Abstract
During infection, parasites evade the host immune system by modulating or exploiting the immune system; e.g., they suppress expression of major histocompatibility complex class II molecules or secrete cytokine-like molecules. However, it is not clear whether helminths disturb the immune responses of their hosts by controlling the antigen-processing pathways of the hosts. In this study, we identified a new cysteine protease inhibitor, nippocystatin, derived from excretory-secretory (ES) products of an intestinal nematode, Nippostrongylus brasiliensis. Nippocystatin, which belongs to cystatin family 2, consists of 144 amino acids and is secreted as a 14-kDa mature form. In vivo treatment of ovalbumin (OVA)-immunized mice with recombinant nippocystatin (rNbCys) profoundly suppressed OVA-specific proliferation of splenocytes but not non-antigen-specific proliferation of splenocytes. OVA-specific cytokine production was also greatly suppressed in rNbCys-treated mice. Although the serum levels of both OVA-specific immunoglobulin G1 (IgG1) and IgG2a were not affected by rNbCys treatment, OVA-specific IgE was preferentially downregulated in rNbCys-treated mice. In vitro rNbCys inhibited processing of OVA by lysosomal cysteine proteases from the spleens of mice. Mice with anti-nippocystatin antibodies became partially resistant to infection with N. brasiliensis. Based on these findings, N. brasiliensis appears to skillfully evade host immune systems by secreting nippocystatin, which modulates antigen processing in antigen-presenting cells of hosts.
To adapt to and survive in hosts, parasites often cleverly modulate host defense systems (3, 6, 15). Before protective immunity is developed, pathogenic antigens must be processed by a set of lysosomal proteases, such as cathepsins in professional antigen-presenting cells (APCs), and then the processed peptides must be presented by major histocompatibility complex molecules expressed on the APCs (30). Many studies have shown that intracellular protozoan parasites block the antigen presentation pathway in the host APCs, especially in hosts with leishmanial infections (2, 3, 6, 15). However, there is little evidence that extracellular parasites, including helminths, modulate the antigen presentation of host APCs.
Natural cysteine protease inhibitors are members of the cystatin superfamily and are subdivided into family 1 (stefin family), family 2 (cystatin family), and family 3 (kininogen family) (26). There are high levels of sequence homology among members of these families, particularly at a highly conserved reactive site consisting of five amino acids, Gln-Val-Val-Ala-Gly (1). Members of family 2 (cystatin family) are secretion type proteins that have a single domain with a molecular mass of 13 to 15 kDa. In nematodes, members of this family have been found in Caenorhabditis elegans (33) and in some species of filariae (7, 8, 9, 13). Recombinant cystatin of the filaria Acanthocheilonema viteae reportedly suppresses T-cell proliferation, but this suppression seems to be nonspecific, since proliferation of T lymphocytes stimulated with concanavalin A (ConA) or anti-CD3 is suppressed (9). Although cystatins from nematodes are thought to suppress the proteases involved in antigen processing through their intrinsic functions, it is not known whether cystatins from nematodes can modulate antigen processing and/or presentation in the host APCs during infection.
We identified a new 14-kDa cystatin family 2 protein, named nippocystatin, in the intestinal nematode Nippostrongylus brasiliensis. Recombinant nippocystatin (rNbCys) selectively inhibited cysteine proteases. Furthermore, treatment of mice with rNbCys modified antigen processing and thereby modulated antigen-specific immune responses, suggesting that N. brasiliensis utilizes this protease inhibitor to evade the host defense system.
MATERIALS AND METHODS
Animals.
Female BALB/c CrSlc mice were purchased from the Japan Shizuoka Laboratory Animal Center (Hamamatsu, Japan). The animals were 8 to 10 weeks old at the start of the experiments.
Parasites.
The strain of N. brasiliensis used was provided by M. Yamada, Kyoto Prefectural University of Medicine, Kyoto, Japan (32). The parasites were maintained by serial passage in SD rats or in B6 mice. For assays, BALB/c mice were subcutaneously infected with 700 infective-stage larvae. The severity of infection was evaluated by determining the number of eggs per gram of feces daily.
Antigen.
Excretory-secretory (ES) products of N. brasiliensis adults were collected by the method described below. Adult N. brasiliensis worms, collected from the small intestines of rats that had been infected 7 days previously with 4,000 infective-stage larvae, were sterilized by repeatedly washing them with phosphate-buffered saline (PBS) containing penicillin and streptomycin. Worms were cultured with PBS at 37°C for 6 h. The culture supernatant was collected, concentrated with a Centricon Plus 20 PL-10 centrifuge (Millipore Corporation, Bedford, Mass.), and used as the ES products.
Immunization.
For ovalbumin (OVA) immunization, mice were each injected intraperitoneally with 3 μg of OVA adsorbed to 4 mg of aluminum hydroxide gel (ALUM) in 0.5 ml of PBS. For rNbCys or ES immunization, mice were each injected intraperitoneally with 3 μg of protein adsorbed to 4 mg of ALUM in 0.5 ml of PBS 4 weeks and 1 week before infection with N. brasiliensis.
cDNA cloning of nippocystatin.
Total RNA was isolated from adult N. brasiliensis worms, prepared by the method described above, by using Trizol reagent (Life Technologies, Rockville, Md.). A fragment of NbCys cDNA was obtained by reverse transcription-PCR. The total RNA was reverse transcribed by using hexanucleotide random primers with Superscript II reverse transcriptase (Life Technologies). Then the cDNA was amplified with Taq DNA polymerase (Takara Shuzo, Shiga, Japan). The thermocycling conditions were 35 cycles of 94°C for 30 s, 52°C for 30 s, and 72°C for 30 s. The sense and antisense primer sequences were 5′-TCATCTCAAGTTGTCGCTGGT-3′ and 5′-AAATTTTCCCATGGTTTCTCCCA-3′ and were based on sequences conserved in previously described cystatins from other nematodes, including Onchocerca volvulus (13), Brugia malayi (8), A. viteae (9), and C. elegans (31). Amplified DNAs were resolved by 2 to 3% agarose gel electrophoresis and stained with ethidium bromide. DNA was extracted with a Qiaex II gel extraction kit (Qiagen, Hilden, Germany) and then subcloned into the pGEM-T Easy sequencing vector (Promega, Madison, Wis.). Plasmids were extracted with a Qiaprep spin miniprep kit (Qiagen). The DNA sequences were determined with an ABI PRISM BigDye terminator cycle sequencing ready reaction kit (Perkin-Elmer, Norwalk, Conn.) and an ABI PRISM 377 DNA sequencer (Perkin-Elmer). On the basis of the nucleotide sequence of the cDNA fragment, specific primers were then synthesized for 3′ and 5′ rapid amplification of cDNA ends (RACE). For 3′ RACE, total RNA from adult N. brasiliensis worms was reverse transcribed by using a NotI primer adapter with deoxyribosylthymine polymer (Life Technologies). The adapter-containing cDNA was amplified with Taq DNA polymerase. The thermocycling conditions were 35 cycles of 94°C for 1 min, 64°C for 1 min, and 72°C for 1 min. The sense primer sequence was 5′-GCAAGCGAACTTACGGCGACGA-3′, and the antisense primer was the adapter primer. For 5′ RACE, total RNA from adult N. brasiliensis worms was reverse transcribed by using the gene-specific primer 5′-TGGCAGTTCGTCGCCGTAAGTTCG-3′. The deoxycytosine polymer was added to the resulting reverse-transcribed product with terminal deoxynucleotidyltransferase and was amplified with Taq DNA polymerase. The thermocycling conditions were 30 cycles of 94°C for 1 min, 54°C for 1 min, and 72°C for 1 min. The sense primer was the adapter primer with deoxyguanine polymer (Life Technologies), and the antisense primer was 5′-TCGCCGTAAGTTCGCTTGCAG-3′. The DNA sequences of the RACE-PCR products were determined by using the sequencing kit mentioned above.
Expression of rNbCys.
The nippocystatin cDNA was subcloned into expression plasmid vector pET and expressed in Escherichia coli BL21 as a protein fused to a leader sequence of the influenza virus hemagglutinin epitope and six histidines. rNbCys was purified from E. coli lysate by affinity chromatography using TALON resin (Clontech Laboratories Inc., Palo Alto, Calif.). The purity of rNbCys was determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and staining with Coomassie brilliant blue (CBB). Purified rNbCys was washed and dialyzed with PBS.
Measurement of protease activities and inhibition of the activities.
The activities of cysteine proteases were measured as follows. Protease was incubated with or without nippocystatin in 0.1 M sodium acetate buffer (pH 5.5) containing 1 mM EDTA, 8 mM cysteine, and 20 μM Z-Phe-Arg-MCA (carbobenzoxy-l-phenylalanyl-l-arginine-4-methyl-coumaryl-7-amide; Peptide Institute, Osaka, Japan) as the substrate at 37°C for 15 min. The reaction was stopped by adding 100 mM monochloroacetate (pH 4.3). The amount of product was monitored fluorometrically with excitation at 370 nm and emission at 460 nm by using a fluorescence spectrometer (Hitachi, Ibaraki, Japan). The activities of the cysteine proteases in the mitochondrion-lysosome (ML) fraction of mouse spleens were measured by a specific assay method using E64 and CA074, as reported previously (10). The activity of cathepsin D was measured by a Folin-Lowry reaction method, as reported previously (25).
OVA processing assay.
OVA was digested at pH 5.0 for 2 h at 37°C with cathepsin B, cathepsin L, or the ML fraction of spleen cells from naive mice in the presence of rNbCys at different concentrations. As a negative control, OVA was also digested with cathepsin D at pH 3.5 for 2 h at 37°C in the presence of 10−6 M rNbCys or in the presence of the same concentration of pepstatin A. After digestion, samples were separated by SDS-PAGE and detected by CBB staining. The ML fraction was prepared by using a previously described protocol (33).
Immunoblotting.
Proteins were separated by SDS-PAGE and detected by CBB staining. The gels were electroblotted onto a polyvinylidene difluoride membrane (Millipore). Rat anti-rNbCys antiserum diluted 1:5,000 was used as the first antibody, and peroxidase-conjugated anti-rat immunoglobulin G (IgG) (Cappel, Durham, N.C.) diluted 1:5,000 was used as the second antibody. Bound antibody was detected by using enhanced chemiluminescence reagents (Amersham Pharmacia Biotech, Buckinghamshire, United Kingdom).
Detection of cysteine protease inhibitor on a gel.
Active cystatin was detected by using a previously described protocol (11). Briefly, proteins were separated by SDS-PAGE, and the gel was washed and absorbed with papain in 0.1 M sodium acetate buffer (pH 5.5) containing 1 mM EDTA and 8 mM cysteine at 37°C for 1 h. The gel was washed and incubated with a substrate, 0.5 mM Z-Phe-Arg-MCA, at 37°C for 30 min. After washing, enzymatic activity was visualized with UV light, and cystatin was detected as a nonfluorescent spot.
Assay of proliferation of splenocytes in vitro.
A total of 4 × 105 splenocytes from mice were stimulated with a solution containing 10 μg of ConA per ml or 400 μg of OVA per ml and were cultured in RPMI medium containing 10% fetal bovine serum for 72 h. For the last 6 h, the cells were cultured with 1 μCi of [3H]thymidine deoxyribose, and incorporation was determined by using a liquid scintillation counter (Aloka, Tokyo, Japan).
Measurement of cytokine production.
A total of 2 × 106 splenocytes from mice were stimulated with a solution containing 400 μg of OVA per ml and cultured in RPMI medium containing 10% fetal bovine serum for 72 h. The amounts of gamma interferon (IFN-γ) and interleukin 4 (IL-4) produced in the supernatants were measured with EM-IFNG and EM-IL4 enzyme-linked immunosorbent assay (ELISA) kits, respectively (Endogen, Cambridge, Mass.).
Measurement of OVA-specific antibody in serum.
Serum levels of OVA-specific antibodies were measured by ELISA by using 96-well plates coated with a solution containing 10 μg of OVA per ml. Serially diluted sera were added to the wells, and the preparations were incubated at 37°C for 2 h and then washed with PBS containing 0.05% Tween 20. Alkaline phosphatase-conjugated anti-mouse IgG1 or IgG2a or peroxidase-conjugated anti-mouse IgE (Southern Biotechnology Associates Inc., Birmingham, Ala.) was diluted 1:1,000 and added to the wells, and then the plates were incubated at 37°C for 2 h. After washing, enzymatic activity was visualized by using p-nitrophosphate or o-phenylenediamine as the substrate. Optical density was measured by using a test wavelength of 415 or 492 nm.
Nucleotide sequence accession number.
The nucleotide sequence of the nippocystatin cDNA has been deposited in the GenBank database under accession number AB050883.
RESULTS
Molecular cloning of nippocystatin and secretion of nippocystatin from N. brasiliensis.
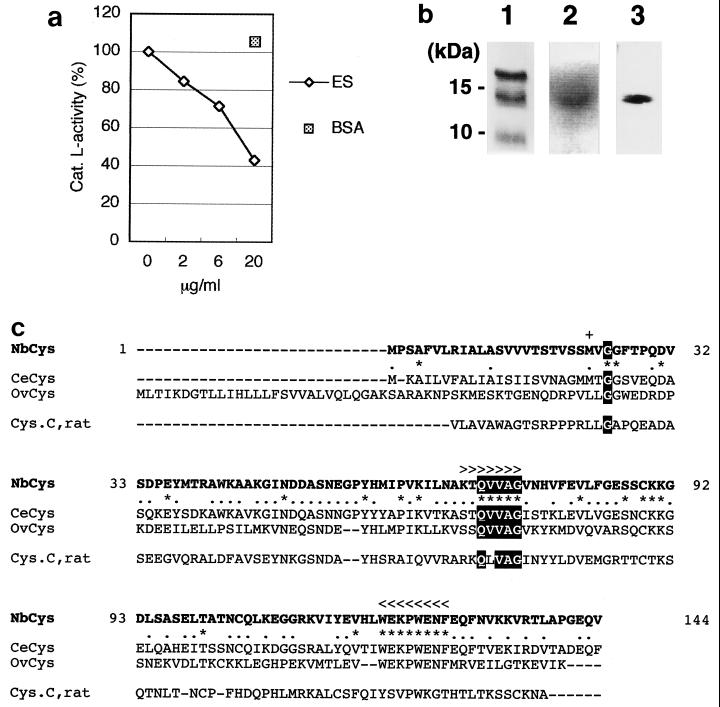
Cysteine proteases, such as cathepsin B and cathepsin L, are major components of proteases in the lysosomes of APCs, and they function in antigen processing, which is essential for inducing antigen-specific immune responses (18). On the other hand, some intracellular parasites express a cystatin-like protein that inhibits cysteine protease activity (11). To determine whether ES products from N. brasiliensis have a component that inhibits cysteine proteases, we examined the ability of ES products to inhibit cathepsin L. When cathepsin L was incubated with ES products, cathepsin L activity was inhibited in a dose-dependent manner (Fig. 1a). This inhibitory activity of ES products was not due to the large amount of protein, since an equivalent amount of bovine serum albumin did not suppress cathepsin L activity. We also investigated the presence of protease-inhibiting activity in ES products by examining the inhibition of another cysteine protease, papain, following separation by SDS-PAGE. After enzymatic reaction on a gel with a substrate, a 14-kDa single band appeared as a nonfluorescent spot under UV light (Fig. 1b, lane 2). These results indicate that ES products have a component that inhibits cysteine protease.
FIG. 1.
Cystatin derived from ES products of N. brasiliensis. (a) Inhibition of cysteine protease by ES products. Purified cathepsin L (cat. L) was incubated with the synthetic substrate Z-Phe-Arg-MCA in the presence of different amounts of ES products from N. brasiliensis or 20 μg of bovine serum albumin. (BSA). Protease activity was assessed by subtracting ES-derived activity. Incubation of cathepsin L without ES products resulted in 100% enzyme activity. (b) Nippocystatin in ES products. ES products were separated by SDS–15% PAGE and detected by CBB staining (lane 1), inhibition of papain (lane 2), and immunoblotting with rat anti-rNbCys antiserum (lane 3). (c) Alignment of the amino acid sequences translated from nippocystatin (NbCys), C. elegans cystatin (CeCys), onchocystatin (OvCys), and rat cystatin C (Cys.C, rat) cDNAs. Sequences conserved in the cystatin superfamily are highlighted. For the three cystatin sequences from nematodes, completely conserved amino acids are indicated by asterisks and partially conserved amino acids are indicated by dots. Gene-specific PCR primers for cloning nippocystatin cDNA were designed on the basis of conserved sequences indicated by more-than signs for the 5′ primer and by less-than signs for the 3′ primer. The first amino acid of mature nippocystatin is indicated by a plus sign and was predicted by using the program SignalP v1.1 (http://www.cbs.dtu.dk/services/SignalP/) (19).
Cystatin is known to be a natural inhibitor of cysteine proteases and to be conserved in eukaryotes. In nematodes, cystatin has been identified in filariae and C. elegans (7, 8, 9, 13, 31). We identified cystatin cDNA in N. brasiliensis by using primers for consensus sequences found in nematode cystatins. The amino acid sequence of the cystatin from N. brasiliensis, nippocystatin, is shown in Fig. 1c. The length of the open reading frame is 435 bp, and the protein consists of 144 amino acids. The cloned protein had a stretch of five amino acids, QVVAG, which is highly conserved in the cystatin superfamily, and it had a hydrophobic signal peptide like other members of cystatin family 2 (Fig. 1c). Nippocystatin was expressed at the mRNA level both in infective third-stage larvae and in adult worms (data not shown). Alignment of the amino acid sequences of nippocystatin and cystatins from other nematodes by using the BLASTP program with sequences in the database of the National Center for Biotechnology Information revealed the following levels of similarity: two cystatins from C. elegans, 72 and 63%; onchocystatin from O. volvulus, 55%; A. viteae cystatin, 33%; and B. malayi cystatin, 30%. The amino acid sequence of nippocystatin showed less similarity to the rat cystatin C sequence (27%). These levels of similarity among amino acid sequences do not contradict taxonomic findings, since both N. brasiliensis and C. elegans belong to the order Rhabditida, while other filarial parasites belong to the order Spiruria.
Since the nippocystatin cDNA encoded a hydrophobic signal peptide with 22 amino acids, nippocystatin was expected to be secreted as a 14-kDa mature form without a signal sequence. To confirm that the 14-kDa inhibitory component in ES products is nippocystatin, we analyzed ES products by immunoblotting with anti-rNbCys antiserum (Fig. 1b, lane 3). We detected nippocystatin as a 14-kDa band in ES products, suggesting that the cysteine protease-inhibiting component in ES products is nippocystatin.
Nippocystatin selectively inhibits cysteine proteases.
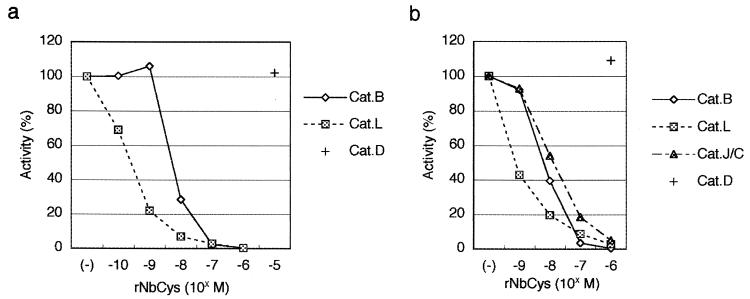
To investigate the function of nippocystatin in the host-parasite relationship, we first used rNbCys to examine the ability of nippocystatin to inhibit several cathepsins (Fig. 2a). rNbCys strongly inhibited the enzymatic activities of two cysteine proteases, cathepsin L and cathepsin B. The concentration of rNbCys at which cathepsin L was inhibited was lower than the concentration of rNbCys at which cathepsin B was inhibited. On the other hand, the aspartic protease cathepsin D was not inhibited by rNbCys even at an extremely high concentration. These findings indicate that nippocystatin can inhibit cysteine proteases, like other members of the cystatin family. In order to investigate the function of nippocystatin in a more physiological condition, a crude mitochondrial lysosomal (ML) fraction obtained from mouse spleens was incubated with a substrate in the presence of rNbCys, and the activities of the cysteine proteases in the ML fraction were determined by a specific assay method (10). The activities of the cysteine proteases cathepsins L, B, and J/C in the ML fraction were specifically inhibited (Fig. 2b).
FIG. 2.
Inhibition of protease activities by rNbCys. (a) Ability of rNbCys to inhibit purified cathepsins. Purified cathepsin B (Cat.B), cathepsin L (Cat.L), and cathepsin D (Cat.D) were incubated with each of the substrates in the presence of different concentrations of rNbCys. Incubation of cathepsins without rNbCys resulted in 100% enzyme activity. (b) Ability of rNbCys to inhibit each of the proteases in the ML fraction obtained from mouse spleens. Different concentrations of rNbCys were incubated with the ML fraction obtained from mouse spleens in the presence of each of the substrates. Cysteine protease activities were determined by subtracting the activities in the presence of specific inhibitors from the total activity. Cat.J/C, cathepsin J/C.
Antigen-specific proliferation of spleen cells is inhibited in OVA-immunized mice treated with nippocystatin in vivo.
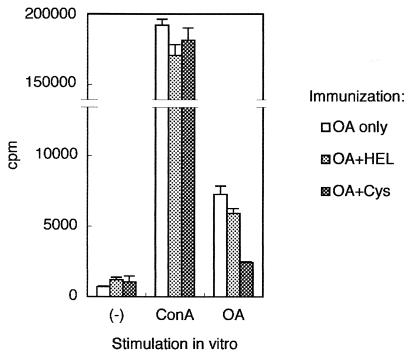
We next investigated whether rNbCys could affect antigen-specific immune responses in antigen-immunized mice. When mice were treated intraperitoneally with rNbCys, the total activity of lysosomal cysteine proteases from the spleens or mesenteric lymph nodes was reduced by 15 to 20% 2 h after treatment (data not shown). We then inoculated mice with rNbCys and simultaneously immunized them with OVA adsorbed to ALUM. Ten days later, spleen cells from these mice were isolated and used for assays. The antigen-specific proliferation of spleen cells from OVA-immunized mice treated with rNbCys was less for rNbCys-treated mice than for mice treated with hen egg lysozyme, a control protein whose molecular size is comparable to the size of rNbCys (Fig. 3). In contrast, the degrees of ConA-induced cellular proliferation in the two groups were comparable, indicating that antigen-specific proliferation was profoundly suppressed by in vivo treatment of mice with rNbCys but that nonspecific proliferation was not suppressed.
FIG. 3.
Antigen-specific proliferation of spleen cells from OVA-immunized mice treated with rNbCys. BALB/c mice were intraperitoneally inoculated with 3 μg of OVA adsorbed to 4 mg of ALUM and were treated with PBS, with 0.2 mg of rNbCys (Cys), or with hen egg lysozyme (HEL). Ten days later, 4 × 105 splenocytes from mice were cultured with a solution containing 10 μg of ConA per ml or 400 μg of OVA per ml for 72 h. For the last 6 h, the cells were incubated with 1 μCi of [3H]thymidine deoxyribose, and incorporation was determined. All in vivo observations were reproduced more than three times. OA, ovalbumin.
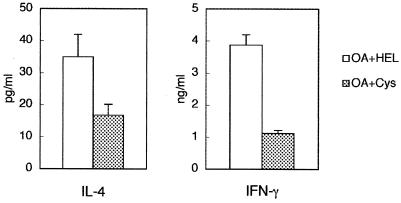
Next, we investigated antigen-specific cytokine production in these mice. Antigen-specific production of IL-4 and IFN-γ from splenocytes was suppressed in mice treated with rNbCys compared to antigen-specific production in mice treated with control protein (Fig. 4). These results suggest that treatment with rNbCys modifies the antigen-specific immune responses.
FIG. 4.
Cytokine production by spleen cells from OVA-immunized mice treated with rNbCys. BALB/c mice were intraperitoneally inoculated with 3 μg of OVA adsorbed to 4 mg of ALUM and were treated with 0.2 mg of rNbCys (Cys) or hen egg lysozyme (HEL). Ten days later, 2 × 106 splenocytes from mice were cultured with a solution containing 400 μg of OVA per ml for 72 h. The IL-4 and IFN-γ in culture supernatants were quantified by cytokine ELISA. OA, ovalbumin.
Profile of antigen-specific antibodies in OVA-immunized mice treated with nippocystatin in vivo.
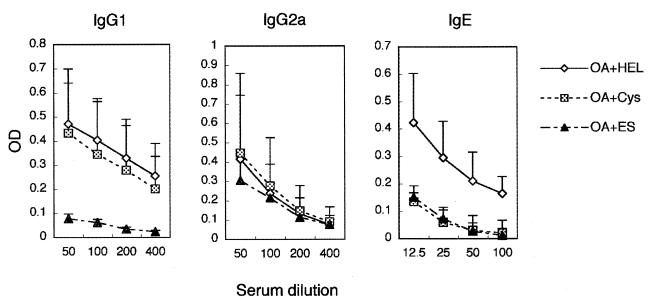
We investigated the serum levels of antigen-specific antibodies in OVA-immunized mice treated with rNbCys (Fig. 5). The levels of OVA-specific IgG1 and IgG2a in rNbCys-treated mice and control mice were not significantly different. However, the level of OVA-specific IgE was markedly suppressed in rNbCys-treated mice. The suppression of antigen-specific IgE was analogous to that in mice treated with ES products of N. brasiliensis (Fig. 5). In the ES-treated mice, OVA-specific IgG1 was suppressed, but IgG2a was not affected. In both the rNbCys- and ES-treated mice, the level of non-antigen-specific total serum IgE was not affected at all (data not shown). These results suggest that rNbCys suppresses the production of antigen-specific IgE.
FIG. 5.
Serum levels of antigen-specific antibodies of OVA-immunized mice treated with rNbCys. BALB/c mice were intraperitoneally inoculated with 3 μg of OVA adsorbed to 4 mg of ALUM and were treated with 0.2 mg of rNbCys (Cys), ES products, or hen egg lysozyme (HEL). Ten days later, sera were collected, and the titers of antigen-specific IgG1, IgG2a, and IgE were determined by ELISA. OA, ovalbumin; OD, optical density.
Nippocystatin inhibits the processing of OVA by lysosomes in vitro.
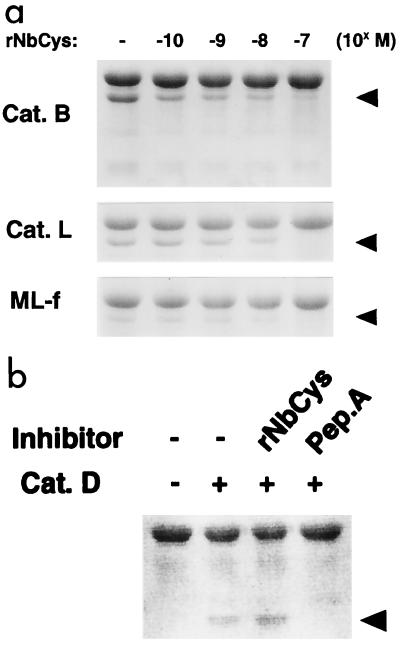
We next investigated whether rNbCys could inhibit OVA processing by cathepsin B and cathepsin L, which are major components of lysosomal proteases. OVA and purified cathepsins were coincubated in the presence or absence of rNbCys, and the processing pattern of OVA was determined by SDS-PAGE. rNbCys clearly inhibited OVA processing by cathepsin B or cathepsin L in vitro (Fig. 6a). Moreover, rNbCys also inhibited processing of OVA by the ML fraction from mouse spleens (Fig. 6a). On the other hand, processing of OVA by cathepsin D was not inhibited by rNbCys at all, whereas pepstatin A, a synthetic cathepsin D inhibitor, completely inhibited cathepsin D activity (Fig. 6b). These results suggest that nippocystatin can actually inhibit the processing of OVA by lysosomal proteases from mouse APCs.
FIG. 6.
(a) Inhibition of OVA processing by rNbCys in vitro. OVA was incubated with cathepsin B (Cat. B), cathepsin L (Cat. L), and the ML fraction of mouse spleens (ML-f) in the presence of different concentrations of rNbCys. Processed OVA was separated by SDS-PAGE and stained with CBB. The arrowheads indicate the positions of processed OVA. (b) Processing of OVA by cathepsin D (Cat. D). OVA was incubated with cathepsin D in the presence of 10−6 M rNbCys or in the presence of the same concentration of pepstatin A (Pep.A), a synthetic inhibitor of cathepsin D. Processed OVA was separated by SDS-PAGE and stained with CBB. Processing was completely inhibited by pepstatin A but not by rNbCys. The arrowhead indicates the position of processed OVA.
Mice immunized with rNbCys become more resistant to infection with N. brasiliensis.
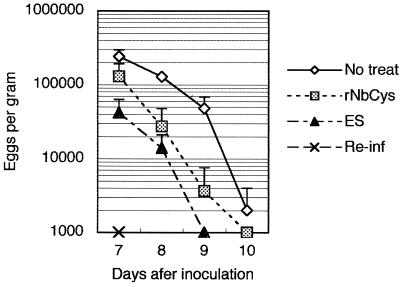
In order to determine whether nippocystatin is actually involved in evasion of the host defenses by N. brasiliensis, we immunized mice repeatedly with rNbCys to produce anti-rNbCys antibodies. Although rNbCys has an immunosuppressive function, as described above, repeated immunization with rNbCys in ALUM induced production of anti-nippocystatin antibody (data not shown). After we confirmed that the anti-rNbCys antibody titer had increased (data not shown), the mice were infected with third-stage larvae. rNbCys-immunized mice were more resistant to infection with N. brasiliensis than nonimmunized mice were, but the resistance was lower than that of ES-immunized mice or mice infected previously (Fig. 7). These findings indicate that N. brasiliensis may prolong its infection by secreting nippocystatin.
FIG. 7.
Effect of immunization of mice with nippocystatin on N. brasiliensis infection. Mice were injected intraperitoneally with 3 μg of rNbCys or ES products adsorbed to 4 mg of ALUM in 0.5 ml of PBS 4 weeks and 1 week before infection with N. brasiliensis. The severity of infection was evaluated by determining the number of eggs per gram of feces daily. As a negative control, untreated mice were infected (No treat). The mice that were immunized with N. brasiliensis were also reinfected to analyze protection of fully immunized mice (Re-inf).
DISCUSSION
We cloned and characterized a 14-kDa protein, named nippocystatin, from N. brasiliensis; this protein is a member of the cystatin family. Nippocystatin was detected in ES products of N. brasiliensis. We also synthesized rNbCys. In vivo treatment of BALB/c mice with rNbCys modulated antigen-specific immune responses to OVA. Moreover, preimmunization with rNbCys made the host more resistant to infection with N. brasiliensis. Alternatively, nippocystatin is a novel candidate for the immunomodulatory effector secreted by N. brasiliensis, and this cystatin is thought to suppress host immunity to infection with N. brasiliensis.
Recently, there have been several reports of modulation of immune responses by members of the cystatin family. For example, chicken cystatin was found to upregulate NO release from IFN-γ-activated peritoneal macrophages of the mouse via cytokine synthesis (27, 28). Cystatins from parasites have also been reported to have immunomodulatory effects. Recombinant cystatin from a filarial parasite, A. viteae, was found to downregulate T-lymphocyte proliferation and enhance IL-10 production in vitro (9). However, this suppressive effect was exerted not only on antigen-specific proliferation but also on ConA-induced nonspecific proliferation (9). In the present study, we found that rNbCys suppressed specific immune responses in vivo without suppressing nonspecific proliferative responses stimulated by ConA. Garraud et al. reported that the recombinant cystatin of O. volvulus induces either polyclonal or antigen-specific IgE and IgG4 antibodies in vitro in an IL-4- and/or IL-13-dependent manner (7). These in vitro results conflict with our in vivo data since treatment of mice with rNbCys suppressed the production of antigen-specific IgE antibody. Thus, nippocystatin appears to have biological functions that are very different from those of the other cystatins mentioned above.
We previously reported that treatment with CA074, a synthetic specific inhibitor of lysosomal cathepsin B, resulted in changes in immune responses from the Th2 type to the Th1 type in BALB/c mice infected with Leishmania major (14); that is, treatment with CA074 suppressed IL-4 production and augmented IFN-γ production. In the present study, on the other hand, treatment of mice with rNbCys reduced both IL-4 and IFN-γ production in response to antigenic stimuli, indicating that this cystatin inhibits multiple cysteine proteases more than CA074 inhibits them. Many studies have suggested that the function of activated CD4 T-cells is greatly affected by the sequences or concentrations of epitopic peptides presented by major histocompatibility complex class II molecules (5, 17). It is generally accepted that the function of lysosomes in APCs is regulated autonomously (12) and might be regulated exogenously by protease inhibitors released from infectious pathogens. Our results strongly suggest that nippocystatin secreted by N. brasiliensis during infection modifies host immune responses by interfering with antigen processing. Another possibility is that nippocystatin inhibits degradation of invariant chains or degradation of other molecules crucial for the development of some types of antigen-specific responses (4, 29, 33). At present, the precise mechanism of immune suppression exerted by the protein is not clear.
In OVA-immunized mice treated with rNbCys, production of antigen-specific IgE was suppressed, while the total serum IgE level was not affected. These findings are compatible with the fact that the concentration of ES product-specific IgE never reached a detectable level in N. brasiliensis-infected mice, although the total serum level of IgE was elevated several hundredfold (20; M. Yamada, personal communication). In general, class switching of antibodies to IgG1 and IgE is dependent on IL-4 (21, 22, 24). In mice immunized with OVA and treated with rNbCys, production of antigen-specific antibodies was suppressed only in the IgE class (Fig. 5), despite the fact that the production of both IL-4 and IFN-γ was downregulated (Fig. 4). This discrepancy is not surprising, since class switching to IgE is thought to require a relatively high dose of a cytokine(s) compared with the doses required for class switching to other immunoglobulin classes (16). Therefore, reduction of the cytokine level should have a greater effect on production of IgE than on production of other classes of antibodies. A recent study suggested that the activity of cathepsin F, a cysteine protease that preferentially locates in macrophages, is intimately correlated with the IgE response to OVA immunization (23). If this lysosomal protease plays an essential role in processing of antigens or contributes to indispensable steps in the IgE response, nippocystatin might also inhibit the activity of cathepsin F.
Does nippocystatin actually function in evasion of host defense systems during N. brasiliensis infection via a mechanism similar to the mechanism by which rNbCys suppresses specific IgE production in OVA-immunized mice? At least nippocystatin is one of the key molecules for residence of N. brasiliensis in hosts, since mice with a high titer of anti-nippocystatin exhibited partial resistance to infection with N. brasiliensis. There has been very little investigation of other physiological functions of cystatins in nematodes. Further experiments are needed to determine whether these cystatins are indispensable for survival of nematodes in their hosts.
ACKNOWLEDGMENTS
We thank Minoru Yamada for providing the strain of N. brasiliensis and for his helpful suggestions. We thank Goro Matsuzaki for his critical reading of the manuscript and for his helpful suggestions.
This work was supported in part by grants-in-aid 10044297, 10470067, 10770140, 11147219, 11153219, 11770131, 11877046, and 12217101 from the Japanese Ministry of Education, Science, and Culture and by research fellowships from the Japan Society for the Promotion of Science for Young Scientists.
REFERENCES
- 1.Abrahamson M, Ritonja A, Brown M A, Grubb A, Machleidt W, Barrett A J. Identification of the probable inhibitory reactive sites of the cysteine proteinase inhibitors human cystatin C and chicken cystatin. J Biol Chem. 1987;262:9688–9694. [PubMed] [Google Scholar]
- 2.Bogdan C, Rölinghoff M. The immune response to Leishmania: mechanisms of parasite control and evasion. Int J Parasitol. 1998;28:121–134. doi: 10.1016/s0020-7519(97)00169-0. [DOI] [PubMed] [Google Scholar]
- 3.Bogdan C, Rölinghoff M. How do protozoan parasites survive inside macrophages? Parasitol Today. 1999;15:22–28. doi: 10.1016/s0169-4758(98)01362-3. [DOI] [PubMed] [Google Scholar]
- 4.Busch R, Doebele R C, Patil N S, Pashine A, Mellins E D. Accessory molecules for MHC class II peptide loading. Curr Opin Immunol. 2000;12:99–106. doi: 10.1016/s0952-7915(99)00057-6. [DOI] [PubMed] [Google Scholar]
- 5.Constant S L, Bottomly K. Induction of Th1 and Th2 CD4+ T cell responses: the alternative approaches. Annu Rev Immunol. 1997;15:297–322. doi: 10.1146/annurev.immunol.15.1.297. [DOI] [PubMed] [Google Scholar]
- 6.Dessaint J P L, Capron A R. Survival strategies of parasites in their immunocompetent hosts. In: Warren K S, editor. Immunology and molecular biology of parasitic infections. 3rd ed. Boston, Mass: Blackwell Scientific Publications; 1993. pp. 87–99. [Google Scholar]
- 7.Garraud O, Nkenfou C, Bradley J E, Perler F B, Nutman T B. Identification of recombinant filarial proteins capable of inducing polyclonal and antigen-specific IgE and IgG4 antibodies. J Immunol. 1995;155:1316–1325. [PubMed] [Google Scholar]
- 8.Gregory W F, Blaxter M L, Maizels R M. Differentially expressed, abundant trans-spliced cDNAs from larval Brugia malayi. Mol Biochem Parasitol. 1997;87:85–95. doi: 10.1016/s0166-6851(97)00050-9. [DOI] [PubMed] [Google Scholar]
- 9.Hartmann S, Kyewski B, Sonnenburg B, Lucius R. A filarial cysteine protease inhibitor down-regulates T cell proliferation and enhances interleukin-10 production. Eur J Immunol. 1997;27:2253–2260. doi: 10.1002/eji.1830270920. [DOI] [PubMed] [Google Scholar]
- 10.Inubushi T, Kakegawa H, Kishino Y, Katunuma N. Specific assay method for the activities of cathepsin L-type cysteine proteinases. J Biochem (Tokyo) 1994;116:282–284. doi: 10.1093/oxfordjournals.jbchem.a124520. [DOI] [PubMed] [Google Scholar]
- 11.Irvine J W, Coombs G H, North M J. Cystatin-like cysteine proteinase inhibitors of parasitic protozoa. FEMS Microbiol Lett. 1992;75:67–72. doi: 10.1016/0378-1097(92)90458-z. [DOI] [PubMed] [Google Scholar]
- 12.Katunuma N. Mechanisms and regulation of lysosomal proteolysis. Rev Biol Cel. 1989;20:35–61. [PubMed] [Google Scholar]
- 13.Lustigman S, Brotman B, Huima T, Prince A M, McKerrow J H. Molecular cloning and characterization of onchocystatin, a cysteine proteinase inhibitor of Onchocerca volvulus. J Biol Chem. 1992;267:17339–17346. [PubMed] [Google Scholar]
- 14.Maekawa Y, Himeno K, Ishikawa H, Hisaeda H, Sakai T, Dainichi T, Asao T, Good R A, Katunuma N. Switch of CD4+ T cell differentiation from Th2 to Th1 by treatment with cathepsin B inhibitor in experimental leishmaniasis. J Immunol. 1998;161:2120–2127. [PubMed] [Google Scholar]
- 15.Mauël J. Intracellular survival of protozoan parasites with special reference to Leishmania spp., Toxoplasma gondii and Trypanosoma cruzi. Adv Parasitol. 1996;38:1–51. doi: 10.1016/s0065-308x(08)60032-9. [DOI] [PubMed] [Google Scholar]
- 16.Moon H B, Severinson E, Heusser C, Johansson S G, Moller G, Persson U. Regulation of IgG1 and IgE synthesis by interleukin 4 in mouse B cells. Scand J Immunol. 1989;30:355–361. doi: 10.1111/j.1365-3083.1989.tb01221.x. [DOI] [PubMed] [Google Scholar]
- 17.Murray J S. How the MHC selects Th1/Th2 immunity. Immunol Today. 1998;19:157–163. doi: 10.1016/s0167-5699(97)01237-1. [DOI] [PubMed] [Google Scholar]
- 18.Nakagawa T Y, Rudensky A Y. The role of lysosomal proteinases in MHC class II-mediated antigen processing and presentation. Immunol Rev. 1999;172:121–129. doi: 10.1111/j.1600-065x.1999.tb01361.x. [DOI] [PubMed] [Google Scholar]
- 19.Nielsen H, Engelbrecht J, Brunak S, von Heijne G. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 1997;10:1–6. doi: 10.1093/protein/10.1.1. [DOI] [PubMed] [Google Scholar]
- 20.Pfeiffer P, König W, Bohn A. Genetic dependence of IgE antibody production in mice infected with the nematode Nippostrongylus brasiliensis. I. Modulation of the IgE antibody response in vivo by serum factors. Int Arch Allergy Appl Immunol. 1983;72:347–355. doi: 10.1159/000234894. [DOI] [PubMed] [Google Scholar]
- 21.Rothman P, Lutzker S, Cook W, Coffman R, Alt F W. Mitogen plus interleukin 4 induction of Cɛ transcripts in B lymphoid cells. J Exp Med. 1988;168:2385–2389. doi: 10.1084/jem.168.6.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Severinson E, Fernandez C, Stavnezer J. Induction of germ-line immunoglobulin heavy chain transcripts by mitogens and interleukins prior to switch recombination. Eur J Immunol. 1990;20:1079–1084. doi: 10.1002/eji.1830200520. [DOI] [PubMed] [Google Scholar]
- 23.Shi G P, Bryant R A, Riese R, Verhelst S, Driessen C, Li Z, Bromme D, Ploegh H L, Chapman H A. Role for cathepsin F in invariant chain processing and major histocompatibility complex class II peptide loading by macrophages. J Exp Med. 2000;191:1177–1186. doi: 10.1084/jem.191.7.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stavnezer J, Radcliffe G, Lin Y C, Nietupski J, Berggren L, Sitia R, Severinson E. Immunoglobulin heavy-chain switching may be directed by prior induction of transcripts from constant-region genes. Proc Natl Acad Sci USA. 1988;85:7704–7708. doi: 10.1073/pnas.85.20.7704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turk V, Lah T, Kregar I. Cathepsin D, cathepsin E. In: Bergmeyer H U, editor. Methods of enzymatic analysis. 3rd ed. Vol. 5. Weinheim, Germany: Verlag Chemie GmbH; 1984. pp. 211–222. [Google Scholar]
- 26.Turk V, Bode W. The cystatins: protein inhibitors of cysteine proteinases. FEBS Lett. 1991;285:213–219. doi: 10.1016/0014-5793(91)80804-c. [DOI] [PubMed] [Google Scholar]
- 27.Verdot L, Lalmanach G, Vercruysse V, Hartmann S, Lucius R, Hoebeke J, Gauthier F, Vray B. Cystatins up-regulate nitric oxide release from interferon-gamma-activated mouse peritoneal macrophages. J Biol Chem. 1996;271:28077–28081. doi: 10.1074/jbc.271.45.28077. [DOI] [PubMed] [Google Scholar]
- 28.Verdot L, Lalmanach G, Vercruysse V, Hoebeke J, Gauthier F, Vray B. Chicken cystatin stimulates nitric oxide release from interferon-gamma-activated mouse peritoneal macrophages via cytokine synthesis. Eur J Biochem. 1999;266:1111–1117. doi: 10.1046/j.1432-1327.1999.00964.x. [DOI] [PubMed] [Google Scholar]
- 29.Villadangos J A, Bryant R A, Deussing J, Driessen C, Lennon-Dumenil A M, Riese R J, Roth W, Saftig P, Shi G P, Chapman H A, Peters C, Ploegh H L. Proteases involved in MHC class II antigen presentation. Immunol Rev. 1999;172:109–120. doi: 10.1111/j.1600-065x.1999.tb01360.x. [DOI] [PubMed] [Google Scholar]
- 30.Watts C. Capture and processing of exogenous antigens for presentation on MHC molecules. Annu Rev Immunol. 1997;15:821–850. doi: 10.1146/annurev.immunol.15.1.821. [DOI] [PubMed] [Google Scholar]
- 31.Wilson R, Ainscough R, Anderson K, Baynes C, Berks M, Bonfield J, Burton J, Connell M, Copsey T, Cooper J, et al. 2.2 Mb of contiguous nucleotide sequence from chromosome III of C. elegans. Nature. 1994;368:32–38. doi: 10.1038/368032a0. [DOI] [PubMed] [Google Scholar]
- 32.Yamada M, Nakazawa M, Matsumoto Y, Arizono N. IgE antibody production in rats against multiple components of excretory-secretory products of the nematode Nippostrongylus brasiliensis. Immunology. 1991;72:104–108. [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang T, Maekawa Y, Hanba J, Dainichi T, Nashed B F, Hisaeda H, Sakai T, Asao T, Himeno K, Good R A, Katunuma N. Lysosomal cathepsin B plays an important role in antigen processing, while cathepsin D is involved in degradation of the invariant chain in ovalbumin-immunized mice. Immunology. 2000;100:13–20. doi: 10.1046/j.1365-2567.2000.00000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]