Abstract
Gingival epithelial cells are a central component of the barrier between oral microflora and internal tissues. Host responses to periodontopathic bacteria and surface components containing fimbriae are thought to be important in the development and progression of periodontal diseases. To elucidate this mechanism, we established immortalized human gingival epithelial cells (HGEC) that were transfected with human papillomavirus. HGEC predominantly expressed Toll-like receptor (TLR) 2, but not TLR4 or CD14. They also induced interleukin-8 (IL-8) production when stimulated with Porphyromonas gingivalis fimbriae and Staphylococcus aureus peptidoglycan, but not Escherichia coli-type synthetic lipid A. Furthermore, an active synthetic peptide composed of residues 69 to 73 (ALTTE) of the fimbrial subunit protein, derived from P. gingivalis and similar to a common component of cell wall peptidoglycans in parasitic bacteria, N-acetylmuramyl-l-alanyl-d-isoglutamine (MDP), significantly induced IL-8 production and NF-κB activation in HGEC, and these cytokine-producing activities were augmented by a complex of soluble CD14 and lipopolysaccharide-binding protein (LBP). IL-8 production in HGEC stimulated with these bacterial components was clearly inhibited by mouse monoclonal antibody to human TLR2. These findings suggest that P. gingivalis fimbrial protein and its active peptide are capable of activating HGEC through TLR2.
Fimbriae, hairlike microfibers, are observable on the cell surface of various bacteria by electron microscopy. Among the various cell surface components, fimbriae are known to be a specific adherence factor, or adhesin, in their microbial etiology (34). Porphyromonas gingivalis has been recognized as a major periodontopathogenic organism (40), and strains of P. gingivalis possessing virulence factors containing fimbriae have been shown to be involved in the development of periodontal diseases (49). Several studies have also described the immunobiological properties of P. gingivalis fimbriae and their active peptides (25–27, 31, 32).
Epithelial cells function as sensors of external stimuli and conduct signals to internal cells (18). Gingival epithelial cells are also thought to play an important role as a first barrier against periodontopathic organisms and their metabolic products. Several bacterial surface components, such as lipopolysaccharide (LPS) and its active center, lipid A, as well as fimbrial protein and peptidoglycan have been implicated in the development and progression of periodontal diseases (13, 51). However, the recognition mechanisms for these potentially pathogenic components in gingival epithelial cells are not well understood.
Toll-like receptors (TLRs) have been identified in monocytes and macrophages based on their homology to Drosophila protein (20, 52). Mammalian TLRs comprise a large family with extracellular leucine-rich repeats and a cytoplasmic Toll/interleukin-1 (IL-1) receptor homology domain and have been implicated in the recognition of bacterial cell wall components (22). Ten members (TLR1 to -10) have been reported (7, 8, 14, 22, 44), and among them, it was recently demonstrated that TLR4 plays an important role as a receptor of bacterial LPS and lipid A (15, 35). TLR2 is also essential for the signaling of various bacterial components, such as Staphylococcus aureus peptidoglycan (43), bacterial lipoproteins (2, 4, 19, 42), lipoteichoic acid (21), and zymosan (46). More recently, TLR9 was found to recognize bacterial DNA (14).
We report here the recognition mechanism of human gingival epithelial cells used to defend against P. gingivalis fimbriae and their peptides.
MATERIALS AND METHODS
Bacteria and fimbrial preparation.
P. gingivalis strain 381 was grown anaerobically in GAM broth (Nissui, Tokyo, Japan) supplemented with hemin and menadione for 26 h at 37°C. Fimbriae were isolated and purified as described previously (28).
Fimbrial synthetic peptide.
In our previous study, we found that ALTTE, residues 69 to 73 of the fimbrillin, functions in the induction of IL-6 production in human peripheral blood mononuclear cells (PBMC) (31). This active peptide was synthesized and purified as described previously (32). The peptide specimen was dissolved in the culture medium described below before the assay.
Bacterial and synthetic components.
Escherichia coli-type lipid A (compound 506), which possesses β-(1-6)-linked glucosamine disaccharide 1, 4′-bisphosphate, with two acyloxyacyl groups at the 2′ and 3′ positions and two 3-hydroxytetradecanoyl groups at the 2 and 3 positions, was synthesized as described by Imoto et al. (17), and the lipid A specimen was dissolved at a concentration of 2 mg/ml in a 0.1% (vol/vol) triethylamine aqueous solution. A cell wall peptidoglycan specimen of Staphylocaccus aureus was prepared in our laboratory as described previously (30). N-Acetylmuramyl-l-alanyl-d-isoglutamine (MDP) was supplied by Sigma (St. Louis, Mo.). These specimens were then dissolved in a pyrogen-free cell culture medium before the assay.
Establishment of HGEC cell lines.
Normal human gingival tissues (ca. 500 mg) were obtained from two patients who required tooth extractions for reasons other than periodontal disease after receiving informed consent. Human gingival epithelial cells were transfected with human papillomavirus 16 (HPV-16) E6 and E7 open reading flames (ORFs) using a retroviral system of HPV-16, which was kindly provided by M. Saitoh (Kanagawa Dental College, Yokosuka, Japan) (23). Immortalized human gingival epithelial cells (HGEC) were subsequently maintained in a long-term culture with HuMedia-KG2 (Kurabo Biomedicals, Osaka, Japan) and exhibited no morphological changes in long-term cultivation. These cells were designated HGEC-1 and HGEC-2, respectively, and used between passages 50 and 60.
Isolation of human monocytes.
Heparinized venous blood drawn from healthy donors was subjected to fractionation using Histopaque-1077 (Sigma) to obtain human PBMC (3). After washing the PBMC, monocytes were isolated by a magnetic cell sorting system using a monocyte isolation kit (Miltenyi Biotech Gmbh, Bergisch Gladbach, Germany).
Immunohistochemistry.
HeLa, an epithelial cell line established from a human cervical cancer specimen, and Gin-1, a gingival fibroblast cell line, were provided by Dainippon Pharmaceutical, Osaka, Japan, and maintained in Dulbecco's modified Eagle's medium (Nikken Biomedicals, Kyoto, Japan) supplemented with 10% fetal bovine serum (FBS; Sigma). HGEC, HeLa, and Gin-1 cells were seeded subconfluently onto plastic tissue culture slides (no. 177437; Nunc Inc.), after which the slides were washed with phosphate-buffered saline (PBS) and fixed in 2% paraformaldehyde in PBS for 10 min. After blocking with 5% normal horse serum in PBS, the fixed cells were incubated for 30 min with mouse monoclonal antibody to human keratin/cytokeratin (AE1/AE3; Nichirei Co., Tokyo, Japan) or mouse immunogloblin G1κ (IgG1κ) isotype (Zymed Labs, Inc., San Francisco, Calif.) as a negative control, followed by staining with a Vectastain Elite ABC kit (Vector Labs, Inc.).
For involucrin detection, cells were incubated with medium containing 2.0 mM calcium for 24 h and then incubated with mouse monoclonal antibody to human involucrin (SY5; Neomarker, Union City, Calif.), followed by staining with a Vectastain Elite ABC kit. Cells were then immediately observed with a microscope.
Reverse transcription-PCR.
Total cellular RNA from HGEC or human monocytes was extracted with RNAzol B (Tel-Test, Friendswood, Tex.) using a single-step isolation method (6) according to the manufacturer's recommendation. RNase-free DNase (Takara Biochemicals, Shiga, Japan) was used to remove genomic DNA based on methods described previously (9). Two micrograms of extracted RNA was reverse-transcribed into first-strand cDNA at 42°C for 40 min, using 100 U/ml of reverse transcriptase (RT; Takara Biomedicals) and 0.1 μM oligo(dT) adapter primer (Takara Biomedicals) in a 50-μl reaction mixture.
PCR amplification of cDNA was performed using oligonucleotide primers specific for CD14 (5′-AGGACTTGCACTTTCCAGCTTG-3′ and 5′-TCCCGTCCAGTGTCAGGTTATC-3′) (37), TLR2 (5′-GCCAAAGTCTTGATTGATTGG-3′ and 5′-TTGAAGTTCTCCAGCTCCTG-3′) (54), TLR4 (5′-GGTGGAAGTTGAACGAATGG-3′ and 5′-CTGTCCTCCCACTCCAGGTA-3′), and β-actin (5′-GTGGGGCGCCCCAGGCACCA-3′ and 5′-CTCCTTAATGTCACGCACGATTTC-3′) (33). The primer sequence for TLR4 mRNA described above was determined based on the sequence data (GenBank accession no. U88880). Five microliters of cDNA from the sample was amplified with 0.2 μM of the sense and antisense primers for the target genes in a 50-μl reaction mixture containing 75 U/ml of Takara Taq (Takara Biomedicals). After an initial denaturation at 94°C for 2 min, various cycles of denaturation (94°C for 45 s), annealing (58 to 60°C for 1 min), and extension (72°C for 2 min) for the respective target genes were performed using a Takara Thermal Cycler MP (Takara Biomedicals). For a negative control, non-RT sample was amplified by PCR.
Following PCR, 10 μl of the total amplified product was electrophoresed on ethidium bromide-stained 1.5% agarose gels and visualized under UV fluorescence. Densitometric analysis of the PCR-amplified bands was performed with NIH Image Software. Each gel image was imported into NIH Image using Photoshop (Adobe Systems), gel-plotting macros were used to outline the bands, and the intensity was calculated using the uncalibrated optical density setting. The relative expression levels were calculated as the density of the product of the respective target genes divided by that of β-actin from the same cDNA.
Flow cytometric analysis.
HGEC or human monocytes were incubated for 15 min at room temperature with mouse monoclonal antibody to human CD14 (Dako, Glostrup, Denmark). In the controls, cells were incubated with mouse IgG2b isotype (Dako). After washing with PBS containing 0.1% azide, the cells were incubated for 15 min at room temperature with fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse immunogloblins (Dako). For TLR4 detection, the cells were stained with goat polyclonal antibody to human TLR4 (Santa Cruz Biotech., Inc., Santa Cruz, Calif.), followed by FITC-conjugated rabbit anti-goat IgG (Zymed Lab., Inc.). Goat IgG (heavy and light chain) (Chemicon International, Inc., Temecula, Calif.) was used for the isotype control. For TLR2 detection, the cells were stained with mouse monoclonal antibody to human TLR2 (TL2.1; Cascade Bioscience Inc., Winchester, Mass.), followed by FITC-conjugated anti-mouse immunogloblins. Mouse IgG2a (Dako) was used for the isotype control. The cells were washed with PBS–0.1% azide, and then fixed with 1% paraformaldehyde. The stained cells were analyzed with a FACSCalibur using Cell Quest software (Becton Dickinson and Co., San Jose, Calif.).
Cytokine production.
HGEC were trypsinized and suspended at a cell density of 2 × 105 cells/ml of HuMedia-KG2 with supplements. A single-cell suspension (2 × 104 cells per well) was seeded in a 96-well flat-bottomed microtiter plate (Falcon 3072; Becton Dickinson and Co., Lincoln Park, N.J.). After incubation for 16 h at 37°C in humidified air containing 5% (vol/vol) CO2, the monolayers were washed three times with PBS and then treated with the indicated doses of the test specimens in 200 μl of HuMedia-KG2 with or without 1% FBS, 0.5 μg of CD14 (Genzyme-Techne, Minneapolis, Minn.), and/or 0.05 μg of LBP (Genzyme-Techne) per ml at 37°C for 24 h. In some experiments, HGEC were incubated with or without 1 μg of TL2.1 or mouse IgG2a per ml for 30 min at room temperature before adding the test specimens. After incubation, the supernatants were collected and stored at −80°C until the assay for cytokine production.
The production of IL-8 was determined in the culture supernatants by enzyme-linked immunosorbent assay (ELISA). The assay was performed according to the manufacturer's instructions (ELISA kit system; Genzyme-Techne), and the results were determined using a standard curve prepared for each assay.
Luciferase assay.
A single-cell suspension (5 × 104 cells per well) was seeded in a 24-well flat-bottomed microtiter plate (Falcon 3047; Becton Dickinson and Co.). After incubation for 24 h at 37°C in humidified air containing 5% (vol/vol) CO2, the monolayers were washed three times with PBS and then transfected with 0.8 μg of pNF-κB-Luc plasmid (Stratagene Co., La Jolla, Calif.) by use of TransFast transfection reagent (Promega Co., Madison, Wis.). The pFC-MEKK plasmid was used as a positive control plasmid in the assay. After an initial incubation for 24 h, the cells were incubated with the indicated doses of the test specimens in 500 μl of HuMedia-KG2, with or without 0.5 μg of CD14 and LBP per ml at 37°C for 7 h. After incubation, the cells were lysed in 80 μl of cell culture lysis reagent (Promega Co.), and then luciferase activity was determined using 20 μl of lysate and 100 μl of luciferase assay substrate (Promega Co.). The luminescence was quantified with a luminometer (Promega Co.).
Statistics.
Data were analyzed by a one-way analysis of variance, using the Bonferroni or Dunn method, and the results are presented as the mean ± standard error of the mean (SEM). When an individual experiment is shown, it is representative of at least three independent experiments.
RESULTS
Detection of total keratin and involucrin.
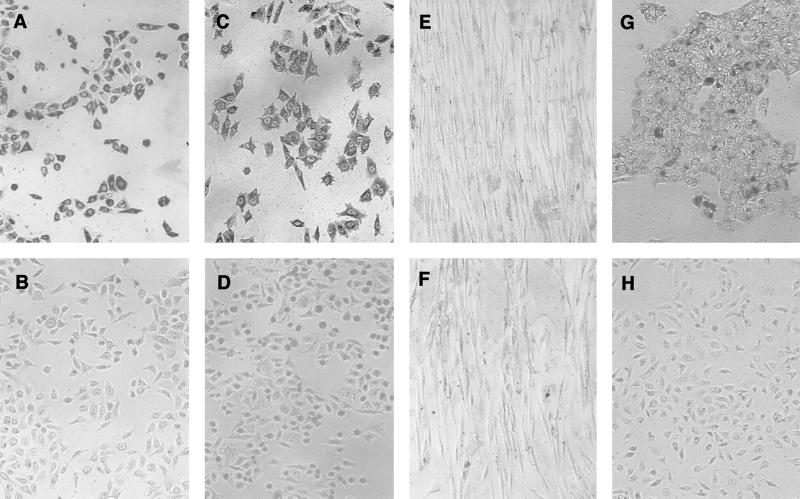
Expression of total keratin and involucrin in HGEC was assessed by immunohistochemical staining. HGEC-1 were clearly stained with mouse monoclonal antibody to human total keratin/cytokeratin, as shown in Fig. 1A. In contrast, these cells were not stained with mouse IgG1κ, an isotype control (Fig. 1B). HeLa cells, which are known sources of total keratin expression (12), also exhibited definite total keratin expression (Fig. 1C), but no staining was shown in Gin-1 (Fig. 1E). Further, HGEC-1 treated with culture medium containing 2.0 mM calcium for 48 h showed the induction of differentiation and obviously expressed involucrin (Fig. 1G), in contrast to the cells treated with 0.15 mM calcium for 24 h (Fig. 1H). HGEC-2, similar to HGEC-1, were shown to express total keratin and involucrin (data not shown).
FIG. 1.
Immunohistochemical staining with anti-total keratin and anti-involucrin antibodies. The stains were used to determine total keratin-positive and -negative cells, respectively, in HGEC-1 (A, B, G, and H), HeLa (C and D), and Gin-1 (E and F) cells. Cells were stained with mouse monoclonal antibody to human total keratin (AE1/AE3) (A, C, and E) and an isotype control, IgG1κ (B, D, and F). HGEC-1 were incubated with medium containing 2.0 mM (G) or 0.15 mM (H) calcium for 24 h or with mouse monoclonal antibody to human involucrin for involucrin detection.
Expression of CD14, TLR2, and TLR4.
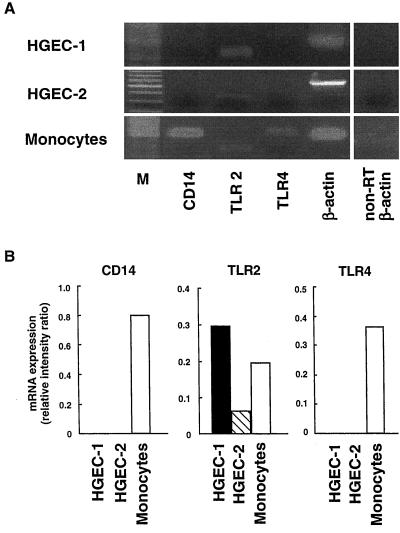
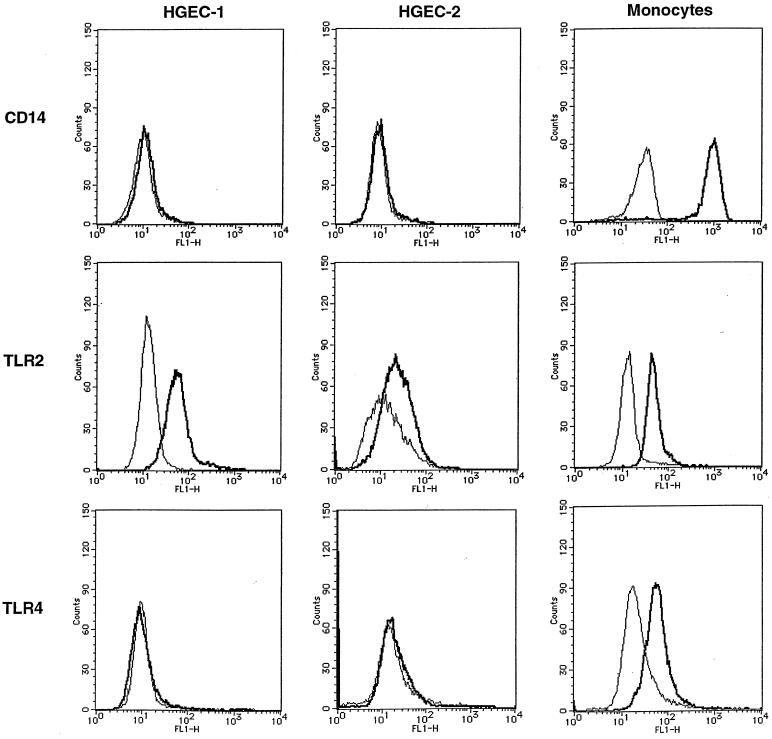
We examined the expression of CD14, TLR2, and TLR4 mRNA in HGEC by RT-PCR (Fig. 2). Both HGEC-1 and HGEC-2 showed TLR2 mRNA expression; however, they expressed no CD14 or TLR4 mRNA. Human peripheral monocytes, as a control, markedly expressed CD14, TLR2, and TLR4 mRNA. RT-PCR analysis of β-actin expression confirmed the quality of all RNA preparations used for RT-PCR. No band was detected in the non-RT sample by PCR. Further, we examined the cell surface expression of CD14, TLR2, and TLR4 gene products in HGEC by flow cytometry analysis (Fig. 3). HGEC-1 clearly expressed TLR2, and HGEC-2 showed weak TLR2 expression. These cells did not express CD14 or TLR4. Human peripheral monocytes, as a positive control, demonstrated specific cell surface CD14, TLR2, and TLR4 staining.
FIG. 2.
CD14, TLR2, and TLR4 mRNA expression in HGEC. (A) Expression of human CD14, TLR2, and TLR4 mRNA was analyzed by RT-PCR as detailed in Materials and Methods. Human monocytes were used as positive sources of CD14, TLR2, and TLR4 mRNA expression to confirm the specificity of the primers and PCR. The β-actin gene was assayed as a positive control. PCR products of non-RT samples were examined as a negative control. Lane M, Size markers. (B) Ethidium bromide-stained PCR products were photographed, and then the images were digitized and analyzed. Results are expressed as the ratio of each PCR product to β-actin band density. Data represent three independent experiments. PCR was performed in duplicate for each assay.
FIG. 3.
CD14, TLR2, and TLR4 expression in HGEC. Expression of CD14, TLR2, and TLR4 in HGEC was determined by staining with specific antibodies (bold line) or their isotype as a control (thin line) as detailed in Materials and Methods. Human monocytes were used as positive sources of CD14, TLR2, and TLR4.
Induction of IL-8 production.
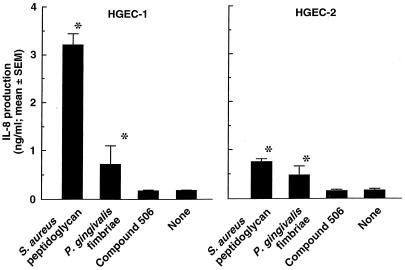
IL-8-producing activities in HGEC after stimulation with test specimens without FBS were examined (Fig. 4). P. gingivalis fimbriae as well as S. aureus peptidoglycan significantly induced IL-8 production in both HGEC-1 and HGEC-2, whereas stimulation of these cells with compound 506 resulted in no induction of IL-8 production. HGEC-1 produced more IL-8 than HGEC-2. The IL-8-producing activities coincided with the level of TLR2 expression and its gene product in each of the HGEC lines.
FIG. 4.
IL-8-producing activities in HGEC by various bacterial components. Cells were stimulated at 37°C for 24 h with 10 μg/ml of each test specimen in HuMedia-KG2 in the absence of FBS. After incubation, the supernatants were collected, and IL-8 production was determined by ELISA. Experiments were done at least three times, and representative results are presented. Each assay was done in triplicate wells, and the data are expressed as the mean ± SEM. *, significant difference between the groups with and without the test specimens (P < 0.01).
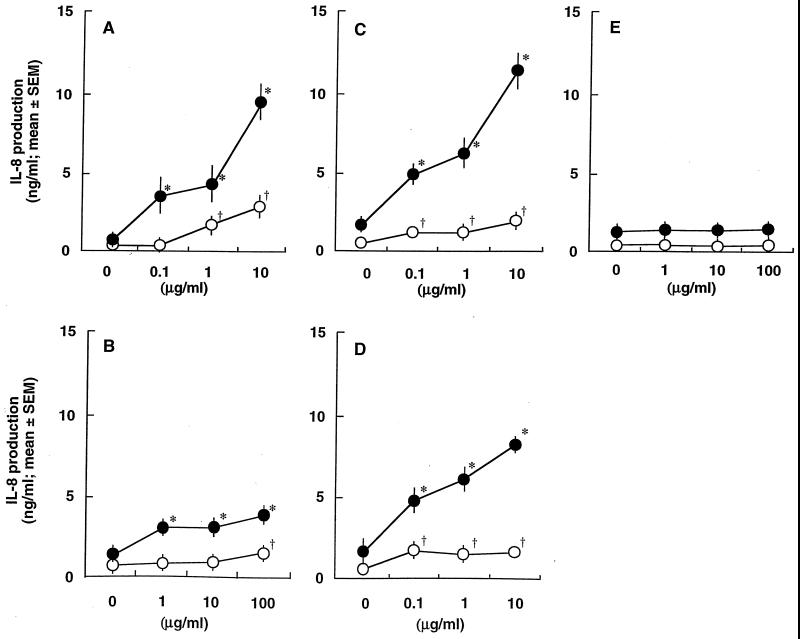
Next, to examine the role of soluble CD14 and/or LBP, we estimated IL-8 production in HGEC-1 stimulated with bacterial components in culture medium with and without FBS (Fig. 5). S. aureus peptidoglycan significantly induced IL-8 production in HGEC-1 in the absence of FBS, and the activity increased with the addition of FBS (Fig. 5A). MDP, a minimum essential structure for the immunopotentiating activities of bacterial cell walls, also exhibited a weak but significant induction of IL-8 production with or without FBS (Fig. 5B). Furthermore, P. gingivalis fimbriae and the active fimbrial peptide ALTTE exhibited a weak induction of IL-8 production in HGEC-1 under FBS-free conditions. The addition of FBS resulted in increased induction of IL-8 production (Fig. 5C and D). On the other hand, E. coli-type synthetic lipid A, compound 506, exhibited no induction of IL-8 production in HGEC-1 with or without FBS (Fig. 5E).
FIG. 5.
Effect of FBS on IL-8-producing activities in HGEC stimulated with S. aureus peptidoglycan (A), MDP (B), P. gingivalis fimbriae (C), ALTTE (D), or compound 506 (E). HGEC-1 were incubated at 37°C for 24 h with the indicated doses of the test specimens with (solid circles) or without (open circles) FBS. The experimental protocols were the same as described in the legend to Fig. 4. Significant differences were seen between the groups with and without the test specimens and with (*, P < 0.01) or without (†, P < 0.01) FBS.
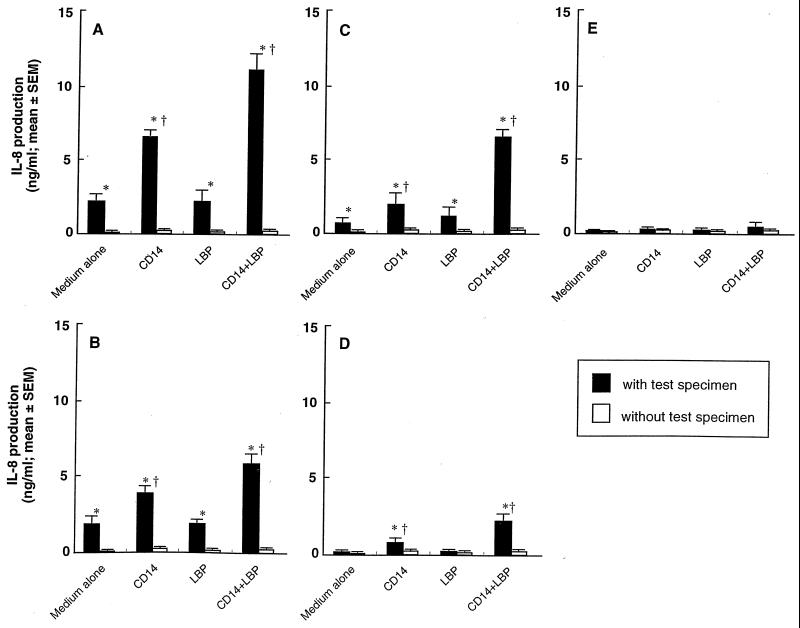
Soluble CD14 is present in serum and facilitates binding of LPS to host cells that do not express membrane CD14 (39), and this binding of LPS to CD14 is enhanced by the presence of LBP (36). We further examined the induction of IL-8 production in HGEC-1 stimulated with these bacterial components, with or without CD14 and/or LBP (Fig. 6). CD14 significantly increased production of IL-8 that was induced by S. aureus peptidoglycan, MDP, P. gingivalis fimbriae, and ALTTE. Additional LBP alone had no effect. However, the addition of LBP together with CD14 resulted in a greater increase in IL-8 production induced by these bacterial components than CD14 alone. On the other hand, compound 506 did no increase IL-8 production with CD14 and LBP. These findings suggest that the activation of HGEC by P. gingivalis fimbriae and their active peptide, ALTTE, similar to S. aureus peptidoglycan and MDP, was dependent on soluble CD14 and LBP in serum, but not on LBP alone.
FIG. 6.
Effect of soluble CD14 and LBP on IL-8-producing activities in HGEC stimulated with (solid bars) or without (open bars) S. aureus peptidoglycan (A), MDP (B), P. gingivalis fimbriae (C), ALTTE (D), or compound 506 (E). HGEC-1 were incubated at 37°C for 24 h with 1 μg/ml of each test specimen supplemented with CD14 (0.5 μg/ml) and/or LBP (0.05 μg/ml). The experimental protocols are the same as described in the legend to Fig. 4. Significant differences were seen between the groups with and without the bacterial specimens (*, P < 0.01), and between the groups with bacterial specimens with and without CD14 and/or LBP (†, P < 0.01).
NF-κB activation in HGEC.
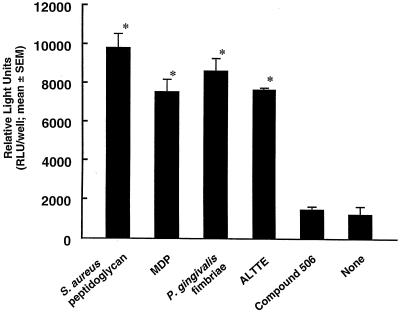
Transcription factor NF-κB is involved in the TLR signaling cascade (54). We examined NF-κB activation in HGEC-1 after stimulation with various bacterial components along with CD14 and LBP by a luciferase assay. P. gingivalis fimbriae and the peptide, ALTTE, as well as S. aureus peptidoglycan and MDP significantly activated NF-κB in HGEC-1 (Fig. 7). These specimens (10 to 100 μg/ml) also clearly activated NF-κB without CD14 or LBP (data not shown). However, compound 506 expressed no activation with CD14 and LBP (Fig. 7). These findings coincided with the pattern of IL-8 production by HGEC (Fig. 6).
FIG. 7.
NF-κB activation in HGEC in response to various bacterial components. HGEC-1 were transfected with 1 μg of pNK-κB-Luc plasmid and then incubated with or without 1 μg/ml of each test specimen with CD14 and LBP in HuMedia-KG2 at 37°C for 7 h. After incubation, the cells were lysed, and then luciferase activity was estimated with a luminometer. The experimental protocols were the same as described in the legend to Fig. 4. Significant differences were seen between the groups with and without the test specimens (*, P < 0.01).
Inhibitory effect of mouse monoclonal antibody to human TLR2 on IL-8 production by HGEC.
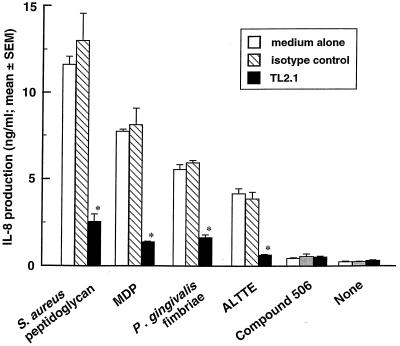
S. aureus peptidoglycan has been reported to be recognized by TLR2 (43). To examine the recognition of HGEC in response to bacterial components, HGEC-1 were treated with mouse monoclonal antibody to human TLR2, TL2.1, or mouse IgG2a as a control before the addition of various bacterial specimens. TL2.1 significantly inhibited IL-8 production induced by P. gingivalis fimbriae, ALTTE, S. aureus peptidoglycan, and MDP in HGEC (Fig. 8). These results indicated that these bacterial components, except for compound 506, activated HGEC through TLR2.
FIG. 8.
Effect of mouse monoclonal antibody to human TLR2 on IL-8-producing activities in HGEC stimulated with various bacterial components. HGEC-1 were incubated with 1 μg/ml of mouse monoclonal antibody to human TLR2, TL2.1, or mouse IgG2a for 30 min at room temperature before the addition of various test specimens (each 1 μg/ml) in the presence of CD14 and LBP. After 24 h of incubation, the supernatants were collected, and IL-8 production was estimated by ELISA. The experimental protocols were the same as described in the legend to Fig. 4. A significant difference was seen from the medium alone (*, P < 0.01).
DISCUSSION
Periodontal diseases are infectious, have a bacterial etiology, and cause inflammatory responses elicited by periodontopathogenic exposure in periodontal tissues. In these responses, epithelial cells are thought to play important roles as the initial point of contact with these pathogens. In the present study, we established immortalized HGEC and examined the expression of total keratin, an epithelial cell marker, by immunohistochemical staining. Oda et al. (23) reported that HPV-16-immortalized oral epithelial cells expressed positive staining with antikeratin antibodies as well as these primary cultured cells. In the present study, HGEC were clearly stained with anti-total keratin antibodies AE1 and AE3. When incubated in culture medium containing 2.0 mM calcium, they also clearly expressed involucrin (Fig. 1). These results demonstrate that immortalized HGEC remained characterized by epithelial cell markers.
Our results also indicated that HGEC express TLR2 but not CD14 or TLR4 (Fig. 2 and 3). Recently, it was shown by RT-PCR that intestinal epithelial cells do not express CD14 mRNA (5). Those cell lines also exhibited a differential expression of TLR2 and TLR4. Faure et al., on the contrary, demonstrated that human dermal umbilical vein and microvessel endothelial cells predominantly expressed TLR4 and showed very weak TLR2 expression. The cells in that study responded to LPS but not Mycobacterium tuberculosis lipoprotein (11). Therefore, it is suggested that various types of cells have different combinations of each TLR to recognize microbial pathogens.
We examined the responses of HGEC using S. aureus peptidoglycan as a TLR2 ligand (44) and E. coli-type synthetic lipid A, compound 506, as a TLR4 ligand (15) in the absence of FBS. HGEC induced IL-8 production stimulated by S. aureus peptidoglycan but not compound 506 (Fig. 4). Wang et al. showed that S. aureus peptidoglycan activated a human embryonic kidney cell line, HEK293, through TLR2 (48). These results suggest that HGEC recognize S. aureus peptidoglycan through TLR2, and TLR4-deficient HGEC are unresponsive to gram-negative bacterial lipid A. It was reported that various cytokines play important roles in the pathogenesis of periodontitis (37, 51). Gingival epithelial cells have been shown to produce cytokines, including IL-8, which are considered the important factor that participates in the initiation and maintenance of inflammatory reactions (16). IL-8 has been shown to be localized in gingival tissues of patients with periodontitis, and IL-8 mRNA was also expressed. IL-8 mRNA levels were shown to correspond to the severity of periodontitis (45).
Many studies have demonstrated that bacterial cell walls and their structural units, whether obtained by use of an enzyme or synthesized, exhibit various biological activities in a variety of host cells (29). These studies have also revealed that MDP is a common component of cell wall peptidoglycan that exhibits various immunobiological properties (41). The present study found that MDP as well as S. aureus peptidoglycan induces IL-8 production and NF-κB activation in TLR2-positive HGEC (Fig. 5 and 7). Furthermore, among various cell surface components, bacterial fimbriae are suspected to be a specific adherence factor. Previously, we demonstrated that fimbriae and their synthetic peptides derived from P. gingivalis, an anaerobic gram-negative periodontopathic bacterium, bind specifically to human gingival fibroblasts (24). It has also been shown that P. gingivalis fimbriae and a synthetic peptide composed of residues 69 to 73 of the fimbrillin, ALTTE, function in the activation of human PBMC (32). In the present study, the active fimbrial peptide, ALTTE, was found to induce IL-8 production in TLR2-positive and TLR4-negative HGEC. To our knowledge, this is the first time that a bacterial fimbrial protein and its active peptide have been indicated as a TLR2 ligand.
CD14, which is present on monocytic cell surfaces, binds to LPS or gram-positive bacterial cell wall components and facilitates signaling (53). It was recently shown that TLRs act as transmembrane coreceptors to CD14 in cellular responses (1). Further, a soluble form of CD14 was found to be able to functionally replace membrane-bound CD14 in CD14-negative cells, such as endothelial cells and astrocytes (38, 39, 50). It was also reported that MDP binds to CD14 molecules (10). Our present results showed that soluble CD14 clearly enhances HGEC activation stimulated with MDP as well as S. aureus peptidoglycan (Fig. 6). Furthermore, LBP with soluble CD14 causes greater cytokine-producing activity. However, LBP alone was not found to elicit an increase of activities. These results suggest that LBP is not associated with direct cell activation. Vesy et al. also reported that LBP promoted rapid binding of purified LPS to CD14 (47).
In the present study, similar results were observed, as P. gingivalis fimbriae and their active peptides appeared to bind to CD14 molecules and augment HGEC activation. This bacterial surface component-induced IL-8 production of HGEC was inhibited by mouse monoclonal antibody to human TLR2 as well as MDP. Taken together, these results demonstrate that bacterial fimbriae and their active peptides are recognized by TLR2 on human gingival epithelial cells and induce IL-8 production via NF-κB activation in HGEC. The recognition of pathogenic factors and their cell responsiveness via this pathway may play an important role in the development and progression of periodontal diseases.
ACKNOWLEDGMENTS
This study was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Science, and Culture of Japan (no. 13470390).
We thank M. Benton for critical reading of the manuscript.
REFERENCES
- 1.Akashi S, Ogata H, Kirikae F, Kirikae T, Kawasaki K, Nishijima M, Shimazu R, Nagai Y, Fukudome K, Kimoto M, Miyake K. Regulatory roles for CD14 and phosphatidylinositol in the signaling via Toll-like receptor 4-MD-2. Biochem Biophys Res Commun. 2000;168:172–177. doi: 10.1006/bbrc.2000.2089. [DOI] [PubMed] [Google Scholar]
- 2.Aliprantis A O, Yang R B, Mark M R, Suggett S, Devaux B, Radolf J D, Klimpel G R, Godowski P, Zychlinsky A. Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science. 1999;285:736–739. doi: 10.1126/science.285.5428.736. [DOI] [PubMed] [Google Scholar]
- 3.Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Scand J Clin Lab Investig. 1968;97:77–89. [PubMed] [Google Scholar]
- 4.Brightbill H D, Libraty D H, Krutzik S R, Yang R B, Belisle J T, Bleharski J R, Maitland M, Norgard M V, Plevy S E, Smale S T, Brennan P J, Bloom B R, Godowski P J, Modlin R L. Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science. 1999;285:732–736. doi: 10.1126/science.285.5428.732. [DOI] [PubMed] [Google Scholar]
- 5.Cario E, Rosenberg I M, Brandwein S L, Beck P L, Reinecker H-C, Podolsky D K. Lipopolysaccharide activates distinct signaling pathways in intestinal epithelial cell lines expressing Toll-like receptors. J Immunol. 2000;164:966–972. doi: 10.4049/jimmunol.164.2.966. [DOI] [PubMed] [Google Scholar]
- 6.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidium thiocyanate-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 7.Chuang T H, Ulevitch R J. Cloning and characterization of a subfamily of human toll-like receptors: hTLR7, hTLR8 and hTLR9. Eur Cytokine Netw. 2000;11:372–378. [PubMed] [Google Scholar]
- 8.Chuang T, Ulevitch R J. Identification of hTLR10: a novel human Toll-like receptor preferentially expressed in immune cells. Biochim Biophys Acta. 2001;1518:157–161. doi: 10.1016/s0167-4781(00)00289-x. [DOI] [PubMed] [Google Scholar]
- 9.Dilworth D, McCarrey J R. Single-step elimination of contaminating DNA prior to reverse transcriptase PCR. PCR Methods Appl. 1992;1:279–282. doi: 10.1101/gr.1.4.279. [DOI] [PubMed] [Google Scholar]
- 10.Dziarski R, Tapping R I, Tobias P S. Binding of bacterial peptidoglycan to CD14. J Biol Chem. 1998;273:8680–8690. doi: 10.1074/jbc.273.15.8680. [DOI] [PubMed] [Google Scholar]
- 11.Faure E, Equils O, Sieling P A, Thomas L, Zhang F X, Kirschning C J, Polentarutti N, Muzio M, Arditi M. Bacterial lipopolysaccharide activates NF-κB through Toll-like receptor 4 (TLR-4) in cultured human dermal endothelial cells. J Biol Chem. 2000;275:11058–11063. doi: 10.1074/jbc.275.15.11058. [DOI] [PubMed] [Google Scholar]
- 12.Fey S J, Larsen P M, Bravo R, Celis A, Celis J E. Differential immunological cross-reactivity of HeLa keratin antibodies with human epidermal keratins. Proc Natl Acad Sci USA. 1983;80:1905–1909. doi: 10.1073/pnas.80.7.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haffajee A D, Socransky S S. Microbial etiological agents of destructive periodontal diseases. Periodontology 2000. 1994;5:78–111. doi: 10.1111/j.1600-0757.1994.tb00020.x. [DOI] [PubMed] [Google Scholar]
- 14.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 15.Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. Toll-Like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749–3752. [PubMed] [Google Scholar]
- 16.Huang G T-J, Haake S K, Kim J-W, Park N-H. Differential expression of interleukin-8 and intercellular adhesion molecule-1 by human gingival epithelial cells in response to Actinobacillus actinomycetemcomitans or Porphyromonas gingivalis infection. Oral Microbiol Immunol. 1998;13:301–309. doi: 10.1111/j.1399-302x.1998.tb00711.x. [DOI] [PubMed] [Google Scholar]
- 17.Imoto M, Yoshimura N, Kusumoto S, Shiba T. Total synthesis of lipid A, active principle of bacterial endotoxin. Proc Jpn Acad Ser B. 1984;60:285–288. [Google Scholar]
- 18.Kagnoff M F, Eckmann L. Epithelial cells as sensors for microbial infection. J Clin Investig. 1997;100:6–10. doi: 10.1172/JCI119522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawai T, Adachi O, Ogawa T, Takeda K, Akira S. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity. 1999;11:115–122. doi: 10.1016/s1074-7613(00)80086-2. [DOI] [PubMed] [Google Scholar]
- 20.Kopp E B, Medzhitov R. The Toll-receptor family and control of innate immunity. Curr Opin Immunol. 1999;11:13–18. doi: 10.1016/s0952-7915(99)80003-x. [DOI] [PubMed] [Google Scholar]
- 21.Lehner M D, Morath S, Michelsen K S, Schumann R R, Hartung T. Induction of cross-tolerance by lipopolysaccharide and highly purified lipoteichoic acid via different Toll-like receptors independent of paracrine mediators. J Immunol. 2001;166:5161–5167. doi: 10.4049/jimmunol.166.8.5161. [DOI] [PubMed] [Google Scholar]
- 22.Medzhitov R, Preston-Hurburt P, Janeway C A., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 23.Oda D, Biglar L, Lee P, Blanton R. HPV immortalization of human oral epithelial cells; a model for carcinogenesis. Exp Cell Res. 1996;266:164–169. doi: 10.1006/excr.1996.0215. [DOI] [PubMed] [Google Scholar]
- 24.Ogawa T, Ogo H, Kinoshita A. Antagonistic effect of synthetic peptides corresponding to the binding regions within fimbrial subunit protein from Porphyromonas gingivalis to human gingival fibroblasts. Vaccine. 1997;15:230–236. doi: 10.1016/s0264-410x(96)00127-2. [DOI] [PubMed] [Google Scholar]
- 25.Ogawa T. The potential protective immune responses to synthetic peptides containing conserved epitopes of Porphyromonas gingivalis fimbrial protein. J Med Microbiol. 1994;41:349–358. doi: 10.1099/00222615-41-5-349. [DOI] [PubMed] [Google Scholar]
- 26.Ogawa T, Ogo H, Uchida H, Hamada S. Humoral and cellular immune response to the fimbriae of Porphyromonas gingivalis and their synthetic peptides. J Med Microbiol. 1994;40:397–402. doi: 10.1099/00222615-40-6-397. [DOI] [PubMed] [Google Scholar]
- 27.Ogawa T, Ogo H, Hamada S. Chemotaxis of human monocytes by synthetic peptides that mimics segments of Porphyromonas gingivalis fimbrial protein. Oral Microbiol Immunol. 1994;9:257–261. doi: 10.1111/j.1399-302x.1994.tb00068.x. [DOI] [PubMed] [Google Scholar]
- 28.Ogawa T, Shimauchi H, Hamada S. Mucosal and systemic immune responses in BALB/c mice to Bacteroides gingivalis fimbriae administered orally. Infect Immun. 1989;57:3466–3471. doi: 10.1128/iai.57.11.3466-3471.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ogawa T, Kotani S, Fukuda K, Tsukamoto Y, Mori M, Kusumoto S, Shiba T. Stimulation of migration of human monocytes by bacterial cell walls and muramyl dipeptides. Infect Immun. 1982;38:817–824. doi: 10.1128/iai.38.3.817-824.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ogawa T, Kotani S, Tsujimoto M, Kusumoto S, Shiba T, Kawata S, Yokogawa K. Contractile effects of bacterial cell walls, their enzymatic digests, and muramyl dipeptides on ileal strips from guinea pigs. Infect Immun. 1982;35:612–619. doi: 10.1128/iai.35.2.612-619.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ogawa T, Kusumoto Y, Uchida H, Nagashima S, Ogo H, Hamada S. Immunobiological activities of synthetic peptide segments of fimbrial protein from Porphyromonas gingivalis. Biochem Biophys Res Commun. 1991;180:1335–1341. doi: 10.1016/s0006-291x(05)81342-7. [DOI] [PubMed] [Google Scholar]
- 32.Ogawa T, Uchida H. A peptide, ALTTE, within the fimbrial subunit protein from Porphyromonas gingivalis induces production of interleukin 6, gene expression and protein phosphorylation in human peripheral blood mononuclear cells. FEMS Immunol Med Microbiol. 1995;11:197–206. doi: 10.1111/j.1574-695X.1995.tb00117.x. [DOI] [PubMed] [Google Scholar]
- 33.Ohyama Y, Nakamura S, Matsuzaki G, Shinoharam M, Hiroki A, Fujimura T, Yamada A, Itoh K, Nomoto K. Cytokine messenger RNA expression in the labial salivary glands of patients with Sjögren syndrome. Arthritis Rheum. 1996;39:1376–1384. doi: 10.1002/art.1780390816. [DOI] [PubMed] [Google Scholar]
- 34.Pearce W A, Buchanan T M. Structure and cell membrane-binding properties of bacterial fimbriae. In: Beachey E H, editor. Receptors and recognition, series B. 6, bacterial adherence. London, England: Chapman & Hall, Ltd.; 1980. pp. 289–344. [Google Scholar]
- 35.Poltorak A, He X, Smirnova I, Liu M-Y, Huffel C V, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 36.Pugin J, Schurer-Maly C C, Leturcq D, Moriarty A, Ulevitch R J, Tobias P S. Lipopolysaccharide activation of human endothelial and epithelial cells is mediated by lipopolysaccharide-binding protein and soluble CD14. Proc Natl Acad Sci USA. 1993;90:2744–2748. doi: 10.1073/pnas.90.7.2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roberts F A, McCaffery K A, Michalek S M. Profile of cytokine mRNA expression in chronic adult periodontitis. J Dent Res. 1997;76:1833–1839. doi: 10.1177/00220345970760120501. [DOI] [PubMed] [Google Scholar]
- 38.Schumann R R, Pfeil D, Lamping N, Kirschning C, Scherzinger G, Schlag P, Karawajew L, Herrmann F. Lipopolysaccharide induces the rapid tyrosine phosphorylation of the mitogen-activated protein kinases erk-1 and p38 in cultured human vascular endothelial cells requiring the presence of soluble CD14. Blood. 1996;87:2805–2814. [PubMed] [Google Scholar]
- 39.Schutt C, Schlling T, Grunwald U, Schoenfeld W, Kruger C. Endotoxin-neutralizing capacity of soluble CD14. Res Immunol. 1992;143:71–78. doi: 10.1016/0923-2494(92)80082-v. [DOI] [PubMed] [Google Scholar]
- 40.Slots J, Listgarten M A. Bacteroides gingivalis, Bacteroides intermedius and Actinobacillus actinomycetemcomitans in human periodontal diseases. J Clin Periodontol. 1988;15:85–93. doi: 10.1111/j.1600-051x.1988.tb00999.x. [DOI] [PubMed] [Google Scholar]
- 41.Takada H, Kotani S. Immunopharmacological activities of synthetic muramyl-peptides. In: Stewart-Tull D, Davies M, editors. Immunology of the bacterial cell envelope. New York, N.Y: John Wiley & Sons; 1985. pp. 119–152. [Google Scholar]
- 42.Takeuchi O, Kaufmann A, Grote K, Kawai T, Hoshino K, Morr M, Muhlradt P F, Akira S. Preferentially the R-stereoisomer of the mycoplasmal lipopeptide macrophage-activating lipopeptide-2 activates immune cells through a toll-like receptor 2- and MyD88-dependent signaling pathway. J Immunol. 2000;164:554–557. doi: 10.4049/jimmunol.164.2.554. [DOI] [PubMed] [Google Scholar]
- 43.Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, Akira S. Differential roles of TLR2 and TLR4 in recognition of Gram-negative and Gram-positive bacterial cell wall components. Immunity. 1999;11:443–451. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 44.Takeuchi O, Kawai T, Sanjo H, Copeland N G, Gilbert D J, Jenkins N A, Takeda K, Akira S. TLR6: a novel member of an expanding toll-like receptor family. Gene. 1999;231:59–65. doi: 10.1016/s0378-1119(99)00098-0. [DOI] [PubMed] [Google Scholar]
- 45.Tonetti M S, Imboden M A, Gerber L, Lang N P, Laissue J, Mueller C. Localized expression of mRNA for phagocyte-specific chemotactic cytokines in human periodontal infections. Infect Immun. 1994;62:4005–4014. doi: 10.1128/iai.62.9.4005-4014.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Underhill D M, Ozinsky A, Hajjar A M, Stevens A, Wilson C B, Bassetti M, Aderem A. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature. 1999;401:811–815. doi: 10.1038/44605. [DOI] [PubMed] [Google Scholar]
- 47.Vesy C J, Kitchens R L, Wolfbauer G, Albers J J, Munford R S. Lipopolysaccharide-binding protein and phospholipid transfer protein release lipopolysaccharides from gram-negative bacterial membranes. Infect Immun. 2000;68:2410–2417. doi: 10.1128/iai.68.5.2410-2417.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang Q, Dziarski R, Kirschning C J, Muzio M, Gupta D. Micrococci and peptidoglycan activate TLR2-MyD88-IRAK-TRAF-NIK-IKK-NF-κB signal transduction pathway that induces transcription of interleukin-8. Infect Immun. 2001;69:2270–2276. doi: 10.1128/IAI.69.4.2270-2276.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.White D, Mayrand D. Association of oral Bacteroides with gingivitis and periodontitis. J Periodont Res. 1981;16:259–265. doi: 10.1111/j.1600-0765.1981.tb00974.x. [DOI] [PubMed] [Google Scholar]
- 50.Willis S A, Nisen P D. Inhibition of lipopolysaccharide-induced IL-1 beta transcription by cyclic adenosine monophosphate in human astrocytic cells. J Immunol. 1995;154:1399–1406. [PubMed] [Google Scholar]
- 51.Wilson M, Reddi K, Henderson B. Cytokine-inducing components of periodontopathic bacteria. J Periodont Res. 1996;31:393–407. doi: 10.1111/j.1600-0765.1996.tb00508.x. [DOI] [PubMed] [Google Scholar]
- 52.Wright S D. Toll, a new piece in the puzzle of innate immunity. J Exp Med. 1999;189:605–609. doi: 10.1084/jem.189.4.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wright S D, Ramos R A, Tobias P S, Ulevitch R J, Mathison J C. CD14, a receptor for complexes of LPS and LPS binding protein. Science. 1990;249:1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- 54.Zhang F X, Kirschning C J, Mancinelli R, Xu X P, Jin Y, Faure E, Mantovani A, Rothe M, Muzio M, Arditi M. Bacterial lipopolysaccharide activates nuclear factor-kappaB through interleukin-1 signaling mediators in cultured human dermal endothelial cells and mononuclear phagocytes. J Biol Chem. 1999;274:7611–7614. doi: 10.1074/jbc.274.12.7611. [DOI] [PubMed] [Google Scholar]