardeshi L., Dong Z., Tan K., Steenwyk J. L. et al. , 2020. The Aspergillus fumigatus transcription factor RglT is important for gliotoxin biosynthesis and self-protection, and virulence. PLoS Pathog 16: e1008645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogozin I. B., Wolf Y. I., Babenko V. N., and Koonin E. V., 2006. Dollo parsimony and the reconstruction of genome evolution.
- Rokas A., Mead M. E., Steenwyk J. L., Raja H. A., and Oberlies N. H., 2020. Biosynthetic gene clusters and the evolution of fungal chemodiversity. Nat Prod Rep 37: 868–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rokas A., Wisecaver J. H., and Lind A. L., 2018. The birth, evolution and death of metabolic gene clusters in fungi. Nat Rev Microbiol 16: 731–744. [DOI] [PubMed] [Google Scholar]
- Schrettl M., Carberry S., Kavanagh K., Haas H., Jones G. W. et al. , 2010. Self-Protection against Gliotoxin—A Component of the Gliotoxin Biosynthetic Cluster, GliT, Completely Protects Aspergillus fumigatus Against Exogenous Gliotoxin. PLOS Pathogens 6: e1000952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimodaira H., 2002. An Approximately Unbiased Test of Phylogenetic Tree Selection. Systematic Biology 51: 492–508. [DOI] [PubMed] [Google Scholar]
- Spikes S., Xu R., Nguyen C. K., Chamilos G., Kontoyiannis D. P. et al. , 2008. Gliotoxin Production in Aspergillus fumigatus Contributes to Host-Specific Differences in Virulence. The Journal of Infectious Diseases 197: 479–486. [DOI] [PubMed] [Google Scholar]
- Steenwyk J. L., Buida T. J. III, Gonçalves C., Goltz D. C., Morales G. et al. , 2022. BioKIT: a versatile toolkit for processing and analyzing diverse types of sequence data. Genetics 221: iyac079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenwyk J. L., Buida III T. J., Labella A. L., Li Y., Shen X.-X. et al. , 2021. PhyKIT: a broadly applicable UNIX shell toolkit for processing and analyzing phylogenomic data. Bioinformatics 37: 2325–2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenwyk J. L., Iii T. J. B., Li Y., Shen X.-X., and Rokas A., 2020. ClipKIT: A multiple sequence alignment trimming software for accurate phylogenomic inference. PLOS Biology 18: e3001007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenwyk J. L., and Rokas A., 2019. Treehouse: a user-friendly application to obtain subtrees from large phylogenies. BMC Research Notes 12: 541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenwyk J. L., Shen X.-X., Lind A. L., Goldman G. H., and Rokas A., 2019. A Robust Phylogenomic Time Tree for Biotechnologically and Medically Important Fungi in the Genera Aspergillus and Penicillium. mBio. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugui J. A., Pardo J., Chang Y. C., Zarember K. A., Nardone G. et al. , 2007. Gliotoxin is a virulence factor of Aspergillus fumigatus: gliP deletion attenuates virulence in mice immunosuppressed with hydrocortisone. Eukaryot Cell 6: 1562–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telford M. J., Lowe C. J., Cameron C. B., Ortega-Martinez O., Aronowicz J. et al. , 2014. Phylogenomic analysis of echinoderm class relationships supports Asterozoa. Proceedings of the Royal Society B: Biological Sciences 281: 20140479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse R. M., Seppey M., Simão F. A., Manni M., Ioannidis P. et al. , 2018. BUSCO Applications from Quality Assessments to Gene Prediction and Phylogenomics. Mol Biol Evol 35: 543–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham H., 2016. ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag; New York. [Google Scholar]
- Wickham H., François R., Henry L., Müller K., and RStudio, 2022. dplyr: A Grammar of Data Manipulation.
- Zdobnov E. M., Kuznetsov D., Tegenfeldt F., Manni M., Berkeley M. et al. , 2021. OrthoDB in 2020: evolutionary and functional annotations of orthologs. Nucleic Acids Research 49: D389–D393. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors affirm that all data necessary for confirming the conclusions of the article are present within the article, figures, tables, and supplemental material.