Abstract
We report an updated analysis from a phase I study of the spleen tyrosine kinase (SYK) and FMS-like tyrosine kinase 3 inhibitor mivavotinib, presenting data for the overall cohort of lymphoma patients, and the subgroup of patients with diffuse large B-cell lymphoma (DLBCL; including an expanded cohort not included in the initial report).
Patients with relapsed/refractory lymphoma for which no standard treatment was available received mivavotinib 60–120 mg once daily in 28-day cycles until disease progression/unacceptable toxicity.
A total of 124 patients with lymphoma, including 89 with DLBCL, were enrolled. Overall response rates (ORR) in response-evaluable patients were 45% (43/95) and 38% (26/69), respectively. Median duration of response was 28.1 months overall and not reached in DLBCL responders. In subgroups with DLBCL of germinal center B-cell (GCB) and non-GCB origin, ORR was 28% (11/40) and 58% (7/12), respectively. Median progression free survival was 2.0 and 1.6 months in the lymphoma and DLBCL cohorts, respectively. Grade ≥3 treatment-emergent adverse events occurred in 96% of all lymphoma patients, many of which were limited to asymptomatic laboratory abnormalities; the most common were increased amylase (29%), neutropenia (27%), and hypophosphatemia (26%).
These findings support SYK as a potential therapeutic target for the treatment of patients with B-cell lymphomas, including DLBCL.
Trial registration: ClinicalTrials.gov number: NCT02000934.
Keywords: Non-Hodgkin’s lymphoma, DLBCL, relapsed/refractory, SYK inhibitor, TAK-659
INTRODUCTION
Diffuse large B-cell lymphoma (DLBCL) is an aggressive histologic subtype of non-Hodgkin’s lymphoma and is the most common adult lymphoid malignancy in the Western world [1–4]. Although first-line R-CHOP treatment (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone) is curative for an estimated 60% of patients, outcomes are poor for those who are refractory to initial treatment or who relapse [4–9].
Multi-agent salvage chemotherapy followed by autologous stem-cell transplant (ASCT) has been the standard treatment for relapsed or refractory DLBCL [8, 10–12]. However, many patients are either ineligible for this intensive approach or do not respond adequately. More recently, several novel salvage therapeutic approaches, including cellular therapies, antibody drug conjugates, and bi-specific antibodies have been developed [13–17]. However, some of these novel therapies, particularly CD19-targeted chimeric antigen receptor (CAR) T-cell therapy, are associated with significant toxicities [14], and therefore additional novel options are needed.
Mivavotinib (TAK-659/CB-659) is an investigational, oral, reversible, potent dual inhibitor of spleen tyrosine kinase (SYK) and FMS-like tyrosine kinase 3 (FLT3) [18]. SYK is an essential component of the B-cell receptor signaling pathway; abnormal SYK signaling has been implicated in the pathogenesis of DLBCL and several other B-cell malignancies [18–22].
The safety, tolerability, and preliminary efficacy of mivavotinib were investigated in a phase I first-in-human dose escalation and expansion study conducted in patients with relapsed or refractory solid tumors or B-cell lymphomas, including DLBCL [23]. The maximum tolerated dose (MTD) was determined to be 100 mg once daily (QD) and anti-tumor activity was observed at doses of 60 mg to 120 mg QD. The most frequently occurring treatment-emergent adverse events (TEAE) were isolated aspartate aminotransferase (AST) elevations, pyrexia, and increased amylase, although abnormalities in clinical laboratory parameters were generally not associated with symptoms and were reversible upon treatment discontinuation.
Mivavotinib demonstrated anti-tumor activity in patients with relapsed or refractory B-cell lymphomas across different histological subtypes, including DLBCL [23]. The overall response rates (ORR) across all B-cell lymphoma subtypes in the intention-to-treat (ITT) population ranged from 20–50%. In patients with DLBCL (n = 53) the ORR was 23% in the ITT population (28% in the response-evaluable population), with a high proportion of those patients (19% of the response-evaluable population) achieving a complete response (CR). At the data cut-off (April 2018), the median treatment duration in patients with DLBCL was 14.3 months. The median duration of response (DOR) was not estimable (NE) due to ongoing responses in several patients. Based on these data, further evaluation was warranted, particularly in the DLBCL cohort [23].
The current analysis provides updated results for these lymphoma patients with extended follow-up. Also included are data for an additional cohort of patients with DLBCL (n = 36) who were enrolled to expand testing of mivavotinib safety and efficacy prior to the opening of a planned phase II study (NCT03123393) [24]. Data from these patients were not included in the initial analysis. Here, we report results for the complete DLBCL cohort (n = 89), including data from both the original and additional DLBCL escalation and expansion cohorts, and updated data for all lymphoma patients in this first-in-human study.
RESULTS
Patients
A total of 124 patients were enrolled; 17 patients were enrolled in the dose escalation phase and received mivavotinib at the following doses: 60 mg QD (n = 4), 80 mg QD (n = 3), 100 mg QD (n = 11), and 120 mg QD (n = 1); 107 patients were enrolled in the expansion phase and received 100 mg QD. Overall, 89 patients had DLBCL (72%), 23 had indolent non-Hodgkins lymphoma (iNHL) (19%), 6 had chronic lymphocytic leukemia (CLL) (5%), 5 had mantle cell lymphoma (MCL) (4%), and 1 patient had Epstein Barr virus-positive post-transplant lymphoproliferative disorder (EBV+PTLD) (1%). The iNHL subgroup included 16 patients with follicular lymphoma, 2 patients with mucosa-associated lymphoid tissue lymphoma, 2 patients with nodal marginal zone B-cell lymphoma, 1 patient with B-cell lymphoplasmacytic lymphoma/immunocytoma, 1 patient with B-cell small lymphocytic lymphoma and 1 patient with splenic marginal zone lymphoma.
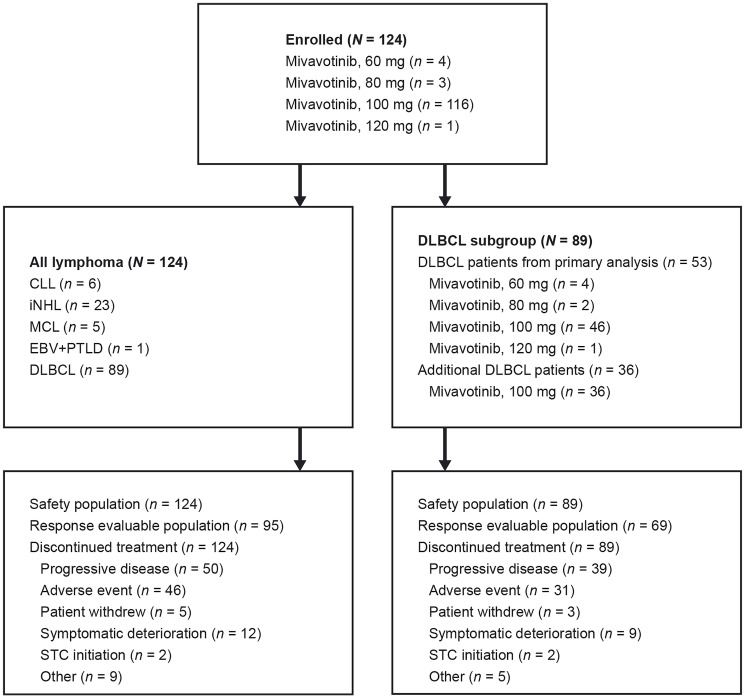
Of the patients with DLBCL, 12 were enrolled during dose escalation, 41 were enrolled to the first expansion cohort, and 36 were enrolled to the second expansion cohort. By data cut-off, treatment had been discontinued in all 124 patients. Patient disposition is summarized in Figure 1.
Figure 1. Patient disposition flow diagram.
Patient baseline demographics and disease characteristics for all lymphoma patients and all DLBCL patients are shown in Table 1. The median age, both in all patients with lymphoma and within the DLBCL subgroup, was 66 years (range 23–91). The median time since diagnosis was 21.8 months (range 0.3–269.3) for all patients with lymphoma and 16.4 months (range 0.3–269.3) for patients with DLBCL. Eighty-one patients with lymphoma (65%), including 60 patients with DLBCL (67%), were Ann Arbor stage III–IV at diagnosis, with 21% and 16%, respectively, having evidence of bone marrow involvement at study entry. Disease was classified as germinal center B-cell (GCB) in 47 patients, non-GCB in 18 patients, and unknown origin in 24 patients. The median number of prior lines of therapy was 3 (range 1–9) in all patients with lymphoma and within the DLBCL subgroup, with 17% and 15%, respectively, having previously undergone ASCT. Disease characteristics for DLBCL patients by dose escalation and expansion cohort are shown in Supplementary Table 1. The baseline characteristics were generally similar between the two DLBCL expansion cohorts, with the exception of median time since diagnosis, which was slightly shorter in the second expansion cohort.
Table 1. Baseline demographic and disease characteristics.
| All DLBCL n = 89 | All lymphomasa,b N = 124 | |
|---|---|---|
| Age (years), median (range) | 66 (23–91) | 66 (23–91) |
| Gender (%), male/female | 62/38 | 61/39 |
| Race (%), white/other | 97/2c | 96/3c |
| Disease characteristics | ||
| Time since diagnosis (months), median (range) | 16.4 (0.3–269.3) | 21.8 (0.3–269.3) |
| Tumor node metastases/Ann Arbor stage at diagnosis, n (%) | 60 (67) | 81 (65) |
| I | 3 (3) | 4 (3) |
| II | 11 (12) | 13 (10) |
| III | 19 (21) | 22 (18) |
| IV | 41 (46) | 59 (48) |
| Bone marrow involvement at entry, n (%) | 14 (16) | 26 (21) |
| Molecular/genetic classification (DLBCL only), n (%) | ||
| GCB/non-GCB | 47 (72d)/18 (28d) | |
| Double/triple hit | 11 (16e) | |
| Treatment history | ||
| Prior lines of therapy, median (range) | 3 (1–9) | 3 (1–9) |
| Prior autologous transplant, n (%) | 13 (15) | 21 (17) |
aDLBCL (n = 89), iNHL (n = 23), CLL (n = 6), MCL (n = 5), EBV+PTLD (n = 1); bIncludes 36 additional patients with DLBCL and 2 additional patients with iNHL in addition to those included in the previously published analysis; cRace not reported for 1 patient; dPercentage of n = 65 patients with known molecular classification (n = 24 patients had unknown classification); ePercentage of n = 67 patients in whom genetic classification was assessed.
Efficacy
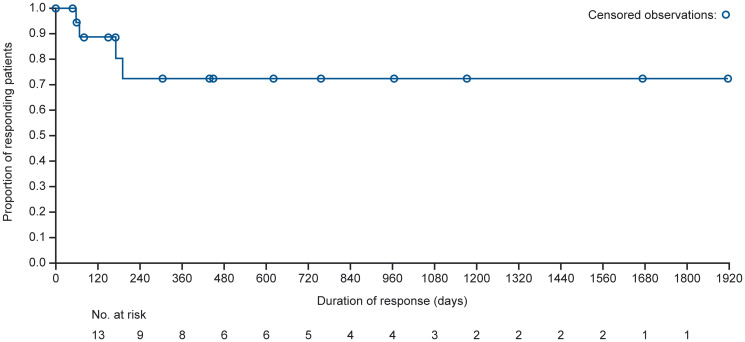
Best overall responses to mivavotinib are shown in Table 2. A total of 95 patients with lymphoma (77%) were evaluable for response; 19 patients (20%) achieved a CR and 24 (25%) achieved a partial response (PR), resulting in an ORR of 45% (95% confidence interval [CI], 35.0–55.8); 40 (42%) patients had a best response of progressive disease (PD). Median time to response among all lymphoma patients was 1.8 months. The median DOR in patients with lymphoma achieving CR or PR was 28.1 months (95% CI, 5.8–NE). Within the DLBCL subgroup, 69 patients (78%) were response-evaluable; of these, 14 patients (20%) achieved CR and 12 (17%) achieved PR, resulting in an ORR of 38% (95% CI, 26.3–50.2). Thirty-five (51%) patients with DLBCL had a best response of PD. In responding patients with DLBCL (n = 26), the median DOR was NE (95% CI, 6.3 months – NE) (Figure 2), 8 patients (31%) had responses lasting >12 months and 5 patients (19%) had responses lasting >24 months. Among DLBCL patients with PR (n = 12), the median DOR was 5.7 months (95% CI, 1.9–NE); among patients with CR, no patients had a PD/relapse event, and data were censored with a range of less than one month (1 day) to 63.0 months with responses ongoing for all 14 patients at the time of data cut.
Table 2. Best overall response.
| Response, n (%) | DLBCL
escalation |
DLBCL
cohort 1 |
DLBCL
cohort 2 |
DLBCL
cohorts (combined) |
All
lymphomas |
|---|---|---|---|---|---|
| ITT population, n | 12 | 41 | 36 | 89 | 124 |
| ORR (CR + PR) | 4 (33) | 8 (20) | 14 (39) | 26 (29) | 43 (35) |
| Response-evaluable population, n | 11 | 33 | 25 | 69 | 95 |
| ORR (CR + PR) | 4 (36) | 8 (24) | 14 (56) | 26 (38) | 43 (45) |
| 95% CI | 10.9–69.2 | 11.1–42.3 | 34.9–75.6 | 26.3–50.2 | 35.0–55.8 |
| Clinical benefit (CR + PR + SD) | 6 (55) | 14 (42) | 14 (56) | 34 (49) | 55 (58) |
| CR | 3 (27) | 6 (18) | 5 (20) | 14 (20) | 19 (20) |
| PR | 1 (9) | 2 (6) | 9 (36) | 12 (17) | 24 (25) |
| SD | 2 (18) | 6 (18) | 0 | 8 (12) | 12 (13) |
| PD | 5 (45) | 19 (58) | 11 (44) | 35 (51) | 40 (42) |
Percentages are based on the total number of patients in the response-evaluable population in each column. 2-sided 95% exact binomial CIs were used. Abbreviation: SD: stable disease.
Figure 2. Kaplan–Meier curve for estimated median DOR in the DLBCL combined cohort (response-evaluable population).
DOR was defined as the time from the date of first documentation of PR or better to the date of first documentation of PD or relapse. Among patients with CR, no patients had a PD/relapse event, and data were censored with a range of less than one month (1 day) to 63.0 months with responses ongoing for all 14 patients at the time of data cut. Among patients with PR, 4 patients had a PD/relapse event, and data were censored for the remaining 8 patients.
Within the subgroup of patients with DLBCL (excluding those enrolled in dose escalation), 33 patients in the first expansion cohort were evaluable for response and 25 in the second cohort were evaluable for response. The ORR was 24% (95% CI, 11.1–42.3) in the first DLBCL cohort (18% with CR and 6% with PR) and 56% (95% CI, 34.9–75.6) in the second DLBCL cohort (20% with CR and 36% with PR). Considering all response-evaluable patients with DLBCL enrolled in the study (n = 69), 40 patients had GCB type DLBCL with an ORR of 28% (including 23% with CR), and 12 patients had non-GCB type DLBCL with an ORR of 58% (8% with CR); 17 patients had unknown GCB type (ORR 47%; CR rate 24%) (Supplementary Table 2). Responses were maintained at data cut-off/last follow up in all responding patients with GCB type DLBCL (n = 11) and in 6/7 responding patients with non-GCB DLBCL; DOR was therefore NE. In the subgroup with unknown cell of origin, median DOR was 6.3 months (95% CI, 1.9–NE). The ORR among DLBCL patients with 1 vs. >1 prior lines of therapy was 50% vs. 35%, while the ORR among DLBCL patients with prior transplant vs. without prior transplant was 33% vs. 39%.
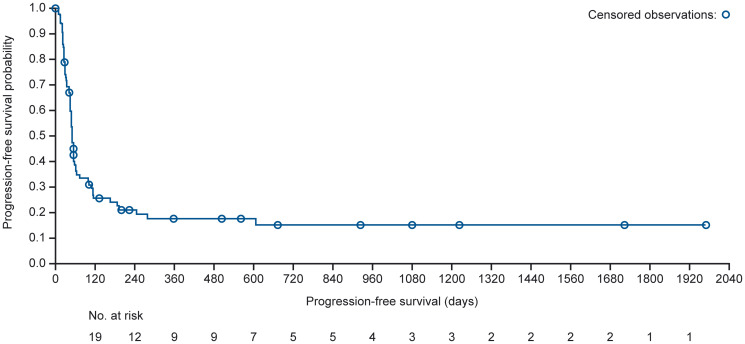
The median progression-free survival (PFS) in all patients with lymphoma was 2.0 months (95% CI, 1.6–3.3); 86 patients (69%) experienced PFS events, of which 53 (62%) were due to progression and 33 (38%) were due to death. In patients with DLBCL, the median PFS was 1.6 months (95% CI, 1.5–1.9), and 67 patients (75%) experienced PFS events; 41 (61%) were due to disease progression and 26 (39%) were due to death (Figure 3). Median time to progression (TTP) was 3.7 months (95% CI, 1.9–7.7) in the overall lymphoma group and 1.8 months (95% CI, 1.6–3.7) in the DLBCL subgroup.
Figure 3. Kaplan–Meier curve for estimated median PFS in the DLBCL combined cohort (safety population).
PFS is defined as the time from the date of the first study treatment administration to the date of the first documentation of progressive disease or death.
The median overall survival (OS) was 8.3 months (95% CI, 3.7–NE) in patients with lymphoma and 3.9 months (95% CI, 2.1–NE) in patients with DLBCL. Overall, 52 patients with lymphoma (42%), including 42 patients in the DLBCL cohorts (47%), died within the time period between the first dose and the last follow-up.
Within the DLBCL response-evaluable population (n = 69), median PFS was 19.9 months (95% CI, 6.3–NE) in responding patients (n = 26 patients with CR or PR), compared with 1.5 months (95% CI, 1.0–1.6) in non-responders (n = 43). Median OS was not reached in responders (95% CI, 8.3–NE) and 3.3 months (95% CI, 2.1–3.9) in non-responders. Considering patient subgroups based on cell of origin, median PFS was similar in patients with GCB (1.6 months [95% CI, 1.3–2.1]), non-GCB (1.7 months [1.1–3.7]) and unknown origin (1.6 months [0.9–3.5]) DLBCL (Supplementary Figure 1A). However, there was a higher proportion of PD events in GCB patients, while there were more deaths in non-GCB patients, and OS differed accordingly, with median OS 19.9 months (95% CI, 2.4–NE) and 2.1 months (95% CI, 1.5–NE), respectively. Median OS in the unknown origin subgroup was 3.7 months (95% CI, 1.2–NE) (Supplementary Figure 1B).
Safety
All patients received at least one dose of mivavotinib and were evaluable for safety. Patients received a median of 2 treatment cycles (range 1–68), and the median treatment duration was 6.8 weeks for all patients with lymphoma and 6.0 weeks for patients with DLBCL. Safety is summarized in Tables 3–5 for the total patients with lymphoma and for the subgroup of patients with DLBCL. Safety was generally consistent between the overall lymphoma population and the DLBCL subgroup and so is only described for patients with lymphoma.
Table 3. Overview of TEAEs (safety population).
| Adverse event n, (%) | DLBCL cohorts (combined) n = 89 | All lymphomas N = 124 |
|---|---|---|
| Any TEAEs | 89 (100) | 124 (100) |
| Related | 82 (92) | 114 (92) |
| Not related | 88 (99) | 122 (98) |
| Grade 1 | 85 (96) | 117 (94) |
| Grade 2 | 83 (93) | 112 (90) |
| Grade ≥3 | 85 (96) | 119 (96) |
| Grade ≥3 related | 65 (73) | 94 (76) |
| Leading to discontinuation | 32 (36) | 48 (39) |
| Serious TEAEs | 65 (73) | 94 (76) |
| Related | 15 (17) | 33 (27) |
| Not related | 58 (65) | 79 (64) |
| Leading to discontinuation | 20 (22) | 32 (26) |
| Deaths | 32 (36) | 39 (31) |
| Related | 2 | 4 |
TEAEs are defined as any adverse event that occurs after administration of the first dose of study treatment through 28 days after last dose of study treatment, or until the start of subsequent antineoplastic therapy. Deaths occurred within 28 days of last treatment dose. Percentages are based on the number of patients in each treatment group for the designated population of this table.
Table 5. Most common grade ≥3 TEAEs occurring in ≥10% of patients by preferred term (safety population).
| Preferred term, n (%) | DLBCL cohorts (combined) n = 89 | All lymphomas N = 124 |
|---|---|---|
| Patients with grade ≥3 TEAE | 85 (96) | 119 (96) |
| Increased amylase | 28 (31) | 36 (29) |
| Neutropenia | 26 (29) | 34 (27) |
| Hypophosphatemia | 19 (21) | 32 (26) |
| Anemia | 15 (17) | 23 (19) |
| Blood CPK increased | 15 (17) | 22 (18) |
| Lipase increased | 15 (17) | 22 (18) |
| Thrombocytopenia | 13 (15) | 19 (15) |
| Pneumonia | 7 (8) | 14 (11) |
| Pyrexia | 8 (9) | 13 (10) |
| AST increased | 11 (12) | 12 (10) |
TEAEs are defined as any adverse event that occurs after administration of the first dose of study treatment through 28 days after the last dose of study treatment, or until the start of subsequent antineoplastic therapy.
All patients with lymphoma experienced at least one TEAE; 96% experienced a grade ≥3 TEAE, and 76% experienced a grade ≥3 TEAE considered by the investigator to be related to mivavotinib (Table 3). The most common TEAEs occurring in ≥10% of all patients were increased AST (60%), pyrexia (56%), and increased amylase (46%) (Table 4). The most frequent grade ≥3 TEAEs occurring in ≥10% of all patients were increased amylase (29%), neutropenia (27%), and hypophosphatemia (26%) (Table 5). Granulocyte colony stimulating factor (G-CSF) was administered to 28 patients for the management of grade ≥3 neutropenia. Overall, 39% of patients with lymphoma experienced TEAEs resulting in mivavotinib discontinuation; the most common TEAEs leading to discontinuation were pneumonia (n = 6), pneumonitis (n = 3), respiratory failure (n = 4), and neutropenia (n = 4). Serious TEAEs were experienced by 76% of patients; 27% of patients had serious TEAEs which were related to mivavotinib, and 26% had serious TEAEs which resulted in discontinuation. Overall, 39 patients with lymphoma died on study; 4 deaths were considered related to mivavotinib and were due to complications from pneumocystis pneumonia, multiorgan failure, respiratory failure and disseminated varicella (n = 1 each).
Table 4. Most frequent TEAEs occurring in ≥10% of patients by preferred term (safety population).
| Preferred term (n [%]) | DLBCL cohorts (combined) n = 89 | All lymphomas N = 124 |
|---|---|---|
| Any TEAE | 89 (100) | 122 (98) |
| AST increased | 56 (63) | 75 (60) |
| Pyrexia | 44 (49) | 69 (56) |
| Amylase increased | 43 (48) | 57 (46) |
| Hypophosphatemia | 34 (38) | 51 (41) |
| Anemia | 35 (39) | 50 (40) |
| Blood CPK increased | 37 (42) | 50 (40) |
| Diarrhea | 33 (37) | 48 (39) |
| Lipase increased | 34 (38) | 45 (36) |
| Fatigue | 30 (34) | 43 (35) |
| ALT increased | 32 (36) | 39 (31) |
| Neutropenia | 29 (33) | 39 (31) |
| Nausea | 25 (28) | 37 (30) |
| Cough | 22 (25) | 34 (27) |
| Thrombocytopenia | 24 (27) | 33 (27) |
| Asthenia | 22 (25) | 32 (26) |
| Decreased appetite | 20 (22) | 28 (23) |
| Periorbital edema | 17 (19) | 27 (22) |
| Blood alkaline phosphate increased | 19 (21) | 26 (21) |
| Constipation | 19 (21) | 26 (21) |
| Vomiting | 17 (19) | 26 (21) |
| Pneumonia | 14 (16) | 25 (20) |
| Edema peripheral | 17 (19) | 24 (19) |
| Hypokalemia | 14 (16) | 23 (19) |
| Abdominal pain | 18 (20) | 22 (18) |
| Headache | 11 (12) | 22 (18) |
| Blood creatine increased | 16 (18) | 20 (16) |
| Dyspnea | 14 (16) | 19 (15) |
| Cytomegalovirus infection reactivation | 14 (16) | 18 (15) |
| Urinary tract infection | 10 (11) | 17 (14) |
| Blood lactate dehydrogenase increased | 12 (13) | 16 (13) |
| Chills | 8 (9) | 16 (13) |
| Gamma-glutamyltransferase increased | 10 (11) | 16 (13) |
| Hypertension | 12 (13) | 16 (13) |
| Stomatitis | 10 (11) | 16 (13) |
| Back pain | 10 (11) | 15 (12) |
| Night sweats | 9 (10) | 14 (11) |
| Oral candidiasis | 6 (7) | 14 (11) |
| Hyponatremia | 8 (9) | 13 (10) |
| Rash maculo-papular | 10 (11) | 13 (10) |
TEAEs are defined as any adverse event that occurs after administration of the first dose of study treatment through 28 days after the last dose of study treatment, or until the start of subsequent antineoplastic therapy. Abbreviations: ALT: alanine aminotransferase; CPK: creatine phosphokinase.
DISCUSSION
Primary data from this phase I, first-in-human study investigating the safety, tolerability, and preliminary efficacy of SYK/FLT3 inhibitor mivavotinib, conducted in patients with advanced solid tumors or lymphoma malignancies, were previously reported [23]. Here we provide updated results for the total cohort of patients with lymphoma (N = 124) and present findings for an expanded subgroup of patients with DLBCL (n = 89). In this updated analysis, mivavotinib demonstrated encouraging efficacy in patients with lymphoma and in the subgroup of patients with DLBCL, with ORRs of 45% and 38%, respectively, and a CR rate of 20% in both groups. The safety profile was consistent with the previous report, with no new signals identified.
The ORR and CR rates reported here for all patients with lymphoma including those with DLBCL are clinically meaningful, given the limited treatment options and poor outcomes for these patients with relapsed or refractory disease [1–4]. In addition, some of the patients were heavily pretreated with a median of 3 prior lines of therapy. Responses were also slightly improved in the second DLBCL expansion cohort (56%) vs. the first expansion cohort (24%), which might be due to differences in eligibility criteria (see Methods section) between the two cohorts. There was also a higher proportion of patients with non-GCB lymphoma in the second cohort (36% vs. 21%; Supplementary Table 1). However, baseline disease characteristics were generally consistent between the two cohorts, except for a shorter median time since diagnosis in the second. Similarly, the ORR was higher in patients with only 1 prior line of therapy (50%) vs. patients with >1 prior line of therapy (35%). Further research would be required to determine whether this reflects a higher benefit of mivavotinib therapy earlier in the treatment paradigm.
Meaningful response rates were observed in both GCB and non-GCB DLBCL subtypes, consistent with the findings of our initial report [23]. There are known biological differences between these two DLBCL subtypes, as activated B-cell-like DLBCL (which comprises the majority of non-GCB) is known to be more dependent on B-cell receptor (BCR) pathway signaling involving SYK than GCB DLBCL [2, 4, 25, 26]. Although the patient numbers were small (n = 12 patients with non-GCB subtype), there was a higher response rate observed in patients with non-GCB DLBCL (58% vs. 28%), which might have been a result of these biological differences; however, the majority were partial responses which did not translate into longer OS. In contrast, patients with GCB subtype had a higher rate of CR (23% vs. 8%) and longer OS (19.9 vs. 2.1 months), which might have been expected since some studies have reported a more aggressive course with non-GCB DLBCL subtypes resulting in worse outcomes for patients [27]. Given the differences in eligibility criteria and the known heterogeneity of DLBCL pathogenesis and presentation, further genomic analyses of non-GCB and GCB DLBCL subtypes to characterize markers for response to targeted treatment could enable the identification of patient populations and DLBCL subtype(s) which may derive therapeutic benefit from a given treatment. For example, mutations in genes for key regulators of the B-cell and/or Toll-like receptor pathways such as MYD88 or CD79B [28] could suggest potential for modification by treatments targeting these pathways, including SYK/FLT3 inhibitors such as mivavotinib.
Although population-level outcomes data are limited for patients with relapsed or refractory DLBCL, in the large retrospective SCHOLAR study of 636 patients, estimated objective response rates were 26% with a CR rate of 7% and a median OS of 6.3 months [29]. The response rates reported in the present study (38% ORR, including 20% CR) are improved compared with those estimated in this retrospective study, despite the median OS of 3.9 months being lower than what might be expected in this patient population. In addition, agents which recently received US Food and Drug Administration accelerated approval for treatment of DLBCL based on encouraging response rates such as selinexor [30], polatuzumab vedotin (in several combinations), tafasitamab (in combination with lenalidomide), and CAR T-cell therapy such as axicabtagene ciloleucel and tisagenlecleucel [14, 31], have achieved improved, or similar response rates than those reported with mivavotinib. However, a subsequent phase II study of mivavotinib monotherapy, which enrolled 49 patients with relapsed or refractory DLBCL (with similar eligibility criteria to the second expansion cohort) was terminated due to lack of efficacy [24]. Given the smaller number of patients in the study and perhaps because not all patients started dosing at 100 mg QD, with 25 patients starting treatments at 60 mg to evaluate a ramp up dosing schedule, it is possible that such differences contributed to the lack of efficacy observed. Altogether, these data suggest that a biomarker selection strategy, further dose refinement, and combinations of mivavotinib with other agents in DLBCL warrant further exploration. An ongoing phase I study is investigating the safety and efficacy of mivavotinib with R-CHOP as first-line treatment for patients with high-risk DLBCL [32], while a phase II study of mivavotinib in patients with relapsed or refractory non-GCB DLBCL is investigating efficacy in subgroups defined according to genetic biomarkers [33].
CAR T-cell therapy is expected to become the new standard of care for relapsed or refractory DLBCL [34, 35]. Several CD19-targeted CAR T-cell therapy agents have been approved for treatment, including axicabtagene ciloleucel [14], lisocabtagene maroleucel [36], and tisagenlecleucel [37, 38]. Considering these recent developments, there may be a role for mivavotinib combinations in the treatment pathway of patients with relapsed or refractory DLBCL, potentially as a bridge to CAR T-cell therapy or as an option post-CAR T-cell therapy.
Overall, safety findings were consistent with the primary analysis and toxicity was manageable despite a high proportion of patients experiencing grade ≥3 TEAEs (96% all cause, and 76% determined by the investigator as being related to mivavotinib). The most common grade ≥3 TEAEs were increased amylase, neutropenia and hypophosphatemia; other common grade ≥3 TEAEs included elevations in clinical laboratory investigations, including AST, amylase, lipase and blood creatine phosphokinase, which were largely asymptomatic and reversible upon dose reduction or discontinuation of the study drug, consistent with the initial analysis. The most common hematologic grade ≥3 TEAE was neutropenia (27%), with most of these patients receiving G-CSF support to manage it; this was largely expected due to the number of patients with bone marrow involvement at study entry. Other hematologic grade ≥3 TEAEs including anemia and thrombocytopenia occurred in 19% and 15% of patients, respectively. The most common metabolic disorder of grade ≥3 was hypophosphatemia (26%). Serious TEAEs were reported in 76% of patients; however, only 27% of patients had serious TEAEs determined by the investigator as being related to mivavotinib, and only 26% of patients had serious TEAEs that led to discontinuation. There were 39 on-study deaths among patients with lymphoma, 4 of which were considered related to mivavotinib and were due to complications from pneumocystis pneumonia, multiorgan failure, respiratory failure and disseminated varicella. The most common reasons for discontinuation were pneumonia and pneumonitis; further intervention to reduce the risk of opportunistic infection (e.g., Pneumocystis jirovecii pneumonia) or viral reactivation could further improve the safety profile of mivavotinib in future studies.
In conclusion, the anti-tumor efficacy of mivavotinib monotherapy observed in the primary analysis of this study was confirmed in our analysis of patients with lymphoma, including an expanded cohort of patients with relapsed or refractory DLBCL, with responses that were deep and durable. These findings support SYK as a potential therapeutic target for the treatment of this population of patients. Further investigation of markers to predict response to SYK inhibition, and research into possible mivavotinib treatment combinations, are needed to develop mivavotinib further, and to provide more extensive therapy options for patients with relapsed or refractory DLBCL who have limited treatment options.
MATERIALS AND METHODS
Study design
This was an open-label, multicenter, phase I, dose escalation and expansion study of QD, oral, single-agent mivavotinib in patients with advanced solid tumors or lymphoid malignancies. The full study design and methods have previously been reported [23]. Here we focus on aspects pertinent to patients with lymphoma, and particularly those with DLBCL.
Briefly, in the dose escalation phase, adults with a confirmed diagnosis of lymphoma for which no standard treatment was available were enrolled to receive escalating doses of oral mivavotinib (60 mg, 80 mg, 100 mg or 120 mg QD) in an accelerated 3+3 dose escalation design. In the expansion phase, patients with lymphoid malignancies received mivavotinib 100 mg QD (the MTD from the dose escalation phase) in one of six disease-specific cohorts: CLL, iNHL, MCL, EBV+PTLD and two separate DLBCL expansion cohorts. All patients in the escalation and expansion phases received oral mivavotinib at their assigned dosage in continuous 28-day cycles until disease progression or unacceptable toxicity.
Patients
Lymphoma patients enrolled in both the escalation and expansion phases had histologically or cytologically confirmed lymphoma, according to the modified International Working Group (IWG) 2007 criteria for malignant lymphoma [39], or the International Workshop on Chronic Lymphocytic Leukemia 2008 criteria. Patients in the expansion cohorts also had at least one site of measurable or evaluable disease confirmed by computed tomography. Additional eligibility criteria included an Eastern Cooperative Oncology Group performance status of 0–1, adequate organ function, a life expectancy longer than 3 months, and recovery from reversible effects of prior anti-cancer therapy.
Patients enrolled in the first DLBCL expansion cohort had pathologically confirmed DLBCL with at least one site of measurable disease based on IWG criteria for malignant lymphoma [39], had relapsed or refractory disease after at least one line of therapy, and were ineligible for or had progressed after receiving high-dose chemotherapy/ASCT. Patients enrolled in the second DLBCL expansion cohort had histologically-confirmed DLBCL, including de novo DLBCL or transformed disease from iNHL, and had relapsed or refractory disease after ≥2 lines of chemotherapy (based on a standard of care which included rituximab plus anthracycline [or equivalent if contraindicated]) and an additional systemic chemotherapy as second-line salvage therapy (that may have included ASCT), but had not failed >4 prior lines of therapy. Patients enrolled in the second DLBCL expansion cohort could also have been previously treated with BCR in-pathway inhibitors not directly targeting SYK. For patients in both DLBCL cohorts, DLBCL cell of origin was determined by immunohistochemistry when available (local laboratory) and was classified as GCB or non-GCB [40]. Cytogenetic profiling was performed using fluorescence in-situ hybridization where available, although this was not mandated, and patients with multiple gene rearrangements (MYC and BCL2 and/or BCL6) were identified as having double- or triple-hit lymphoma.
Assessments
Efficacy endpoints including ORR, DOR, PFS, TTP and OS were analyzed for all patients with lymphoma, including the additional DLBCL expansion cohort, and for the full DLBCL subgroup (both expansion cohorts and the escalation cohort combined), based on data collected up to June 29, 2021. This report includes extended follow-up data for patients included in the initial analysis, as well as data for additional DLBCL patients. DOR was also analyzed among the separate DLBCL cohorts, and assessments of ORR were made in the GCB, non-GCB, or unknown GCB classified DLBCL subgroups.
Responses were assessed in patients who received at least one dose of study drug and had at least one post-baseline disease assessment (response-evaluable population). Assessments were performed at cycles 2, 4, 6, then every 3 cycles through cycle 24, and thereafter every 6 cycles (until disease progression or the start of alternative therapies). PFS, TTP and OS were evaluated in the ITT population, based on the time from the date of first study drug administration to the date of first documentation of PD (PFS/TTP) or death (PFS/OS).
The safety population was defined as all patients in any lymphoma cohort receiving at least one dose of study drug. Adverse events and toxicity were assessed continually during treatment and were graded in accordance with the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03.
Statistical analyses were primarily descriptive without formal hypothesis testing. Median DOR, PFS and OS were estimated using the Kaplan–Meier method.
Data sharing statement
Requests for de-identified datasets for the results reported in this publication will be made available to qualified researchers following submission of a methodologically sound proposal. Data will be made available for such requests following online publication of this article and for 1 year thereafter in compliance with applicable privacy laws, data protection, and requirements for consent and anonymization. Calithera does not share identified participant data or a data dictionary.
SUPPLEMENTARY MATERIALS
ACKNOWLEDGMENTS
This study was sponsored by Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited. We thank all of the patients and their families, and the investigators and staff at all clinical sites, for their participation in the trial. The authors acknowledge Tori Gordon, BSc, of Ashfield MedComms, an Inizio Company, for medical writing support for the development of this manuscript under the direction of the authors, which was funded by Takeda Pharmaceuticals U.S.A., Inc., and complied with the Good Publication Practice 3 ethical guidelines (Battisti WP, et al. Ann Intern Med 2015. 163: 461–4). R. Popat and W. Townsend acknowledge support from the National Institute for Health Research University College London Hospitals Biomedical Research Centre.
Abbreviations
- ALT
alanine aminotransferase
- ASCT
autologous stem-cell transplant
- AST
aspartate aminotransferase
- BCR
B-cell receptor
- CAR
CD19-targeted chimeric antigen receptor
- CI
confidence interval
- CLL
chronic lymphocytic leukemia
- CPK
creatine phosphokinase
- CR
complete response
- DLBCL
diffuse large B-cell lymphoma
- DOR
duration of response
- EBV+PTLD
Epstein Barr Virus-positive post-transplant lymphoproliferative disorder
- FLT3
FMS-like tyrosine kinase 3
- GCB
germinal center B-cell
- G-CSF
granulocyte colony stimulating factor
- iNHL
indolent non-Hodgkin’s lymphoma
- ITT
intention-to-treat
- IWG
International Working Group
- MCL
mantle cell lymphoma
- MTD
maximum tolerated dose
- NE
not estimable
- ORR
overall response rate
- OS
overall survival
- PD
progressive disease
- PFS
progression-free survival
- PR
partial response
- QD
once daily
- R-CHOP
rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone
- SD
stable disease
- SYK
spleen tyrosine kinase
- TEAE
treatment-emergent adverse events
- TTP
time to progression
Author contributions
LIG, SM, IP, YS and FB were involved in the conception and design of the study. LIG, RK, JBK, RP, HAB, SF, MRP, GG, DES, FIC, JR, JPO, PLZ, SPI, WT, HM, IP, SW, SK, YY, JZ, KS, YS, CC, and FB made substantial contributions to the acquisition, analysis and interpretation of data. All authors drafted the work or revised it critically for important intellectual content. All authors approved of the final version to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
CONFLICTS OF INTEREST
LIG is on the Data Safety and Monitoring Board (DSMB) for Janssen and is a consultant for Ono Pharmaceuticals. RK received research support from Takeda. RP received honoraria and consultancy fees from Takeda, Janssen, BMS, GSK and Roche. HAB is employed by Sarah Cannon and HCA Healthcare; owns stocks/shares at HCA Healthcare; holds a non-compensated consulting position for AstraZeneca, Bayer, Bristol-Myers Squibb, Boehringer Ingelheim, GRAIL, Incyte, Novartis, TG Therapeutics, Vincerx Pharma; and reports grants or funds paid to their institution from AbbVie, Agios, ARMO Biosciences, Array BioPharma, AstraZeneca, Bayer, BeiGene, BioAtla, BioMed Valley Discoveries, Boehringer Ingelheim, Bristol-Myers Squibb, CALGB, CicloMed, eFFECTOR Therapeutics, Lilly, EMD Serono, Roche/Genentech, GlaxoSmithKline, Harpoon Therapeutics, Hengrui Therapeutics, Incyte, Janssen, Jounce Therapeutics, Kymab, Macrogenics, MedImmune, Merck, Millennium, Moderna, Pfizer, Revolution Medicine, Foundation Medicine, SeaGen, Tesaro, TG Therapeutics, Verastem, Vertex Pharmaceuticals, Xbiotech, Zymeworks, Arch Oncology, Arvinas, Coordination Pharmaceuticals, NGM Biopharmaceuticals, Gossamer Bio, Ryvu Therapeutics, and BioTheryX. SM received honoraria from Karyopharm, GSK, BMS, Janssen and Oncopeptides. MRP reports leadership at ION Pharma; honoraria from Pfizer, Pharmacyclics, Bayer, Janssen, Genentech, and Adaptive Biotechnologies; consulting/advisory role at Pharmacyclics, Janssen, Pfizer, and EMD Serono; and speakers’ bureaus at Exelixis, Genentech, Roche, Taiho Pharmaceutical, and Celgene. GG reports participation in advisory boards for Roche, IQVIA, Kite-Gilead, Italfarmaco, Takeda, Ideogen, and Genmab; support for attending meetings from Roche, and Sandoz; and training activities for Roche, Takeda, Clinigen, Ideogen, Beigene, and Incyte; and individual scientific consultancy for Takeda. DES reports honoraria from AbbVie, AstraZeneca, Beigene, Janssen, Lily, Roche, and Takeda; consulting fees from AbbVie, ASTEX, AstraZeneca, Beigene, Janssen, and Kyowa Kirin; and financial support for conference/travel from AbbVie, Novartis, and Roche. FIC reports participation in advisory council/committees for Eli-Lilly, Bristol Meyers Squibb, MSD, Roche, Merck-Serono, Astra-Zeneca, OncXerna, Pierre Fabre, Boehringer Ingelheim, Incyte, Astella, GSK, Sotio, Eisai, Daiichi-Sankyo, Taiho, Servier; honoraria from Eli-Lilly, Eisai, Servier, Roche; and grants or funds from Eli-Lilly, Janssen-Cilag. JPO reports participation in advisory council/committees for Janssen, Takeda, and Roche; and grants/funds from Takeda. PLZ reports participation in advisory council/committees for Takeda, MSD, AbbVie, BMS, and ADC Therapeutics; and honoraria from Takeda, MSD, BMS, Gilead, Novartis, Kyowa Kirin, Sanofi, Incyte, Roche, Eusapharma, Janssen, Incyte, and AstraZeneca. SPI reports honoraria from Curebio, MD Education, and Target Oncology; consulting fees from Seagen, Yingli, Legend, and Securabio; and grants/funds from Merck, Seagen, CRISPRx, Legend, Myeloid, Innate, Rhizen, Spectrum, Affimed, Takeda, Yingli, and Ono. WT reports participation in advisory council/committees for Roche, Incyte, and Takeda; honoraria and consulting fees from Roche, Incyte, Takeda, Gilead, and BMS. SK reports employment and ownership of stocks/shares at Labcorp Drug Development. KS reports ownership of stock at AstraZeneca and Teva Pharmaceuticals. HM, IP, SW, SK, YY, JZ, KS and YS are all employees of Takeda; IP and SW also report the ownership of stocks/shares at Takeda. CC reports honoraria and consulting fees from Takeda and Regeneron; and honoraria from BMS, AstraZeneca, Gilead, and Novartis. FB reports participation in advisory council/committees, honoraria, consulting fees, and grants or funds from Roche, Genentech, AbbVie, Janssen, Lilly, AstraZeneca, Novartis, Kite, BMS, Takeda, TG therapeutics, BeiGene, Advantage, Allogene, LAVA therapeutics, Enterome. SF, JBK, and JR report no conflicts of interest.
Ethical statement and consent
All patients provided written informed consent. The study was conducted in accordance with the protocol, the ethical principles that have their origin in the Declaration of Helsinki, in accordance with the International Conference on Harmonization Good Clinical Practice standards, and applicable regulatory requirements. Relevant institutional review boards or ethics committees approved all aspects of the study, and all authors had access to primary clinical trial data. This trial is registered at ClinicalTrials.gov (NCT02000934).
FUNDING
This study was funded by Takeda Development Center Americas, Inc. (TDCA), Lexington, MA, USA.
REFERENCES
- 1. Jiang S, Zhen H, Jiang H. Second primary malignancy in diffuse large B-cell lymphoma patients: A SEER database analysis. Curr Probl Cancer. 2020; 44:100502. 10.1016/j.currproblcancer.2019.100502. [DOI] [PubMed] [Google Scholar]
- 2. Carbone A, Gloghini A, Kwong YL, Younes A. Diffuse large B cell lymphoma: using pathologic and molecular biomarkers to define subgroups for novel therapy. Ann Hematol. 2014; 93:1263–77. 10.1007/s00277-014-2116-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cheng S, Coffey G, Zhang XH, Shaknovich R, Song Z, Lu P, Pandey A, Melnick AM, Sinha U, Wang YL. SYK inhibition and response prediction in diffuse large B-cell lymphoma. Blood. 2011; 118:6342–52. 10.1182/blood-2011-02-333773. [DOI] [PubMed] [Google Scholar]
- 4. Chan A, Dogan A. Prognostic and Predictive Biomarkers in Diffuse Large B-cell Lymphoma. Surg Pathol Clin. 2019; 12:699–707. 10.1016/j.path.2019.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cheson BD. Predicting the future for DLBCL. Blood. 2020; 135:1308–9. 10.1182/blood.2020005002. [DOI] [PubMed] [Google Scholar]
- 6. Koff JL, Flowers CR. Prognostic modeling in diffuse large B-cell lymphoma in the era of immunochemotherapy: Where do we go from here? Cancer. 2017; 123:3222–25. 10.1002/cncr.30740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Raut LS, Chakrabarti PP. Management of relapsed-refractory diffuse large B cell lymphoma. South Asian J Cancer. 2014; 3:66–70. 10.4103/2278-330X.126531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gisselbrecht C, Van Den Neste E. How I manage patients with relapsed/refractory diffuse large B cell lymphoma. Br J Haematol. 2018; 182:633–43. 10.1111/bjh.15412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liu Y, Barta SK. Diffuse large B-cell lymphoma: 2019 update on diagnosis, risk stratification, and treatment. Am J Hematol. 2019; 94:604–16. 10.1002/ajh.25460. [DOI] [PubMed] [Google Scholar]
- 10. Crump M, Kuruvilla J, Couban S, MacDonald DA, Kukreti V, Kouroukis CT, Rubinger M, Buckstein R, Imrie KR, Federico M, Di Renzo N, Howson-Jan K, Baetz T, et al. Randomized comparison of gemcitabine, dexamethasone, and cisplatin versus dexamethasone, cytarabine, and cisplatin chemotherapy before autologous stem-cell transplantation for relapsed and refractory aggressive lymphomas: NCIC-CTG LY.12. J Clin Oncol. 2014; 32:3490–96. 10.1200/JCO.2013.53.9593. [DOI] [PubMed] [Google Scholar]
- 11. Arboe B, Olsen MH, Gørløv JS, Duun-Henriksen AK, Dalton SO, Johansen C, de Nully Brown P. Treatment intensity and survival in patients with relapsed or refractory diffuse large B-cell lymphoma in Denmark: a real-life population-based study. Clin Epidemiol. 2019; 11:207–16. 10.2147/CLEP.S178003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Susanibar-Adaniya S, Barta SK. 2021 Update on Diffuse large B cell lymphoma: A review of current data and potential applications on risk stratification and management. Am J Hematol. 2021; 96:617–29. 10.1002/ajh.26151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Caimi PF, Ai W, Alderuccio JP, Ardeshna KM, Hamadani M, Hess B, Kahl BS, Radford J, Solh M, Stathis A, Zinzani PL, Havenith K, Feingold J, et al. Loncastuximab tesirine in relapsed or refractory diffuse large B-cell lymphoma (LOTIS-2): a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2021; 22:790–800. 10.1016/S1470-2045(21)00139-X. [DOI] [PubMed] [Google Scholar]
- 14. Locke FL, Miklos DB, Jacobson CA, Perales MA, Kersten MJ, Oluwole OO, Ghobadi A, Rapoport AP, McGuirk J, Pagel JM, Muñoz J, Farooq U, van Meerten T, et al. , and All ZUMA-7 Investigators and Contributing Kite Members. Axicabtagene Ciloleucel as Second-Line Therapy for Large B-Cell Lymphoma. N Engl J Med. 2022; 386:640–54. 10.1056/NEJMoa2116133. [DOI] [PubMed] [Google Scholar]
- 15. Salles G, Duell J, González Barca E, Tournilhac O, Jurczak W, Liberati AM, Nagy Z, Obr A, Gaidano G, André M, Kalakonda N, Dreyling M, Weirather J, et al. Tafasitamab plus lenalidomide in relapsed or refractory diffuse large B-cell lymphoma (L-MIND): a multicentre, prospective, single-arm, phase 2 study. Lancet Oncol. 2020; 21:978–88. 10.1016/S1470-2045(20)30225-4. [DOI] [PubMed] [Google Scholar]
- 16. Dickinson M, Carlo-Stella C, Morschhauser F, Bachy E, Corradini P, Iacoboni G, Khan C, Wrobel T, Offner F, Trneny M, Wu SJ, Cartron G, Hertzberg M, et al. Glofitamab in patients with relapsed/refractory (R/R) diffuse large B-cell lymphoma (DLBCL) and ≥ 2 prior therapies: Pivotal phase II expansion results. J Clin Oncol. 2022. (Suppl 16); 40:7500. 10.1200/JCO.2022.40.16_suppl.7500. [DOI] [Google Scholar]
- 17. Thieblemont C, Phillips T, Ghesquieres H, Cheah CY, Clausen MR, Cunningham D, Do YR, Feldman T, Gasiorowski R, Jurczak W, Kim TM, Lewis DJ, van der Poel M, et al. Epcoritamab, a Novel, Subcutaneous CD3xCD20 Bispecific T-Cell-Engaging Antibody, in Relapsed or Refractory Large B-Cell Lymphoma: Dose Expansion in a Phase I/II Trial. J Clin Oncol. 2022. [Epub ahead of print]. 10.1200/JCO.22.01725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lam B, Arikawa Y, Cramlett J, Dong Q, de Jong R, Feher V, Grimshaw CE, Farrell PJ, Hoffman ID, Jennings A, Jones B, Matuszkiewicz J, Miura J, et al. Discovery of TAK-659 an orally available investigational inhibitor of Spleen Tyrosine Kinase (SYK). Bioorg Med Chem Lett. 2016; 26:5947–50. 10.1016/j.bmcl.2016.10.087. [DOI] [PubMed] [Google Scholar]
- 19. Leseux L, Hamdi SM, Al Saati T, Capilla F, Recher C, Laurent G, Bezombes C. Syk-dependent mTOR activation in follicular lymphoma cells. Blood. 2006; 108:4156–62. 10.1182/blood-2006-05-026203. [DOI] [PubMed] [Google Scholar]
- 20. Li J, Yin W, Jing Y, Kang D, Yang L, Cheng J, Yu Z, Peng Z, Li X, Wen Y, Sun X, Ren B, Liu C. The Coordination Between B Cell Receptor Signaling and the Actin Cytoskeleton During B Cell Activation. Front Immunol. 2018; 9:3096. 10.3389/fimmu.2018.03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Monti S, Savage KJ, Kutok JL, Feuerhake F, Kurtin P, Mihm M, Wu B, Pasqualucci L, Neuberg D, Aguiar RC, Dal Cin P, Ladd C, Pinkus GS, et al. Molecular profiling of diffuse large B-cell lymphoma identifies robust subtypes including one characterized by host inflammatory response. Blood. 2005; 105:1851–61. 10.1182/blood-2004-07-2947. [DOI] [PubMed] [Google Scholar]
- 22. Gururajan M, Jennings CD, Bondada S. Cutting edge: constitutive B cell receptor signaling is critical for basal growth of B lymphoma. J Immunol. 2006; 176:5715–19. 10.4049/jimmunol.176.10.5715. [DOI] [PubMed] [Google Scholar]
- 23. Gordon LI, Kaplan JB, Popat R, Burris HA 3rd, Ferrari S, Madan S, Patel MR, Gritti G, El-Sharkawi D, Chau I, Radford JA, Pérez de Oteyza J, Zinzani PL, et al. Phase I Study of TAK-659, an Investigational, Dual SYK/FLT3 Inhibitor, in Patients with B-Cell Lymphoma. Clin Cancer Res. 2020; 26:3546–56. 10.1158/1078-0432.CCR-19-3239. [DOI] [PubMed] [Google Scholar]
- 24. Millennium Pharmaceuticals Inc. TAK-659 in participants with relapsed or refractory diffuse large B-cell lymphoma (DLBCL). https://clinicaltrials.gov/ct2/show/NCT03123393. ClinicalTrials.gov Identifier: NCT03123393. Accessed November 12, 2021.
- 25. Efremov DG, Turkalj S, Laurenti L. Mechanisms of B Cell Receptor Activation and Responses to B Cell Receptor Inhibitors in B Cell Malignancies. Cancers (Basel). 2020; 12:1396. 10.3390/cancers12061396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Davis RE, Ngo VN, Lenz G, Tolar P, Young RM, Romesser PB, Kohlhammer H, Lamy L, Zhao H, Yang Y, Xu W, Shaffer AL, Wright G, et al. Chronic active B-cell-receptor signalling in diffuse large B-cell lymphoma. Nature. 2010; 463:88–92. 10.1038/nature08638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Scott DW, Mottok A, Ennishi D, Wright GW, Farinha P, Ben-Neriah S, Kridel R, Barry GS, Hother C, Abrisqueta P, Boyle M, Meissner B, Telenius A, et al. Prognostic Significance of Diffuse Large B-Cell Lymphoma Cell of Origin Determined by Digital Gene Expression in Formalin-Fixed Paraffin-Embedded Tissue Biopsies. J Clin Oncol. 2015; 33:2848–56. 10.1200/JCO.2014.60.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Akhter A, Masir N, Elyamany G, Phang KC, Mahe E, Al-Zahrani AM, Shabani-Rad MT, Stewart DA, Mansoor A. Differential expression of Toll-like receptor (TLR) and B cell receptor (BCR) signaling molecules in primary diffuse large B-cell lymphoma of the central nervous system. J Neurooncol. 2015; 121:289–96. 10.1007/s11060-014-1655-3. [DOI] [PubMed] [Google Scholar]
- 29. Crump M, Neelapu SS, Farooq U, Van Den Neste E, Kuruvilla J, Westin J, Link BK, Hay A, Cerhan JR, Zhu L, Boussetta S, Feng L, Maurer MJ, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood. 2017; 130:1800–8. 10.1182/blood-2017-03-769620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kalakonda N, Maerevoet M, Cavallo F, Follows G, Goy A, Vermaat JSP, Casasnovas O, Hamad N, Zijlstra JM, Bakhshi S, Bouabdallah R, Choquet S, Gurion R, et al. Selinexor in patients with relapsed or refractory diffuse large B-cell lymphoma (SADAL): a single-arm, multinational, multicentre, open-label, phase 2 trial. Lancet Haematol. 2020; 7:e511–22. 10.1016/S2352-3026(20)30120-4. [DOI] [PubMed] [Google Scholar]
- 31. US Food and Drug Administration. Novartis Pharmaceuticals Corporation. KYMRIAH (tisagenlecleucel). https://www.fda.gov/media/107296/download. Revised May 2018. Accessed December 6, 2021.
- 32. Northwestern University. Combination chemotherapy and TAK-659 as front-line treatment in treating patients with high-risk diffuse large B cell lymphoma. https://clinicaltrials.gov/ct2/show/NCT03742258. ClinicalTrials.gov Identifier: NCT03742258. Accessed December 6, 2021.
- 33. Calithera Biosciences, Inc. Study of Mivavotinib (CB-659) in Relapsed/Refractory Diffuse Large B-Cell Lymphoma (DLBCL). https://clinicaltrials.gov/ct2/show/NCT05319028. ClinicalTrials.gov Identifier: NCT05319028. Accessed July 19, 2022.
- 34. Al-Mansour M, Al-Foheidi M, Ibrahim E. Efficacy and safety of second-generation CAR T-cell therapy in diffuse large B-cell lymphoma: A meta-analysis. Mol Clin Oncol. 2020; 13:33. 10.3892/mco.2020.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chavez JC, Bachmeier C, Kharfan-Dabaja MA. CAR T-cell therapy for B-cell lymphomas: clinical trial results of available products. Ther Adv Hematol. 2019; 10:2040620719841581. 10.1177/2040620719841581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kamdar M, Solomon SR, Arnason J, Johnston PB, Glass B, Bachanova V, Ibrahimi S, Mielke S, Mutsaers P, Hernandez-Ilizaliturri F, Izutsu K, Morschhauser F, Lunning M, et al. , and TRANSFORM Investigators. Lisocabtagene maraleucel versus standard of care with salvage chemotherapy followed by autologous stem cell transplantation as second-line treatment in patients with relapsed or refractory large B-cell lymphoma (TRANSFORM): results from an interim analysis of an open-label, randomised, phase 3 trial. Lancet. 2022; 399:2294–308. 10.1016/S0140-6736(22)00662-6. [DOI] [PubMed] [Google Scholar]
- 37. US Food & Drug Administration. Kite Pharma, Inc. Yescarta (axicabtagene ciloleucel). https://www.fda.gov/media/108377/download. Revised May 2020. Accessed December 6, 2021.
- 38. Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, Jäger U, Jaglowski S, Andreadis C, Westin JR, Fleury I, Bachanova V, Foley SR, et al. , and JULIET Investigators. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N Engl J Med. 2019; 380:45–56. 10.1056/NEJMoa1804980. [DOI] [PubMed] [Google Scholar]
- 39. Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, Coiffier B, Fisher RI, Hagenbeek A, Zucca E, Rosen ST, Stroobants S, Lister TA, et al. , and International Harmonization Project on Lymphoma. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007; 25:579–86. 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 40. Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, Ott G, Müller-Hermelink HK, Campo E, Braziel RM, Jaffe ES, Pan Z, Farinha P, Smith LM, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004; 103:275–82. 10.1182/blood-2003-05-1545. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.