Abstract
Background:
This study aims to evaluate the effect of Letrozole (LE) in reducing ovarian hyperstimulation syndrome (OHSS) in high-risk participants with polycystic ovary syndrome (PCOS) treated with In vitro fertilization (IVF).
Methods:
This study was a randomized clinical trial in which participants were randomly divided into two groups (n= 25 per group). Based on GnRH-antagonist protocol, recombinant follicle-stimulating hormone 150 units/day subcutaneously and human menopausal gonadotropin 75 units/ day intramuscularly used from day 2 of the menstrual cycle. In the study group, Letrozole 5 mg daily was added simultaneously with gonadotropin during the first five days of the IVF cycle and in the control group placebo was added.
Results:
There were statistically significant differences among the groups in terms of Estradiol level on Trigger Day (p= 0.04). The total days of stimulation and cumulative Gonadotropin dose were significantly lower in the Letrozole group (p= 0.00). There were no significant differences between the groups in terms of the number of oocytes retrieved, numbers of implanted embryos, and clinical pregnancy rates (p-value> 0.05). There was only one moderate case in the intervention group and 9 moderate symptoms in the control group (p= 0.04).
Discussion
Administration of Letrozole with GnRH antagonist protocol, conventional protocol in PCOS cases in IVF cycle, had a significant effect on reducing the incidence of OHSS. So, if the future studies prove LE co-administration may lessen the incidence of OHSS, LE will be a highly potent drug for preventing OHSS in PCOS cases.
Key Words: In vitro fertilization, Infertility, Letrozole, Ovarian hyperstimulation syndrome
Introduction
Ovulation disorders are one of the challenges for modern medicine due to their effects on fertility. Polycystic ovary syndrome (PCOS) is one of the most common endocrine disorders and the most common cause of infertility in women, affecting 4- 6% of women of childbearing age (1- 3). Multiple pathophysiological factors are associated with PCOS, such as genetic variants, environmental factors, and transgenerational components (4).Assisted Reproductive Techniques (ART) and ovulation induction are common treatment approaches in the management of PCOS (5). However, These treatments have several complications, including ovarian hyperstimulation syndrome (OHSS), caused by an oversized ovary and increased vascular permeability and protein permeability from the intravascular space to the third space, such as the pericardium, pleura, or peritoneum (6, 7). The mortality rate associated with the condition is estimated at 3-10% (8, 9), and several factors such as age, low body weight, some ovulation protocols, and high or rapidly elevated levels of estradiol can increase this rate to 20% (10, 11).
There is no definitive treatment for this syndrome, and many complications in severe forms such as kidney failure, respiratory failure, and thromboembolic disorders can be life-threatening, so identifying people at risk of developing this syndrome will be the best treatment (12). The presence of more than 13 of 11 mm follicles during the In vitro fertilization (IVF) cycle is a predictor of severe overstimulation syndrome. If there are 20 follicles before ovulation, the incidence of severe overstimulation syndrome reaches 15% (13).
Due to the dangers of this syndrome in assisted reproductive techniques, methods that can reduce the incidence of this syndrome in these participants have always been the focus of researchers. One of these cases is the use of Letrozole. Letrozole is a reversible aromatase inhibitor that is useful in inducing ovulation (14). This drug inhibits estradiol production by preventing the conversion of androgens to estrogens. Following the increase in serum androgen levels and then a decrease in serum estrogen levels by negative feedback, this drug produces Follicle-stimulating hormone (FSH), and the ovaries become sensitive to the effects of FSH (14). On the other hand, it is proved that administration of Letrozole in the luteal phase reduces the incidence of OHSS by reducing serum estradiol (E2) levels (15). Therefore, in this study, we aimed to investigate the effect of Letrozole administration in the follicular phase in reducing OHSS in high-risk participants with PCOS treated with assisted reproductive techniques.
Materials and Methods
Trial design
The present study was a prospective randomized controlled clinical trial (RCT) that was performed at the Shahid Beheshti Hospital (Isfahan, Iran) from March 2020 to December 2021.
Participants
Women with primary or secondary infertility who seek infertility treatment due to polycystic ovaries were included in the present study.
Inclusion criteria included: 1) age between 20-40 years, 2) candidate for treatment with ovarian hyper stimulation and IVF, 3) history of at least one year of infertility, 4) body mass index (BMI)< 25 kg/m² and, 5) Antimullerian hormone (AMH) levels> 5 ng/ml.
Exclusion criteria were history of any hormone therapy during the previous three months, history of allergy to Letrozole and other aromatase inhibitors, history of heart disease, kidney failure, liver disease, and other endocrine diseases.
Sampling was non-probabilistic and easy. Fifty cases were equally divided into two groups by the balanced block randomization method (n= 25 per group). In this randomization method, quintuple therapy blocks, with ten probabilities, were randomly moved back, and a balanced random list of two treatment groups was obtained with the aim that if the total number of samples were not complete, both groups would be equal.
Diagnosis of polycystic ovaries was made according to Rotterdam criteria (16). After the approval of the ethics committee of Isfahan University, the eligible participants were admitted to the study after obtaining written consent based on the individual's desire to participate in the research project. Then the participants were randomly divided into two groups. BMI, age, history for infertility duration, hormonal evaluation including follicular (FSH), Luteinizing hormone (LH), progesterone, estradiol (E2), and AMH was checked in appropriate laboratory methods and collecting the blood sample on the second day of the menstrual cycle was done for all participants. Ultrasound was performed to evaluate the number of ovarian follicles with size 2-10 nm, antral follicular count-AFC, and to check endometrial thickness on the second day of the menstrual cycle. The Gonadotropin-releasing hormone (GnRH) antagonist treatment protocol was performed for participants in both study and control groups. The Ethics Committee of Isfahan University of Medical Sciences approved this study (IR.MUI.MED.REC.1399.1031). In addition, the study protocol was approved by the Iranian Registry of Clinical Trial (IRCT) (code: IRCT20180313039085N1).
Ovarian Stimulation
All participants underwent the same ovulation induction protocol with six doses of recombinant follicle-stimulating hormone (r-hFSH; Gonal-F®) 150 IU/day subcutaneously from the second day of the menstrual cycle (days 2, 3, 4, 5, 6, and 7) and Intramuscular hMG (Menogon®, Ferring Pharmaceuticals A/S, Copenhagen, Denmark) 75 IU/day from day four of the menstrual cycle until the trigger day. Then, transvaginal sonography was carried out by two experienced gynecologists from day 7 menstrual cycle and then it was repeated every other day. After confirming at least three mature follicles (≥ 14 mm), Cetrotide (Merck Serono, Germany) 250 μg/day subcutaneously was administrated until the trigger day. When more than three mature follicles≥ 17 mm diameter were observed by transvaginal sonography, 0.2 mg subcutaneous GnRH analog triptorelin (Decapeptyl: Ipsen, Paris, France) was prescribed. Oocyte retrieval was performed 35-36 hours post-HCG using 17 G ovum-aspiration needle (Cook Ireland Ltd, Limerick, Ireland) under general anesthesia.
Interventions
Participants received Letrozole (Femara; Novartis), 5 mg daily in the case group (n= 25) on the second day of the menstrual cycle for five consecutive days, and a placebo was administered in the control group in an identical manner. The retrieved oocytes were fertilized at the laboratory using IVF or ICSI with fresh semen. Finally, all blastocysts were cryopreserved on day four using a vitrification protocol.
Participants were trained about the symptoms of OHSS and were asked to come to the hospital if they developed any symptoms. The OHSS symptoms were classified based on Golan criteria, Mild = patients with laboratory evidence of OHSS and enlargement of the ovaries, Moderate = the feature of mild OHSS plus abdominal distention, nausea, vomiting, and diarrhea, and Sever=the feature of moderate OHSS plus ascites (17).
Participants with mild symptoms were treated on an outpatient basis, and individuals with moderate to severe symptoms were hospitalized.
Outcomes
Finally, the number of oocytes obtained per case, frequency of cases and severity of OHSS, frequency of clinical symptoms in participants with OHSS, such as nausea and vomiting, abdominal pain, diarrhea, abdominal ascites (using ultrasound), pleural effusion (with the use of ultrasound), the duration of hospitalization and the frequency of side effects of Letrozole in case of occurrence were recorded in the specific profile of each case.
Statistical methods
Statistical analysis was carried out using the SPSS software version 25. Analysis was performed at descriptive (mean±SD, percentages, and Kolmogorov test) and inferential (analysis of variance, T-student test, Mann–Whitney U-test, chi-square test) levels. A p-value< 0.05 is considered as the significant level as appropriate.
Results
Participant flow
Sixty-five participants were assessed for eligibility (n= 65). Six participants had no meeting inclusion criteria, four participants declined to participate, and five did not receive allocation intervention. Finally, a total of 50 women (25 pergroup) were completed study. The first group was considered the intervention group and the second group as the control group. Figure 1 is shown details of the enrollment, allocation, follow-up, and analysis of participants through the trial according to the CONSORT 2010 criteria (18) (Fig. 1).
Fig. 1.
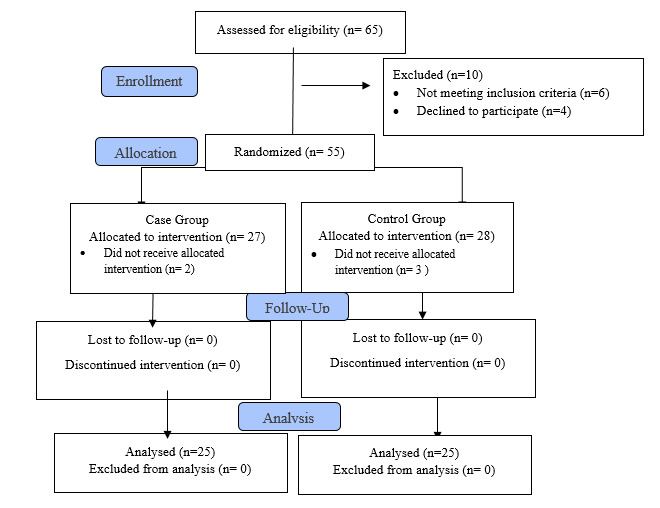
CONSORT 2010 Flow Diagram of the study.
Outcomes and estimation
Socio-demographic and characteristics of the study population were illustrated in Table 1. In this study, 50 women were evaluated. The mean age of the study population was 30.06±4.25 years, and the BMI was 23.02±0.85 kg/m2. According to the analysis, no significant difference was found between the studied groups regarding age, BMI, baseline hormones levels, Endometrial thickness, and antral follicle count, p-value> 0.05 (Table 1).
Table 1.
Demographic and clinical baseline data of the groups.
| Variable | Study Group | Control Group | p -Value | |
|---|---|---|---|---|
| (N: 25) | (N: 25) | |||
| Age (years) † | 30.80±4.67 | 29.32±3.73 | 0.22 | |
| BMI (kg/m2)† | 22.87±0.90 | 23.18±0.79 | 0.29 | |
| AMH (ng/mL) (days 2-3)† | 10.41±4.98 | 9.55±4.03 | 0.50 | |
| LH (mIU/ml) (days 2-3)† | 10.69±5.57 | 11.54±4.73 | 0.56 | |
| FSH (mIU/ml) (days 2-3)† | 5.70±1.82 | 4.87±2.11 | 0.14 | |
| Estradiol(pg/mg) (days 2-3)† | 58.34±23.83 | 68.64±24.56 | 0.13 | |
| Progesterone (days 2-3)† | 0.56±0.26 | 0.61±0.25 | 0.46 | |
| AFC (days 1-3) ‡ | 1-7 | 0 | 0 | 0.26 |
| 7-12 | 11 | 10 | ||
| 13-18 | 14 | 15 | ||
| Endometrial Thickness (mm) (days 1-3) ‡ | < 5mm | 22 | 24 | 0.29 |
| >5mm | 3 | 1 | ||
Values show median±SD and percentage. Statistical significance was tested with
T-test
Chi-square test
*p< 0.05 shows statistical significance (bold)). AMH: Anti-Müllerian Hormone; FSH: Follicle-stimulating hormone; LH: Luteinizing hormone; AFC: antral follicle count
Table 2 shows the stimulation characteristics and cycle outcomes. There were statistically significant differences among the groups in terms of Estradiol level on Trigger Day (p= 0.04). In addition, the total days of stimulation and cumulative Gonadotropin dose were significantly lower in the Letrozole group (p= 0.00) (Table 2). There were no significant differences between the groups in terms of the number of oocytes retrieved, numbers of implanted embryos, and clinical pregnancy rates (p-value> 0.05).
Table 2.
Stimulation characteristics and cycle outcomes.
| Variable | Study Group | Control Group | p -Value |
|---|---|---|---|
| (N: 25) | (N: 25) | ||
| Estradiol (Trigger Day)† | 4902.48±1252.944 | 5744.92±1621.78 | 0.04 * |
| Progesterone (Trigger Day) † | 1.74±1.40 | 1.39±0.32 | 0.227 |
| Day of Stimulation§ | 8.20±0.91 | 9.60±1.41 | 0.00 * |
| Number of Oocyte Retrieved† | 8.52±1.96 | 9.08±1.97 | 0.32 |
| Numbers of Implanted Embryos † | 4.04±1.27 | 4.60±1.19 | 0.11 |
| Gonadotropin Dose (IU) § | 20.24±1.92 | 22.00±2.84 | 0.01 * |
| Clinical Pregnancy rates ‡ | 15(60%) | 13(52%) | 0.56 |
Values show median±SD and percentage. Statistical significance was tested with
T-test
Chi-square test
Mann-Whitney
p< 0.05 shows statistical significance (bold).
The OHSS symptoms based on Golan criteria were evaluated in this study, and as presented in Table 3 there was only one Moderate case in the intervention group and 9 Moderate symptoms in the control group (p= 0.04) (Table 3).
Table 3.
Classification of OHSS symptoms between groups.
| Variable | Study Group | Control Group | p -Value |
|---|---|---|---|
| (N: 25) | (N: 25) | ||
| Mild | 6 | 4 | 0.01 * |
| Moderate | 1 | 9 | |
| Severe | 0 | 0 |
Values show median±SD and percentage. Statistical significance was tested with Chi-square test
p< 0.05 shows statistical significance (bold).
Discussion
Ovarian hyperstimulation syndrome (OHSS) is an iatrogenic complication of controlled ovarian stimulation during Assisted Reproductive Technology (ART) and is characterized by increased multi follicular, high E2, and ovarian enlargement. In this case, excessive secretion of body fluids from the blood vessels may lead to fluid accumulation in the pleura, ascites,electrolyte disturbances, impaired liver and kidney function, and thrombosis. With the development of ART and the use of ovulation stimuli, the rate of OHSS is increasing (10, 23). Vascular endothelial growth factor (VEGF) is a significant mediator of hCG and a potent endothelial cell mitogen and vascular endothelial growth factor that can increase vascular permeability. This factor increases after hCG-induced ovulation, and its inhibition can relieve the symptoms of OHSS. The VEGF factor is considered the main regulator of OHSS and is associated with the occurrence and severity of OHSS (19). Letrozole can significantly reduce the level of VEGF in residual tissue in participants with breast cancer (20).
In the present study, none of the participants had severe OHSS. In the Letrozole group, one case had moderate OHSS, and six women had mild OHSS. While in the control group, nine participants had moderate OHSS, and four participants had mild OHSS. As in previous studies investigating the use of Letrozole combined with GnRH-agonist trigger (10, 21), the results of the present study did not report serious adverse reactions of OHSS and also cycle cancellations in both groups. This is in good agreement with previous findings (10, 22) which highlighted that Letrozole therapy in patients with OHSS is associated with a lower rate of serious adverse reactions of OHSS. The different results in the incidence of OHSS may be related to the therapeutic dose of Letrozole. In addition, In contrast to previous research in which 2.5 mg of Letrozole was administered for five days (23, 24), the therapeutic dose used in the present study was 5 mg. In a similar study (11) high-risk OHSS participants were treated with 2.5 mg, 5 mg, and 7.5 mg of Letrozole. The level of E2 was significantly lower in all three groups of Letrozole compared with the control group on the fifth, eighth and tenth days after hCG administration. The researchers indicated that in high-risk participants with OHSS, treatment with 7.5 mg of Letrozole could help decrease the incidence of moderate and severe OHSS. The higher dose of Letrozole used, the lower the amount of E2. This can reduce the incidence of OHSS.
Our experiments are in line with previous results. In a similar study, Letrozole was evaluated for its effect on E2, and LH levels during the luteal phase. Based on this study, administration of 2.5 mg of Letrozole in the luteal phase can affect corpus luteum function which improves LH levels faster. Furthermore, it is mentioned that women at risk for OHSS can benefit from this treatment approach (24). A similar pattern of results was obtained in Qiaohua et al. who highlighted that in women with high-risk OHSS undergoing IVF, treatment with Letrozole could significantly limit OHSS (11). Letrozole is a non-steroidal oral aromatase inhibitor that can prevent the conversion of androgens to estrogens and thus reduce strain levels (10). It was first used in E2-dependent tumors such as breast cancer. Letrozole can decrease E2 levels, reverse E2-induced hypothalamic-pituitary-negative feedback, and enhance gonadotropin secretion. Therefore, it is used to induce ovulation (25, 26). This trend justifies the reduction of days of stimulation and cumulative Gonadotropin dose in the intervention group because by increasing the level of FSH after administration of Letrozole, the prescribed dose necessary to induce ovulation decreases.
In addition, in this study, the total days of stimulation and cumulative Gonadotropin dose were significantly lower in the intervention group. These findings suggest that by reducing the amount of time required for controlled ovarian hyperstimulation, the patient's drug intake is reduced, which not only averts the side effects of higher doses, including OHSS incidence but also reduces treatment costs. This lends support to previous findings in the literature (10, 27, 28). Based on the present study findings, administration of Letrozole at a dose of 5 mg daily had a significant effect on reducing the incidence of OHSS.
Funding
This study was supported by Isfahan University of Medical Sciences (Grant NO: 399738).
Conflict of interest
All authors declare no conflict of interest.
Acknowledgements
The authors would like to thank the patients and their families for their participation in this study. Also, we would like to express our gratitude to the Shahid Beheshti infertility center in Isfahan for their great insights and invaluable assistance to the present research project.
References
- 1.Akbari Nasrekani Z, Fathi M. Efficacy of 12 weeks aerobic training on body composition, aerobic power and some women-hormones in polycystic ovary syndrome infertile women. Iran J Obstet Gynecol Infertil. 2016;19(5):1–10. [Google Scholar]
- 2.Nasseh A, Sarvaideo J. In: Sex- and Gender-Based Women's Health. Tilstra SA, Kwolek D, Mitchell JL, Dolan BM, Carson MP, et al., editors. Springer, Cham.; 2020. pp. 83–98. Polycystic Ovary Syndrome. [Google Scholar]
- 3.Chauhan AR, Prasad M, Chamariya S, Achrekar S, Mahale SD, Mittal K. Novel FSH receptor mutation in a case of spontaneous ovarian hyperstimulation syndrome with successful pregnancy outcome. J Hum Reprod Sci. 2015;8(4):230–3. doi: 10.4103/0974-1208.170410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoeger KM, Dokras A, Piltonen T, et al. Update on PCOS: consequences, challenges, and guiding treatment. J Clin Endocrinol Metab. 2021;106(3):e1071–e1083. doi: 10.1210/clinem/dgaa839. [DOI] [PubMed] [Google Scholar]
- 5.Nagori C. Ovulation Induction in PCOS. In: Handbook of Infertility & Ultrasound for Practicing Gynecologists. Jaypee Brothers Medical Publishers Ltd. 2021:106–119. [Google Scholar]
- 6.Smith V, Osianlis T, Vollenhoven B. Prevention of ovarian hyperstimulation syndrome: a review. Obstet Gynecol Int. 2015;2015:514159. doi: 10.1155/2015/514159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu F, Jiang Q, Sun X, Huang Y, Zhang Z, Han T, et al. Lipid metabolic disorders and ovarian hyperstimulation syndrome: a retrospective analysis. . Front physiol. 2020;11:491892. doi: 10.3389/fphys.2020.491892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orvieto R, Vanni VS. Ovarian hyperstimulation syndrome following GnRH agonist trigger—think ectopic. J Assist Reprod Genet. 2017;34(9):1161–1165. doi: 10.1007/s10815-017-0960-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Y, Yang T, Hao C, Zhao J. A retrospective study of letrozole treatment prior to human chorionic gonadotropin in women with polycystic ovary syndrome undergoing in vitro fertilization at risk of ovarian hyperstimulation syndrome. Med Sci Monit. 2018;24:4248–4253. doi: 10.12659/MSM.910743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tshzmachyan R, Hambartsoumian E. The role of Letrozole (LE) in controlled ovarian stimulation (COS) in patients at high risk to develop ovarian hyper stimulation syndrome (OHSS). A prospective randomized controlled pilot study. J Gynecol Obstet Hum Reprod. 2020;49(2):101643. doi: 10.1016/j.jogoh.2019.101643. [DOI] [PubMed] [Google Scholar]
- 11.He Q, Liang L, Zhang C, Li H, Ge Z, Wang L, et al. Effects of different doses of letrozole on the incidence of early-onset ovarian hyperstimulation syndrome after oocyte retrieval. Syst Biol Reprod Med. 2014;60(6):355–60. doi: 10.3109/19396368.2014.957879. [DOI] [PubMed] [Google Scholar]
- 12.Jellad S, Hassine AH, Basly M, Mrabet A, Chibani M, Rachdi R. Vascular endothelial growth factor antagonist reduces the early onset and the severity of ovarian hyperstimulation syndrome. J Gynecol Obstet Hum Reprod. 2017;46(1):87–91. doi: 10.1016/j.jgyn.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 13.Choudhary RA, Vora PH, Darade KK, Pandey S, Ganla KN. A prospective randomised comparative clinical trial study of luteal phase letrozole versus ganirelix acetate administration to prevent severity of early onset OHSS in ARTs. Int J Fertil Steril. 2021;15(4):263–268. doi: 10.22074/IJFS.2021.139562.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lamb HM, Adkins JC. Letrozole. Drugs. 1998;56(6):1125–40. doi: 10.2165/00003495-199856060-00020. [DOI] [PubMed] [Google Scholar]
- 15.Marguerie M, Bedaiwy M. In: Textbook of Assisted Reproduction. Allahbadia GN, Ata B, Lindheim SR, Woodward BJ, Bhagavath B, et al., editors. Singapore: Springer Nature.; 2020. pp. 53–68. Letrozole in fertility therapy. [Google Scholar]
- 16.EsHRE R. ASRM-Sponsored PCOS Consensus Workshop Group Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil steril. 2004;81(1):19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 17.Golan A, Ron-El R, HERMAN A, Soffer Y. Ovarian hyperstimulation syndrome: an update review. Obstet Gynecol Surv. 1989;44(6):430–40. doi: 10.1097/00006254-198906000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Moher D, Hopewell S, Schulz KF, Montori V, G∅tzsche PC, Devereaux P, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. International Journal of Surgery. 2012;10(1):28–55. doi: 10.1016/j.ijsu.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 19.Dumesic DA, Oberfield SE, Stener-Victorin E, Marshall JC, Laven JS, Legro RS. Scientific statement on the diagnostic criteria, epidemiology, pathophysiology, and molecular genetics of polycystic ovary syndrome. Endocr Rev. 2015;36(5):487–525. doi: 10.1210/er.2015-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagini S. Breast cancer: current molecular therapeutic targets and new players. Anticancer Agents Med Chem. 2017;17(2):152–163. doi: 10.2174/1871520616666160502122724. [DOI] [PubMed] [Google Scholar]
- 21.Bülow NS, Skouby SO, Warzecha AK, Udengaard H, Andersen CY, Holt MD, et al. Impact of letrozole co-treatment during ovarian stimulation with gonadotrophins for IVF: a multicentre, randomized, double-blinded placebo-controlled trial. Hum Reprod. 2022;37(2):309–321. doi: 10.1093/humrep/deab249. [DOI] [PubMed] [Google Scholar]
- 22.Sopa N, Larsen EC, Nyboe Andersen A. Low dose HP-hMG in an antagonist protocol for IVF in ovulatory and anovulatory patients with high AMH. Gynecol Endocrinol. 2018;34(7):623–626. doi: 10.1080/09513590.2018.1428302. [DOI] [PubMed] [Google Scholar]
- 23.Fatemi H, Kyrou D, Papanikolaou E, Al Buarki H, Garcia Velasco J. Letrozole and cabergoline co-administration in the early luteal phase for prevention of OHSS in a high risk patient undergoing ovarian stimulation for IVF. J Fert In Vitro. 2012;2(4) [Google Scholar]
- 24.Khadem Ghaebi N, Amirian M, Zarmehri B, Zabihi H. Comparison of the effect of letrozole versus cabergoline for prevention of ovarian hyperstimulation syndrome (OHSS) in patients under ovulation induction treatments and IVF cycles. Iran J Obstet Gynecol Infertil. 2016;18(184):1–8. [Google Scholar]
- 25.Mai Q, Hu X, Yang G, Luo Y, Huang K, Yuan Y, et al. Effect of letrozole on moderate and severe early-onset ovarian hyperstimulation syndrome in high-risk women: a prospective randomized trial. Am J Obstet Gynecol. 2017;216(1):42. e1–. e10. doi: 10.1016/j.ajog.2016.08.018. [DOI] [PubMed] [Google Scholar]
- 26.Franik S, Kremer JA, Nelen WL, Farquhar C. Aromatase inhibitors for subfertile women with polycystic ovary syndrome. Cochrane Database Syst Rev. 2014;2:CD010287. doi: 10.1002/14651858.CD010287.pub2. [DOI] [PubMed] [Google Scholar]
- 27.Hu S, Yu Q, Wang Y, Wang M, Xia W, Zhu C. Letrozole versus clomiphene citrate in polycystic ovary syndrome: a meta-analysis of randomized controlled trials. Arch Obstet Gynaecol. 2018;297(5):1081–1088. doi: 10.1007/s00404-018-4688-6. [DOI] [PubMed] [Google Scholar]
- 28.Legro RS. Ovulation induction in polycystic ovary syndrome: current options. Best Pract Res Clin Obstet Gynaecol. 2016;37:152–159. doi: 10.1016/j.bpobgyn.2016.08.001. [DOI] [PubMed] [Google Scholar]


