Abstract
Human Leishmania infantum infection results in a spectrum of clinical expressions ranging from cutaneous to either asymptomatic or fatal visceral disease. In this context, characterization of parasite virulence appears to be relevant as a biological marker of intrinsic parasitic factors that can affect the pathology of leishmaniasis. Since parasite populations in naturally infected hosts are likely to be composed of multiclonal associations, we first explored the biodiversity of parasite virulence at the intrastrain level in vitro and in vivo by using 11 clones isolated from three strains previously known to express different virulence phenotypes in mice. Subsequently, we studied the course of infection in mice inoculated simultaneously or successively with strains or clones showing various virulence phenotypes. Analysis of in vitro growth characteristics showed no differences among clones from the different parental strains. By contrast, in vivo experiments evidenced a marked intrastrain heterogeneity of virulence to mice. One out of five clones obtained from a virulent strain showed a typical virulence phenotype, while the remaining four clones had low-virulence profiles, as did the six clones isolated from two low-virulence strains. In mixed multiclonal infections, the virulence phenotype was expressed as a dominant character over the associated low-virulence clones. After a challenge with either a homologous or a heterologous strain or clone, virulence phenotypes were conserved and expressed as in naive mice independently from the preexisting population. These results strongly suggest that parasite virulence in L. infantum visceral leishmaniasis is clonal and dominant in nature.
Parasitic infection with Leishmania spp. results in a broad spectrum of clinical diseases in humans. Leishmania infantum is responsible for most cases of human leishmaniasis in southern Europe and reflects this diversity with proteiform clinical expressions ranging from cutaneous leishmaniasis (CL) to either asymptomatic or fatal visceral leishmaniasis (VL).
The varying clinical expression observed in VL depends on complex relationships in which not only the genetic potential (3, 4) and/or immunological status of the host (10, 15, 20) but also the proper parasite biodiversity (15, 16, 19, 26) appear as determinant factors. Characterization of Leishmania polymorphism at the species and subspecies levels is currently based on isoenzyme and biomolecular analysis. However, identification of biological markers like parasite tropism or virulence remains crucial as a complementary tool to account for the considerable biodiversity of the parasite.
In this context, characterization of parasite virulence, i.e., the ability to develop and multiply in vitro and/or in vivo appears particularly relevant for analysis of parasitic factors that can affect the pathology of VL. Using 21 Mediterranean strains of L. infantum isolated from humans, we previously showed that there is a remarkable intraspecific heterogeneity in experimental virulence expression of the parasite in BALB/c mice (29). Three major infection profiles were characterized: a visceralization (V) profile with persistent heavy liver parasite burdens and marked progressive spleen involvement, a regulation (R) profile in which the infection is finally contained after an initial phase involving both organs, and an undetermined (U) profile with undetectable, low-level, or poorly characterized infection. Both the V and R profile types were maintained in C57BL/6 mice (cure haplotype) (17) and also in CB-17 congenic immunodeficient scid mice, although heavier parasite loads were observed in scid mice than in BALB/c mice in the late phase of infection (12). Thus, as these infection profiles are observed in various genetic and immunological host contexts, they actually characterize parasite virulence phenotypes in vivo.
Because the population structure of Leishmania spp. and other kinetoplastids is mainly clonal (8, 18, 30) and because strains isolated from naturally infected hosts are likely composed of multiclonal parasite associations (7, 25), such virulence profiles have to be investigated at the clonal level before any conclusion about the genotypic nature of parasite virulence can be drawn. Thus, this work was undertaken to explore the polymorphism of L. infantum at the intrastrain level and its possible impact on the basis of virulence phenotyping. Using a mouse model of VL, we studied the virulence biodiversity of L. infantum clonal populations issuing from strains with various levels of virulence. We also experimentally investigated the interaction between strain and clone populations with diverse levels of virulence when simultaneously or successively present in the same host.
MATERIALS AND METHODS
Parasites.
The strains and clones used in this study were from the International Leishmania Cryobank and Identification Center in Montpellier, France. Three L. infantum strains were selected for their different infection profiles, as defined in previous experimental studies (29): (i) strain MHOM/FR/91/LEM 2259 zymodeme MON-1 expressing a virulence (V) profile, in which parasite burdens rise continuously in the spleen (106 to107 ml−1 at day 100) while persisting at high levels in the liver (LEM 2259/V); (ii) strain MHOM/FR/91/LEM 2176 (MON-33), previously known as having a regulation (R) profile in which liver and spleen involvement is observed at the early phase of infection and followed by regulation with parasite burdens decreasing to low or undetectable levels in the liver while remaining moderate in the spleen (LEM 2176/R); and (iii) strain MHOM/FR/94/LEM 2859 (MON-1), expressing an uncharacterized (U) phenotype consisting of low-level or even undetectable infection (LEM 2859/U). Both strains LEM 2259/V and LEM 2176/R were isolated from the bone marrow of human immunodeficiency virus-infected patients with VL. Strain LEM 2859/U originated from the skin of an immunocompetent patient.
Clones were derived from these strains by the hanging-drop method using promastigotes at the mid-log phase of growth (1). Five, four, and two clones were obtained from strains LEM 2259, LEM 2176, and LEM 2859, respectively.
In vitro culture.
For in vitro growth characteristic study, parasites were cultivated at 27°C in Schneider's drosophila medium (SDM) (GIBCO BRL) and HOSMEM liquid medium (2) supplemented with hemin 10 μM (Sigma) and 20% heat-inactivated fetal calf serum (GIBCO). Parasites were inoculated into 25-ml culture flasks at day 0 at a final concentration of 105 ml−1. Parasite concentrations were evaluated by means of nucleoside hydrolase activity determination using p-nitrophenyl-β-d-ribofuranoside substrate. Nucleoside hydrolase activity was measured at day 4 and day 7 in duplicate samples of culture supernatants in 96-well microplates as previously described (13). Parasite concentrations (per milliliter) in samples were calculated from a standard curve established with a serial twofold dilution of a promastigote suspension made in duplicate from 2 × 107 to 5 × 103 parasites ml−1. For animal inoculation, mass cultures were produced in SDM.
Experimental animal infection.
Female 8-week-old BALB/c mice (IFFA CREDO) housed under standard conditions were used. Groups of mice were inoculated intravenously (i.v.) with 6-day-old promastigotes of the different strains or clones. The kinetics of infection was monitored throughout the experiment by determining the parasite burdens in the livers and spleens of three to five mice for each group at each measurement point.
Parasite burdens.
Parasites in organs were quantified by means of a culture microtitration assay as previously described (5). Briefly, organs were excised, weighed, and then homogenized with a tissue grinder (ULTRATURRAX, Stauffen, Germany) in 4 ml of SDM supplemented with 20% heat-inactivated fetal calf serum, penicillin (100 U/ml), and streptomycin (50 μg/ml) (Bio-Merieux). Serial fourfold dilutions of organ homogenates, ranging from 1/1 to 1/4 × 106, were made in duplicate under sterile conditions in 96-well microtitration plates containing 225 μl of culture medium. Plates were examined for the presence of mobile promastigotes under an inverted microscope after 10 days of incubation at 26°C. The final titer was the last dilution containing at least one parasite. Results were expressed as the logarithmic mean number of parasites per gram established from three to five mice for each group. The detection threshold of the method is 5 × 102 parasites g−1.
Experimental design.
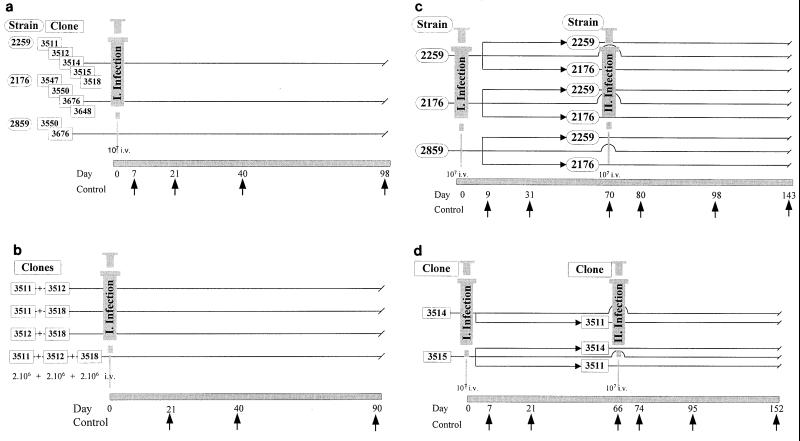
Three sets of experiments were carried out (Fig 1). (i) The first set of experiments was carried out in order to explore intrastrain virulence biodiversity. For that study, the virulence phenotypes of strains LEM 2259/V, LEM 2176/R, and LEM 2859/U and 11 clones were characterized. Groups of 16 mice were infected at day 0 with each strain or clone, and the kinetics of infection was monitored from day 7 to day 98 as shown in Fig 1a. (ii) A second set of experiments was designed to determine the kinetics of infection in mice injected simultaneously with clones with various virulence phenotypes. Infections were done with mixtures of clones that expressed different virulence profiles in the first experiment. Three clones originating from strain LEM 2259/V were selected, virulent clone 3511/V and less pathogenic clones 3518/R and 3512/R. An equal mixture of two or three clones (2 × 106 promastigotes each) was injected into mice at day 0, and the evolution of the infection was monitored in their livers and spleens at day 21, day 40, and day 90 (Fig 1b). (iii) The third set of experiments was carried out to study the interactions between successive primary and challenge infections with strains or clones expressing various pathogenic phenotypes.
FIG. 1.
Experimental design of single or concurrent animal infections. Panels: a, single infections with strains or clones; b, single infections with mixed clones; c, successive infections with strains; d, successive infections with clones.
Groups of mice (n = 48) were first infected at day 0 with 107 promastigotes of strain LEM 2259/V, LEM 2176/R, or LEM 2859/U. The animals in each group were then either challenged at day 70 with 107 promastigotes of the same strain or a heterologous strain or not reinfected (n = 12 each). Naive uninfected mice were also inoculated at day 70 as a control for the challenge inoculum. Liver and spleen parasite burdens were measured at day 9, day 31, and day 70 and then at day 80, day 98, and day 143 in order to monitor the kinetics of infection before and after the challenge infection (Fig 1c).
Similarly, cross-infections were carried out with three clones of strain LEM 2259/V. Two groups of animals were infected at day 0 with nonvirulent clone 3514/R or 3515/U. Six to eight mice in both groups were then either challenged at day 66 with clone 3511/V or not reinfected. A group of eight mice infected with clone 3515/U was also challenged at day 66 with clone 3514/R. Naive mice were also used at day 66 as a control for the secondary infection. Animals were sacrificed at day 7, day 21, and day 66 and then at day 74, day 95, and day 152 (Fig 1d).
Serological studies.
Serum antibody levels were monitored in cross-infections (experiment iii) before the challenge infection and at the end of the experiment.
Antibody titers were determined with an enzyme-linked immunosorbent assay method using a crude extract of strain LEM 2259/V as the coating reagent (5 μg ml−1 in 0.1 M carbonate buffer, pH 9.4) as described by Honoré et al. (17). Pools of sera were made for each group of mice at each control point, i.e., day 70 or day 66 after the primary infection and then after a challenge (day 98 or day 152). Pooled sera diluted 1/200 were tested in duplicate. Anti-Leishmania antibodies were detected with biotin-labeled antibodies against mouse immunoglobulin G (1/2,000; Dako), streptavidin/peroxidase (1/2,000; Boehringer), and then 2,2′-azino-di-[3-ethylbenzthiazoline sulfonate] diammonium (ABTS) substrate (Boehringer).
Results were expressed as arbitrary units (AU). The reaction cutoff was determined in a preliminary assay as the mean number of AU + 2 standard deviations established from five uninfected mice.
Statistical analysis.
Data were analyzed with GraphPad Prism 3.0 statistical software (GraphPad Software). For better comparison of data from separate experiments in which there were mild variations in the dates of examination, we chose to present data extrapolated from the regression curves calculated by using Pearson's correlation analysis. Thus, for in vitro studies, parasite concentrations (per milliliter) at day 4 and day 7 were calculated from the regression equation of the promastigote growth curve. Similarly, for in vivo experiments, liver and spleen parasite burdens (per gram) were extrapolated from the corresponding regression lines of parasite burdens. Thus, parasite loads were calculated at day 7 and day 100 for single infections and at day 7 and day 65 or day 70 (before reinfection) and day 75 or day 80 and then day 150 in challenge experiments. Comparisons were made by one-way analysis of variance (ANOVA) with Bonferroni's posttests or by two-way ANOVA. For two-way-ANOVA comparisons, the area under the curve was chosen as a global estimate of parasite growth in vitro or virulence in animals (24).
RESULTS
Characterization of clones.
The in vitro growth characteristics of the three strains and their respective clones are summarized in Table 1. All of the strains and clones grew readily, with a 1- to nearly 3-log increase in parasite concentration from day 0 to day 7, except for strains LEM 2259 and LEM 2859 in SDM. Promastigote growth rates in the two media were not correlated (r2 = 0.296, P = 0.32). Promastigote growth was significantly better in HOS culture medium than in SDM (P = 0.005).
TABLE 1.
In vitro growth characteristics of strains and clones of L. infantum
| Strain | Clone | Zb | SDM
|
HOS
|
||
|---|---|---|---|---|---|---|
| Day 4 | Day 7 | Day 4 | Day 7 | |||
| MHOM/FR/91 2259 | 1 | 5.5 ± 2.2 | 5.3 ± 3.4 | 6.5 ± 3.8 | 7.2 ± 5.8 | |
| 3511 | 1 | 5.9 ± 0.1 | 6.1 ± 0.2 | 6.9 ± 2.3 | 7.8 ± 3.5 | |
| 3512 | 1 | 5.9 ± 0.5 | 6.2 ± 0.7 | 7.0 ± 4.8 | 7.9 ± 0.6 | |
| 3514 | 1 | 6.7 ± 3.5 | 7.4 ± 5.3 | 7.0 ± 0.4 | 7.9 ± 4.5 | |
| 3515 | 1 | 6.8 ± 3.8 | 7.5 ± 7.5 | 6.6 ± 3.0 | 7.2 ± 7.2 | |
| 3518 | 1 | 6.1 ± 0.9 | 6.4 ± 1.3 | 7.1 ± 4.7 | 8.1 ± 8.2 | |
| MHOM/FR/91 2176 | 33 | 6.6 ± 2.8 | 7.1 ± 4.2 | 7.2 ± 5.4 | 8.1 ± 8.0 | |
| 3547 | 33 | 6.8 ± 3.7 | 7.5 ± 5.6 | 6.9 ± 4.1 | 7.7 ± 6.3 | |
| 3550 | 33 | 6.5 ± 1.6 | 7.0 ± 2.5 | 6.8 ± 4.1 | 7.6 ± 6.3 | |
| 3576 | 33 | 6.9 ± 4.2 | 7.6 ± 6.4 | 6.9 ± 4.1 | 7.7 ± 6.3 | |
| 3648 | 33 | 6.6 ± 2.6 | 7.2 ± 4.0 | 6.5 ± 3.1 | 7.1 ± 4.7 | |
| MHOM/FR/94 2859 | 1 | 5.0 ± 4.7 | 4.6 ± 7.2 | 6.2 ± 1.2 | 6.5 ± 1.9 | |
| 3646 | 1 | 6.2 ± 1.7 | 6.5 ± 2.6 | NDc | ND | |
| 3721 | 1 | 6.8 ± 2.9 | 7.5 ± 4.4 | 7.1 ± 3.5 | 8.1 ± 5.3 | |
Results are given as the logarithm of the number of parasites per milliliter ± the standard error at days 4 and 7 of culture in SDM and HOS media calculated from the equation of the growth regression line. Two-way ANOVA of the area under the curve: strain factor (clones of strain 2259 versus 2176 versus 2859, P = 0.59 (no significant difference); medium (SDM versus HOS), P = 0.005; interaction, P = 0.14 (no significant difference).
Z, zymodeme.
ND, not done.
Characterization of virulence in vivo (experiment i) is shown in Table 2 and Fig. 2. No deaths or clinical symptoms were observed in infected mice. However, based on the number of parasites recovered from their livers and spleens, a great heterogeneity in the strain and clone virulence profiles was evidenced.
TABLE 2.
Virulence characteristics of strains and clones of L. infantum in micea
| Strain | Clone | Zb | Parasite
burden
|
|||
|---|---|---|---|---|---|---|
| Liver
|
Spleen
|
|||||
| Day 7 | Day 100 | Day 7 | Day 100 | |||
| MHOM/FR/91 2259 | 1 | 6.1 ± 0.6 | 5.2 ± 0.4 | 5.3 ± 0.7 | 7.1 ± 0.2 | |
| 3511 | 1 | 5.6 ± 0.4 | 5.5 ± 0.6 | 4.3 ± 0.5 | 6.9 ± 0.5 | |
| 3512 | 1 | 3.8 ± 0.3 | 2.5 ± 0.0 | 2.7 ± 0.0 | 3.1 ± 0.4 | |
| 3514 | 1 | 4.6 ± 0.2 | 2.9 ± 0.3 | 3.2 ± 0.3 | 3.5 ± 0.5 | |
| 3515 | 1 | 3.0 ± 0.2 | 2.8 ± 0.1 | 2.6 ± 0.1 | 3.1 ± 0.4 | |
| 3518 | 1 | 3.7 ± 0.8 | 2.6 ± 0.0 | 2.9 ± 0.2 | 3.6 ± 0.9 | |
| MHOM/FR/91 2176 | 33 | 3.9 ± 0.4 | 2.9 ± 0.3 | 2.7 ± 0.3 | 2.8 ± 0.2 | |
| 3547 | 33 | 5.6 ± 0.6 | 2.0 ± 0.9 | 4.6 ± 0.4 | 2.6 ± 0.6 | |
| 3550 | 33 | 2.9 ± 0.2 | 2.6 ± 0.4 | 2.0 ± 0.0 | 2.0 ± 0.0 | |
| 3576 | 33 | 5.6 ± 0.3 | 2.2 ± 0.6 | 5.2 ± 0.3 | 2.5 ± 0.4 | |
| 3648 | 33 | 3.5 ± 0.4 | 2.5 ± 0.6 | 2.7 ± 0.2 | 2.9 ± 0.3 | |
| MHOM/FR/94 2859 | 1 | 2.0 ± 0.0 | 2.0 ± 0.0 | 2.0 ± 0.0 | 2.0 ± 0.0 | |
| 3646 | 1 | 2.8 ± 0.2 | 2.6 ± 0.3 | 2.0 ± 0.0 | 2.0 ± 0.0 | |
| 3721 | 1 | 4.4 ± 0.3 | 2.7 ± 0.5 | 3.3 ± 0.4 | 2.9 ± 0.5 | |
Results are given as the logarithm of the number of parasites per gram ± the standard error calculated at days 7 and 100 postinfection from the equation of the liver and spleen regression lines. Two-way ANOVA of the area under the curve: strain factor (clones of strain 2259 versus 2176 versus 2859, P = .23 (no significant difference); organ (liver versus spleen), P = 0.69 (no significant difference).
Z, zymodeme.
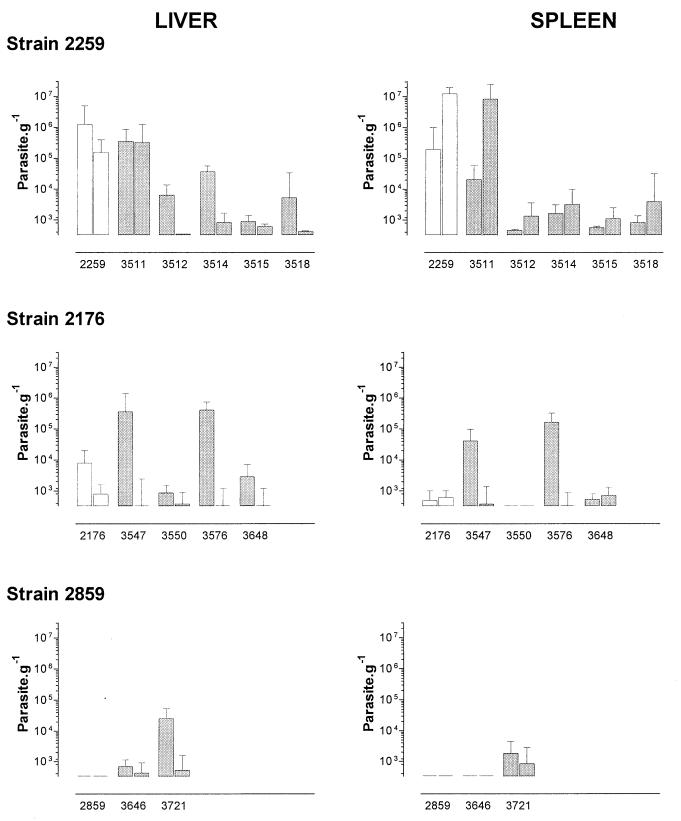
FIG. 2.
Experiment i. Calculated parasite loads ± the standard errors at day 7 (column 1) and day 100 (column 2). Mice were inoculated i.v. at day 0 with 107 promastigotes of L. infantum LEM 2259/V, LEM 2176/R, and LEM 2859/U (□) or their respective clones (▩).
Mice inoculated with strains LEM 2259/V, LEM 2176/R, and LEM 2859/U showed the expected type of infection profile observed previously. Mice injected with strain LEM 2259/V developed a V type of infection with parasite burdens persisting at high levels in the liver at day 100 (1.6 × 105 g−1) and increasing from 2 × 105 to 1.4 × 107 g−1 in the spleen. Inoculation with strain LEM 2176/R resulted in a controlled infection with parasite burdens decreasing from 104 g−1 at day 7 to undetectable levels at day 100 in the liver while remaining at low levels (<103 g−1) in the spleen. No parasite was detected in the livers and spleens of mice inoculated with strain LEM 2859/U.
Among the five clones obtained from strain LEM 2259/V, clone 3511 showed a typical V phenotype. With this clone, liver parasite burdens remained at levels of >1.5 × 105 g−1 throughout the experiment. In the spleen, a 3-log increase was observed from day 7 to day 100, reaching 8.3 × 106 g−1. This profile contrasted strongly with those observed with the other clones originating from strain LEM 2259/R. In mice inoculated with clones 3512, 3514, and 3518, a controlled R type of infection was observed. In these mice, parasite burdens decreased to very low or undetectable levels in the liver while not exceeding 104 g−1 in the spleen. Infection with the last clone, 3515, resulted in low involvement of both the liver and spleen (U). The virulence profile difference between clone 3511/V and each of the four other clones of strain LEM 2259 was highly significant in both the liver (ANOVA, P = 0.0004; Bonferroni's posttest, P = 0.01 to 0.0001) and the spleen (ANOVA, P = 0.004; Bonferroni's posttest, P <0.05 to 0.01).
A great variability in virulence expression was also noted between clones of strains LEM 2176/R and LEM 2859/U. However, no clone from these two strains expressed a virulence phenotype in mice. An R profile of infection was observed in mice inoculated with three clones from strain LEM 2176/R (clones 3547, 3576, and 3648) and one from strain LEM 2859/U (clone 3721), whereas a U profile was obtained with the last two clones, 3550 (strain LEM 2176/R) and 3646 (strain LEM 2859/U).
Mixed infections with clones.
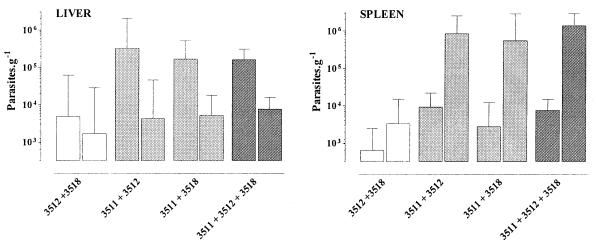
Evolution of infection in mice injected with mixtures of clones (experiment ii) is represented in Fig. 3. Inoculation with a mixture of clones 3512/R and 3518/R resulted in a controlled R type of infection. The parasite burden decreased in the liver from 4.7 × 103 at day 7 to 1.7 × 103 g−1 at day 100 while remaining at a low level in the spleen (3.3 × 103 g−1 at day 100). This contrasted strongly with the evolution of infection in mice inoculated simultaneously with clone 3511/V and clone 3512/R, 3518/R, or both (P <0.001). Despite a reduction in parasite burdens in the liver, a straightforward (2- to 2.5-log) increase occurred constantly in the spleen. At day 100, parasite loads reached 5.5 × 105 to 1.6 × 106 g−1. Thus, the virulence phenotype of clone 3511/V was expressed in the three groups of mice, whatever the R clone coinjected.
FIG. 3.
Experiment ii. Calculated parasite burdens ± the standard errors at day 7 (column 1) and day 100 (column 2). Mice were inoculated i.v. simultaneously at day 0 with 106 promastigotes of each of two or three clones of L. infantum strain LEM 2259/V. The three clones used expressed either a V (clone 3511) or an R (clones 3512 and 3518) phenotype when inoculated separately. Combinations of clones 3212 and 3218 (R and R phenotypes, respectively; □), 3511 and 3512 or 3518 (V and R phenotypes, respectively; ▩), and 3511, 3512, and 3518 (V, R, and R phenotypes, respectively; ░⃞) were used.
Cross-infections.
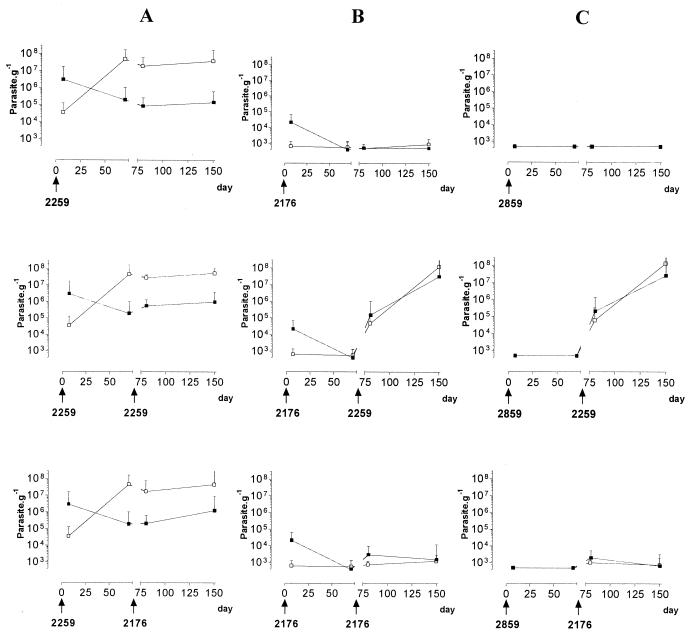
The kinetics of infection in mice inoculated with strains with various levels of virulence (experiment iiia) are shown in Fig. 4. Single inoculations with strains LEM 2259, LEM 2176, and LEM 2811 resulted in V, R, and U infection profiles, respectively, as already described. In mice primarily infected with strain LEM 2259/V (Fig. 4A), the infection profile was not modified after a challenge at day 70 with the same strain or heterologous strain LEM 2176/R, except for a weak increase in the liver burden at day 80. By contrast, profound changes in the kinetics of infection were observed after reinfection in mice previously infected with strain LEM 2176/R (Fig. 4B) or LEM 2859/U (Fig. 4C). Similar modifications occurred in both groups of mice. A challenge infection with strain LEM 2259/V resulted in massive organ involvement, with parasite burdens reaching levels of >106 g−1 in the liver and >107 g−1 in the spleen at day 150. By contrast, a new infection at day 70 with strain LEM 2176/R resulted in only a weak increase in the hepatic and splenic burdens at day 80, followed by control of the challenge infection (Fig. 4B and C). Thus, the virulence phenotype of strain LEM 2259/V was constantly expressed in both primary and challenge infections. All groups of mice primarily infected and/or challenged with this strain showed very heavy parasite burdens at day 150 in the liver (105 to 106 g−1) and especially in the spleen (>107 g−1).
FIG. 4.
Experiment iii. Kinetics of parasite burdens in the livers (▪) and spleens (□) of mice successively inoculated i.v. at day 0 and then at day 70 with 107 promastigotes of three strains of L. infantum. The primary inoculation was done with strain LEM 2259/V (A), LEM 2176/R (B), or LEM 2859/U (C) expressing a V, R, or U phenotype, respectively. The challenge inoculation at day 70 was done with either the primary infecting strain or a heterologous strain.
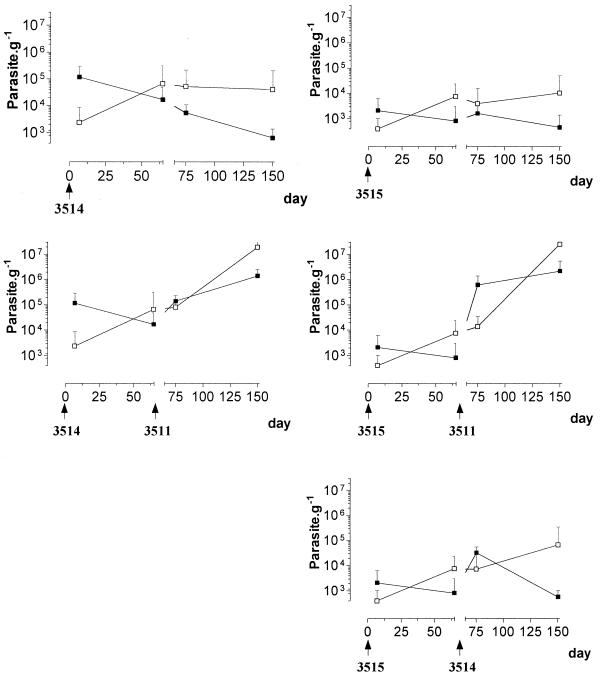
Similar results were obtained in cross-infection experiments with three different clones of strain LEM 2259/V (experiment iiib) (Fig. 5). In mice infected at day 0 by low-virulence clone 3514/R or 3515/U, the challenge infection at day 66 with virulent clone 3511/V resulted in a massive infection, with parasite burdens reaching 1.5 × 106 to 1.9 × 106 g−1 in the liver and progressively increasing to a level of 2 × 107 g−1 in the spleen at the end of the experiment. By contrast, mice infected at day 0 with clone 3515/U showed a controlled R type of infection after a challenge with clone 3514/R, as previously observed in single infections with this clone. In these mice, after the challenge infection, parasite burdens remained quite low (maximum, 5.7 × 104 g−1) until day 150 in the spleen while decreasing to undetectable levels after a transient rise at day 80 (2.7 × 104 g−1) in the liver.
FIG. 5.
Experiment iii. Evolution of parasite burdens in the livers (▪) and spleens (□) of mice successively inoculated i.v. at day 0 and then at day 66 with 107 promastigotes of three clones of L. infantum strain LEM 2259/V. The clones used expressed V (clone 3511), R (clone 3514), and U (clone 3515) phenotypes.
Serological studies.
In uninfected mice, the mean titer was 158 ± 8.6 AU, determining a positive cutoff of 175 AU. On this basis, a significant antibody response was observed in all groups of infected mice (Table 3).
TABLE 3.
Anti-Leishmania immunoglobulin G antibody response in pooled sera from mice cross-infected with L. infantum strains and clones with various levels of virulencea
| Primary infectionb strain or clone (phenotype) | No. of AU
|
Strain (phenotype)c | Clone (phenotype)d | No. of AU
|
||
|---|---|---|---|---|---|---|
| Day 70 | Day 66 | Day 198 | Day 152 | |||
| Strains | ||||||
| 2259 (V) | 683 | 2259 (V) | 681 | |||
| 2176 (R) | 677 | |||||
| 2176 (R) | 343 | 2259 (V) | 476 | |||
| 2176 (R) | 366 | |||||
| 2859 (U) | 267 | 2259 (V) | 346 | |||
| 2176 (R) | 260 | |||||
| Clones | ||||||
| 3511 (V) | 580 | None | 477 | |||
| 3514 (R) | 395 | None | 244 | |||
| 3511 (V) | 450 | |||||
| 3515 (R) | 260 | None | 213 | |||
| 3511 (V) | 410 | |||||
| 3514 (R) | 175 | |||||
Results are expressed in arbitrary units as determined in an enzyme-linked immunosorbent assay using a crude antigen from virulent L. infantum strain 2259/V.
Primary infection was done on day 0.
Used for secondary infection on day 70.
Used for secondary infection on day 66.
A strong antibody response was elicited by strain LEM 2259/V at day 70, before the challenge infection (683 AU). This contrasted with the low antibody levels observed at the same time after infection with strain LEM 2176/R, i.e., 343 AU. At day 70, a significant antibody response (267 AU) was also noted in mice inoculated with strain LEM 2859/U, although no parasites were detected in this group of mice. Mice primarily inoculated with strain LEM 2176/R or LEM 2859/U showed a net antibody level increase (≥30%) after a challenge with strain LEM 2259/V (476 and 346 AU at day 98, respectively), while no change was observed after a challenge infection with strain LEM 2176/R. In mice first infected with strain LEM 2259/V, the antibody response remained unchanged until the end of the experiment whatever the strain used for the secondary infection.
Similar results were observed in mice infected with clones. A weak antibody response was elicited after inoculation with clones 3514/R (395 AU) and 3515/U (260 AU). By contrast, infection with V clone 3511/V was accompanied with a strong and persistent serological response, 580 and 477 AU at day 66 and day 152, respectively. In mice preinoculated with clone 3514/R or 3515/U and then reinfected with clone 3511/V at day 66, a straightforward antibody level increase was observed after the challenge, reaching 470 and 410 AU at day 152, respectively. By contrast, in mice infected with clone 3515/U and then challenged with clone 3514/R, antibodies decreased to an insignificant level (175 AU) at day 152.
DISCUSSION
Genetic diversity is a crucial parameter that must be taken into account in human VL. In particular, polymorphism of parasite virulence and tropism is likely to have a profound impact on disease transmission and/or pathology, as suggested by experimental studies on L. guyanensis CL in hamsters and Trypanosoma cruzi trypanosomiasis in mice (11, 22).
Phenotypic characterization is a prerequisite for further identification of genetic loci and/or mechanisms involved in parasite tropism and virulence. Phenotypic analysis of Leishmania isolates is currently based largely on isoenzyme polymorphism (27). Thus, dermotropic and viscerotropic human L. infantum zymodemes have been identified (15). However, we have previously shown that this method cannot discriminate between parasites with various levels of virulence since strains of L. infantum belonging to the same zymodeme exhibit a large heterogeneity of virulence profiles in mice (29).
In a first attempt to investigate the biodiversity of virulence expression of L. infantum parasites at the intrastrain level, we analyzed the in vitro and in vivo growth characteristics of three strains (isolated from humans) that exhibit different virulence patterns in mice, as well as those of 11 clones originating from these strains. Analysis of parasite growth characteristics in vitro showed no differences among clones from the different parental strains. By contrast, a marked intrastrain heterogeneity was evidenced by in vivo virulence phenotype analysis. One out of five clones obtained from the V strain generated a typical V profile, with marked and prolonged involvement of the liver and spleen until the end of the experiment, while the four remaining clones showed low-virulence infection profiles (three R and one U). No virulent clone was obtained from the two strains selected for their nonvirulence or undetermined phenotype. Clones obtained from these strains gave four R and two U types of infection. Thus, we can conclude that strains are composed of multiclonal parasite populations that are heterogeneous on the basis of experimental virulence in mice.
As a consequence, we examined the course of infection in cases of concurrent infections with clones in order to investigate the character of dominance of the V or R phenotype. First, we used several combinations of two or three clones issuing from the same parental strain, LEM 2259/V, but displaying various virulence profiles when injected alone (one V and two R clones). In this experiment, the presence of the V clone in the inoculum consistently resulted in a V type of infection whatever the R clone(s) coinjected. This shows that virulence is expressed as a dominant character in experimental multiclonal infections without any apparent interaction with the associated low-virulence clonal populations.
Second, we examined the outcome of infection in cases of sequential inoculations with strains or clones with different virulence phenotypes. We found that the expression of strain or clone virulence phenotypes was not modified in successive infections of the same host. The course of infection in previously infected animals was identical to that observed in naive mice, whatever the combination of virulence phenotypes used for the primary and secondary inoculations.
To our knowledge, this is the first data concerning the interactions of parasites varying in virulence in successive experimental VL infections. Using the same L. infantum strain for primary and challenge infections, Rousseau et al. (28) found that a primary infection induced a protective effect that was organ dependent, protection being achieved in the liver but not in the spleen. In CL, previous experiments on the protective role of a primary infection against a challenge have led to contradictory results. Li et al. (21) observed that infection of BALB/c mice with a low-virulence clone of L. major protected mice against a challenge infection with a highly virulent clone. Our results are more in agreement with those of Da Fonseca et al. (9), who showed that a primary infection with a totally avirulent strain of L. amazonensis did not protect mice against a virulent strain.
Serological studies showed that the type of infection significantly influenced the humoral response in mice. Indeed, a serological response was detected in all infected mice, including those inoculated with low-virulence strains or clones. However, mice inoculated with a virulent strain or clone showed higher antibody levels than did animals infected with less virulent parasite populations. Similarly, a challenge infection with a V strain or clone was followed by a marked increase in antibody levels while no changes were observed after a challenge with an R strain or clone. This shows that progression of infection is accompanied by a humoral response in both primarily infected or superinfected animals. This correlates with observations made in human VL, where patent infections are frequently associated with a strong specific antibody response and T-cell anergy to Leishmania antigen (14), while a cure is accompanied by an antibody level decrease and restoration of the cellular response (6, 23).
Our results clearly show that (i) L. infantum strains isolated from humans are composed of clonal populations displaying a marked polymorphism of experimental virulence to mice, (ii) populations with various levels of virulence do not interact in vivo in experimental multiclonal infections and strain and/or clone virulence phenotypes are conserved in simultaneous or successive infections, and (iii) the virulence phenotype is expressed as a dominant character in such experimental infections with different parasite populations. This may have a profound impact on the epidemiology and immunopathology of L. infantum VL. Characterization of parasite virulence is a crucial step that must be done in parallel with immunological or biomolecular research studies on VL.
ACKNOWLEDGMENTS
We thank M. Lambert for cloning of the strains.
The Centre National de Référence des Leishmanioses received financial support from the Ministry of Health.
REFERENCES
- 1.Bastien P, Wahba M. A simplification of the technique for cloning Leishmania. Ann Trop Med Parasitol. 1989;83:435–437. doi: 10.1080/00034983.1989.11812369. [DOI] [PubMed] [Google Scholar]
- 2.Berens R L, Marr J J. An easily prepared defined medium for cultivation of Leishmania donovanipromastigotes. J Parasitol. 1978;64:160. [PubMed] [Google Scholar]
- 3.Blackwell J M. Genetic susceptibility to Leishmaniainfections: studies in mice and man. Parasitology. 1996;112:S67–S74. [PubMed] [Google Scholar]
- 4.Bradley D. Regulation of Leishmania populations within the host. II. Genetic control of acute susceptibility of mice to Leishmania donovaniinfections. Clin Exp Immunol. 1977;30:130–140. [PMC free article] [PubMed] [Google Scholar]
- 5.Buffet P, Sulahian A, Garin Y J F, Nassar N, Derouin F. Culture microtitration: a sensitive method for quantifying Leishmania infantumin tissues of infected mice. Antimicrob Agents Chemother. 1995;39:2167–2168. doi: 10.1128/aac.39.9.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carvalho E M, Bacellar O, Brownell C, Regis T, Coffman R L, Reed S G. Restoration of the IFN-γ production and lymphocyte proliferation in visceral leishmaniasis. J Immunol. 1994;152:5949–5956. [PubMed] [Google Scholar]
- 7.Cuba-Cuba C A, Evans D, de C. Rosa A, Mardsen P D. Clonal variation within a mucosal isolate derived from a patient with Leishmania (Viannia) braziliensisinfection. Rev Inst Med Trop São Paulo. 1991;33:343–350. doi: 10.1590/s0036-46651991000500002. [DOI] [PubMed] [Google Scholar]
- 8.Cupolillo E, Grimaldi G, Momen H. Genetic diversity among Leishmania (Viannia) parasites. Ann Trop Med Parasitol. 1997;91:617–626. doi: 10.1080/00034989760716. [DOI] [PubMed] [Google Scholar]
- 9.Da Fonseca A L S, Vallochi A L, de Furtado G, de Abrahamsohn I, Lima G M. Immune response and protection in mice inoculated with Leishmania amazonensisclones expressing different degrees of virulence. Parasitol Res. 1997;83:690–697. doi: 10.1007/s004360050321. [DOI] [PubMed] [Google Scholar]
- 10.Dedet J P, Pratlong F, Lanotte G, Ravel C. Cutaneous leishmaniasis. The parasite. Clin Dermatol. 1999;17:261–268. doi: 10.1016/s0738-081x(99)00044-9. [DOI] [PubMed] [Google Scholar]
- 11.De Lana M, da Silveira Pinto A, Bastrenta B, Barnabé C, Noël S, Tibayrenc M. Trypanosoma cruzi: infectivity of clonal genotype infections in acute and chronic phase in mice. Exp Parasitol. 2000;96:61–66. doi: 10.1006/expr.2000.4552. [DOI] [PubMed] [Google Scholar]
- 12.Gangneux J P, Sulahian A, Honore S, Meneceur P, Derouin F, Garin Y J F. Evidence for determining parasitic factors in addition to host genetics and the immune status in the outcome of murine Leishmania infantumvisceral leishmaniasis. Parasite Immunol. 2000;22:515–519. doi: 10.1046/j.1365-3024.2000.00332.x. [DOI] [PubMed] [Google Scholar]
- 13.Garin Y J F, Sulahian A, Meneceur P, Gangneux J P, Pannier-Stockman C, Derouin F. Assessment of Leishmaniapromastigote growth in vitro by means of nucleoside hydrolase activity determination. Parasitol Res. 2001;87:145–148. doi: 10.1007/pl00008567. [DOI] [PubMed] [Google Scholar]
- 14.Ghalib H W, Piuvezam M R, Skeiky Y A W, Siddig M, Hashim F A, El Hassan A M, Russo D M, Reed S G. Interleukin 10 production correlates with pathology in human Leishmania donovaniinfections. J Clin Investig. 1993;92:324–329. doi: 10.1172/JCI116570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gradoni L, Gramiccia M. Leishmania infantum tropism: strain genotype or host immune status? Parasitol Today. 1994;10:264–267. doi: 10.1016/0169-4758(94)90142-2. [DOI] [PubMed] [Google Scholar]
- 16.Gramiccia M, Gradoni L, Angelici M C. Epidemiology of Mediterranean leishmaniasis by Leishmania infantum: isoenzyme and kDNA analysis for the identification of parasites from man, vectors and reservoirs. NATO-ASI Ser Ser A Life Sci. 1989;1:21–37. [Google Scholar]
- 17.Honoré S, Garin Y J F, Sulahian A, Gangneux J P, Derouin F. Influence of the host and parasite strain in a mouse model of visceral Leishmania infantuminfection. FEMS Immunol Med Microbiol. 1998;21:231–239. doi: 10.1111/j.1574-695X.1998.tb01170.x. [DOI] [PubMed] [Google Scholar]
- 18.Jiménez M, Alvar J, Tibayrenc M. Leishmania infantumis clonal in AIDS patients too: epidemiological implications. AIDS. 1997;11:569–573. doi: 10.1097/00002030-199705000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Jiménez M, Ferrer-Dufol M, Cañavate C, Gutiérrez-Solar B, Molina R, Laguna F, López-Vélez R, Cercenado E, Daudén E, Blázquez J, Ladrón de Guevara C, Gómez J, De la Torre J, Barros C, Altés J, Serra T, Alvar J. Variability of Leishmania (Leishmania) infantumamong stocks from immunocompromised, immunocompetent patients and dogs in Spain. FEMS Microbiol Lett. 1995;131:197–204. doi: 10.1016/0378-1097(95)00259-8. [DOI] [PubMed] [Google Scholar]
- 20.Kubar J, Marty P, Lelievre A, Quaranta J F, Staccini P, Caroli-Bosc C, Le Fichoux Y. Visceral leishmaniosis in HIV-positive patients: primary infection, reactivation and latent infection. Impact of the CD4+T-lymphocyte counts. AIDS. 1998;12:2147–2153. doi: 10.1097/00002030-199816000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Li J, Nolan T J, Farrell J P. Leishmania major. A clone with low virulence for BALB/c mice elicits a Th1 type response and protects against infection with a highly virulent clone. Exp Parasitol. 1997;87:47–57. doi: 10.1006/expr.1997.4183. [DOI] [PubMed] [Google Scholar]
- 22.Martinez J E, Valderrama L, Gama V, Leiby D A, Saravia N G. Clonal diversity in the expression and stability of the metastatic capability of Leishmania guyanensisin the golden hamster. J Parasitol. 2000;86:792–799. doi: 10.1645/0022-3395(2000)086[0792:CDITEA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 23.Mary C, Lamouroux D, Dunan S, Quilici M. Western blot analysis of antibodies to Leishmania infantumantigens: potential of the 14-kD and 16-kD antigens for diagnosis and epidemiologic purposes. Am J Trop Med Hyg. 1992;47:764–771. doi: 10.4269/ajtmh.1992.47.764. [DOI] [PubMed] [Google Scholar]
- 24.Motulsky, H. 10 July 1998. Comparing dose-response or kinetic curves with GraphPad Prism. HMS Beagle BioMedNet Magazine 34. [Online.] http: //news.bma.com/hmsbeagle.
- 25.Pacheco R S, Grimaldi G, Momen J R, H, Morel C M. Population heterogeneity among clones of New World Leishmaniaspecies. Parasitology. 1990;100:393–398. doi: 10.1017/s0031182000078677. [DOI] [PubMed] [Google Scholar]
- 26.Pratlong F, Dedet J P, Marty P, Portus M, Deniau M, Dereure J, Abranches P, Reynes J, Martini A, Lefebvre M, Rioux J A. Leishmania-human immunodeficiency virus coinfection in the Mediterranean basin: isoenzymatic characterization of 100 isolates of the Leishmania infantum complex. J Infect Dis. 1995;172:323–326. doi: 10.1093/infdis/172.1.323. [DOI] [PubMed] [Google Scholar]
- 27.Rioux J A, Lanotte G, Serres E, Pratlong F, Bastien P, Perieres J. Taxonomy of Leishmania. Use of isoenzymes. Suggestions for a new classification. Ann Parasitol Hum Comp. 1990;65:111–125. doi: 10.1051/parasite/1990653111. [DOI] [PubMed] [Google Scholar]
- 28.Rousseau, D., S. Demartino, F. Anjuère, B. Ferrua, K. Fragaki, Y. Le Fichoux, and J. Kubar. Sustained parasite burden in spleen of Leishmania infantum infected BALB/c mice is accompanied by expression of MCP-1 transcripts and lack of protection against challenge. Eur. Cytokine Netw., in press. [PubMed]
- 29.Sulahian A, Garin Y J F, Pratlong F, Dedet J P, Derouin F. Experimental pathogenicity of viscerotropic and dermotropic isolates of Leishmania infantumfrom immunocompromised and immunocompetent patients in a murine model. FEMS Immunol Med Microbiol. 1997;17:131–138. doi: 10.1111/j.1574-695X.1997.tb01005.x. [DOI] [PubMed] [Google Scholar]
- 30.Tibayrenc M, Kjellberg F, Ayala F J. A clonal theory of parasitic protozoa: the population structures of Entamoeba, Giardia, Leishmania, Naegleria, Plasmodium, Trichomonas, and Trypanosomaand their medical and taxonomical consequences. Proc Natl Acad Sci USA. 1990;87:2414–2418. doi: 10.1073/pnas.87.7.2414. [DOI] [PMC free article] [PubMed] [Google Scholar]