Abstract
Rationale:
Inflammation contributes to lung function decline and the development of chronic obstructive pulmonary disease. Omega-3 fatty acids have anti-inflammatory properties and may benefit lung health.
Objectives:
Investigate associations of omega-3 fatty acids with lung function decline and incident airway obstruction in adults of diverse races/ethnicities from general population cohorts.
Methods:
Complementary study designs: (1) longitudinal study of plasma phospholipid omega-3 fatty acids and repeated FEV1 and FVC measures in the National Heart, Lung, and Blood Institute Pooled Cohorts Study, and (2) two-sample Mendelian Randomization (MR) study of genetically predicted omega-3 fatty acids and lung function parameters.
Measurements and Main Results:
The longitudinal study found that higher omega-3 fatty acid concentrations were associated with attenuated lung function decline in 15,063 participants, with the largest effect sizes for docosahexaenoic acid (DHA). One standard deviation higher DHA was associated with an attenuation of 1.8 mL/year for FEV1 (95% confidence interval [CI] 1.3–2.2) and 2.4 mL/year for FVC (95% CI 1.9–3.0). One standard deviation higher DHA was also associated with a 9% lower incidence of spirometry-defined airway obstruction (95% CI 0.86–0.97). DHA associations persisted across sexes, smoking histories, and Black, white and Hispanic participants, with the largest magnitude associations in former smokers and Hispanics. The MR study showed positive associations of genetically predicted omega-3 fatty acids with FEV1 and FVC, with statistically significant findings across multiple MR methods.
Conclusions:
The longitudinal and MR studies provide evidence supporting beneficial effects of higher circulating omega-3 fatty acids, especially DHA, on lung health.
Keywords: spirometry, nutrition, epidemiology, Mendelian Randomization
INTRODUCTION
Lung function increases through early adulthood, plateaus, and then declines with aging. Accelerated lung function decline increases the risk of chronic obstructive pulmonary disease (COPD), a leading cause of death in the United States. The primary risk factor for both accelerated lung function decline and COPD is cigarette smoking,1 which increases inflammation in the lungs. Nutrients like omega-3 fatty acids with anti-inflammatory properties represent biologically plausible agents for slowing the rate of lung function decline and preventing the development of chronic airway obstruction.
The omega-3 fatty acids include alpha-linolenic acid (ALA), eicosapentaenoic acid (EPA), docosapentaenoic acid (DPA), and docosahexaenoic acid (DHA). ALA—present in some vegetable oils—must be obtained through the diet, while EPA, DPA, and DHA—found mainly in fish—can also be derived endogenously from ALA.2 Metabolism of EPA, DPA, and DHA contributes to total body anti-inflammatory function. Metabolites of these fatty acids help resolve inflammation by blocking recruitment and inducing clearance of immune cells from sites of inflammation and by downregulating the expression of transcriptional factors like NF-κB that increase expression of inflammatory cytokines.3,4
Cross-sectional and case-control studies have reported that higher circulating omega-3 fatty acids correlate with higher lung function and lower risk of airway impairment.5–9 To our knowledge, no studies have investigated the relationship of circulating omega-3 fatty acids with subsequent decline in lung function and development of COPD in generally healthy adult populations, limiting causal inference. Randomized controlled trials of nutrition interventions for chronic disease outcomes like COPD are challenging, as most chronic diseases have long latency periods and the optimal window for interventions is unknown.10, 11 Alternative study designs to investigate the role of nutrition in the etiology of COPD are needed.
Longitudinal prospective cohorts and Mendelian Randomization (MR) provide two study designs to test whether observational data are consistent with the expectations of a causal relationship. Longitudinal prospective cohorts measure exposures prior to the occurrence of the outcome, thus mitigating concerns about reverse causality. MR studies, which examine genotypes associated with an exposure for relation to an outcome, help mitigate confounding bias by leveraging the inherent randomization of genotypes. We conducted a longitudinal observational study of circulating omega-3 fatty acids and lung function decline and a two-sample MR study of genetically predicted omega-3 fatty acids and lung function parameters. By triangulating evidence from these two study designs, our goal was to improve causal inference for associations of omega-3 fatty acids with longitudinal decline in lung function.
METHODS
We applied two complementary study designs: 1) a longitudinal observational study of omega-3 fatty acids and repeated measures of forced expiratory volume in the first second (FEV1) and forced vital capacity (FVC) in the NHLBI Pooled Cohorts Study, and 2) a two-sample MR study using publicly available genome-wide association study (GWAS) data of omega-3 fatty acid biomarkers, FEV1, FVC, and spirometry-defined airway obstruction. An overview of the study design is shown in Figure E1.
Longitudinal Observational Study
Population
The NHLBI Pooled Cohorts Study harmonized spirometry data from nine U.S. population-based cohorts.12 Of these, four cohorts—the Atherosclerosis Risk in Communities (ARIC) Study, Coronary Artery Risk Development in Young Adults (CARDIA) Study, Cardiovascular Health Study (CHS), and the Multi-Ethnic Study of Atherosclerosis (MESA)— measured omega-3 fatty acids in plasma phospholipids in some or all participants. We included all NHLBI Pooled Cohorts Study participants identifying as Black, white, Asian, or Hispanic with plasma phospholipid omega-3 fatty acids and lung function measurements.
Omega-3 Fatty Acid Measurements
Details on omega-3 fatty acid measurements in each cohort are presented in Table E1. Concentrations of ALA, EPA, DPA and DHA were expressed as a percentage of total plasma phospholipid fatty acids.
Lung Function Measurements
FEV1 and FVC were measured by spirometry. Details on cohort-specific spirometry are shown in Table E1. Harmonization of spirometry data across cohorts was previously described.12 Airway obstruction was defined as FEV1/FVC < 70%, following the criteria for persistent airflow limitation described in the Global Initiative for Chronic Obstructive Lung Disease 2022 report.13
Statistical Analysis
Baseline characteristics were calculated for the overall sample and by cohort. Differences in omega-3 fatty acid concentrations between participants who did or did not develop airway obstruction during follow-up were evaluated with Student’s t-tests. Correlations between fatty acid concentrations were evaluated with Pearson’s correlations.
Associations of omega-3 fatty acids with FEV1 and FVC decline were evaluated with linear mixed effects modeling. Associations of omega-3 fatty acids with incident airway obstruction were evaluated with Cox proportional hazards modeling. Details on statistical models, including covariate adjustments, are provided in the online data supplement.
Effect modification by sex, race/ethnicity, and smoking was investigated with three-way interaction terms. The Benjamini and Hochberg false discovery rate correction for multiple testing was applied to interaction analyses separately for each lung function phenotype; both unadjusted and false discovery rate adjusted p-values are presented. Stratified estimates were derived from interaction terms and covariances.
Analyses were performed in SAS version 9.4.
MR Study
Two-sample MR was performed to estimate associations of genetically predicted EPA, DPA, DHA, and total omega-3 fatty acids with FEV1, FVC, and spirometry-defined airway obstruction.
Omega-3 Fatty Acid Genetic Instruments
Genetic instruments were based on single nucleotide polymorphisms (SNPs) in distinct genetic loci identified in published omega-3 fatty acid GWAS.14–17 The F-statistic, a measure of instrument strength and validity, was calculated for all instruments.18
SNP–Omega-3 Fatty Acid Associations
Primary analyses for each fatty acid used SNP—fatty acid associations from the GWAS explaining, to our knowledge, the greatest proportion of variation in that fatty acid.14,15 Analyses were replicated using SNP—fatty acid associations from additional, independent GWAS.16,17 Summary data for all SNP—fatty acid associations are shown in Tables E2–E5.
SNP–Lung Function Associations
Publicly available data from lung function GWAS in European ancestry UK Biobank participants were used for all analyses.19,20 Data were extracted through the MR-Base platform (study IDs ukb-b-11141 for FEV1, ukb-b-14713 for FVC, and ieu-b-106 for airway obstruction). Further details on the genetic instruments and the omega-3 fatty acid and lung function GWAS are presented in the online data supplement.
Statistical Analysis
MR analyses used the inverse variance weighted (IVW) method, which assumes pleiotropy is either nonexistent or balanced, and the MR-Egger, weighted-median, and weighted mode methods, which can provide valid estimates when the assumption of zero or balanced pleiotropy is violated.21–24 Analyses were performed in R Studio version 1.2.1335 using the “TwoSampleMR” R package (https://github.com/MRCIEU/TwoSampleMR).25
RESULTS
Longitudinal Analysis
Participant Characteristics
Analyses of lung function decline included 15,063 participants from four cohorts (Table 1). The mean age at first spirometry visit was 56 years. The total sample was comprised of 55% female and 69% white, 20% Black, 4% Asian, and 7% Hispanic participants. Forty-eight percent were never smokers, 36% were former smokers, and 16% were current smokers. Mean concentrations for ALA, EPA, DPA, and DHA were 0.2%, 0.7%, 0.9%, and 3.3%, respectively, of total plasma phospholipid fatty acids. Participants had a mean of 2.6 spirometry measurements; 82% had at least two measurements and 49% had at least three measurements. The median (interquartile range) follow-up time among participants with at least two spirometry measurements was 7(16) years. Twenty-three percent of participants had spirometry-defined airway obstruction at baseline. Characteristics of participants without airway obstruction at baseline are shown in Table E6.
Table 1.
Characteristics of NHLBI Pooled Cohorts Study participants at first spirometry measurement
| Overall | ARIC | CARDIA | CHS | MESA | |
|---|---|---|---|---|---|
|
| |||||
| Participants, n (%) | 15063 (100) | 3545 (24) | 3303 (22) | 3727 (25) | 4488 (30) |
|
| |||||
| Age, years | 56.3 (18.8) | 54.7 (5.8) | 25.6 (3.9) | 72.5 (5.3) | 66.8 (9.9) |
|
| |||||
| Female sex, n (%) | 8224 (55) | 1836 (52) | 1867 (57) | 2196 (59) | 2325 (52) |
|
| |||||
| Race/ethnicity, N (%) | |||||
| white | 10434 (69) | 3545 (100) | 1792 (54) | 3341 (90) | 1756 (39) |
| Black | 3008 (20) | -- | 1511 (46) | 383 (10) | 1114 (25) |
| Asian | 641 (4) | -- | -- | 3 (0) | 638 (14) |
| Hispanic | 980 (7) | -- | -- | 980 (22) | |
|
| |||||
| Height, cm | 167.4 (19.9) | 169.3 (9.5) | 170.3 (9.5) | 164.8 (9.3) | 165.8 (10) |
|
| |||||
| Weight, kg a | 75.1 (16.5) | 77.9 (16.4) | 71.3 (16.1) | 72.3 (14.2) | 78 (17.6) |
|
| |||||
| Smoking status, n (%) | |||||
| Never | 7169 (48) | 1303 (37) | 2004 (61) | 1785 (48) | 2077 (46) |
| Former | 5440 (36) | 1422 (40) | 446 (14) | 1566 (42) | 2006 (45) |
| Current | 2454 (16) | 820 (23) | 853 (26) | 376 (10) | 405 (9) |
|
| |||||
| Smoking pack-years, median (IQR) b | 15.3 (30.2) | 24.8 (27.0) | 2.3 (5.2) | 27.0 (36.4) | 12.2 (26.5) |
|
| |||||
| Smoking cigarettes per day, median (IQR) c | 15 (10) | 20 (10) | 10 (14) | 19.5 (10) | 10 (15) |
|
| |||||
| FEV1, mL | 2712 (930) | 3051 (780) | 3549 (796) | 2089 (656) | 2345 (732) |
|
| |||||
| FVC, mL d | 3584 (1118) | 4113 (983) | 4303 (1032) | 2964 (867) | 3147 (955) |
|
| |||||
| FEV1/FVC < 0.7, n (%) b | 3405 (23) | 787 (22) | 119 (4) | 1461 (39) | 1045 (23) |
|
| |||||
| Follow-up time, yr, median (IQR) e | 4 (16.0) | 3.0 (20.1) | 20 (1.0) | 6.8 (3.0) | 11.0 (6.0) |
|
| |||||
| PFT measurements, n | 2.6 (1.2) | 2.2 (0.6) | 4.3 (0.9) | 2.3 (0.7) | 2 (0.8) |
|
| |||||
| ALA, % f | 0.2 (0.1) | 0.1 (0.1) | 0.2 (0.1) | 0.2 (0.1) | 0.2 (0.1) |
|
| |||||
| EPA, % f | 0.7 (0.6) | 0.6 (0.3) | 0.8 (0.5) | 0.6 (0.4) | 0.9 (0.8) |
|
| |||||
| DPA, % f | 0.9 (0.2) | 0.9 (0.2) | 0.9 (0.2) | 0.8 (0.2) | 0.9 (0.2) |
|
| |||||
| DHA, % f | 3.3 (1.2) | 2.8 (0.9) | 3.2 (1.1) | 3.0 (1.0) | 3.9 (1.5) |
All values reported as mean (SD) unless otherwise noted.
Six participants were missing baseline weight.
Smoking pack-years calculated among former and current smokers (N = 7,894).
Smoking cigarettes per day calculated among current smokers (N = 2,454).
N = 15,022 for FVC and FEV1/FVC due to failure to meet spirometry reproducibility criteria.
Follow-up time calculated among participants with at least two spirometry measurements (N = 12,375).
Percent of total fatty acids in plasma phospholipids.
Correlations of omega-3 fatty acids
Correlations of ALA with EPA, DPA, and DHA were evaluated in the full sample and by sex, race/ethnicity, and smoking status (Table E7). In the full sample, ALA was positively correlated with EPA, DPA, and DHA, with decreasing magnitudes (Pearson’s correlation coefficients [r] = 0.18, 0.10, and 0.03 for EPA, DPA, and DHA respectively, p < 0.0001 for all). Correlation patterns were similar in male participants, white participants, and never and former smokers. In contrast, ALA and DHA were not correlated in female participants (r = 0.01, p = 0.255) and were negatively correlated in Black participants (r = −0.04, p = 0.034), Hispanic participants (r = −0.09, p = 0.004), and current smokers (r = −0.04, p = 0.067).
Lung Function Decline
The mean rate of lung function decline was 36.8 mL per year for FEV1 and 35.8 mL per year for FVC. In covariate-adjusted models, higher concentrations of ALA, EPA, and DHA were associated with attenuated decline for both FEV1 and FVC (Table 2). One SD higher ALA, EPA, and DHA was associated with an attenuation of 0.77, 0.62, and 1.77 mL/year for FEV1 and 1.30, 0.84, and 2.43 mL/year for FVC (p < 0.005 for all). In extended models that accounted for the effects of sex, smoking, and race/ethnicity on lung function decline, the associations of ALA with FEV1 decline and of EPA with both FEV1 and FVC decline were no longer statistically significant (p > 0.05), while the association of DPA with FEV1 decline was strengthened (p = 0.03). The associations of DHA with FEV1 and FVC decline remained statistically significant (p < 0.05) in all models.
Table 2.
Associations of omega-3 fatty acids with FEV1 and FVC decline
| FA | FA SD |
BASE MODELA
|
EXTENDED MODELB
|
||||
|---|---|---|---|---|---|---|---|
| B | SE | Pvalue | B | SE | Pvalue | ||
|
| |||||||
| FEV1 DECLINE (ML/YEAR) | |||||||
|
| |||||||
| ALA | 0.07 | 0.77 | 0.20 | < 0.0001 | 0.29 | 0.20 | 0.144 |
| EPA | 0.59 | 0.62 | 0.21 | 0.003 | 0.30 | 0.21 | 0.155 |
| DPA | 0.20 | 0.15 | 0.21 | 0.484 | 0.45 | 0.21 | 0.030 |
| DHA | 1.22 | 1.77 | 0.22 | < 0.0001 | 0.94 | 0.23 | < 0.0001 |
|
| |||||||
| FVC DECLINE (ML/YEAR) | |||||||
|
| |||||||
| ALA | 0.07 | 1.30 | 0.24 | < 0.0001 | 0.74 | 0.24 | 0.002 |
| EPA | 0.59 | 0.84 | 0.26 | 0.001 | 0.39 | 0.26 | 0.131 |
| DPA | 0.20 | −0.22 | 0.26 | 0.398 | 0.13 | 0.25 | 0.598 |
| DHA | 1.22 | 2.43 | 0.27 | < 0.0001 | 1.37 | 0.28 | < 0.0001 |
FA = fatty acid; SD = standard deviation. .Associations with P < 0.05 are shown in bold. Beta coefficients, B, are for time × FA interactions, presented as mL/year per SD higher FA. Bolded results are significant at p < 0.05.
Base model adjusted for age and age2, sex, race/ethnicity, height and height2, weight (FVC only), smoking status, baseline pack-years of cigarette smoking, cigarettes smoked per day at each spirometry measurement, and cohort.
Extended model further adjusted for sex × time, race/ethnicity × time, and baseline smoking status × time interaction terms, to account for the effect of these variables on decline in lung function.
Three-way interaction terms revealed nominally statistically significant modification by sex, smoking, and/or race/ethnicity (interaction p < 0.05) of some omega-3 fatty acid—lung function decline associations (Table E8). All interaction terms remained significant after correction for multiple testing (adjusted p < 0.05), except for the “sex by ALA by elapsed time” and the “race by EPA by elapsed time” interaction terms for FEV1 (adjusted p = 0.053 and 0.059, respectively).
Where there was evidence for differences by biological sex, associations were observed primarily in male participants. For example, one SD higher ALA was associated with attenuated FEV1 decline of 1.01 (95% confidence interval [CI] = 0.32–1.71) mL/year in males; there was little to no association in females (beta = 0.08, 95% CI = −0.38–0.54) (Figure 1A). Similar sex-specific patterns were observed for associations of EPA and DPA with FEV1 decline (Figure 1A and 1B) and ALA with FVC decline (Figure E2A).
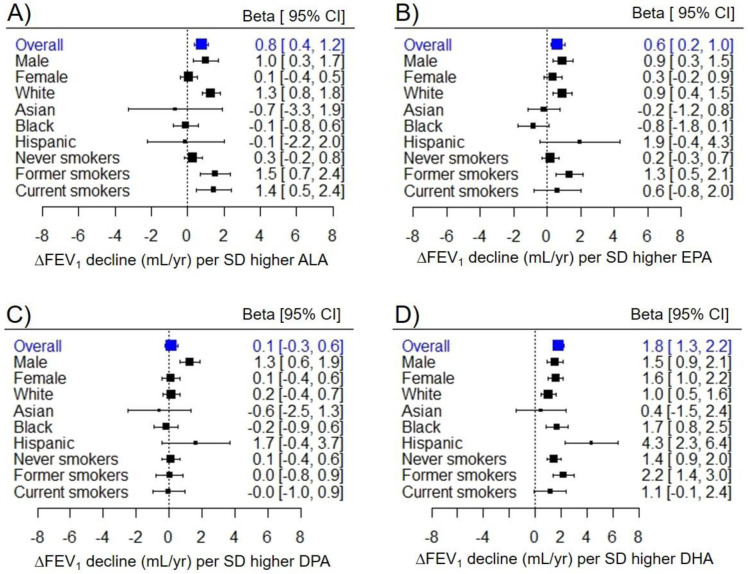
Figure 1. Stratification of omega-3 fatty acid–FEV1 decline associations by sex, race/ethnicity, and baseline smoking status.
Sex, race/ethnicity, and baseline smoking status differences in omega-3 fatty acid–FEV1 decline associations for A) ALA, B) EPA, C) DPA, and D) DHA. Estimates (Betas) and 95% confidence intervals (CIs) for each group were derived from three-way interaction terms and covariances. All estimates are presented per standard deviation (SD) higher fatty acid.
Where there was evidence for differences by smoking, associations were observed primarily or strongest in former smokers. For example, one SD higher EPA was associated with attenuated FEV1 decline of 1.3 mL/year (95% CI = 0.5–2.1) in former smokers, 0.6 mL/year (95% CI = −0.8–2.0) in current smokers, and 0.2 mL/year (95% CI = −0.3–0.7) in never smokers (Figure 1B). There were similar patterns for associations of ALA and DHA with FEV1 decline (Figure 1A and 1D) and for ALA, EPA and DHA with FVC decline (Figure E2A, E2B and E2D).
Effect modification by race/ethnicity varied depending on the omega-3 fatty acid. The positive associations of ALA and EPA with lung function decline were observed primarily in white participants (Figure 1A and 1B). In contrast, the positive associations of the DHA with lung function decline were largest in Black and Hispanic participants, still positive and statistically significant in white participants, and non-significant in Asian participants (Figure 1D). Race/ethnicity specific results for FVC decline followed a similar pattern (Figure E2).
Incident Airway Obstruction
There were 1,455 (12.5%) cases of incident airway obstruction during a median (interquartile range) follow-up period of 5(6) years. Characteristics of cases and participants who did not develop airway obstruction during follow-up are shown in Table E6. Concentrations of ALA, EPA and DPA were similar between cases and those who did not develop airway obstruction, while DHA concentration was slightly lower in cases (3.23% vs 3.29% of total fatty acids, Pdiff = 0.05).
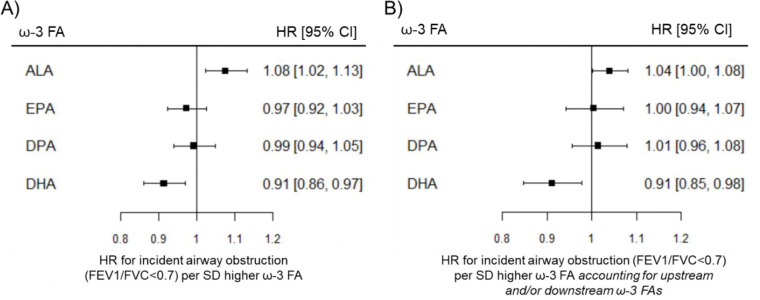
In covariate-adjusted models, higher ALA was associated with higher rates of airway obstruction (hazard ratio = 1.08, 95% CI = 1.03–1.13), while higher DHA was associated with lower rates of airway obstruction (hazard ratio = 0.91, 95% CI = 0.87–0.97) (Figure 2). When all omega-3 fatty acids were included in the same model, the association of ALA with rates of incident airway obstruction was attenuated (hazard ratio = 1.04, 95% CI = 1.00–1.08), while the association of DHA with rates of incident airway obstruction was unchanged (hazard ratio = 0.91, 95% CI = 0.85–0.98).
Figure 2. Associations of omega-3 fatty acids with incident airway obstruction in participants without airway obstruction at baseline.
Estimates from Cox proportional hazards models. HR = hazard ratio. CI = confidence interval. SD = standard deviation. A) Associations of omega-3 fatty acids with incident airway obstruction adjusted baseline age, sex, race/ethnicity, height, smoking status, pack-years of cigarette smoking, and cohort. B) Associations of omega-3 fatty acids with incident airway obstruction further adjusted for other upstream and/or downstream omega-3 fatty acids.
There was little evidence for associations of EPA or DPA with incident airway obstruction. There was no statistically significant evidence for effect modification by sex, race/ethnicity, or smoking for any omega-3 fatty acid (Table E7).
Two-Sample MR Analysis
Genetic Instruments for Omega-3 Fatty Acids
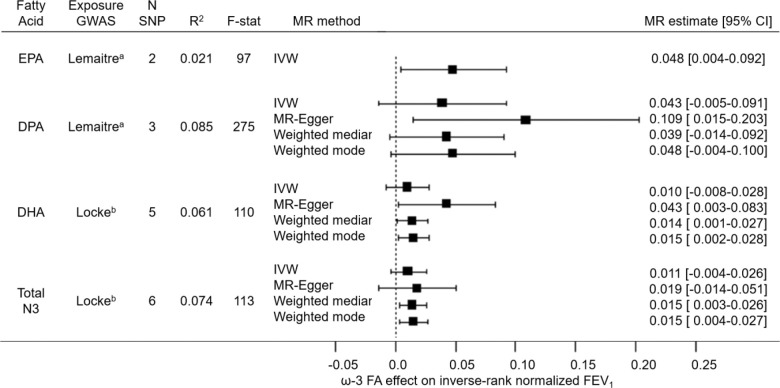
Genetic instruments for EPA, DPA, DHA, and total omega-3 fatty acids explained 2.1%, 8.5%, 6.5%, and 7.4% of the variance, respectively. In the primary analyses, F-statistics for the omega-3 fatty acid genetic instruments ranged from 97 for EPA to 275 for DPA, exceeding the MR standard for instrument strength of F-statistic > 10 (Figure 3). F-statistics were generally lower in the replication analyses, ranging from 6 for DHA to 44 for total omega-3 fatty acids (Table E9).
Figure 3. Forest plot of two-sample MR estimates from primary analysis of EPA, DPA, and DHA and total omega-3 fatty acids on FEV1.
Forest plot showing MR estimates and 95% confidence intervals (CIs) for associations of genetically predicted omega-3 fatty acids with FEV1 using different MR methods. N SNP: number of SNPs contributing to the omega-3 fatty acid genetic instrument. Exposure GWAS: GWAS that SNP–omega-3 fatty acid association data were extracted from. R2: proportion of omega-3 fatty acid variance explained by the genetic instrument. F-stat: F statistic, a measure of genetic instrument strength where the MR standard for validity is F > 10. IVW: Inverse variance weighted MR.
a Lemaitre study: GWAS fatty acid units in % of total fatty acid composition.
b Locke study: GWAS fatty acid units in standardized fatty acid concentration (mmol/L).
MR of Omega-3 Fatty Acids and Lung Function
Associations of genetically predicted omega-3 fatty acids with FEV1 were consistently positive across different MR methods (Figure 3). Results were statistically significant (p < 0.05) for the following: IVW estimate for EPA; the MR-Egger estimate for DPA; the MR-Egger, weighted median, and weighted mode methods for DHA; and the weighted median and weighted mode methods for total omega-3 fatty acids. Results for FVC were also consistently positive, with statistically significant results (p <0.05) for the MR-Egger estimate for DPA (Figure E3). There was no evidence for an association of genetically predicted omega-3 fatty acids with the odds of airway obstruction (Figure E4).
Results were similar in replication analyses (Table E9). MR estimates for FEV1 and FVC were consistently positive across different methods, with statistically significant results (p <0.05) for some methods. Again, there was no statistically significant evidence for associations of genetically predicted omega-3 fatty acids with the odds of airway obstruction.
DISCUSSION
Our longitudinal pooled individual participant data analysis in a large, multiracial U.S. population-based sample found that higher levels of omega-3 fatty acids in circulation were associated with an attenuated rate of lung function decline, with the strongest associations seen for DHA. Higher DHA was also associated with decreased risk of incident airway obstruction. Results from the MR study, although not significant across all MR methods, were consistent with a positive association of genetically predicted omega-3 fatty acids with lung function. Together, the findings from the longitudinal and MR studies support a protective effect of higher circulating omega-3 fatty acids—most significantly DHA—on lung health.
Circulating omega-3 fatty acids correlate with lung function parameters cross-sectionally,5, 6, 8, 9 and longitudinal studies of dietary intake have shown protective associations of higher omega-3 fatty acid intake with lung function decline and the odds of COPD.26, 27 To our knowledge, this is the first study to longitudinally evaluate the association of omega-3 fatty acid biomarkers with lung function decline in a generally healthy adult population, and the first study to apply MR methodology to the omega-3 fatty acid–lung function relationship. Triangulation of our longitudinal and MR findings helps to address limitations of reverse causality and confounding in previous studies, and contributes to the evidence base for a beneficial effect of omega-3 fatty acids on long-term lung outcomes. Beneficial effects of omega-3 FAs in the lungs have also been shown experimentally.28–32 In vitro, the omega-3 fatty acid-derived small lipid molecule, resolvin E1, reduced the production of proinflammatory cytokines and stress-related proteins in human alveolar epithelial cells.28 In animal studies, dietary or pharmacological supplementation with omega-3 fatty acids protected against experimentally-induced airway inflammation and resulting lung pathology, and accelerated lung tissue recovery following lung injury.29–32 In clinical studies of acute lung injury, omega-3 fatty acid supplementation decreased inflammation in the lungs and accelerated respiratory function recovery, as recently reviewed.33 Altogether, the anti-inflammatory effects of omega-3 fatty acids in the lungs are well established, providing strong biologic plausibility that support our findings.
Previous studies of omega-3 fatty acids and lung outcomes have not consistently investigated effect modification by important biological and lifestyle factors. In our longitudinal study, we investigated whether associations of omega-3 fatty acids with lung function decline and incident airway obstruction differed by sex, smoking, and race/ethnicity, and found both qualitative and quantitative differences. We found that the positive associations of ALA, EPA, and DPA with lung function decline was observed primarily in males, white participants, and/or those with a history of smoking. In contrast, the positive associations of DHA with lung function decline was observed in males and females; in Black, white and Hispanic participants; and in all smoking groups, with the largest magnitude associations in Black and Hispanic participants and former smokers. Correlations between fatty acid levels also differed by sex, race/ethnicity, and smoking. ALA and DHA were positively correlated in males, white participants, and former smokers, but were uncorrelated or negatively correlated in females, Black and Hispanic participants, and current smokers. These patterns are consistent with reported differences in the efficiency of omega-3 fatty acid metabolism by sex, race/ethnicity, and smoking.34–41 Unfortunately, we were unable to test these differences in the MR analyses because omega-3 fatty acid GWAS stratified by sex or smoking are unavailable and the limited sample sizes of lung function GWAS of non-European populations are underpowered for MR analyses.
In our longitudinal study, the protective associations of omega-3 fatty acids with incident airway obstruction were limited to DHA, with little evidence for differences by sex, smoking status, or race/ethnicity. In our MR study, we found no evidence for associations of omega-3 fatty acids with odds of airway obstruction. The weaker associations and lack of interaction effects for the omega-3 fatty acid–airway obstruction associations could be explained by a loss of power due to assessment of a binary (vs. continuous) outcome and, for the longitudinal study, smaller sample size (limited to participants without airway obstruction at baseline). Larger sample sizes, more precise COPD phenotyping, and increased statistical power may be necessary to detect long-term effects of omega-3 fatty acids on obstructive airway disease.
This study has several strengths and novel aspects. Our longitudinal study pooled individual participant data from multiple cohorts, leading to a large, diverse sample with repeated lung function measurements (up to 25 years of follow-up in some participants). These data also enabled investigation of effect modification by important biological and lifestyle factors. For the exposure, we used omega-3 fatty acids biomarkers, which reflect dietary intake while minimizing reporting bias and measurement error in dietary intake assessed from diet recalls or food frequency questionnaires. Similar or identical laboratory methods were used to measure omega-3 fatty acids across the cohorts, increasing confidence that the data were suitable for pooling. Our MR study used publicly available GWAS summary statistics, which allowed us to maximize sample size, instrument strength, and statistical power. Our MR findings were consistent in direction across multiple MR methods designed to address potential violation in the assumptions of MR and support our longitudinal findings.
Our analyses also have limitations. For our longitudinal study, plasma phospholipids, which represent short-term fatty acid status, were measured only once in each cohort. Multiple spirometry measurements were taken in all cohorts, but the number of spirometry measurements and intervals between spirometry measurements varied. Repeated omega-3 fatty acids generally correlate over time, but one-time measurements may not reflect fluctuations in omega-3 fatty acids bioavailability due to dietary or lifestyle changes, especially in participants with longer total follow-up times. Our findings may have been strengthened if repeated measurements of omega-3 fatty acids and additional spirometry measurements were taken at consistent intervals over longer follow-up periods across the cohorts. This may be especially true for the analysis of incident airway obstruction, since censoring and the identification of cases in each cohort was limited to the cohort clinic visits where spirometry was performed. Additionally, omega-3 fatty acids were reported as percentages of total plasma phospholipids rather than absolute concentrations. Fatty acids proportions and absolute concentrations are generally well correlated, suggesting the use of relative versus absolute concentrations is unlikely to influence interpretation of our results.42, 43 Finally, our sample had limited numbers of Asian and Hispanic participants; although we found significant positive associations of DHA with lung function decline in Hispanic participants, our analyses may have been underpowered to detect other associations in these groups. For our MR study, the genetic instruments for the different omega-3 fatty acids included SNPs in genetic loci associated with multiple fatty acids, limiting the specificity of the results. Our analysis relied on publicly available GWAS summary statistics; stratified GWAS of omega-3 FAs were not available and that limited our ability to confirm the sex- and smoking-specific effects seen in our longitudinal cohort study. Our MR study was also limited to European ancestry; larger GWAS of omega-3 FAs and lung function parameters, specifically in non-European populations, are needed to confirm our longitudinal findings.
Overall, this study represents the first longitudinal study of omega-3 fatty acid biomarkers and lung function decline and the first MR study of genetically predicted omega-3 fatty acids and lung function parameters. Our findings are consistent in direction across the longitudinal and MR studies and provide evidence supporting a protective effect of omega-3 fatty acids—specifically DHA—on lung health. Further research into biological and lifestyle factors influencing omega-3 fatty acid metabolism and utilization in the lungs is warranted.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the staff and participants of the Atherosclerosis Risk in Communities Study, the Coronary Artery Risk Development in Young Adults Study, the Cardiovascular Health Study, and the Multi-Ethnic Study of Atherosclerosis for their important contributions.
Funding:
Research reported in this publication was supported by the National Institutes of Health (NIH), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) award T32DK007158 and National Heart, Lung, and Blood Institute (NHLBI) award R01HL149352. The content is solely the responsibility of the authors and does not represent the official views of the NIH. The NHLBI Pooled Cohorts Study was supported by NIH/NHLBI awards R21HL121457, R21HL129924, K23HL130627. The Atherosclerosis Risk in Communities (ARIC) study has been funded in whole or in part with Federal funds from the NHLBI, NIH, Department of Health and Human Services, under contract numbers 75N92022D00001, 75N92022D00002, 75N92022D00003, 75N92022D00004, and 75N92022D00005. The Coronary Artery Risk Development in Young Adults Study (CARDIA) is conducted and supported by the NHLBI in collaboration with the University of Alabama at Birmingham (HHSN268201800005I and HHSN268201800007I), Northwestern University (HHSN268201800003I), University of Minnesota (HHSN268201800006I), and Kaiser Foundation Research Institute (HHSN268201800004I). The Cardiovascular Health Study (CHS) was supported by contracts HHSN268201200036C, HHSN268200800007C, HHSN268201800001C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, 75N92021D00006, and grants U01HL080295 and U01HL130114 from the NHLBI, with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by R01AG023629 from the National Institute on Aging (NIA). The Multi-Ethnic Study of Atherosclerosis (MESA) was supported by contracts 75N92020D00001, HHSN268201500003I, N01-HC-95159, 75N92020D00005, N01-HC-95160, 75N92020D00002, N01-HC-95161, 75N92020D00003, N01-HC-95162, 75N92020D00006, N01-HC-95163, 75N92020D00004, N01-HC-95164, 75N92020D00007, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the NHLBI, and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from the National Center for Advancing Translational Sciences (NCATS).
Footnotes
This study demonstrates associations of higher circulating omega-3 fatty acids—most significantly DHA—with attenuated decline in FEV1 and FVC, and reduced rates of incident spirometry-defined airway obstruction. Positive associations of genetically predicted omega-3 fatty acids with FEV1 and FVC were also demonstrated. Together these findings support a role of nutrients that increase anti-inflammatory function in the maintenance of lung health and suggest that omega-3 fatty acid nutritional status may be a promising target for COPD prevention.
Data availability statement:
Pre-existing data access policies for each of the parent cohort studies specify that research data requests can be submitted to each steering committee; these will be promptly reviewed for confidentiality or intellectual property restrictions and will not unreasonably be refused. Individual-level patient data may be further restricted by consent, confidentiality or privacy laws/considerations.
REFERENCES
- 1.NIH National Heart, Lung, and Blood Institute. COPD - Causes and Risk Factors. Updated March 22. Accessed December 8, 2022. https://www.nhlbi.nih.gov/health/copd/causes
- 2.Burdge GC. Metabolism of alpha-linolenic acid in humans. Prostaglandins Leukot Essent Fatty Acids. Sep 2006;75(3):161–8. Proceedings of the 7th Fatty Acid and Cell Signaling (FACS)workshop held in Paris on September 28–30, 2005. doi: 10.1016/j.plefa.2006.05.013 [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez-Cruz M, Serna DS. Nutrigenomics of omega-3 fatty acids: regulators of the master transcription factors. Nutrition. Sep 2017;41:90–96. doi: 10.1016/j.nut.2017.04.012 [DOI] [PubMed] [Google Scholar]
- 4.Serhan CN, Petasis NA. Resolvins and protectins in inflammation resolution. Chem Rev. Oct 12 2011;111(10):5922–43. doi: 10.1021/cr100396c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu J, Gaddis NC, Bartz TM, et al. Omega-3 fatty acids and genome-wide interaction analyses reveal DPP10-pulmonary function association. Am J Respir Crit Care Med. Mar 1 2019;199(5):631–642. doi: 10.1164/rccm.201802-0304OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shahar E, Boland LL, Folsom AR, Tockman MS, McGovern PG, Eckfeldt JH. Docosahexaenoic acid and smoking-related chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999 1999;159(6):1780–1785. [DOI] [PubMed] [Google Scholar]
- 7.Kompauer I, Demmelmair H, Koletzko B, Bolte G, Linseisen J, Heinrich J. Association of fatty acids in serum phospholipids with lung function and bronchial hyperresponsiveness in adults. Eur J Epidemiol. 2008/03/01/ 2008;23(3):175–90. doi: 10.1007/s10654-007-9218-y [DOI] [PubMed] [Google Scholar]
- 8.Novgorodtseva TP, Denisenko YK, Zhukova NV, Antonyuk MV, Knyshova VV, Gvozdenko TA. Modification of the fatty acid composition of the erythrocyte membrane in patients with chronic respiratory diseases. Lipids Health Dis. Jul 30 2013;12:117. doi: 10.1186/1476-511X-12-117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Csanky E, Ruhl R, Scholtz B, Vasko A, Takacs L, Hempel WM. Lipid metabolite levels of prostaglandin D2 and eicosapentaenoic acid recovered from bronchoalveolar lavage fluid correlate with lung function of chronic obstructive pulmonary disease patients and controls. Electrophoresis. Apr 2009;30(7):1228–34. doi: 10.1002/elps.200800722 [DOI] [PubMed] [Google Scholar]
- 10.Lynch J, Smith GD. A life course approach to chronic disease epidemiology. Annu Rev Public Health. 2005 2005;26(1):1–35. doi: 10.1146/annurev.publhealth.26.021304.144505 [DOI] [PubMed] [Google Scholar]
- 11.Barker DJ. In utero programming of chronic disease. Clin Sci (Lond). Aug 1998;95(2):115–28. doi: 10.1042/CS0950115 [DOI] [PubMed] [Google Scholar]
- 12.Oelsner EC, Balte PP, Cassano PA, et al. Harmonization of respiratory data from 9 US population-based cohorts: the NHLBI Pooled Cohorts Study. Am J Epidemiol. Nov 1 2018;187(11):2265–2278. doi: 10.1093/aje/kwy139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for Prevention, Diagnosis and Management of COPD: 2022 Report. 2021. Global Initiative for Chronic Obstructive Lung Disease, Inc. Accessed 2022/03/14. https://goldcopd.org/wp-content/uploads/2021/12/GOLD-REPORT-2022-v1.1-22Nov2021_WMV.pdf
- 14.Lemaitre RN, Tanaka T, Tang W, et al. Genetic loci associated with plasma phospholipid n-3 fatty acids: a meta-analysis of genome-wide association studies from the CHARGE Consortium. PLoS Genet. Jul 2011;7(7):e1002193. doi: 10.1371/journal.pgen.1002193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Locke AE, Steinberg KM, Chiang CWK, et al. Exome sequencing of Finnish isolates enhances rare-variant association power. Nature. Aug 2019;572(7769):323–328. doi: 10.1038/s41586-019-1457-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shin SY, Fauman EB, Petersen AK, et al. An atlas of genetic influences on human blood metabolites. Nat Genet. Jun 2014;46(6):543–550. doi: 10.1038/ng.2982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kettunen J, Demirkan A, Würtz P, et al. Genome-wide study for circulating metabolites identifies 62 loci and reveals novel systemic effects of LPA. Nat Commun. 2016/03/23/ 2016;7(1):11122. doi: 10.1038/ncomms11122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burgess S, Davies NM, Thompson SG. Bias due to participant overlap in two-sample Mendelian randomization. Genet Epidemiol. 2016/11/01/ 2016;40(7):597–608. doi: 10.1002/gepi.21998@10.1002/(ISSN)1098-2272.GEPI-top-cited [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitchell R, Gibran H, Dudding T, Corbin L, Harrison S, Paternoster L. Data from: UK Biobank Genetic Data: MRC-IEU Quality Control, version 2. University of Bristol. 2019. [Google Scholar]
- 20.Millard LAC, Davies NM, Gaunt TR, Davey Smith G, Tilling K. Software Application Profile: PHESANT: a tool for performing automated phenome scans in UK Biobank. Int J Epidemiol. Feb 2018;47(1):29–35. doi: 10.1093/ije/dyx204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hemani G, Bowden J, Davey Smith G. Evaluating the potential role of pleiotropy in Mendelian randomization studies. Hum Mol Genet. Aug 1 2018;27(R2):R195–R208. doi: 10.1093/hmg/ddy163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bowden J, Davey Smith G, Burgess S . Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. Apr 2015;44(2):512–25. doi: 10.1093/ije/dyv080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. May 2016;40(4):304–14. doi: 10.1002/gepi.21965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hartwig FP, Davey Smith G, Bowden J. Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int J Epidemiol. Dec 1 2017;46(6):1985–1998. doi: 10.1093/ije/dyx102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hemani G, Zheng J, Elsworth B, et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife. May 30 2018;7:e34408. doi: 10.7554/eLife.34408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leng S, Picchi MA, Tesfaigzi Y, et al. Dietary nutrients associated with preservation of lung function in Hispanic and non-Hispanic white smokers from New Mexico. Int J Chron Obstruct Pulmon Dis. 2017/10/30/ 2017;12:3171–3181. doi: 10.2147/COPD.S142237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Varraso R, Barr RG, Willett WC, Speizer FE, Camargo CA, Jr. Fish intake and risk of chronic obstructive pulmonary disease in 2 large US cohorts. Am J Clin Nutr. Feb 2015;101(2):354–61. doi: 10.3945/ajcn.114.094516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mayer K, Sommer N, Hache K, et al. Resolvin E1 improves mitochondrial function in human alveolar epithelial cells during severe inflammation. Lipids. Jan 2019;54(1):53–65. doi: 10.1002/lipd.12119 [DOI] [PubMed] [Google Scholar]
- 29.Dominguez EC, Heires AJ, Pavlik J, et al. A high docosahexaenoic acid diet alters the lung inflammatory response to acute dust exposure. Nutrients. 2020/08/04/ 2020;12(8):2334. doi: 10.3390/nu12082334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ulu A, Burr A, Heires AJ, et al. A high docosahexaenoic acid diet alters lung inflammation and recovery following repetitive exposure to aqueous organic dust extracts. J Nutr Biochem. Nov 2021;97:108797. doi: 10.1016/j.jnutbio.2021.108797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kocherlakota C, Nagaraju B, Arjun N, Srinath A, Kothapalli KSD, Brenna JT. Inhalation of nebulized omega-3 fatty acids mitigate LPS-induced acute lung inflammation in rats: Implications for treatment of COPD and COVID-19. Prostaglandins Leukot Essent Fatty Acids. Apr 2022;179:102426. doi: 10.1016/j.plefa.2022.102426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee KP, Park SJ, Kang S, et al. Omega-3 Polyunsaturated fatty acids accelerate airway repair by activating FFA4 in club cells. Am J Physiol Lung Cell Mol Physiol. Jun 1 2017;312(6):L835–L844. doi: 10.1152/ajplung.00350.2016 [DOI] [PubMed] [Google Scholar]
- 33.Huang Z, Zheng J, Huang W, et al. The effects and safety of omega-3 fatty for acute lung injury: a systematic review and meta-analysis. World J Surg Oncol. Sep 3 2020;18(1):235. doi: 10.1186/s12957-020-01916-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burdge GC, Jones AE, Wootton SA. Eicosapentaenoic and docosapentaenoic acids are the principal products of alpha-linolenic acid metabolism in young men*. Br J Nutr. Oct 2002;88(4):355–63. doi: 10.1079/BJN2002662 [DOI] [PubMed] [Google Scholar]
- 35.Pawlosky RJ, Hibbeln JR, Salem N Jr. Compartmental analyses of plasma n-3 essential fatty acids among male and female smokers and nonsmokers. J Lipid Res. Apr 2007;48(4):935–43. doi: 10.1194/jlr.M600310-JLR200 [DOI] [PubMed] [Google Scholar]
- 36.Burdge GC, Wootton SA. Conversion of alpha-linolenic acid to eicosapentaenoic, docosapentaenoic and docosahexaenoic acids in young women. Br J Nutr. Oct 2002;88(4):411–20. doi: 10.1079/BJN2002689 [DOI] [PubMed] [Google Scholar]
- 37.Harris DN, Ruczinski I, Yanek LR, et al. Evolution of hominin polyunsaturated fatty acid metabolism: from Africa to the New World. Genome Biol Evol. May 1 2019;11(5):1417–1430. doi: 10.1093/gbe/evz071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mathieson S, Mathieson I. FADS1 and the timing of human adaptation to agriculture. Mol Biol Evol. Dec 1 2018;35(12):2957–2970. doi: 10.1093/molbev/msy180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang C, Hallmark B, Chai JC, et al. Impact of Amerind ancestry and FADS genetic variation on omega-3 deficiency and cardiometabolic traits in Hispanic populations. Commun Biol. Jul 28 2021;4(1):918. doi: 10.1038/s42003-021-02431-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marangoni F, Colombo C, De Angelis L, et al. Cigarette smoke negatively and dose-dependently affects the biosynthetic pathway of the n-3 polyunsaturated fatty acid series in human mammary epithelial cells. Lipids. Jul 2004;39(7):633–7. doi: 10.1007/s11745-004-1276-5 [DOI] [PubMed] [Google Scholar]
- 41.Risé P, Ghezzi S, Manzoni C, Colombo C, Galli C. The in vitro effects of cigarette smoke on fatty acid metabolism are partially counteracted by simvastatin. Prostaglandins Leukot Essent Fatty Acids. Jan 2009;80(1):71–5. doi: 10.1016/j.plefa.2008.11.005 [DOI] [PubMed] [Google Scholar]
- 42.Song X, Diep P, Schenk JM, et al. Changes in relative and absolute concentrations of plasma phospholipid fatty acids observed in a randomized trial of Omega-3 fatty acids supplementation in Uganda. Prostaglandins Leukot Essent Fatty Acids. Nov 2016;114:11–16. doi: 10.1016/j.plefa.2016.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Song X, Schenk JM, Diep P, et al. Measurement of circulating phospholipid fatty acids: association between relative weight percentage and absolute concentrations. J Am Coll Nutr. Sep-Oct 2016;35(7):647–656. doi: 10.1080/07315724.2015.1116417 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Pre-existing data access policies for each of the parent cohort studies specify that research data requests can be submitted to each steering committee; these will be promptly reviewed for confidentiality or intellectual property restrictions and will not unreasonably be refused. Individual-level patient data may be further restricted by consent, confidentiality or privacy laws/considerations.