Abstract
It has been previously reported that although inducible nitric oxide synthase (iNOS) gene knockout (NOS2−/−) mice resolve Chlamydia trachomatis genital infection, the production of reactive nitrogen species (RNS) via iNOS protects a significant proportion of mice from hydrosalpinx formation and infertility. We now report that higher in vivo RNS production correlates with mouse strain-related innate resistance to hydrosalpinx formation. We also show that mice with a deletion of a key component of phagocyte NADPH oxidase (p47phox−/−) resolve infection, produce greater amounts of RNS in vivo, and sustain lower rates of hydrosalpinx formation than both wild-type (WT) NOS2+/+ and NOS2−/− controls. When we induced an in vivo chemical block in iNOS activity in p47phox−/− mice using NG-monomethyl-l-arginine (L-NMMA), a large proportion of these mice eventually succumbed to opportunistic infections, but not before they resolved their chlamydial infections. Interestingly, when compared to WT and untreated p47phox−/− controls, L-NMMA-treated p47phox−/− mice resolved their infections more rapidly. However, L-NMMA-treated p47phox−/− mice lost resistance to chronic chlamydial disease, as evidenced by an increased rate of hydrosalpinx formation that was comparable to that for NOS2−/− mice. We conclude that phagocyte oxidase-derived reactive oxygen species (ROS) regulate RNS during chlamydial urogenital infection in the mouse. We further conclude that while neither phagocyte oxidase-derived ROS nor iNOS-derived RNS are essential for resolution of infection, RNS protect from chronic chlamydial disease in this model.
Female mice resolve chlamydial urogenital infection by utilizing immune responses that require T cells, major histocompatibility complex (MHC) class II antigen processing, macrophage activation, and CD4 coreceptors (hereafter referred to as type 1 immune responses) (34). This is evidenced by chronic infection in both congenitally athymic nude mice (35), MHC class II knockout (KO) mice (24), and TCR α/β KO mice (29). The exact mechanism used by type 1 immune response components to restrict chlamydial growth is not yet known, although effective immune responses are at least partially dependent upon functional CD4+ T cells (24, 39) and the production of type 1 cytokines, such as gamma interferon (IFN-γ) and interleukin-12 (8, 29). The production of reactive nitrogen species (RNS) through the cytokine-inducible nitric oxide synthase (iNOS) likely plays a contributing role in elimination of chlamydial infection in vitro, though it is not necessary for resolution of mouse urogenital infection (17, 20, 28, 31). The IFN-γ-inducible tryptophan decyclizing enzyme 2,3-indoleamine dioxygenase has been shown to restrict chlamydial growth in human systems in vitro but does not appear to be important in the mouse in response to infection (2, 31). Other IFN-γ-dependent mechanisms are likely to play a role, especially in the absence of iNOS. These mechanisms may include restriction of iron or other key nutrients or the production of antimicrobial peptides (36, 43).
Although some information is known about protective immune mechanisms in the murine model of chlamydial genital infection, much less is known about mechanisms related to immunopathogenesis as a result of infection. It is accepted that immune responses during chlamydial infection can be both protective and pathogenic (23). In the mouse model of urogenital chlamydial infection, disease outcomes include infertility, histopathological changes, and hydrosalpinx formation (10, 13, 14, 27). The latter has been accepted as a surrogate marker of chronic chlamydial disease and infertility (40). There are distinct strain differences with regard to susceptibility to chronic chlamydial disease in mice. In general, C3H/HeN (H-2k) mice are classified as susceptible, BALB/c (H-2d) mice as intermediately susceptible, and C57BL/6 (H-2b) as resistant (4, 10, 14). Susceptibility to disease appears to be related to undefined innate immune reactivity because athymic nude mice as well as β2-microglobulin, MHC class II, and IFN-γ gene KO mice all develop hydrosalpinx subsequent to infection (8, 9, 24, 33). We have recently reported a role for iNOS in protection from chronic chlamydial disease in mice (32). In addition, Darville et al. have shown a positive correlation between the production of tumor necrosis factor alpha early in infection and resistance to disease (11). In contrast, increased and sustained macrophage inflammatory protein 2 production and the corresponding neutrophil influx were associated with susceptibility to disease (12).
In the present study, we sought to determine the role of free radicals derived from phagocyte oxidase and iNOS in protective and pathological immune responses in the mouse model of chlamydial urogenital infection. We compared the in vivo capacity to generate iNOS-derived RNS in resistant and susceptible strains of mice and used mice with specific deletions in the genes encoding iNOS (NOS2−/−) and in a key component of phagocyte NADPH oxidase (p47phox−/−). We also assessed infection outcome and chronic disease development in a chemically induced double knockout by inhibiting RNS production with the l-arginine analogue NG-monomethly-l-arginine (L-NMMA) in p47phox−/− mice.
MATERIALS AND METHODS
Mice.
Mice with a targeted disruption in the iNOS gene (NOS2−/−) were obtained under a materials transfer agreement with John Mudgett (Merck & Co., Rahway, N.J.), and a colony was initiated at the Midwestern University Animal Resource Facility. The KO in these mice was confirmed, and all mice were housed as described elsewhere (31, 32). Mice with a targeted deletion in the cytosolic p47(phox) gene, which is essential for effective superoxide production by the NADPH oxidase (p47phox−/−), were obtained from a colony at National Institutes of Allergy and Infectious Diseases (19). Wild-type C57BL/6 and C3H/HeN mice (NOS2+/+) were purchased from Jackson Laboratories (Bar Harbor, Maine). In some experiments, a parallel set of mice was housed in rodent metabolism cages in order to collect urine for nitrite determination as described below. A note on gene KO mouse nomenclature in the text: all −/− symbols denote a homozygous KO of a targeted gene; +/+ refers to a homozygous gene-intact mouse; and ± refers to a mouse that is heterozygous for the targeted gene.
Chlamydiae.
Chlamydia trachomatis mouse pneumonitis (MoPn) strain (Weiss) was grown in HeLa 229 cells and maintained as previously described (5) with minor modifications (18).
Infection.
For primary infection, mice were pretreated with DepoProvera (P4) (Upjohn, Kalamazoo, Mich.) and inoculated intravaginally with 200 50% infective doses of C. trachomatis MoPn, exactly as described elsewhere (6, 7). Shedding of viable MoPn chlamydiae from the lower urogenital tract was assessed by the collection of cervical-vaginal swabs and subsequent culturing of swab-collected material in HeLa 229 cell monolayers (8). Inclusion-forming units (IFU) were enumerated by indirect fluorescent microscopy (8). To assess upper genital tract infection by culture, at sacrifice uterine horns and oviducts were excised and frozen at −80°C for later processing. After thawing, tissues were homogenized, sonicated, and cleared of large debris by low-speed centrifugation (10 min at 500 × g at 4°C). Diluted supernatants of homogenates were plated on HeLa 229 monolayers in 24-well plates as described elsewhere (8), and culturing was done as described above for swab samples.
Assessment of opportunistic infections in p47phox−/− mice.
Four to five weeks following initiation of treatment with L-NMMA, some p47phox−/− mice became moribund or showed obvious signs of localized inflammation (e.g., erythema and edema). These animals were sacrificed and necropsied, and organs were removed and processed for chlamydial isolation as described above. Aliquots of homogenates were also streaked on blood agar plates prior to freezing and incubated at 36°C in a humidified atmosphere containing 5% CO2. Isolated pathogens were broadly categorized by colony morphology and/or Gram stain as yeast, gram-positive cocci, actinomycetales, or gram-negative rods.
Assessment of pathological outcome.
Necropsy was performed with most animals at day 56 postinfection unless mice were sacrificed earlier due to becoming moribund as a result of opportunistic infections. At necropsy, hydrosalpinx formation (a surrogate marker of infertility) was assessed by gross macroscopic or 10× microscopic observation as previously described (32, 40). Organ changes suggestive of disseminated chlamydial or opportunistic infection (iliac lymph node adenopathy, splenomegaly, visceral adhesions, or lung and uterus disease) were noted.
Assessment and inhibition iNOS-derived RNS.
RNS production in vivo in response to the infection was assessed as described elsewhere (16). Briefly, mice were housed, three or four per cage, in Plexiglas metabolism cages (Nalgene, Rochester, N.Y.) beginning 6 days prior to infection and fed nitrate- and nitrite-free amino acid rodent chow (Ziegler Brothers, Gardners, Pa.) and Millipore water ad libitum. We have found that a minimum of 5 days on a nitrate- and nitrite-free diet is needed to allow urinary clearance of dietary nitrates and nitrites from mice. This can be observed in Fig. 1 (see also Fig. 3) as the relatively high levels of urine nitrite 5 to 7 days prior to infection which declines markedly after several days on a nitrate- and nitrite-free diet. Prior experimentation has determined that urine nitrite remains low indefinitely on a nitrate-free, nitrite-free diet in the absence of infection or other stimuli. Hence, increases in urine nitrite following infection on day zero are taken as an infection response. These observations have also been described in detail by others (16). In some experiments, groups received either 50 mM L-NMMA (an iNOS inhibitor; CYCLOPPS Corp., Salt Lake City, Utah) or 50 mM l-arginine (as a control) in their drinking water. Food and water was changed and replenished daily, and urine was collected daily in receptacles containing isopropanol (approximately 1:5 isopropanol/urine). Following centrifugation to clear debris, urine was stored at −20°C until nitrite concentrations could be assessed.
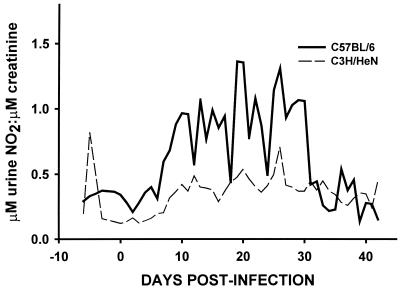
FIG. 1.
Urine nitrite excretion in response to C. trachomatis MoPn urogenital infection in disease-susceptible C3H/HeN mice and resistant C57BL/6 mice. Urine was collected daily from 6 days prior to infection until day 42 postinfection. Nitrite levels were assessed by the Greiss reaction and standardized according to urine creatinine concentration. The solid line represents the response for C57BL/6 mice. The dashed line represents that for C3H/HeN mice. Following clearance of dietary nitrates and nitrites (day −7 to day zero), a significantly higher level of excretion of nitrite was observed with C57BL/6 mice when the main effects of strain and time were compared (P = 0.0006; Kruskal-Wallis one-way ANOVA on ranks).
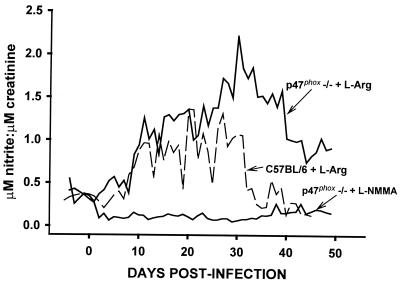
FIG. 3.
Urine nitrite excretion in response to C. trachomatis MoPn urogenital infection in phagocyte oxidase deficient mice. Urine was collected daily from 6 days prior to infection until day 55 postinfection. Nitrite levels were assessed by the Greiss reaction and standardized according to urine creatinine concentration. The solid line represents the response for p47phox−/− mice treated with either 50 mM L-NMMA (as labeled) or 50 mM l-arginine (labeled p47phox−/−) given in their drinking water. The dashed line represents that of C57BL/6 mice similarly treated with 50 mM l-arginine. Significantly elevated excretion of nitrite was observed with the l-arginine treated p47phox−/− mice compared to similarly treated C57BL/6 mice (P < 0.00001; Kruskal-Wallis one-way ANOVA on ranks). This response was significantly blunted by treatment with L-NMMA compared to that of the C57BL/6 or p47phox−/− controls (P = 0.00001; Kruskal-Wallis one-way ANOVA on ranks).
Urine samples were batch assessed for nitrite content, an indicator of in vivo iNOS activity, by adaptation of the Greiss reaction (38). Stored urine was thawed, and supernatants were diluted 1:5 or 1:10 in Dulbecco's minimal essential media without phenol red (GIBCO, Long Island, N.Y.). Aliquots were incubated with standardized amounts of Pseudomonas oleovarans (American Type Culture Collection, Manassas, Va.) (ATCC no. 8062) to reduce nitrate to nitrite. Diluted samples were then assessed for nitrite concentration by the Greiss reaction and standardized according to creatinine content using a manual picric acid method (Sigma Aldrich, St. Louis, Mo.).
Statistics.
Infection course was analyzed by a two-factor (treatment group and days) repeated measures analysis of variance (ANOVA) and posthoc analysis was completed with a Tukey-Kramer test. Rates of hydrosalpinx formation were compared by Fisher's exact test. The mean urine nitrite elevation over time in response to infection was compared using the Kruskal-Wallis one-way ANOVA on ranks.
RESULTS
Disease-resistant mouse strains sustain higher levels of in vivo iNOS activity than susceptible strains. Though an essential role for iNOS-derived RNS in resolution of chlamydial infection has been ruled out (28, 31), a role for RNS in attenuating pathological changes in the murine upper genital tract subsequent to infection has been established (28, 32). To further elaborate on this observation, we assessed RNS production in vivo in two mouse strains that have been characterized as to their disparate susceptibilities to chlamydial infection, subsequent chronic disease, or lethality in an intraperitoneal infection model (4, 10, 14). C57BL/6 is a resistant strain, whereas C3H/HeN is a susceptible strain. A typical infection was observed as has been previously described (7) and as depicted for C57BL/6 in Fig. 2. This included confirmation of a slightly protracted infection course in the C3H/HeN when compared to the C57BL/6 (data not shown). In general, 104 to 106 IFU are isolated through day 14, and the infection declines thereafter, with most animals resolving infection by 35 days postinfection. However, as can be seen in Fig. 1, the resistant C57BL/6 mice respond to infection by producing significantly greater amounts of urine nitrite, a measure of in vivo iNOS-derived RNS production, than the susceptible strains. These data indicate that a positive correlation exists between iNOS activity and innate strain resistance to chronic chlamydial disease.
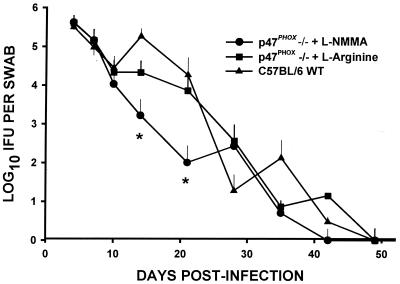
FIG. 2.
Course of infection as assessed by quantitation of viable C. trachomatis chlamydia shed from the urogenital tract. C. trachomatis MoPn was isolated from cervical-vaginal swabs collected at 4, 7, 10, and 14 days and every 7 days thereafter through day 56. Each data point represents the mean and standard deviation of IFU enumerated in HeLa 229 cultures of swabs from culture-positive mice. Overall, a significantly lower IFU count was observed when the main effects of treatment group and time were compared (P = <0.0001, two-factor ANOVA). The asterisks designate significant differences in the quantitative recovery of viable MoPn chlamydia at the indicated time points postinfection (two-tailed t test).
Mice resolve urogenital chlamydial infection independent of phagocyte oxidase-derived oxygen free radicals.
It has previously been demonstrated that mice resolve chlamydial infection in the absence of iNOS. This was proved with both NOS2−/− (28, 31, 32) and WT C57BL/6 mice treated with the iNOS inhibitor, L-NMMA (31). To further explore the role of free radicals in immune protection in this model, NOS2−/−, p47phox−/−, or WT C57BL/6 control mice were infected as before. To induce a chemical double KO, beginning 1 to 2 days prior to infection, a group of p47phox−/− mice was given a 50 mM dose of the iNOS inhibitor L-NMMA in their drinking water while parallel control groups of either p47phox−/− or WT C57BL/6 mice received 50 mM l-arginine. Urine nitrite excretion was monitored in all groups except the NOS2−/−. Chlamydial shedding was monitored in all mice by sequential collection of cervical-vaginal swabs and subsequent isolation in cell culture.
Figure 2 shows the course of infection for various mice as measured by the number of IFU isolated from cervical-vaginal swabs over time. Table 1 depicts the infection course in the same mice as a ratio of culture-positive mice to the total number in each group over time. Although all groups described above were assessed, the NOS2−/− infection course data are not shown here because identical results have been published elsewhere (28, 31, 32). Despite genetic deletion of phagocyte oxidase-derived oxygen free radicals, p47phox−/− mice were able to resolve their infections in a time frame similar to that for the WT and NOS2−/− mice. Interestingly, p47phox−/− L-NMMA mice also resolved their infections but did so more rapidly than the p47phox−/−, NOS2−/−, and WT C57BL/6 controls. This was evidenced by comparing the ratio of animals resolving infection to those mice remaining culture positive (Table 1, day 28) and by comparing the number of IFU isolated in the culture-positive animals over the whole time course of the infection (Fig. 2).
TABLE 1.
Infection course as a function of the ratio of number positive to total number infected
| Group | No. positive/total no. infected on day postinfectiond
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 4 | 7 | 10 | 14 | 21 | 28 | 35 | 42 | 49 | |
| p47phox−/− + L-NMMAa | 13/13 | 13/13 | 12/13 | 12/13 | 6/13 | 1/13e | 2/13 | 0/4 | 0/4 |
| p47phox−/− + l-arginineb | 13/13 | 13/13 | 13/13 | 12/13 | 10/12 | 6/11 | 4/11 | 1/11 | 0/11 |
| WT C57BL/6 + l-argininec | 10/10 | 10/10 | 10/10 | 10/10 | 8/10 | 8/10 | 4/10 | 1/10 | 0/10 |
p47phox−/− mice treated with 50 mM L-NMMA in drinking water.
p47phox−/− mice treated with 50 mM l-arginine in drinking water.
Wild-type C57BL/6 mice treated with 50 mM l-arginine in drinking water.
Numbers represent numbers of animals culture-positive for C. trachomatis by genital swab of the total number tested.
Statistical significance was achieved when L-NMMA-treated p47phox−/− mice were compared to either l-arginine-treated p47phox−/− mice (P = 0.0007) or l-arginine-treated C57BL/6 mice (P = 0.023) by Fisher's exact test.
By days 38 to 42, several mice in the group of p47phox−/− mice treated with 50 mM L-NMMA became cachectic or appeared moribund. These mice were sacrificed and necropsied. In some cases, they showed obvious signs (erythema or edema) of localized infections or inflammatory reactions including involvement of the footpads and knee joints. Upon necropsy, hepatosplenomegaly and signs of multiple organ system involvement were observed. This included discoloration and focal necrotic lesions in the liver, spleen, and lungs. Culture and/or Gram stain of exudates and homogenates of various tissues confirmed disseminated opportunistic infections with staphylococci, actinomycetales, yeast, and gram-negative lactose-nonfermenting rods in many but not all of these animals. However, viable disseminated chlamydiae were only isolated in low numbers from the lung (one mouse, day 42) and the upper genital tract (two mice, days 38 and 39). These findings indicate that while deletion and inhibition of two major free radical-generating mechanisms left these mice susceptible to opportunistic pathogens, they did not alter local resolving chlamydia-specific immune responses or lead to significant disseminated chlamydial infection as has been observed with other immunosuppressed mice (8, 29).
To confirm the effectiveness of the chemical iNOS inhibition, parallel groups of mice were housed in metabolism cages for daily urine collection and monitoring of nitrite levels as a measure of in vivo RNS production. Figure 3 verifies the effectiveness of the administration of L-NMMA to p47phox−/− mice and demonstrates that the treatment abrogated RNS elevation in response to the infection. An additional finding of interest was that p47phox−/− control mice had significantly elevated and protracted RNS production in response to chlamydial infection compared to WT controls throughout the 55-day monitoring period. From this we conclude that a disinhibition of reactive nitrogen free radical production occurs in the absence of phagocyte oxidase-derived oxygen free radicals.
A role for RNS and ROS interaction in the development of chronic chlamydial disease.
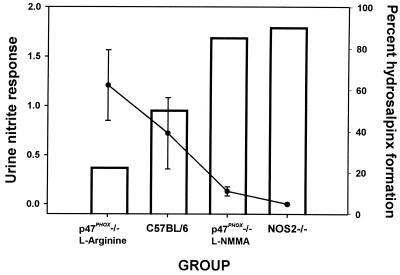
On day 56 postinfection, all remaining mice were sacrificed and necropsied. Table 2 summarizes the rates of hydrosalpinx formation in each group. These data also include eight mice in the group of p47phox−/− mice treated with 50 mM L-NMMA that were sacrificed prior to day 56 (38 to 42 days postinfection) due to opportunistic infections as described above. We have observed that when present, hydrosalpinx develops between 21 and 35 days postinfection in WT mice. Confirming our previous reports, Table 2 shows that progesterone-pretreated WT C57BL/6 mice sustained a 50% rate of hydrosalpinx formation (32). We have also observed that the rate of hydrosalpinx formation in C57BL/6 mice is lower in the absence of progesterone pretreatment (data not shown). Though this observation remains unexplained, it may be due to progesterone-mediated alteration of host response factors in this mouse or increases in the chlamydial burden within the host. Also confirming previous observations, the incidence of hydrosalpinx formation increased significantly to 90% with NOS2−/− mice. Interestingly, the rate of hydrosalpinx formation declined significantly to 22.2% with the p47phox−/− control mice that were treated with l-arginine. This trend was reversed to near the rate observed with NOS2−/− mice when iNOS was chemically blocked in p47phox−/− mice. Data summarizing Table 2 and Fig. 3 are depicted in Fig. 4. These data show a negative correlation between mean daily urine nitrite production (days 7 through 54 postinfection) and hydrosalpinx formation. We conclude from these observations that iNOS-derived RNS give protection from hydrosalpinx formation, and though not critical, reactive oxygen species (ROS) may be at least partially responsible for its induction.
TABLE 2.
Gross pathological outcome subsequent to infection
| Group | Status | Hydrosalpinx formation (%)a | P value(s)b |
|---|---|---|---|
| WT (C57BL/6) | Infected | 14/28 (50.0) | <0.0001c |
| Uninfected | 0/20 (0.0) | ||
| NOS2−/− | Infected | 18/20 (90) | 0.005d |
| Uninfected | 0/20 (0.0) | ||
| p47phox−/− | Infected | 8/36 (22.2) | 0.041c, 0.033d |
| Uninfected | 0/20 (0.0) | ||
| p47phox−/− + L-NMMA | Infected | 17/20 (85) | 0.016d, <0.0001e |
| Uninfected | NDe |
Represents the total number of hydrosalpinges formed of the total number of oviducts assessed.
P values were determined using Fisher's exact test.
Compared to uninfected mice in the same group.
Compared to WT infected C57BL/6 mice.
Compared to p47phox−/− controls.
ND, not done.
FIG. 4.
Protection from chronic chlamydial disease correlates with higher in vivo iNOS activity. The mean urine nitrite response (day 7 through day 54 postinfection) was calculated for each experimental group from the data displayed in Fig. 3 and is shown here as the solid line graph. The percent hydrosalpinx formation for each group from Table 2 is displayed as the open bars. A negative correlation exists between the mean nitrite excretion during infection and hydrosalpinx formation (correlation coefficient = −0.99952).
DISCUSSION
Our present data indicate that while neither phagocyte oxidase-derived ROS nor iNOS-derived RNS are essential for resolution of infection in this model (Table 1 and Fig. 2), iNOS-derived RNS protect mice from development of chronic disease as assessed by hydrosalpinx formation (Table 2 and Fig. 4). These data support and extend previous findings with NOS2−/− mice and WT mice receiving chemical inhibitors of iNOS-derived RNS (28, 31, 32). In the previous studies, mice deficient in iNOS resolved culture-apparent infection but developed exacerbated disease outcomes as assessed by hydrosalpinx formation and infertility. These observations were concurrent with persistent detection of chlamydial DNA subsequent to resolution of infection in both NOS2−/− mice and WT mice. However, reactivated shedding of viable chlamydiae upon immunosuppression was observed with NOS2−/− mice only (32). While we did not attempt to establish a link to persisting chlamydiae and chronic disease in the present study, this possibility cannot be ruled out and remains under active investigation.
Our observation of opportunistic infections in p47phox−/− mice dually inhibited in production of RNS supports the findings of Shiloh et al., who used mice with a genetic double KO in iNOS and phagocyte oxidase (37). This indicates a compensatory influence of one free radical-generating system in the absence of the other for host defense against indigenous flora. Also similar to our results, Shiloh et al. observed killing of frank (or obligate) pathogens in macrophages derived from the double-KO mice, albeit at a lower rate than that for controls. Thus, this indicates a third undefined antibacterial activity for the killing of frank pathogens. A finding that appears to be unique to chlamydial infection, however, was that of a shortened infection course in L-NMMA-treated p47phox−/− mice (Table 1 and Fig. 2). Abbreviated infections in the absence of iNOS have also been reported (28). The reason for these observations remains unexplained. However, nitrogen free radicals have been shown to have an adverse effect on T-lymphocyte responses in other model systems (1, 21, 25, 42), and T lymphocytes are critical to resolution of infection in this model (34). It is also conceivable that the presence of higher numbers of opportunistic pathogens competing for nutrients in the doubly compromised mice reduced the capacity of chlamydia to maintain an identifiable presence, although other work suggests that competition for nutrients would to lead to chlamydial persistence rather than irradication (2).
While we have concluded that the two major free radical-generating systems are not essential to resolution of infection, a role for RNS in protection from chronic chlamydial disease outcomes can now be theorized. These conclusions are supported by several observations. First, we have shown a positive correlation between RNS production in vivo in response to infection and innate resistance to hydrosalpinx formation (Fig. 1 and 4). Second, in the absence of phagocyte oxidase activity, RNS production was enhanced and the subsequent pathological outcome was mollified to levels lower than those observed in WT mice (Fig. 3 and Table 2, respectively). Finally, when an additional block in RNS production was implemented in the absence of phagocyte oxidase, resistance to hydrosalpinx formation was reversed to mirror results observed with NOS2−/− mice (Table 2). From these observations, we conclude that oxygen and nitrogen free radicals play disparate roles in the development of chronic chlamydial disease. Reactive nitrogen intermediates protect from chronic disease, whereas pathological damage may occur, at least partially, through reactive oxygen-dependent mechanisms. It should be pointed out, however, that ROS generated through the phagocyte oxidase pathway cannot fully account for induction of pathological immune responses to the same degree that RNS serve to mollify them, because in the absence of both systems, a high rate of hydrosalpinx still occurs.
While the exact mechanisms of disease protection afforded by RNS in this model remain enigmatic, it is rewarding to know that similar roles for free radicals have been reported using different models of inflammatory diseases (42). The development of several inflammatory diseases of both infectious and noninfectious etiology have been attributed to ROS and RNS individually (as reviewed in references 22 and 41). The effects of these free radicals on host tissues are multifactoral and include, but are not limited to, enhancement of inflammation through the upregulation of leukocyte adhesion molecules, disruption of normal cellular metabolism, membrane lipid peroxidation, and damage to nucleic acids (as reviewed in reference 41). When they are considered with the work of Darville et al. (12) that showed an early and prolonged neutrophil influx and associated cytokine production with susceptibility to disease, a plausible hypothesis for our present results could be formed. This would include excessive or protracted inflammatory responses, oxidative tissue damage, and the ensuing chronic disease in the host repair phase in susceptible mice. An increase in RNS production for a resistant strain over that for the susceptible strain (Fig. 1) could protect from chronic disease development by mollifying the effect of ROS. Indeed, interaction between the two free radical-generating systems seems to provide counterregulatory effects. For example, nitric oxide reacts rapidly with oxygen free radicals to form peroxynitrite and thus modulate the effects of ROS on several cellular systems (41). Our data showing heightened iNOS activity in the absence of ROS for the p47phox−/− mice support a regulatory role for ROS in controlling the protective activity of iNOS or its product in the murine model. Additionally, ROS and RNS may have opposing and dose-dependent effects on chlamydia-induced fibrosis through the activation and inhibition of matrix metalloproteinases, which are responsible for extracellular matrix modification and the induction of scarring responses. In general, ROS tend to activate latent matrix metalloproteinases, while RNS may bind the zinc in the their active site, thus inactivating this class of enzymes (3, 15, 26, 30). Taken together, our results provide support for the hypothesis that the overall balance between superoxide and nitric oxide generation is critical in development of disease subsequent to chlamydial infection in the mouse model.
ACKNOWLEDGMENTS
We thank Ferric C. Fang for suggesting the use of the p47phox−/− mouse rendered deficient in RNS by L-NMMA in our system.
This work was supported by Public Health Service grants AI37807 (to K.H.R.) and AI19782 (to G.I.B.).
REFERENCES
- 1.Allione A, Bernabei P, Bosticardo M, Ariotti S, Forni G, Novelli F. Nitric oxide suppresses human T lymphocyte proliferation through IFN-γ-dependent and IFN-γ-independent induction of apoptosis. J Immunol. 1999;163:4182–4191. [PubMed] [Google Scholar]
- 2.Beatty W L, Morrison R P, Byrne G I. Persistent Chlamydiae: from cell culture to a paradigm for chlamydial pathogenesis. Microbiol Rev. 1994;58:686–699. doi: 10.1128/mr.58.4.686-699.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burkhardt H, Hartmann F, Schwingel M L. Activation of latent collagenase from polymorphonuclear leukocytes by oxygen radicals. Enzyme. 1986;36:221–231. doi: 10.1159/000469298. [DOI] [PubMed] [Google Scholar]
- 4.Byrne G I, Padilla M, Lacey D, Paulnock D, Xiu L G. Mouse model for protective immunity to chlamydia. In: Bowie W R, Caldwell H D, Jones R P, Mardh P A, Ridgway G L, Schachter J, Stamm W E, Ward M E, editors. Chlamydial infections: proceedings of the Seventh International Symposium on Human Chlamydial Infections. Cambridge, United Kingdom: Cambridge University Press; 1990. pp. 236–240. [Google Scholar]
- 5.Caldwell H D, Kromhout J, Schachter J. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect Immun. 1981;31:1161–1176. doi: 10.1128/iai.31.3.1161-1176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cotter T W, Meng Q, Shen Z L, Zhang Y X, Su H, Caldwell H D. Protective efficacy of major outer membrane protein-specific immunoglobulin A (IgA) and IgG monoclonal antibodies in a murine model of Chlamydia trachomatis genital tract infection. Infect Immun. 1995;63:4704–4714. doi: 10.1128/iai.63.12.4704-4714.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cotter T W, Miranpuri G S, Ramsey K H, Poulsen C E, Byrne G I. Reactivation of chlamydial genital tract infection in mice. Infect Immun. 1997;65:2067–2073. doi: 10.1128/iai.65.6.2067-2073.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cotter T W, Ramsey K H, Miranpuri G S, Poulsen C E, Byrne G I. Dissemination of Chlamydia trachomatis chronic genital tract infection in gamma interferon gene knockout mice. Infect Immun. 1997;65:2145–2152. doi: 10.1128/iai.65.6.2145-2152.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Darville T, Andrews C W, Kishen L R, Rank R G, Williams D M. Transforming growth factor-β is associated with increased pathology in γ-interferon gene knockout mice infected with Chlamydiae. In: Stephens R S, Byrne G I, Christiansen G, Clarke I N, Grayston J T, Rank R G, Saikku P, Schachter J, Stamm W E, editors. Chlamydial infections. Proceedings of the Ninth International Symposium on Human Chlamydial Infections, San Francisco, Calif. 1998. pp. 407–410. [Google Scholar]
- 10.Darville T, Andrews C W, Laffoon K K, Shymasani W, Kishen L R, Rank R G. Mouse strain-dependent variation in the course and outcome of chlamydial genital tract infection is associated with differences in host response. Infect Immun. 1997;65:3065–3073. doi: 10.1128/iai.65.8.3065-3073.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Darville T, Andrews C W, Jr, Rank R G. Does inhibition of tumor necrosis factor alpha affect chlamydial genital tract infection in mice and guinea pigs? Infect Immun. 2000;68:5299–5305. doi: 10.1128/iai.68.9.5299-5305.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Darville T, Andrews C W, Jr, Sikes J D, Fraley P L, Rank R G. Early local cytokine profiles in strains of mice with different outcomes from chlamydial genital tract infection. Infect Immun. 2001;69:3556–3561. doi: 10.1128/IAI.69.6.3556-3561.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Darville T, Laffoon K K, Kishen L R, Rank R G. Tumor necrosis factor-α activity in genital tract secretions of guinea pigs infected with chlamydiae. Infect Immun. 1995;63:4675–4681. doi: 10.1128/iai.63.12.4675-4681.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De La Maza L M, Pal S, Khamesipour A, Peterson E M. Intravaginal inoculation of mice with the Chlamydia trachomatis mouse pneumonitis biovar results in infertility. Infect Immun. 1994;62:2094–2097. doi: 10.1128/iai.62.5.2094-2097.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eberhardt W, Huwiler A, Beck K F, Walpen S, Pfeilschifter J. Amplification of IL-1 beta-induced matrix metalloproteinase-9 expression by superoxide in rat glomerular mesangial cells is mediated by increased activities of NF-kappa B and activating protein-1 and involves activation of the mitogen-activated protein kinase pathways. J Immunol. 2000;165:5788–5797. doi: 10.4049/jimmunol.165.10.5788. [DOI] [PubMed] [Google Scholar]
- 16.Granger D L, Hibbs J B, Broadnax L M. Urinary nitrate excretion in relation to murine macrophage activation: influence of dietary L-arginine and oral NG-monomethyl-L-arginine. J Immunol. 1991;146:1294–1302. [PubMed] [Google Scholar]
- 17.Igietseme J U. The molecular mechanism of T-cell control of Chlamydia in mice: role of nitric oxide. Immunology. 1996;87:1–8. [PMC free article] [PubMed] [Google Scholar]
- 18.Ingalls R R, Rice P A, Qureshi N, Takayama K, Lin J S, Golenbock D T. The inflammatory cytokine response to Chlamydia trachomatis infection is endotoxin mediated. Infect Immun. 1995;63:3125–3130. doi: 10.1128/iai.63.8.3125-3130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jackson S H, Gallin J I, Holland S M. The p47phox mouse knock-out model of chronic granulomatous disease. J Exp Med. 1995;182:751–758. doi: 10.1084/jem.182.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mayer J, Woods M L, Vavrin Z, Hibbs J B., Jr Gamma IFN-induced nitric oxide production reduces Chlamydia trachomatis infectivity in McCoy cells. Infect Immun. 1993;61:491–498. doi: 10.1128/iai.61.2.491-497.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Millar A E, Sternberg J, McSharry C, Wei X Q, Liew F Y, Turner C M. T-cell responses during Trypanosoma brucei infections in mice deficient in inducible nitric oxide synthase. Infect Immun. 1999;67:3334–3338. doi: 10.1128/iai.67.7.3334-3338.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller R A, Britigan B E. Role of oxidants in microbial pathophysiology. Clin Microbiol Rev. 1997;10:1–18. doi: 10.1128/cmr.10.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morrison R P. Immune responses to Chlamydia are protective and pathogenic. In: Bowie W R, Caldwell H D, Jones R P, Mardh P A, Ridgway G L, Schachter J, Stamm W E, Ward M E, editors. Chlamdyial infections—proceedings of the Seventh International Symposium on Human Chlamydial Infections. Cambridge, United Kingdom: Cambridge University Press; 1990. pp. 163–172. [Google Scholar]
- 24.Morrison R P, Feilzer K, Tumas D B. Gene knockout mice establish a primary protective role for major histocompatibility complex class II-restricted responses in Chlamydia trachomatis genital tract infection. Infect Immun. 1995;63:4661–4668. doi: 10.1128/iai.63.12.4661-4668.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nabeshima S, Nomoto M, Matsuzaki G, Kishihara K, Taniguchi H, Yoshida S, Nomoto K. T-cell hyporesponsiveness induced by activated macrophages through nitric oxide production in mice infected with Mycobacterium tuberculosis. Infect Immun. 1999;67:3221–3226. doi: 10.1128/iai.67.7.3221-3226.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Owens M W, Milligan S A, Jourd'heuil D, Grisham M B. Effects of reactive metabolites of oxygen and nitrogen on gelatinase A activity. Am J Physiol. 1997;273:L445–L450. doi: 10.1152/ajplung.1997.273.2.L445. [DOI] [PubMed] [Google Scholar]
- 27.Pal S, Peterson E M, De La Maza L M. Intranasal immunization induces long-term protection in mice against a Chlamydia trachomatis genital challenge. Infect Immun. 1996;64:5341–5348. doi: 10.1128/iai.64.12.5341-5348.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perry L L, Feilzer K, Caldwell H D. Neither interleukin-6 nor inducible nitric oxide synthase is required for clearance of Chlamydia trachomatis from the murine genital tract epithelium. Infect Immun. 1998;66:1265–1269. doi: 10.1128/iai.66.3.1265-1269.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perry L L, Feilzer K, Caldwell H D. Immunity to Chlamydia trachomatis is mediated by T helper 1 cells through IFN-γ-dependent and -independent pathways. J Immunol. 1999;158:3344–3352. [PubMed] [Google Scholar]
- 30.Rajagopalan S, Meng X P, Ramasamy S, Harrison D G, Galis Z S. Reactive oxygen species produced by macrophage-derived foam cells regulate the activity of vascular matrix metalloproteinases in vitro. Implications for atherosclerotic plaque stability. J Clin Investig. 1996;98:2572–2579. doi: 10.1172/JCI119076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramsey K H, Miranpuri G S, Poulsen C E, Marthakis N B, Braune L M, Byrne G I. Inducible nitric oxide synthase does not affect resolution of murine chlamydial genital tract infections or eradication of chlamydiae in primary murine cell culture. Infect Immun. 1998;66:835–838. doi: 10.1128/iai.66.2.835-838.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramsey K H, Miranpuri G S, Sigar I M, Ouellette S, Byrne G I. Chlamydia trachomatis persistence in the female mouse genital tract: inducible nitric oxide synthase and infection outcome. Infect Immun. 2001;69:5131–5137. doi: 10.1128/IAI.69.8.5131-5137.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramsey K H, Rank R G. Resolution of chlamydial genital infection with antigen-specific T-lymphocyte lines. Infect Immun. 1991;59:925–931. doi: 10.1128/iai.59.3.925-931.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rank R G. Models of immunity. In: Stephens R S, editor. Chlamydia: intracellular biology, pathogenesis, and immunity. Washington, D.C.: American Society for Microbiology; 1999. pp. 239–295. [Google Scholar]
- 35.Rank R G, Soderberg L S F, Barron A L. Chronic chlamydial genital infection in congenitally athymic nude mice. Infect Immun. 1985;48:847–849. doi: 10.1128/iai.48.3.847-849.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raulston J E. Response of Chlamydia trachomatis serovar E to iron restriction in vitro and evidence for iron-regulated chlamydial proteins. Infect Immun. 1997;65:4539–4547. doi: 10.1128/iai.65.11.4539-4547.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shiloh M U, MacMicking J D, Nicholson S, Brause J E, Potter S, Marino M, Fang F, Dinauer M, Nathan C. Phenotype of mice and macrophages deficient in both phagocyte oxidase and inducible nitric oxide synthase. Immunity. 1999;10:29–38. doi: 10.1016/s1074-7613(00)80004-7. [DOI] [PubMed] [Google Scholar]
- 38.Stuehr D J, Marletta M A. Mammalian nitrate biosynthesis: mouse macrophages produce nitrite and nitrate in response to Escherichia coli lipopolysaccharide. Proc Natl Acad Sci USA. 1985;82:7738–7742. doi: 10.1073/pnas.82.22.7738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Su H, Caldwell H D. CD4+ T cells play a significant role in adoptive immunity to Chlamydia trachomatis infection of the mouse genital tract. Infect Immun. 1995;63:3302–3308. doi: 10.1128/iai.63.9.3302-3308.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Su H, Messer R, Whitmire W, Fischer E, Portis J C, Caldwell H D. Vaccination against chlamydial genital tract infection after immunization with dendritic cells pulsed ex vivo with nonviable Chlamydiae. J Exp Med. 1998;188:809–818. doi: 10.1084/jem.188.5.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Usmar-Darley V, Wiseman H, Halliwell B. Nitric oxide and oxygen radicals: a question of balance. Fed Eur Biochem Soc Lett. 1995;369:131–135. doi: 10.1016/0014-5793(95)00764-z. [DOI] [PubMed] [Google Scholar]
- 42.van der Veen R C, Dietlin T A, Hofman F M, Pen L, Segal B H, Holland S M. Superoxide prevents nitric oxide-mediated suppression of helper T lymphocytes: decreased autoimmune encephalomyelitis in nicotinamide adenine dinucleotide phosphate oxidase knockout mice. J Immunol. 2000;164:5177–5183. doi: 10.4049/jimmunol.164.10.5177. [DOI] [PubMed] [Google Scholar]
- 43.Yasin B, Harwig S S L, Lehrer R I, Wagar E A. Susceptibility of Chlamydia trachomatis to protegrins and defensins. Infect Immun. 1996;64:709–713. doi: 10.1128/iai.64.3.709-713.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]