Abstract
Objectives
Hospital-acquired infections (HAIs) are significant problems as public health issues which need attention. Such infections are significant problems for society and healthcare organizations. This study aimed to carry out a systematic review and a meta-analysis to analyze the prevalence of HAIs globally.
Methods
We conducted a comprehensive search of electronic databases including EMBASE, Scopus, PubMed and Web of Science between 2000 and June 2021. We found 7031 articles. After removing the duplicates, 5430 studies were screened based on the titles/ abstracts. Then, we systematically evaluated the full texts of the 1909 remaining studies and selected 400 records with 29,159,630 participants for meta-analysis. Random-effects model was used for the analysis, and heterogeneity analysis and publication bias test were conducted.
Results
The rate of universal HAIs was 0.14 percent. The rate of HAIs is increasing by 0.06 percent annually. The highest rate of HAIs was in the AFR, while the lowest prevalence were in AMR and WPR. Besides, AFR prevalence in central Africa is higher than in other parts of the world by 0.27 (95% CI, 0.22–0.34). Besides, E. coli infected patients more than other micro-organisms such as Coagulase-negative staphylococci, Staphylococcus spp. and Pseudomonas aeruginosa. In hospital wards, Transplant, and Neonatal wards and ICU had the highest rates. The prevalence of HAIs was higher in men than in women.
Conclusion
We identified several essential details about the rate of HAIs in various parts of the world. The HAIs rate and the most common micro-organism were different in various contexts. However, several essential gaps were also identified. The study findings can help hospital managers and health policy makers identify the reason for HAIs and apply effective control programs to implement different plans to reduce the HAIs rate and the financial costs of such infections and save resources.
Introduction
Hospital-acquired infections (HAIs) are significant problems which need serious attention worldwide. HAIs refer to a group of infections a patient does not have before admission to the hospital. HAIs do not even exist in the latency period; they occur upon arrival at the hospital or within 48–72 hours after admission to the hospital [1–4]. Nowadays, such infections are significant problems for societies and healthcare organizations. They prolong the treatment period and make both patients and health centers pay excessive costs, including increased drug intakes and tests [5]. Therefore, by preventing and reducing nosocomial infections, significant savings will be made in the costs imposed on health centers, the health system and society consequently [6].
Due to financial constraints, there are many problems in controlling HAIs in emerging countries. Besides the problems caused by the extension of hospital stay for the patient, HAIs can be transmitted to the patient’s relatives through casual contacts and jeopardize their physical conditions [6]. Such infections are not limited to specific patients. They may occur to every patient or hospital employee and increase the mortality rate of hospitals [7].
According to studies, the most prevalent causes of HAIs include urinary tract infections (UTIs), respiratory tract infections (RTIs), circulatory system infections, and surgical site infections [8–10]. According to a report of the World Health Organization (WHO) on 55 hospitals in 14 countries, 8.7% of the hospitalized patients had HAIs, which were more prevalent in the Eastern Mediterranean Region and less prevalent in the West of the Pacific [11–13]. The prevalence rate of these infections was reported to be 5% in the North of America and some parts of Europe, and was about 40% in some Asian, Latin American, and African countries [14, 15]. According to the findings of a study conducted in Europe, the prevalence of HAIs was nearly 2.9%. Medical interventions, poor health standards of the hospital environment, and poor personal hygiene of hospital staff and patients poor practice of personal hygiene among hospital staff and patients can cause HAIs [16]. However, the major/leading cause of HAIs is lack of compliance to health and safety guidelines of hospitals [17]. Although it is impossible to eliminate such infections even in the most advanced hospitals, standards and guidelines can be complied with the intention of reducing or managing them [18, 19]. Nowadays, with technological advances and high expectations of high quality care services, it is highly essential to analyze the frequency and causes of HAIs [20]. Therefore, it is necessary to know the prevalence rate of different HAIs to devise infection control programs in hospitals and help develop a reliable and effective plan. Lack of accurate data on the prevalence of HAIs makes the execution of control plans challenging and causes higher costs for health systems and patients [21, 22].
Due to the presence of developing and underdeveloped countries in the EMRO (the Eastern Mediterranean Regional Office of the World Health Organization), AFRO (African Regional Office of the World Health Organization) and other countries with high prevalence of HAIs, the issue of HAIs is a significant concern, thereby spending hefty sums for controlling and reducing such infections by governments [23].
Although a number of studies have been conducted on different parts of WHO regions to determine the prevalence rate of HAIs, no systematic review has been conducted globally. This study aimed to carry out a systematic review and a meta-analysis to analyze the prevalence of HAIs globally. The research findings will contribute to the development of effective control programs by managers and policymakers of the health sector to reduce the financial costs of HAIs and save financial resources.
Methods
Databases and search terms
We conducted a comprehensive search of electronic databases including EMBASE, Scopus, PubMed and Web of Science between 2000 and June 2021. Search terms included (“infection cross”[Title] OR “cross infections”[Title] OR “healthcare associated infections”[Title] OR “healthcare associated infection”[Title] OR “health care associated infection”[Title] OR “health care associated infections”[Title] OR “hospital infection”[Title] OR “infections hospital”[Title] OR “nosocomial infection”[Title] OR “nosocomial infections”[Title] OR “hospital infections”[Title]). We found 7031 articles through searching the databases. After entering the records into EndNote software and removing the duplicates, 5430 studies were screened on the basis of their titles/ abstracts. We reviewed the reference list of all included articles to ensure the comprehensiveness of the search.
Inclusion and exclusion criteria
On the basis of the research keywords, we included studies reporting quantitative data on HAIs prevalence and their determining factors among the general population. Different observational studies, including cross-sectional, prospective, case-study, and cohort, were included. We considered articles with available full texts published in English between 2000 and June 2021 for further consideration in this review. The reason we included articles from 2000 was to estimate the trend of the current century. We excluded interventional studies, reviews, reports, letters to the editor, books, case-control, and commentaries. We also excluded the review studies using invalid methods or containing insufficient data focused on diagnostic approaches, treatment methods, and medication.
Study selection
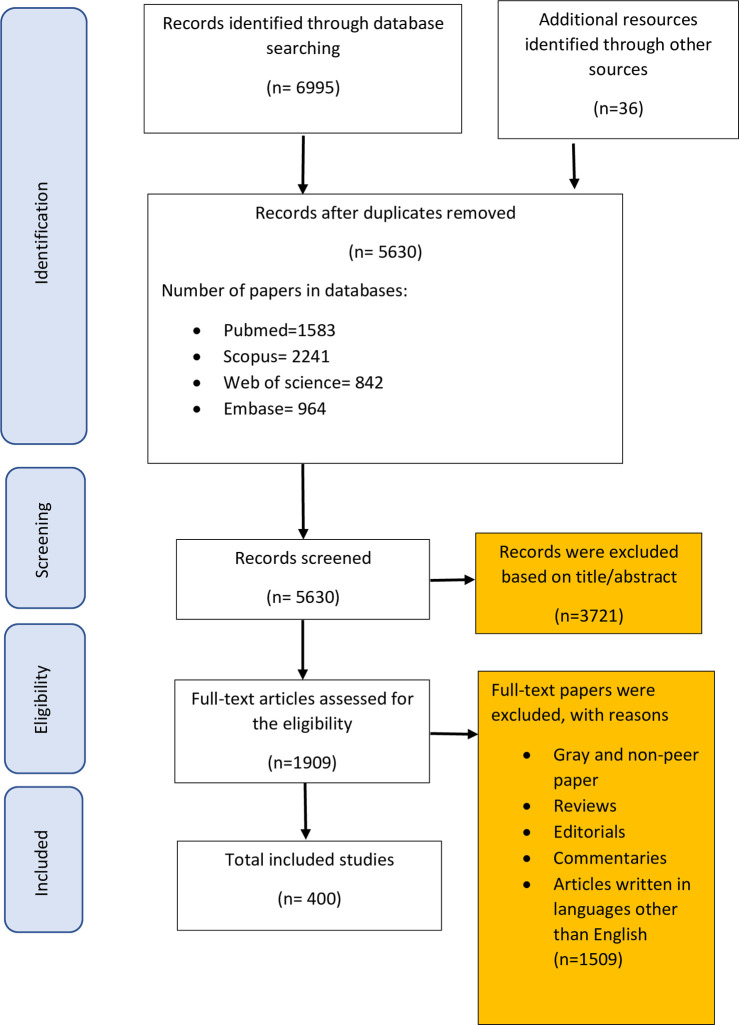
Searching electronic databases resulted in 7031 articles. After removing the duplicates, two researchers reviewed the remaining 5630 records independently, based on the titles and abstracts. In the next step, we systematically evaluated the full texts of the 1909 remaining studies to determine whether they met the eligibility criteria defined in the study. Finally, we selected 400 records with 29159630 participants to evaluate in this meta-analysis (Fig 1).
Fig 1. Flow diagram of our review process (PRISMA).
Quality assessment
We evaluated the methodological quality of the articles, using the Newcastle-Ottawa Scale (NOS) based on the procedures suggested in the Cochrane Handbook of Systematic Reviews. The NOS comprises a star system in which a study is evaluated in three areas, including four items regarding the selection of study groups, two items regarding the comparability of groups, and three items in terms of exposure or outcome ascertainment. If any of the items in the NOS were not reported in the article, a zero score was assigned; and for each of the areas addressed in the study, one was given. We categorized studies based on their methodological quality in different groups, from poor (score between 0 and 3) to high quality (score between 7 and 9). Two independent reviewers performed the quality assessment process; in case of any disagreement, a third investigator resolved the issues [24].
Data extraction
One of the reviewers used a data extraction form to enter data of the included studies. The form included items such as author/ authors’ name, the title of the study, publication year, study setting, sample size, characteristics of the study population including their age and gender, the total prevalence of hospital-acquired infection, the prevalence of hospital-acquired infection based on the infection type and related organisms (S1 File).
Statistical analysis
We used a random-effects model to estimate the pooled prevalence of HAIs, measuring the effect size with a 95% confidence interval (CI) and illustrating the graphical results with Forest plots. The I2 test quantified the statistical heterogeneity, and the Egger test was applied to assess publication bias. We used subgroup analyses due to the variability of estimates based on different study settings, type of infection, and socio-demographic characteristics of study populations. We carried out all analyses, using the Comprehensive Meta-Analysis and R software. All figures with p<0.05 were considered statistically significant.
Patient and public involvement
We considered articles with available full texts published in English between 2000 and June 2021 for further consideration in this review.
Results
Overview
According to the inclusion and exclusion criteria and PRISMA checklist (Preferred reporting items for systematic reviews and meta-analyses) [25], we selected 400 articles for the final review stage (see Fig 1). The total number of patients participating in these studies was 29,159,630, of which 5,441,722 had various HAIs. On the basis of the data analysis, we estimated the rate of the global HAIs to be 0.14 (95% CI, 0.12–0.15) (Table 1).
Table 1. The pooled analysis of global prevalence of nosocomial infection.
| Models | Number Studies | Effect size and 95% interval | Test of null (2-Tail) | |||
|---|---|---|---|---|---|---|
| Point estimate | Lower limit | Upper limit | Z-value | P-value | ||
| Random effects | 400 | 0.141 | 0.127 | 0.156 | -32.219 | 0.000 |
As Fig 2 shows, the prevalence of nosocomial infections is increasing, as with a one-year increase, 0.06 would be added to nosocomial infections (Fig 2). Moreover, to clarify the findings, we divided it into five range. The findings show that the highest prevalence of nosocomial infections was 0.20 (95% CI, 0.11–0.32) between 2011–2015, but it decreased to 0.17 (95% CI, 0.08–0.23) between 2016–2011 (Table 6).
Fig 2. Meta-regression based on year of publication and sample size.
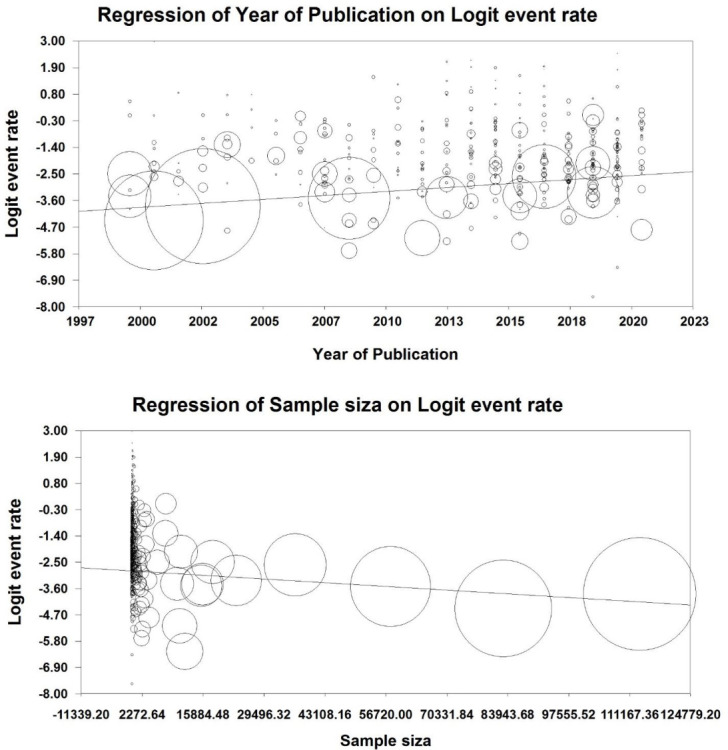
Table 6. The results of subgroups-analysis.
| Variables | Number of studies | Prevalence (95% CI) | I2 | P |
|---|---|---|---|---|
| Age | ||||
| 0–5 | 20 | 0.21 (0.5–0.22) | 97.6% | 0.001 |
| 5–25 | 13 | 0.14 (0.5–0.17) | 98.2% | 0.001 |
| 25–50 | 88 | 0.13 (0.14–0.21) | 98.7% | 0.001 |
| >50 | 67 | 0.17 (0.08–0.19) | 97.4% | 0.001 |
| Length of stay (Day) | ||||
| ≤15 | 85 | 0.12 (0.6–0.28) | 96.6% | 0.001 |
| >15 | 91 | 0.15 (0.11–0.34) | 93.2% | 0.001 |
| Year of Publication | ||||
| 2000–2005 | 78 | 0.11 (0.6–0.19) | 97.2% | 0.001 |
| 2006–2010 | 93 | 0.14 (0.3–0.25) | 96.4% | 0.001 |
| 2011–2015 | 105 | 0.20 (0.11–0.32) | 98.2% | 0.001 |
| 2016–2021 | 124 | 0.16 (0.08–0.23) | 95.7% | 0.001 |
| Countries based on income | ||||
| Low income | 27 | 0.32 (0.15–0.49) | 95.1% | 0.001 |
| Middle income | 26 | 0.16 (0.11–0.26) | 98.3% | 0.001 |
| High income | 29 | 0.06 (0.03–0.12) | 96.5% | 0.001 |
| Quality of Study | ||||
| Low | 26 | 0.16 (0.06–0.19) | 96.4% | 0.001 |
| Medium | 177 | 0.12 (0.05–0.016) | 92.5% | 0.001 |
| High | 197 | 0.14 (0.10–0.16) | 91.1% | 0.001 |
Abbreviations: Confidence Interval (CI).
Since we included 400 studies in this study and there were different sample sizes, we performed a pooled analysis based on the sample size. The results revealed no significant relationship between sample size and HAIs, and with changing the sample size, we observed no significant difference in the rate of HAIs (Fig 2).
Meta-analysis based on WHO regions and countries
Total
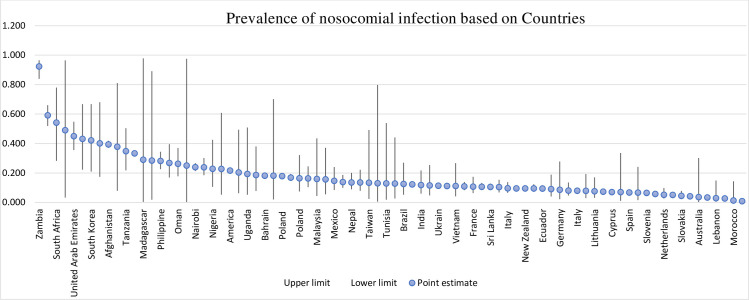
As illustrated in Table 2, the highest rate of HAIs was in the AFR, and based on 94 studies analyzed, this rate was equal to 0.27 (95% CI, 0.22–0.34). The lowest infection rates were in AMR and WPR which were 0.09 (95% CI, 0.07–0.11) and 0.09 (95% CI, 0.06–0.13), respectively. Fig 3 demonstrates the distribution map of HAIs. The map shows that the rate of HAIs in central Africa is higher than anywhere else in the world (Fig 3).
Table 2. Meta-analysis based on WHO regions.
| WHO Regions | Number Studies | Effect size and 95% interval | Test of null (2-Tail) | |||
|---|---|---|---|---|---|---|
| Point estimate | Lower limit | Upper limit | Z-value | P-value | ||
| AFRO | 94 | 0.270 | 0.222 | 0.324 | -7.574 | 0.000 |
| AMRO | 18 | 0.096 | 0.079 | 0.117 | -20.059 | 0.000 |
| EMRO | 103 | 0.125 | 0.098 | 0.159 | -13.945 | 0.000 |
| EURO | 114 | 0.114 | 0.096 | 0.134 | -21.394 | 0.000 |
| SEARO | 24 | 0.129 | 0.086 | 0.188 | -8.270 | 0.000 |
| WPRO | 47 | 0.097 | 0.069 | 0.136 | -11.437 | 0.000 |
Fig 3. Distribution of the global prevalence of nosocomial infection in patients based on countries, 2000–2021.
Map created with PhotoshopCC, using political borders.
Meta-analysis based on micro-organism and infection types
Based on the analysis of microorganisms and various HAIs, the findings showed that among all major microorganisms responsible for the HAIs, patients were infected by E. coli more than other microorganisms, 0.18 (95% CI, 0.16–0.20). However, according to WHO regions, Coagulase-negative staphylococci was the most common microorganisms in WPRO and EURO with 0.21 (95% CI, 0.11–0.36) and 0.14 (95% CI, 0.10–0.20). Also, in South-East Asian Region Office (SEARO) and EMRO, the highest rate of infection was related to E. coli with 0.19 (95% CI, 0.13–0.26) and 0.16 (95% CI, 0.13–0.20). Finally, Pseudomonas aeruginosa and Staphylococcus spp. microorganisms were the most common infectious agents in AMRO and AFRO, respectively (Table 3).
Table 3. Meta-analysis based on micro-organism and infection types.
| Sub-groups | WHO Regions | Total | ||||||
|---|---|---|---|---|---|---|---|---|
| WPRO | SEARO | AMRO | EURO | EMRO | AFRO | |||
| Micro-organism | Acinetobacter spp | 0.14 | 0.10 | 0.08 | 0.11 | 0.15 | 0.10 | 0.13 |
| Candida spp | 0.07 | 0.17 | 0.09 | 0.06 | 0.07 | 0.06 | 0.07 | |
| Coagulase-negative staphylococci | 0.21 | 0.04 | 0.15 | 0.14 | 0.15 | 0.11 | 0.14 | |
| E.Coli | 0.10 | 0.19 | 0.12 | 0.05 | 0.16 | 0.24 | 0.18 | |
| Enterobacter spp | 0.08 | 0.15 | 0.11 | 0.07 | 0.09 | 0.07 | 0.08 | |
| Enterococcus spp | 0.05 | 0.12 | 0.08 | 0.06 | 0.07 | 0.04 | 0.07 | |
| Klebsiella spp | 0.13 | 0.14 | 0.12 | 0.12 | 0.12 | 0.14 | 0.13 | |
| Pseudomonas aeruginosa | 0.07 | 0.18 | 0.17 | 0.10 | 0.10 | 0.09 | 0.11 | |
| Staphylococcus spp | 0.14 | 0.17 | 0.63 | 0.13 | 0.13 | 0.26 | 0.14 | |
| Streptococcus spp | 0.03 | 0.01 | 0.06 | 0.05 | 0.06 | 0.04 | 0.05 | |
| Other | 0.04 | 0.17 | 0.03 | 0.08 | 0.12 | 0.04 | 0.05 | |
| Infections | Bacteraemia | 0.17 | 0.26 | 0.05 | 0.11 | 0.12 | 0.97 | 0.17 |
| Bloodstream infection | 0.18 | 0.51 | 0.26 | 0.19 | 0.32 | 0.61 | 0.25 | |
| Gastrointestinal infection | 0.08 | 0.91 | 0.46 | 0.07 | 0.08 | 0.93 | 0.12 | |
| Pneumonia | 0.21 | 0.28 | 0.33 | 0.24 | 0.26 | 0.26 | 0.25 | |
| Respiratory tract infection | 0.52 | 0.90 | 0.12 | 0.17 | 0.22 | 0.38 | 0.22 | |
| Surgical site infection | 0.06 | 0.92 | 0.22 | 0.15 | 0.24 | 0.89 | 0.26 | |
| Urinary tract infection | 0.10 | 0.79 | 0.23 | 0.22 | 0.25 | 0.88 | 0.25 | |
| Wound infection | 0.06 | 0.68 | 0.03 | 0.06 | 0.39 | 0.96 | 0.34 | |
| Other | 0.45 | 0.89 | 0.37 | 0.14 | 0.10 | 0.59 | 0.21 | |
The results of analyzes based on the type of infections showed that the highest type of infection among all HAIs was wound infection, with a rate of 0.34 (95% CI, 0.24–0.47). Regarding the WHO regions, the analyses showed that each region was more involved with a particular infection. For example, in the WPRO and SEARO, respiratory tract infections and surgical site infections were the most common infections. However, wound infection was more prevalent in the EMRO and AFRO than in other infections (Table 3).
Meta-analysis based on hospital ward
The findings showed that the highest prevalence of HAIs in hospital wards was related to the transplant wards with the prevalence rate of 0.77 (95% CI, 0.38–0.90), followed by Neonatal and ICU wards, with a prevalence rate of 0.69 (95% CI, 0.47–0.85) and 0.68 (95% CI, 0.61–0.73), respectively (Table 4).
Table 4. Meta-analysis based on hospital ward.
| Hospital Wards | Number Studies | Effect size and 95% interval | Test of null (2-Tail) | |||
|---|---|---|---|---|---|---|
| Point estimate | Lower limit | Upper limit | Z-value | P-value | ||
| Burns | 19 | 0.25 | 0.15 | 0.38 | -3.60 | 0.00 |
| Cardiology | 2 | 0.07 | 0.06 | 0.08 | -35.16 | 0.00 |
| CCU | 3 | 0.10 | 0.01 | 0.04 | -12.66 | 0.00 |
| Emergency | 9 | 0.22 | 0.09 | 0.46 | -2.28 | 0.02 |
| Hematology | 6 | 0.05 | 0.03 | 0.10 | -7.96 | 0.00 |
| ICU | 140 | 0.68 | 0.61 | 0.73 | 5.37 | 0.00 |
| Internal medicine | 23 | 0.22 | 0.13 | 0.35 | -3.87 | 0.00 |
| Labour & postpartum | 8 | 0.54 | 0.48 | 0.97 | 1.88 | 0.06 |
| Medical wards | 25 | 0.33 | 0.28 | 0.40 | -5.10 | 0.00 |
| Medical wards | 14 | 0.28 | 0.21 | 0.36 | -5.06 | 0.00 |
| Neonatal | 10 | 0.69 | 0.47 | 0.85 | 1.68 | 0.09 |
| Nephrology | 7 | 0.05 | 0.03 | 0.07 | -12.17 | 0.00 |
| NICU | 43 | 0.44 | 0.35 | 0.52 | -1.44 | 0.15 |
| Obstetrics and gynecology | 34 | 0.07 | 0.04 | 0.12 | -9.58 | 0.00 |
| Oncology | 6 | 0.23 | 0.07 | 0.98 | 0.33 | 0.74 |
| Orthopedic | 8 | 0.09 | 0.07 | 0.11 | -15.72 | 0.00 |
| Pediatric | 39 | 0.21 | 0.14 | 0.29 | -5.65 | 0.00 |
| PICU | 21 | 0.28 | 0.19 | 0.39 | -3.83 | 0.00 |
| Rehabilitation | 10 | 0.07 | 0.04 | 0.10 | -11.63 | 0.00 |
| Surgery | 101 | 0.43 | 0.37 | 0.50 | -1.87 | 0.06 |
| Transplant | 5 | 0.77 | 0.38 | 0.90 | 1.72 | 0.09 |
| Trauma | 4 | 0.34 | 0.04 | 0.87 | -0.51 | 0.61 |
| Other wards | 58 | 0.16 | 0.11 | 0.21 | -9.19 | 0.00 |
Meta-analysis based on gender
Overall, the prevalence of HAIs is higher in men than women. However, the prevalence of this type of infection is higher in women in AMR and EMR. In AFR, EUR and SEAR, men showed higher prevalence rate, while the rates were the same for both genders in the WPR (Table 5).
Table 5. Meta-analysis based on gender.
| Gender | WHO Regions | Effect size and 95% interval | Test of null (2-Tail) | |||
|---|---|---|---|---|---|---|
| Point estimate | Lower limit | Upper limit | Z-value | P-value | ||
| Female | AFRO | 0.243 | 0.192 | 0.304 | -7.321 | 0.000 |
| AMRO | 0.103 | 0.021 | 0.374 | -2.571 | 0.010 | |
| EMRO | 0.252 | 0.176 | 0.348 | -4.652 | 0.000 | |
| EURO | 0.088 | 0.069 | 0.113 | -16.846 | 0.000 | |
| SEARO | 0.145 | 0.103 | 0.199 | -9.028 | 0.000 | |
| WPRO | 0.104 | 0.051 | 0.202 | -5.448 | 0.000 | |
| Overall | 0.030 | 0.030 | 0.030 | -1027.257 | 0.000 | |
| Male | AFRO | 0.260 | 0.198 | 0.332 | -5.895 | 0.000 |
| AMRO | 0.067 | 0.024 | 0.177 | -4.722 | 0.000 | |
| EMRO | 0.242 | 0.186 | 0.308 | -6.701 | 0.000 | |
| EURO | 0.114 | 0.091 | 0.140 | -16.751 | 0.000 | |
| SEARO | 0.180 | 0.123 | 0.256 | -6.606 | 0.000 | |
| WPRO | 0.104 | 0.049 | 0.207 | -5.201 | 0.000 | |
Meta-regression on other sub-groups
Age
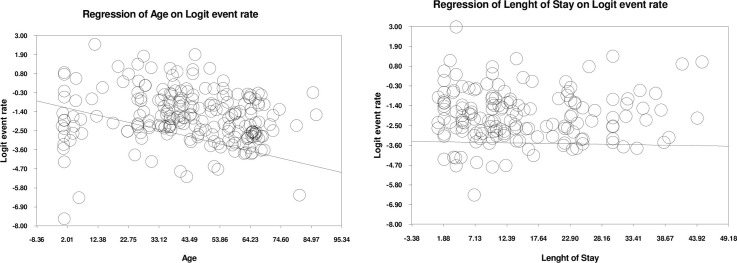
The results of the analysis showed that the prevalence of HAIs decreases with increasing age. For every one year increase in age, the prevalence decreases by 0.04 (Fig 4). We categorized the participants by age to make our results clear, and we noticed that the highest prevalence of nosocomial infections was in the age range of 0–5 years 0.21 (95% CI, 0.5–0.22) (Table 6).
Fig 4. Meta-regression based on age and length of saty.
Length of stay
According to our findings, No significant relationship was found between length of stay and the prevalence of HAIs (Fig 4). days, We divided the length of stay in the hospital into more than 15 days and less than 15, based on the division of other studies [26]. The results showed that the prevalence rate was estimated to be 0.15 (95% CI, 0.11–0.34) in people who were in the hospital for more than 15 days and 0.12 (95% CI, 0.6–0.28) for those who were in the hospital for 15 days or less than 15 days (Table 6).
Countries based on income
According to the findings of the analysis, countries with lower incomes had higher prevalence of infection. For example, in low-income countries, the prevalence was 0.32 (95% CI, 0.15–0.49) and the prevalence of high-income countries was estimated 0.06 (95% CI, 0.03–0.12) (Table 6)
Quality of study
The findings revealed that the prevalence rate in lower quality studies was 0.16 (95% CI, 0.06–0.19) whereas it was 0.14 (95% CI, 0.10–0.16) in high quality studies (Table 6).
Publication bias
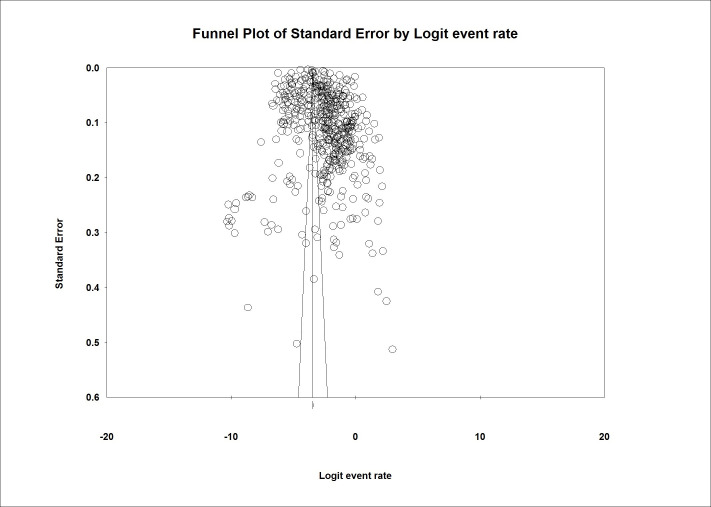
Based on Fig 5, the analysis showed that this study has a Publication bias. This claim is true since the result of the Egger test was greater than 0.01. (P-value 2-tailed = 0.091).
Fig 5. Funnel plot of publication bias.
Discussion
HAIs are one of the most severe public health issues with high morbidity, mortality, and costs [27]. This study aimed to conduct a systematic review and meta-analysis to determine the prevalence rate of HAIs globally. This is the first comprehensive SLR investigating HAIs from all key aspects. In this systematic review, we screened 7031 journal articles and selected 400 articles that contained quantitative information about the global prevalence of HAIs for evaluation in the meta-analysis.
On the basis of the findings of this study, the rate of universal HAIs is estimated to be 0.14 with an annual increasing rate of 0.06 worldwide. According to our findings, the highest rate of HAIs was in the AFR, while the lowest rates were in AMRO and WPRO, at 0.09. Besides, the central Africa had higher rate than other parts of the world. This may be due to the lack of health facilities and resources in this area. The continent is also facing natural crises such as water shortages and droughts, which in turn are increasing nosocomial infections. On the other hand, economic conditions in this region are one of the most important causes of these infections. Another study revealed that the HAIs rate is 7.5% in high-income countries, while it varies between 5.7 and 19.2 in low-income countries percent [28]. According to the WHO data, the HAIs rate is 25% in developing countries and 5–15% in developed countries [29, 30]. Another study estimated the HAIs rate at 16 percent in the Eastern Mediterranean Region [8]. In Roberts et al. study, 159 patients (12.7%) developed HAIs from among 1,253 patients in the United States [31]. The findings of our study suggest that patients are at a higher risk of nosocomial infections due to lack of facilities and poor conditions of hospitals and medical centers in low-income and underdeveloped countries than developed countries.
According to the analysis of microorganisms, the E. coli infected patients with HAIs more than other microorganisms (0.18). Based on the WHO regions, Coagulase-negative staphylococci were the most common microorganisms in WPRO and EURO with the incidence of 0.21 and 0.14, respectively. In SEARO and EMRO, the highest infectivity was E. coli, with 0.19 and 0.16. Moreover, Pseudomonas aeruginosa and Staphylococcus spp. microorganisms were the most common infectious agents in AMRO and AFRO, respectively. One of the studies on this issue revealed that Staphylococcus aureus, Pseudomonas aeroginosa, and Klebsiella species are the most common pathogens in Africa and South America [32]. Another review in Africa reported Klebsiella, Staphylococcus aureus, Pseudomonas aeroginosa, and E.coli the most common microorganisms in HAIs. These three microorganisms, in addition to being easier to transport than others, have significant resistance to antibiotics. On the other hand, they are more resistant to sterilization and disinfection methods than the others. Due to these characteristics, these microorganisms have a higher prevalence rate than others. Because these bacteria are more resistant to antibiotics than others [33].
Analysis based on the type of infections revealed that the highest type of infection in all HAIs was wound infection, with a rate of 0.34. In terms of the WHO regions, each region represents a specific type of infection; in the WPRO and SEARO. Respiratory tract infections and surgical site infections were the most widespread infections while wound infection was more prevalent in the EMRO and AFRO. A similar study showed that lower respiratory tract infections were the leading cause of HAIs [34]. Other common infections were urinary tract infections, surgical site infections, and bloodstream infections [9].
In terms of the prevalence of HAIs in hospital wards, transplant unit had the highest rate at 0.77, followed by neonatal and ICU wards 0.69 and 0.68, respectively. Nonetheless, a study in Ethiopia found that the infection rate at the surgical site was high % [35]. Another study found the surgical site as the most frequent type of HAI in Low and Middle-Income Countries [9].
Regarding HAIs in terms of gender, the prevalence of HAIs was higher in men than in women. In line with our study, the HAIs burden was shown to be greater in men in another study [36]. In the WHO regions, the rate was higher in women in AMRO and EMRO, whereas, in AFRO, EURO and SEARO, men were reported to have a higher rate. However, in the WPRO, the rates were the same for both sexes. Another study of about 633,000 people in China reported that the prevalence rate was higher in men than in women, supporting our findings [37]. Similarly, another study in the United States on about 530,000 people showed similar results to our findings [10].
With the increasing length of stay in the hospital and despite the fact that we divided the patients into two groups of more than 15 LoS and less than 15 LoS to determine the effect of length of stay on NI, the difference was not too large and we did not find any significant changes in the HAIs rate. However, the AlemkereI’sstudy found that the HAIs risk in patients with a longer stay was 24 times more than in patients with a shorter stay [38]. But in another study, the findings showed that an increase in length of stay could affect the rate of nosocomial infection, but this effect was considered significant after 9 days [39]. In another study of 65,000 people, the findings showed that although longer stays could affect the prevalence of nosocomial infections, the effect was not significant [40]. We think that this variable can affect nosocomial infections, but this effect can manifest itself after a long time stay in the hospital. A short time stay in the hospital does not have much effect and cannot increase the prevalence. According to studies reviewed, this effect becomes more severe after 15 days and increases the prevalence.
The study showed that with the increasing age, the prevalence of nosocomial infections decreases. On the other hand, by age classification, we found that the prevalence of infection is higher in the age group of 0–5 years and in the age group over 50 years. In a study conducted in Argentina on people under the age of five, the prevalence rate of nosocomial infections was reported to be 50%, which was much higher than the average for our study and the global average [41]. In another study conducted in Turkey on people of similar age range, the prevalence rate was about 25 percent [42].
Conclusion
This systematic and meta-analysis review was conducted to determine the rate of HAIs worldwide. The review identified a number of essential details about the rate of HAIs in various parts of the world. It revealed that the rate of universal HAIs and the number of publications in this regard has risen in recent years. The HAIs rate and the most common micro-organism were different in various regions. However, several important gaps were identified such as lack of data in different regions and territories and different domains like the cause of HAIs. The study findings can help managers and policymakers of the health sector identify the reason for HAIs and apply effective control programs to implement different plans to reduce the HAIs rate and the financial costs of such infections and save resources. We recommend that more studies be carried out to identify strategies and plans for preventing HAIs in all countries, particularly in Low and Middle-Income Countries. Nosocomial infection is one of the most important indicators of hospitals to evaluate the performance of the hospital in terms of patient safety. Our study is done on a global scale so it can be very generalizable and help health decision makers to plan to prevent these types of infections. By reducing nosocomial infections, in addition to improving the patient’s safety index, a large amount of the costs incurred by the hospital due to these types of infections will be reduced.
We suggest to decision makers that by focusing on different aspects of nosocomial infections such as age, gender, causes, etc. that we mentioned in this study, comprehensive and practical programs can be used to prevent these infections.
Limitations
There are some limitations that should be considered when interpreting our study results. First, there might be a language bias in the study as we only included the studies published in English. We focused on peer-reviewed articles; thus, grey literatures and unpublished articles were not included in this review. In addition, in some countries, reliable and published data was not available, so we could not analyze all countries in the world. Finally, studies reviewed did not address many of the variables directly related to nosocomial infections such as type of hospital, number of hospital beds, etc. We also did not include Covid-19 disease in nosocomial infections because they have different definitions, and if we included Covid-19 infections in our study, it would falsely increase the prevalence of nosocomial infections in recent years, it would be a significant bias.
We suggest that researchers work on the gaps in our study. For example, conduct studies in countries where no articles on nosocomial infections have been found. On the other hand, studies on the cause and transmission of these infections can greatly help the health system to reduce these types of diseases.
Supporting information
(PDF)
(DOCX)
Acknowledgments
Our research team would like to thank all those who are trying to improve the fields related to health service management, especially the (@health.care.management) team (hcmanagers.ir), who have made great efforts to increase the credibility of this field in the Iranian health system.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Hazard D., et al., Predicting Potential Prevention Effects on Hospital Burden of Nosocomial Infections: A Multistate Modeling Approach. Value in Health, 2021. 24(6): p. 830–838. doi: 10.1016/j.jval.2021.02.002 [DOI] [PubMed] [Google Scholar]
- 2.Šuljagić V., et al., A nationwide assessment of the burden of healthcare-associated infections and antimicrobial use among surgical patients: results from Serbian point prevalence survey, 2017. Antimicrob Resist Infect Control, 2021. 10(1): p. 47. doi: 10.1186/s13756-021-00889-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.König E., et al., Prospective Surveillance of Healthcare-Associated Infections in Residents in Four Long-Term Care Facilities in Graz, Austria. Antibiotics (Basel), 2021. 10(5). doi: 10.3390/antibiotics10050544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoxha A., Duysburgh E., and Mortgat L., Healthcare-associated infections in home healthcare: an extensive assessment, 2019. Euro Surveill, 2021. 26(5). doi: 10.2807/1560-7917.ES.2021.26.5.1900646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gozel M.G., et al., National Infection Control Program in Turkey: The healthcare associated infection rate experiences over 10 years. American Journal of Infection Control, 2021. [DOI] [PubMed] [Google Scholar]
- 6.Bedir Demirdağ T., et al., The prevalence and diagnostic criteria of health-care associated infections in neonatal intensive care units in Turkey: A multicenter point- prevalence study. Pediatrics and Neonatology, 2021. 62(2): p. 208–217. [DOI] [PubMed] [Google Scholar]
- 7.Vidaković S., et al., Risk factors for healthcare associated infections and in-hospital mortality in a neurological intensive care unit in a tertiary hospital in Belgrade, Serbia: A prospective cohort study. Vojnosanitetski Pregled, 2020. 77(10): p. 1060–1066. [Google Scholar]
- 8.Ghashghaee A., et al., The Prevalence of Hospital-Acquired Infections in the EMRO: A Systematic Review and Meta-Analysis from 2000 to 2018. 2019. [Google Scholar]
- 9.Ketata N., et al., Point prevalence survey of health-care associated infections and their risk factors in the tertiary-care referral hospitals of Southern Tunisia. Infection, Disease & Health, 2021. 26(4): p. 284–291. doi: 10.1016/j.idh.2021.06.004 [DOI] [PubMed] [Google Scholar]
- 10.Hu Y., et al., Epidemiology and outcomes of bloodstream infections in severe burn patients: a six-year retrospective study. Antimicrobial Resistance & Infection Control, 2021. 10(1): p. 1–8. doi: 10.1186/s13756-021-00969-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sax H., et al., Preventing healthcare-associated infection in Switzerland: Results of a national survey. Infect Control Hosp Epidemiol, 2020. 41(5): p. 597–600. doi: 10.1017/ice.2019.351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenthal V.D., et al., Six-year multicenter study on short-term peripheral venous catheters-related bloodstream infection rates in 246 intensive units of 83 hospitals in 52 cities of 14 countries of Middle East: Bahrain, Egypt, Iran, Jordan, Kingdom of Saudi Arabia, Kuwait, Lebanon, Morocco, Pakistan, Palestine, Sudan, Tunisia, Turkey, and United Arab Emirates-International Nosocomial Infection Control Consortium (INICC) findings. J Infect Public Health, 2020. 13(8): p. 1134–1141. doi: 10.1016/j.jiph.2020.03.012 [DOI] [PubMed] [Google Scholar]
- 13.Rangelova V., et al., Surveillance of Nosocomial Infections in a Bulgarian Neonatal Intensive Care Unit. Folia Med (Plovdiv), 2020. 62(4): p. 753–761. doi: 10.3897/folmed.62.e50437 [DOI] [PubMed] [Google Scholar]
- 14.Lakhani K., et al., Nosocomial infection with SARS-CoV-2 and main outcomes after surgery within an orthopaedic surgery department in a tertiary trauma centre in Spain. Int Orthop, 2020. 44(12): p. 2505–2513. doi: 10.1007/s00264-020-04798-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iordanou S., et al., Device-associated health care-associated infections: The effectiveness of a 3-year prevention and control program in the Republic of Cyprus. Nurs Crit Care, 2020. doi: 10.1111/nicc.12581 [DOI] [PubMed] [Google Scholar]
- 16.Gugliotta C., et al., Prevalence study on health-care associated infections and on the use of antimicrobials carried out with the light protocol of the European Centre for Disease Prevention and Control. Ann Ig, 2020. 32(4): p. 357–367. doi: 10.7416/ai.2020.2359 [DOI] [PubMed] [Google Scholar]
- 17.Gozel M.G., et al., National Infection Control Program in Turkey: The healthcare associated infection rate experiences over 10 years. Am J Infect Control, 2020. doi: 10.1016/j.ajic.2020.12.013 [DOI] [PubMed] [Google Scholar]
- 18.Gazzarata R., et al., Healthcare Associated Infections: An Interoperable Infrastructure for Multidrug Resistant Organism Surveillance. Int J Environ Res Public Health, 2020. 17(2). doi: 10.3390/ijerph17020465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duszynska W., et al., Device associated -health care associated infections monitoring, prevention and cost assessment at intensive care unit of University Hospital in Poland (2015–2017). BMC Infect Dis, 2020. 20(1): p. 761. doi: 10.1186/s12879-020-05482-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chalapa V.I., et al., Epidemiological surveillance of healthcare-associated infections and the problem of underestimating cases: Results of sociological study in medical organizations of khanty-mansiysk autonomous Okrug—Ugra. Profilakticheskaya Meditsina, 2020. 23(3): p. 48–55. [Google Scholar]
- 21.Bjark P.H., Hansen E., and Lingaas E., In-hospital deaths attributable to healthcare-associated infections. Tidsskr Nor Laegeforen, 2020. 140(6). [DOI] [PubMed] [Google Scholar]
- 22.Berglund Kristiansson E. and Källman U., Healthcare staff’s views on the patients’ prerequisites to be co-creator in preventing healthcare-associated infections. Scand J Caring Sci, 2020. 34(2): p. 314–321. doi: 10.1111/scs.12730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scamardo M.S., et al., Trends, risk factors and outcomes of healthcare-associated infections in a neonatal intensive care unit in Italy during 2013–2017. Ital J Pediatr, 2020. 46(1): p. 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wells G.A., et al., The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses, 2000, Oxford. [Google Scholar]
- 25.Moher D., et al., Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS medicine, 2009. 6(7): p. e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Magal P. and McCluskey C., Two-group infection age model including an application to nosocomial infection. SIAM Journal on Applied Mathematics, 2013. 73(2): p. 1058–1095. [Google Scholar]
- 27.Manoukian S., et al., Estimating excess length of stay due to healthcare-associated infections: a systematic review and meta-analysis of statistical methodology. Journal of Hospital Infection, 2018. 100(2): p. 222–235. [DOI] [PubMed] [Google Scholar]
- 28.Voidazan S., et al., Healthcare Associated Infections—A New Pathology in Medical Practice? International journal of environmental research and public health, 2020. 17(3): p. 760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Larypoor M. and Frsad S., Evaluation of nosocomial infections in one of hospitals of Qom, 2008. Iranian Journal of Medical Microbiology, 2011. 5(3): p. 7–17. [Google Scholar]
- 30.Olise C. and Simon-Oke I., Fomites: Possible vehicle of nosocomial infections. Journal of Public Health and Nutrition, 2018. 1(1). [Google Scholar]
- 31.Roberts R.R., et al., Costs attributable to healthcare-acquired infection in hospitalized adults and a comparison of economic methods. Medical care, 2010: p. 1026–1035. doi: 10.1097/MLR.0b013e3181ef60a2 [DOI] [PubMed] [Google Scholar]
- 32.Yallew W.W., Kumie A., and Yehuala F.M., Point prevalence of hospital-acquired infections in two teaching hospitals of Amhara region in Ethiopia. Drug, healthcare and patient safety, 2016. 8: p. 71. doi: 10.2147/DHPS.S107344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Irek E.O., et al., A systematic review of healthcare-associated infections in Africa: An antimicrobial resistance perspective. African journal of laboratory medicine, 2018. 7(2): p. 1–9. doi: 10.4102/ajlm.v7i2.796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jagt E.W. and Short S., Healthcare-Associated Infections, in Pediatric Critical Care. 2021, Springer. p. 1105–1143. [Google Scholar]
- 35.Ayala D., et al., Magnitude and factors associated with surgical site infection among mothers underwent cesarean delivery in Nekemte town public hospitals, western Ethiopia. Plos one, 2021. 16(4): p. e0250736. doi: 10.1371/journal.pone.0250736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bordino V., et al., Burden of healthcare-associated infections in Italy: incidence, attributable mortality and disability-adjusted life years (DALYs) from a nationwide study, 2016. Journal of Hospital Infection, 2021. 113: p. 164–171. doi: 10.1016/j.jhin.2021.04.023 [DOI] [PubMed] [Google Scholar]
- 37.Zhang F., et al., Acute effects of ambient air pollution on clinic visits of college students for upper respiratory tract infection in Wuhan, China. Environmental Science and Pollution Research, 2021. 28(23): p. 29820–29830. doi: 10.1007/s11356-021-12828-7 [DOI] [PubMed] [Google Scholar]
- 38.Babatola A.O., et al., Addressing antimicrobial resistance in Nigerian hospitals: exploring physicians prescribing behavior, knowledge, and perception of antimicrobial resistance and stewardship programs. Expert review of anti-infective therapy, 2021. 19(4): p. 537–546. doi: 10.1080/14787210.2021.1829474 [DOI] [PubMed] [Google Scholar]
- 39.Boncea E.E., et al., Association between intrahospital transfer and hospital-acquired infection in the elderly: a retrospective case–control study in a UK hospital network. BMJ Quality & Safety, 2021. 30(6): p. 457–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stewart S., et al., Impact of healthcare-associated infection on length of stay. Journal of Hospital Infection, 2021. 114: p. 23–31. [DOI] [PubMed] [Google Scholar]
- 41.Böncüoğlu E., et al., Upward trend in the frequency of community-acquired methicillin-resistant Staphylococcus aureus as a cause of pediatric skin and soft tissue infections over five years: a cross-sectional study. The Turkish journal of pediatrics, 2021. 63(2): p. 200–205. [DOI] [PubMed] [Google Scholar]
- 42.Noah N. and Potas N., Association between nursing work stress, burnout and nosocomial infection rate in a neonatal intensive care unit in Hargeisa, Somaliland. Tropical Doctor, 2021: p. 00494755211055250. [DOI] [PubMed] [Google Scholar]