Abstract
We motivate and present biVI, which combines the variational autoencoder framework of scVI with biophysically motivated, bivariate models for nascent and mature RNA distributions. In simulated benchmarking, biVI accurately recapitulates key properties of interest, including cell type structure, parameter values, and copy number distributions. In biological datasets, biVI provides a route for the identification of the biophysical mechanisms underlying differential expression. The analytical approach outlines a generalizable strategy for representing multimodal datasets generated by single-cell RNA sequencing.
1. Introduction
Advances in experimental methods for single-cell RNA sequencing (scRNA-seq) allow for the simultaneous quantification of multiple cellular species at a time, such as nascent and mature transcriptomes [1, 2], surface [3-5] and nuclear [6] proteomes, and chromatin accessibility [7, 8]. The biophysical “integration” of such datasets requires the parameterization of interpretable mechanistic models [9]. Such modeling is challenging because mechanistic models tend to be intractable for more than a few co-regulated genes; in practice, single-cell genomics measurements are made for thousands of genes in tens of thousands of cells [10].
One approach to integration of multimodal single-cell genomics data has been to leverage recent advances in machine learning [11-13] and matrix factorization techniques [14]. This approach aims to summarize multimodal single cell measurements in a common low-dimensional space, which purports to reflect the cell state structure. Specifically, single-cell variational inference (scVI), a popular machine learning framework for analyzing scRNA-seq data [12, 15], has previously been adapted to multimodal data with protein [11] and chromatin [16] measurements. However, methods based on this approach are neither informed by nor lead to specific biophysical interpretations, and omit the intrinsic causal relationships between “upstream” and “downstream” parts of the central dogma [17, 18].
Consider, for example, the joint modeling of nascent and mature RNA counts, which are readily available by realigning existing scRNA-seq reads [1, 2], via the scVI framework. While scVI has not been considered for such integration, one can conceive of utilizing it for this purpose as follows: each cell could be encoded by a neural network to a latent low-dimensional vector z, which could be decoded into a set of cell- and gene-specific parameters by another neural network. These parameters would define a negative binomial distribution used to evaluate and optimize the likelihoods of RNA count matrices. In principle, nascent and mature RNA molecules can be “integrated” with scVI by concatenating the two matrices and treating nascent and mature transcripts as distinct “genes.” Latent representations of cells would then be decoded to produce parameters for independent distributions of nascent and mature molecules, as diagrammed in Figure 1a. However, the likelihoods and inferred parameters would have no biophysical interpretation and would merely be represented as part of a neural “black box” used to reduce data dimensionality.
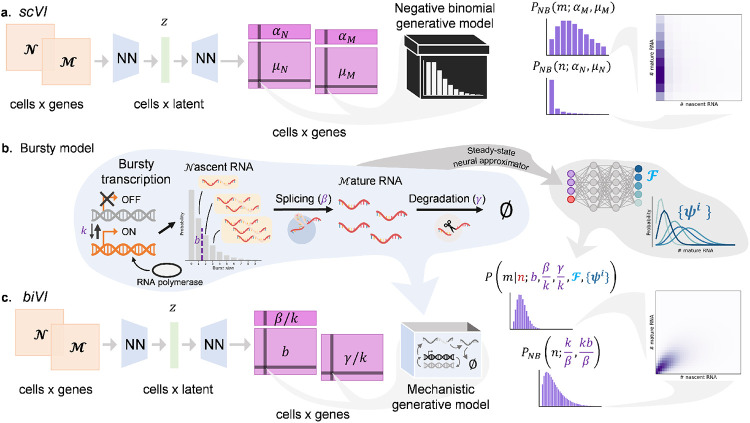
Figure 1:
biVI reinterprets and extends scVI to infer biophysical parameters rather than statistical summaries. a. scVI can take in concatenated nascent (𝒩) and mature (ℳ) RNA count matrices, encode each cell with a common low-dimensional representation z, and learn the per-cell parameters μN and μM and per-gene parameters αN and αM that encode formally independent nascent and mature count distributions. This approach is descriptive and not motivated by any specific model of physiology. b. A schematic of the telegraph model of transcription: a gene locus has the on rate k, the off rate koff, and the RNA polymerase binding rate kRNAP. In the bursty regime, where kRNAP and koff are relatively high, nascent RNA molecules are produced in geometrically distributed bursts with mean b = kRNAP/koff. After production, molecules are spliced at a constant rate β and degraded at a constant rate γ. Although the two-stage telegraph model does not have a closed-form solution, its steady-state distribution can be approximated with the combination of a pre-trained neural network ℱ and a set of basis functions {ψi}. c. biVI can take in nascent and mature count matrices, produce a common low-dimensional representation for each cell, and output per-cell parameters b and γ/k, as well as the per-gene parameters β/k, for a mechanistically motivated joint distribution of nascent and mature counts.
Here, we introduce biVI, a strategy that adapts the variational autoencoder (VAE) framework to work with well-characterized stochastic models of transcription, and use it to fit simulated and biological datasets that include nascent and mature RNA counts. First, we propose several stochastic models of transcription, formalized by chemical master equations (CMEs), that could give rise to count distributions consistent with scVI. For example, Figure 1b illustrates the bursty model of transcription, in which nascent RNA molecules are produced in geometrically distributed bursts with mean b, which arrive at a constant rate k. Nascent molecules undergo splicing at rate β to produce mature molecules; these are, in turn, degraded with constant rate γ. The bursty model has been extensively experimentally validated in mammalian cells [19-21], and gives rise to a joint distribution of nascent and mature molecules parameterized by the burst size and the relative rates of transcription, splicing, and degradation. While the joint steady-state distribution induced by the bursty model is analytically intractable [22], we have previously shown that it can be efficiently and accurately approximated by the combination of judiciously chosen basis functions and a pre-trained neural network designed to predict their weights [23]. biVI replaces scVI’s independent nascent and mature likelihoods with mechanistically motivated, biologically interpretable bivariate likelihoods. The method encodes a concatenated matrix of nascent and mature counts to a low-dimensional representation, which in turn decodes to parameters which have biophysically meaningful interpretations under a particular model (Figure 1c). Using biVI, we can thus infer biophysical parameters for mechanistic models of transcription for tens of thousands of single cells and genes. While we focus our analysis on the bursty model, biVI implements two alternative descriptions of single-molecule variation, the constitutive and extrinsic noise models previously discussed in the literature [9,24,25]. The descriptions and derivations of each biophysical model are available in Section S1, with diagrams for the constitutive model in Figure S1 and extrinsic model in Figure S2.
2. Results and discussion
Before using biVI to infer biophysical parameters with experimental data, we sought to evaluate its ability to reconstruct distributions from a known mechanistic ground truth. By showing that biVI can accurately recapitulate simulated data distributions, we can increase confidence in the validity of biophysical parameters inferred from experimental data. The simulation procedure is outlined in Section 4.5, with further details in Section S2. For the bursty model, we generated parameters b, β/k, and γ/k for 2,000 genes, increasing burst sizes for marker genes to produce twenty distinct cell types (Section S2). Next, we sampled nascent and mature RNA counts for 10,000 cells, approximately equally distributed across cell types. We trained biVI with the bivariate bursty likelihood and scVI with independent negative binomial likelihoods on 80% of the simulated cells (Section 4.5), holding out 20% for testing. To reconstruct distributions for a given gene in a specific cell type, we took the average of the probability laws obtained for that cell type (Section 4.5.3). We reproduced the analysis for the constitutive and extrinsic models, with results shown in Figures S4 and S5.
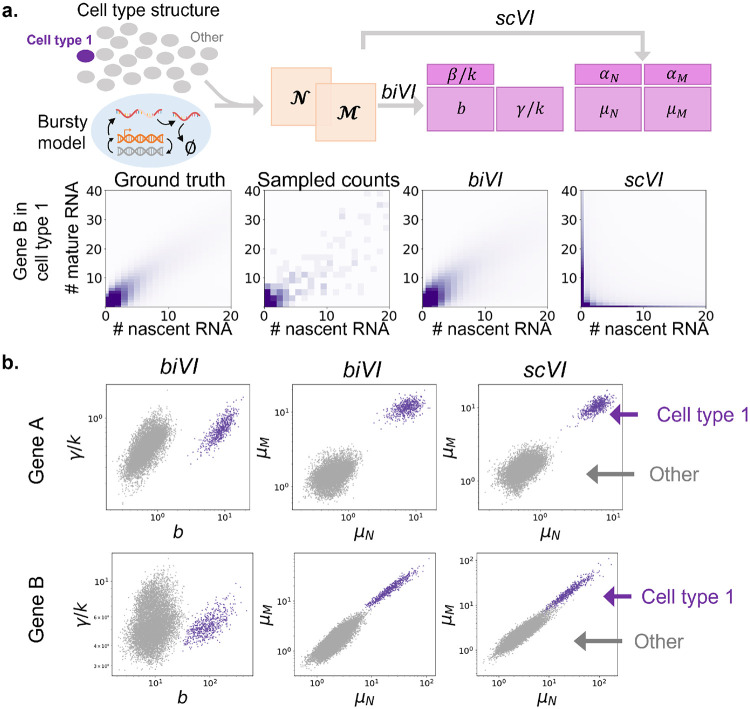
On simulated data generated with the bursty model, the negative log-likelihood, or reconstruction loss, of the 2,000 held-out testing cells was lower for biVI (3,885.8) than for scVI (3,953.6) (Figure S3). Applying biVI to fit the model used to generate the data set gave lower reconstruction losses than scVI for all three simulated data sets (Figure S4, Figure S5). Furthermore, the Kullback-Liebler divergence (KLD) between true and reconstructed count distributions, separately computed for each cell type, was lower for biVI (0.014 on average) than scVI (0.212 on average) (Figure S3). Figure 2a qualitatively illustrates biVI’s improved ability to reconstruct distributions based on noisy sampled observations. If the model is correct, biVI achieves better reconstruction performance on several metrics. Conversely, the suboptimal results under the incorrect models suggest that biVI may provide a route for model selection.
Figure 2:
biVI reflects the global structure and stochasticity of simulated bursty data. a. We generated parameters for 2,000 genes under the bursty model of transcription, and sampled nascent and mature counts from the resulting joint distribution to yield 10,000 cells across 20 cell types. Next, we trained biVI with the bivariate bursty likelihood and scVI with independent negative binomial likelihoods. For a sample gene, biVI accurately reconstructs the underlying bivariate distribution (darker color: higher; lighter color: lower probability mass or number of observations). b. Cell-specific parameters inferred by biVI and scVI for two marker genes for cell type 1, Gene A and Gene B. biVI yields nascent and mature means, as well as parameter values, which are related to the means through a transformation. scVI only yields the means.
A primary use of scVI is to reduce the dimensionality of similar cells, such as multiple observations of the same cell type, to similar low-dimensional latent representations, removing noise and redundancies. biVI facilitates this application for multimodal data. To characterize how well cell types are preserved in the latent space, we applied several clustering metrics (Section S4) to the latent representation of 2,000 cells (simulated, bursty model, held-out set) encoded by biVI and scVI (Section S2), using the ground truth cell types as cluster assignments. The average percent of latent-space nearest neighbors in the same cell type as each cell, computed over all cells, was similar for biVI (90.0%) and scVI (90.4%). The silhouette score, a measure of how similar each cell is to other cells in its cluster compared to other clusters, achieved analogous performance, yielding 0.383 for biVI and 0.387 for scVI. Finally, scaled inter-cluster distance (average inter-cluster distance divided by average intra-cluster distance, with larger values corresponding to better cluster separation), was 2.75 for biVI and 2.60 for scVI. These clustering metrics are evidence that cells can be similarly well represented in a low dimensional space using biVI and scVI, and that biVI modifications do not impede the process of variational inference or downstream analyses.
By incorporating nascent data, biVI enables the detection of interesting differences in gene expression that may be subtle or poorly identifiable from mature RNA counts alone. By using a specific biophysical model, it provides an explanatory framework. Figure 2b shows inferred parameters across all simulated cells for two marker genes, which have higher burst sizes in the simulated cell type 1. The first column plots the biVI inferred burst size b against relative degradation rate γ/k for every cell, the second column displays biVI inferred mature means μM and nascent means μN, whereas the third displays the scVI inferred means. The biVI means and parameters are related through the transformations outlined in Section 4.9. Gene A shows a clear separation of cell type 1 in all workflows. In contrast, Gene B shows a clearer separation of parameter values than averages, and correctly ascribes it to a change in burst size.
With these simulation benchmarks suggesting that biVI can perform as well as or better than scVI, we proceeded to use the two pipelines to analyze experimental data. We selected a single scRNA-seq library generated from mouse brain tissue [26] and processed it with kallisto∣bustools [2] to obtain spliced and unspliced count matrices (Section 4.7). These count matrices contained the data for 6,418 previously annotated cells across 19 cell subtypes with expression measurements for the 2,000 most highly variable genes (Section 4.7). Next, we fit the counts with biVI (Section 4.8), making the key assumption that unspliced and spliced molecules may be treated as the nascent and mature species of the bursty generative model. 4,622 cells were used to train both VAE models with 513 validation cells, with 1,283 testing cells held out for assessing performance.
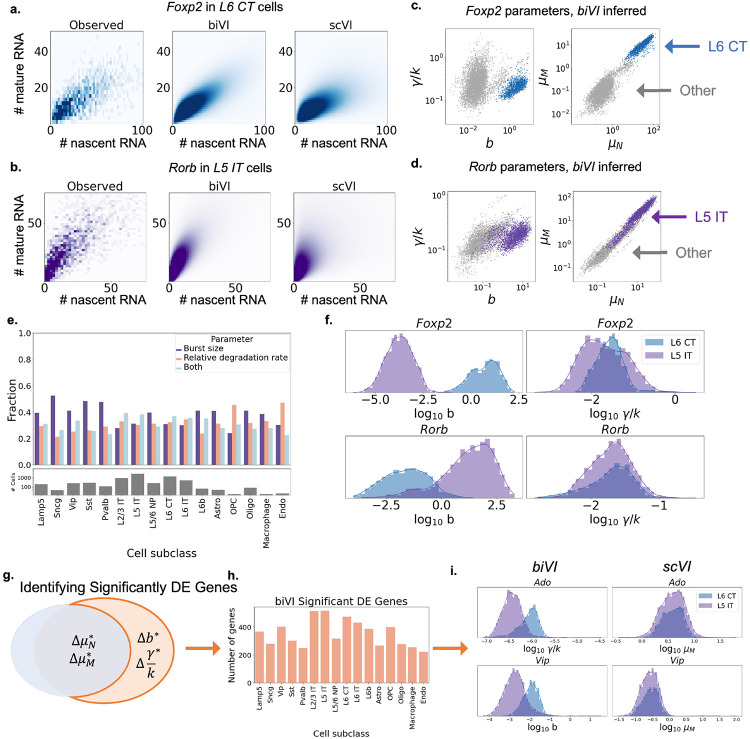
Figure 3a-b shows the observed and reconstructed distributions of Foxp2 (an L6 CT neuron marker), and Rorb (an L5 IT neuron marker), restricted to their respective cell types. Consistently with simulations, qualitative visual inspection suggests that biVI recapitulates distribution shapes better than scVI.
Figure 3:
biVI successfully fits single-cell neuron data and suggests the biophysical basis for expression differences. a.-b. Observed and inferred distributions of Foxp2, a marker gene for L6 CT cells, and Rorb, a marker gene for L5 IT cells (conventions as in Figure 2a). c.-d. Cell-specific parameters inferred for Foxp2 and Rorb demonstrate identifiable differences in means and parameters in the marked cell types. e. Cell subclasses show different modulation patterns, with especially pronounced distinctions in non-neuronal cells (top: fractions of genes exhibiting differences in each parameter; bottom: number of cells in each subclass). f. Inferred parameters for Foxp2 and Rorb in L5 IT (purple) and L6 CT (blue) cells show consistent differences in burst size. g. biVI allows the identification of cells which exhibit differences in burst size or relative degradation rate, without necessarily demonstrating differences in mean expression. h. Hundreds of genes demonstrate this modulation behavior, albeit with variation across cell subclasses. i. Histograms of biVI parameters and scVI mature means for two genes that exhibit parameter modulation without identifiable mean modulation. Ado appears to show differences in the degradation rate, whereas Vip appears to show differences in the burst size (top: results for Ado; bottom: results for Vip; purple: L5 IT cells; blue: L6 CT cells).
In addition to accurately capturing observed distributions, we can interpret the inferred parameters to determine how genes are regulated. Figure 3c-d shows the decoded averages and parameters for Foxp2 and Rorb across all cells. The average nascent and mature counts are higher in the cell types they mark, highlighted in corresponding colors. However, additionally, the parametric biVI results suggest these genes are upregulated at the transcriptional level, by increasing the burst size rather than decreasing the degradation rate.
We can leverage these differences to compare gene regulation trends across numerous cell types. We identified genes with significant differences in the parameters of one cell type versus all others using a two-sided t-test (Section 4.9), using a Bonferroni-corrected p-value threshold of 0.05 and a log2 fold change threshold of 1. To stay consistent with scVI [15], and avoid the conflation of “systematic” and “sequencing depth”-like effects, we evaluated differences in the compositional, “normalized” versions of inferred burst sizes, nascent means, and mature means, independent of the scVI cell size factor. We note that Figure 3 demonstrates the “scaled,” or non-normalized versions of these quantities. The relative degradation rate is independent of sequencing depth, so differential expression analysis was performed directly on the inferred values. To avoid the potential pitfalls of small sample sizes, we excluded all cell types with fewer than 10 barcodes (L5 ET, L6 IT Car3, VLMC, SMC) from this analysis. Figure 3e shows the fraction of identified genes in each cell subclass that exhibited differences in burst size, relative degradation rate, or both. While cell subclasses modify expression of genes through a variety of regulatory strategies (modifying burst size, degradation rate, or both), some interesting patterns can be observed. For example, the GABAergic subclasses (first five categories) appeared to more consistently exhibit deviation in burst size than in degradation rate. These coarse-grained differences may suggest common regulatory behaviors are present in similar cell types, although more sophisticated statistical methods are necessary to draw rigorous conclusions about these trends.
Finally, biVI identified novel genes that were significantly differential between cell subclasses in burst size or relative degradation rate but not nascent or mature inferred means (Section 4.9, Figure 3g-h). For some cell types, there were several hundred such genes, potentially interesting targets for follow-up experimental investigation. For example, the gene Ado demonstrated statistically significant differences in the biVI inferred relative degradation rate, but not scVI inferred mature means, in the neuronal subclass L6 CT (Figure 3i, top row). Ado has been associated with gliablastoma metabolism in non-neuronal cells [27]; the observation that it shows significant differences in inferred relative degradation rates in a neuronal cell subclass could lead to a broader understanding of the role its noise behaviors play in the growth and progression of malignant gliomas. As another example, burst size of Vip, the gene that codes for the peptide hormone vasoactive intestinal peptide (VIP), is statistically significantly different in neuronal cells of subclass L5 IT (Figure 3i, bottom row). While VIP is known to act as a hormone receptor and marks a GABAergic neuronal subclass (Vip cells) [28], the regulation of its burst size in (glutamatergic) L5 IT cells suggests functions in neurons outside of the subclass it marks, a suggestive area for follow-up investigation. Such mechanistic description provides a framework for characterizing the connection between the gene’s role and the cell’s regulatory strategies beyond the mean expression [29, 30].
3. Conclusion
We have demonstrated that a mechanistically interpretable autoencoder can be defined in a self-consistent way for bivariate data. This autoencoder, implemented in the biVI framework, combines the functional and distributional assumptions of scVI with the mechanistic hypotheses of transcriptional biophysics. When applied to simulated data, it correctly recovers three properties of interest. (1) Recapitulation of the gene-specific distributions, as characterized quantitatively through the KLD and qualitatively through the distribution shape. (2) Maintenance of cell type structure, as quantified by a variety of clustering metrics. (3) Recovery of the physical parameters used to generate count matrices, including the biophysical basis of differences in marker genes. When applied to real sequencing data, it achieves similar performance, and attains a degree of interpretability: we can begin to attribute differences in expression to specific regulatory mechanisms.
This interpretability is both enabled and limited by the modeling decisions: the biological findings are only as meaningful as the model. For simplicity and consistency with scVI, we have made several key assumptions. Most crucially, we propose that the model has been correctly specified, and that only the burst sizes and degradation rates are modulated between different cell types or states. It is likely that the latter assumption can be made somewhat more physically realistic by proposing that regulation primarily occurs at the gene locus, i.e., allowing the burst size b and burst frequency k to vary while keeping the processing rates β and γ constant. At this time, the most appropriate way to mathematically formulate this model while maintaining compatibility with neural networks is not yet clear. On the other hand, controlling for misspecification of the generative model appears to be a much more challenging problem, whether in terms of establishing a goodness-of-fit criterion or in terms of performing model selection simultaneously with inference. Preliminary comparisons, wherein we use multiple models to fit simulated datasets, suggest that the correct model may be identifiable, but the appropriate statistical procedures are not yet clear. Relaxing model assumptions and implementing these extensions are the next natural steps for the interpretable summary of large datasets.
More fundamentally, we have assumed that a variational autoencoder framework is a priori appropriate for single-cell RNA sequencing datasets. However, this framework is incompatible with certain common axioms [31]. For example, scVI and biVI cannot represent truly discrete cell types, i.e., sets of cells that have an identical latent representation but may have distinct count distributions due to single-molecule stochasticity. In addition, steady-state probability distributions are intrinsically incompatible with transient phenomena, such as differentiation (as discussed in [31]) and cell cycling, limiting the scope of this approach to stationary systems. Finally, we have merely asserted that the biVI mean parameters can be represented in a neural, compositional form, and demonstrated that this formulation produces acceptably faithful data summaries; however, we have not taken the next step of justifying or critiquing this assertion. To improve the prediction of nascent and mature count matrices, we have imposed biophysical constraints on the species’ joint distributions. Although the generative model is interpretable, the neural component is still a computational heuristic. In a similar spirit, but the orthogonal direction, previous work in the summary of single-cell data has used linear decoders, which constrain the compositional gene abundance parameters to be linear transformations of the latent embedding vector [32]. We anticipate that broader improvements in interpretability will require considerable further investigation and formalization, potentially with analogous, hypothesis-driven constraints on the variational autoencoder structure.
In spite of the limitations imposed by these assumptions, the approach we present in this report provides a fairly general, principled toolbox for the treatment of multimodal datasets. As we discuss in the mathematical treatment, the analytical procedure involves instantiating a set of biophysical hypotheses, analyzing the resulting stochastic system to obtain its distribution, and using this distribution as a multivariate generative model. As single-cell technologies evolve to provide larger-scale and more precise measurements of biomolecules, we anticipate that this approach can be self-consistently extended to provide a more comprehensive picture of biophysical processes in living cells.
4. Methods
In order to extend the scVI method to work with multimodal molecule count data in a way that is coherent with biology as well as the autoencoder, we define bivariate likelihood functions that (i) encode a specific, precedented mechanistic model of transcriptional regulation and (ii) are admissible under the assumptions made in the standard scVI pipeline. On a high level, our method entails the following steps:
Choose one of the scVI univariate generative models (Section 4.2), including the functional form of its likelihood and any assumptions about its distributional parameters.
Identify a one-species chemical master equation (CME) that produces this distribution as its steady state, and translate assumptions about distributional parameters into assumptions about the biophysical quantities that parameterize the CME (Section 4.3). The one-species system and its assumptions will typically not be uniquely determined.
Identify a two-species CME and derive assumptions about parameter values consistent with the one-species system. There will typically be multiple ways to preserve the assumptions but only a single CME.
Modify the autoencoder architecture to output the variables that parameterize the CME solution under the foregoing assumptions, and use this solution as the generative model.
4.1. Statistical preliminaries
We use the standard parameterization of the Poisson distribution:
| (1) |
We use the shape-mean parameterization of the univariate negative binomial distribution:
| (2) |
We use mean parameterization of the geometric distribution on :
| (3) |
4.2. scVI models
A brief summary of the generative process of the standard, univariate scVI pipeline is useful to contextualize the options and constraints of the bivariate model. Each cell is represented by a low-dimensional vector z, which is formally a random variable. scVI uses the “decoder” neural network to generate the quantities ρcg, which describe the compositional abundance of gene g in cell c as a function of z, such that . Furthermore, it either fits or uses a plug-in estimate for ℓc, a cell-specific “size factor,” such that the mean expression of a gene in a given cell is μcg = ρcgℓc.
The univariate workflow provides the options of three discrete generative models: Poisson with mean μcg, negative binomial with mean μcg and gene-specific dispersion parameter αg, and zero-inflated negative binomial, with an additional Bernoulli mixture parameter. We report the master equation models consistent with the first two generative laws below, and discuss a potential basis for and reservations about the zero-inflated model in Section S1.4
4.3. Master equation models
The one-species CMEs encode reaction schema of the following type:
| (4) |
where is a generic transcript species used to instantiate a univariate scVI generative model, γ is the transcript’s Markovian degradation rate, and the specific dynamics of the transcription process (first arrow) are deliberately left unspecified for now. Such systems induce univariate probability laws of the form P(x).
The two-species CMEs encode reaction schema of the following type:
| (5) |
where 𝒩 denotes a nascent species, ℳ denotes a mature species, and β denotes the nascent species’ Markovian conversion rate. Such systems induce bivariate probability laws of the form P(n,m). We typically identify the nascent species with unspliced transcripts and the mature species with spliced transcripts. We use the nascent/mature nomenclature to simplify notation and emphasize that this identification is natural for scRNA-seq data, but not mandatory in general.
Formalizing a model in terms of the CME requires specifying the precise mechanistic meaning of ρcg and ℓc. Previous reports equivocate regarding the latter [11], appealing either to cell-wide effects on the biology (in the spirit of [24, 25]) or technical variability in the sequencing process (in the spirit of [33]). For completeness, we treat both cases.
Below, we present the theoretical results, including the biophysical models, the functional forms of bivariate distributions consistent with the standard scVI models, and the consequences of introducing further assumptions. The full derivations are given in Section S1.
4.3.1. Constitutive: The Poisson model and its mechanistic basis
The Poisson generative model can be recapitulated by the following schema:
| (6) |
where k is a constant transcription rate. This process converges to the bivariate Poisson stationary distribution, with the following likelihood:
| (7) |
where μN = k/β and μM = k/γ. If we suppose each gene’s β and γ are constant across cell types, the likelihoods involve a single compositional parameter ρcg, such that
| (8) |
where is a gene-specific parameter that can be fit or naïvely estimated by the ratio of the unspliced and spliced averages. On the other hand, if the downstream processes’ kinetics can also change between cell types, we must use two compositional parameters:
| (9) |
We refer to this model as “Poisson,” reflecting its functional form, or “constitutive,” reflecting its biophysical basis.
4.3.2. Extrinsic: The negative binomial model and a possible mixture basis
The negative binomial generative model can be recapitulated by the following schema:
| (10) |
where k is the transcription rate, a realization of K, a gamma random variable with shape α, scale η, and mean ⟨K⟩ = αη. This process converges to the bivariate negative binomial (BVNB) stationary distribution, with the following likelihood:
| (11) |
where μN = ⟨K⟩/β and μM = ⟨K⟩/γ. If we suppose that cell type differences only involve changes in the transcription rate scaling factor η, with constant α, β, and γ, the likelihoods involve a single compositional parameter ρcg. The mean parameters are identical to Equation 8, with an analogous parameter γg/βg, as well as a gene-specific shape parameter αg. On the other hand, if the downstream processes’ kinetics can also change between cell types, we must use two compositional parameters, as in Equation 9.
We refer to this model as “extrinsic” to reflect its biophysical basis in extrinsically stochastic rates of transcriptional initiation.
4.3.3. Bursty: The negative binomial model and a possible bursty basis
The negative binomial generative model may be recapitulated by the alternative schema [22]:
| (12) |
where k is the burst frequency and B is a geometric random variable with mean b (Equation 3). This system converges to the following stationary distribution:
| (13) |
where μN = kb/β, μM = kb/γ, and α is arbitrarily set to k/β for simplicity.
Although the nascent marginal is known to be negative binomial, the joint P(n,m) and conditional P(m∣n) distributions are not available in closed form. For a given set of parameters, the joint distribution can be approximated over a finite microstate domain n, m ∈ [0, ßN – 1] × [0, ßM – 1], with total state space size ßN ßM. This approach is occasionally useful, if intensive, for evaluating the likelihoods of many independent and identically distributed samples. The numerical procedure entails using quadrature to calculate values of the generating function on the complex unit sphere, then performing a Fourier inversion to obtain a probability distribution [22]. However, this strategy is inefficient in the variational autoencoder framework, where each observation is associated with a distinct set of parameters. Furthermore, it is likely incompatible with automatic differentiation.
In [23], we demonstrated that the numerical approach can be simplified by approximating P(m∣n) with a learned mixture of negative binomial distributions: the weights are given by the outputs of a neural network, whereas the negative binomial bases are constructed analytically. The neural network is trained on the outputs of the generating function procedure. Although the generative model does not have a simple closed-form expression, it is represented by a partially neural, pre-trained function that is a priori compatible with the VAE.
If we suppose cell type differences only involve changes in the burst size b, with constant k, β and γ, we use Equation 13 to evaluate likelihoods. These likelihoods involve a single compositional parameter ρcg, with mean parameters identical to Equation 8, with an analogous parameter γg/βg, as well as a gene-specific shape parameter αg. On the other hand, if kinetics of the degradation process can also change between cell types, we must use two compositional parameters, as in Equation 9. There is no admissible way to allow modulation in the burst frequency.
We refer to this model as “bursty,” reflecting its biophysical basis.
4.4. biVI modifications
We built modifications upon scVI version 0.18.0 [34]. The scVI framework already supports the constitutive model. By setting conditional likelihood to “poisson,” no modification of scVI architecture is necessary. The conditional likelihood is the product of two Poisson distributions (Equation 7).
For the extrinsic and bursty models, mean parameters for nascent and mature counts, μN and μM, and a single shape parameter α are necessary. The default scVI architecture returns two independent parameters for nascent and mature counts of the same gene. biVI thus modifies the scVI architecture to update vectors rather than , where NG is the number of genes. For the extrinsic model, the conditional likelihood is set to the extrinsic likelihood Pextrinsic(n, m; α, μN, μM) (Equation 11). For the bursty model, the conditional likelihood is set to the bursty likelihood Pbursty(n, m; α, μN, μM) (Equation 13).
4.5. Simulated data
To validate our implementation of biVI, and to understand potential pitfalls inherited from the standard scVI autoencoder workflow, we generated ground truth data by simulation. Simulated ground truth is limited in important ways, as it omits known and unknown sources of biological and technical variability. However, to begin to understand the limitations of integrating descriptive autoencoders with mechanistic models, it is helpful to have simple and well-understood simulated datasets. By judiciously choosing simulation scenarios, we can characterize the relative and absolute accuracy of the procedures under ideal-case conditions, which describe a natural upper bound on their performance.
The simulation procedures are described in Section S2. For each of the three models (constitutive, extrinsic, and bursty), we simulated a data set of nascent and mature RNA counts for 2,000 genes across 10,000 cells, spread across 20 cell types. The cell types were distinguished by low-magnitude variation in all genes’ parameters, as well as higher-magnitude upregulation of expression in a small set of cell type-specific marker genes. In the extrinsic noise model, upregulation was effected by increasing the transcription rate scale η. In the analogous constitutive case, it was effected by increasing the transcription rate k. In the bursty model, it was effected by increasing the burst size b.
4.5.1. Fitting simulated data
We fit the three simulated data sets using biVI with the three generative physical models, as well as standard scVI with the negative binomial distribution as its conditional likelihood. All models were trained on 7,200 cells with 800 validation cells for 400 epochs with a learning rate of 0.001. The encoder and decoder networks consisted of 3 layers with 128 nodes each, with a latent space of dimension 10. As in standard scVI, a standard normal prior was used for the latent space.
4.5.2. Performance metrics on simulated data
Performance of the trained models was tested on 2,000 held-out cells for each simulated data set. In order to characterize the absolute reconstruction of ground truth cell types, the mean squared error (MSE) between biVI inferred means, μN and μM, and ground truth simulated means was calculated for each cell. Furthermore, we assessed how well different models reduced cells of the same cell type to similar latent spaces while maintaining good separation of cells in different cell types. To compare clustering accuracy for different models, we applied several metrics to the latent spaces obtained for testing data, using simulated ground truth cell type as cluster assignment. We calculated average intra-cluster distance (ICD), or the Euclidean distance between each cell’s latent representation and the mean of its assigned cluster in the latent space averaged over all cells. We also calculated average intra-cluster variance (ICV), or the variance of ICDs for each cell in a cluster averaged over all clusters. Inter-cluster distance was also calculated: distance between cluster means averaged over all pair-wise clusters (excluding the cluster’s distance to its own mean). Finally, scaled inter-cluster distance, or inter-cluster distance divided by average intra-cluster distance, was found. We also report nearest neighbor percentages (Figures S3, S4, S5), or the percent of the nk nearest neighbors in the same cell type as each cell, where nk is the number of cells in the given cell’s cell type. A quantitative description of clustering metrics is provided in Section S4. Broadly speaking, these metrics characterize the methods’ utility for discovering discrete cell populations using typical clustering algorithms, which perform best when low-dimensional clusters are relatively condensed, homogeneous, and reflect the high-dimensional structure.
4.5.3. Reconstructing gene distributions
A simulated cell type is defined by cell type-specific parameters for each gene, θkg, where k indexes over cell types, g indexes over genes, and θ contains the model parameters. Each cell in a cell type has identical parameters. biVI and scVI, however, infer parameters for every cell and gene: θcg, where c indexes over cells and g indexes over genes. To compare reconstructed distributions to simulated ground truth distributions for a given gene of a given cell type, we first select all cells of that cell type. Next, we define the distribution under a given model for each cell using biVI or scVI inferred parameters. We average over the cell-specific probabilities for a given gene to produce a cell-type distribution for that gene:
| (14) |
where nk is the total number of cells in cell type k, and ck indexes over all cells in that cell type. This identity follows immediately from defining the cell type’s distribution as the mixture of the distributions of its constituent cells. In the case of biVI, we plug in Equation 7, 11, or 13 for P(n, m; θckg). In the case of scVI, we use a product of two independent negative binomial laws:
| (15) |
where μN and μM are cell- and gene-specific, whereas αN and αM are fit separately and take on different values (Section 4.4). For simplicity, this comparison omits uncertainty associated with θcg, which is formally inherited from the uncertainty in the latent representation z for each cell c.
4.5.4. Kullback-Leibler divergence between simulated and reconstructed distributions
We compare biVI and scVI’s ability to accurately reconstruct ground truth gene distributions by calculating the truncated Kullback-Leibler divergence (KLD) between ground truth simulated distributions and reconstructed distributions for all genes in all cell types. We reconstruct gene and cell-type specific biVI and scVI distributions as described above in Section 4.5.3. We also calculate true distributions Qkg(n, m; θkg) using the gene and cell-type specific ground truth simulated parameters θkg (parameter generation and sampling are described in Section S2). We calculate both true and reconstructed model (biVI or scVI) distributions over a 50 by 50 grid of nascent and mature values, then normalize both to sum to 1.0 over the grid by dividing all probabilities by their sum over the grid. Normalization ensures that KL divergences are well-defined. We then calculate the KL divergence over the restricted grid:
| (16) |
4.6. Connecting the models to transcriptome data
Thus far, we have used “nascent” and “mature” as shorthands for the discrete species in a two-stage model of RNA processing. In other words, Equation 5 is axiomatic for this nomenclature. We have gone one step further and named the rate of conversion β the splicing rate, explicitly identifying the nascent species with unspliced RNA and the mature species with spliced RNA. This identification is a modeling decision that elides the considerable simplification of biological complexities. In the current section, we elaborate on the assumptions.
In the field of microbiology, “nascent” RNA is often, but not always, used to characterize the mRNA molecules in the process of synthesis, associated to a DNA strand via an RNA polymerase complex [35-38]. In this framework, the “mature” transcriptome is simply the complement of the nascent transcriptome, i.e., all molecules that are not chemically associated to a DNA strand. Therefore, the canonical definition of “nascent” RNA is equivalent to transcribing RNA, which is a polymeric structure with a particular sequence.
Transcribing RNA can be observed directly through electron micrography [37]. However, more typically, it is investigated through more or less direct experimental proxies that can be scaled to many genes and cells at a time. In the single-cell fluorescence subfield, DNA or membrane staining can be used to identify bright spots localized to the nucleus, which is treated as signal from RNA at the transcription site [29, 39]; this signal may include contributions from RNA incidentally, or mechanistically, retained at a DNA locus [36]. In this strategy, “nascent” molecules are DNA-associated. Alternatively, and more commonly, transcribing molecules have been studied by using probes targeted to the 5′ and 3′ regions [40-43], or to intronic and exonic regions [44-47]. In this strategy, “nascent” molecules contain a particular region, either synthesized earlier or removed later in the RNA life-cycle.
The use of intron data as a proxy for active transcription is reminiscent of, but distinct from sequence census [48] strategies that directly study RNA sequences. These strategies, in turn, typically use chemical methods to enrich for newly transcribed RNA. For example, Reimer et al. isolate chromatin, then deplete sequences that have been post-transcriptionally poly(A) tailed [49]. Analogously, Drexler et al. use 4-thiouridine (4sU) labeling to enrich for newly synthesized molecules [50]. These approaches may produce conflicting results; for example, introns may be rich both in poly(A) handles [1] and 4sU targets [49], giving rise to obscure technical effects. Therefore, these “processed” or “temporally labeled” proxies are coarsely representative of transcriptional dynamics, and their quantitative interpretability is unclear as of yet.
The sequence content may be used more directly, by conceding that DNA association or localization are not easily accessible by sequence census methods, and treating splicing per se. This approach has a fairly long history. Intronic quantification has been used to characterize transcriptional mechanisms in microarray datasets [51], and to characterize differentiation programs in RNA sequencing [52,53]. In single-cell RNA sequencing, intronic content has been leveraged to identify transient behaviors from snapshot data [1], albeit with some outstanding theoretical concerns and caveats [54]. Briefly, it is, in principle, possible to coarsely classify molecules with intronic content as “unspliced” or “pre-mRNA” and aggregate all others as “spliced,” “mature,” or simply “mRNA.” By applying this binary classification, and defining a simple first-order model of splicing, data may be successfully fit. The quantification of transcripts so classified is a relatively straightforward genomic alignment problem. The multiple available implementations [1, 2, 55, 56] tend to disagree on the appropriate assignment of ambiguous sequencing reads [54,56], obscuring a more fundamental problem: the binary classification is mechanistically limited [50,57-59], and it is likely that detailed splicing graph models will be necessary in the future [31,54]. The focus on sequence is yet another step removed from the transcriptional dynamics, particularly since some of the splicing processes occur after transcriptional elongation is complete [60]. However, in spite of its limitations and assumptions, the simple Markovian two-stage model has been successful in the past [61,62]. Mechanistic evidence does not suggest that, e.g., deterministic delayed elongation is necessary to represent “unspliced” distributions [63]: under this model, we appear to be able to treat splicing and degradation as Markovian, without representing the elongation process at all, and obtain reasonable fits to the data. Somewhat surprisingly [64], the geometric-Poisson distribution, which describes bursty transcription coupled to deterministic elongation [65], is a particularly poor fit for unspliced RNA counts [63].
Adding yet more complexity to the modeling, “mature” – whether “off-template,” “spliced,” or “processed” – molecules are not immediately available for degradation; first, the process of nuclear export must take place. Studies that presuppose access to imaging data tend to model it explicitly [22, 29, 66-68]. However, this approach has not been applied in sequencing assays, as current technologies do not distinguish nuclear and cytoplasmic molecules. Furthermore, comparisons of paired single-cell and single-nucleus datasets are hampered by the limited characterization of the noise sources in the latter technology. Yet again, we have generally found that omitting this effect in single-cell data produces acceptable fits [61-63].
Pending the development of more sophisticated sequencing and alignment technologies, as well as the implementation of tractable models of biology, the data exploration portion of our study focuses on the “spliced” and “unspliced” matrices generated by kallisto ∣bustools [2]. This choice is a compromise, and we adopt it after considering the following factors:
Availability of quantification workflows: spliced and unspliced matrices are straightforward to generate.
Model tractability: the two-stage models can be evaluated [23]; more sophisticated models require new algorithms to be integrated with variational autoencoders.
The scope of sequencing data: single-cell protocols do not yet give access to sub-cellular information, so inference of elongation or nuclear retention dynamics is acutely underspecified.
Self-consistency and compatibility: a priori, we seek to recapitulate the data types (spliced RNA counts) and distributions (negative binomial and Poisson) already implemented in scVI, leaving improvements to further investigation.
We use the terms “nascent” and “mature” to identify the unspliced and spliced RNA matrices. This choice of nomenclature is deliberate. Although it somewhat conflicts with the established microbiology literature, this terminology is intended to emphasize the models’ generality, both here and elsewhere [9, 23, 69]. The two-stage Markovian process is axiomatic. The specific identities assigned to the mathematical objects may range beyond counts identified by sequence census methods. They may represent the discretized and subtracted intensities of 3’, 5’, intron, or exon fluorescent probes, the counts of molecules within and outside the nuclear envelope, or polymerase counts obtained by micrography. Therefore, the terminology should be taken in the sense used for similar non-delayed models in [64, 66, 70, 71].
4.7. Preprocessing Allen data
Raw 10x v3 single-cell data were originally generated by the Allen Institute for Brain Science [26]. The raw reads in FASTQ format [72] and cluster metadata [73] were obtained from the NeMO Archive. We selected mouse library B08 (donor ID 457911) for analysis.
To obtain spliced and unspliced counts, we first obtained the pre-built mm10 mouse genome released by 10x Genomics (https://support.10xgenomics.com/single-cell-gene-expression/software/downloads/latest, version 2020-A). We used kallisto∣bustools 0.26.0 [2] to build an intronic/exonic reference (kb ref with the option --lamanno). Next, we pseudoaligned the reads to this reference (kb count with the option --lamanno). We used the outputs produced by the standard bustools filter. This filter was relatively permissive: all (8,424) barcodes given cell type annotations in the Allen metadata were present in the output count matrix (10,975 barcodes).
Based on previous clustering results, we selected cells that were given cell type annotations, and omitted “low quality” or “doublet” barcodes [26], for a total of 6,418 cells. Although any choice to retain or omit cells from analysis is arbitrary, our work models the generating process that produced cells’ nascent and mature counts by presupposing each barcode corresponds to a single cell. Therefore, we propose that cells identified as low-quality (empty cells) or as doublets (two cells measured in one observation) [26] have a fundamentally different data-generating process than individual single cells, and therefore remove them before fitting VAE models. However, we stress that the stochastic nature of transcription and sequencing, the intrinsic uncertainties associated with read alignment, and the numerical compromises made in clustering large datasets mean that previous annotations are not “perfect,” merely a reasonable starting point for comparing alternative methods.
We used Scanpy [74] to restrict our analysis to several thousand variable genes, which presumably reflect the cell type signatures of interest. The mature count matrix for the 6,418 retained cells was normalized to sum to 10,000 counts per cell, then transformed with log1p. The top 2,000 most highly variable genes were identified using scanpy.pp.highly_variable_genes with minimum mean of 0.0125, maximum mean of 3, and minimum dispersion of 0.5 [74].
4.8. Fitting Allen data
We applied biVI with the three generative models (bursty, constitutive, and extrinsic) and scVI with negative binomial likelihoods to the count matrices obtained by the filtering procedures outlined above. 4,622 cells were used for training with 513 validation cells, and 1,283 cells were held out for testing performance. All models were trained for 400 epochs with a learning rate of 0.001. Encoders and decoder consisted of 3 layers of 128 nodes, and each model employed a latent dimension of 10.
4.9. Differential parameter values and gene expression
After fitting the VAE models, we sought to identify meaningful statistical differences that distinguish cell types. In order to avoid instabilities, we excluded cell subclasses “L6 IT Car3,” “L5 ET,” “VLMC,” and “SMC” from this analysis, as they contained fewer than ten annotated cells, necessitating more sophisticated statistical models that take into account their small sample sizes. The following analysis thus considers 6,398 cells in 16 unique subclasses. We only computed differential expression metrics under the bursty model.
Differential parameter values and gene expression were tested for each assigned subclass label (as annotated in [26]) versus all others using two-sided t-tests implemented in scipy.stats.ttest_ind [75] under the null hypothesis that there was no difference in the average of the tested values. We applied Bonferroni corrections for the two-tailed test, the 16 subclass labels, and the 2,000 genes: values were considered significantly differential if the p-value under the t-test was below 0.05 × (2 × 16 × 2, 000)−1 = 7.813 × 10−7. To assign significance, we further required that the magnitude of log2 fold change to be greater than 1, i.e., the arithmetic mean of the tested value was doubled or halved in one cell subclass versus the rest. We adopted this effect size threshold due to the relatively large number of cells and corresponding prevalence of genes with significant p-values.
The t-tests were performed on normalized burst size and means, i.e., the inferred burst size or inferred means divided by observed sequencing depth for that cell. The observed sequencing depth of a cell ℓc was set to the sum of all observed counts, nascent and mature, in that cell. Normalization was thus done to ensure that identified differences in parameters was not due to sequencing depth but was biologically meaningful. Relative degradation rate is independent of sequencing depth: t-tests were performed directly on inferred relative degradation rates.
As discussed in Section 4.4, scVI fits the cell-specific parameters and , as well as gene-specific parameters and . To compute effect sizes and p-values, we simply compared the distributions of ρcg in distinct cell populations, using for differences in nascent expression and for differences in mature expression.
As discussed in Section S1.3 biVI fits , and αg under the bursty generative model. To calculate parameters, we use the definitions outlined in the section, enforcing αg = kg/βg. This yields the following relationships:
| (17) |
where C is a large multiplicative constant shared across cells and genes. We set kg to unity with no loss of generality at steady state. Finally, we compare the inferred values of γz/kg and bz/C to identify differentially expressed genes.
Supplementary Material
6. Acknowledgments
M.C., G.G., T.C., and L.P. were partially funded by IGVF-1-UCI.IGVF and NIH U19MH114830. Y.C. was partially funded by T32 GM007377. G.G. thanks Drs. Ido Golding and Heng Xu for the inspiration leading to the explanatory model for the zero-inflated negative binomial distribution in Section S1.4. The RNA illustrations used in Figures 1, 2, S1, and S2 were derived from the DNA Twemoji by Twitter, Inc., used under the CC-BY 4.0 license. We thank the Caltech Bioinformatics Resource Center for GPU resources that helped in performing the analyses.
5. Data availability
Simulated datasets, simulated parameters used to generate them, and Allen dataset B08 and its associated metadata are available in the Zenodo package 7497222. All analysis scripts and notebooks are available at https://github.com/pachterlab/CGCCP_2023. The repository also contains a Google Colaboratory demonstration notebook applying the methods to a small human blood cell dataset.
References
- [1].Manno Gioele La, Soldatov Ruslan, Zeisel Amit, Braun Emelie, Hochgerner Hannah, Petukhov Viktor, Lidschreiber Katja, Kastriti Maria E., Lönnerberg Peter, Furlan Alessandro, Fan Jean, Borm Lars E., Liu Zehua, van Bruggen David, Guo Jimin, He Xiaoling, Barker Roger, Sundström Erik, Castelo-Branco Gonçalo, Cramer Patrick, Adameyko Igor, Linnarsson Sten, and Kharchenko Peter V.. RNA velocity of single cells. Nature, 560(7719):494–498, August 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Melsted Páll, Booeshaghi A. Sina, Liu Lauren, Gao Fan, Lu Lambda, Min Kyung Hoi, Beltrame Eduardo da Veiga, Hjörleifsson Kristján Eldjárn, Gehring Jase, and Pachter Lior. Modular, efficient and constant-memory single-cell RNA-seq preprocessing. Nature Biotechnology, 39(7):813–818, July 2021. [DOI] [PubMed] [Google Scholar]
- [3].Peterson Vanessa M, Zhang Kelvin Xi, Kumar Namit, Wong Jerelyn, Li Lixia, Wilson Douglas C, Moore Renee, McClanahan Terrill K, Sadekova Svetlana, and Klappenbach Joel A. Multiplexed quantification of proteins and transcripts in single cells. Nature Biotechnology, 35(10):936–939, October 2017. [DOI] [PubMed] [Google Scholar]
- [4].Mimitou Eleni P., Cheng Anthony, Montalbano Antonino, Hao Stephanie, Stoeckius Marlon, Legut Mateusz, Roush Timothy, Herrera Alberto, Papalexi Efthymia, Ouyang Zhengqing, Satija Rahul, Sanjana Neville E., Koralov Sergei B., and Smibert Peter. Multiplexed detection of proteins, transcriptomes, clonotypes and CRISPR perturbations in single cells. Nature Methods, 16(5):409–412, May 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Stoeckius Marlon, Hafemeister Christoph, Stephenson William, Houck-Loomis Brian, Chattopadhyay Pratip K, Swerdlow Harold, Satija Rahul, and Smibert Peter. Simultaneous epitope and transcriptome measurement in single cells. Nature Methods, 14(9):865–868, September 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Chung Hattie, Parkhurst Christopher N., Magee Emma M., Phillips Devan, Habibi Ehsan, Chen Fei, Yeung Bertrand Z., Waldman Julia, Artis David, and Regev Aviv. Joint single-cell measurements of nuclear proteins and RNA in vivo. Nature Methods, 18(10):1204–1212, October 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Reyes M., Billman K., Hacohen N., and Blainey P.C.. Simultaneous profiling of gene expression and chromatin accessibility in single cells. Advanced Biosystems, 3,11, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].De Rop Florian, Ismail Joy N, González-Blas Carmen Bravo, Hulselmans Gert J, Flerin Christopher Campbell, Janssens Jasper, Theunis Koen, Christiaens Valerie M, Wouters Jasper, Marcassa Gabriele, de Wit Joris, Poovathingal Suresh, and Aerts Stein. HyDrop enables droplet based single-cell ATAC-seq and single-cell RNA-seq using dissolvable hydrogel beads. eLife, 11:e73971, February 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gorin Gennady, Vastola John J., Fang Meichen, and Pachter Lior. Interpretable and tractable models of transcriptional noise for the rational design of single-molecule quantification exper-iments. Nature Communications, 13(1):7620, December 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Svensson Valentine, Vento-Tormo Roser, and Teichmann Sarah A. Exponential scaling of single-cell RNA-seq in the past decade. Nature Protocols, 13(4):599–604, April 2018. [DOI] [PubMed] [Google Scholar]
- [11].Gayoso Adam, Steier Zoë, Lopez Romain, Regier Jeffrey, Nazor Kristopher L., Streets Aaron, and Yosef Nir. Joint probabilistic modeling of single-cell multi-omic data with totalVI. Nature Methods, 18(3):272–282, March 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gayoso Adam, Lopez Romain, Xing Galen, Boyeau Pierre, Wu Katherine, Jayasuriya Michael, Melhman Edouard, Langevin Maxime, Liu Yining, Samaran Jules, Misrachi Gabriel, Nazaret Achille, Clivio Oscar, Xu Chenling, Ashuach Tal, Lotfollahi Mohammad, Svensson Valentine, da Veiga Beltrame Eduardo, Talavera-Lépez Carlos, Pachter Lior, Theis Fabian J., Streets Aaron, Jordan Michael I., Regier Jeffrey, and Yosef Nir. scvi-tools: a library for deep proba-bilistic analysis of single-cell omics data. Preprint, bioRxiv: 2021.04.28.441833, April 2021. [Google Scholar]
- [13].Lin Xiang, Tian Tian, Wei Zhi, and Hakonarson Hakon. Clustering of single-cell multi-omics data with a multimodal deep learning method. Nature Communications, 13(1):7705, December 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Argelaguet R., Arnol D., Bredikhin D., Deloro Y., Velten B., Marioni J. C., and Stegle O.. Mofa+: a statistical framework for comprehensive integration of multi-modal single-cell data. Genome Biology, 21, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lopez Romain, Regier Jeffrey, Cole Michael B., Jordan Michael I., and Yosef Nir. Deep generative modeling for single-cell transcriptomics. Nature Methods, 15(12):1053–1058, December 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ashuach Tal, Reidenbach Daniel A., Gayoso Adam, and Yosef Nir. PeakVI: A deep generative model for single-cell chromatin accessibility analysis. Cell Reports Methods, 2(3):100182, March 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Crick Francis. Central Dogma of Molecular Biology. Nature, 227(5258):561–563, August 1970. [DOI] [PubMed] [Google Scholar]
- [18].Li Gene-Wei and Xie X. Sunney. Central dogma at the single-molecule level in living cells. Nature, 475(7356):308–315, July 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Raj Arjun, Peskin Charles S, Tranchina Daniel, Vargas Diana Y, and Tyagi Sanjay. Stochastic mRNA Synthesis in Mammalian Cells. PLoS Biology, 4(10):e309, September 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Dar R. D., Razooky B. S., Singh A., Trimeloni T. V., McCollum J. M., Cox C. D., Simpson M. L., and Weinberger L. S.. Transcriptional burst frequency and burst size are equally modulated across the human genome. Proceedings of the National Academy of Sciences, 109(43):17454–17459, October 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Sanchez A. and Golding I.. Genetic Determinants and Cellular Constraints in Noisy Gene Expression. Science, 342(6163):1188–1193, December 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Singh Abhyudai and Bokes Pavol. Consequences of mRNA Transport on Stochastic Variability in Protein Levels. Biophysical Journal, 103(5):1087–1096, September 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Gorin Gennady, Carilli Maria, Chari Tara, and Pachter Lior. Spectral neural approximations for models of transcriptional dynamics. Preprint, bioRxiv: 2022.06.16.496448, June 2022. [DOI] [PubMed] [Google Scholar]
- [24].Ham Lucy, Brackston Rowan D., and Stumpf Michael P. H.. Extrinsic Noise and Heavy-Tailed Laws in Gene Expression. Physical Review Letters, 124(10):108101, March 2020. [DOI] [PubMed] [Google Scholar]
- [25].Elowitz Michael B, Levine Arnold J, Siggia Eric D, and Swain Peter S. Stochastic Gene Expression in a Single Cell. Science, 297(5584):1183–1186, 2002. [DOI] [PubMed] [Google Scholar]
- [26].Yao Zizhen, Liu Hanqing, Xie Fangming, Fischer Stephan, Adkins Ricky S., Aldridge Andrew I., Ament Seth A., Bartlett Anna, Behrens M. Margarita, Van den Berge Koen, Bertagnolli Darren, de Bézieux Hector Roux, Biancalani Tommaso, Booeshaghi A. Sina, Bravo Héctor Corrada, Casper Tamara, Colantuoni Carlo, Crabtree Jonathan, Creasy Heather, Crichton Kirsten, Crow Megan, Dee Nick, Dougherty Elizabeth L., Doyle Wayne I., Dudoit Sandrine, Fang Rongxin, Felix Victor, Fong Olivia, Giglio Michelle, Goldy Jeff, Hawrylycz Mike, Herb Brian R., Hertzano Ronna, Hou Xiaomeng, Hu Qiwen, Kancherla Jayaram, Kroll Matthew, Lathia Kanan, Li Yang Eric, Lucero Jacinta D., Luo Chongyuan, Mahurkar Anup, McMillen Delissa, Nadaf Naeem M., Nery Joseph R., Nguyen Thuc Nghi, Niu Sheng-Yong, Ntranos Vasilis, Orvis Joshua, Osteen Julia K., Pham Thanh, Pinto-Duarte Antonio, Poirion Olivier, Preissl Sebastian, Purdom Elizabeth, Rimorin Christine, Risso Davide, Rivkin Angeline C., Smith Kimberly, Street Kelly, Sulc Josef, Svensson Valentine, i Michael, Torkelson Amy, Tung Herman, Vaishnav Eeshit Dhaval, Vanderburg Charles R., van Velthoven Cindy, Wang Xinxin, White Owen R., Huang Z. Josh, Kharchenko Peter V., Pachter Lior, Ngai John, Regev Aviv, Tasic Bosiljka, Welch Joshua D., Gillis Jesse, Macosko Evan Z., Ren Bing, Ecker Joseph R., Zeng Hongkui, and Mukamel Eran A.. A transcriptomic and epigenomic cell atlas of the mouse primary motor cortex. Nature, 598(7879):103–110, October 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Shen D., Tian L., Yang F., Li J., Li X., Yao Y., Lam E. W. F., Gao P., Jin B., and Wang R.. Ado/hypotaurine: a novel metabolic pathway contributing to glioblastoma development. Cell Death Discovery, 7, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Gozes I. and Brenneman D. E.. Vip: molecular biology and neurobiological function. Molecular Neurobiology, 3:201–236, 1989. [DOI] [PubMed] [Google Scholar]
- [29].Munsky Brian, Li Guoliang, Fox Zachary R., Shepherd Douglas P., and Neuert Gregor. Dis[u-tribution shapes govern the discovery of predictive models for gene regulation. Proceedings of the National Academy of Sciences, 115(29):7533–7538, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Munsky Brian, Trinh Brooke, and Khammash Mustafa. Listening to the noise: random fluctuations reveal gene network parameters. Molecular Systems Biology, 5:318, October 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Gorin Gennady and Pachter Lior. Modeling bursty transcription and splicing with the chemical master equation. Biophysical Journal, 121(6):1056–1069, February 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Svensson Valentine, Gayoso Adam, Yosef Nir, and Pachter Lior. Interpretable factor models of single-cell RNA-seq via variational autoencoders. Bioinformatics, 36(11):3418–3421, June 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Wang Jingshu, Huang Mo, Torre Eduardo, Dueck Hannah, Shaffer Sydney, Murray John, Raj Arjun, Li Mingyao, and Zhang Nancy R.. Gene expression distribution deconvolution in single-cell RNA sequencing. Proceedings of the National Academy of Sciences, 115(28):E6437–E6446, July 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Gayoso Adam, Lopez Romain, Xing Galen, Boyeau Pierre, Amiri Valeh Valiollah Pour, Hong Justin, Wu Katherine, Jayasuriya Michael, Mehlman Edouard, Langevin Maxime, Liu Yining, Samaran Jules, Misrachi Gabriel, Nazaret Achille, Clivio Oscar, Xu Chenling, Ashuach Tal, Gabitto Mariano, Lotfollahi Mohammad, Svensson Valentine, da Veiga Beltrame Eduardo, Kleshchevnikov Vitalii, Talavera-López Carlos, Pachter Lior, Theis Fabian J., Streets Aaron, Jordan Michael I., Regier Jeffrey, and Yosef Nir. A Python library for probabilistic analysis of single-cell omics data. Nature Biotechnology, February 2022. [DOI] [PubMed] [Google Scholar]
- [35].Rodriguez Joseph and Larson Daniel R.. Transcription in Living Cells: Molecular Mechanisms of Bursting. Annual Review of Biochemistry, 89(1):189–212, June 2020. [DOI] [PubMed] [Google Scholar]
- [36].Xu Heng, Skinner Samuel O., Sokac Anna Marie, and Golding Ido. Stochastic Kinetics of Nascent RNA. Physical Review Letters, 117(12):128101, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Choubey Sandeep, Kondev Jane, and Sanchez Alvaro. Deciphering Transcriptional Dynamics In Vivo by Counting Nascent RNA Molecules. PLOS Computational Biology, 11(11):e1004345, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Choubey Sandeep. Nascent RNA kinetics: Transient and steady state behavior of models of transcription. Physical Review E, 97(2):022402, 2018. [DOI] [PubMed] [Google Scholar]
- [39].Gómez-Schiavon Mariana, Chen Liang-Fu, West Anne E., and Buchler Nicolas E.. BayFish: Bayesian inference of transcription dynamics from population snapshots of single-molecule RNA FISH in single cells. Genome Biology, 18(1):164, December 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Wang Mengyu, Zhang Jing, Xu Heng, and Golding Ido. Measuring transcription at a single gene copy reveals hidden drivers of bacterial individuality. Nature Microbiology, 4:2118–2127, September 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Xu Heng, Sepúlveda Leonardo A, Figard Lauren, Sokac Anna Marie, and Golding Ido. Combining protein and mRNA quantification to decipher transcriptional regulation. Nature Methods, 12(8):739–742, August 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Zenklusen Daniel, Larson Daniel R, and Singer Robert H. Single-RNA counting reveals alternative modes of gene expression in yeast. Nature Structural & Molecular Biology, 15(12):1263–1271, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Senecal Adrien, Munsky Brian, Proux Florence, Ly Nathalie, Braye Floriane E., Zimmer Christophe, Mueller Florian, and Darzacq Xavier. Transcription Factors Modulate c-Fos Transcriptional Bursts. Cell Reports, 8(1):75–83, July 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Halpern Keren Bahar, Tanami Sivan, Landen Shanie, Chapal Michal, Szlak Liran, Hutzler Anat, Nizhberg Anna, and Itzkovitz Shalev. Bursty Gene Expression in the Intact Mammalian Liver. Molecular Cell, 58(1):147–156, April 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Skinner Samuel O, Xu Heng, Nagarkar-Jaiswal Sonal, Freire Pablo R, Zwaka Thomas P, and Golding Ido. Single-cell analysis of transcription kinetics across the cell cycle. eLife, 5:e12175, January 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Shah Sheel, Takei Yodai, Zhou Wen, Lubeck Eric, Yun Jina, Eng Chee-Huat Linus, Koulena Noushin, Cronin Christopher, Karp Christoph, Liaw Eric J., Amin Mina, and Cai Long. Dynamics and Spatial Genomics of the Nascent Transcriptome by Intron seqFISH. Cell, 174(2):363–376.e16, July 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Wan Yihan, Anastasakis Dimitrios G., Rodriguez Joseph, Palangat Murali, Gudla Prabhakar, Zaki George, Tandon Mayank, Pegoraro Gianluca, Chow Carson C., Hafner Markus, and Larson Daniel R.. Dynamic imaging of nascent RNA reveals general principles of transcription dynamics and stochastic splice site selection. Cell, 184(11):2878–2895.e20, May 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Wold Barbara and Myers Richard M. Sequence census methods for functional genomics. Nature Methods, 5(1):19–21, January 2008. [DOI] [PubMed] [Google Scholar]
- [49].Reimer Kirsten A., Mimoso Claudia A., Adelman Karen, and Neugebauer Karla M.. Co-transcriptional splicing regulates 3’ end cleavage during mammalian erythropoiesis. Molecular Cell, 81(5):998–1012.e7, March 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Drexler Heather L., Choquet Karine, and Churchman L. Stirling. Splicing Kinetics and Co-ordination Revealed by Direct Nascent RNA Sequencing through Nanopores. Molecular Cell, 77(5):985–998.e8, March 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Zeisel A., Kostler W. J., Molotski N., Tsai J. M., Krauthgamer R., Jacob-Hirsch J., Rechavi G., Soen Y., Jung S., Yarden Y., and Domany E.. Coupled pre-mRNA and mRNA dynamics unveil operational strategies underlying transcriptional responses to stimuli. Molecular Systems Biology, 7(1):529–529, September 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Pimentel Harold, Conboy John G., and Pachter Lior. Keep Me Around: Intron Retention Detection and Analysis. Preprint, arXiv: 1510.00696, October 2015. [Google Scholar]
- [53].Pimentel Harold, Parra Marilyn, Gee Sherry L., Mohandas Narla, Pachter Lior, and Conboy John G.. A dynamic intron retention program enriched in RNA processing genes regulates gene expression during terminal erythropoiesis. Nucleic Acids Research, 44(2):838–851, January 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Gorin Gennady, Fang Meichen, Chari Tara, and Pachter Lior. RNA velocity unraveled. PLOS Computational Biology, 18(9):e1010492, September 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Hjörleifsson Kristján Eldjárn, Sullivan Delaney K., Holley Guillaume, Melsted Páll, and Pachter Lior. Accurate quantification of single-nucleus and single-cell RNA-seq transcripts. Preprint, bioRxiv: 2022.12.02.518832, December 2022. [Google Scholar]
- [56].Soneson Charlotte, Srivastava Avi, Patro Rob, and Stadler Michael B.. Preprocessing choices affect RNA velocity results for droplet scRNA-seq data. PLOS Computational Biology, 17(1):e1008585, January 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Mazille Maxime, Buczak Katarzyna, Scheiffele Peter, and Mauger Oriane. Stimulus-specific remodeling of the neuronal transcriptome through nuclear intron-retaining transcripts. The EMBO Journal, 41(21):e110192, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Sina Booeshaghi A., Yao Zizhen, van Velthoven Cindy, Smith Kimberly, Tasic Bosiljka, Zeng Hongkui, and Pachter Lior. Isoform cell-type specificity in the mouse primary motor cortex. Nature, 598(7879):195–199, October 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Kessler O, Jiang Y, and Chasin L A. Order of intron removal during splicing of endogenous adenine phosphoribosyltransferase and dihydrofolate reductase pre-mRNA. Molecular and Cellular Biology, 13(10):6211–6222, October 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Coté Allison, Coté Chris, Bayatpour Sareh, Drexler Heather L, Alexander Katherine A, Chen Fei, Wassie Asmamaw T, Boyden Edward S, Berger Shelley, Churchman L Stirling, and Raj Arjun. pre-mRNA spatial distributions suggest that splicing can occur post-transcriptionally. Preprint, bioRxiv: 2020.04.06.028092, June 2021. [Google Scholar]
- [61].Gorin Gennady and Pachter Lior. Length Biases in Single-Cell RNA Sequencing of pre-mRNA. Preprint, bioRxiv: 2021.07.30.454514, July 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Gorin Gennady and Pachter Lior. Monod: mechanistic analysis of single-cell RNA sequencing count data. Preprint, bioRxiv: 2022.06.11.495771, June 2022. [Google Scholar]
- [63].Gorin Gennady, Yoshida Shawn, and Pachter Lior. Transient and delay chemical master equations. Preprint, bioRxiv: 2022.10.17.512599, October 2022. [Google Scholar]
- [64].Cao Zhixing and Grima Ramon. Analytical distributions for detailed models of stochastic gene expression in eukaryotic cells. Proceedings of the National Academy of Sciences, 117(9):4682–4692, March 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Jiang Qingchao, Fu Xiaoming, Yan Shifu, Li Runlai, Du Wenli, Cao Zhixing, Qian Feng, and Grima Ramon. Neural network aided approximation and parameter inference of non-Markovian models of gene expression. Nature Communications, 12(1):2618, December 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Filatova Tatiana, Popovic Nikola, and Grima Ramon. Modulation of nuclear and cytoplasmic mRNA fluctuations by time-dependent stimuli: Analytical distributions. Mathematical Biosciences, 347:108828, May 2022. [DOI] [PubMed] [Google Scholar]
- [67].Hansen Maike M.K., Desai Ravi V., Simpson Michael L., and Weinberger Leor S.. Cytoplasmic Amplification of Transcriptional Noise Generates Substantial Cell-to-Cell Variability. Cell Systems, 7(4):384–397.e6, October 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Battich Nico, Stoeger Thomas, and Pelkmans Lucas. Control of Transcript Variability in Single Mammalian Cells. Cell, 163(7):1596–1610, December 2015. [DOI] [PubMed] [Google Scholar]
- [69].Gorin Gennady and Pachter Lior. Special function methods for bursty models of transcription. Physical Review E, 102(2):022409, August 2020. [DOI] [PubMed] [Google Scholar]
- [70].Fu Xiaoming, Patel Heta P, Coppola Stefano, Xu Libin, Cao Zhixing, Lenstra Tineke L, and Grima Ramon. Quantifying how post-transcriptional noise and gene copy number variation bias transcriptional parameter inference from mRNA distributions. eLife, 11:e82493, October 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Fu Xiaoming, Patel Heta P., Coppola Stefano, Xu Libin, Cao Zhixing, Lenstra Tineke L., and Grima Ramon. Accurate inference of stochastic gene expression from nascent transcript heterogeneity. Preprint, bioRxiv: 2021.11.09.467882, November 2021. [Google Scholar]
- [72].Allen Institute for Brain Science. FASTQ files for Allen v3 mouse MOp samples, February 2020. [Google Scholar]
- [73].Allen Institute for Brain Science. Analyses for Allen v3 mouse MOp samples, February 2020. [Google Scholar]
- [74].Alexander Wolf F., Angerer Philipp, and Theis Fabian J.. SCANPY: large-scale single-cell gene expression data analysis. Genome Biology, 19(1):15, December 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Virtanen Pauli, Gommers Ralf, Oliphant Travis E., Haberland Matt, Reddy Tyler, Cournapeau David, Burovski Evgeni, Peterson Pearu, Weckesser Warren, Bright Jonathan, van der Walt Stéfan J., Brett Matthew, Wilson Joshua, Millman K. Jarrod, Mayorov Nikolay, Nelson Andrew R. J., Jones Eric, Kern Robert, Larson Eric, Carey C J, Polat Ilhan, Feng Yu, Moore Eric W., VanderPlas Jake, Laxalde Denis, Perktold Josef, Cimrman Robert, Hen-riksen Ian, Quintero E. A., Harris Charles R., Archibald Anne M., Ribeiro Antônio H., Pedregosa Fabian, van Mulbregt Paul, SciPy 1.0 Contributors, Vijaykumar Aditya, Bardelli Alessandro Pietro, Rothberg Alex, Hilboll Andreas, Kloeckner Andreas, Scopatz Anthony, Lee Antony, Rokem Ariel, Woods C. Nathan, Fulton Chad, Masson Charles, Häggström Christian, Fitzgerald Clark, Nicholson David A., Hagen David R., Pasechnik Dmitrii V., Olivetti Emanuele, Martin Eric, Wieser Eric, Silva Fabrice, Lenders Felix, Wilhelm Florian, Young G., Price Gavin A., Ingold Gert-Ludwig, Allen Gregory E., Lee Gregory R., Audren Hervé, Probst Irvin, Dietrich Jörg P., Silterra Jacob, Webber James T, Slavič Janko, Nothman Joel, Buchner Johannes, Kulick Johannes, Schönberger Johannes L., de Miranda Cardoso José Vinícius, Reimer Joscha, Harrington Joseph, Rodríguez Juan Luis Cano, Nunez-Iglesias Juan, Kuczynski Justin, Tritz Kevin, Thoma Martin, Newville Matthew, Kümmerer Matthias, Bolingbroke Maximilian, Tartre Michael, Pak Mikhail, Smith Nathaniel J., Nowaczyk Nikolai, Shebanov Nikolay, Pavlyk Oleksandr, Brodtkorb Per A., Lee Perry, McGibbon Robert T., Feldbauer Roman, Lewis Sam, Tygier Sam, Sievert Scott, Vigna Sebastiano, Peterson Stefan, More Surhud, Pudlik Tadeusz, Oshima Takuya, Pingel Thomas J., Robitaille Thomas P., Spura Thomas, Jones Thouis R., Cera Tim, Leslie Tim, Zito Tiziano, Krauss Tom, Upadhyay Utkarsh, Halchenko Yaroslav O., and Vázquez-Baeza Yoshiki. SciPy 1.0: fundamental algorithms for scientific computing in Python. Nature Methods, 17(3):261–272, March 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Jahnke Tobias and Huisinga Wilhelm. Solving the chemical master equation for monomolecular reaction systems analytically. Journal of Mathematical Biology, 54:1–26, September 2006. [DOI] [PubMed] [Google Scholar]
- [77].Perez-Carrasco Ruben, Beentjes Casper, and Grima Ramon. Effects of cell cycle variability on lineage and population measurements of messenger RNA abundance. Journal of The Royal Society Interface, 17(168):20200360, July 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Gorin Gennady and Pachter Lior. Intrinsic and extrinsic noise are distinguishable in a synthesis – export – degradation model of mRNA production. Preprint, bioRxiv: 2020.09.25.312868, September 2020. [Google Scholar]
- [79].Jiang Ruochen, Sun Tianyi, Song Dongyuan, and Li Jingyi Jessica. Statistics or biology: the zero-inflation controversy about scRNA-seq data. Genome Biology, 23:31, January 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Svensson Valentine. Droplet scRNA-seq is not zero-inflated. Nature Biotechnology, 38(2):147–150, February 2020. [DOI] [PubMed] [Google Scholar]
- [81].Jia Chen. Kinetic Foundation of the Zero-Inflated Negative Binomial Model for Single-Cell RNA Sequencing Data. SIAM Journal on Applied Mathematics, 80(3):1336–1355, January 2020. [Google Scholar]
- [82].Pedregosa Fabian, Varoquaux Gael, Gramfort Alexandre, Michel Vincent, Thirion Bertrand, Grisel Olivier, Blondel Mathieu, Prettenhofer Peter, Weiss Ron, Dubourg Vincent, Van-derplas Jake, Passos Alexandre, and Cournapeau David. Scikit-learn: Machine Learning in Python. Journal of Machine Learning Research, 12:2825–2830, October 2011. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Simulated datasets, simulated parameters used to generate them, and Allen dataset B08 and its associated metadata are available in the Zenodo package 7497222. All analysis scripts and notebooks are available at https://github.com/pachterlab/CGCCP_2023. The repository also contains a Google Colaboratory demonstration notebook applying the methods to a small human blood cell dataset.