Abstract
Background:
Use of magnetic resonance imaging (MRI) as a tool to aid in neuroprognostication after cardiac arrest (CA) has been described, yet details of specific indications, timing, and sequences are unknown. We aim to define the current practices in use of brain MRI in prognostication after pediatric CA.
Methods:
A survey was distributed to pediatric institutions participating in three international studies. Survey questions related to center demographics, clinical practice patterns of MRI after CA, neuroimaging resources, and details regarding MRI decision support.
Results:
Response rate was 31% (44 of 143). Thirty-four percent (15 of 44) of centers have a clinical pathway informing the use of MRI after CA. Fifty percent (22 of 44) of respondents reported that an MRI is obtained in nearly all patients with CA, and 32% (14 of 44) obtain an MRI in those who do not return to baseline neurological status. Poor neurological examination was reported as the most common factor (91% [40 of 44]) determining the timing of the MRI. Conventional sequences (T1, T2, fluid-attenuated inversion recovery, and diffusion-weighted imaging/apparent diffusion coefficient) are routinely used at greater than 97% of centers. Use of advanced imaging techniques (magnetic resonance spectroscopy, diffusion tensor imaging, and functional MRI) were reported by less than half of centers.
Conclusions:
Conventional brain MRI is a common practice for prognostication after CA. Advanced imaging techniques are used infrequently. The lack of standardized clinical pathways and variability in reported practices support a need for higher-quality evidence regarding the indications, timing, and acquisition protocols of clinical MRI studies.
Keywords: Child, Cardiac arrest, Neuroimaging, MRI, Hypoxia-ischemia, Brain, Surveys and questionnaires
Introduction
Pediatric cardiac arrest (CA) is a relatively uncommon event yet continues to be a major public health concern. Epidemiologic studies have described a prevalence of nontraumatic out-of-hospital CA (OHCA) of eight per 100,000 person years and a prevalence if in-hospital CA (IHCA) of 0.77 per 1000 hospital admissions. Mortality rates range from 40% to 60% for IHCA with greater than 90% for OHCA.1–5 In those who survive to hospital discharge, rates of survival with favorable neurological outcome are 4% to 16%6,7 for OHCA and 80% to 96%3,5 for IHCA.
Multiple monitoring modalities including electroencephalography, laboratory tests, and neuroimaging are used to aid management and prognostication after CA.8–11 However, no single modality has sufficient accuracy to facilitate neurological prognostication after return of spontaneous circulation (ROSC).12 In recent years, brain magnetic resonance imaging (MRI)-based biomarkers have been increasingly recognized as promising tools to evaluate children after CA. Recent studies have shown that conventional MRI sequences (T1, T2, and diffusion-weighted imaging [DWI]) can accurately identify hypoxic-ischemic injury and can be used to assist in prognostication.13–19 In addition, more advanced MRI techniques, such as MR spectroscopy (MRS), are able to measure the concentration of metabolites that serve as markers of injury and may also have prognostic value.10,20,21 Quantitative whole brain white matter fractional anisotropy measurements utilizing diffusion tensor imaging are associated with long-term neurological outcome in patients with CA.18 Resting-state functional MRI has also been shown to have prognostic information in patients with CA who are comatose and have indeterminate prognosis in patients with higher functional connectivity who recovered consciousness.19
Despite these advances, there is lack of evidence-based consensus regarding the indications, timing, and MRI sequences most useful in this population, and little is known about current practice. The objective of this study was to assess current practices of post-CA MRI for neuroprognostication at international tertiary care pediatric centers. To achieve our objective, we surveyed pediatric centers to assess practices in four areas: neuroimaging resources, rationale for MRI, timing of MRI acquisition following CA, and specific MRI sequences used.
Methods
Study design
This was a survey of current practices regarding brain MRI use in children post-CA. The survey was conducted between May and August 2020. Pediatric hospitals participating in the Personalizing Outcomes after Child Cardiac Arrest (POCCA) study, the Approaches and Decisions after Pediatric Traumatic Brain Injury (ADAPT) trial, and the Prevalence of Acute critical Neurological disease in children: a Global Epidemiological Assessment (PANGEA) study were invited to participate in the survey. All participating sites have expertise in critical care and imaging-related research.22–24
Survey development and distribution
The 16-item survey was developed in consultation with experts in the field of Pediatric Critical Care and Neuroradiology at the University of Pittsburgh and Oregon Health and Science University. After the initial survey was developed, the remainder of the study group provided feedback. The final survey consisted of a multiple choice questionnaire divided into two sections. The first section contained general information about each institution, the characteristics of each intensive care unit (total number of pediatric intensive care unit beds, estimated annual number of pediatric CA), and neuroimaging resources (number and type of MRI scanners, availability of experts in neuroradiology). The second section focused on MRI practices and presence of an institutional clinical pathway to support clinical decisions surrounding indications of MRI after CA, timing, and MRI sequences used in these patients.
The survey was distributed by the POCCA data coordinating center to all the participating sites. Each site’s medical director or principal investigator was contacted via e-mail and asked to complete the survey, with the assistance of the institution’s neuroradiologist if needed. Follow-up e-mails were sent every two weeks up to four times. A single survey was completed per institution. If an individual site participated in more than one of the studies used to generate the survey distribution list, the site principal investigator of the most contemporary study was selected to receive this survey. Site principal investigators were physicians in Pediatric Neurology or Pediatric Critical Care.
Statistical analysis
Descriptive statistics were summarized as frequencies for categorical data and median and interquartile range for continuous data. Data from survey results were dichotomized based on the presence or absence of a clinical pathway for use of brain MRI as a prognostication tool following pediatric CA. Submitted surveys that did not include a response to this question were excluded from data analysis. Statistical analyses were performed using SAS software, Version 9.4 of the SAS System for Windows. Copyright 2015, Cary, NC, USA.
Results
Cohort characteristics
The survey was distributed to 143 individual institutions. Of those, 32% (46 of 143) completed the survey. Two surveys did not include a response to the survey question: “Does your center have a clinical pathway for the use of brain MRI as a prognostication tool following pediatric cardiac arrest?” and thus were excluded. The respondents included 30 institutions from North America (68.2%) and 14 international institutions (31.8%). Fifty-nine percent (26 of 44) of respondents were from freestanding children’s hospitals, 36% (16 of 44) were from hospitals annexed to an adult hospital, and 5% (two of 44) responded “other” regarding hospital type. Centers identifying as “other” regarding hospital type indicated pediatric beds within an adult facility. Participating sites had a median of 23 (interquartile range 12 to 36) PICU beds. A minority of sites (34% [15 of 44]) reported a clinical pathway to guide MRI use in CA. The number of CA cases at responding sites varied substantially (Table 1).
TABLE 1.
Site Characteristics
| n (%) or Median (25th, 75th Percentiles) | All (N = 44) | Clinical Pathway for MRI |
|
|---|---|---|---|
| No (n = 29) | Yes (n = 15) | ||
|
| |||
| Center’s location | N = 44 | n = 29 | n = 15 |
| North America | 30 (68.2) | 21 (72.4) | 9 (60) |
| International | 14(31.8) | 8 (27.6) | 6 (40) |
| Type of hospital | N = 44 | n = 29 | n = 15 |
| Freestanding children’s hospital | 26 (59) | 15 (51.7) | 11 (73.3) |
| Children’s hospital annexed to an adult hospital | 16 (36.3) | 13 (44.8) | 3 (20) |
| Other | 2 (4.55) | 1 (3.45) | 1 (6.67) |
| Number of PICU beds | N = 43 | n = 28 | n = 15 |
| 23 (12–36) | 19 (13.5–32.5) | 36 (10–48) | |
| Estimated no. pediatric in-hospital cardiac arrests per year | N = 43 | n = 29 | n = 14 |
| <10 | 10 (23.2) | 8 (27.5) | 2 (14.2) |
| 10–19 | 11 (25.5) | 9 (31.0) | 2 (14.2) |
| 20–29 | 12 (27.9) | 7 (24.1) | 5 (35.7) |
| 30–39 | 6 (13.9) | 2 (6.9) | 4 (28.0) |
| ≥ 40 | 3 (6.9) | 2 (6.9) | 1 (7.1) |
| Unknown | 1 (2.3) | 1 (3.4) | 0 (0.0) |
| Estimated no. pediatric out-of-hospital cardiac arrests per year | N = 43 | n = 28 | n = 15 |
| <10 | 11 (25.5) | 8 (28.5) | 3 (20.0) |
| 10–19 | 13 (30.2) | 9 (32.1) | 4 (26.6) |
| 20–29 | 13 (30.2) | 7 (25.0) | 6 (40.0) |
| 30–39 | 1 (2.3) | 1 (3.5) | 0 (0.0) |
| ≥ 40 | 4 (9.3) | 2 (7.1) | 2 (13.3) |
| Unknown | 1 (2.3) | 1 (3.5) | 0 (0.0) |
Abbreviations:
MRI = Magnetic resonance imaging
PICU = Pediatric intensive care unit
Neuroimaging resources
The majority (75% [33 of 44]) of respondents reported that their institution employs a neuroradiologist to interpret brain MRIs (Table 2). Of centers with neuroradiology expertise, 55% (18 of 33) report these subspecialists are available to interpret MRIs 24/7. A higher percentage of institutions with a clinical pathway for post-CA MRI reported having 24/7 neuroradiology availability (57% vs 34%). Eighty-one percent (35 of 43) of responding centers have a 3-T MRI available, although 19% (eight of 43) are limited to 1.5-T MRI.
TABLE 2.
Neuroimaging Resources
| n (%) or Median (25th, 75th Percentiles) | All (N = 44) | Clinical Pathway for MRI |
|
|---|---|---|---|
| No (n = 29) | Yes (n = 15) | ||
|
| |||
| Neuroradiologist for MRI interpretation available | N = 44 | n = 29 | n = 15 |
| Yes | 33 (75.0) | 23 (79.3) | 10 (66.7) |
| Neuroradiologist available 24/7 | N = 43 | n = 29 | n = 14 |
| Yes | 18 (41.9) | 10 (34.5) | 8 (57.1) |
| MRI scanner within the ICU | N = 44 | n = 29 | n = 15 |
| Yes | 1 (2.3) | 0 (0.0) | 1 (6.7) |
| MRI field strength | N = 43 | n = 28 | n = 15 |
| 1.5 T only | 8 (18.6) | 7 (25.0) | 1 (6.7) |
| 3.0 T only | 3 (7.0) | 2 (7.1) | 1 (6.7) |
| More than one field strength among 1.5 T, 3.0 T, and other | 32 (74.4) | 19 (67.9) | 13 (86.7) |
Abbreviations:
ICU = Intensive care unit
MRI = Magnetic resonance imaging
Practices regarding MRI use in cardiac arrest
Of the participating institutions, 45% (20 of 44) have a dedicated pediatric neurocritical care team/service (Table 3). Institutions with a pediatric neurocritical care team/service were almost twice as likely to have a clinical pathway for MRI after CA (66.7% vs 34.5%). Fifty percent of institutions (22 of 44) obtain an MRI in nearly all pediatric patients with CA, and 31.8% (14 of 44) of institutions reported performing MRI in patients who are not back to their baseline. Centers with a clinical pathway for MRI use after CA were less likely to obtain an MRI on a “case-by-case” basis (6.7% [one of 15]) compared with centers without a clinical pathway (24.1% [seven of 29]).
TABLE 3.
Clinical Practices Regarding the Use of MRI in pediatric CA
| n (%) or Median (25th, 75th Percentiles) | All (N = 44) | Pathway |
|
|---|---|---|---|
| No (n = 29) | Yes(n = 15) | ||
|
| |||
| Pediatric neurocritical care team/service | N = 44 | n = 29 | n = 15 |
| Yes | 20 (45.5) | 10 (34.5) | 10 (66.7) |
| Typical practice for MRI prognostication after CA | N = 44 | n = 29 | n = 15 |
| Brain MRI is performed for nearly all patients (with some exception such as DNR) | 22 (50.0) | 15 (51.7) | 7 (46.7) |
| Brain MRI is performed for patients who are not back to their baseline neurological status | 14(31.8) | 7 (24.1) | 7 (46.7) |
| Brain MRI is performed for patients only on a “case-by-case” basis | 8 (18.2) | 7 (24.1) | 1 (6.7) |
| Typical timing of MRI for prognostication after CA | N = 44 | n = 29 | n = 15 |
| Within the first 72 hours after ROSC (acute) | 12 (27.3) | 6 (20.7) | 6 (40.0) |
| After 72 hours from ROSC, but before transfer to ward (subacute) | 28 (63.6) | 19 (65.5) | 9 (60.0) |
| Before hospital discharge or transfer to inpatient rehabilitation | 0 (0) | - | - |
| Other | 4(9.1) | 4 (13.8) | 0 (0.0) |
| Factors determining timing of MRI Intubation status: MRI timing is adjusted to accommodate/prioritize extubation |
N = 44 | n = 29 | n = 15 |
| Yes | 31 (70.5) | 20 (69.0) | 11 (73.3) |
| Poor neurological examination: the team decides to obtain MRIs earlier | N = 44 | n = 29 | n = 15 |
| Yes | 40 (90.9) | 25 (86.2) | 15 (100.0) |
| Favorable neurological examination: the team waits longer to obtain MRI | N = 44 | n = 29 | n = 15 |
| Yes | 27 (61.4) | 16 (55.2) | 11 (73.3) |
Abbreviations:
CA = Cardiac arrest
DNR = Do not resuscitate
MRI = Magnetic resonance imaging
ROSC = Return of spontaneous circulation
The majority of institutions (63.6% [28 of 44]) perform an MRI after 72 hours of ROSC but before transfer to the inpatient ward, 27.3% of institutions (12 of 44) obtain an MRI within 72 hours of ROSC, and 9% (four of 44) report the timing of MRI for prognostication after CA as “other.” All centers with a clinical pathway reported obtaining an MRI either within the first 72 hours after ROSC (40% [six of 15]) or greater than 72 hours after ROSC and before transfer to the inpatient ward (60% [nine of 15]). None of the centers indicating the timing of MRI as “other” reported having a clinical pathway. When asked to indicate what factors are used to determine timing of brain MRI for prognostication, 90% of centers (40 of 44) reported poor neurological examination as the main factor determining the timing of the MRI. Other clinical factors determining timing of imaging for prognostication included intubation status (70% [31 of 44]) and favorable neurological examination (61% [27 of 44]). All centers with a clinical pathway for MRI after CA indicated at least one clinical factor is used to determine timing of imaging for prognostication. Only one center reported using no clinical factors to determine timing of MRI.
Types of MRI sequences used in children with cardiac arrest
Sites were asked to indicate which sequences were included in the imaging protocol used to evaluate children with CA. The percentage of centers using each specific MRI sequence, stratified by centers with and without a pathway, is illustrated in Fig. Over 97% (43 of 44) of institutions use T1, T2, and fluid-attenuated inversion recovery (FLAIR). All centers reported obtaining DWI. Sequences sensitive to hemorrhage such as gradient echo and susceptibility-weighted imaging are used in 43% and 67% of centers, respectively. Less than half of the institutions (40% and 31% of institutions with and without a pathway, respectively) report use of spectroscopy (MRS). Other techniques, such as functional MRI, magnetic resonance venogram, and postcontrast T1 are used by less than 30% of institutions, regardless of the presence or absence of a pathway.
FIGURE.
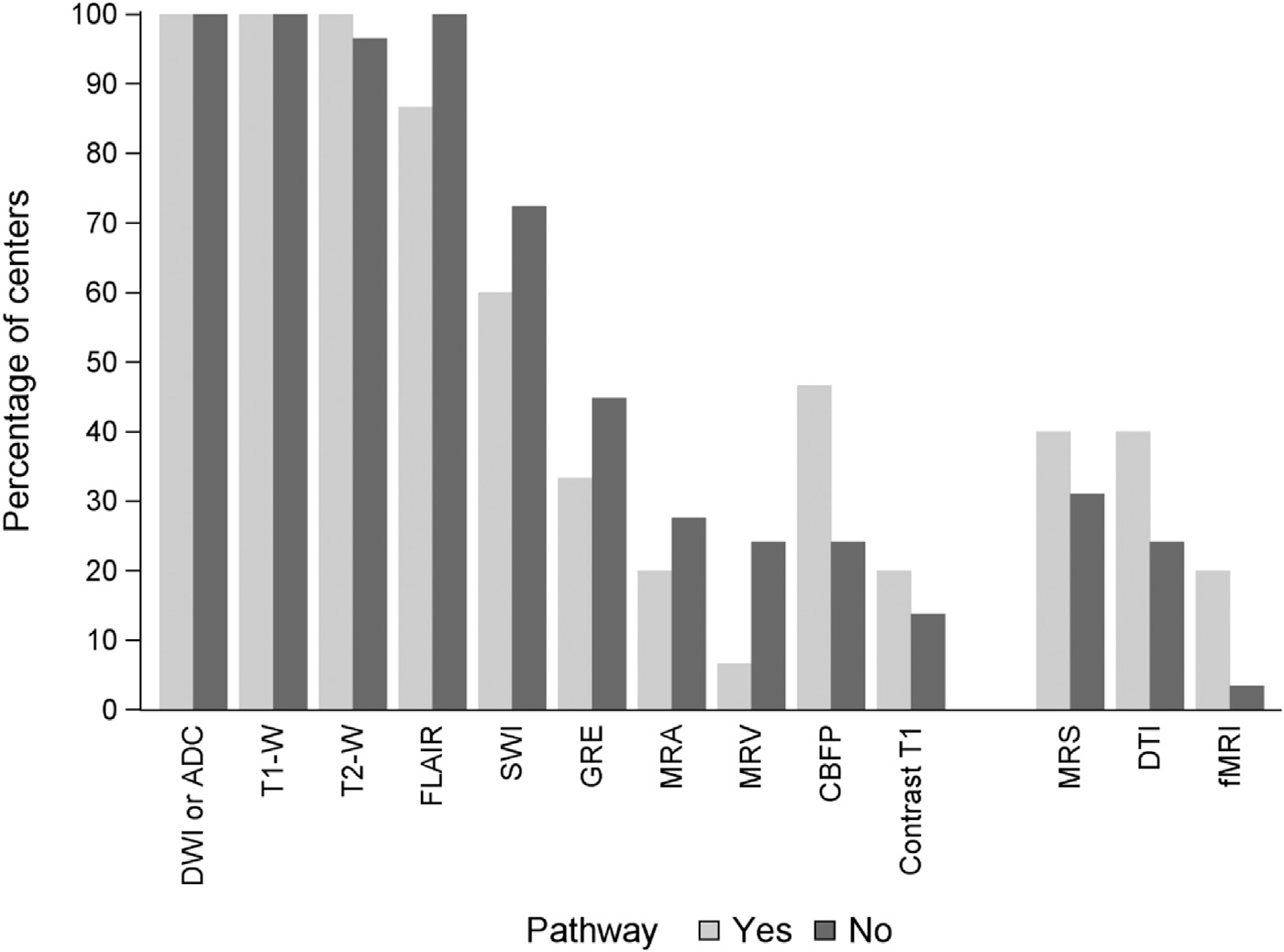
Type of MRI sequences used in pediatric cardiac arrest. DWI, diffusion-weighted imaging; ADC, apparent diffusion coefficient; FLAIR, fluid-attenuated inversion recovery; GRE, gradient echo; SWI, susceptibility-weighted imaging; CBFP, cerebral blood flow/perfusion; MRS, magnetic resonance spectroscopy; DTI, diffusion tensor imaging; MRA, magnetic resonance angiogram; MRV, magnetic resonance venogram; fMRI, functional MRI.
Discussion
Our study describes current practices in use of brain MRI as a neuroprognostic tool in children with CA from 44 national and international pediatric tertiary care centers. The results suggest that clinicians often perform MRI as part of their routine care in children with CA, with half of the responding institutions indicating that they obtain MRI in nearly all patients post-CA, and an additional 30% indicating that they obtain an MRI in those who are not back to their neurological baseline. Nearly all institutions routinely incorporate conventional sequences (i.e., T1, T2, DWI, and FLAIR). Reported use of sequences sensitive to hemorrhage is 43% (gradient echo) and 67% (susceptibility-weighted imaging). These rates appear low, but it is likely that centers choose to utilize a single sequence to evaluate for hemorrhage, resulting in little overlap in these values, thus indicating that this practice is also widely used at surveyed centers. However, there is still significant heterogeneity and less use of advanced MRI sequences post-CA. We found that only a minority of the protocols incorporate advanced imaging sequences such as MRS. There are several potential reasons or explanations that may contribute to these observations. First, although promising, data regarding the clinical value of sequences such as MRS are limited to single-center, retrospective studies. Second, the interpretation of these images requires particular expertise. In our study, 25% of centers do not have a member of the radiology team with specific training in neuroradiology.
In our study, only 34% of centers indicated that they have an established clinical pathway for the use of MRI in pediatric CA. Institutions with CA pathways informing MRI utilization reported less variation in patient selection or timing of MRIs. The lack of pathway-driven practices is likely due to a number of factors including sparse and low-quality evidence in pediatrics and practice guidelines developed by data extrapolated from adult patients or by expert opinion.25 In addition, an increasing number of institutions have incorporated pediatric neurocritical care teams to build institutional expertise and provide standardized, evidence-based care for children with acute brain injury.26,27 Although less than half of the responding institutions have a pediatric neurocritical care team available, the presence of a this team doubled the likelihood of pathway-driven care.
Taken together, these observations further highlight the need for evidence to guide clinical practice in the area of pediatric CA. Recent guidelines published by the American Heart Association cite insufficient evidence to support the use of MRI as a standard of care in pediatric CA and state that “MRI during the first seven days post-arrest may be valuable” in assisting with prognostication.12 Beyond this, no recommendations are made regarding indications, optimal timing, or acquisition protocol. The guidelines cite retrospective study design, small sample size, selection bias, lack of blinding in imaging interpretation, variable outcome measures, and follow-up times as limitations to the current evidence.
Conventional MRI protocols designed for clinical use include sequences such as T1- and T2-weighted images, FLAIR, and diffusion-weighted imaging.28 Prior studies have investigated the utility of these sequences in the assessment of children with CA. In a single-center study of 28 children who received a brain MRI within the first 14 days post-ROSC, T2 signal intensity abnormalities in the basal ganglia and diffusion restriction in the cerebral lobes were associated with unfavorable outcomes.15 A separate study evaluated the correlation between imaging abnormalities and unfavorable outcomes in 23 children with CA who underwent MRI within 14 days after ROSC.10 The injured areas most commonly identified by T2-weighted imaging were the lentiform nucleus (68%), the caudate (55%), and the thalamus (50%). T2 abnormalities in the lentiform nuclei were associated with unfavorable outcomes at hospital discharge. Diffusion restriction was most commonly seen in the lentiform nucleus (41%), thalamus (32%), frontal (30%), and parietal (30%) lobes. More recently, a single-center study of 77 pediatric patients after OHCA who had an MRI at a median of day 4 post-CA found that the extent of diffusion restriction and T2/FLAIR abnormalities was associated with unfavorable outcomes with good discrimination (area under the receiver operator curve of 0.96 [95% confidence interval 0.91, 0.99] for diffusion and 0.92 [0.85, 0.97] for T2/FLAIR injury).17 In addition toT2-weighted imaging and DWI, more advanced imaging modalities such as MRS may have prognostic value in this population. Increased lactate concentrations in the parieto-occipital gray matter and decreased N-acetyl-aspartate in the parietal white matter using MRS have been observed in children with CA and unfavorable outcomes.10 Decreased N-acetyl-aspartate and increased lactate were also observed in a study of children with acute brain injury (including CA) and unfavorable outcomes.20
Given the evolution of the appearance of brain lesions on MRI, both the composition of the MRI protocol and the timing of the initial MRI after presentation are critical to capture the extent of brain injury accurately. MRI sequences sensitive to ischemia and cytotoxic edema such as DWI/apparent diffusion coefficient often identify lesions in children with CA. Unlike abnormalities on T1 and T2 MRI sequences, diffusion abnormalities can be seen within minutes in acute ischemia (i.e., arterial ischemic stroke). However, diffusion abnormalities can also be delayed in CA possibly due to the variability of hemodynamic compromise over the course of the resuscitation and postresuscitation period.29–32 Thus, to ensure appropriate evaluation of the presence or absence of hypoxicischemic brain injury after CA, the timing of MRI is very important. The American Heart Association guidelines recommend considering use of MRI “within the first seven days after injury.” 12 In our survey, most centers stated that they perform MRIs after 72 hours post-ROSC. Ninety percent stated that poor neurological examination is a main determinant for obtaining an MRI early, and 61% stated that a favorable neurological examination would lead to clinical teams waiting longer to obtain an MRI.
Our survey has a number of limitations that should be considered when interpreting these results. First, our survey response rate was relatively low at 30%. Second, a limitation common to survey studies with a single respondent per center is that the responses are based on the individual’s understanding of current institutional practices and may represent her or his opinion and practice over fact. Third, specific details regarding post-CA patient volume at surveyed centers was not requested. Instead, estimates of patient volume were provided to reduce the potential burden for the survey respondent. The values of patient volumes provided resulted in a way to stratify centers by estimated volume, but did not allow for precise understanding of the exact patient load at each center. The authors believe the estimated post-CA patient volumes are sufficient to make necessary conclusions regarding practice variation with respect to MRI use in neuroprognostication and center volume. Fourth, the survey question asked the respondent to describe the center’s typical practice for use of MRI for neuroprognostication after CA. The goal of the question was to determine if centers uniformly obtain a brain MRI in all cases or if other factors influenced the decision to obtain an MRI. The responses were the expert opinion of the clinician who completed the survey. There were no specific prompts within the survey question to define neurological examination findings that might describe details of return to neurological baseline status or poor neurological examination thus limiting the precision of these data. Fifth, the survey did not capture clinical details at the patient level. The survey was seeking details of general institutional practices with regard to MRI use for neuroprognostication after CA. Owing to the inherent limitations of a survey, as well as the fine balance of creating a thorough detailed survey while not overburdening the respondent, questions focused on etiology of arrest or contraindications for MRI were not included. Sixth, the surveyed institutions represent tertiary care facilities with an interest in clinical research in pediatric CA, which may not be generalizable to all pediatric intensive care settings. Imaging resources and practices may differ from institutions not participating in large research consortia, limiting the external validity of our findings. Of note, the cohort was not limited to large centers and included institutions with a broad spectrum of size and number of CA cases per year. Last, the correlation between the indications for MRI, timing, and acquisition protocols and patient outcomes was beyond the scope of this survey. Prospective, adequately powered, multicenter studies with standardized, patient-oriented outcomes are needed to bridge this knowledge gap.
Our survey reveals prevalent use of MRI for neuroprognostication in pediatric CA. Responses suggest a wide variability in the timing, MRI sequences, and neuroradiology resources, as well as a dearth of centers with clinical pathways to guide MRI utilization after pediatric CA. Studies to ultimately determine the optimal use of MRI in pediatric CA will need to account for and implement strategies to reduce this variation.
Acknowledgments
We acknowledge members of the POCCA Steering Committee: Patrick Kochanek, MD, MCCM; Robert Clark, MD; Hulya Bayir, MD; Ashok Panigrahy, MD; Rachel Berger, MD, MPH; Sue Beers, PhD; and Tony Fabio, PhD, MPH; POCCA investigators: Karen Walson, MD (Children’s Healthcare of Atlanta); Alexis Topjian, MD, MSCE (Children’s Hospital of Philadelphia); Christopher JL Newth, MD, FRCPC (Children’s Hospital of Los Angeles); Elizabeth Hunt, MD, MPH, PhD, Jordan Duval-Arnould, DrPH, MPH (Johns Hopkins Children’s Center); Binod Balakrishnan, MD, Michael T. Meyer, MD, MS, FCCM (Children’s Hospital of Wisconsin); Melissa G. Chung, MD (Nationwide Children’s Hospital); Anthony Willyerd, MD (Phoenix Children’s Hospital); Lincoln Smith, MD, Jesse Wenger, MD (Seattle Children’s); Stuart Friess, MD, Jose Pineda, MD, MS (St. Louis Children’s); Ashley Siems, MD, Jason Patregnani, MD, John Diddle, MD (Children’s National Hospital); Aline Maddux, MD, MSCS, and Craig Press, MD, PhD (Children’s Hospital of Colorado); Lesley Doughty, MD (Cincinnati Children’s Hospital Medical Center); and Juan Piantino, MD (Doernbecher Children’s Hospital); and the POCCA coordinators: David Maloney, BS, Pamela Rubin, RN (UPMC Children’s Hospital of Pittsburgh); Beena Desai, BS, CCRC, Maureen G. Richardson, BSN, RN, CPN, Cynthia Bates, CCRP (Children’s Healthcare of Atlanta); Darshana Parikh, Janice Prodell, Maddie Winters, Katherine Smith, MPH, BSN, RN, CPN (Children’s Hospital of Philadelphia); Jeni Kwok, JD, Adriana Cabrales, BA (Children’s Hospital of Los Angeles); Ronke Adewale, Pam Melvin(Johns Hopkins Children’s Center); Sadaf Shad, Katherine Siegel, Katherine Murkowski, Mary Kasch (Children’s Hospital of Wisconsin); Josey Hensley RN, BSN, Lisa Steele, RN, BSN (Nationwide Children’s Hospital); Danielle Brown, Brian Burrows, Lauren Hlivka (Phoenix Children’s Hospital); Deana Rich (Seattle Children’s Hospital); Amila Tutundzic, Tina Day, Lori Barganier (St. Louis Children’s Hospital); Ashley Wolfe, Mackenzie Little, Elyse Tomanio, Neha Patel, Diane Hession (Children’s National Hospital); Yamila Sierra MPH, CCRP (Children’s Hospital of Colorado); and Rhonda Jones, Laura Benken (Cincinnati Children’s).
We also thank Jonathan Elmer, MD, MS; Subramanian Subramanian, MD; Srikala Narayanan, MD; Julia Wallace; Tami Robinson; Andrew Frank; Stefan Bluml, PhD; Jessica Wisnowski, PhD; Keri Feldman; Avinash Vemulapalli; Linda Ryan; Scott Szypulski, MBA; Christopher Keys, and all the children and families who generously participated in POCCA. We are grateful to the all ICU staff, nurses, and physicians for their efforts in study recruitment and provision of excellent clinical care to families.
Funding:
Juan Piantino: National Heart, Lung, and Blood Institute (NHLBI) K23HL150217. Ericka Fink: National Institute of Neurological Disorders and Stroke (NINDS) R01NS096714.
Footnotes
Declarations of Interest: Dr. Fink receives funding from National Institute of Neurological Disorders and Stroke (NINDS) R01NS096714 and Neurocritical Care Society Incline Grant.
Dr. Piantino receives support from the National Institute of Health, National Heart, Lung, and Blood Institute, grant K23HL150217.
Dr. Press has performed paid expert testimony and provides consulting for Marinus Pharmaceuticals.
The remaining authors have no relevant interests to disclose.
References
- 1.Atkins DL, Everson-Stewart S, Sears GK, et al. Epidemiology and outcomes from out-of-hospital cardiac arrest in children: the resuscitation outcomes consortium epistry-cardiac arrest. Circulation. 2009;119:1484–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Topjian AA, Berg RA. Pediatric out-of-hospital cardiac arrest. Circulation. 2012;125:2374–2378. [DOI] [PubMed] [Google Scholar]
- 3.Berg RA, Sutton RM, Holubkov R, et al. Ratio of PICU versus ward cardiopulmonary resuscitation events is increasing. Crit Care Med. 2013;41:2292–2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knudson JD, Neish SR, Cabrera AG, et al. Prevalence and outcomes of pediatric in-hospital cardiopulmonary resuscitation in the United States: an analysis of the Kids’ Inpatient Database. Crit Care Med. 2012;40:2940–2944. [DOI] [PubMed] [Google Scholar]
- 5.Girotra S, Spertus JA, Li Y, et al. Survival trends in pediatric in-hospital cardiac arrests: an analysis from get with the guidelines-resuscitation. Circ Cardiovasc Qual Outcomes. 2013;6:42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meert KL, Telford R, Holubkov R, et al. Pediatric out-of-hospital cardiac arrest characteristics and their association with survival and neurobehavioral outcome. Pediatr Crit Care Med. 2016;17:e543–e550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donoghue AJ, Nadkarni V, Berg RA, et al. Out-of-hospital cardiac arrest: an epidemiologic review and assessment of current knowledge. Ann Emerg Med. 2005;46:512–522. [DOI] [PubMed] [Google Scholar]
- 8.Fink EL, Berger RP, Clark RSB, et al. Exploratory study of serum ubiquitin carboxyl-terminal esterase L1 and glial fibrillary acidic protein for outcome prognostication after pediatric cardiac arrest. Resuscitation. 2016;101:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fink EL, Berger RP, Clark RSB, et al. Serum biomarkers of brain injury to classify outcome after pediatric cardiac arrest. Crit Care Med. 2014;42:664–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fink EL, Wisnowski J, Clark R, et al. Brain MR imaging and spectroscopy for outcome prognostication after pediatric cardiac arrest. Resuscitation. 2020;157:185–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abend NS, Topjian AA, Kessler SK, et al. Outcome prediction by motor and pupillary responses in children treated with therapeutic hypothermia after cardiac arrest. Pediatr Crit Care Med. 2012;13:32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Topjian AA, de Caen A, Wainwright MS, et al. Pediatric post-cardiac arrest care: a scientific statement from the American Heart Association. Circulation. 2019;140:e194–e233. [DOI] [PubMed] [Google Scholar]
- 13.Högler S, Sterz F, Sipos W, et al. Distribution of neuropathological lesions in pig brains after different durations of cardiac arrest. Resuscitation. 2010;81:1577–1583. [DOI] [PubMed] [Google Scholar]
- 14.Back T, Hemmen T, Schüler OG. Lesion evolution in cerebral ischemia. J Neurol. 2004;251:388–397. [DOI] [PubMed] [Google Scholar]
- 15.Fink EL, Panigrahy A, Clark RSB, et al. Regional brain injury on conventional and diffusion weighted MRI is associated with outcome after pediatric cardiac arrest. Neurocrit Care. 2013;19:31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Christophe C, Fonteyne C, Ziereisen F, et al. Value of MR imaging of the brain in children with hypoxic coma. AJNR Am J Neuroradiol. 2002;23:716–723. [PMC free article] [PubMed] [Google Scholar]
- 17.Kirschen MP, Licht DJ, Faerber J, et al. Association of MRI brain injury with outcome after pediatric out-of-hospital cardiac arrest. Neurology. 2021;96:e719–e731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Velly L, Perlbarg V, Boulier T, et al. Use of brain diffusion tensor imaging for the prediction of long-term neurological outcomes in patients after cardiac arrest: a multicentre, international, prospective, observational, cohort study. Lancet Neurol. 2018;17:317–326. [DOI] [PubMed] [Google Scholar]
- 19.Pugin D, Hofmeister J, Gasche Y, et al. Resting-state brain activity for early prediction outcome in postanoxic patients in a coma with indeterminate clinical prognosis. AJNR Am J Neuroradiol. 2020;41:1022–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ashwal S, Holshouser BA, Tomasi LG, et al. 1H-magnetic resonance spectroscopy-determined cerebral lactate and poor neurological outcomes in children with central nervous system disease. Ann Neurol. 1997;41:470–481. [DOI] [PubMed] [Google Scholar]
- 21.Barkovich AJ, Baranski K, Vigneron D, et al. Proton MR spectroscopy for the evaluation of brain injury in asphyxiated, term neonates. AJNR Am J Neuroradiol. 1999;20:1399–1405. [PMC free article] [PubMed] [Google Scholar]
- 22.Fink EL, Clark RSB, Panigrahy A, et al. Personalising Outcomes after Child Cardiac Arrest (POCCA): design and recruitment challenges of a multicentre, observational study. BMJ Open. 2020;10, e039323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Larsen GY, Schober M, Fabio A, et al. Structure, process, and culture differences of pediatric trauma centers participating in an international comparative effectiveness study of children with severe traumatic brain injury. Neurocrit Care. 2016;24:353–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fink EL, Kochanek PM, Tasker RC, et al. International survey of critically Ill children with acute neurologic insults: the prevalence of acute critical neurological disease in children: a global epidemiological assessment study. Pediatr Crit Care Med. 2017;18:330–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cronin M, Wainwright MS. Underpowered and too heterogenous: a humbling assessment of the literature supporting neuroprognostication after pediatric cardiac arrest. Pediatr Crit Care Med. 2020;21:915–916. [DOI] [PubMed] [Google Scholar]
- 26.Wainwright MS, Grimason M, Goldstein J, et al. Building a pediatric neurocritical care program: a multidisciplinary approach to clinical practice and education from the intensive care unit to the outpatient clinic. Semin Pediatr Neurol. 2014;21:248–254. [DOI] [PubMed] [Google Scholar]
- 27.Wainwright MS, Hansen G, Piantino J. Pediatric neurocritical care in the 21st century: from empiricism to evidence. Curr Opin Crit Care. 2016;22:106–112. [DOI] [PubMed] [Google Scholar]
- 28.Arbelaez A, Castillo M, Mukherji SK. Diffusion-weighted MR imaging of global cerebral anoxia. AJNR Am J Neuroradiol. 1999;20:999–1007. [PMC free article] [PubMed] [Google Scholar]
- 29.Beauchamp NJ Jr, Bryan RN. Acute cerebral ischemic infarction: a pathophysiologic review and radiologic perspective. AJR Am J Roentgenology. 1998;171:73–84. [DOI] [PubMed] [Google Scholar]
- 30.Chien D, Kwong KK, Gress DR, Buonanno FS, Buxton RB, Rosen BR. MR diffusion imaging of cerebral infarction in humans. AJNR Am J Neuroradiol. 1992;13:1097–1102 [discussion 1103–1105]. [PMC free article] [PubMed] [Google Scholar]
- 31.Gray L, MacFall J. Overview of diffusion imaging. Magn Reson Imaging Clin N Am. 1998;6:125–138. [PubMed] [Google Scholar]
- 32.Reith W, Hasegawa Y, Latour LL, Dardzinski BJ, Sotak CH, Fisher M. Multislice diffusion mapping for 3-D evolution of cerebral ischemia in a rat stroke model. Neurology. 1995;45:172–177. [DOI] [PubMed] [Google Scholar]


