Abstract
IMPORTANCE
RCC encompasses a set of histologically distinct cancers with a high estimated genetic heritability, of which only a portion is currently explained. Previous rare germline variant studies in RCC have usually pooled clear and non-clear cell RCCs and have not adequately accounted for population stratification that may significantly impact the interpretation and discovery of certain candidate risk genes.
OBJECTIVE
To evaluate the enrichment of germline PVs in established cancer-predisposing genes (CPGs) in clear cell and non-clear cell RCC patients compared to cancer-free controls using approaches that account for population stratification and to identify unconventional types of germline RCC risk variants that confer an increased risk of developing RCC.
DESIGN, SETTING, AND PARTICIPANTS
In 1,436 unselected RCC patients with sufficient data quality, we systematically identified rare germline PVs, cryptic splice variants, and copy number variants (CNVs). From this unselected cohort, 1,356 patients were ancestry-matched with 16,512 cancer-free controls, and gene-level enrichment of rare germline PVs were assessed in 143 CPGs, followed by an investigation of somatic events in matching tumor samples.
MAIN OUTCOMES AND MEASURES
Gene-level burden of rare germline PVs, identification of secondary somatic events accompanying the germline PVs, and characterization of less-explored types of rare germline PVs in RCC patients.
RESULTS
In clear cell RCC (n = 976 patients), patients exhibited significantly higher prevalence of PVs in VHL compared to controls (OR: 39.1, 95% CI: 7.01–218.07, p-value:4.95e-05, q-value:0.00584). In non-clear cell RCC (n = 380 patients), patients carried enriched burden of PVs in FH (OR: 77.9, 95% CI: 18.68–324.97, p-value:1.55e-08, q-value: 1.83e-06) and MET (OR: 1.98e11, 95% CI: 0-inf, p-value: 2.07e-05, q-value: 3.50e-07). In a CHEK2-focused analysis with European cases and controls, clear cell RCC patients (n=906 European patients) harbored nominal enrichment of the previously reported low-penetrance CHEK2 variants, p.Ile157Thr (OR:1.84, 95% CI: 1.00–3.36, p-value:0.049) and p.Ser428Phe (OR:5.20, 95% CI: 1.00–26.40, p-value:0.045) while non-clear cell RCC patients (n=295 European patients) exhibited nominal enrichment of CHEK2 LOF germline PVs (OR: 3.51, 95% CI: 1.10–11.10, p-value: 0.033). RCC patients with germline PVs in FH, MET, and VHL exhibited significantly earlier age of cancer onset compared to patients without any germline PVs in CPGs (Mean: 46.0 vs 60.2 years old, Tukey adjusted p-value < 0.0001), and more than half had secondary somatic events affecting the same gene (n=10/15, 66.7%, 95% CI: 38.7–87.0%). Conversely, patients with rare germline PVs in CHEK2 exhibited a similar age of disease onset to patients without any identified germline PVs in CPGs (Mean: 60.1 vs 60.2 years old, Tukey adjusted p-value: 0.99), and only 30.4% of the patients carried secondary somatic events in CHEK2 (n=7/23, 95% CI: 14.1–53.0%). Finally, rare pathogenic germline cryptic splice variants underexplored in RCC were identified in SDHA and TSC1, and rare pathogenic germline CNVs were found in 18 patients, including CNVs in FH, SDHA, and VHL.
CONCLUSIONS AND RELEVANCE
This systematic analysis supports the existing link between several RCC risk genes and elevated RCC risk manifesting in earlier age of RCC onset. Our analysis calls for caution when assessing the role of germline PVs in CHEK2 due to the burden of founder variants with varying population frequency in different ancestry groups. It also broadens the definition of the RCC germline landscape of pathogenicity to incorporate previously understudied types of germline variants, such as cryptic splice variants and CNVs.
Keywords: Renal cell carcinoma, population stratification, germline pathogenic variants, cryptic splice variant, copy number variant, CHEK2 associated cancer risk
INTRODUCTION
Renal cell carcinoma (RCC) is the ninth most common neoplasm in the United States, accounting for 2% of all cancers worldwide1. The Nordic Twin study has placed the genetic heritability of RCC as high as 38%2, however, only a fraction of the heritability is explained by currently identified rare and common RCC risk loci. Moving beyond known RCC risk genes (eTable 1)3,4, several pan-RCC studies have reported rare germline PVs in DNA damage repair (DDR) genes such as CHEK2, ATM, or BRCA1/2 5–10, suggesting that inherited defects in DDR may contribute to RCC risk. However, most studies lacked ancestry-matched cancer-free controls to formally test these hypotheses.
Two recent studies performed case-control gene-level burden analyses, in RCC alone11 and across cancer types, finding a higher burden of germline PVs in CHEK2 in RCC patients compared to matched controls12. However, these studies pooled all RCC subtypes together as one phenotype for association testing, although clear cell RCC (ccRCC) and non-clear cell RCCs (nccRCC, e.g., papillary, chromophobe) have distinct molecular and clinical features13,14. Furthermore, additional analyses are necessary to account for fine-level population stratification within Europe to mitigate spurious association15, especially when evaluating genes like CHEK2, which is known to harbor many putative PVs that are founder variants from bottlenecked populations (e.g., Ashkenazi Jewish) with highly variable allele frequencies between different European sub-populations.
Here, we first performed a germline variant discovery analysis of 1,436 unselected RCC patients to characterize several types of genomic variation. Next, we performed a case-control association study of ccRCC and nccRCC in a subset (n=1,356) that was successfully ancestry-matched with 16,512 cancer-free controls, and we utilized an ancestry-informed generalized linear model (GLM) to evaluate the major germline drivers of RCC risk. Furthermore, we performed a sub-European ancestry-focused meta-analysis of CHEK2 to address finer-level population stratification within European populations, and we evaluated associated tumor genomic data for concomitant somatic assessments of candidate PVs. Finally, we evaluated potential clinically relevant but underexplored germline variant types (cryptic splice and copy number variants) by using integrative genomic and transcriptomic analyses, all toward expanding and refining the landscape of germline pathogenic variation in RCC.
METHODS
RCC Case Cohort
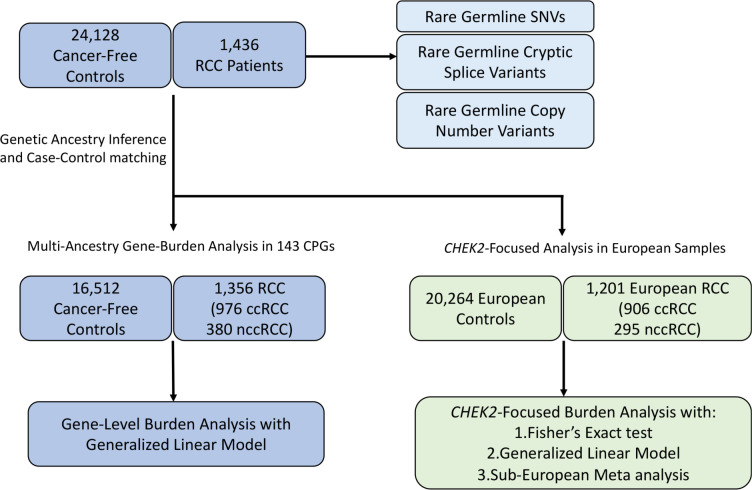
Whole-exome sequencing (WES) Binary Alignment Maps (BAMs) aligned to Genome Research Consortium human build 37 (GRCh37) from 1,436 RCC patients were collected from 8 different RCC studies (Figure 1; Table 1). Samples from TCGA-KIRC16, TCGA-KIRP17, and TCGA-KICH18 studies were analyzed using the germline BAMs stored in the controlled access TCGA workspaces in the Terra analysis platform (https://terra.bio/). Germline BAMs from CheckMate-02519 (NCT01668784, EGAD00001006029), CheckMate-01020 (NCT01354431, EGAS00001004291), CheckMate-00921 (NCT01358721, EGAD00001006027) were downloaded from European Genome-Phenome Archive (EGA) data repository. Whole genome sequencing (WGS) BAMs of ccRCC patients were downloaded from the International Network of Cancer Genome Consortium (ICGC) PanCancer Analysis of Whole Genomes (PCAWG) Dataset22: Renal Cell Cancer – EU/FR (RECA-EU) PCAWG WGS (Dataset ID: EGAD00001002131) and were sliced using a custom exome-target intervals to only include coding regions captured in the WES samples. Data from papillary and chromophobe RCC patients from Genentech were downloaded from EGA (Dataset ID: EGAD0000100102323). All samples underwent identical quality control procedures and were processed using the same analytical methods. This study was approved by the participating institutions where written consent from participants was collected. This study conforms to the Declaration of Helsinki.
Figure 1. Overview of the study.
Rare germline PVs, cryptic splice variants and copy number variants were characterized in 1,436 RCC patients. Gene-level burden analysis was restricted to 1,356 RCC patients ancestry-matched with 16,512 cancer-free controls after genetic ancestry inference and case-control pair-matching (Method). CHEK2 focused analysis was restricted to 1,201 European RCC patients and 20,264 European cancer-free controls.
Table 1.
Patient characteristics of all 1,436 renal cell carcinoma patients
| Renal Cell Carcinoma case cohort (N=1436) | |||
|---|---|---|---|
| n | % | ||
| Age at Diagnosis | 10~19 | 1 | 0.1 |
| 20~29 | 10 | 0.7 | |
| 30~39 | 46 | 3.2 | |
| 40~49 | 191 | 13.3 | |
| 50~59 | 397 | 27.6 | |
| 60~69 | 469 | 32.7 | |
| 70~79 | 249 | 17.3 | |
| 80< | 55 | 3.8 | |
| Age Unknown | 18 | 1.3 | |
| Self-identified Gender | Male | 980 | 68.2 |
| Female | 456 | 31.8 | |
| Histology | Clear Cell RCC | 1031 | 71.8 |
| Papillary RCC | 302 | 21.0 | |
| Chromophobe RCC | 103 | 7.2 | |
| Original Study | CHECKMATE_025 | 446 | 31.1 |
| TCGA_KIRC | 372 | 25.9 | |
| TCGA_KIRP | 285 | 19.8 | |
| ICGC_RECA_EU | 95 | 6.6 | |
| TCGA_KICH | 76 | 5.3 | |
| CHECKMATE_010 | 61 | 4.2 | |
| CHECKMATE_009 | 57 | 4.0 | |
| GENETECH_Non_ccRCC | 44 | 3.1 | |
| Inferred Ancestry | European | 1201 | 83.6 |
| African | 131 | 9.1 | |
| Admixed American | 79 | 5.5 | |
| East Asian | 19 | 1.3 | |
| South Asian | 6 | 0.4 | |
Cancer-Free Control Cohort
WES BAMs aligned to GRCh37 from a total of 24,128 adult unrelated individuals without known cancer diagnosis were collected from the following studies: Autism Sequencing Consortium (ASC) (Database of Genotypes and Phenotypes (dbGAP):phs000298.v4.p3), Framingham Cohort (dbGAP:phs000007.v32.p1), Multi-Ethnic Study of Atherosclerosis (MESA) Cohort (dbGAP: phs000209.v13.p3), National Heart, Lung and Blood Institute (NHLBI) GO-ESP: Lung Cohorts Exome Sequencing Project (dbGAP: phs000291.v2.p1), 1000 Genomes Project24. All control samples were processed using methods identical to those used for the RCC case cohort.
Evaluation of Exome Sequencing Coverage
The average sample level sequencing coverage was calculated using Genome Analysis Toolkit (GATK)25 (version 3.7) tool “DepthofCoverage” to ensure that all BAMs of cases and controls had sufficient read counts to confidently call germline variants. Exome-wide mean coverage of 10X was considered the minimum acceptable coverage to ensure confident germline variant detection; samples below this threshold were excluded from our final analysis.
Germline Variant Detection from WES Data
Germline variants were called from the BAM files using a deep learning-based variant discovery method, DeepVariant (version 0.8.0, docker: gcr.io/deepvariant-docker/deepvariant:0.8.0)26 that had demonstrated superior sensitivity and specificity than GATK based joint-genotyping27,28. All germline variants annotated with “PASS” in the FILTER column of the Variant Call Format (VCF) files were selected. Variants with low read coverage (<10 Read Depth) and low variant allele frequency (VAF <0.20) were excluded. Final sets of high-quality variants were then merged into cohort-level VCF files using GATK (version 3.7) tool “CombineVariants”. Subsequently, the ‘vt’ tool (version 3.13) was used on the cohort VCF files to normalize and decompose multiallelic variants.
Genetic Relatedness Analysis
We performed a genetic relatedness analysis on the cohort VCF files in two steps. In the first step, we implemented GENESIS (version 2.12.0) tool PC-AiR29 to perform a principal components analysis (PCA) on the detected germline variants for the detection of population structure in the case and control cohort, respectively. We then used GENESIS tool “PC-Relate”30 implemented in “Hail”31 (version 0.2.11, https://github.com/hail-is/hail) to estimate kinship coefficients between every possible pair within a cohort. We removed one sample out of each pair that had a kinship coefficient above 0.125 which indicates genetic relatedness within second-degree relatives. Related samples were removed from the cohort VCF using GATK’s (version 3.7) tool “SelectVariants”.
Genetic Ancestry Inference
First, cohort VCF files for cases and controls were combined with a cohort VCF file of 1000 Genomes Project24 samples (n=2,504) with known continental ancestries of Admixed American (AMR), African (AFR), European (EUR), East Asian (EAS), and South Asian (SAS), and the resulting VCF file was filtered for germline variants identified in the exome intervals well-covered (>15X read coverage) in over 95% of all samples. Next, the filtered VCF file was loaded into a matrix table using Hail (version 0.2.11, https://github.com/hail-is/hail), and rare germline variants with a cohort allele frequency below 1% and deviating from Hardy-Weinberg equilibrium (chi-squared p-value <1×10−6) were excluded. We next performed Linkage Disequilibrium (LD) pruning using the “ld_prune” method in Hail to prune out variants with Spearman correlation coefficient greater than 0.1 within a 1 million base pair window, and the resulting filtered germline variants were used for PCA using Hail “hwe_normalized_pca” method. Finally, Sklearn (version 0.20.0) “RandomForestClassifier” function was applied to the top 10 global principal components (PCs) of reference samples from the 1000 Genomes Project to train random forest classifiers for the 5 continental ancestries, which were used to uniformly assign continental ancestry to the cases and controls (Supplementary Figure 1A).
Ancestry Pair-Matching of Cases and Controls
Once continental ancestry was assigned, cases and controls were divided into each continental ancestry group, and the second round of PCA was performed on each group to identify continental ancestry-specific PCs. We then used the R optmatch (version 0.9–14) package’s “pairmatch” function to identify control samples that were closest to each case based on the top 10 PCs. To ensure an equivalent representation of each ancestry group, we applied a fixed 1:12 ratio between the number of cases and controls across all continental ancestry groups, and AMR cases and controls were excluded in the gene-level burden analysis due to the limited number of AMR control samples failing to meet the case-control ratio. We also removed RCC patients with subtypes other than clear cell, papillary, or chromophobe RCC (Supplementary Figure 1B, C).
Sub-European Ancestry Inference and Ashkenazi Jewish Inference
For sub-European ancestry inference, the same variant filtering and PCA were repeated as in the continental ancestry inference, but only using samples identified as Europeans. The top 10 PCs and sub-European ancestry labels (Finnish in Finland (FIN), Iberian Population in Spain (IBS), Toscani in Italia (TSI), Utah Residents with Northern and Western European Ancestry (CEU), and British in England and Scotland (GBR)) from the 1000 Genomes European samples were used to train a random forest classifier. The classifier was applied to the case and control samples to infer sub-European ancestry.
Since Ashkenazi Jewish (ASJ) individuals were unable to be identified using the above approach, we used SNPweights32 software with pre-calculated SNP weights from Ashkenazi Jewish reference samples to identify ASJ individuals (Supplementary Figure 3). Samples with ASJ proportion >0.5 were defined as ASJ. In the end, European cases and controls were divided into Northwestern Europeans (including CEU and GBR), Southern Europeans (IBS and TSI excluding ASJ), Finnish, and Ashkenazi Jewish.
Functional and Clinical Annotation and Prioritization of Germline Variants
Germline variants in the cohort VCF files were annotated using Variant Effect Predictor (VEP, version 104.3)33 with dbNSFP34 (version 4.1a, GRCh37) and ClinVar35 (Release 20220829, GRCh37) plug-ins. A curated list of 143 CPGs (eTable 2) was used to identify candidate rare (Minor Allele Frequency (MAF) <1%) germline PVs. All identified variants were then classified into five categories: benign, likely benign, variants of unknown significance, likely pathogenic, and pathogenic, using the American College of Medical Genetics (ACMG) classification36 provided by VarSome37 website (accessed between September-November 2022). Variants classified as likely pathogenic or pathogenic are collectively referred to as pathogenic variants (PVs). The presence of the identified PVs was further validated by manual examination of the BAM files using Integrative Genomics Viewer (IGV, version 2.11.1).
Gene-level Burden Analysis with a Generalized Linear Model
To perform gene-level burden analysis, a null model based on a generalized linear model (GLM), as implemented in the Python “statsmodel” library (version 0.13.2)38 was first constructed using the top 10 global PCs from ancestry inference as covariates and RCC case status as the dependent variable. For each gene with at least one pathogenic variant in RCC cases or controls, a corresponding extended model incorporating a burden indicator variable representing the presence of a pathogenic variant in the gene for every sample was constructed. A likelihood ratio test was then performed between the null model and each extended model, and the resulting test statistics were adjusted for the false discovery rate (FDR) using the Benjamini-Hochberg procedure with FDR=0.05. The burden of three low-penetrance CHEK2 variants defined by a recent study12 - CHEK2 p.Ile157Thr, p.Ser428Phe, and p.Thr476Met – were evaluated separately from the pathogenic LOF variants identified in CHEK2.
Statistical Analysis and Data Visualization
Odds ratios, 95% confidence intervals, and p-values for two-sided Fisher’s Exact test were computed as implemented in the exact2×2 R package. Adjusted p-values (q-values) were computed based on the Benjamini-Hochberg procedure with FDR=0.05. A one-way ANOVA test was run using the “f_oneway” function from Python “scipy” library (version 1.5.2)39, and post hoc pairwise comparisons were performed where applicable using the “pairwise_tukeyhsd” function from Python “statsmodels” library. Sample proportion confidence intervals were calculated using the R “prop.test” function. For the meta-analysis, association statistics from sub-European groups were combined using a fixed-effects meta-analysis implemented using the “metafor” R package40. All figures were generated using Python “Seaborn” (version 0.11.0, Waskom M) and “Matplotlib” (version 3.3.2) packages and were further refined using Adobe Photoshop 2021. The commutation plot (Figure 4) summarizing the germline and somatic variants in RCC cases was generated using Python “CoMut” package41 (version 0.0.3, https://github.com/vanallenlab/comut).
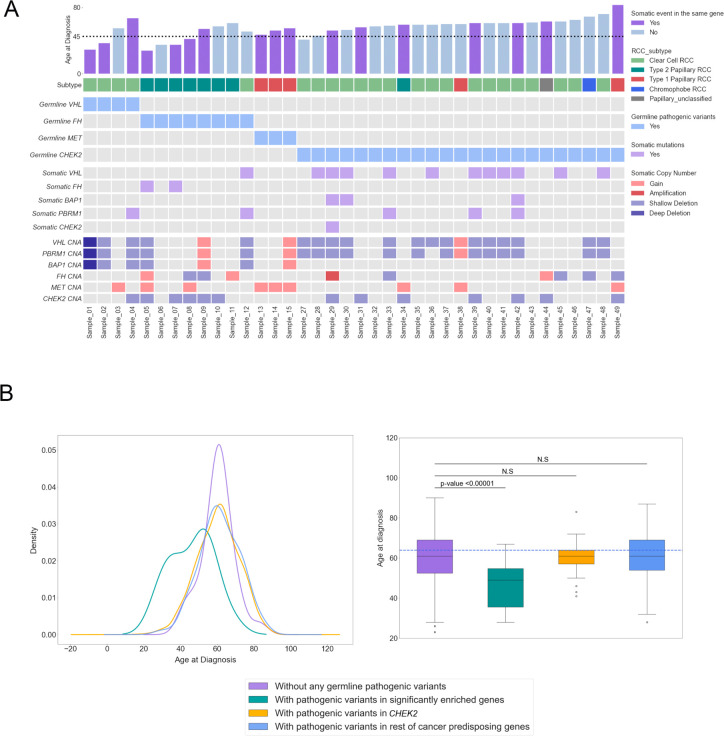
Figure 4. Somatic events in the carriers of pathogenic variants in genes with enrichment.
A) Comutation plot summarizing germline and somatic variants in RCC patients with germline pathogenic variants in the three significantly enriched genes as well as CHEK2. For the somatic events, only variants in relevant genes are featured. The dotted horizontal line on the bar plot indicates the age of onset at 45. CNA indicates copy number alterations
B) Density plot (left) and Box plot (right) for the distribution of age of disease onset for the different pathogenic variant carrier groups. Adjusted p-value was calculated after a one-way ANOVA with post hoc Tukey’s HDS test.
Identification of Somatic Variants and Copy Number Events
For carriers of germline pathogenic variants in VHL, MET, FH, and CHEK2, somatic variants data provided from TCGA and ICGC were downloaded from the GDC data portal (https://portal.gdc.cancer.gov/) and ICGC data portal (https://dcc.icgc.org/) respectively (accessed: Oct. 2022) and was analyzed to identify somatic variants and copy number alterations involving the genes of interest. For somatic variants, LOF truncating variants including frameshift, splice site, or nonsense variants as well as missense variants with oncogenic annotation from OncoKB were included. For samples from CheckMate studies, somatic variants and copy number alterations were identified with the CGA WES Characterization pipeline (https://github.com/broadinstitute/CGA_Production_AnalysisPipleline), which detects, filters, and annotates somatic variants and copy number alterations. The CGA pipeline employs the following tools: MuTect42, ContEst43, Strelka44, Orientation Bias Filter45, DeTiN46, AllelicCapSeq47, MAFPoNFilter48, RealignmentFilter, ABSOLUTE49, GATK25, PicardTools (https://software.broadinstitute.org/gatk/documentation/tooldocs/4.0.1.0/picard_fingerprint_CrosscheckFingerprints.php), Variant Effect Predictor33,and Oncotator50. Samples were excluded for quality control as previously described51. Briefly, tumor samples with ≥ 5% contamination43, normal samples contaminated with ≥ 20% tumor nuclei46, samples with poor sequencing coverage (tumor mean target coverage < 25x, normal mean target coverage < 15x), and samples identified as a tumor/normal swap based on the copy number profile (lane mix-up > 1) were excluded from further analyses. To assess tumor purity, both ABSOLUTE49 and FACETS52 were used. ASBOLUTE solutions were reviewed for tumor samples with < 20% estimated purity from FACETS and samples were subsequently excluded if < 20% purity was estimated from ABSOLUTE. To ensure adequate power for detecting somatic variants, a power calculation for each tumor sample was performed as previously described and samples with < 80% power were excluded53.
Identification and Validation of Cryptic Splice Variants
SpliceAI (version 1.3.1, https://github.com/Illumina/SpliceAI)54 was used to identify cryptic splice variants among the called germline variants detected by DeepVariant. The “SpliceAI” INFO column provided Delta scores ranging from 0 to 1 for the probability of acceptor gain/loss and donor gain/loss. The maximum Delta score was defined as the “SpliceAI” score in the annotated cohort VCF files, and the putative cryptic splice variants were further categorized according to the official cutoff recommendations: 0.2 (high recall), 0.5 (recommended), and 0.8 (high precision). Rare germline variants with SpliceAI score over 0.5 were defined as putative cryptic splice variants. For samples carrying a cryptic splice variant identified from SpliceAI, available tumor or germline mRNA BAM files were manually reviewed using IGV to visualize and evaluate their splicing patterns.
CNV Detection from Whole Exome Sequencing Data
We applied GATK-gCNV to detect rare germline CNVs from exome sequencing data55. To minimize the discrepancy among the different exome sequencing baits used for different sources, GATK (version 4.1.9.0) tool “CollectReadCounts” was used to gather read counts on the 8441 sequencing bait regions unique to 7 major capture kits, and PCA was run to make batches of samples sequenced using the same sequencing bait (Supplementary Figure 5A). From each identified batch, germline CNVs were detected using GATK-gCNV55 following the best practices on the Terra platform (https://app.terra.bio/#workspaces/help-gatk/Germline-CNVs-GATK4). The detected CNVs were harmonized and filtered using the gCNV filtering R scripts downloaded from the gCNV repository (https://github.com/theisaacwong/talkowski/tree/master/gCNV), which combines CNVs from multiple batches and identifies high-quality rare CNVs determined by assessing 3 quality control measures – Quality Score (QS), the rarity of a CNV (PASS_Freq, for site frequency of 1% or less), and the number of samples carrying a rare CNV (PSS_Sample, Samples carrying more than 100 unfiltered or more than 10 filtered calls are excluded).
RESULTS
Patient Characteristics of RCC Discovery Case Cohort
We collected whole-exome sequencing (WES) data from 1,436 RCC patients unselected for earlier age of disease onset or positive family history from 8 independent RCC studies (Figure 1, Table 1). 71.8% of patients had clear cell RCC (ccRCC, n=1,031), while the rest had non-clear cell RCCs (nccRCC), including papillary (n=302, 21.0%) and chromophobe RCC (n=103, 7.2%). Broad continental-level genetic ancestry inference (Supplementary Figure 1) identified most of the cohort as being of predominantly European ancestry (83.6%, n=1,200) followed by African (9.1%, n=131), Admixed American (5.6%, n=80), East Asian (1.3%, n=19), and South Asian (0.4%, n=6).
Prevalence of Rare Germline Pathogenic Variants in ccRCC and nccRCC
We first evaluated rare (MAF<1%) germline variants that met existing clinical interpretation guidelines36 as pathogenic or likely pathogenic in 1,031 ccRCC patients (Figure 2). In known RCC risk genes, we identified rare germline PVs in VHL (n=4, 0.38%, 95% CI: 0.12–1.1%), BAP1, MITF (n=3 each, 0.29%, 95% CI: 0.075–0.92%), FLCN, FH, and SDHD (n=1 each, 0.097%, 95% CI: 0.0051–0.63%). When evaluating DNA damage repair (DDR) genes (eTable 3), we identified 52 ccRCC patients that harbored rare germline PVs in homologous recombination or Fanconi Anemia genes such as CHEK2, RECQL4, FANCA, or BRCA1/2 (5.04%, 95% CI: 3.82–6.60%), 26 in base excision repair genes MUTYH and NTHL1 (2.52%, 95% CI:1.69–3.73%), 8 in nucleotide excision repair genes; ERCC1, ERCC2, ERCC3, XPA, and XPC (0.77%, 95% CI:0.36–1.59%), and 2 in mismatch repair genes MLH1 and PMS2 (0.19%, 95% CI:0.033–0.79%). Overall, 131 ccRCC patients carried one or more heterozygous rare germline PVs (12.71%, 95% CI:10.77–14.93%; eTable 4); 13 in previously established RCC risk genes (1.26%, 95% CI: 0.71–2.21%), 86 in DDR genes (8.34%, 95% CI:6.76–10.24%), and 37 in rest of the germline cancer predisposition genes (CPGs, 3.59%, 95% CI:2.57–4.96%). Among these, 9 ccRCC patients carried rare germline PVs in two different CPGs (0.87%, 95% CI: 0.43–1.71%; eTable 6).
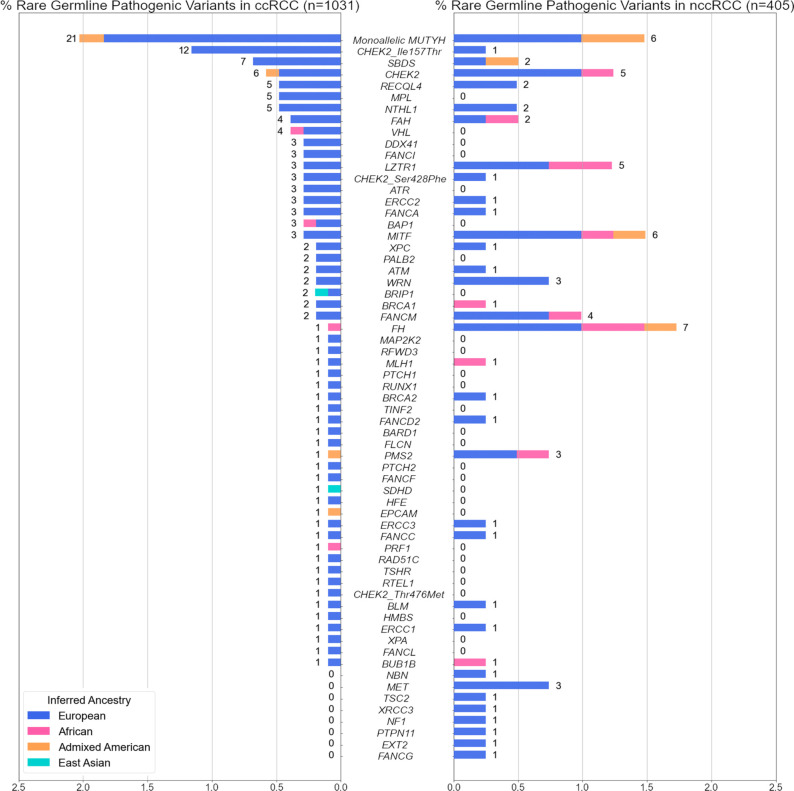
Figure 2. Rare germline pathogenic variants identified in the 1,436 RCC patients.
The proportion of ccRCC or nccRCC patients carrying rare (MAF <1%) germline pathogenic variants in one of the 143 germline cancer predisposition genes tested. 3 known CHEK2 low-penetrance variants were treated separately from the rest of the PVs in CHEK2. Numbers next to the bars indicate counts of RCC patients carrying PVs in each gene.
In parallel, we also characterized rare germline PVs in 405 nccRCC patients (Figure 2). Rare germline PVs were found in the following known kidney cancer risk genes: 7 in FH (1.72%, 95% CI:0.76–3.69%), 6 in MITF (1.48%, 95% CI:0.60–3.36%), 3 in MET (0.74%, 95% CI:0.19–2.33%), and 1 in TSC2 (0.25%, 0.013–1.59%). Regarding DDR genes, 24 germline PVs were detected in homologous recombination or Fanconi Anemia genes (5.93%, 95% CI:3.91–8.81%), 8 in base excision repair genes with PVs in ccRCC - MUTYH and NTHL1 (1.98%, 95% CI:0.92–4.01%), and 4 each in mismatch repair and nucleotide excision repair genes (0.99%, 95% CI:0.32–2.69% each). Altogether, one or more rare pathogenic germline PVs were detected in 68 nccRCC patients (16.79%, 95% CI:13.35–20.87%; eTable 5) – 17 in known RCC risk genes (4.20%, 95% CI: 2.54–6.77%), 39 in DNA damage repair genes (9.63%, 95% CI:7.02–13.03%), and 13 in other CPGs (3.21%, 95% CI:1.79–5.57%). Four patients were identified with rare germline PVs in two different CPGs (0.99%, 95% CI:0.32–2.69% eTable 6). Thus, the relatively higher proportion of patients with identified germline PVs in DDR genes, which was mainly driven by rare germline PVs in CHEK2 and MUTYH (3.90%, n=56/1436 across RCC subtypes, 95% CI: 2.98–5.07%) was consistent with observations in the pan-RCC patients from previous RCC studies6,8–10.
Gene-Level Enrichment of Rare Germline Pathogenic Variants in ccRCC and nccRCC Patients
To investigate whether the identified PVs predispose individuals to an increased risk of RCC, we performed genetic ancestry inference and case-control pair matching to link 1,356 RCC patients (of the original 1,436; 94.4% of the RCC cohort) with 16,512 cancer-free controls (Method; Supplementary Figure 1) and compared the gene-level burden of rare germline PVs in ccRCC (n=976) and nccRCC (n=380) separately against the cancer-free controls. To further account for residual population stratification not captured in our ancestry matching procedure, we conducted gene-level burden analysis for each gene using a generalized linear model (GLM) that accounts for continental ancestry (Methods). As expected, ccRCC patients exhibited a significantly higher enrichment of germline PVs in VHL compared to the ancestry-matched controls (OR: 39.1, 95% CI: 7.01–218.07, p-value:4.95e-05, q-value:0.00584), and in the low-penetrance CHEK2 p.Ser428Phe variant (OR: 31.96, 95% CI: 6.23–163.89, p-value:0.000385, q-value:0.0227) after multiple-hypothesis correction (Table 2). ccRCC patients also carried a nominally higher frequency of PVs in the two other known kidney cancer risk genes, BAP1 and SDHD, as well as in the common low penetrance CHEK2 p.Ile157Thr variant (p-values <0.05, q-values > 0.05; eTable 7).
Table 2. Gene-level burden analysis of clear cell and non-clear cell RCCs.
Test statistics from the generalized linear model for genes with nominal enrichment. Genes with adjusted p-value less than 0.05 were considered significant and highlighted. A table of results for all genes tested can be found in eTables 7 and 8
| Gene/Variant | FDR_Adjusted_p-value | p-value | Odds_ratio | OR_95CI_low | OR_95CI_high | case_PV_carrier | control_PV_carrier | |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Clear Cell RCC | VHL | 0.00584 | 4.95E-05 | 39.1 | 7.01 | 218.07 | 4 | 2 |
| CHEK2 _Ser428Phe | 0.0227 | 0.000385 | 31.96 | 6.23 | 163.89 | 3 | 3 | |
| SDHD | 0.506 | 0.0141 | 1.72E+10 | 0 | inf | 1 | 0 | |
| BAP1 | 0.526 | 0.0314 | 5.57 | 1.44 | 21.52 | 3 | 9 | |
| CHEK2 _Ile157Thr | 0.526 | 0.0356 | 2.04 | 1.11 | 3.73 | 12 | 122 | |
|
| ||||||||
| Non Clear Cell RCC | FH | 1.83E-06 | 1.55E-08 | 77.9 | 18.68 | 324.97 | 6 | 3 |
| MET | 2.07E-05 | 3.50E-07 | 1.98E+11 | 0 | inf | 3 | 0 | |
| LZTR1 | 0.244 | 0.00619 | 4.96 | 1.92 | 12.85 | 5 | 42 | |
| PMS2 | 0.653 | 0.0241 | 5.58 | 1.65 | 18.84 | 3 | 25 | |
| MITF | 0.653 | 0.0277 | 3.38 | 1.34 | 8.53 | 5 | 62 | |
| CHEK2 _Ser428Phe | 0.707 | 0.036 | 27.73 | 2.78 | 276.8 | 1 | 3 | |
| EXT2 | 0.79 | 0.0483 | 22.36 | 2 | 250.27 | 1 | 2 | |
For nccRCC, patients carried a significantly higher prevalence of germline PVs compared to the controls in FH (OR: 77.9, 95% CI: 18.68–324.97, p-value:1.55e-08, q-value: 1.83e-06) and MET (OR: 1.98e11, 95% CI: 0-inf, p-value: 2.07e-05, q-value: 3.50e-07). LZTR1, PMS2, MITF, EXT2, and CHEK2 p.Ser428Phe also exhibited nominal enrichment of germline PVs but did not pass multiple hypothesis correction (p-values <0.05, q-values > 0.05, Table 2). In contrast to prior studies9,11,12, LOF variants in CHEK2 were not significantly enriched in ccRCC after excluding low-penetrance variants (OR: 1.01, CI: 0.41–2.52; p-value of 0.980, q-value: 0.997) or nccRCC (OR: 2.82, CI: 1.13–7.05; p-value of 0.0536, q-value: 0.79). No other DDR genes were enriched with PVs in ccRCC or nccRCC patients compared to the cancer-free controls (eTables 7 and 8).
Evaluation of CHEK2 in RCC Risk Via Accounting for Fine-Scale Genetic Differences in European Sub-populations
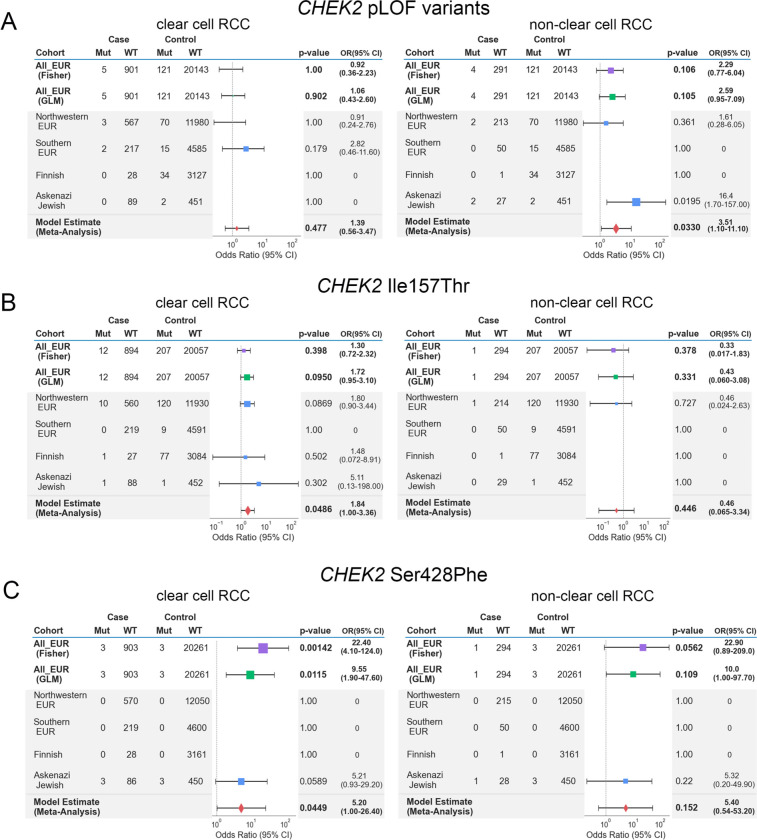
The three main germline PVs identified in CHEK2 in our study – c.1100del, Ser428Phe, and Ile157Thr – are all founder variants from different European sub-populations, with substantial variation in population minor allele frequencies across different European populations (eTable 9)56. These variants were also identified at different frequencies in our cases and controls of different sub-European ancestries (Supplementary Figure 2). Therefore, to explore whether subtle ancestry differences in European populations may have confounded CHEK2 rare variant analyses, we performed three additional CHEK2 burden analyses restricted to European samples to evaluate the impact of addressing fine-level population stratification on the role of CHEK2 as an RCC risk gene: (1) a Fisher’s Exact-based association study on all Europeans pooled together; (2) a GLM-based burden-analysis using the top 10 genetic principal components from a European-only principal components analysis; (3) a meta-analysis combining test statistics from different sub-European populations (Figure 3; Methods). CHEK2 germline LOF PVs did not demonstrate enrichment in ccRCC cases in all three tests, though they exhibited a nominal enrichment in nccRCC only in the meta-analysis (OR: 3.51, 95% CI:1.10–11.10, combined p-value: 0.0330, Figure 3A). In contrast, only ccRCC patients exhibited a nominally higher burden of CHEK2 p.Ile157Thr variant in the meta-analysis (OR: 1.84, 95% CI:1.00–3.36, combined p-value: 0.0486) (Figure 3B). Finally, this expanded statistical framework demonstrated that the CHEK2 p.Ser428Phe variant that was significantly enriched in the above multi-ancestry GLM-based burden analysis were only modestly enriched in these ccRCC patients (OR:5.20, 95% CI:1.00–26.40, combined p-value:0.0449), reflecting the localized burden of the variant in the inferred Ashkenazi Jewish RCC individuals in cases and cancer-free controls (Figure 3C). Taken together, the results demonstrate that the risk assessment of CHEK2 germline variants requires careful consideration of population stratification due to the varying frequency of founder variants in this gene.
Figure 3. Meta-Analysis of CHEK2 risk in ccRCC and nccRCC in European samples.
Tables and forest plots summarize the model estimates summary statistics from the sub-European meta-analysis for CHEK2 germline variants. The area of squares is proportional to the −log10 of the p-values, and the horizontal bars indicate 95% confidence intervals for the estimated odds ratio. Test statistics from Fisher’s Exact and GLM tests were plotted for comparison.
A) Summary tables for CHEK2 LOF excluding the low-penetrance variants
B) Summary tables for CHEK2 founder variant p.Ile157Thr
C) Summary tables for CHEK2 founder variant p.Ser428Phe
Prevalence of Somatic Second-Hit Variants in Carriers of Germline Pathogenic Variants
To further clarify the potential roles of the rare germline PVs identified in these analyses, we next investigated the available tumor samples from the RCC patients in our study to identify somatic events (truncating somatic variants or copy number alterations; Methods) accompanying the germline PVs identified in our analyses (Figure 4A; eTable 10). Tumors from ccRCC patients carrying germline PVs in VHL had a somatic copy number deletion in chromosome 3 spanning VHL (n=3/4, 75.0%, 95% CI: 21.94–98.68%).57 Regarding nccRCC, all 3 carriers of germline MET PVs were patients with type 1 papillary RCC (pRCC) whose tumors had somatic copy number gains at chromosome 7 (spanning the MET locus), while 87.5% (n=7/8, 95% CI: 46.68–99.34%) of patients with germline PVs in FH were from type 2 pRCC whose tumors often had somatic variants or copy number deletions in FH (n=4/7, 57.1%, 95% CI: 20.24–88.19%). Overall, 10 patients (66.7%, 95% CI: 38.69–87.01%) carrying germline PVs in FH, MET, or VHL harbored identifiable secondary somatic events in the same genes, further indicating the importance of these genes in the RCC oncogenesis. In contrast, patients carrying CHEK2 germline variants were not limited to a specific RCC subtype, and only 7 of 23 (30.4%, 95% CI: 14.06–53.01%) RCC patients with germline variants in CHEK2 had secondary somatic variants in CHEK2 (1 patient with both somatic variant and copy number deletion in CHEK2; 6 patients with CHEK2 copy number deletions).
Age of RCC Presentation for Carriers of Germline Pathogenic Variants in RCC Risk Genes
Rare germline PVs in genes known to cause hereditary cancer syndromes have been associated with an earlier onset of disease in different cancers including RCC58–62, and detection of these variants with genetic testing can guide clinical management in at elevated genetic risk for cancer63. To further characterize the clinical impact of the rare germline PVs identified in the genes with a significantly higher burden of PVs in RCC patients, we compared the age of disease onset between the groups defined by genetic status: (1) patients carrying germline PVs in the known RCC risk genes; FH, MET, and VHL; (2) patients carrying germling PVs in CHEK2; (3) patients carrying germline PVs in other CPGs without enrichment; (4) patients carrying no germline PVs (Figure 4B; eTable 11). The carriers of rare germline PVs in FH, MET, and VHL presented with disease at a significantly earlier age compared to the other three groups (Mean: 46.0, Median: 49.0 years old, Tukey post hoc adjusted p-values < 0.01 for all three pairwise comparisons), and patients with germline and somatic biallelic events presented with disease at an earlier age (n=10/15, Mean: 44.6, Median: 44.5 years old, Figure 4A). However, the age of disease onset for the patients carrying germline CHEK2 PVs showed no evidence of age difference from that of patients carrying no germline PVs (Mean: 60.1, Median: 61 vs Mean: 60.2, Median: 61 years old, Tukey post hoc adjusted p-value =1.0) or patients carrying PVs in the rest of CPGs that did not exhibit enrichment in RCC (Mean: 60.1, Median: 61 vs Mean: 61.3, Median: 61 years old, Tukey post hoc adjusted p-value =0.949). Similarly, RCC patients carrying both germline and somatic variants in CHEK2 did not present at an earlier age of onset compared to patients without any germline PVs (n=7/23, Mean: 62.1, Median: 61.0 years old). These results, taken together with the only modest enrichment of germline PVs in CHEK2, suggests caution for considering CHEK2 as a RCC predisposition gene.
Identifying Additional Forms of Inherited Genomic Alterations in RCC Risk Genes
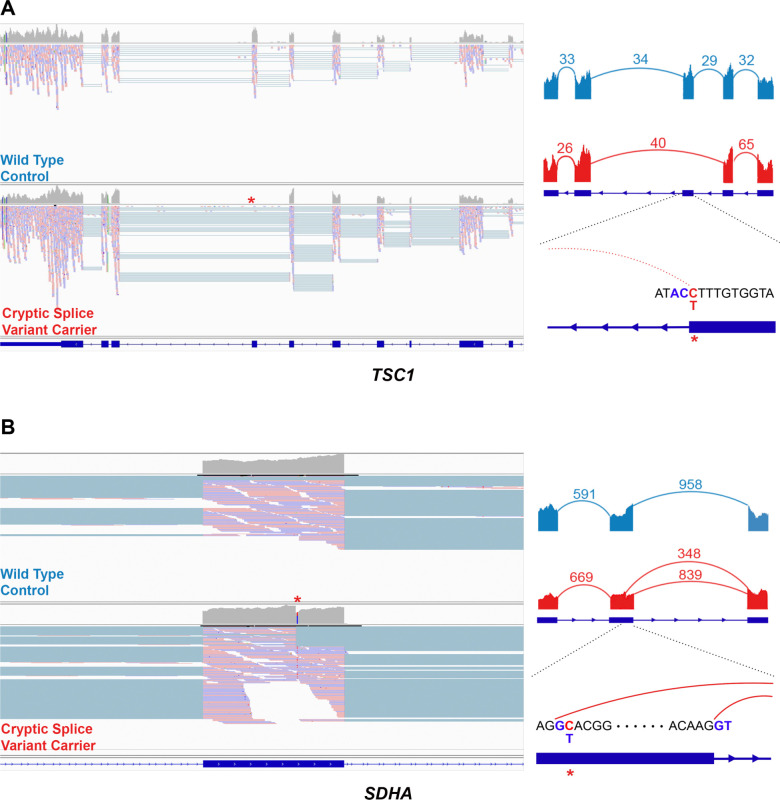
While 13.9% (n=199/1436, 95% CI: 12.13–15.78%) of total RCC (ccRCC and nccRCC) patients carried rare germline PVs in CPGs, only 15 RCC patients (1.04%, 95% CI: 0.61–1.76%) harbored germline PVs in known RCC risk genes (FH, MET, and VHL), but the rest of the CPGs did not exhibit increased burden of PVs in RCC patients in our analyses and thus are of uncertain biological significance in RCC pathogenesis. Thus, we hypothesized that RCC risk genes may also be disrupted through mechanisms that can escape the detection of conventional germline variant detection methods commonly used in clinical and research contexts, such as cryptic splice variants outside of the canonical splice sites and germline copy number variants (CNVs). Using existing computational methods to predict cryptic splice variants, we identified 109 candidate rare germline cryptic splice variants in CPGs in 102 RCC patients (Supplementary Figure 4A; eTable 12; Methods). Of these, 86 patients had tumor and/or germline mRNA sequencing data available to validate these predicted splice variants. The available RNA sequencing data showed no evidence of aberrant splicing for 82 variants (95.35%, 95% CI: 87.87–98.50%). However, two cryptic splice variants in two RCC risk genes, TSC1 and SDHA, demonstrated a clear pattern of aberrant splicing (Figure 5). The cryptic splice variant in TSC1 in a chromophobe RCC patient changed antisense cytosine upstream of a splice donor motif to thymine, leading to complete exon-skipping of exon 21. In the papillary RCC patient with a variant in SDHA, the cryptic splice variant introduced a cryptic donor motif inside exon 13 and removed 15 amino acids at the end of the exon. Two other cryptic splice variants in TP53 and LZTR1 showed aberrant splicing, but the number of splice junction reads was too low to confidently conclude them as clear splice variants (Supplementary Figure 4B; Methods).
Figure 5. Example cryptic splice variants in established RCC risk genes.
Left: IGV screenshot of the tumor mRNA sequencing data of the wildtype control (above) and the carrier of cryptic splice variant (bottom). Right: Sashimi plot showing the pattern of splicing with the numbered split junction reads.
A) Disruption of splice donor motif led to complete exon skipping in TSC1
B) The cryptic splice variant in SDHA introduced a new splice donor motif GT inside an exon
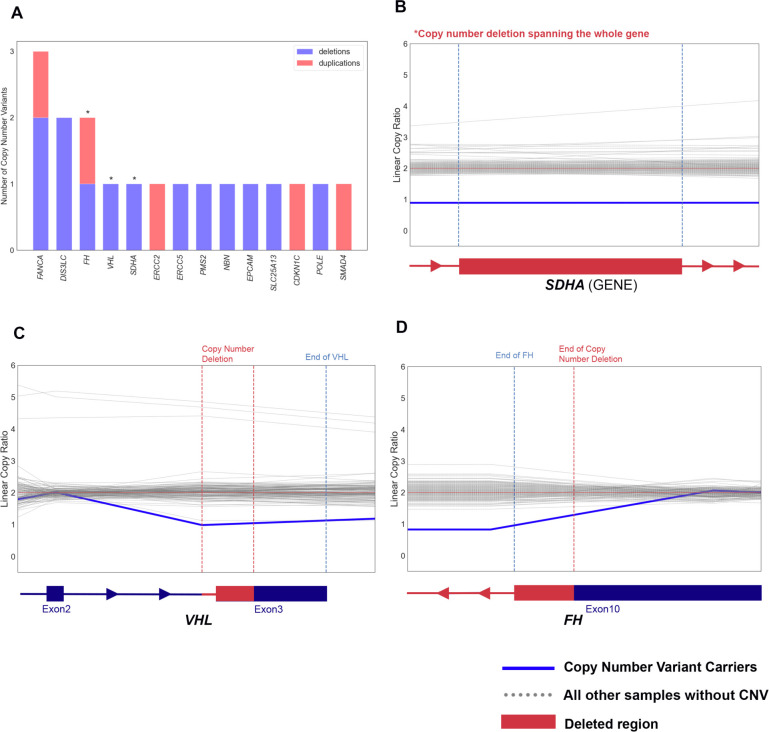
We next evaluated germline CNVs (Methods). Collectively, we identified 2,503 high-quality rare germline CNVs in 888 RCC samples (1,211 deletions and 1,292 duplications; Supplementary Figure 5B–C; Methods). Of these, 18 heterozygous CNVs in 18 (1.25%, 95% CI: 0.77–2.02%) RCC patients affected 14 CPGs including RCC risk genes FH, VHL, and SDHA (Figure 6A; eTable 13). For example, we found a ccRCC patient harboring a deletion spanning part of the last exon of VHL (Chr3:10191124–10192282, GRCh37, Figure 6C), and another deletion was identified in a papillary RCC patient overlapped the last 761bp of FH (Chr1:240070386–241661618, Figure 6D). We also identified a large 215 kbp deletion completely spanning SDHA in a different papillary RCC patient (Chr5:139251–354374, Figure 6B), which disrupted a region similar to a variant previously described in the gnomAD structural variant (SV) database (gnomAD ID: DEL_5_54065, Chr5:135575–308762)64. Thus, by characterizing underappreciated variant types such as cryptic splice and CNVs, we identified 6 additional putative pathogenic variants in the established RCC risk genes, increasing the diagnostic yield of rare germline PVs in risk genes from 2.1% (n=30/1436, 95% CI: 1.44–3.01%) to 2.5% (n=36/1436, 95% CI: 1.79–3.49%).
Figure 6. Example rare germline copy number variants (CNVs) in established cancer-predisposing genes.
A) Bar-plot summarizing the counts of deletions and duplications including the germline cancer predisposing genes
B-D) Denoised linear copy ratio (DCR) plot indicating heterozygous copy number deletion with copy ratio of 1 for the carriers of CNV. The blue line indicates DCR for the CNV carrier, and the dotted grey lines indicate DCR for the rest of wild type samples.
DISCUSSION
Thus far, fourteen genes in the HIF, PI3K/mTOR, chromatin regulation, and cell cycle pathways have been identified as established RCC risk genes65, but the estimated high genetic heritability of RCC is not fully explained by germline PVs detected in these genes. Multiple recent studies have built on these foundational discoveries to report frequencies of rare germline PVs in DDR genes in large RCC cohorts. However, analyses with proper cancer-free controls and statistical models accounting for population structure are necessary to determine whether these PVs are significantly enriched in RCC populations. Attempts to demonstrate association by comparing frequencies of PVs in RCC cases against public databases such as ExAC66 or gnomAD56 are fraught with major technical limitations, including that the samples are likely not sequenced using the same sequencing platform, variants were not called using the same variant discovery pipeline and were not processed identically, and cases and controls were not ancestry-matched to ensure a robust statistical comparison. Recently, two studies leveraged case-control approaches to report an association of rare germline PVs in CHEK2 with an elevated risk of RCC11,12. However, both studies treated different subtypes of RCC together as a single phenotype and included CHEK2 variants with distinct population properties. To address the gaps of knowledge in the field, we performed histology-specific and case-control analyses of rare germline PVs in RCC, finding that PVs in the three significantly enriched genes – VHL, MET, and FH – had no significant overlap in different RCC subtypes. This molecular difference was also clearly demonstrated when we investigated companion somatic variants and copy number events stratified by histological subtype.
Furthermore, while adjusting for finer-scale population stratification is the current standard in GWAS studies, many case-control rare germline variant studies in cancer (including most RCC studies) only adjusted for population stratification by limiting their association analysis to cases and controls of European descent under the assumption that individuals of broad continental European ancestry would have a similar genetic background. Consistent with prior observations that population stratification can confound rare variant association studies in other disease contexts67–70, we found that a standard Fisher’s Exact test-based association model without addressing finer-scale population stratification can lead to a false association in this context, especially for CHEK2 that has several founder variants with varying population frequency within Europe. For example, the most well-studied CHEK2 c.1100del variant’s population frequency substantially varies in different regions of Europe, ranging from ~0% in Spain to 1.6% in the Netherlands and has a relatively lower population frequency in North America compared to Europe71–77. These factors may explain why in our study, the CHEK2 variant was detected in only 0.35% of RCC cases, where most patients were from the U.S. and patients were unselected for family history. Meanwhile, this variant was identified in more than 1% of RCC cases in a UK-based RCC association study11 and significant enrichment of CHEK2 germline PVs was reported in studies with patients selected for positive family history9 or positive CHEK2 germline variant carrier status in panel sequencing12. The variation in population frequency of CHEK2 variants combined with prior studies lacking robust genetic ancestry inference and case-control matching may partially explain the disagreeing risk assessment within and across cancer types for this gene78. Our result warrants caution against the commonly used practice of treating all “white” or “Caucasian” individuals with predominantly European ancestry as one group in cancer genetic studies. This practice can substantially confound association studies, particularly for studies including participants from the U.S. population which consists of different European ancestry groups as well as non-European ancestry groups.
Furthermore, in this study, RCC patients with germline PVs in CHEK2 did not have frequent secondary somatic events, whereas tumors from carriers of PVs in bona fide RCC predisposition genes like FH, MET, or VHL largely exhibited secondary somatic events in these genes. In addition, in this cohort of RCC patients unselected for the age of disease onset, RCC patients with germline PVs in CHEK2 did not exhibit earlier age of RCC onset, contrasting the relatively early age of breast cancer onset for germline CHEK2 variant carriers reported in several studies71,73,79–81, which suggests uncertainty regarding the role of CHEK2 germline PVs in RCC risk. Unlike in breast cancer, the biological role of DDR genes in Fanconi anemia or homologous recombination pathways such as CHEK2 has not definitively been demonstrated in RCC, and we did not identify an enrichment of germline PVs in other DDR genes besides CHEK2. Given these observations, we suggest caution in including CHEK2 or any other DDR genes as RCC risk genes. Critically, our analysis does not preclude the association of CHEK2 with RCC but advises for addressing population stratification in larger cohorts that include different sub-European ancestry groups is warranted to clarify the role of this gene in RCC risk and heritability.
Moreover, the general focus on germline small nucleotide variants and small indels in coding sequences in prior studies may limit our understanding of potential PVs in established RCC risk genes or other candidate genes. Thus, we also investigated rare germline cryptic splice variants and germline CNVs that have not been well-characterized in RCC or cancer germline studies. Cryptic splice variants can introduce or remove splicing donor or acceptor motifs inducing aberrant mRNA splicing and loss of protein function82,83. However, they can be easily overlooked as non-pathogenic because they are usually annotated as non-truncating when using conventional annotation approaches. In this study, we identified two rare germline cryptic splice variants which induced aberrant splicing in RCC risk genes SDHA and TSC1, which appear to reduce wild-type transcript abundance based on our investigation of matched transcriptome sequencing data. To our knowledge, this is the first description of germline cryptic splice variants in RCC and may warrant incorporation of them into comprehensive clinical genetic testing strategies.
Lastly, to further augment the search space for germline inherited risk events, we systematically characterized rare germline CNVs in RCC patients. Rare germline CNVs are known to increase susceptibility for different cancers84–89 and a few RCC studies reported CNVs detected in FLCN and VHL in RCC patients90,91. However, a systematic characterization of germline CNVs using WES data has not been fully explored in RCC despite the wider availability of whole exome sequencing data and improved methods for germline CNV detection. Here, we successfully identified 18 rare germline copy number duplications and deletions in CPGs from the whole-exome sequenced samples, including 4 CNVs in RCC risk genes FH, SDHA, and VHL. With the widespread use of WES and improvement in CNV identification methods, investigation of germline SNVs and short insertions and deletions together with cryptic splice and CNVs should be considered as a routine testing strategy for RCC inherited risk assessment and possibly across cancer types.
LIMITATIONS
The current study has several limitations. First, the findings from our gene-burden analyses merit validation in additional independent case and control cohorts, particularly in larger and more diverse patient populations. Indeed, even for the sub-European ancestry identification, we did not have the means to distinguish Northwestern Europeans from Eastern or central Europeans who might have clustered together with the Northwestern Europeans in the 1000 Genomes Project-based inference, let alone for the myriad subpopulations on other non-European continents. In the future, more refined meta-analyses might take advantage of reference panels representing diverse ancestry groups to better address such subtle sub-continental differences. In addition, we had to constrain the analysis to 143 CPGs instead of a whole-exome-wide analysis due to the limited study power, further emphasizing the need for larger and more diverse patient cohorts. Finally, further studies comparing chromophobe RCC as distinct from papillary RCC as well as studies of other rare subtypes such as medullary or collecting duct RCCs are needed to better characterize inherited risk events in heterogeneous nccRCC subtypes.
CONCLUSIONS AND RELEVANCE
This systematic population stratification-aware analysis supports the link between several RCC risk genes and elevated risk and describes distinct patterns of inherited germline and somatic variants in different RCC subtypes. Our results also call for caution when assessing the risk conferred by germline PVs in CHEK2. Finally, it broadens the definition of the RCC germline landscape of pathogenicity to incorporate previously underutilized germline variations.
Supplementary Material
KEY Points.
Question:
Can we improve the assessment of germline genetic risk determinants for clear cell and non-clear cell renal cell carcinoma (RCC) with approaches that are aware to population-stratification and RCC histological subtypes?
Findings:
In this systematic case-control study of 1,356 RCC patients and 16,512 ancestry-matched cancer-free controls strictly controlling for population stratification, clear-cell RCC patients exhibited a significantly higher prevalence of rare germline pathogenic variants (PVs) in VHL, and non-clear cell RCC patients carried significantly more rare germline PVs in FH and MET. European clear-cell RCC patients harbored a nominally significant enrichment of two low-penetrance CHEK2 variants (p.Ser428Phe and p.Ile157Thr) while European non-clear RCC patients carried a nominally significant enrichment of rare germline loss-of-function (LOF) variants in CHEK2. Subsequent somatic analyses identified secondary somatic events in genes significantly enriched for germline PVs (VHL, FH, MET), and these variant carriers presented with earlier age of disease onset, but CHEK2 germline variant carriers harbored relatively fewer somatic events in CHEK2 and did not present with earlier age of onset. Finally, we identified 6 RCC patients with rare germline cryptic splice and copy number variants that impacted known kidney cancer risk genes, increasing the diagnostic yield of pathogenic variants in RCC risk genes from 2.1% to 2.5%.
Meaning:
Clear and non-clear RCCs have distinct germline pathogenic variant enrichment patterns and somatic variants. Accurate risk assessment of CHEK2 in RCC requires careful adjustment for population stratification. In addition, previously underappreciated forms of germline variants may explain a portion of the missing heritability in RCC.
ACKNOWLEDGMENTS
We would like to thank all individuals who participated in the multiple studies from which we collected our raw sequencing data. We also would like to thank Isaac Wong and Jack Fu in Talkowski Lab for helping us trouble-shooting the GATK-gCNV. Finally, we would like to thank Dr. Alexander (Sasha) Gusev for his input on the Jewish Inference analysis using SNPWEIGHTs. This work was supported by The National Institutes of Health R37CA222574 (E.M.V), R01CA227388 (E.M.V), R50CA265182 (J.P.), Mark Foundation Emerging Leader Award, the Department of Defense Physician Research Award (W81XWH-21-1-0084, PC200150) (S.H.A), and the Department of Defense Idea Development Award - Early-Career Investigator (KC210042/W81XWH-22-1-0455) (S.H.A), Alex’s Lemonade Stand Foundation Young Investigator Grant (R.G.), and the Wong Family Award in Translational Oncology (R.G.). The funding organizations were not responsible for the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
COMPETING INTERESTS
E.M.V.A. holds consulting roles with Tango Therapeutics, Genome Medical, Genomic Life, Enara Bio, Manifold Bio, Monte Rosa, Novartis Institute for Biomedical Research, Riva Therapeutics and Serinus Bio; he receives research support from Novartis, Bristol-Myers Squibb and Sanofi; he has equity in Tango Therapeutics, Genome Medical, Genomic Life, Syapse, Enara Bio, Manifold Bio, Microsoft, Monte Rosa, Riva Therapeutics and Serinus Bio; he has filed institutional patents on chromatin mutations, immunotherapy response, and methods for clinical interpretation. T.K.C. reports institutional and personal, paid and/or unpaid support for research, advisory boards, consultancy, and honoraria from: Alkermes, AstraZeneca, Aravive, Aveo, Bayer, Bristol Myers-Squibb, Calithera, Circle Pharma, Eisai, EMD Serono, Exelixis, GlaxoSmithKline, IQVA, Infinity, Ipsen, Jansen, Kanaph, Lilly, Merck, Nikang, Nuscan, Novartis, Pfizer, Roche, Sanofi/Aventis, Surface Oncology, Takeda, Tempest, Up-To-Date, CME events (Peerview, OncLive, MJH and others), outside the submitted work. Institutional patents filed on molecular alterations and immunotherapy response/toxicity, and ctDNA. Equity: Tempest, Pionyr, Osel, Precede Bio. CureResponse. Committees: NCCN, GU Steering Committee, ASCO/ESMO, ACCRU, KidneyCan. Medical writing and editorial assistance support may have been funded by Communications companies in part. No speaker’s bureau. Mentored several non-US citizens on research projects with potential funding (in part) from non-US sources/Foreign Components. The institution (Dana-Farber Cancer Institute) may have received additional independent funding of drug companies or/and royalties potentially involved in research around the subject matter. T. K. C is also supported in part by the Dana-Farber/Harvard Cancer Center Kidney SPORE (2P50CA101942-16) and Program 5P30CA006516-56, the Kohlberg Chair at Harvard Medical School and the Trust Family, Michael Brigham, Pan Mass Challenge, Hinda and Arthur Marcus Fund and Loker Pinard Funds for Kidney Cancer Research at DFCI. D.A.B. reports nonfinancial support from Bristol Myers Squibb, honoraria from LM Education/Exchange Services, advisory board fees from Exelixis and AVEO, personal fees from Charles River Associates, Schlesinger Associates, Imprint Science, Insight Strategy, Trinity Group, Cancer Expert Now, Adnovate Strategies, MDedge, CancerNetwork, Catenion, OncLive, Cello Health BioConsulting, PWW Consulting, Haymarket Medical Network, Aptitude Health, ASCO Post/Harborside, Targeted Oncology, AbbVie, and research support from Exelixis and AstraZeneca, outside of the submitted work. R.G. has equity in Google, Microsoft, Amazon, Apple, Moderna, Pfizer, and Vertex Pharmaceuticals. B.R. has filed institutional patents on methods for clinical interpretation. Z.B. receives research support from Bristol Myers Squibb and imCORE/Genentech. He also reports honoraria from UpToDate. C.L. receives research funding from imCORE/Genentech outside the submitted work. The other authors declare no competing interests.
ETHICS DECLARATION
All individuals in this study consented to institutional review board-approved protocol (IRB# 20-293) that allowed for comprehensive genetic analysis of germline samples (Methods). This study conforms to the Declaration of Helsinki.
DATA AND CODE AVAILABILITY
All computation tools and packages in this study are publicly available. The docker image containing all GATK tools is available at (https://hub.docker.com/r/broadinstitute/gatk/). The docker image containing the germline variant detection tool, DeepVariant can be found at (https://hub.docker.com/r/google/deepvariant). Tools and detailed usage for SpliceAI (https://github.com/Illumina/SpliceAI) and GATK-gCNV (https://github.com/theisaacwong/talkowski/tree/master/gCNV) can be found on the respective GitHub pages. All raw sequencing data for TCGA studies can be accessed with controlled access on the GDC data portal (https://portal.gdc.cancer.gov/) with approval. All raw sequencing data for the ICGC study can be accessed with controlled access on the ICGC data portal (https://dcc.icgc.org/) with approval. All raw sequencing data for CHECKMATE clinical studies and Genentech study can be downloaded from European Genome-Phenome Archive (Dataset ID: EGAD00001001023) with approval. All raw sequencing data for cancer-free control samples can be accessed on dbGAP– Autism Sequencing Consortium (ASC) (dbGAP:phs000298.v4.p3), Framingham Cohort (dbGAP:phs000007.v32.p1), MESA Cohort (dbGAP: phs000209.v13.p3), NHLBI GO-ESP: Lung Cohorts Exome Sequencing Project (dbGAP: phs000291.v2.p1). In-house exome data for controls is available upon request.
REFERENCES
- 1.Padala SA, Barsouk A, Thandra KC, et al. Epidemiology of Renal Cell Carcinoma. World J Oncol. Jun 2020;11(3):79–87. doi: 10.14740/wjon1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mucci LA, Hjelmborg JB, Harris JR, et al. Familial Risk and Heritability of Cancer Among Twins in Nordic Countries. JAMA. Jan 5 2016;315(1):68–76. doi: 10.1001/jama.2015.17703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haas NB, Nathanson KL. Hereditary kidney cancer syndromes. Adv Chronic Kidney Dis. Jan 2014;21(1):81–90. doi: 10.1053/j.ackd.2013.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmidt LS, Linehan WM. Genetic predisposition to kidney cancer. Semin Oncol. Oct 2016;43(5):566–574. doi: 10.1053/j.seminoncol.2016.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nguyen KA, Syed JS, Espenschied CR, et al. Advances in the diagnosis of hereditary kidney cancer: Initial results of a multigene panel test. Cancer. Nov 15 2017;123(22):4363–4371. doi: 10.1002/cncr.30893 [DOI] [PubMed] [Google Scholar]
- 6.Carlo MI, Mukherjee S, Mandelker D, et al. Prevalence of Germline Mutations in Cancer Susceptibility Genes in Patients With Advanced Renal Cell Carcinoma. JAMA Oncol. Sep 1 2018;4(9):1228–1235. doi: 10.1001/jamaoncol.2018.1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu J, Wang H, Ricketts CJ, et al. Germline mutations of renal cancer predisposition genes and clinical relevance in Chinese patients with sporadic, early-onset disease. Cancer. Apr 1 2019;125(7):1060–1069. doi: 10.1002/cncr.31908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hartman TR, Demidova EV, Lesh RW, et al. Prevalence of pathogenic variants in DNA damage response and repair genes in patients undergoing cancer risk assessment and reporting a personal history of early-onset renal cancer. Sci Rep. Aug 11 2020;10(1):13518. doi: 10.1038/s41598-020-70449-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abou Alaiwi S, Nassar AH, Adib E, et al. Trans-ethnic variation in germline variants of patients with renal cell carcinoma. Cell Rep. Mar 30 2021;34(13):108926. doi: 10.1016/j.celrep.2021.108926 [DOI] [PubMed] [Google Scholar]
- 10.Truong H, Sheikh R, Kotecha R, et al. Germline Variants Identified in Patients with Early-onset Renal Cell Carcinoma Referred for Germline Genetic Testing. Eur Urol Oncol. Dec 2021;4(6):993–1000. doi: 10.1016/j.euo.2021.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yngvadottir B, Andreou A, Bassaganyas L, et al. Frequency of pathogenic germline variants in cancer susceptibility genes in 1336 renal cell carcinoma cases. Hum Mol Genet. Aug 25 2022;31(17):3001–3011. doi: 10.1093/hmg/ddac089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bychkovsky BL, Agaoglu NB, Horton C, et al. Differences in Cancer Phenotypes Among Frequent CHEK2 Variants and Implications for Clinical Care-Checking CHEK2. JAMA Oncol. Sep 22 2022;doi: 10.1001/jamaoncol.2022.4071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sato Y, Yoshizato T, Shiraishi Y, et al. Integrated molecular analysis of clear-cell renal cell carcinoma. Nat Genet. Aug 2013;45(8):860–7. doi: 10.1038/ng.2699 [DOI] [PubMed] [Google Scholar]
- 14.Motzer RJ, Jonasch E, Boyle S, et al. NCCN Guidelines Insights: Kidney Cancer, Version 1.2021. J Natl Compr Canc Netw. Sep 2020;18(9):1160–1170. doi: 10.6004/jnccn.2020.0043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Persyn E, Redon R, Bellanger L, Dina C. The impact of a fine-scale population stratification on rare variant association test results. PLoS One. 2018;13(12):e0207677. doi: 10.1371/journal.pone.0207677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cancer Genome Atlas Research N. Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature. Jul 4 2013;499(7456):43–9. doi: 10.1038/nature12222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cancer Genome Atlas Research N, Linehan WM, Spellman PT, et al. Comprehensive Molecular Characterization of Papillary Renal-Cell Carcinoma. N Engl J Med. Jan 14 2016;374(2):135–45. doi: 10.1056/NEJMoa1505917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davis CF, Ricketts CJ, Wang M, et al. The somatic genomic landscape of chromophobe renal cell carcinoma. Cancer Cell. Sep 8 2014;26(3):319–330. doi: 10.1016/j.ccr.2014.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med. Nov 5 2015;373(19):1803–13. doi: 10.1056/NEJMoa1510665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Motzer RJ, Rini BI, McDermott DF, et al. Nivolumab for Metastatic Renal Cell Carcinoma: Results of a Randomized Phase II Trial. J Clin Oncol. May 1 2015;33(13):1430–7. doi: 10.1200/JCO.2014.59.0703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choueiri TK, Fishman MN, Escudier B, et al. Immunomodulatory Activity of Nivolumab in Metastatic Renal Cell Carcinoma. Clin Cancer Res. Nov 15 2016;22(22):5461–5471. doi: 10.1158/1078-0432.CCR-15-2839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Consortium ITP-CAoWG. Pan-cancer analysis of whole genomes. Nature. Feb 2020;578(7793):82–93. doi: 10.1038/s41586-020-1969-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Durinck S, Stawiski EW, Pavia-Jimenez A, et al. Spectrum of diverse genomic alterations define non-clear cell renal carcinoma subtypes. Nat Genet. Jan 2015;47(1):13–21. doi: 10.1038/ng.3146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Genomes Project C, Auton A, Brooks LD, et al. A global reference for human genetic variation. Nature. Oct 1 2015;526(7571):68–74. doi: 10.1038/nature15393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McKenna A, Hanna M, Banks E, et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. Sep 2010;20(9):1297–303. doi: 10.1101/gr.107524.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poplin R, Chang PC, Alexander D, et al. A universal SNP and small-indel variant caller using deep neural networks. Nat Biotechnol. Nov 2018;36(10):983–987. doi: 10.1038/nbt.4235 [DOI] [PubMed] [Google Scholar]
- 27.AlDubayan SH, Conway JR, Camp SY, et al. Detection of Pathogenic Variants With Germline Genetic Testing Using Deep Learning vs Standard Methods in Patients With Prostate Cancer and Melanoma. JAMA. Nov 17 2020;324(19):1957–1969. doi: 10.1001/jama.2020.20457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Camp SY, Kofman E, Reardon B, et al. Evaluating the molecular diagnostic yield of joint genotyping-based approach for detecting rare germline pathogenic and putative loss-of-function variants. Genet Med. May 2021;23(5):918–926. doi: 10.1038/s41436-020-01074-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Conomos MP, Miller MB, Thornton TA. Robust inference of population structure for ancestry prediction and correction of stratification in the presence of relatedness. Genet Epidemiol. May 2015;39(4):276–93. doi: 10.1002/gepi.21896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Conomos MP, Reiner AP, Weir BS, Thornton TA. Model-free Estimation of Recent Genetic Relatedness. Am J Hum Genet. Jan 7 2016;98(1):127–48. doi: 10.1016/j.ajhg.2015.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hail Team H. 0.2. 2021:https://github.com/hail-is/hail. [Google Scholar]
- 32.Chen CY, Pollack S, Hunter DJ, Hirschhorn JN, Kraft P, Price AL. Improved ancestry inference using weights from external reference panels. Bioinformatics. Jun 1 2013;29(11):1399–406. doi: 10.1093/bioinformatics/btt144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McLaren W, Gil L, Hunt SE, et al. The Ensembl Variant Effect Predictor. Genome Biol. Jun 6 2016;17(1):122. doi: 10.1186/s13059-016-0974-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu X, Li C, Mou C, Dong Y, Tu Y. dbNSFP v4: a comprehensive database of transcript-specific functional predictions and annotations for human nonsynonymous and splice-site SNVs. Genome Med. Dec 2 2020;12(1):103. doi: 10.1186/s13073-020-00803-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Landrum MJ, Lee JM, Benson M, et al. ClinVar: improving access to variant interpretations and supporting evidence. Nucleic Acids Res. Jan 4 2018;46(D1):D1062–D1067. doi: 10.1093/nar/gkx1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. May 2015;17(5):405–24. doi: 10.1038/gim.2015.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kopanos C, Tsiolkas V, Kouris A, et al. VarSome: the human genomic variant search engine. Bioinformatics. Jun 1 2019;35(11):1978–1980. doi: 10.1093/bioinformatics/bty897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perktold J S, Skipper. statsmodels: Econometric and statistical modeling with python. presented at: Proceedings of the 9th Python in Science Conference; 2010; [Google Scholar]
- 39.Virtanen P, Gommers R, Oliphant TE, et al. SciPy 1.0: fundamental algorithms for scientific computing in Python. Nat Methods. Mar 2020;17(3):261–272. doi: 10.1038/s41592-019-0686-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Viechtbauer W. Conducting Meta-Analyses in R with the metafor Package. 2010;
- 41.Crowdis J, He MX, Reardon B, Van Allen EM. CoMut: visualizing integrated molecular information with comutation plots. Bioinformatics. Aug 1 2020;36(15):4348–4349. doi: 10.1093/bioinformatics/btaa554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cibulskis K, Lawrence MS, Carter SL, et al. Sensitive detection of somatic point mutations in impure and heterogeneous cancer samples. Nat Biotechnol. Mar 2013;31(3):213–9. doi: 10.1038/nbt.2514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cibulskis K, McKenna A, Fennell T, Banks E, DePristo M, Getz G. ContEst: estimating cross-contamination of human samples in next-generation sequencing data. Bioinformatics. Sep 15 2011;27(18):2601–2. doi: 10.1093/bioinformatics/btr446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saunders CT, Wong WS, Swamy S, Becq J, Murray LJ, Cheetham RK. Strelka: accurate somatic small-variant calling from sequenced tumor-normal sample pairs. Bioinformatics. Jul 15 2012;28(14):1811–7. doi: 10.1093/bioinformatics/bts271 [DOI] [PubMed] [Google Scholar]
- 45.Costello M, Pugh TJ, Fennell TJ, et al. Discovery and characterization of artifactual mutations in deep coverage targeted capture sequencing data due to oxidative DNA damage during sample preparation. Nucleic Acids Res. Apr 1 2013;41(6):e67. doi: 10.1093/nar/gks1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taylor-Weiner A, Stewart C, Giordano T, et al. DeTiN: overcoming tumor-in-normal contamination. Nat Methods. Jul 2018;15(7):531–534. doi: 10.1038/s41592-018-0036-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Landau DA, Carter SL, Stojanov P, et al. Evolution and impact of subclonal s in chronic lymphocytic leukemia. Cell. Feb 14 2013;152(4):714–26. doi: 10.1016/j.cell.2013.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lawrence MS, Stojanov P, Mermel CH, et al. Discovery and saturation analysis of cancer genes across 21 tumour types. Nature. Jan 23 2014;505(7484):495–501. doi: 10.1038/nature12912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carter SL, Cibulskis K, Helman E, et al. Absolute quantification of somatic DNA alterations in human cancer. Nat Biotechnol. May 2012;30(5):413–21. doi: 10.1038/nbt.2203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ramos AH, Lichtenstein L, Gupta M, et al. Oncotator: cancer variant annotation tool. Hum Mutat. Apr 2015;36(4):E2423–9. doi: 10.1002/humu.22771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Braun DA, Street K, Burke KP, et al. Progressive immune dysfunction with advancing disease stage in renal cell carcinoma. Cancer Cell. May 10 2021;39(5):632–648 e8. doi: 10.1016/j.ccell.2021.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shen R, Seshan VE. FACETS: allele-specific copy number and clonal heterogeneity analysis tool for high-throughput DNA sequencing. Nucleic Acids Res. Sep 19 2016;44(16):e131. doi: 10.1093/nar/gkw520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu D, Schilling B, Liu D, et al. Integrative molecular and clinical modeling of clinical outcomes to PD1 blockade in patients with metastatic melanoma. Nat Med. Dec 2019;25(12):1916–1927. doi: 10.1038/s41591-019-0654-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jaganathan K, Kyriazopoulou Panagiotopoulou S, McRae JF, et al. Predicting Splicing from Primary Sequence with Deep Learning. Cell. Jan 24 2019;176(3):535–548 e24. doi: 10.1016/j.cell.2018.12.015 [DOI] [PubMed] [Google Scholar]
- 55.Babadi M, Fu JM, Lee SK, et al. GATK-gCNV: A Rare Copy Number Variant Discovery Algorithm and Its Application to Exome Sequencing in the UK Biobank. bioRxiv. 2022;doi: 10.1101/2022.08.25.504851 [DOI] [Google Scholar]
- 56.Karczewski KJ, Francioli LC, Tiao G, et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature. May 2020;581(7809):434–443. doi: 10.1038/s41586-020-2308-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jonasch E, Walker CL, Rathmell WK. Clear cell renal cell carcinoma ontogeny and mechanisms of lethality. Nat Rev Nephrol. Apr 2021;17(4):245–261. doi: 10.1038/s41581-020-00359-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Melhem-Bertrandt A, Bojadzieva J, Ready KJ, et al. Early onset HER2-positive breast cancer is associated with germline TP53 mutations. Cancer. Feb 15 2012;118(4):908–13. doi: 10.1002/cncr.26377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pearlman R, Frankel WL, Swanson B, et al. Prevalence and Spectrum of Germline Cancer Susceptibility Gene Mutations Among Patients With Early-Onset Colorectal Cancer. JAMA Oncol. Apr 1 2017;3(4):464–471. doi: 10.1001/jamaoncol.2016.5194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu YL, Cadoo KA, Maio A, et al. Early age of onset and broad cancer spectrum persist in MSH6- and PMS2-associated Lynch syndrome. Genet Med. Jun 2022;24(6):1187–1195. doi: 10.1016/j.gim.2022.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reckamp KL, Behrendt CE, Slavin TP, et al. Germline mutations and age at onset of lung adenocarcinoma. Cancer. Aug 1 2021;127(15):2801–2806. doi: 10.1002/cncr.33573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.ZK Stadler AM, Padunan A, et al. Germline mutation prevalence in young adults with cancer. Presented at: American Association for Cancer Research Virtual Annual Meeting II. 2020; [Google Scholar]
- 63.Weitzel JN, Blazer KR, MacDonald DJ, Culver JO, Offit K. Genetics, genomics, and cancer risk assessment: State of the Art and Future Directions in the Era of Personalized Medicine. CA Cancer J Clin. Sep-Oct 2011;61(5):327–59. doi: 10.3322/caac.20128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Collins RL, Brand H, Karczewski KJ, et al. A structural variation reference for medical and population genetics. Nature. May 2020;581(7809):444–451. doi: 10.1038/s41586-020-2287-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ricketts CJ, Crooks DR, Sourbier C, Schmidt LS, Srinivasan R, Linehan WM. SnapShot: Renal Cell Carcinoma. Cancer Cell. Apr 11 2016;29(4):610–610 e1. doi: 10.1016/j.ccell.2016.03.021 [DOI] [PubMed] [Google Scholar]
- 66.Karczewski KJ, Weisburd B, Thomas B, et al. The ExAC browser: displaying reference data information from over 60 000 exomes. Nucleic Acids Res. Jan 4 2017;45(D1):D840–D845. doi: 10.1093/nar/gkw971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tintle N, Aschard H, Hu I, Nock N, Wang H, Pugh E. Inflated type I error rates when using aggregation methods to analyze rare variants in the 1000 Genomes Project exon sequencing data in unrelated individuals: summary results from Group 7 at Genetic Analysis Workshop 17. Genet Epidemiol. 2011;35 Suppl 1(Suppl 1):S56–60. doi: 10.1002/gepi.20650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mathieson I, McVean G. Differential confounding of rare and common variants in spatially structured populations. Nat Genet. Feb 5 2012;44(3):243–6. doi: 10.1038/ng.1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jiang Y, Epstein MP, Conneely KN. Assessing the impact of population stratification on association studies of rare variation. Hum Hered. 2013;76(1):28–35. doi: 10.1159/000353270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang Y, Guan W, Pan W. Adjustment for population stratification via principal components in association analysis of rare variants. Genet Epidemiol. Jan 2013;37(1):99–109. doi: 10.1002/gepi.21691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Consortium CBCC-C. CHEK2*1100delC and susceptibility to breast cancer: a collaborative analysis involving 10,860 breast cancer cases and 9,065 controls from 10 studies. Am J Hum Genet. Jun 2004;74(6):1175–82. doi: 10.1086/421251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kenneth Offit HP, Tomas Kirchhoff, Prema Kolachana, Beth Rapaport, Peter Gregersen, Steven Johnson, Orit Yossepowitch, Helen Huang Jaya Satagopan, Mark Robson, Lauren Scheuer, Khedoudja Nafa and Nathan Ellis. Frequency of CHEK2*1100delC in New York breast cancer cases and controls. BMC Medical Genetics. 2003; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mateus Pereira LH, Sigurdson AJ, Doody MM, et al. CHEK2:1100delC and female breast cancer in the United States. Int J Cancer. Nov 10 2004;112(3):541–3. doi: 10.1002/ijc.20439 [DOI] [PubMed] [Google Scholar]
- 74.Neuhausen S, Dunning A, Steele L, et al. Role of CHEK2*1100delC in unselected series of non-BRCA1/2 male breast cancers. Int J Cancer. Jan 20 2004;108(3):477–8. doi: 10.1002/ijc.11385 [DOI] [PubMed] [Google Scholar]
- 75.Osorio A, Rodriguez-Lopez R, Diez O, et al. The breast cancer low-penetrance allele 1100delC in the CHEK2 gene is not present in Spanish familial breast cancer population. Int J Cancer. Jan 1 2004;108(1):54–6. doi: 10.1002/ijc.11414 [DOI] [PubMed] [Google Scholar]
- 76.Vahteristo P, Bartkova J, Eerola H, et al. A CHEK2 genetic variant contributing to a substantial fraction of familial breast cancer. Am J Hum Genet. Aug 2002;71(2):432–8. doi: 10.1086/341943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yael Laitman BK, Ephrat Levy Lahad, Moshe Z. Papa and Eitan Friedman. Germline CHEK2 Mutations in Jewish Ashkenazi Women at High Risk for Breast Cancer. Israel Medical Association Journal. 2007; [PubMed] [Google Scholar]
- 78.Stolarova L, Kleiblova P, Janatova M, et al. CHEK2 Germline Variants in Cancer Predisposition: Stalemate Rather than Checkmate. Cells. Dec 12 2020;9(12)doi: 10.3390/cells9122675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Margolin S, Eiberg H, Lindblom A, Bisgaard ML. CHEK2 1100delC is prevalent in Swedish early onset familial breast cancer. BMC Cancer. Aug 17 2007;7:163. doi: 10.1186/1471-2407-7-163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rashid MU, Jakubowska A, Justenhoven C, et al. German populations with infrequent CHEK2*1100delC and minor associations with early-onset and familial breast cancer. Eur J Cancer. Dec 2005;41(18):2896–903. doi: 10.1016/j.ejca.2005.04.049 [DOI] [PubMed] [Google Scholar]
- 81.Rogier A. Oldenburg KK-J, Jaennelle Kraan,Hans Morreau, Jan G. M. Klijn,Nicoline Hoogerbrugge,Marjolein J. L. Ligtenberg, Christi J. van Asperen, Hans F. A. Vasen, Carel Meijers,, Hanne Meijers-Heijboer THdB, Cees J. Cornelisse, and Peter Devilee. The CHEK2*1100delC Variant Acts as a Breast Cancer Risk Modifier in Non-BRCA1/BRCA2 Multiple-Case Families. CANCER RESEARCH. 2003; [PubMed] [Google Scholar]
- 82.Lee M, Roos P, Sharma N, et al. Systematic Computational Identification of Variants That Activate Exonic and Intronic Cryptic Splice Sites. Am J Hum Genet. May 4 2017;100(5):751–765. doi: 10.1016/j.ajhg.2017.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cooper TA, Wan L, Dreyfuss G. RNA and disease. Cell. Feb 20 2009;136(4):777–93. doi: 10.1016/j.cell.2009.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Walker LC, Pearson JF, Wiggins GA, Giles GG, Hopper JL, Southey MC. Increased genomic burden of germline copy number variants is associated with early onset breast cancer: Australian breast cancer family registry. Breast Cancer Res. Mar 16 2017;19(1):30. doi: 10.1186/s13058-017-0825-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Laitinen VH, Akinrinade O, Rantapero T, Tammela TL, Wahlfors T, Schleutker J. Germline copy number variation analysis in Finnish families with hereditary prostate cancer. Prostate. Feb 15 2016;76(3):316–24. doi: 10.1002/pros.23123 [DOI] [PubMed] [Google Scholar]
- 86.Yoshihara K, Tajima A, Adachi S, et al. Germline copy number variations in BRCA1-associated ovarian cancer patients. Genes Chromosomes Cancer. Mar 2011;50(3):167–77. doi: 10.1002/gcc.20841 [DOI] [PubMed] [Google Scholar]
- 87.Brea-Fernandez AJ, Fernandez-Rozadilla C, Alvarez-Barona M, et al. Candidate predisposing germline copy number variants in early onset colorectal cancer patients. Clin Transl Oncol. May 2017;19(5):625–632. doi: 10.1007/s12094-016-1576-z [DOI] [PubMed] [Google Scholar]
- 88.Shi J, Zhou W, Zhu B, et al. Rare Germline Copy Number Variations and Disease Susceptibility in Familial Melanoma. J Invest Dermatol. Dec 2016;136(12):2436–2443. doi: 10.1016/j.jid.2016.07.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Park RW, Kim TM, Kasif S, Park PJ. Identification of rare germline copy number variations over-represented in five human cancer types. Mol Cancer. Feb 3 2015;14:25. doi: 10.1186/s12943-015-0292-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schneider M, Dinkelborg K, Xiao X, et al. Early onset renal cell carcinoma in an adolescent girl with germline FLCN exon 5 deletion. Fam Cancer. Jan 2018;17(1):135–139. doi: 10.1007/s10689-017-0008-8 [DOI] [PubMed] [Google Scholar]
- 91.Matsuda D, Khoo SK, Massie A, et al. Identification of copy number alterations and its association with pathological features in clear cell and papillary RCC. Cancer Lett. Dec 18 2008;272(2):260–7. doi: 10.1016/j.canlet.2008.06.015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All computation tools and packages in this study are publicly available. The docker image containing all GATK tools is available at (https://hub.docker.com/r/broadinstitute/gatk/). The docker image containing the germline variant detection tool, DeepVariant can be found at (https://hub.docker.com/r/google/deepvariant). Tools and detailed usage for SpliceAI (https://github.com/Illumina/SpliceAI) and GATK-gCNV (https://github.com/theisaacwong/talkowski/tree/master/gCNV) can be found on the respective GitHub pages. All raw sequencing data for TCGA studies can be accessed with controlled access on the GDC data portal (https://portal.gdc.cancer.gov/) with approval. All raw sequencing data for the ICGC study can be accessed with controlled access on the ICGC data portal (https://dcc.icgc.org/) with approval. All raw sequencing data for CHECKMATE clinical studies and Genentech study can be downloaded from European Genome-Phenome Archive (Dataset ID: EGAD00001001023) with approval. All raw sequencing data for cancer-free control samples can be accessed on dbGAP– Autism Sequencing Consortium (ASC) (dbGAP:phs000298.v4.p3), Framingham Cohort (dbGAP:phs000007.v32.p1), MESA Cohort (dbGAP: phs000209.v13.p3), NHLBI GO-ESP: Lung Cohorts Exome Sequencing Project (dbGAP: phs000291.v2.p1). In-house exome data for controls is available upon request.