Abstract
Aim
Primary liver cancer is usually diagnosed at advanced stages with distant metastasis, underlying the high metastatic rate and mortality in patients. This study aimed to analyse the metastatic patterns and prognosis of primary liver cancer, and its relationship with age and several other factors, such as histological variants, TNM stage, and grade.
Methods
We included data from 5274 patients from the Surveillance, Epidemiology, and End Results (SEER) database of the American National Cancer Institute diagnosed with primary liver cancer with metastatic disease between 2010 and 2015. The correlation between the metastatic patterns of primary liver cancer and age was evaluated. The hazard ratio (HR) and 95% confidence intervals (CI) for overall survival were calculated by applying univariate Cox analysis, while the correlation between the metastatic patterns and age was analysed by applying multivariate Cox analysis. We also plotted Kaplan-Meier curves to illustrate the correlation between overall survival (OS) and various factors.
Results
Several factors were associated with poorer prognosis, including age>60 years, histologic type of spindle cell variant, higher grade, no surgery, tumour size ≥ 1 cm, and lung metastasis. The rate of metastasis increased with age. Older patients (> 50 years) were prone to bone metastasis, while less likely to have lung metastasis compared with younger patients (< 50 years). Patients with lung metastasis had a higher risk of being diagnosed with metastasis in other locations. Furthermore, surgery significantly reduced mortality and primary site surgery in particular, mitigated the risk of bone and lung metastases.
Conclusions
Our study shows the correlation of prognosis and metastatic patterns with age and several other factors. The findings can hopefully provide knowledge that will allow a better diagnosis and management of elderly patients with primary liver cancer.
Introduction
Liver cancer is the fourth most common cause of cancer-related deaths (CRD) worldwide [1]. It exhibits the second highest mortality rate globally, with a 5-year survival rate of 18% [2, 3]. Hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma (iCCA) are the two major types of primary liver cancers, accounting for over 90% of all cases [4]. Primary liver cancers are usually diagnosed in patients with chronic liver diseases, including hepatocellular damage attributed to fatty liver disease and viral infections. However, the application of HBV vaccination and hepatitis C direct antiviral drugs have significantly reduced the number of virus-induced liver cancers; hence, non-alcoholic fatty liver disease (NAFLD) is becoming the main cause of liver cancer [5].
In the last decade, the mortality rate of liver cancer among men and women in the United States has increased, especially among those aged over 50 years [6]. The treatment of most liver cancers consists of trans-arterial chemoembolization, oral administration of sorafenib, or immunotherapy. However, long-term use of chemotherapy, such as sorafenib, commonly exerts toxic effects [7], while surgical treatment is an option only for 5% to 15% of patients, diagnosed at an early stage [8]. Moreover, elderly patients are prone to postoperative complications [9]. Thus, given the delicate balance between risks and benefits, the selection of therapeutic regimens that benefit elderly liver cancer patients represents a dilemma.
Aging has been associated with a poorer prognosis in liver cancer [10, 11]. A significantly higher mortality rate has been observed in the foreign-born population compared with the US-born population [6]. Different histologic variants, surgical treatments, and location of metastasis also result in different prognoses and metastatic patterns of liver cancer [12, 13]. Such differences could be due to changes in lifestyle, presence of HBV infection, or the metabolic changes of aging. Furthermore, the poorer prognosis related to age and to some histologic variants, such as the spindle cell variant, could be related to the rate of metastasis. Thus, we believe it is worth evaluating the metastatic patterns, survival outcomes, and their association with age.
Existing studies have mostly focused on the pathogenesis and mechanisms of liver cancer. A study by Wu et al. demonstrated that different liver cancer histological variants had different metastatic patterns [14]. However, to the best of our knowledge, the correlation between liver cancer metastasis, mortality, and aging has not been investigated. Based on the data of adult primary liver cancer patients in the Surveillance, Epidemiology, and End and Result (SEER) database, this study was performed on factors including age, sex, race, grade, T stage, N stage, and histological type, etc. There are two main aims: 1) determine which parameters influence metastatic pattern of either bone, brain or lung; 2) determine which parameters influence overall survival (OS) The present study attempted to provide insights into the prognosis of primary liver cancer among patients with different conditions, thereby seeking to inspire future treatment groups.
Materials and methods
Ethics
All data of cancer patients were acquired from the SEER database (https://seer.cancer.gov/data/access.html) with the reference number: 13003-Nov2019. SEER is a public database, and no ethical approval is needed. Informed consent was not required. The data of individual participants was accessible from the SEER database.
Data collection
SEER* Stat version 8.3.4 was used to filter the data of the patients from the SEER database (http://seer.cancer.gov/). Patient selection complied with the SEER 18 Regs Research Data, as maintained by the National Cancer Institute. The inclusion criteria of this study were as follows: 1) patient age ≥ 18 years old; 2) primary site: liver; 3) availability of information regarding histological subtypes (histologic type ICD-O-3: 8170–8174); 4) malignant behaviour; 5) primary liver cancer confirmed by biopsy or microscopy; and 6) diagnosis performed between 2010 and 2015. Patients with unknown survival status and survival times were excluded, as well as those not at the M1 stage, according to the TMN staging system. Individual information can be assessed in the SEER database. As listed in Table 1, the clinical characteristics analyzed in the current study included age, race, sex, year of diagnosis, histologic type, grade, laterality, T stage, N stage, primary site surgery, other site surgery, lymph node surgery, bone metastasis, lung metastasis, liver metastasis. Laterality was defined as location of primary tumor. OS was defined as the period between diagnosis and death by any cause as described in SEER database.
Table 1. Characteristics of patients.
| Clinicopathological Characteristics | All patients n = (5,274) | Bone n = (1551) | Brain n = (116) | Lung n = (2140) |
|---|---|---|---|---|
| No (%) | No (%) | No(%) | No(%) | |
| Age | ||||
| Age<50 | 374(7) | 71(5) | 11(10) | 191(9) |
| Age50-59 | 1615(31) | 472(30) | 38(32) | 650(30) |
| age≥60 | 3285(62) | 1008(65) | 67(58) | 1299(61) |
| Sex | ||||
| Male | 4281(81) | 1326 (85) | 94(81) | 1687(79) |
| Female | 993(19) | 225(15) | 22(19) | 453(21) |
| Race | ||||
| Black | 842(16) | 276(18) | 19(16) | 372(17) |
| White | 3591(68) | 1079(69) | 82(71) | 1356(63) |
| Other | 824(15) | 191(12) | 14(12) | 403(19) |
| Unknown | 17(1) | 5(1) | 1(1) | 9(1) |
| Year diagnosis | ||||
| 2010 | 802(15) | 203(13) | 18(16) | 303(14) |
| 2011 | 810(15) | 232(15) | 21(18) | 326(15) |
| 2012 | 900(17) | 284(18) | 14(12) | 362(17) |
| 2013 | 912(17) | 277(18) | 24(21) | 373(17) |
| 2014 | 897(17) | 253(16) | 17(14) | 382(18) |
| 2015 | 953(19) | 302(20) | 22(19) | 394(18) |
| Histologic type | ||||
| 8170(NOS) | 5188(98) | 1532(99) | 115(99) | 2100(98) |
| 8171(fibrolamellar) | 27(<1) | 3(<1) | 0 | 13(1) |
| 8172(scirrhous) | 6(<1) | 1(<1) | 0 | 2(<1) |
| 8173(spindle cell variant) | 18(<1) | 4(<1) | 0 | 7(<1) |
| 8174(clear cell variant) | 35(1) | 11(1) | 1(1) | 18(8) |
| Grade | ||||
| I | 312(6) | 100(6) | 11(10) | 104(5) |
| II | 595(11) | 149(10) | 21(18) | 246(12) |
| III | 610(12) | 143(9) | 9(8) | 278(13) |
| IV | 61(1) | 9(1) | 3(3) | 22(1) |
| Unknown | 3696(70.0) | 1150(74) | 72(62) | 1490(70) |
| Laterality | ||||
| Paired | 10(<1) | 0 | 0 | 5(<1) |
| Not paired | 5264(100) | 1551(100.0) | 116(100.0) | 2135(100) |
| T stage | ||||
| T0 | 28(<1) | 16(1) | 2(2) | 11(1) |
| T1 | 1071(20) | 330(21) | 34(29) | 433(20) |
| T2 | 547(10) | 184(12) | 14(12) | 196(9) |
| T3a | 1058(20) | 328(21) | 19(16) | 425(20) |
| T3b | 870(17) | 194(13) | 10(9) | 327(15) |
| T3NOS | 11(<1) | 2(<1) | 0(0) | 5(<1) |
| T4 | 544(10) | 97(6) | 7(6) | 256(12) |
| TX | 1145(22) | 400(26) | 30(26) | 487(23) |
| N stage | ||||
| N0 | 3132(59) | 984(63) | 78(67) | 1321(62) |
| N1 | 1236(24) | 286(19) | 15(13) | 425(20) |
| NX | 906(17) | 281(18) | 23(20) | 394(18) |
| Primary site surgery | ||||
| Yes | 178(3) | 39(3) | 5(4) | 45(2) |
| No | 5096(97) | 1512(97) | 111(96) | 2095(98) |
| Lymph node surgery | ||||
| Yes | 46(1) | 7(1) | 0(0) | 10(1) |
| No | 5228(99) | 1544(99) | 116(100) | 2130(99) |
| Other surgery | ||||
| Yes | 209(4) | 110(7) | 12(10) | 52(3) |
| No | 5065(96) | 1441(93) | 104(90) | 2088(97) |
| Tumor size | ||||
| <1cm | 3730(71) | 1069(69) | 78(67) | 1472(69) |
| ≥1cm | 4(<1) | 1(<1) | 0(0) | 2(<1) |
| Unknown | 1540(29) | 481(31) | 38(33) | 666(31) |
| Bone | ||||
| Yes | 1551(29) | 44(38) | 353(17) | |
| No | 3491(66) | 69(60) | 1721(80) | |
| Unknown | 232(5) | 3(2) | 66(3) | |
| Brain | ||||
| Yes | 116(2) | 44(3) | 47(2) | |
| No | 4871(92) | 1446(93) | 2015(94) | |
| Unknown | 287(6) | 61(4) | 78(4) | |
| Lung | ||||
| Yes | 2140(41) | 353(23) | 47(41) | |
| No | 2886(55) | 1135(73) | 66(57) | |
| Unknown | 248(4) | 63(4) | 3(3) |
Note: NOS, non-specific hepatocellular carcinomas.
Statistical analysis
The effect of each clinical feature on overall survival (OS) was identified using hazard ratio (HR) and 95% confidence intervals (CIs). For nomogram model construction, univariate Cox regression was performed to screen the factors that affected the overall survival, and multivariate Cox regression was performed to further screen the independent prognostic factors. Then, the factors with P < 0.05 in the multivariate Cox regression analysis were included in the nomogram model. The predictive performance of nomogram model was assessed by the consistency index (C-index). C-index of 0.5 represent a random probability, indicating that this model has no predictive effect, while C-index of 1 represent a complete consistent, indicating that the predicted results of the model are completely consistent with the reality [15]. Age as a clinical feature was categorized into three groups (≤ 50 years, 51–59 years, and >60 years). This grouping pattern follows the same general grouping pattern used in other cancer studies [14]. Additionally, these groupings lead to the outcome which were identified as more statistically significant. Logistic regression models were adopted to assess the correlation between the metastatic patterns and clinical features. The relationship between the risk of metastasis with a variety of factors was analysed using the odds ratio (OR). The curve of the metastatic pattern was generated using SPSS 21.0. The Kaplan-Meier analysis method was employed for survival curves, followed by chi-square test for statistical significance. Statistical significance was defined as P < 0.05 for all tests. The multivariate Cox regression and all calculations were carried out using R version 3.2.3. The R packages used in this study including RMS, foreign, and survival package.
Results
Demographic characteristics
After applying the aforementioned inclusion criteria, a total of 5274 liver cancer patients were included in the study (Table 1), of which 4281 were men (81.1%) and 993 women (18.8%). The majority of the patients were aged over 60 years (<50 years, 374, [7%]; 50–59 years, 1615, [30.6%]; ≥60 years, 3285, [62.1%]) and White (White, 3591, [68%]; Black, 842, [15.9%]; Other, 824, [15.6%]; Unknown, 17, [0.3%]). Regarding histological variants, 98.3% of patients were diagnosed with non-specific hepatocellular carcinomas (NOS). For tumor size, there were only 4 samples (< 1%) with tumor size ≥ 1cm among the 5274 samples. The majority of samples had tumor size < 1cm. Additionally, most of the patients did not undergo surgical treatment (91.8%). The distant metastasis sites were primarily the lungs (2140, 40.6%), followed by the bones (1551, 29.4%); brain metastases were rare (n = 116, 2.2%).
Metastasis patterns
The analysis of different metastasis sites showed that the risk of developing metastatic disease in bones increased with age, whereas it is opposite in brain and lungs (Fig 1). Age ≥ 60 years was found to be associated with the maximum risk of metastasis at bones, and age at 50–59 and ≥ 60 had lower risk of metastasis at lung. Whereas brain metastasis was found to be the lowest and similar among the 3 age groups. Table 2 showed the proportion of metastases patterns in different age group. It could been seen that the proportion of patients with bone metastases showed an increasing trend with age (0.190, 0.290 and 0.310 in different age groups). There was no difference in the proportion of patients with brain metastasis among different age groups. Patients (< 50 years) had a relatively high proportion of lung metastases (0.510 vs. 0.40).
Fig 1. Rate of distant metastatic sites associated with age.
The line chart shows the metastasis rate of bone, brain and lung at three age groups (< 50 years, 50–59 years and ≥ 60 years). Y-axis represent metastasis rate and X-axis represent age groups.
Table 2. The proportion of metastases patterns in different age group.
| Age | Bone metastasis | Brain metastasis | Lung metastasis | |||
|---|---|---|---|---|---|---|
| Yes | Proportoin | Yes | Proportoin | Yes | Proportoin | |
| <50 | 71 | 0.19 | 11 | 0.03 | 191 | 0.51 |
| 50–59 | 472 | 0.29 | 38 | 0.02 | 650 | 0.4 |
| ≥60 | 1008 | 0.31 | 67 | 0.02 | 1299 | 0.4 |
According to the results of logistic regression, various factors were relevant to the metastatic patterns (Table 3). Patients older than 50 years are more likely to develop bone metastases (for 50–59 year: OR = 1.505, p = 0.007; for ≥ 60 years: OR = 1.669, p<0.001), while had a low risk of lung metastases (for 50–59 year: OR = 0.743, p = 0.017; for ≥ 60 years: OR = 0.716, p = 0.005). Compared with Blacks, Whites and other ethnicities had a low risk to develop bone metastases (for Whites: OR = 0.773, p = 0.003; for Other: OR = 0.629, p<0.001), and whites also had a low risk to develop lung metastases (OR = 0.730, p<0.001). Males are more likely to develop bone metastases (OR = 1.484, p<0.001) and had a low risk to develop lung metastases (OR = 0.846, p = 0.026) than females. Patients with advanced grade had increased risk to develop lung metastasis (OR for Grade II, III and V were 1.378, 1.656, 1.341, respectively; P<0.050), however, there was no statistical significance for Grade IV (P = 0.970). In terms of T stage, T1-T4 showed a relatively low risk of bone metastasis than T0 (OR<1, P<0.050). Compared with N0, N1 had a low risk of bone metastasis (OR = 0.649, P<0.001) and lung metastasis (OR = 0.651, P<0.001). The risk of bone metastases (OR = 1.868, P = 0.002) and lung metastases (OR = 2.326, P<0.001) is relatively high in those who have not undergone primary site surgery, while the risk of bone metastases (OR = 0.362, P<0.001) and brain metastases (OR = 0.374, P = 0.003) is decreased in those who have not undergone other site surgery. In addition, the patients without brain metastasis had an high risk to develop bone metastasis (OR = 3.387, P<0.001), while the patients without lung metastasis had low risk to develop bone metastasis (OR = 0.698, P = 0.010).
Table 3. Logistic regression of metastasis patterns.
| Clinicopathological Characteristics | Bone metastasis | Brain metastasis | Lung metastasis | |||
|---|---|---|---|---|---|---|
| OR | Pr(>|z|) | OR | Pr(>|z|) | OR | Pr(>|z|) | |
| Age | ||||||
| <50 | reference | reference | reference | reference | reference | reference |
| 50–59 | 1.5047 | 0.0074 | 0.7218 | 0.3578 | 0.7429 | 0.0169 |
| ≥60 | 1.6692 | 0.0005 | 0.5717 | 0.0968 | 0.7164 | 0.0048 |
| Race | ||||||
| Black | reference | reference | reference | reference | reference | reference |
| White | 0.7731 | 0.0033 | 1.0189 | 0.943 | 0.7301 | 0.0001 |
| Other | 0.629 | 0.0001 | 0.7907 | 0.5159 | 1.0738 | 0.4947 |
| Unknown | 0.8261 | 0.7416 | 2.4161 | 0.4125 | 1.2515 | 0.665 |
| Sex | ||||||
| Female | reference | reference | reference | reference | reference | reference |
| Male | 1.4835 | <0.0001 | 0.9551 | 0.8503 | 0.8455 | 0.0258 |
| Histologic types | ||||||
| NOS | reference | reference | reference | reference | reference | reference |
| Fibrolamellar | 0.6785 | 0.5512 | 0 | 0.99 | 1.1842 | 0.698 |
| Scirrhous | 0.998 | 0.9986 | 0 | 0.9954 | 0.6125 | 0.5799 |
| Spindle cell variant | 0.8412 | 0.7729 | 0 | 0.9923 | 1.0899 | 0.8669 |
| Clear cell type | 1.5719 | 0.2459 | 1.04 | 0.9698 | 1.7199 | 0.1363 |
| Grade | ||||||
| Grade I | reference | reference | reference | reference | reference | reference |
| Grade II | 0.7704 | 0.1078 | 1.0383 | 0.9221 | 1.3781 | 0.0364 |
| Grade III | 0.8254 | 0.2398 | 0.4319 | 0.0685 | 1.6559 | 0.0009 |
| Grade IV | 0.3854 | 0.0165 | 1.4404 | 0.5903 | 0.9888 | 0.9704 |
| Grade V | 1.0619 | 0.6525 | 0.5309 | 0.0579 | 1.3414 | 0.0245 |
| Laterality | ||||||
| Paired | reference | reference | reference | reference | reference | reference |
| Not Paired | NA | 0.9366 | NA | 0.9941 | 0.9939 | 0.9925 |
| T Stage | ||||||
| T0 | reference | reference | reference | reference | reference | reference |
| T1 | 0.3697 | 0.0156 | 0.4193 | 0.2576 | 0.8162 | 0.6237 |
| T2 | 0.4174 | 0.0359 | 0.3427 | 0.1778 | 0.7306 | 0.4538 |
| T3a | 0.4025 | 0.0269 | 0.2566 | 0.082 | 0.8311 | 0.6549 |
| T3b | 0.2379 | 0.0005 | 0.1732 | 0.032 | 0.6722 | 0.3399 |
| T3NOS | 0.1877 | 0.0645 | 0 | 0.9935 | 0.9609 | 0.9581 |
| T4 | 0.2081 | 0.0002 | 0.1972 | 0.0553 | 0.9693 | 0.9407 |
| TX | 0.4528 | 0.0582 | 0.3415 | 0.1807 | 0.8994 | 0.8005 |
| N stage | ||||||
| N0 | reference | reference | reference | reference | reference | reference |
| N1 | 0.6491 | <0.0001 | 0.606 | 0.0848 | 0.6509 | <0.0001 |
| NX | 1.031 | 0.7416 | 1.1396 | 0.6143 | 1.0836 | 0.3476 |
| Primary site surgery | ||||||
| Yes | reference | reference | reference | reference | reference | reference |
| No | 1.8678 | 0.0019 | 0.9655 | 0.9427 | 2.3264 | <0.0001 |
| Lymphnode surgery | ||||||
| Yes | reference | reference | reference | reference | reference | reference |
| No | 2.3642 | 0.053 | NA | 0.9871 | 1.8866 | 0.0963 |
| Other site surgery | ||||||
| Yes | reference | reference | reference | reference | reference | reference |
| No | 0.3623 | <0.0001 | 0.3738 | 0.0028 | 1.4976 | 0.0199 |
| Bone metastasis | ||||||
| Yes | reference | reference | reference | reference | ||
| No | 0.9597 | 0.8431 | 3.3995 | 0 | ||
| Unknown | 0.5857 | 0.4313 | 1.4101 | 0.0875 | ||
| Brain metastasis | ||||||
| Yes | reference | reference | reference | reference | ||
| No | 3.387 | <0.0001 | 0.8338 | 0.2071 | ||
| Unknown | 1.9211 | 0.0005 | 1.4464 | 0.7267 | ||
| Lung metastasis | ||||||
| Yes | reference | reference | reference | reference | ||
| No | 0.6977 | 0.01 | 0.795 | 0.2825 | ||
| Unknown | 0.8043 | 0.8612 | 0.6233 | 0.4911 | ||
| Tumour size | ||||||
| <1cm | reference | reference | reference | reference | reference | reference |
| ≥1cm | 1.067 | 0.4899 | NA | 0.9965 | 1.121 | 0.175 |
| Unknown | NA | 0.9325 | 1.0869 | 0.7572 | 0.1193 | 0.0213 |
Note: NOS, non-specific hepatocellular carcinomas.
Survival analysis
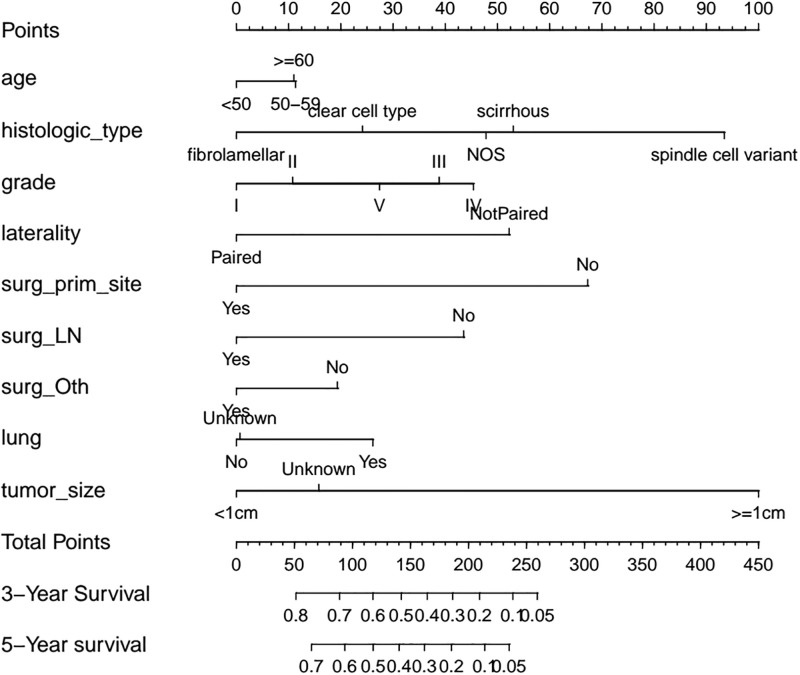
In the univariate Cox regression analysis, a variety of factors were found to be associated with OS of patients, including histologic types, grade, T stage, N stage, primary site surgery, lymph node surgery, other site surgery, bone metastasis, lung metastasis and tumor size (Table 4). For the multivariate Cox regression analysis, a variety of factors were identified as independent risk factors (p<0.05) correlated to OS, including age, histological subtype, grade, laterality, N stage, primary site surgery, lymph node surgery, other surgery, lung metastasis, and primary tumour size (Table 5). The C-index of the prediction result reached 0.617, which indicated weak consistence [15]. A nomogram was generated based on the multivariate-Cox analysis (Fig 2). The predicted value of the nomogram resembled the observed value, in which tumour size ≥ 1 cm, scirrhous and spindle cell variant subtype, and no primary site surgery demonstrated high risks, with a score >50, as shown in the nomogram. The risk scores of the patients were calculated and then divided into a high risk group and a low risk group according to the median risk score (1.032198). The low risk group had better survival outcomes than the high risk group (p<0.050, Fig 3A).
Table 4. Univariate-Cox regression analysis of the cohort survival.
| Clinical Characteristics | HR | HR.95L | HR.95H | Pvalue |
|---|---|---|---|---|
| Age | 1.026499 | 0.98239 | 1.072589 | 0.243167 |
| Race | 0.991507 | 0.944034 | 1.041367 | 0.733314 |
| Sex | 1.028881 | 0.957975 | 1.105035 | 0.434501 |
| Year of_diagnosis | 0.994404 | 0.978163 | 1.010914 | 0.504169 |
| Histologic_type | 0.91162 | 0.845244 | 0.983207 | 0.016438 |
| Grade | 1.073388 | 1.051498 | 1.095734 | 1.62E-11 |
| Laterality | 1.50432 | 0.782187 | 2.893142 | 0.221047 |
| T stage | 1.046208 | 1.033332 | 1.059244 | 8.71E-13 |
| N stage | 1.077906 | 1.039928 | 1.11727 | 4.14E-05 |
| Primary site surgery | 2.782811 | 2.350981 | 3.293961 | 1.25E-32 |
| Lymph node surgery | 2.842242 | 2.027431 | 3.98452 | 1.36E-09 |
| Other site surgery | 1.540951 | 1.33266 | 1.781797 | 5.35E-09 |
| Bone metastasis | 1.084886 | 1.029385 | 1.143379 | 0.002359 |
| Brain metastasis | 1.044228 | 0.941435 | 1.158244 | 0.413051 |
| Lung metastasis | 0.768457 | 0.730299 | 0.808609 | 3.85E-24 |
| Tumor_size | 1.125901 | 1.092126 | 1.160721 | 2.33E-14 |
Table 5. Multivariate-Cox regression analysis of the cohort survival.
| Clinicopathological Characteristics | coef | Exp(HR) | lower0.95 | upper0.95 | Pr(>|z|) |
|---|---|---|---|---|---|
| Age | |||||
| Age<50 | reference | ||||
| Age50-59 | 0.162 | 1.18 | 1.0414 | 1.3277 | 0.008925** |
| Age≥60 | 0.174 | 1.19 | 1.0593 | 1.3371 | 0.003385** |
| Race | |||||
| Black | reference | ||||
| White | -0.0312 | 0.969 | 0.8967 | 1.0478 | 0.432537 |
| Other | -0.0156 | 0.985 | 0.8904 | 1.0887 | 0.761277 |
| Unknown | -0.189 | 0.828 | 0.4871 | 1.4068 | 0.484953 |
| Sex | |||||
| Female | reference | ||||
| Male | 0.0311 | 1.03 | 0.9594 | 1.1093 | 0.400637 |
| Year of diagnosis | |||||
| 2010 | reference | ||||
| 2011 | -0.0428 | 0.958 | 0.8668 | 1.059 | 0.402142 |
| 2012 | 0.00733 | 1.01 | 0.9134 | 1.111 | 0.883298 |
| 2013 | -0.0325 | 0.968 | 0.8773 | 1.068 | 0.516716 |
| 2014 | -0.0305 | 0.97 | 0.8782 | 1.0714 | 0.548106 |
| 2015 | -0.0925 | 0.912 | 0.8248 | 1.0076 | 0.069973 |
| Histologic type | |||||
| NOS | reference | ||||
| Fibrolamellar | -0.618 | 0.539 | 0.3527 | 0.824 | 0.004318** |
| Scirrhous | 0.0522 | 1.05 | 0.4679 | 2.3724 | 0.899702 |
| Spindle cell variant | 0.566 | 1.76 | 1.1032 | 2.8135 | 0.017728* |
| Clear cell variant | -0.267 | 0.765 | 0.5393 | 1.0861 | 0.134265 |
| Grade | |||||
| I | reference | ||||
| II | 0.124 | 1.13 | 0.9813 | 1.3065 | 0.088888. |
| III | 0.477 | 1.61 | 1.3963 | 1.8586 | <0.001*** |
| IV | 0.565 | 1.76 | 1.3316 | 2.3259 | <0.001*** |
| Unknown | 0.342 | 1.41 | 1.2478 | 1.5886 | <0.001*** |
| Laterality | |||||
| Paired | reference | ||||
| Not Paired | 0.63 | 1.88 | 0.9732 | 3.6228 | 0.040269. |
| T stage | |||||
| T0 | reference | ||||
| T1 | -0.0985 | 0.906 | 0.6169 | 1.3311 | 0.615519 |
| T2 | -0.0411 | 0.96 | 0.6504 | 1.4162 | 0.835877 |
| T3a | 0.1 | 1.11 | 0.7526 | 1.6239 | 0.609127 |
| T3b | 0.144 | 1.16 | 0.7845 | 1.6996 | 0.465908 |
| T3NOS | 0.205 | 1.23 | 0.6061 | 2.4869 | 0.568891 |
| T4 | 0.102 | 1.11 | 0.7496 | 1.6372 | 0.607582 |
| TX | -0.0363 | 0.964 | 0.6525 | 1.4252 | 0.855385 |
| N stage | |||||
| N0 | reference | ||||
| N1 | 0.0993 | 1.1 | 1.0297 | 1.1844 | 0.005433** |
| NX | 0.0183 | 1.02 | 0.9372 | 1.1067 | 0.666113 |
| Primary site surgery | |||||
| Yes | reference | ||||
| No | 0.809 | 2.25 | 1.8874 | 2.6737 | <0.001*** |
| Lymph node surgery | |||||
| Yes | reference | ||||
| No | 0.588 | 1.8 | 1.2707 | 2.5505 | <0.001*** |
| Other site surgery | |||||
| Yes | reference | ||||
| No | 0.206 | 1.23 | 1.0596 | 1.426 | 0.006445** |
| Bone metastasis | |||||
| Yes | reference | ||||
| No | 0.0261 | 1.03 | 0.9615 | 1.0958 | 0.433311 |
| Unknown | -0.0283 | 0.972 | 0.7913 | 1.1942 | 0.787558 |
| Brain metastasis | |||||
| Yes | reference | ||||
| No | -0.153 | 0.859 | 0.7107 | 1.0373 | 0.113941 |
| Unknown | -0.061 | 0.941 | 0.7169 | 1.2348 | 0.660246 |
| Lung metastasis | |||||
| Yes | reference | ||||
| No | -0.33 | 0.719 | 0.6767 | 0.7643 | <0.001*** |
| Unknown | -0.367 | 0.693 | 0.5773 | 0.8319 | <0.001*** |
| Tumour size | |||||
| <1cm | reference | ||||
| ≥1cm | 1.03 | 2.79 | 1.0389 | 7.5086 | 0.041795* |
| Unknown | 0.24 | 1.27 | 1.1715 | 1.3806 | <0.001*** |
Significance codes: <0.001’***’ 0.001’**’ 0.01’*’ 0.05’.’
Concordance = 0.617 (se = 0.005)
Likelihood ratio test = 554.3 on 41 df, p = <2e-16
Wald test = 487.3 on 41 df, p = <2e-16
Score (logrank) test = 504.8 on 41 df, p = <2e-16
Fig 2. Nomogram model.
The Nomogram model was established by 9 independent prognostic factors, which could predict the 3-year and 5-year survival for patients. In which histologic type 8173 (spindle cell variant) corresponds to maximum points for risk of death, followed by no primary site surgery.
Fig 3. Kaplan-Meier curves based on different factors.
The Kaplan-Meier survival curves show the differences on survival probabilities within high and low risk (A), among three age groups (B), among five histologic types (C) and whether received primary site surgery or not (D).
Regarding the different age groups, results showed that HR increased with age. Patients aged between 50 and 59 years (HR = 1.180, 95%CI:1.041–1.328, p<0.050) and age≥60 (HR = 1.190, 95%CI:1.060–1.337, p<0.050) had a worse prognosis than patients aged <50 years. Consistently, survival analysis suggested that patients aged 50–59 years and ≥ 60 years achieved similar survival outcomes, while those aged < 50 years had better probability of a longer OS (p<0.050, Fig 3B). Additionally, compared with other metastatic sites, the absence of lung metastasis was exclusively associated with a better prognosis (HR = 0.719, 95%CI: 0.677–0.764, p<0.001), which indicated that lung metastasis result in poorer prognosis; regarding different histological types, patients diagnosed with spindle cell type had the worst prognosis (HR = 1.760, 95%CI: 1.103–2.814, p<0.050) while fibrolamellar had the best prognosis (HR = 0.539, 95%CI: 0.353–0.824, p<0.050). Also, Kaplan-Meier curve confirmed these results (Fig 3C). However, among the 5274 samples, about 98% samples were NOS types, and other types were less. Effective statistical analysis cannot be performed due to the large difference in sample size. Therefore, the results should be further confirmed. Moreover, no primary-surgery-treated patients showed the second highest HR related to survival (HR = 2.250, 95%CI, 1.887–2.673, p<0.001), which may identify surgical treatment as the second most important factor affecting OS. Primary site surgery had a prominent effect on improving survival prognosis (p<0.050, Fig 3D). The most important factor affecting survival was tumour size≥1 (HR = 2.790, 95%CI, 1.039–7.509, p<0.050). However, due to the insufficiency of cases, there is only 3 tumours with size≥1cm. Therefore, this result is controversial. It is noteworthy that patients diagnosed in 2015 exhibited a lower risk of death compared to other years, which might be explained by the insufficiency of the follow-up period. However, this outcome was not statistically significant (p = 0.07).
Other statistically significant factors related to poorer prognosis included: no other site surgery (HR = 1.230, 95%CI, 1.057–1.426, p<0.010), no lymph node surgery (HR = 1.800, 95%CI, 1.271–2.551, p<0.001), N1 stage (HR = 1.100, 95%CI, 1.030–1.184, p<0.010), no paired laterality (HR = 1.880, 95%CI, 0.973–3.623, p<0.050), advanced grade (Grade III: HR = 1.610, 95%CI, 1.396–1.859, p<0.001; Grade IV: HR = 1.760, 95%CI, 1.332–2.326, p<0.001; unknown: HR = 1.760, 95%CI, 1.248–1.589, p<0.010). The reference of the HR can be found in Table 5.
Kaplan-Meier analysis
The relationship between different metastatic sites and survival is shown in Fig 4. The unknown metastasis category included patients with intrahepatic metastasis or extrahepatic metastasis with an unknown location. The presence of metastasis at any of these sites was identified as a risk factor for death. Whereas bone metastasis exhibited better short-term prognosis than lung metastasis, within the 20 months follow-up period, but the long-term prognosis was worse. Additionally, the comparison of OS between patients from different age groups with different metastasis patterns is illustrated in Fig 5; while the comparison of OS between patients from different metastatic site groups with different ages, is shown in Fig 6. From Fig 5, lung metastasis exhibited worse prognosis within 20 months among all patients and in patients above the age of 60. In patients age 51–60, lung metastasis had the worst prognosis before approximate 40 months, overweighted by bone metastasis after 40 months. Shown by Fig 6, the prognosis of patients with lung metastasis showed significant correlation to the age. Patients younger than 50 had better prognosis than elderly patients (P < 0.05).
Fig 4. Kaplan-Meier curves among different metastasis status.
The Kaplan-Meier survival curves show the differences on survival probabilities among metastasis, non-metastasis and unknown metastasis status at bone (A), brain (B) and lung (C).
Fig 5. Kaplan-Meier curves for different age groups.
The Kaplan-Meier survival curves show the differences on survival probabilities among bone metastasis, brain metastasis, lung metastasis and unknown metastasis site for all patients (A), patients < 50 years (B), patients in 50–59 years (C) and patients ≥ 60 years (D).
Fig 6. Kaplan-Meier curves for different metastatic sites.
The Kaplan-Meier survival curves show the differences on survival probabilities among < 50 years, 50–59 years and ≥ 60 years patients with bone metastasis (A), brain metastasis (B), lung metastasis (C) and unknown metastasis (D).
What is the value of primary site surgery?
As illustrated in Fig 7, except for the T0 stage patients, a primary site surgery was performed on patients at all stages, including advanced stages. In all stages of the disease, the overall mean survival of patients with a primary site surgery (mean = 17.4, sd = 17.6) seemed to surpass that of those without a primary site surgery (mean = 4.9, sd = 8.2). This is certified by our results of cox regression (Shown in Tables 4 and 5). Table 6 showed that there was a significant decrease in the number of patients undergoing surgery for patients with T3a and T3b tumors.
Fig 7. Outcome of the primary site surgery.
In the bar chart of mean survival time (months), the bars representing patients undergone primary site surgery were on the left and non-primary site surgery were on the right.
Table 6. Chi-square test of patients undergone primary site surgery at different T stages.
| PSS | NPSS | p value | |
|---|---|---|---|
| T1 | 51 | 1020 | |
| T2 | 32 | 515 | 0.4125 |
| T3a | 29 | 1029 | 0.0194 |
| T3b | 16 | 854 | 0.0007 |
| T3NOS | 2 | 9 | 0.1771 |
| T4 | 30 | 514 | 0.593 |
| TX | 18 | 1127 | <0.0001 |
Note: PSS, Patients with primary site surgery; NPSS, Patients with no primary site surgery.
Discussion
In the present study, the correlation between metastatic patterns and age were investigated in metastatic HCC patients based on the data in SEER database. Age was identified as a risk factor for bone metastasis and death. Among the 5274 patients, 4281 were males (81.1%) and 993 were females (18.8%). It is a known fact that males are more often affected with liver cancer [16]. Multiple underlying mechanisms had been proposed to explain the gender disparity in live cancer. For example, Naugler et al. suggested that estrogen-mediated inhibition of interleukin-6 production by Kupffer cells (KCs) decreased the risk of liver cancer in women [16]. Manieri et al. indicated that the reduced levels of adiponectin was responsible for the elevated risk of liver cancer in male [17]. When analysing the different distant metastatic sites, lung metastases were associated with the poorest prognosis. We also verified that the lung was the leading site of distant metastasis. Previous studies reported that the most favourable sites for HCC extrahepatic metastasis were the lung, lymph nodes, bone, and adrenal gland, in that order [18, 19]. Although HCC is considered to have a lower metastatic rate than other solid tumours such as lung, breast, and colonic tumours, 25% of HCC patients will develop lung metastasis in 5 years [20]. According to our results, several factors were significantly correlated with an increase of both distant metastasis and mortality, including age ≥ 50 years, spindle cell variant, grade, no primary site surgery, no lymph node surgery, no other surgery, N1 stage, tumour size, and presence of lung metastases. In 2005, Natsuizaka M et al. reported that 73.8% patients with metastatic HCC, had HCC at T3 and T4 stages [21]. However, this was not observed in our present study. This difference may be explained by the fact that a more frequent use of advanced imaging methods may have helped detect liver cancer at an earlier stage.
Our results show that most cases of liver cancer with extrahepatic metastases were diagnosed in patients above the age of 50 years. Moreover, older patients were associated with a higher risk of distant metastases and an elevated mortality rate. This can be explained by immune infiltration and the formation of a unique microenvironment that is critical for tumorigenesis and metastasis in the liver [22, 23]. During the process of tumorigenesis, the accumulation of mutations in these hepatocytes, which is correlated to age, underpins the invasive behaviour of the tumour [13, 24–27]. Moreover, the liver is a highly metabolically active organ, and liver cancer is significantly related to the metabolic disruption occurring during chronic inflammation, with increased glycolysis and decreased TCA cycle activity [28–30]. Therefore, we believe that age-related mutations and age-related variations in metabolism may cause metastasis and death. Furthermore, this study illustrated that survival outcomes were correlated with the different metastatic sites independently. It has been demonstrated that cause of death in liver cancer patients is not usually attributed to extrahepatic metastatic lesions [19]. However, our study showed patients with lung metastases have a worse prognosis than other metastases patterns. This could be explained by the current insufficient methods for the management of lung metastasis. The lung metastasis group had the highest proportion of patients and was more likely to be diagnosed with other metastases. For this reason, the aforementioned outcomes suggest that physicians should be more aggressive when managing patients with lung metastasis.
The analysis revealed that patients with distant metastases who underwent surgery showed a significantly lower mortality rate. In particular, those who underwent primary site surgery showed reduced metastasis risk to bones and lungs, than those who did not. But this may also imply that the patients with bones or lungs metastases are more unlikely to choose surgical treatment. Patients at T1-2 stages have a 5-year survival rate of 50% [31]. According to the guidelines, surgery is the first-line option for early HCC, whereas it is only optimal for patients classified as very early stage, and as early stage only if certain conditions are present (e.g. well-preserved liver function, sufficient amount of parenchyma, optimal portal pressure, and acceptable for laparoscopic invasive approach) [8]. Lee et al. reported that the portal lymph node is one of the leading sites of primary liver cancer metastasis [12]. Nevertheless, there are controversies regarding whether surgery should be performed in patients at advanced stages, as a surgical procedure may stimulate regeneration, elevating the risk of recurrence and mortality. It has been reported that as many as 65% of patients experience recurrence within 5 years after surgery in patients at advanced stages [32]. These are potential hazards for relapse and postoperative death, in patients with decreased liver function. Our analysis verified the availability and importance of surgery in the treatment of liver cancer with distant metastasis in patients at all stages.
There were some limitations to this study. First, the limited data regarding co-morbidities and management provided by the SEER database, which might have introduced confounding factors affecting the prognosis of patients. Second, intrahepatic metastases and metastases to other sites were not included in the survival analysis. Third, patients with unknown metastatic patterns may have affected the results.Fourth, management and nursing should also be defined as indicators of prognosis for follow-up research. Lastly, among the 5274 samples, only 4 samples had tumor size ≥ 1cm, while 3730 samples had tumor size < 1cm. For histologic types, about 98% samples were NOS, and other types were less. Effective statistical analysis on tumor size and histologic types cannot be performed due to the large difference in sample size. Therefore, the statistical results on tumor size and histologic types were unreliable. Further effective statistical analysis analysis based on large sample size are needed to evaluate the results. the data provided by the database allowed the analysis of the metastatic patterns of primary liver cancer; however, further research is needed to obtain a better characterization of these patterns.
In conclusion, this population-based study revealed a potential correlation between metastatic patterns and clinical features such as age, histologic type, grade, and TNM stage. This study also defined that primary site surgery could significantly reduce the risk of metastasis and extend overall survival. This indicates that patients diagnosed with N1 stage, spindle cell variant, or higher-grade cancer may benefit more from surgery than what is currently believed. Moreover, a considerable number of elderly patients were not prescribed satisfactory surgery (Among the 5274 patients, the majority of patients did not receive surgery. For primary site surgery: 97%; for lymph node surgery: 99%; and for other surgery: 96%), of which those aged ≥ 60 years had the highest mortality rate. Many liver cancer patients are diagnosed at an advanced stage with distant metastasis; thus, these findings will improve physicians’ ability to manage liver cancer patients with a more personalised approach.
Data Availability
All the patients data files are available from the SEER database (accession number(s) 13003-Nov2019).
Funding Statement
This study was funded by the National Natural Science Foundation of China (700865002) which provided funds for the research project and participated in the supervision of the research. The design of the study, data collection, analysis, and interpretation, writing of the report, and the decision to submit the article for publication were not controlled by the foundation.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, et al. (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68: 394–424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Ward EM, Johnson CJ, Cronin KA, Ma J, et al. (2017) Annual Report to the Nation on the Status of Cancer, 1975–2014, Featuring Survival. J Natl Cancer Inst 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Organization WH Projections of mortality and causes of death, 2016 to 2060 (http://www.who.int/healthinfo/ global_burden_disease/projections/en/).
- 4.Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, et al. (2020) The 2019 WHO classification of tumours of the digestive system. Histopathology 76: 182–188. doi: 10.1111/his.13975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Younossi Z, Stepanova M, Ong JP, Jacobson IM, Bugianesi E, et al. (2019) Nonalcoholic Steatohepatitis Is the Fastest Growing Cause of Hepatocellular Carcinoma in Liver Transplant Candidates. Clin Gastroenterol Hepatol 17: 748–755.e743. doi: 10.1016/j.cgh.2018.05.057 [DOI] [PubMed] [Google Scholar]
- 6.Schulze K, Nault JC, Villanueva A (2016) Genetic profiling of hepatocellular carcinoma using next-generation sequencing. J Hepatol 65: 1031–1042. doi: 10.1016/j.jhep.2016.05.035 [DOI] [PubMed] [Google Scholar]
- 7.El-Serag HB, Marrero JA, Rudolph L, Reddy KR (2008) Diagnosis and treatment of hepatocellular carcinoma. Gastroenterology 134: 1752–1763. doi: 10.1053/j.gastro.2008.02.090 [DOI] [PubMed] [Google Scholar]
- 8.Liver. EAftSot (2018) EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol 69: 182–236. doi: 10.1016/j.jhep.2018.03.019 [DOI] [PubMed] [Google Scholar]
- 9.Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, et al. (2017) Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int 11: 317–370. doi: 10.1007/s12072-017-9799-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nishikawa H, Kimura T, Kita R, Osaki Y (2013) Treatment for hepatocellular carcinoma in elderly patients: a literature review. J Cancer 4: 635–643. doi: 10.7150/jca.7279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Islami F, Miller KD, Siegel RL, Fedewa SA, Ward EM, et al. (2017) Disparities in liver cancer occurrence in the United States by race/ethnicity and state. CA Cancer J Clin 67: 273–289. doi: 10.3322/caac.21402 [DOI] [PubMed] [Google Scholar]
- 12.Lee YT, Geer DA (1987) Primary liver cancer: pattern of metastasis. J Surg Oncol 36: 26–31. doi: 10.1002/jso.2930360107 [DOI] [PubMed] [Google Scholar]
- 13.Villanueva A (2019) Hepatocellular Carcinoma. N Engl J Med 380: 1450–1462. doi: 10.1056/NEJMra1713263 [DOI] [PubMed] [Google Scholar]
- 14.Wu W, He X, Andayani D, Yang L, Ye J, et al. (2017) Pattern of distant extrahepatic metastases in primary liver cancer: a SEER based study. J Cancer 8: 2312–2318. doi: 10.7150/jca.19056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harrell FE Jr., Califf RM, Pryor DB, Lee KL, Rosati RA (1982) Evaluating the yield of medical tests. Jama 247: 2543–2546. [PubMed] [Google Scholar]
- 16.Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, et al. (2007) Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science 317: 121–124. doi: 10.1126/science.1140485 [DOI] [PubMed] [Google Scholar]
- 17.Manieri E H-ML, Mora A, Tomás-Loba A, Leiva-Vega L, Fernández DI, Rodríguez E, et al. (2019) Adiponectin accounts for gender differences in hepatocellular carcinoma incidence. J Exp Med 216: 1108–1119. doi: 10.1084/jem.20181288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katyal S, Oliver JH, Peterson MS, Ferris JV, Carr BS, et al. (2000) Extrahepatic metastases of hepatocellular carcinoma. Radiology 216: 698–703. doi: 10.1148/radiology.216.3.r00se24698 [DOI] [PubMed] [Google Scholar]
- 19.Uka K, Aikata H, Takaki S, Shirakawa H, Jeong SC, et al. (2007) Clinical features and prognosis of patients with extrahepatic metastases from hepatocellular carcinoma. World J Gastroenterol 13: 414–420. doi: 10.3748/wjg.v13.i3.414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin SC, Shih SC, Kao CR, Chou SY (2003) Transcatheter arterial embolization treatment in patients with hepatocellular carcinoma and risk of pulmonary metastasis. World J Gastroenterol 9: 1208–1211. doi: 10.3748/wjg.v9.i6.1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Natsuizaka M, Omura T, Akaike T, Kuwata Y, Yamazaki K, et al. (2005) Clinical features of hepatocellular carcinoma with extrahepatic metastases. J Gastroenterol Hepatol 20: 1781–1787. doi: 10.1111/j.1440-1746.2005.03919.x [DOI] [PubMed] [Google Scholar]
- 22.Sia D, Jiao Y, Martinez-Quetglas I, Kuchuk O, Villacorta-Martin C, et al. (2017) Identification of an Immune-specific Class of Hepatocellular Carcinoma, Based on Molecular Features. Gastroenterology 153: 812–826. doi: 10.1053/j.gastro.2017.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seki E, Schwabe RF (2015) Hepatic inflammation and fibrosis: functional links and key pathways. Hepatology 61: 1066–1079. doi: 10.1002/hep.27332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zucman-Rossi J, Villanueva A, Nault JC, Llovet JM (2015) Genetic Landscape and Biomarkers of Hepatocellular Carcinoma. Gastroenterology 149: 1226–1239.e1224. doi: 10.1053/j.gastro.2015.05.061 [DOI] [PubMed] [Google Scholar]
- 25.Kaur A, Webster MR, Marchbank K, Behera R, Ndoye A, et al. (2016) sFRP2 in the aged microenvironment drives melanoma metastasis and therapy resistance. Nature 532: 250–254. doi: 10.1038/nature17392 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Bianchi-Frias D, Vakar-Lopez F, Coleman IM, Plymate SR, Reed MJ, et al. (2010) The effects of aging on the molecular and cellular composition of the prostate microenvironment. PLoS One 5. doi: 10.1371/journal.pone.0012501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferrara R, Mezquita L, Auclin E, Chaput N, Besse B (2017) Immunosenescence and immunecheckpoint inhibitors in non-small cell lung cancer patients: Does age really matter? Cancer Treat Rev 60: 60–68. doi: 10.1016/j.ctrv.2017.08.003 [DOI] [PubMed] [Google Scholar]
- 28.Ridgway WM, Gershwin ME (2014) Prometheus unbound: NKT cells inhibit hepatic regeneration. Hepatology 60: 1133–1135. doi: 10.1002/hep.27214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kettner NM, Voicu H, Finegold MJ, Coarfa C, Sreekumar A, et al. (2016) Circadian Homeostasis of Liver Metabolism Suppresses Hepatocarcinogenesis. Cancer Cell 30: 909–924. doi: 10.1016/j.ccell.2016.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang Q, Tan Y, Yin P, Ye G, Gao P, et al. (2013) Metabolic characterization of hepatocellular carcinoma using nontargeted tissue metabolomics. Cancer Res 73: 4992–5002. doi: 10.1158/0008-5472.CAN-13-0308 [DOI] [PubMed] [Google Scholar]
- 31.Ishii H, Furuse J, Kinoshita T, Konishi M, Nakagohri T, et al. (2004) Extrahepatic spread from hepatocellular carcinoma: who are candidates for aggressive anti-cancer treatment? Jpn J Clin Oncol 34: 733–739. doi: 10.1093/jjco/hyh135 [DOI] [PubMed] [Google Scholar]
- 32.Suh SW, Lee KW, Lee JM, You T, Choi Y, et al. (2014) Prediction of aggressiveness in early-stage hepatocellular carcinoma for selection of surgical resection. J Hepatol 60: 1219–1224. doi: 10.1016/j.jhep.2014.01.027 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the patients data files are available from the SEER database (accession number(s) 13003-Nov2019).