Abstract
Vaginal infection with the mouse pneumonitis agent of Chlamydia trachomatis (MoPn) produces shorter courses of infection in C57BL/6 and BALB/c mice than in C3H/HeN mice, while C57BL/6 mice are more resistant to oviduct pathology. A robust Th1 response is extremely important in host defense against chlamydia. In this study we examined gamma interferon (IFN-γ), interleukin 10 (IL-10), and the T-cell-regulatory chemokines macrophage inflammatory protein-1α (MIP-1α) and monocyte chemoattractant protein-1 (MCP-1) to determine if differences in these responses were associated with the differential courses of infection seen in these three strains of mice. Increased and prolonged IFN-γ responses and lower IL-10 responses were observed in the C57BL/6 strain compared to BALB/c and C3H. Examination of genital tract chemokines revealed a marked predominance of MIP-1α over MCP-1 only in the C57 strain. Thus, a pattern of high MIP-1α and low MCP-1 levels during the first week of infection is associated with an increased Th1 response and a shorter, more benign chlamydial infection. Inhibition of the MCP-1 response in C3H mice increased their later T-cell production of IFN-γ but decreased their early IFN-γ response and had no effect on the course or outcome of infection. Inhibition of MCP-1 is not beneficial in chlamydial infection because of its pleiotropic effects.
A key issue for the study of chlamydial infection is to understand why individuals infected with Chlamydia trachomatis experience different clinical outcomes. Cytokine patterns elicited by infection are critical in the regulation of the adaptive immune response and in the resolution of infection. We used a mouse model of ascending chlamydial genital tract infection to explore genetically controlled differences in the host response that lead to differential outcomes of chlamydial infection. Vaginal infection of C57BL/6 (C57), BALB/c, and C3H/HeN (C3H) mice with the mouse pneumonitis biovar of C. trachomatis (MoPn) results in genital tract infections that differ with respect to duration and outcome (5, 6, 8). The C57 strain exhibits a short duration of infection and the least oviduct pathology. The BALB/c strain also exhibits an infection of relatively short duration, but despite this, the mice develop severe oviduct pathology. The C3H strain exhibits a longer course of infection and also develops severe oviduct pathology (5, 6, 8). Thus, the C57 strain exhibits the least susceptible phenotype, the BALB/c strain exhibits an intermediate phenotype, and the C3H strain exhibits the most susceptible phenotype (5, 6, 8). All three strains develop high levels of cells producing antigen-specific gamma interferon (IFN-γ) and low to undetectable levels of interleukin 4 (IL-4)-producing-cells in the genital tract and iliac nodes after infection (3, 6). Thus, a predominant chlamydia-specific Th1 response is induced by genital tract infection in all three strains.
Recent data have shed some light on regulation of the Th1 response as regards chlamydial infection. Yang et al. (44) demonstrated in a murine chlamydial pneumonia model that IL-10 production inhibits IFN-γ expression and delays clearance of lung infection in BALB/c mice compared to C57. Data from Igietseme et al. indicate that IL-10 acts at the level of the dendritic cell (DC) to inhibit efficient induction of a Th1 response against chlamydial infection (12).
Induction of chemokines is important in recruiting appropriate effector cells to sites of inflammation, including differential recruitment of Th1 and Th2 cells (28, 35). Increasing evidence suggests that differential expression of the chemokines macrophage inflammatory protein-1α (MIP-1α) and monocyte chemoattractant protein-1 (MCP-1) influences the direction of T-cell differentiation, with MIP-1α being a Th1 promoter (18, 19, 23, 25, 36) and MCP-1 being a Th2 promoter (9, 18, 19, 24–26).
In this study we examined the patterns of IFN-γ and IL-10 responses in C57, BALB/c, and C3H mice to further explore their potential relationship to differential disease outcomes after genital chlamydial infection. We also examined the relevant chemokines, MIP-1α and MCP-1. In addition, we examine the effects of inhibition of MCP-1 in C3H mice. An important question is whether inhibition of specific chemokine mediators leads to a decrease in the sequelae of chlamydial infection or, alternatively, promotes the spread of infection and increases tissue damage in the host.
(This work was presented in part at the 5th International Chlamydia Meeting, Helsinki, Finland, 2000.)
MATERIALS AND METHODS
Animals.
Female C57BL/6 (H-2b), BALB/c (H-2d), and C3H/HeN (H-2k) mice (6 weeks old) were purchased from Jackson Labs, Bar Harbor, Maine. Mice were given food and water ad libitum in an environmentally controlled room with a cycle of 12 h of light and 12 h of darkness. Female mice 7 to 10 weeks of age were used throughout the study.
Infection with chlamydiae and measurement of chlamydial shedding.
MoPn, a C. trachomatis biovar, was used for infection. This agent was originally obtained from American Type Culture Collection and is maintained in McCoy cells (32). Mice were infected by placing 30 μl of 250 mM sucrose–10 mM sodium phosphate–5 mM l-glutamic acid (SPG) containing 107 inclusion-forming units (IFU) of MoPn into the vaginal vault. Infection was performed with the mice under sodium pentobarbital anesthesia. In selected experiments, control mice of each strain were inoculated with SPG. The mice received 2.5 mg of progesterone (Depo-Provera; Upjohn, Kalamazoo, Mich.) subcutaneously 7 days before vaginal infection; the progesterone was given to synchronize all mice in a state of anestrus. The course of infection was followed by swabbing the vaginal vault and ectocervix with a Calgiswab (Spectrum Medical Industries, Los Angeles, Calif.) at various times following infection and by enumerating IFU by isolation on McCoy cell monolayers (21).
Collection of genital tract secretions for cytokine analysis.
Genital tract secretions were collected from mice on multiple days throughout the course of infection and analyzed by enzyme-linked immunosorbent assay (ELISA) for various cytokines and chemokines of interest. At intervals before and after infection or inoculation of buffer, an aseptic surgical sponge (ear wicks, 2 by 5 mm) (DeRoyal, Powell, Tenn.) was inserted into the vagina of an anesthetized animal and retrieved 30 min later. The sponges were held at −70°C until the day of the cytokine assay. Each sponge was placed in a Spin-X microcentrifuge tube (Fisher Scientific) containing a 0.2-μm cellulose acetate filter and incubated in 300 μl of sterile phosphate-buffered saline (PBS) with 0.5% bovine serum albumin and 0.05% Tween 20 for 1 h on ice and then centrifuged for 5 min. Spin-X filters were preblocked with 0.5 ml of sterile PBS with 2% bovine serum albumin and 0.05% Tween-20 for 30 min at 25°C, centrifuged, and washed twice with 0.05 ml of sterile PBS. Samples were kept on ice and promptly loaded into an ELISA plate prepared for a specific cytokine assay.
Cytokine analysis of iliac node supernatants.
Mononuclear cells (MNCs) were prepared by gentle teasing of the pooled nodes from five mice in RPMI 1640, and mononuclear cells were enriched over a Ficoll-Hypaque gradient. MNCs plated in 96-well plates at 2 × 106 cells per well were stimulated with 5 μg of UV-inactivated MoPn antigen at 5 μg/ml (34). Control wells received no antigen. After 72 h of incubation, plates were centrifuged and cell supernatants were removed and stored for later cytokine assays.
Cytokine and chemokine protein assays.
Samples from sponges and iliac node supernatants were assayed individually for cytokine or chemokine (IFN-γ, IL-10, IL-4, MIP-1α, or MCP-1) activity by ELISA using commercial cytokine ELISA kits (R & D Systems, Minneapolis, Minn.).
Antibody inhibition of MCP-1 in C3H mice.
Groups of five C3H mice were injected intravaginally with 150 μg of polyclonal rabbit anti-murine MCP-1 antibodies (Endogen, Woburn, Mass.) twice a day on days −1, 1, 3, and 5 of infection. Control infected mice were injected with 150 μg of normal rabbit immunoglobulin G (IgG) (Endogen). Each mouse was anesthetized by methoxyflurane inhalation, and 75 μl of antibody was injected into each side of the vagina using a 28-gauge needle attached to an insulin syringe. The needle was advanced approximately 2 to 3 mm into the vaginal wall.
Histopathology.
Genital tract tissues from the anti-MCP-1 antibody-treated and control IgG-treated mice were removed en bloc and processed as previously described for histopathology (6). A pathologist blinded to the experimental groups scored the ectocervix, endocervix, uterine horns, and oviducts for inflammation and dilatation as previously described (6).
Statistics.
Statistical comparisons between the groups for level of infection and cytokine production over the course of infection were made by a two-factor (days and murine strain) analysis of variance with the post hoc Tukey test as a multiple-comparison procedure. Differences in grouped cytokine data were analyzed with the t test with correction for unequal variances. The Wilcoxon rank sum test was used to compare the duration of infection in the respective groups over time. The Kruskal-Wallis one-way analysis of variance (ANOVA) on ranks was used to determine significant differences in the pathological data between groups. All experiments were repeated at least once.
RESULTS
IFN-γ responses are higher in C57 mice than in BALB/c and C3H.
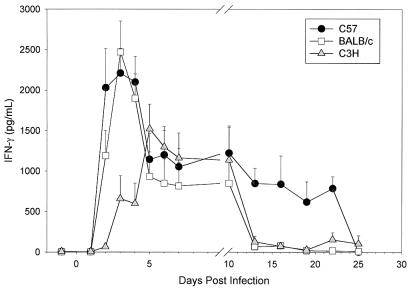
Since IFN-γ plays a critical role in host defense against chlamydiae (16, 17, 29, 32, 39), we hypothesized that differences might exist in the production of IFN-γ that would help to explain the relative susceptibilities seen in C57, BALB/c, and C3H mice. Genital tract secretions tested by ELISA for IFN-γ revealed increased levels of IFN-γ in all three strains over the first 10 days of infection, after which IFN-γ responses fell in the BALB/c and C3H mice but remained increased in the C57 strain through day 22 (Fig. 1). In fact, IFN-γ levels remained elevated in C57 mice for a significantly prolonged period compared to BALB/c and C3H (P < 0.05 by two-way ANOVA).
FIG. 1.
IFN-γ levels in genital tract secretions of C57, BALB/c, and C3H mice during primary infection with C. trachomatis MoPn. Levels were determined with ELISA kits specific for murine IFN-γ. Data are means plus standard errors of the means and are the combined results of three separate experiments (five or six mice per strain per experiment). Each sample was tested in duplicate.
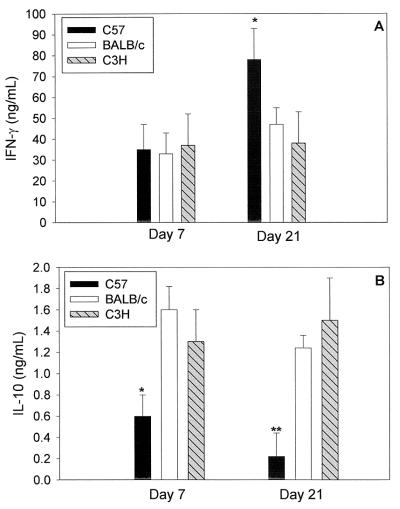
We next quantified IFN-γ production from MNCs taken from iliac nodes on days 7 and 21 of infection. Only low levels of IFN-γ (<0.2 ng/ml) were seen from cells cultured with medium alone on both days 7 and 21 (data not shown). In all three strains, high levels of IFN-γ were detected on days 7 and 21 when MNCs were stimulated with UV-inactivated antigen (Fig. 2A). The levels were not different among the strains on day 7; however, on day 21, MoPn-specific IFN-γ responses were significantly higher (P = 0.03) in C57 mice than in BALB/c and C3H (Fig. 2A).
FIG. 2.
Increased IFN-γ and decreased IL-10 production by MNCs in C57 versus BALB/c and C3H mice. Mice were infected intravaginally with MoPn (107 IFU), and iliac node MNCs were cultured on day 7 or 21 postinfection. IFN-γ (A) or IL-10 (B) production by MNCs (72 h supernatants) after UV-inactivated MoPn-specific stimulation was measured by ELISA. Data are means plus standard deviations for each group of mice from one of three experiments with similar results. ∗∗, P < 0.01; ∗, P < 0.05.
IL-10 responses are lower in C57 mice than in BALB/c and C3H.
IL-10 and IL-4 levels were low in genital tract secretions, with no differences between the strains (data not shown). The production of IL-4 from antigen-stimulated iliac node MNCs was nearly undetectable in all three strains (data not shown). In addition, IL-10 production was minimal (Fig. 2). IL-10 levels were significantly lower in C57 mice than in BALB/c and C3H on both days 7 and 21 (Fig. 2B).
MIP-1α is associated with an increased Th1 response in C57 mice, and MCP-1 is associated with a diminished Th1 response in C3H mice.
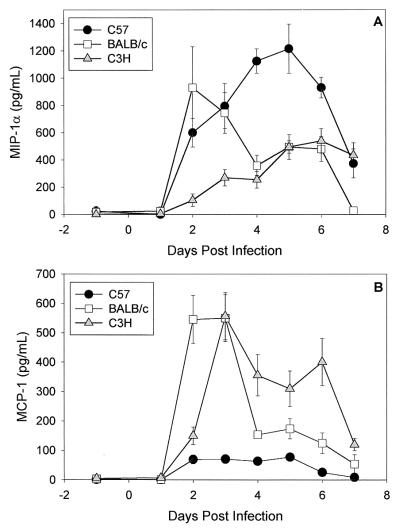
When we compared MIP-1α and MCP-1 levels in the strains, striking differences between C57 and C3H mice during the first week of infection were found (Fig. 3). We found high levels of MIP-1α and low levels of MCP-1 in C57 mice. In contrast, in the C3H strain, we found a significant MCP-1 response and low levels of MIP-1α. The MIP-1α response was significantly greater in C57 than C3H mice and the MCP-1 response was significantly greater in C3H than C57 mice over the first week of infection (P < 0.05 by two-way ANOVA). We detected increased levels of both chemokines in the BALB/c strain during the first few days of infection. After the first week of infection, MIP-1α and MCP-1 were absent from secretions in all three strains except for increases in MCP-1 in C3H mice on day 21 (mean ± standard error of the mean, 215 ± 65 pg/ml) and 28 (168 ± 42 pg/ml). All secretions from progesterone-treated mock-infected mice were negative for chemokines (data not shown).
FIG. 3.
MIP-1α (A) and MCP-1 (B) levels in genital tract secretions of C57, BALB/c, and C3H mice during the first week of primary infection with C. trachomatis MoPn. Levels were determined with ELISA kits specific for the respective chemokines. Data are means ± standard errors of the means and are the combined results of three separate experiments (five or six mice per strain per experiment). Each sample was tested in duplicate.
We also examined MIP-1α and MCP-1 production from the iliac nodes on days 7 and 21 after in vitro stimulation with MoPn antigen. We found high levels of MIP-1α but no detectable MCP-1 in all three strains (data not shown). Thus, although a MIP-1α response was predominant in the draining nodes of all three strains, early increases of MCP-1 occurred in the genital tracts of BALB/c and C3H mice (Fig. 3B), the strains with lower Th1 responses.
Inhibition of early MCP-1 in C3H mice increases their Th1 response.
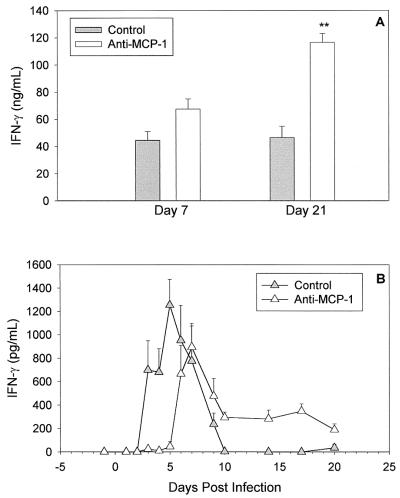
Anti-MCP-1 administration resulted in a >90% reduction in local MCP-1 levels for the first 5 days of infection; on days 6 and 7, levels rebounded to those seen in C3H mice treated with control IgG (data not shown). Groups of mice were sacrificed on days 7 and 21, and we determined the IFN-γ and IL-10 responses from iliac node MNCs stimulated with MoPn antigen. The IL-10 response was not significantly different on day 7 or day 21 (day 7, 1.8 ± 0.2 ng/ml in control IgG-treated mice and 1.4 ± 0.3 ng/ml in anti-MCP-treated mice; day 21, 1.5 ± 0.3 ng/ml in control mice and 1.9 ± 0.4 ng/ml in anti-MCP-treated mice). However, IFN-γ levels were significantly increased on day 21 in the nodes from anti-MCP-1-treated mice (Fig. 4A). Higher IFN-γ levels were also seen on day 7 in the nodes from anti-MCP-1-treated mice than in those from control IgG-treated mice, although the difference was not statistically significant (Fig. 4A).
FIG. 4.
Increased IFN-γ responses by CD4 T cells in C3H mice treated with anti-MCP-1. Groups of MoPn-infected C3H mice (five per group) were treated with normal rabbit IgG or anti-MCP-1 on days −1, 1, 3, and 5 of infection. (A) Iliac node MNCs were cultured on day 7 or 21 postinfection. IFN-γ production by MNCs (72-h supernatants) after UV-inactivated MoPn-specific stimulation was measured by ELISA. Data are means plus standard deviations for each group of mice from one of two experiments with similar results. ∗∗, P < 0.01. (B) IFN-γ levels determined by ELISA in genital tract secretions of control rabbit IgG-treated and anti-MCP-1 antibody-treated C3H mice over 3 weeks of infection. Data are means plus standard errors of the means and are the combined results of two separate experiments. Each sample was tested in duplicate.
When local production of IFN-γ was examined, interesting kinetics were seen in C3H mice treated with anti-MCP-1. IFN-γ was not detected on days 2 to 5 in the anti-MCP-1-treated mice but then increased on day 6, and levels remained steady through day 20 (Fig. 4B). The prolonged detection of IFN-γ in genital tract secretions was similar to what we had observed previously in infected C57 mice (Fig. 1). Although inhibition of the early MCP-1 response resulted in a prolonged IFN-γ response in the genital tract, the course of infection, as determined from isolations, and the outcome of infection, as determined by histopathology, were unchanged (data not shown).
DISCUSSION
Prior studies of C. trachomatis genital tract infection in mice have shown the C57 strain to be more resistant to upper tract disease than BALB/c and C3H (5, 6, 8), and control of infection occurs earlier in C57 than C3H mice (5, 6). If one considers the IFN-γ response a measure of the level of Th1 immunity, our studies show that C57 mice have a prolonged and increased Th1 response compared to BALB/c and C3H. Lower levels of IFN-γ and higher levels of IL-10 (a Th2-like cytokine) were previously found in BALB/c than C57 mice in a lung model of MoPn infection (44); we confirmed those relationships in genital tract infection and extended the observation to the C3H strain. Many studies indicate that early resolution of primary C. trachomatis genital tract infection in mice is highly dependent on strong Th1-type immune responses (12–16, 21, 29, 39–41). Our data support that conclusion and suggest that genetically controlled quantitative differences in the Th1 response contribute to differential outcomes of infection in the genital tract.
Chemokines play established roles as attractants of cell types important in the development of T-cell immune responses. Recent reports indicate that they can also influence the nature of the developing T-cell response, with multiple in vitro and in vivo studies documenting MIP-1α as promoting a Th1 response (18, 19, 23, 36) and MCP-1 as promoting a Th2 response (4, 9, 18, 19, 22, 25, 26). Determination of the patterns of these two chemokines in C57, BALB/c, and C3H mice after infection with chlamydiae suggest that a local predominance of MIP-1α rather than MCP-1 is beneficial as regards chlamydial genital tract infection. Increased MIP-1α levels were associated with a stronger Th1 response in C57 mice, and increased MCP-1 levels were detected in BALB/c and C3H mice, strains with weaker Th1 responses. A recent study by Shaw et al. (37) revealed up-regulation of chemokine mRNA for MIP-1α, MIP-3α, and MIP-2 but not for MCP-1 in DCs pulsed with nonviable chlamydiae that generate a potent protective Th1 immune response following intravenous adoptive transfer.
Inhibition of the early MCP-1 response in C3H mice resulted in late increases in the IFN-γ response in the genital tract and significant increases in their antigen-specific IFN-γ response in the iliac nodes. Since CD4+ T cells are prominent in the genital tract and iliac nodes after days 7 to 10 of infection (3, 20, 27), it is most likely that this reflects increased CD4+-T-cell production of IFN-γ. Matsukawa et al. (26) demonstrated the ability of MCP-1, when present during the sensitization phase of T-cell antigen stimulation, to decrease the development of an ensuing Th1 response to purified protein derivative. However, in this animal model of chlamydial genital tract infection, late increases in genital tract IFN-γ occurred in the face of significant inhibition of the early genital tract IFN-γ response. MCP-1 has been shown to actively recruit natural killer (NK) cells to a site of inflammation (1, 42), and NK cell activity is high in the genital tract on days 2 and 4 of infection (43). Thus, inhibition of early local MCP-1 may have led to decreased NK cell recruitment and consequent decreased early IFN-γ, despite later enhanced T-cell production of IFN-γ. This implies that MCP-1 has a direct role in inhibition of the Th1 response in the genital tract independent of its activities on NK cells.
There are many possible reasons why we were unsuccessful in altering the course of infection in C3H mice despite inhibition of the local MCP-1 response. First, we were successful in blocking the MCP-1 response for only the first 5 days of infection, after which levels rebounded to those seen in control IgG-treated mice. Second, the coincident early inhibition of IFN-γ in the genital tract may have frustrated our efforts to heighten the strength of the Th1 response to the level seen in C57 mice. Third, and perhaps most important, is that although we were successful in significantly increasing the antigen-specific IFN-γ response in the iliac nodes, inhibition of MCP-1 did not change the IL-10 response. Yang et al. (44) demonstrated the importance of lower IL-10 production in C57 than BALB/c mice in the MoPn pneumonia model. A recent report by Igietseme et al. (12) showed that IL-10 is a key down-regulator of the potency with which DCs induce a Th1 response in chlamydial infection. Finally, it is also possible that although the late IFN-γ response was increased in the genital tract and iliac nodes, other IL-12-dependent activities important for eradication of MoPn from the genital tract (29, 31) were not increased by inhibition of MCP-1 alone. Perry et al. demonstrated that although IL-12-dependent CD4+ T cells are necessary for control of chlamydial genital tract infection in the mouse, the functional activity of these cells does not depend on secretion of IFN-γ (29), particularly when the murine biovar of C. trachomatis is used for infection (31).
Clearly, other mechanisms besides the intensity of the Th1 response play a role in the differential rate of resolution of chlamydial genital tract infection seen in these three strains of mice. BALB/c mice have a shorter duration of infection (similar to the C57 strain) and yet a diminished Th1 response similar in magnitude to that of C3H mice. We have previously reported higher levels of tumor necrosis factor alpha (TNF-α) and IL-1β in the C57 and BALB/c strains than in C3H (5, 6). However, depletion of TNF-α in C57 mice did not alter their level of infection (7), and TNF-α-receptor knockout mice have only a marginal delay in their resolution of infection (31). We have found no difference in IL-6 responses among the three strains (5), and IL-6 knockout mice resolve infection at a rate equal to controls (30). Early MIP-2 and polymorphonuclear leukocyte responses are high in the BALB/c strain compared to C57 and C3H (5), perhaps contributing to their ability to eradicate genital infection in a time course comparable to that of C57 in spite of their relative deficiency in Th1 immunity.
In conclusion, these studies have determined that an increased Th1 response occurs with chlamydial genital tract infection in C57 mice and this likely contributes to their lower susceptibility to primary infection compared to BALB/c and C3H. These data support a role for genetically controlled differences in the Th1 response as contributing to different outcomes of infection observed in humans (2, 10, 11, 38). The increased Th1 response in C57 mice was associated with high MIP-1α and low MCP-1 responses. We determined that although MCP-1 helps to establish the intensity of the Th1 response, it is not a strong determinant of the T-cell response in chlamydial genital tract infection in contrast to its predominance in other models of infection and inflammation (9, 18, 26). This may explain the lack of an effect of inhibition of MCP-1 on the course of chlamydial infection or resulting pathology. This and other studies (12, 44) indicate that IL-10 is more important in this regard; in future studies we will further examine the potential role of MIP-1α. Elaborating the immune mechanisms that determine whether an infected individual will develop benign resolution of infection or chronic disease can aid in developing treatment and prevention strategies.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grant AI43337 and by the Horace C. Cabe Foundation and the Bates-Wheeler Foundation, Arkansas Children's Hospital Research Institute. Support was also provided by a grant from the University of Arkansas for Medical Sciences Foundation Fund.
REFERENCES
- 1.Allavena P, Bianchi G, Zhou D, Van Damme J, Jilek P, Sozzani S, Mantovani A. Induction of natural killer cell migration by monocyte chemotactic protein-1, -2 and -3. Eur J Immunol. 1994;24:3233–3236. doi: 10.1002/eji.1830241249. [DOI] [PubMed] [Google Scholar]
- 2.Bailey R L, Holland M J, Whittle H C, Mabey D C. Subjects recovering from human ocular chlamydial infection have enhanced lymphoproliferative responses to chlamydial antigens compared with those of persistently diseased controls. Infect Immun. 1995;63:389–392. doi: 10.1128/iai.63.2.389-392.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cain T K, Rank R G. Local Th1-like responses are induced by intravaginal infection of mice with the mouse pneumonitis biovar of Chlamydia trachomatis. Infect Immun. 1995;63:1784–1789. doi: 10.1128/iai.63.5.1784-1789.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chensue S W, Warmington K S, Ruth J H, Sanghi P S, Lincoln P, Kunkel S L. Role of monocyte chemoattractant protein-1 (MCP-1) in Th1 (mycobacterial) and Th2 (schistosomal) antigen-induced granuloma formation: relationship to local inflammation, Th cell expression, and IL-12 production. J Immunol. 1996;157:4602–4608. [PubMed] [Google Scholar]
- 5.Darville T, Andrews C W, Jr, Sikes J D, Fraley P L, Rank R G. Early local cytokine profiles in strains of mice with different outcomes from chlamydial genital tract infection. Infect Immun. 2001;69:3556–3561. doi: 10.1128/IAI.69.6.3556-3561.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Darville T, Andrews C W, Jr, Laffoon K K, Shymasani W, Kishen L R, Rank R G. Mouse strain-dependent variation in the course and outcome of chlamydial genital tract infection is associated with differences in host response. Infect Immun. 1997;65:3065–3073. doi: 10.1128/iai.65.8.3065-3073.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Darville T, Andrews C W, Jr, Rank R G. Does inhibition of tumor necrosis factor alpha affect chlamydial genital tract infection in mice and guinea pigs? Infect Immun. 2000;68:5299–5305. doi: 10.1128/iai.68.9.5299-5305.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de la Maza L M, Pal S, Khamesipour A, Peterson E. Intravaginal inoculation of mice with the Chlamydia trachomatis mouse pneumonitis biovar results in infertility. Infect Immun. 1994;62:2094–2097. doi: 10.1128/iai.62.5.2094-2097.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gu L, Tseng S, Horner R M, Tam C, Loda M, Rollins B J. Control of TH2 polarization by the chemokine monocyte chemoattractant protein-1. Nature. 2000;404:407–411. doi: 10.1038/35006097. [DOI] [PubMed] [Google Scholar]
- 10.Holland M J, Bailey R L, Conway D J, Culley F, Miranpuri G, Byrne G I, Whittle H C, Mabey D C. T helper type-1 (Th1)/Th2 profiles of peripheral blood mononuclear cells (PBMC): responses to antigens of Chlamydia trachomatis in subjects with severe trachomatous scarring. Clin Exp Immunol. 1996;105:429–435. doi: 10.1046/j.1365-2249.1996.d01-792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holland M J, Bailey R L, Hayes L J, Whittle H C, Mabey D C. Conjunctival scarring in trachoma is associated with depressed cell-mediated immune responses to chlamydial antigens. J Infect Dis. 1993;168:1528–1531. doi: 10.1093/infdis/168.6.1528. [DOI] [PubMed] [Google Scholar]
- 12.Igietseme J U, Ananaba G A, Bolier J, Bowers S, Moore T, Belay T, Eko F O, Lyn D, Black C M. Suppression of endogenous IL-10 gene expression in dendritic cells enhances antigen presentation for specific Th1 induction: potential for cellular vaccine development. J Immunol. 2000;164:4212–4219. doi: 10.4049/jimmunol.164.8.4212. [DOI] [PubMed] [Google Scholar]
- 13.Igietseme J U, Ramsey K H, Magee D M, Williams D M, Kincy T J, Rank R G. Resolution of murine chlamydial genital infection by the adoptive transfer of a biovar-specific, Th1 lymphocyte clone. Reg Immunol. 1993;5:317–324. [PubMed] [Google Scholar]
- 14.Igietseme J U, Rank R G. Susceptibility to reinfection after a primary chlamydial genital infection is associated with a decrease of antigen-specific T cells in the genital tract. Infect Immun. 1991;59:1346–1351. doi: 10.1128/iai.59.4.1346-1351.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Igietseme J U, Uriri I M, Kumar S N, Ananaba G A, Ojior O O, Momodu I A, Candal D H, Black C M. Route of infection that induces a high intensity of gamma interferon-secreting T cells in the genital tract produces optimal protection against Chlamydia trachomatis infection in mice. Infect Immun. 1998;66:4030–4035. doi: 10.1128/iai.66.9.4030-4035.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johansson M, Schon K, Ward M, Lycke N. Genital tract infection with Chlamydia trachomatis fails to induce protective immunity in gamma interferon receptor-deficient mice despite a strong local immunoglobulin A response. Infect Immun. 1997;65:1032–1044. doi: 10.1128/iai.65.3.1032-1044.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johansson M, Schon K, Ward M, Lycke N. Studies in knockout mice reveal that anti-chlamydial protection requires TH1 cells producing IFN-γ: is this true for humans? Scand J Immunol. 1997;46:546–552. doi: 10.1046/j.1365-3083.1997.d01-167.x. [DOI] [PubMed] [Google Scholar]
- 18.Karpus W J, Kennedy K J. MIP-1alpha and MCP-1 differentially regulate acute and relapsing autoimmune encephalomyelitis as well as Th1/Th2 lymphocyte differentiation. J Leukoc Biol. 1997;62:681–687. [PubMed] [Google Scholar]
- 19.Karpus W J, Lukacs N W, Kennedy K J, Smith W S, Hurst S D, Barrett T A. Differential CC chemokine-induced enhancement of T helper cell cytokine production. J Immunol. 1997;158:4129–4136. [PubMed] [Google Scholar]
- 20.Kelly K A, Rank R G. Identification of homing receptors that mediate the recruitment of CD4 T cells to the genital tract following intravaginal infection with Chlamydia trachomatis. Infect Immun. 1997;65:5198–5208. doi: 10.1128/iai.65.12.5198-5208.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelly K A, Robinson E A, Rank R G. Initial route of antigen administration alters the T-cell cytokine profile produced in response to the mouse pneumonitis biovar of Chlamydia trachomatis following genital infection. Infect Immun. 1996;64:4976–4983. doi: 10.1128/iai.64.12.4976-4983.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu B, Rutledge B J, Gu L, Fiorillo J, Lukacs N W, Kunkel S L, North R, Gerard C, Rollins B J. Abnormalities in monocyte recruitment and cytokine expression in monocyte chemoattractant protein 1-deficient mice. J Exp Med. 1998;187:601–608. doi: 10.1084/jem.187.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu Y, Xin K Q, Hamajima K, Tsuji T, Aoki I, Yang J, Sasaki S, Fukushima J, Yoshimura T, Toda S, Okada E, Okuda K. Macrophage inflammatory protein-1α (MIP-1α) expression plasmid enhances DNA vaccine-induced immune response against HIV-1. Clin Exp Immunol. 1999;115:335–341. doi: 10.1046/j.1365-2249.1999.00793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lukacs N W, Chensue S W, Karpus W J, Lincoln P, Keefer C, Strieter R M, Kunkel S L. C-C chemokines differentially alter interleukin-4 production from lymphocytes. Am J Pathol. 1997;150:1861–1868. [PMC free article] [PubMed] [Google Scholar]
- 25.Luther S A, Cyster J G. Chemokines as regulators of T cell differentiation. Nat Immunol. 2001;2:102–107. doi: 10.1038/84205. [DOI] [PubMed] [Google Scholar]
- 26.Matsukawa A, Lukacs N W, Standiford T J, Chensue S W, Kunkel S L. Adenoviral-mediated overexpression of monocyte chemoattractant protein-1 differentially alters the development of Th1 and Th2 type responses in vivo. J Immunol. 2000;164:1699–1704. doi: 10.4049/jimmunol.164.4.1699. [DOI] [PubMed] [Google Scholar]
- 27.Morrison S G, Morrison R P. In situ analysis of the evolution of the primary immune response in murine Chlamydia trachomatis genital tract infection. Infect Immun. 2000;68:2870–2879. doi: 10.1128/iai.68.5.2870-2879.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Garra A, McEvoy L M, Zlotnik A. T-cell subsets: chemokine receptors guide the way. Curr Biol. 1998;8:R646–R649. doi: 10.1016/s0960-9822(07)00413-7. [DOI] [PubMed] [Google Scholar]
- 29.Perry L L, Feilzer K, Caldwell H D. Immunity to Chlamydia trachomatis is mediated by T helper 1 cells through IFN-gamma-dependent and -independent pathways. J Immunol. 1997;158:3344–3352. [PubMed] [Google Scholar]
- 30.Perry L L, Feilzer K, Caldwell H D. Neither interleukin-6 nor inducible nitric oxide synthase is required for clearance of Chlamydia trachomatis from the murine genital tract epithelium. Infect Immun. 1998;66:1265–1269. doi: 10.1128/iai.66.3.1265-1269.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perry L L, Su H, Feilzer K, Messer R, Hughes S, Whitmire W, Caldwell H D. Differential sensitivity of distinct Chlamydia trachomatis isolates to IFN-γ-mediated inhibition. J Immunol. 1999;162:3541–3548. [PubMed] [Google Scholar]
- 32.Ramsey K H, Soderberg L S F, Rank R G. Resolution of chlamydial genital infection in B-cell-deficient mice and immunity to reinfection. Infect Immun. 1988;56:1320–1325. doi: 10.1128/iai.56.5.1320-1325.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rank R G, Ramsey K H, Pack E A, Williams D M. Effect of gamma interferon on resolution of murine chlamydial genital infection. Infect Immun. 1992;60:4427–4429. doi: 10.1128/iai.60.10.4427-4429.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rank R G, Batteiger B E, Soderberg L S F. Immunization against chlamydial genital infection in guinea pigs with UV-inactivated and viable chlamydiae administered by different routes. Infect Immun. 1990;58:2599–2605. doi: 10.1128/iai.58.8.2599-2605.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sallusto F. The role of chemokines and chemokine receptors in T cell priming and Th1/Th2-mediated responses. Haematologica. 1999;84:28–31. [PubMed] [Google Scholar]
- 36.Schrum S, Probst P, Fleischer B, Zipfel P F. Synthesis of the CC-chemokines MIP-α, MIP-1β, and RANTES is associated with a type 1 immune response. J Immunol. 1996;157:3598–3604. [PubMed] [Google Scholar]
- 37.Shaw J H, Grund V R, Durling L, Caldwell H D. Expression of genes encoding Th1 cell-activating cytokines and lymphoid homing chemokines by chlamydia-pulsed dendritic cells correlates with protective immunizing efficacy. Infect Immun. 2001;69:4667–4672. doi: 10.1128/IAI.69.7.4667-4672.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stamm W E. Chlamydia trachomatis infections: progress and problems. J Infect Dis. 1999;179(Suppl. 2):S380–S383. doi: 10.1086/513844. [DOI] [PubMed] [Google Scholar]
- 39.Su H, Messer R, Whitmire W, Fischer E, Portis J C, Caldwell H D. Vaccination against chlamydial genital tract infection after immunization with dendritic cells pulsed ex vivo with nonviable chlamydiae. J Exp Med. 1998;188:809–818. doi: 10.1084/jem.188.5.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Su H, Messer R, Whitmire W, Hughes S, Caldwell H D. Subclinical chlamydial infection of the female mouse genital tract generates a potent protective immune response: implications for development of live attenuated chlamydial vaccine strains. Infect Immun. 2000;68:192–196. doi: 10.1128/iai.68.1.192-196.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Su H, Morrison R, Messer R, Whitmire W, Hughes S, Caldwell H D. The effect of doxycycline treatment on the development of protective immunity in a murine model of chlamydial genital infection. J Infect Dis. 1999;180:1252–1258. doi: 10.1086/315046. [DOI] [PubMed] [Google Scholar]
- 42.Taub D D, Sayers T J, Carter C R, Ortaldo J R. Alpha and beta chemokines induce NK cell migration and enhance NK-mediated cytolysis. J Immunol. 1995;155:3877–3888. [PubMed] [Google Scholar]
- 43.Tseng C T, Rank R G. Role of NK cells in early host response to chlamydial genital infection. Infect Immun. 1998;66:5867–5875. doi: 10.1128/iai.66.12.5867-5875.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang X, Hayglass K T, Brunham R C. Genetically determined differences in IL-10 and IFN-γ responses correlate with clearance of Chlamydia trachomatis mouse pneumonitis infection. J Immunol. 1996;156:4338–4344. [PubMed] [Google Scholar]