Abstract
Chronic inflammation is a major cause of cancer worldwide. Interleukin 33 (IL-33) is a critical initiator of cancer-prone chronic inflammation; however, its induction mechanism by the environmental causes of chronic inflammation is unknown. Herein, we demonstrate that Toll-like receptor (TLR)3/4-TBK1-IRF3 pathway activation links environmental insults to IL-33 induction in the skin and pancreas. FDA-approved drug library screen identified pitavastatin as an effective IL-33 inhibitor by blocking TBK1 membrane recruitment/activation through the mevalonate pathway inhibition. Accordingly, pitavastatin prevented chronic pancreatitis and its cancer sequela in an IL-33-dependent manner. IRF3-IL-33 axis was highly active in chronic pancreatitis and its associated pancreatic cancer in humans. Interestingly, pitavastatin use correlated with a significantly reduced risk of chronic pancreatitis and pancreatic cancer in patients. Our findings demonstrate that blocking the TBK1-IRF3 signaling pathway suppresses IL-33 expression and cancer-prone chronic inflammation. Statins present a safe and effective therapeutic strategy to prevent chronic inflammation and its cancer sequela.
Keywords: Interleukin-33, TBK1-IRF3 pathway, pitavastatin, chronic inflammation, cancer prevention, chronic pancreatitis, pancreatic cancer
Introduction
Chronic inflammation accounts for 20% of cancers worldwide1–3. Cancer-prone chronic inflammation, such as pancreatitis, inflammatory bowel disease (IBD), and hepatitis, have risen in recent decades, highlighting the urgent need for improved cancer prevention strategies in at-risk populations4–6. Several immune cells and factors, including M2 macrophages, mast cells, TGF-β, interleukin (IL)-10, and IL-13, have been identified to promote carcinogenesis in chronic inflammation7–11. However, inhibiting these effectors alone or combined to prevent cancer has proven challenging due to their redundant function in cancer promotion12–14. Furthermore, anti-inflammatory medications, including dexamethasone, which can reduce the risk of cancer development by broadly suppressing immune responses15, have severe side effects, including coagulopathy and immune hyper-activation, limiting their use as cancer-preventive agents16,17. To overcome these challenges, it is essential to develop safe agents that can block the development of chronic inflammation and thereby prevent its cancer sequela.
IL-33 is an epithelium-derived alarmin cytokine that is a member of the IL-1 cytokine family and drives type 2 immune responses in allergic inflammation by triggering T helper 2 (Th2) cell and type 2 innate lymphoid cell (ILC2) activation18,19. IL-33 is a critical initiator of chronic inflammation20,21. By binding to its receptor, suppressor of tumorigenesis 2 (ST2, also known as interleukin-1 receptor-like 1 (IL1RL1)), IL-33 promotes the development of a chronic inflammatory environment in damaged tissues19. IL-33 and ST2 are highly expressed in chronic inflammatory diseases, including colitis, pancreatitis, and chronic obstructive pulmonary disease20–24. IL-33 plays a complex role in cancer development. IL-33 induction suppresses colon tumor growth and activates CD8+ T and natural killer (NK) cells to inhibit lung metastasis in mice25,26. In contrast, the upregulation of IL-33 during the transition from acute to chronic inflammation initiates the development of a tumor-promoting immune environment20,27–29. Importantly, IL-33 also acts as a nuclear protein and promotes tumorigenesis by regulating SMAD signaling in chronic inflammation21. Thus, blocking IL-33 expression instead of its cytokine function alone is essential to achieve cancer prevention in chronic inflammation.
Chronic inflammation is triggered by innate recognition of damage-associated molecular patterns (DAMPs) and pathogen-associated molecular patterns (PAMPs) in injured tissues30,31. Mitogen-activated protein kinase (MAPK), Nuclear factor-κB (NF-κB), and TANK-binding kinase 1 (TBK1) signaling are the cardinal intracellular pathways activated by DAMPs and PAMPs upon binding to pattern recognition receptors (PRRs), including Toll-like receptors (TLRs), retinoid acid-inducible gene I (RIG-I)-like receptors (RLRs), and nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs)30,32–34. NF-κB, activator protein 1 (AP-1), cAMP-response element binding protein (CREB), interferon regulatory factor 3 (IRF3), and IRF7 are among the major transcription factors activated by PRRs, which can regulate downstream inflammatory cytokines35–40. Several of these signaling pathways can induce IL-33 expression41,42. Knockdown of Glutaredoxin-1 inhibits induction of IL-33 in macrophage cell lines42. RLRs and CREB signaling induce IL-33 expression in mouse embryonic fibroblasts and macrophage cell lines41. However, the mechanism of IL-33 induction in epithelial cells during the development of chronic inflammation remains unknown.
Herein, we identify TLR3/4-TBK1-IRF3 signaling as the key regulator of IL-33 expression and discover statin as a novel IL-33 inhibitor by regulating TBK1 signaling in cancer-prone chronic inflammation. TLR3/4-TBK1-IRF3 signaling was highly activated in the skin and pancreas chronic inflammation, and knockdown of IRF3 blocked Il33 expression in vitro and in vivo. Pitavastatin, an IL-33 inhibitor identified from the FDA-approved drug library screening, reduced membrane-bound phosphorylated TBK1 (p-TBK1) through mevalonate pathway inhibition, which resulted in the suppression of p-IRF3 and IL-33 expression. Accordingly, pitavastatin reduced the risk of pancreatitis and PDAC in mice and humans. We conclude that blocking the TBK1-IRF3-IL-33 axis by statin represents a novel and actionable strategy to prevent chronic inflammation and its cancer sequela.
Results
Chronic inflammatory insults in the skin and pancreas activate the TLR3/4-TBK1-IRF3 signaling pathway
To determine the mechanism of IL-33 induction in chronic inflammation, we subjected wild-type (WT) mice to established models of chronic inflammation in the skin and pancreas. To induce chronic dermatitis, WT mice received topical 2,4-Dinitro-1-fluorobenzene (DNFB, a contact allergen) in acetone or acetone alone (control) on the back skin three times a week for 22 days20,21. Epidermal thickness and mast cell numbers were significantly increased in DNFB-treated skin (Extended Data Fig. 1a, b). To induce chronic pancreatitis, mice received intraperitoneal caerulein injections in phosphate-buffered saline (PBS) or PBS alone hourly for 6 hours per day, three days per week for three weeks43,44. Caerulein treatment led to inflammation and fibrosis in the pancreas, which was associated with a significant CD45+ leukocyte infiltration into the pancreas (Extended Data Fig. 1c, d). Consistent with previous reports22,23, IL-33 was highly expressed in the epithelial cells of DNFB-treated skin and caerulein-treated pancreas compared with acetone-treated skin and PBS-treated pancreas, respectively (Fig. 1a). IL-33 RNA and protein levels were significantly increased in the inflamed compared with control tissues (Extended Data Fig. 1e-h). To identify the signaling pathway that induced IL-33 in chronic inflammation, we performed RNA sequencing on the epidermal keratinocytes isolated from the back skin of WT mice treated with DNFB versus acetone and the pancreas of WT mice treated with caerulein versus PBS. Among differentially expressed genes, nine common genes were increased in DNFB-treated skin and caerulein-treated pancreas (Extended Data Fig. 1i, j). Among them, S100a8 and S100a9, well-known DAMPs and TLR 3/4 ligands45,46, were highly enriched in chronic inflammation. Consistently, Gene Set Enrichment Analysis (GSEA) revealed the activation of the TLR3/4 signaling pathway in chronic pancreatitis (Fig. 1b, c). Thus, TLR3/4 signaling may induce IL-33 expression in chronic inflammation.
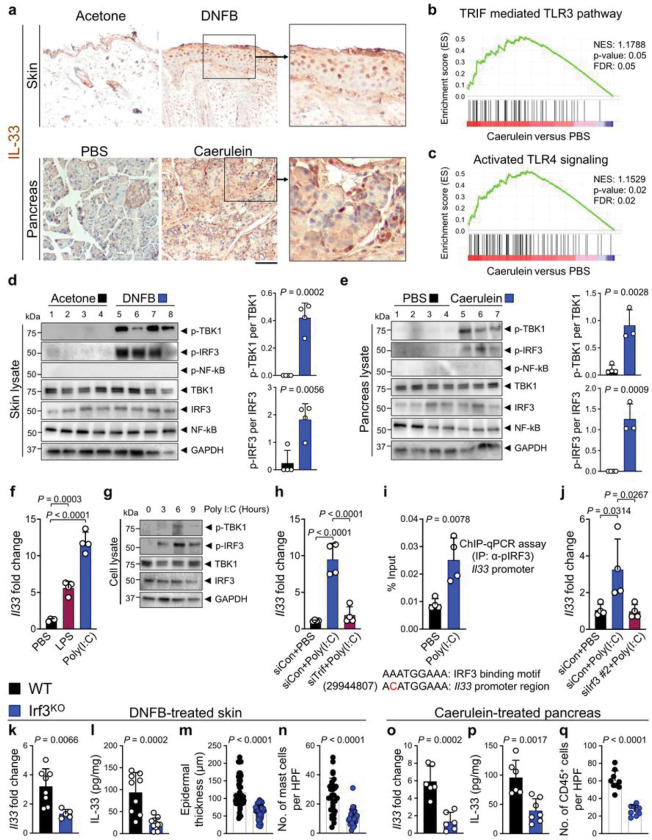
Figure 1. TBK1-IRF3 signaling induces IL-33 expression in chronic inflammation.
a, Representative images of IL-33 immunostaining on DNFB-treated skin and caerulein-treated pancreas compared with the acetone and PBS-treated controls, respectively. Note the nuclear IL-33 stains in the epithelial cells of the inflamed organs.
b, The enrichment plot of TRIF mediated TLR3 signaling gene set in caerulein-treated compared with PBS-treated pancreas.
c, The enrichment plot of activated TLR4 signaling gene set in caerulein-treated compared with PBS-treated pancreas.
d, (Left) Immunoblot of p-TBK1, p-IRF3, p-NF-κB, TBK1, IRF3, NF-κB and GAPDH proteins in DNFB- versus acetone-treated WT skin (n=4 in each group). (Right) The ratio of p-TBK1/TBK1 and p-IRF3/IRF3 protein band intensity quantified from the immunoblot shown on the left.
e, (Left) Immunoblot of p-TBK1, p-IRF3, p-NF-κB, TBK1, IRF3, NF-κB and GAPDH proteins in caerulein-versus PBS-treated WT pancreas (n=3 in caerulein and n=4 in PBS group). (Right) The ratio of p-TBK1/TBK1 and p-IRF3/IRF3 protein band intensity quantified from the immunoblot shown on the left.
f, Il33 expression in Pam212 cells at 6 hours after treatment with poly(I:C), LPS and PBS (n=4 in each group).
g, Time course of p-TBK1, p-IRF3, TBK1, IRF3 and GAPDH protein expression in Pam212 cells after poly(I:C) treatment.
h, Il33 expression in Trif siRNA (siTrif)- versus siRNA control (siCon)-treated Pam212 cells in response to poly(I:C) versus PBS treatment (n=4 in each group).
i, Chromatin Immunoprecipitation (ChIP)-qPCR assay for p-IRF3 binding to Il33 promoter region after the treatment of Pam212 cells with poly(I:C) versus PBS (n=4 in each group). Note the presence of a sequence very similar to the IRF3 binding motif in the Il33 promoter region.
j, Il33 expression in siIrf3- versus siCon-treated Pam212 cells in response to poly(I:C) versus PBS treatment (n=4 in each group).
k, Il33 expression levels in DNFB-treated WT versus Irf3KO skin (n=8 in WT and n=5 in Irf3KO group).
l, IL-33 protein levels in DNFB-treated WT versus Irf3KO skin (n=9 in WT and n=10 in Irf3KO group).
m, Epidermal thickness in DNFB-treated WT versus Irf3KO skin. Each dot represents the average of three measurements in an HPF image. Ten random HPF images per skin sample are included (n=6 in each group).
n, Mast cell counts in DNFB-treated WT versus Irf3KO skin. Each dot represents cell counts from an HPF image. Three randomly selected HPF images are included per sample (n=10 in each group).
o, Il33 expression levels in caerulein-treated WT versus Irf3KO pancreas (n=6 in each group).
p, IL-33 protein levels in caerulein-treated WT versus Irf3KO pancreas (n=6 in WT and n=7 in Irf3KO group).
q, CD45+ immune cell counts in caerulein-treated WT versus Irf3KO pancreas. Each dot represents cell counts from an HPF image. Three randomly selected HPF images are included per sample (n=3 in each group).
Graphs show mean + SD, Fig. 1d, e, i, k-q: unpaired t test, Fig 1f, h, j: one-way ANOVA, scale bar: 100 μm
TRIF-mediated TBK1-IRF3 signaling regulates IL-33 expression
To determine the nature of TLR3/4 signaling in chronic inflammation, we examined the activation of TLR3/4 downstream targets, TRIF and MyD88 adaptor proteins30. TRIF-mediated TBK1 and IRF3 phosphorylation (i.e., activated forms of TBK1 and IRF3) were markedly increased in DNFB-treated skin and caerulein-treated pancreas compared with acetone and PBS-treated controls, respectively (Fig. 1d, e). Likewise, the known IRF3 target genes, Tnf, Il1b, and Cxcl10, were significantly induced in DNFB-treated skin and caerulein-treated pancreas (Extended Data Fig.2a-f). However, MyD88-mediated NF-kB signaling was not activated in either chronic inflammatory condition. To determine whether Il33 was regulated by TBK1-IRF3 signaling, we treated epithelial cells with polyinosinic-polycytidylic acid (poly(I:C)), a TLR3 agonist, and Lipopolysaccharides (LPS), a TLR4 agonist41,47. Poly(I:C) and LPS significantly increased Il33 expression in a mouse keratinocyte cell line, Pam212 (Fig. 1f). Likewise, poly(I:C) increased phosphorylated TBK1 and IRF3 in Pam212 cells after 6 hours and markedly induced the known IRF3 target genes expression (Fig. 1g and Extended Data Fig. 2g-i). Knocking down Trif expression by siRNA completely blocked poly(I:C)-induced Il33 expression (Fig. 1h). Il33 promoter region contained a sequence very similar to IRF3 binding motif (mm10;chr19+:29944807) (Fig. 1i, bottom). Importantly, poly(I:C) promoted the binding of phosphorylated IRF3 to the Il33 promoter region (Fig. 1i, top). In addition, knocking down Irf3 by siRNA markedly reduced poly(I:C)-induced Il33 expression (Fig. 1j and Extended Data Fig. 2j). These findings demonstrate that IL-33 is a downstream target of TRIF-mediated TBK1-IRF3 signaling.
IRF3 is required for the induction of IL-33 and chronic inflammation in the skin and pancreas
To determine whether Il33 expression is mediated by TBK1-IRF3 signaling in chronic inflammation, we subjected Irf3 knockout (Irf3KO) mice to chronic inflammatory conditions in the skin and pancreas. IL-33 RNA and protein levels were significantly reduced in DNFB-treated Irf3KO compared with WT skin (Fig. 1k, l). Moreover, epidermal thickness and mast cell numbers were decreased markedly in DNFB-treated Irf3KO compared with WT skin (Fig. 1m, n and Extended Data Fig. 3a, b). Consistent with these results, IL-33 levels were reduced in DNFB-treated skin of Trif and Myd88 double knockout (Trif,Myd88DKO) mice but not in Myd88KO mice (Extended Data Fig. 3c), which indicates that TRIF adapter protein is the primary upstream activator of the IRF3 signaling pathway to induce IL-33 in chronic inflammation. IL-33 RNA and protein levels decreased in caerulein-treated Irf3KO compared with WT pancreas (Fig. 1o, p). Likewise, caerulein-treated Irf3KO pancreas showed less inflammation and reduced CD45+ leukocyte infiltration compared with WT pancreas (Fig. 1q and Extended Data Fig. 3d). Collectively, these findings demonstrate that TBK1-IRF3 regulates IL-33 expression in chronic dermatitis and pancreatitis.
Pitavastatin blocks TBK1 phosphorylation and IL-33 expression via mevalonate pathway inhibition
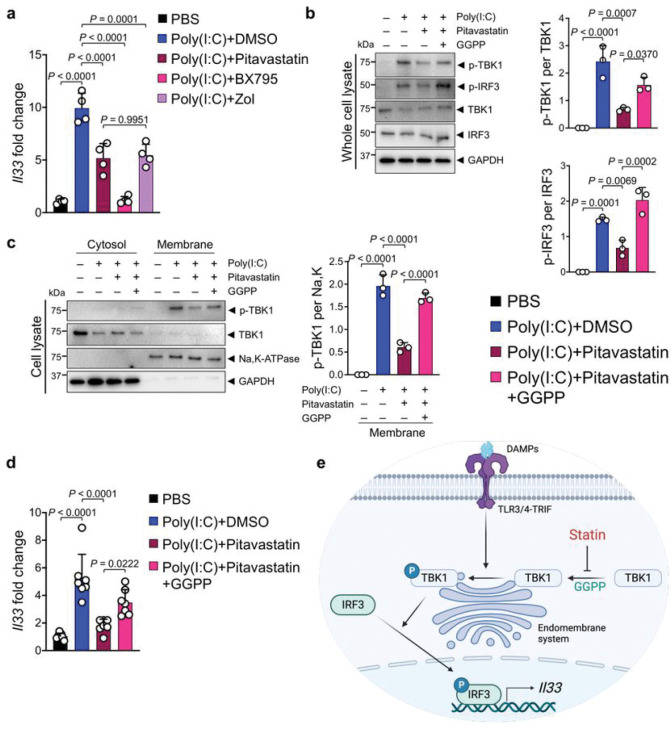
To identify a small molecule IL-33 inhibitor that can be safely used to alleviate chronic inflammation and its cancer sequela, we screened an FDA-approved drug library in a luciferase-based Il33 expression assay (Extended Data Fig. 4a, b). Among 1018 FDA-approved small molecules that were screened, we found five candidates, which decreased Il33/control luminescence absorbance to less than 40% while having no effect on absorbance in a control luminescence assay (Extended Data Fig. 4c). Among these candidates, pitavastatin calcium (labeled as O16 in the screen) suppressed poly(I:C)-induced Il33 and endogenous Il33 levels in Pam212 and PyMttg breast cancer cell line, respectively (Extended Data Fig. 4d, e). Pitavastatin is a lipophilic statin that inhibits β-Hydroxy β-methylglutaryl-CoA (HMG-CoA) reductase, an intermediate reaction in the mevalonate pathway48. Pitavastatin and zoledronic acid, another mevalonate pathway inhibitor, equally suppressed poly(I:C)-induced Il33 expression, while a TBK1 inhibitor, BX795, completely blocked poly(I:C)-induced Il33 expression in Pam212 cells (Fig. 2a). Interestingly, lipophilic statins, pitavastatin and atorvastatin, inhibited Il33 expression more potently compared with a hydrophilic statin, rosuvastatin (Extended Data Fig. 4f). Statins inhibit HMG-CoA reductase, which leads to the reduction in geranylgeranyl diphosphate (GGPP), a product of the mevalonate pathway48,49. GGPP plays a critical role in the membrane localization of intracellular proteins50. Mevalonate pathway inhibition by pitavastatin blocked poly(I:C)-induced activation of the TBK1-IRF3 signaling pathway in Pam212 cells (Fig. 2b). Importantly, poly(I:C) treatment led to the recruitment of TBK1 to the membrane for phosphorylation (i.e., activation), and pitavastatin markedly reduced membrane-bound p-TBK1 (Fig. 2c). The addition of exogenous GGPP to Pam212 cells reversed pitavastatin effect and restored TBK1-IRF3 signaling pathway activation and membrane-bound p-TBK1 levels (Fig. 2b, c). Accordingly, pitavastatin suppression of poly(I:C)-induced Il33 expression was reversed by exogenous GGPP (Fig. 2d). Thus, pitavastatin inhibits Il33 expression by blocking GGPP-dependent membrane recruitment and activation of TBK1 (Fig. 2e).
Figure 2. Pitavastatin inhibits IL-33 expression by blocking GGPP-dependent TBK1 activation.
a, Il33 expression in poly(I:C)-treated Pam212 cells that received pitavastatin, BX795, Zoliadic acid (Zol) or PBS (n=4 in each group).
b, (Left) Immunoblot of p-TBK1, p-IRF3, TBK1, IRF3, and GAPDH proteins in whole cell lysates of poly(I:C)-treated Pam212 cells that received pitavastatin alone or in combination with GGPP. (Right) The ratio of p-TBK1/TBK1 and p-IRF3/IRF3 protein band intensity from immunoblots (n=3 in each group).
c, (Left) Immunoblot of p-TBK1, TBK1, Na-K-ATPase, and GAPDH proteins in membrane and cytosol fraction of poly(I:C)-treated Pam212 cells that received pitavastatin alone or in combination with GGPP. (Right) The ratio of membrane-bound p-TBK1/Na,K-ATPase protein band intensity from the immunoblots (n=3 in each group).
d, Il33 expression in poly(I:C)-treated Pam212 cells that received pitavastatin alone or in combination with GGPP (n=7 in each group).
e, Schematic diagram of pitavastatin mechanism of action in blocking mevalonate pathway-GGPP mediated TBK1-IRF3 signaling activation.
Graphs show mean + SD, one-way ANOVA.
Pitavastatin suppresses chronic inflammation and its cancer sequela in an IL-33-dependent manner
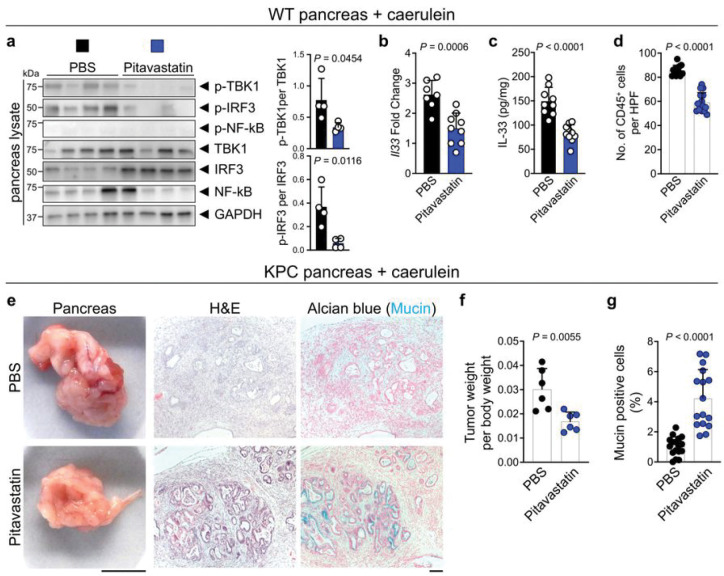
Next, we investigated the impact of pitavastatin treatment on suppressing IL-33 and chronic Inflammation in vivo. To test the pitavastatin effect on skin inflammation, mice were treated with topical DNFB on the back skin for 22 days together with topical pitavastatin versus carrier control (acetone). Pitavastatin treatment dramatically blocked p-TBK1 and p-IRF3 levels compared to acetone-treated mice (Extended Data Fig. 5a). Accordingly, IL-33 RNA and protein levels were markedly decreased in pitavastatin- compared with acetone-treated mice (Extended Data Fig. 5b, c). Skin inflammation, as marked by epidermal thickness and mast cell numbers in the skin, was significantly reduced in pitavastatin- compared to acetone-treated mice (Extended Data Fig. 5d-f). To examine the effect of pitavastatin on chronic pancreatitis, mice were treated with caerulein for three weeks together with intraperitoneal pitavastatin versus carrier control (PBS). Pitavastatin treatment significantly reduced p-TBK1 and p-IRF3 levels in the pancreas with no effect on NF-kB signaling (Fig. 3a). Likewise, pitavastatin significantly reduced IL-33 RNA and protein levels in the caerulein-treated pancreas (Fig. 3b, c). Moreover, pitavastatin treatment preserved the normal architecture of the caerulein-treated pancreas and reduced CD45+ leukocyte infiltration into the pancreas compared with PBS-treated mice (Fig. 3d and Extended Data Fig. 6a). Importantly, pitavastatin had no significant impact on the severity of pancreatitis in Il33KO mice (Extended Data Fig. 6b, c). Thus, pitavastatin prevents chronic inflammation by suppressing the TBK1-IRF3-IL-33 signaling axis in vivo.
Figure 3. Pitavastatin blocks chronic pancreatitis and its cancer sequela.
a, (Left) Immunoblot of p-TBK1, p-IRF3, p-NF-kB, TBK1, IRF3, NF-kB and GAPDH proteins in pitavastatin-versus PBS-treated WT pancreas at the completion of caerulein treatment protocol (n=4 in each group). (Right) The ratio of p-TBK1/TBK1 and p-IRF3/IRF3 protein band intensity quanti ed from the immunoblot shown on the left.
b, Il33 expression in pitavastatin- versus PBS-treated WT pancreas at the completion of caerulein treatment protocol (n=9 in pitavastatin and n=7 in PBS group).
c, IL-33 protein levels in pitavastatin- versus PBS-treated WT pancreas at the completion of caerulein treatment protocol (n=10 in pitavastatin and n=9 in PBS group).
d, CD45+ immune cell counts in pitavastatin- versus PBS-treated WT pancreas at the completion of the caerulein treatment protocol. Each dot represents cell counts from an HPF image. Three randomly selected HPF images are included per sample (n=5 in pitavastatin and n=4 in PBS group).
e, Representative images of macroscopic, hematoxylin and eosin (H&E), and Alcian blue-stained pancreatic tumors from pitavastatin- versus PBS-treated KPC mice that underwent caerulein-induced pancreatic cancer protocol.
f, Ratio of pancreatic tumor per body weight in pitavastatin- versus PBS-treated KPC mice at the completion of caerulein-induced pancreatic cancer protocol (n=6 in each group).
g, Percent mucin-positive cells in pitavastatin- versus PBS-treated KPC tumors at the completion of caerulein-induced pancreatic cancer protocol. Each dot represents % mucin-positive cells in an HPF image. Two to three randomly selected HPF images are included per sample (n=6 in each group).
Graphs show mean + SD, unpaired t test, scale bars: 1cm (macroscopic images), 100 μm (microscopic images).
Chronic pancreatitis is a risk factor for the development of pancreatic cancer51,52. To establish a chronic pancreatitis-associated pancreatic cancer model in mice, we treated pancreas-specific Kras and Tp53 mutant (KrasLSL-G12D, Tp53ox/+, p48-Cretg or KPC) mice with hourly intraperitoneal injections of caerulein for 7 hours per day for two consecutive days (Extended Data Fig. 6d)53,54. This pancreatic carcinogenesis protocol led to a significant induction of IL-33 expression in the pancreas (Extended Data Fig. 6e). KPC mice were treated with pitavastatin versus PBS control after the caerulein injection protocol. Pitavastatin treatment significantly reduced pancreatic tumor weight per body weight ratio compared with PBS-treated KPC mice (Fig. 3e, f). Moreover, pitavastatin treatment blocked the progression of pancreatic tumors and retained the tumor cells in a pre-cancerous stage with high mucin production compared with PBS-treated tumors (Fig. 3e, g). In contrast, there was no significant difference in pancreatic tumor per body weight ratio or mucin production by tumor cells in Il33KO KPC mice treated with pitavastatin versus PBS control (Extended Data Fig. 6f-h). These findings demonstrate that pitavastatin blocks chronic pancreatitis-associated pancreatic cancer in an IL-33-dependent manner.
IRF3-IL-33 axis is highly active in chronic pancreatitis and pancreatic cancer in humans
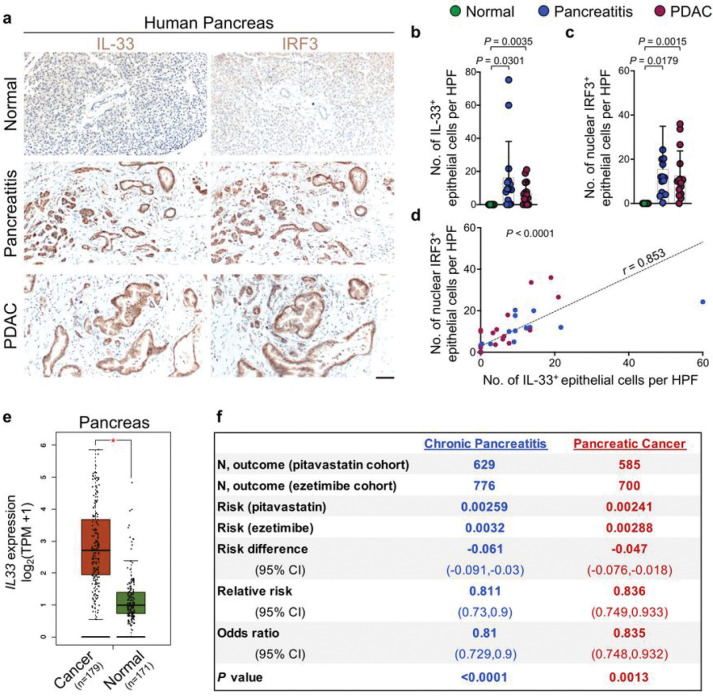
To extend our findings to cancer-prone chronic inflammation in humans, we examined IL-33 and IRF3 expression in the epithelial cells across 15 matched samples of the normal pancreas, pancreatitis, and pancreatitis-associated pancreatic ductal adenocarcinoma (PDAC). IL-33 and IRF3 were highly expressed in the nucleus of epithelial cells in pancreatitis and pancreatitis-associated PDAC samples (Fig. 4a–c). Moreover, the number of IL-33+ epithelial cells was positively correlated with the number of IRF3+ epithelial cells across the samples (Fig. 4d). Expression of IL33 and other IRF3 target genes, TNF, IL1B, and CXCL10, were highly upregulated in pancreatic cancer compared to the normal pancreas in a large collection of samples represented in TCGA and GTEx databases (Fig. 4e and Extended Data Fig. 7).
Figure 4. IRF3-IL-33 signaling axis is highly active in human chronic pancreatitis-associated pancreatic cancer, and pitavastatin reduces the risk of chronic pancreatitis and pancreatic cancer in patients.
a, Representative images of IL-33 and IRF3 immunostaining on adjacent sections of matched normal pancreas, chronic pancreatitis, and PDAC collected from pancreatic cancer patients.
b, IL-33+ epithelial cell counts per HPF in the matched samples from human pancreatic tissues. Each dot represents the average cell counts across three randomly selected HPF images per sample (n=15 patients, paired t-test).
c, IRF3+ epithelial cell counts per HPF in the matched samples from human pancreatic tissues. Each dot represents the average cell counts across three randomly selected HPF images per sample (n=15 patients, paired t-test).
d, The correlation between IL-33+ and IRF3+ cell counts across normal pancreas, chronic pancreatitis, and PDAC samples (n=45 matched samples from 15 patients, t-test for the Pearson correlation coefficient).
e, Box plot of IL33 expression in pancreatic cancer versus normal pancreas across TCGA/GTEx datasets (* P < 0.01, one-way ANOVA, Gene Expression Pro ling Interactive Analysis database).
f, A retrospective cohort analysis of chronic pancreatitis and pancreatic cancer risk in matched cohorts of patients treated with pitavastatin (test) versus ezetimibe (control).
Graphs show mean + SD, scale bar: 100 μm
Pitavastatin treatment is associated with reduced risk of chronic pancreatitis and pancreatic cancer in patients
Finally, we investigated the effect of pitavastatin on the risk of chronic pancreatitis and pancreatic cancer in humans using an epidemiologic approach. We compared matched cohorts of patients from the TriNetX Diamond Network, a global health network containing electronic medical record-derived data from more than 200 million patients across 92 healthcare organizations in North America and Europe (Supplementary Table S1)55. The risk of chronic pancreatitis was significantly decreased in patients treated with pitavastatin compared to those treated with ezetimibe, another cholesterol-lowering agent commonly used in the clinic, which does not affect the mevalonate pathway (control, OR 0.81; 95% CI (0.729–0.9); P<0.0001). Furthermore, the risk of pancreatic cancer was markedly decreased in the pitavastatin-treated group compared with the ezetimibe-treated control (OR 0.835; 95% CI (0.748–0.932); P=0.0013) (Fig. 4f). Collectively, these outcomes indicate that blocking TBK1-IRF3-IL-33 signaling axis by statins may prevent chronic inflammation and its cancer sequela in high-risk patients.
Discussion
Our findings reveal that lipophilic statins suppress cancer-prone chronic inflammation by blocking the TBK1-IRF3-IL-33 signaling axis induced by chronic exposure to environmental insults. Cellular damage and release of DAMPs lead to TLR3/4-mediated activation of the TBK1-IRF3 signaling pathway. Phosphorylated IRF3 directly binds to the Il33 promoter to drive IL-33 expression during the initiation of chronic inflammation. Importantly, pitavastatin blocks Il33 expression by inhibiting the mevalonate pathway-mediated TBK1 binding to the membrane, which is required for its phosphorylation and downstream IRF3 activation. By inhibiting Il33 expression, pitavastatin blocks the cytokine and nuclear functions of IL-33 in chronic inflammation, effectively reducing the risk of chronic pancreatitis and pancreatic cancer in mice and humans. Therefore, blocking the TBK1-IRF3-IL-33 signaling axis with statins represents a safe, effective, and readily accessible strategy to prevent chronic inflammation and its cancer sequela, which can impact many individuals at high risk of developing cancer-prone chronic inflammation.
Several malignancies are associated with activated TBK1-IRF3 signaling pathway within the cancer cells, which can play a critical cell-autonomous role in cancer progression56,57. In particular, TBK1 activation has been linked to skin and pancreatic cancer development58,59. Furthermore, high TBK1 expression has been shown to induce an immunosuppressive tumor microenvironment by increasing PD-L1 expression and inhibiting CD8+ T cell infiltration in lung and liver cancer60,61. Accordingly, TBK1 inhibitors have tumor inhibitory effects associated with improved sensitivity to immunotherapy in several cancer types, including melanoma and liver cancer59,60,62. Moreover, activated TBK1-IRF3 signaling leads to the induction of angiogenesis factors, which is associated with poor prognosis in several cancers, including pancreatic cancer57,63–65. Our findings demonstrate that IL-33 is a target of TBK1-IRF3 signaling in cancer-prone tissues. Likewise, IL-33’s pro-tumor function can play an integral role in tumor promotion by TBK1-IRF3 signaling27–29. Thus, targeting the TBK1-IRF3-IL-33 signaling axis is an attractive strategy to block cancer development.
The mevalonate pathway has recently emerged as an important regulator of cancer development. Blocking the mevalonate pathway product, GGPP, inhibits cancer cells’ amino acid uptake by regulating macropinocytosis66. Moreover, the inhibition of the mevalonate pathway by statins induces a strong antitumor T cell immunity by promoting antigen presentation50. Importantly, our findings reveal a previously unknown mechanism by which the mevalonate pathway regulates TBK1-IRF3 signaling through its essential role in the membrane recruitment of TBK1, which is required for its phosphorylation/activation. Our findings may also provide a novel explanation for how GGPP regulates PI3K/MAPK pathway activation by promoting the membrane localization of the critical signaling molecules in the pathway67,68. Thus, the elucidation of statins’ mechanism of action in blocking the TBK1-IRF3-IL-33 signaling axis has far-reaching implications in revealing a fundamental aspect of TBK1-IRF3 and other major intracellular signaling pathways.
Our work uncovers an unprecedented role for statins as a novel class of chemopreventive agents for suppressing chronic inflammation and its cancer sequela. Statins are commonly used for long-term control of hyperlipidemia, and 56 million adults are taking statins in the United States alone69. Statins are well-tolerated over many years of treatment with minimal side effects70, which signifies their high potential as effective agents for cancer prevention. Unlike TBK1 inhibitors that are in development with a high cost and potential side effects, statins are affordable FDA-approved medications that can be safely prescribed for long-term use, which are essential requirements for an ideal chemopreventive agent. Furthermore, our findings highlight the topical application of statins as a novel treatment strategy for cancer-prone chronic inflammation in the skin. Likewise, statins may present a breakthrough for pancreatic cancer prevention. Pancreatic cancer is an insidious cancer type known for its unresponsiveness to current treatments, including immunotherapies, due to its highly immunosuppressive tumor microenvironment with dense desmoplastic stroma71,72. Although statins’ impact on an established tumor microenvironment is context-dependent73–77, our findings strongly indicate that statin use can block the development of cancer-prone chronic inflammation and the formation of an immunosuppressive tumor microenvironment in high-risk patients. Importantly, statin use is associated with an increased survival rate in pancreatic cancer patients78,79. Moreover, we demonstrate that pitavastatin markedly reduces the risk of pancreatic cancer development compared with ezetimibe in a large population study. Finally, the beneficial effect of statins in blocking the TBK1-IRF3-IL-33 axis may also extend to other IL-33-dependent chronic inflammatory conditions, including chronic obstructive pulmonary disease (COPD), atopic dermatitis, and asthma80–82.
Materials And Methods
Human samples
De-identified formalin-fixed paraffin-embedded human pancreas tissue sections were obtained from the Department of Pathology at Massachusetts General Hospital.
Animal studies
All mice were housed under pathogen-free conditions in an animal facility at Massachusetts General Hospital in accordance with animal care regulations. Irf3KO mice were purchased from the Riken BioResource Research Center (Ibaraki, Japan). Il33KO mice were a gift from Dr. Marco Colonna, and p53f/f (Trp53tm1Brnn/J), P48-Cre (Ptf11atm1(cre)Hnak/RschJ), LSL-KrasG12D (Krastm4Tyj/J), Myd88KO (Myd88tm1.1Defr/J), TrifKO (Ticam1Lps2/J) and C57BL/6 WT mice were purchased from the Jackson Laboratory (Bar Harbor, ME).
Skin chronic inflammation
Four- to six-week-old male and female mice were shaved on their abdomen and sensitized to 50 μL 0.5% 1-Fluoro-2,4-dinitrobenzene (DNFB, Millipore Sigma, St. Louis, MO, catalog no. 42085) dissolved in acetone with olive oil at 3:1 ratio (refer to as acetone). Two days after the first sensitization, mice were sensitized to 50 μL 0.25% DNFB on their abdomen again. After five days, mice were challenged with 100 μL 0.25% DNFB on their back skin, which was repeated three times per week for 22 days. Skin rash was monitored over the duration of the study.
Chronic pancreatitis
Mice were weighed and injected with 50 μg/kg caerulein (BACHEM, Torrance, CA, catalog no. 4030451) in 100 μL of PBS intraperitoneally every hour for 6 hours, three days per week for three weeks. Mice were harvested for analysis at the completion of the three-week treatment protocol.
Caerulein-mediated pancreatic cancer
Mice were weighed and injected with 50 μg/kg caerulein in 100 μL of PBS intraperitoneally every hour for 7 hours, for two consecutive days. Mice were harvested 30 days after the last injection.
Pitavastatin treatment
For chronic inflammation in the skin, mice were treated topically with 0.25 mM pitavastatin (Selleck Chemicals LLC, Houston, TX, catalog no. S1759) in 200 μL acetone or 200 μL acetone alone on their back skin twice a week. Pitavastatin treatments were given at the time of DNFB applications. For chronic pancreatitis and caerulein-mediated pancreatic cancer, mice were treated intraperitoneally with 2 mg/kg pitavastatin in 100 uL PBS or PBS alone. Pitavastatin was given once every three days until harvest.
Cell lines and transfection
Pam212 cells were grown at 37°C in DMEM (Thermo Fisher Scientific, Waltham, MA, catalog no.11995065) supplemented with 10% fetal bovine serum (Thermo Fisher Scientific, catalog no. 26140079), 1X penicillin-streptomycin-glutamine (Thermo Fisher Scientific, catalog no. 10378016), 1X MEM non-essential amino acids solution (Thermo Fisher Scientific, catalog no. 11140050), 1X HEPES (Thermo Fisher Scientific, catalog no. 15630080) and 0.1% 2-Mercaptoethanol (Thermo Fisher Scientific, catalog no. 21985023). PyMttg cell line (derived from a primary breast tumor of MMTV-PyMTtg mouse on a C57BL/6 background) was grown at 37 °C in RPMI Medium 1640 (Thermo Fisher Scientific, catalog no. 11875093) supplemented with 10% fetal bovine serum, 1X penicillin-streptomycin-glutamine and 0.1% 2-Mercaptoethanol. Transfections of LPS (Millipore Sigma, catalog no. L6529) or poly(I:C) (Invivogen, San Diego, CA, catalog no. tlrl-pic) in Pam212 cells were performed using Lipofectamine 2000 (Thermo Fisher Scientific, catalog no. 11668019) according to the manufacturer’s instructions. For gene knock down, Pam212 cells were transfected with siRNA construct using Lipofectamine RNAiMax Transfection reagent (Thermo Fisher Scientific, catalog no. 13778075) according to the manufacturer’s instructions. Specific siRNA constructs are listed in Supplementary Table S2.
Stable cell lines
After transfection of mouse Il33 promoter clone (GeneCopoeia, Rockville, MD, catalog no. MPRM34949-PG02) or mouse control promoter clone (deleted promoter region from mouse Il33 promoter clone) with Lipofectamine 2000 in Pam212 cells, cells were incubated with 3 μg/ml of puromycin (Invivogen, catalog no. ant-pr-1) for positive transfected cell selection. After two days, the medium was changed with a new puromycin-containing medium for further selection. After the second two days, cells were transferred to a new plate with a puromycin-containing medium. Puromycin selection was repeated five more times. Then, cells were passaged in regular media to ensure optimal growth.
Small compound screening with Luciferase assay
One thousand stable cells were seeded in 384 wells (Corning, Glendale, AZ, catalog no. 3570), and each plate was incubated for 24 hours. The following day, 10 mM of FDA-approved Drug Library compounds (Selleckchem, catalog no. L1300, 2019 version) were added to each well. After 24 hours, 5 mL of luciferase buffer from Pierce Gaussia luciferase glow assay kit (Thermo Fisher Scientific, catalog no.16160) was added to each well and incubated for 5 min. Each plate was read and measured with luminescence by EnVision 2014 plate reader (Perkin Elmer, Waltham, MA, catalog no. 2014 EnVision).
Western blot
Cell lysates were prepared in LIPA buffer (Thermo Fisher Scientific, catalog no. 89900) consisting of 1X protease inhibitor cocktail, EDTA-free (Thermo Fisher Scientific, catalog no. A32955). Mice tissues were meshed and lysed by 0.1% TWEEN-20 (Millipore Sigma, catalog no. P1379) in PBS. Mice tissues were frozen in liquid nitrogen and thawed by incubation at 37 °C for further lysis. Tissue lysates were sonicated for 10–20 sec and centrifugated at 13,200 rpm. After checking the protein concentration in each sample, the identical amounts of total proteins were loaded onto Mini-PROTEIN TGX™ Gels (BIO-RAD, Hercules, CA, catalog no.456–1083 or 456–1086) with 1X Tris/Glycine/SDS buffer (BIO-RAD, catalog no.1610732). A few minutes later (according to protein size, ~30 min with 200 Voltage), they were transferred to Immobilon–P membrane (Millipore Sigma, catalog no. IPVH00010) with Transfer buffer (Boston Bioproducts, Ashland, MA, catalog no. BP-190). Then, the samples were incubated with 3% bovine serum albumin (Thermo Fisher Scientific, catalog no. BP1600) or 5% Skim milk (BD biosciences, San Jose, CA, catalog no. 232100) in 1X Tris-Buffered Saline (Boston Bioproducts, catalog no. BM301X) containing 0.1% TWEEN, called TBS-T for 30 min. After washing with TBS-T three times, the membranes were subjected to immunoblot with proper antibodies overnight at 4 °C. The following day, the membranes were incubated with appropriate secondary antibodies after washing. Membranes were developed with Pierce ECL Western blotting substrate kit (Thermo Fisher Scientific, catalog no. 32106). First and secondary antibodies are listed in Supplementary Table S2.
Histology, immunohistochemistry, and immunofluorescence
Tissue samples were collected and fixed in 4% paraformaldehyde (Millipore Sigma, catalog no. P6148) overnight at 4°C. Next, tissues were dehydrated in PBS and ethanol, processed, and embedded in paraffin. Five to seven μm sections of paraffin-embedded tissues were placed on slides, deparaffinized, and stained with H&E, toluidine blue (for mast cell) (Millipore Sigma, catalog no. T3260), or Alcian blue (for mucin) (VECTOR Laboratories, Burlingame, CA, catalog no. H3501). For immunohistochemistry, antigen retrieval was performed in 500 μL of antigen unmasking solution (VECTOR Laboratories, catalog no. H3300) diluted in 50 mL distilled water using a Cuisinart pressure cooker for 20 min at high pressure. Slides were washed three times for three minutes each in 1X TBS with 0.025% Triton X-100. Sections were blocked with 3% bovine serum albumin (Thermo Fisher Scientific, catalog no. BP1600) and 5% goat serum (Millipore Sigma, catalog no. G9023) for 1 hour. Slides were incubated overnight at 4°C with a primary antibody diluted in TBS containing 3% BSA (Supplementary Table 2). The following day, slides were washed as above and incubated in 100 μL biotinylated secondary antibody (VECTOR Laboratories, catalog no. PK-6200) for 30 min. After washing, slides were stained with a 100 μL mixture of reagents A and B from VECTASTAIN Elite ABC universal kit Peroxidase (VECTOR Laboratories, catalog no. PK-6200) for 30 min. After washing again, slides were incubated with 100 μL ImmPACT DAB chromogen staining (VECTOR Laboratories, catalog no. SK-4105) for a few minutes (depending on the signal). Finally, slides were dehydrated in ethanol and xylene and mounted with a coverslip using three drops of mounting media. For immunofluorescence staining, rehydrated tissue sections were permeated with 1X PBS supplemented with 0.2% Triton X-100 for 5 min. Antigen retrieval was performed similarly to immunohistochemistry. Slides were washed three times for 3 min each in 1X PBS with 0.1% Tween-20. Sections were blocked with 3% bovine serum albumin and 5% goat serum for 1 hour. The slides were incubated overnight at 4°C with primary antibodies. The following day, slides were washed as above and stained for 2 hours at room temperature with secondary antibodies conjugated to fluorochromes. Next, slides were incubated with 1:2,000 DAPI (Thermo Fisher Scientific, catalog no. D3571) for 3 min at room temperature, then washed as above. Slides were mounted with Prolong Gold Antifade Reagent (Thermo Fisher Scientific, catalog no. P36930). The number of positive cells was counted in randomly selected high-power field (HPF, 200x magnification) images in a blinded manner by a trained investigator. A pathologist reviewed clinical samples.
Quantitative PCR
Mouse dorsal skin and pancreas samples were homogenized and lysed in RLT lysis solution (QIAGEN, Hilden, Germany, catalog no. 79216)/0.1% MeOH using Mini-BeadBeater-8 (BioSpec Products, Inc., Bartlesville, OK). Trizol reagent was added to tissue samples and cell pellets to extract RNA (Thermo Fisher Scientific, catalog no. 15-596-018). Total RNA was extracted using an RNeasy micro kit and quantified using NanoDrop ND-1100 (NanoDrop Technologies, Wilmington, DE). cDNA was synthesized from 1 mg of total RNA using Invitrogen SuperScripts III Reverse Transcriptase (Thermo Fisher Scientific, catalog no. 18080085). The gene expression levels from cDNA samples were determined using QuantStudio 3 system (Thermo Fisher Scientific) using SYBR Select Master Mix (Thermo Fisher Scientific, catalog no. 4472908) or TaqMan Universal Master Mix II (Thermo Fisher Scientific, catalog no. 44-400-40). Primer sequences for SYBR green and Taqman assays are listed in Supplementary Table S2. Quantitative real-time PCR for SYBR green analyses was performed in a final reaction volume of 20 mL consisting of 5 mL of cDNA of the respective sample and 10 mL of SYBR green master mix mixed with the corresponding primers (2 mM) for each gene. TaqMan analysis was performed in a 10 mL final reaction, including 4.5 mL cDNA and 5.5 mL TaqMan master mix and corresponding primers (20 mM). All assays were conducted in triplicate and normalized to Gapdh expression.
RNA-Seq
WT mice underwent DNFB-induced skin inflammation and caerulein-induced pancreatitis protocols. For skin RNA-seq, the epidermis was isolated from the mice’s back skin. Epidermis and pancreas tissues were lysed in RLT buffer (Qiagen, catalog no. 79216) supplemented with 1% β-mercaptoethanol (Thermo Fisher Scientific, 21-985-023). Libraries were generated and sequenced using the Smart-Seq2 protocol as previously described using Novaseq 6000 (Illumina) on the Broad Genomics Platform83. The raw files were mapped to the mouse genome/mm10 by STAR-2.5.384. Aligned transcripts were quantified using RSEM-1.3.185. Differentially expressed genes (DEG) were analyzed by DESEq286. Original data are available in the NCBI Gene Expression Omnibus (GEO) with accession number GSE207956 (RNA-Seq).
ChIP-qPCR assay
Pam212 cells that had been transfected with poly(I:C) were fixed in 1% formaldehyde (Millipore Sigma, catalog no. F8775) for 10 min and were washed with cold PBS. Cells were lysed with buffer contained with 2.5% of glycerol, 50 mM HEPES (pH 7.5), 150 mM NaCl, 0.5 mM EDTA, 0.5% NP-40, and 0.25% Triton X-100. After centrifugation at 2,000 rpm, lysates were resuspended in buffer A contained with 1 mM Tris/HCl (pH 7.9), 20 mM NaCl and 0.5 mM EDTA and incubated at room temperature for 10 min. Following the second centrifugation at 2,000 rpm, cells were sonicated in a sonication buffer containing 10 mM HEPES, 1 mM EDTA, and 0.5% SDS for 30 minutes to achieve chromatin fragmentation. Following centrifugation at 13,200 rpm, proteins were immunoprecipitated with the p-IRF3 antibody (Cell Signaling Technology, Danvers, MA, catalog no. 29047) at 4 °C overnight. The following day, the samples were incubated with a 30 μL 50% slurry of ChIP-Grade Protein-G agarose beads (Cell Signaling Technology, catalog no. 9007S) for 3 hours. Samples were precipitated and washed three times with wash buffer containing 20 mM Tris/HCl (pH 7.9), 10 mM NaCl, 2 mM EDTA, 0.1% SDS, and Triton X-100. After the last wash step, samples were eluted with 100 μL of TE buffer contained with 100 mM Tris/HCl (pH 8), 1 mM EDTA, and 1% SDS three times. De-crosslinking was conducted by overnight incubation with 15 μL of 3 M NaCl at 65°C. Precipitated DNA was eluted by ChIP DNA Clean & Concentrator (ZYMO research, Irvine, CA, catalog no. D5205) and subjected to quantitative PCR.
PCR
DNA was extracted from mouse tissue using KAPA Express Extract buffer, and KAPA Express Extract enzyme from Kapa Genotyping kit (Kapa Biosystems Inc, Wilmington, MA, catalog no. KK7302). After tissue lysis, PCR was performed using 2X KAPA2G Fast genotyping mix from the Genotyping kit and the primers that are listed in Supplementary Table S2.
Membrane extraction
Pam212 cells were transfected with poly(I:C) using Lipofectamine 2000 according to the manufacturer’s instructions, and cells were treated with pitavastatin (10 mM) and GGPP (3 mM) (Echelon Biosciences, Salt Lake City, UT, catalog no. I-0200). After 6 hours of incubation, cells were washed with Cell Wash Solution (Thermo Fisher Scientific, catalog no. 89842) and collected with a cell scraper. After centrifugation at 2,000 rpm, only pellets were resuspended in 100 mL of Permeabilized Buffer (Thermo Fisher Scientific, catalog no. 89842) consisting of 1X EDTA-free protease inhibitor cocktail. Samples were incubated for 10 min at 4°C with constant mixing. After incubation, permeabilized cells were centrifugated for 15 min at 13,200 rpm, and only the supernatant was carefully transferred to a new tube (Cytosol fraction). Next, the pellets were resuspended in Solubilization Buffer (Thermo Fisher Scientific, catalog no. 89842) and incubated for 30 min at 4°C with constant mixing. After incubation, samples were centrifugated for 15 min at 13,200 rpm, and only the supernatant was transferred to a new tube (membrane fraction).
Epidemiological analysis
A retrospective cohort analysis was performed using de-identified data from the TriNetX Diamond Network. A search query was used to identify the cohort of patients within the network who had received pitavastatin. Eligible patients were identified based on the presence of corresponding RxNorm concept unique identifiers (RXCUI) in the patients’ electronic medical records. Using International Classification of Diseases Tenth Revision (ICD-10) codes, all patients with a history of chronic pancreatitis and pancreatic cancer prior to statin initiation were excluded from the cohorts to reduce confounding. The control cohort for each analysis included all patients within the network who had received ezetimibe but had no recorded statin use and patients with a history of any of the diagnoses mentioned above before ezetimibe initiation were also excluded.
The index event for all analyses was the initiation of pitavastatin for study cohorts and the initiation of ezetimibe for the control cohort. Cases and controls were matched using 1:1 propensity score-matching for age at index event, sex, race, and ethnicity using “greedy nearest neighbor matching” and a caliper of 0.1 pooled standard deviations. Baseline characteristics were reported by count and percentage of the total for categorical variables and means and standard deviations (SD) for continuous variables. Relative risks are presented with 95% confidence intervals. P-values are uncorrected and based on Z-tests or Fisher’s exact tests. Statistical analyses were performed in real-time using the TriNetX platform.
Statistical analysis
A paired t-test was used for comparing IL-33+ and IRF3+ cell counts, and a paired t-test for the Pearson correlation coefficient was used for correlation between IL-33+ and IRF3+ counts across matched human pancreatic samples. Statistical differences between the three groups were analyzed using one-way ANOVA. Tukey multiple comparison tests were used to examine the differences in the mean ranks among all three possible pairwise comparisons. Unpaired t-test was used as the test of significance for tumor per body weight ratio, epidermal thickness, mast cell and leukocyte counts, RNA and protein expression levels, and other quantitative measurements. P-value < 0.05 is considered significant. Bar graphs show mean + SD.
Study approval
Massachusetts General Hospital IRB approved the analysis of de-identified clinical tissue samples. Massachusetts General Hospital IACUC approved the animal studies.
ACKNOWLEDGMENTS
We thank Dr. Marco Colonna for Il33KO mice. We thank Minyoung Kim for assistance with clinical tissue collection. Il33KO mice were generated with support from Mucosal Immunology Studies Team (MIST) (U01; RFA-AI-15-023). SD holds a Career Award for Medical Scientists from the Burroughs Wellcome Fund. JHP, MM, HGS, MA, and SD were supported by grants from the Burroughs Wellcome Fund, Sidney Kimmel Foundation, and NIH (K08AR068619 and R01AR076013).
Footnotes
COMPETING OF INTEREST
JHP and SD are coinventors on a filed patent for the use of IL-33 inhibition in the treatment of cancer, fibrosis, and inflammation (PCT/US21/40725), which is licensed to DermBiont Inc. AM is an equity holder of DermBiont Inc. The remaining authors state no conflict of interest.
Contributor Information
Jong Ho Park, Massachusetts General Hospital.
Mahsa Mortaja, Massachusetts General Hospital.
Heehwa Son, Massachusetts General Hospital.
Marjan Azin, Massachusetts General Hospital.
Jun Wang, Massachusetts General Hospital.
Michael Collier, Massachusetts General Hospital.
Anna Mandinova, Massachusetts General Hospital.
Yevgeniy Semenov, Massachusetts General Hospital.
Mari Mino-Kenudson, Massachusetts General Hospital.
Shadmehr Demehri, Massachusetts General Hospital.
References
- 1.Chen L. et al. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 9, 7204–7218, doi: 10.18632/oncotarget.23208 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grivennikov S. I., Greten F. R. & Karin M. Immunity, inflammation, and cancer. Cell 140, 883–899, doi: 10.1016/j.cell.2010.01.025 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greten F. R. & Grivennikov S. I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 51, 27–41, doi: 10.1016/j.immuni.2019.06.025 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGuigan A. et al. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol 24, 4846–4861, doi: 10.3748/wjg.v24.i43.4846 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Axelrad J. E., Lichtiger S. & Yajnik V. Inflammatory bowel disease and cancer: The role of inflammation, immunosuppression, and cancer treatment. World J Gastroenterol 22, 4794–4801, doi: 10.3748/wjg.v22.i20.4794 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang X. et al. Risk Factors and Prevention of Viral Hepatitis-Related Hepatocellular Carcinoma. Front Oncol 11, 686962, doi: 10.3389/fonc.2021.686962 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Little A. C. et al. IL-4/IL-13 Stimulated Macrophages Enhance Breast Cancer Invasion Via Rho-GTPase Regulation of Synergistic VEGF/CCL-18 Signaling. Front Oncol 9, 456, doi: 10.3389/fonc.2019.00456 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tian Z., Hou X., Liu W., Han Z. & Wei L. Macrophages and hepatocellular carcinoma. Cell Biosci 9, 79, doi: 10.1186/s13578-019-0342-7 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maciel T. T., Moura I. C. & Hermine O. The role of mast cells in cancers. F1000Prime Rep 7, 09, doi: 10.12703/P7-09 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Padua D. & Massague J. Roles of TGFbeta in metastasis. Cell Res 19, 89–102, doi: 10.1038/cr.2008.316 (2009). [DOI] [PubMed] [Google Scholar]
- 11.Mirlekar B. Tumor promoting roles of IL-10, TGF-beta, IL-4, and IL-35: Its implications in cancer immunotherapy. SAGE Open Med 10, 20503121211069012, doi: 10.1177/20503121211069012 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duan Z. & Luo Y. Targeting macrophages in cancer immunotherapy. Signal Transduct Target Ther 6, 127, doi: 10.1038/s41392-021-00506-6 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Teixeira A. F., Ten Dijke P. & Zhu H. J. On-Target Anti-TGF-beta Therapies Are Not Succeeding in Clinical Cancer Treatments: What Are Remaining Challenges? Front Cell Dev Biol 8, 605, doi: 10.3389/fcell.2020.00605 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weiss S. A., Wolchok J. D. & Sznol M. Immunotherapy of Melanoma: Facts and Hopes. Clin Cancer Res 25, 5191–5201, doi: 10.1158/1078-0432.CCR-18-1550 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zappavigna S. et al. Anti-Inflammatory Drugs as Anticancer Agents. Int J Mol Sci 21, doi: 10.3390/ijms21072605 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dinarello C. A. Anti-inflammatory Agents: Present and Future. Cell 140, 935–950, doi: 10.1016/j.cell.2010.02.043 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bonifant C. L., Jackson H. J., Brentjens R. J. & Curran K. J. Toxicity and management in CAR T-cell therapy. Mol Ther Oncolytics 3, 16011, doi: 10.1038/mto.2016.11 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cayrol C. & Girard J. P. IL-33: an alarmin cytokine with crucial roles in innate immunity, inflammation and allergy. Current opinion in immunology 31, 31–37, doi: 10.1016/j.coi.2014.09.004 (2014). [DOI] [PubMed] [Google Scholar]
- 19.Griesenauer B. & Paczesny S. The ST2/IL-33 Axis in Immune Cells during Inflammatory Diseases. Frontiers in immunology 8, 475, doi: 10.3389/fimmu.2017.00475 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ameri A. H. et al. IL-33/regulatory T cell axis triggers the development of a tumor-promoting immune environment in chronic inflammation. Proceedings of the National Academy of Sciences of the United States of America 116, 2646–2651, doi: 10.1073/pnas.1815016116 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park J. H. et al. Nuclear IL-33/SMAD signaling axis promotes cancer development in chronic inflammation. EMBO J 40, e106151, doi: 10.15252/embj.2020106151 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gabryelska A., Kuna P., Antczak A., Bialasiewicz P. & Panek M. IL-33 Mediated Inflammation in Chronic Respiratory Diseases-Understanding the Role of the Member of IL-1 Superfamily. Front Immunol 10, 692, doi: 10.3389/mmu.2019.00692 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kelsen S. G. et al. Astegolimab (anti-ST2) efficacy and safety in adults with severe asthma: A randomized clinical trial. J Allergy Clin Immunol 148, 790–798, doi: 10.1016/j.jaci.2021.03.044 (2021). [DOI] [PubMed] [Google Scholar]
- 24.Pushparaj P. N. et al. Interleukin-33 exacerbates acute colitis via interleukin-4 in mice. Immunology 140, 70–77, doi: 10.1111/imm.12111 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luo P. et al. The IL-33/ST2 pathway suppresses murine colon cancer growth and metastasis by upregulating CD40 L signaling. Biomed Pharmacother 127, 110232, doi: 10.1016/j.biopha.2020.110232 (2020). [DOI] [PubMed] [Google Scholar]
- 26.Gao K. et al. Transgenic expression of IL-33 activates CD8(+) T cells and NK cells and inhibits tumor growth and metastasis in mice. Cancer Lett 335, 463–471, doi: 10.1016/j.canlet.2013.03.002 (2013). [DOI] [PubMed] [Google Scholar]
- 27.Pastille E. et al. The IL-33/ST2 pathway shapes the regulatory T cell phenotype to promote intestinal cancer. Mucosal Immunol 12, 990–1003, doi: 10.1038/s41385-019-0176-y (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang W., Wu J., Ji M. & Wu C. Exogenous interleukin-33 promotes hepatocellular carcinoma growth by remodelling the tumour microenvironment. J Transl Med 18, 477, doi: 10.1186/s12967-020-02661-w (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alonso-Curbelo D. et al. A gene-environment-induced epigenetic program initiates tumorigenesis. Nature 590, 642–648, doi: 10.1038/s41586-020-03147-x (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kawasaki T. & Kawai T. Toll-like receptor signaling pathways. Front Immunol 5, 461, doi: 10.3389/fimmu.2014.00461 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nie L., Cai S. Y., Shao J. Z. & Chen J. Toll-Like Receptors, Associated Biological Roles, and Signaling Networks in Non-Mammals. Front Immunol 9, 1523, doi: 10.3389/fimmu.2018.01523 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang G. & Ghosh S. Toll-like receptor-mediated NF-kappaB activation: a phylogenetically conserved paradigm in innate immunity. J Clin Invest 107, 13–19, doi: 10.1172/JCI11837 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wicherska-Pawlowska K., Wrobel T. & Rybka J. Toll-Like Receptors (TLRs), NOD-Like Receptors (NLRs), and RIG-I-Like Receptors (RLRs) in Innate Immunity. TLRs, NLRs, and RLRs Ligands as Immunotherapeutic Agents for Hematopoietic Diseases. Int J Mol Sci 22, doi: 10.3390/ijms222413397 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fitzgerald K. A. & Kagan J. C. Toll-like Receptors and the Control of Immunity. Cell 180, 1044–1066, doi: 10.1016/j.cell.2020.02.041 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park J. M. et al. Signaling pathways and genes that inhibit pathogen-induced macrophage apoptosis--CREB and NF-kappaB as key regulators. Immunity 23, 319–329, doi: 10.1016/j.immuni.2005.08.010 (2005). [DOI] [PubMed] [Google Scholar]
- 36.Kempe S., Kestler H., Lasar A. & Wirth T. NF-kappaB controls the global pro-inflammatory response in endothelial cells: evidence for the regulation of a pro-atherogenic program. Nucleic Acids Res 33, 5308–5319, doi: 10.1093/nar/gki836 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang A. et al. Transcription factor complex AP-1 mediates inflammation initiated by Chlamydia pneumoniae infection. Cell Microbiol 15, 779–794, doi: 10.1111/cmi.12071 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wen A. Y., Sakamoto K. M. & Miller L. S. The role of the transcription factor CREB in immune function. J Immunol 185, 6413–6419, doi: 10.4049/jimmunol.1001829 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jefferies C. A. Regulating IRFs in IFN Driven Disease. Front Immunol 10, 325, doi: 10.3389/fimmu.2019.00325 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu X., Li J., Fu M., Zhao X. & Wang W. The JAK/STAT signaling pathway: from bench to clinic. Signal Transduct Target Ther 6, 402, doi: 10.1038/s41392-021-00791-1 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Polumuri S. K. et al. Transcriptional regulation of murine IL-33 by TLR and non-TLR agonists. J Immunol 189, 50–60, doi: 10.4049/jimmunol.1003554 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weinberg E. O. et al. IL-33 induction and signaling are controlled by glutaredoxin-1 in mouse macrophages. PLoS One 14, e0210827, doi: 10.1371/journal.pone.0210827 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin W. R. et al. Granulocyte colony-stimulating factor reduces fibrosis in a mouse model of chronic pancreatitis. PLoS One 9, e116229, doi: 10.1371/journal.pone.0116229 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Komar H. M. et al. Inhibition of Jak/STAT signaling reduces the activation of pancreatic stellate cells in vitro and limits caerulein-induced chronic pancreatitis in vivo. Sci Rep 7, 1787, doi: 10.1038/s41598-017-01973-0 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsai S. Y. et al. Regulation of TLR3 Activation by S100A9. J Immunol 195, 4426–4437, doi: 10.4049/jimmunol.1500378 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pruenster M., Vogl T., Roth J. & Sperandio M. S100A8/A9: From basic science to clinical application. Pharmacol Ther 167, 120–131, doi: 10.1016/j.pharmthera.2016.07.015 (2016). [DOI] [PubMed] [Google Scholar]
- 47.Natarajan C., Yao S. Y. & Sriram S. TLR3 Agonist Poly-IC Induces IL-33 and Promotes Myelin Repair. PLoS One 11, e0152163, doi: 10.1371/journal.pone.0152163 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mullen P. J., Yu R., Longo J., Archer M. C. & Penn L. Z. The interplay between cell signalling and the mevalonate pathway in cancer. Nat Rev Cancer 16, 718–731, doi: 10.1038/nrc.2016.76 (2016). [DOI] [PubMed] [Google Scholar]
- 49.Goldstein J. L. & Brown M. S. Regulation of the mevalonate pathway. Nature 343, 425–430, doi: 10.1038/343425a0 (1990). [DOI] [PubMed] [Google Scholar]
- 50.Xia Y. et al. The Mevalonate Pathway Is a Druggable Target for Vaccine Adjuvant Discovery. Cell 175, 1059–1073 e1021, doi: 10.1016/j.cell.2018.08.070 (2018). [DOI] [PubMed] [Google Scholar]
- 51.Guerra C. et al. Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer cell 11, 291–302, doi: 10.1016/j.ccr.2007.01.012 (2007). [DOI] [PubMed] [Google Scholar]
- 52.Wang S. et al. The molecular biology of pancreatic adenocarcinoma: translational challenges and clinical perspectives. Signal Transduct Target Ther 6, 249, doi: 10.1038/s41392-021-00659-4 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kong B. et al. Dynamic landscape of pancreatic carcinogenesis reveals early molecular networks of malignancy. Gut 67, 146–156, doi: 10.1136/gutjnl-2015-310913 (2018). [DOI] [PubMed] [Google Scholar]
- 54.Chen S. M., Chieng W. W., Huang S. W., Hsu L. J. & Jan M. S. The synergistic tumor growth-inhibitory effect of probiotic Lactobacillus on transgenic mouse model of pancreatic cancer treated with gemcitabine. Scientific reports 10, 20319, doi: 10.1038/s41598-020-77322-5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tang K. et al. Association of Cutaneous Immune-Related Adverse Events With Increased Survival in Patients Treated With Anti-Programmed Cell Death 1 and Anti-Programmed Cell Death Ligand 1 Therapy. JAMA Dermatol 158, 189–193, doi: 10.1001/jamadermatol.2021.5476 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shen R. R. & Hahn W. C. Emerging roles for the non-canonical IKKs in cancer. Oncogene 30, 631–641, doi: 10.1038/onc.2010.493 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Muvaffak A. et al. Evaluating TBK1 as a therapeutic target in cancers with activated IRF3. Mol Cancer Res 12, 1055–1066, doi: 10.1158/1541-7786.MCR-13-0642 (2014). [DOI] [PubMed] [Google Scholar]
- 58.Cruz V. H., Arner E. N., Du W., Bremauntz A. E. & Brekken R. A. Axl-mediated activation of TBK1 drives epithelial plasticity in pancreatic cancer. JCI Insight 5, doi: 10.1172/jci.insight.126117 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moller M. et al. The Specific IKKepsilon/TBK1 Inhibitor Amlexanox Suppresses Human Melanoma by the Inhibition of Autophagy, NF-kappaB and MAP Kinase Pathways. Int J Mol Sci 21, doi: 10.3390/ijms21134721 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jiang Y. et al. TANK-Binding Kinase 1 (TBK1) Serves as a Potential Target for Hepatocellular Carcinoma by Enhancing Tumor Immune Infiltration. Front Immunol 12, 612139, doi: 10.3389/fimmu.2021.612139 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhu L. et al. TBKBP1 and TBK1 form a growth factor signalling axis mediating immunosuppression and tumourigenesis. Nat Cell Biol 21, 1604–1614, doi: 10.1038/s41556-019-0429-8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bai L. Y. et al. BX795, a TBK1 inhibitor, exhibits antitumor activity in human oral squamous cell carcinoma through apoptosis induction and mitotic phase arrest. Eur J Pharmacol 769, 287–296, doi: 10.1016/j.ejphar.2015.11.032 (2015). [DOI] [PubMed] [Google Scholar]
- 63.Korherr C. et al. Identification of proangiogenic genes and pathways by high-throughput functional genomics: TBK1 and the IRF3 pathway. Proc Natl Acad Sci U S A 103, 4240–4245, doi: 10.1073/pnas.0511319103 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu J., Leng X., Pan Z., Xu L. & Zhang H. Overexpression of IRF3 Predicts Poor Prognosis in Clear Cell Renal Cell Carcinoma. Int J Gen Med 14, 5675–5692, doi: 10.2147/IJGM.S328225 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cruz V. H. & Brekken R. A. Assessment of TANK-binding kinase 1 as a therapeutic target in cancer. J Cell Commun Signal 12, 83–90, doi: 10.1007/s12079-017-0438-y (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jiao Z. et al. Statin-induced GGPP depletion blocks macropinocytosis and starves cells with oncogenic defects. Proc Natl Acad Sci U S A 117, 4158–4168, doi: 10.1073/pnas.1917938117 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang T. et al. Simvastatin-induced breast cancer cell death and deactivation of PI3K/Akt and MAPK/ERK signalling are reversed by metabolic products of the mevalonate pathway. Oncotarget 7, 2532–2544, doi: 10.18632/oncotarget.6304 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nan X. et al. Ras-GTP dimers activate the Mitogen-Activated Protein Kinase (MAPK) pathway. Proc Natl Acad Sci U S A 112, 7996–8001, doi: 10.1073/pnas.1509123112 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Salami J. A. et al. National Trends in Statin Use and Expenditures in the US Adult Population From 2002 to 2013: Insights From the Medical Expenditure Panel Survey. JAMA Cardiol 2, 56–65, doi: 10.1001/jamacardio.2016.4700 (2017). [DOI] [PubMed] [Google Scholar]
- 70.Collins R. et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet 388, 2532–2561, doi: 10.1016/S0140-6736(16)31357-5 (2016). [DOI] [PubMed] [Google Scholar]
- 71.Bear A. S., Vonderheide R. H. & O’Hara M. H. Challenges and Opportunities for Pancreatic Cancer Immunotherapy. Cancer Cell 38, 788–802, doi: 10.1016/j.ccell.2020.08.004 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Looi C. K. et al. Therapeutic challenges and current immunomodulatory strategies in targeting the immunosuppressive pancreatic tumor microenvironment. J Exp Clin Cancer Res 38, 162, doi: 10.1186/s13046-019-1153-8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lei A. et al. Atorvastatin promotes the expansion of myeloid-derived suppressor cells and attenuates murine colitis. Immunology 149, 432–446, doi: 10.1111/imm.12662 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tang T. T. et al. Atorvastatin upregulates regulatory T cells and reduces clinical disease activity in patients with rheumatoid arthritis. J Lipid Res 52, 1023–1032, doi: 10.1194/jlr.M010876 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Meng X. et al. Statins induce the accumulation of regulatory T cells in atherosclerotic plaque. Mol Med 18, 598–605, doi: 10.2119/molmed.2011.00471 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sokalska A., Hawkins A. B., Yamaguchi T. & Duleba A. J. Lipophilic statins inhibit growth and reduce invasiveness of human endometrial stromal cells. J Assist Reprod Genet 36, 535–541, doi: 10.1007/s10815-018-1352-9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mira E. et al. A lovastatin-elicited genetic program inhibits M2 macrophage polarization and enhances T cell infiltration into spontaneous mouse mammary tumors. Oncotarget 4, 2288–2301, doi: 10.18632/oncotarget.1376 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tamburrino D. et al. Statin use improves survival in patients with pancreatic ductal adenocarcinoma: A meta-analysis. Dig Liver Dis 52, 392–399, doi: 10.1016/j.dld.2020.01.008 (2020). [DOI] [PubMed] [Google Scholar]
- 79 <j/>79.Lee H. S. et al. Statin Use and Its Impact on Survival in Pancreatic Cancer Patients. Medicine (Baltimore) 95, e3607, doi: 10.1097/MD.0000000000003607 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sjoberg L. C. et al. Interleukin 33 exacerbates antigen driven airway hyperresponsiveness, inflammation and remodeling in a mouse model of asthma. Sci Rep 7, 4219, doi: 10.1038/s41598-017-03674-0 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Prefontaine D. et al. Increased expression of IL-33 in severe asthma: evidence of expression by airway smooth muscle cells. J Immunol 183, 5094–5103, doi: 10.4049/jimmunol.0802387 (2009). [DOI] [PubMed] [Google Scholar]
- 82.Schmitz J. et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 23, 479–490, doi: 10.1016/j.immuni.2005.09.015 (2005). [DOI] [PubMed] [Google Scholar]
- 83.Picelli S. et al. Full-length RNA-seq from single cells using Smart-seq2. Nat Protoc 9, 171–181, doi: 10.1038/nprot.2014.006 (2014). [DOI] [PubMed] [Google Scholar]
- 84.Dobin A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21, doi: 10.1093/bioinformatics/bts635 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li B. & Dewey C. N. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics 12, 323, doi: 10.1186/1471-2105-12-323 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Love M. I., Huber W. & Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15, 550, doi: 10.1186/s13059-014-0550-8 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]