Graphical Abstract:

INTRODUCTION
The field of metabolomics continues to grow exponentially, as it contributes significantly to numerous disciplines including biomedicine, pharmacology, drug development, toxicology, as well as environmental, plant, food and nutrition science. Metabolomics investigations are widely applied to both basic and applied sciences with a majority focused on biomedicine, necessitated by the urgent need to address major human diseases including cancer. Many studies are focused on the mechanistic understanding of biochemical processes, early disease diagnosis, and therapeutic management of human diseases.
Nuclear magnetic resonance (NMR) spectroscopy and mass spectrometry (MS) are the two most widely used analytical platforms in the metabolomics field. MS offers significantly higher sensitivity than NMR, and hence, it enables analysis of many more metabolites compared to NMR. Most MS studies (~80%) utilize liquid chromatography (LC) to separate metabolites prior to detection,1 but even so, major challenges for LC-MS analysis include ion suppression and formation of adducts, which cause variation in peak intensities and increased spectral complexity, respectively. Stringent analytical protocols and/or isotope labeled internal standards are therefore commonly required to help alleviate these challenges. NMR, on the other hand, is less sensitive than MS and exhibits somewhat limited spectral resolution for complex biological samples. Generally, chromatography is not used in NMR analysis unlike in MS because it reduces sensitivity and increases variability. However, NMR exhibits numerous unique characteristics that are beneficial to the field of metabolomics.1–4 For instance, NMR is highly reproducible and quantitative. It enables absolute quantitation of metabolites using a single internal standard or even without the need for an internal standard5 on a routine basis. NMR is a gold standard for the establishment of the identity of unknown metabolites unambiguously. It enables the analysis of samples with little to no need for sample preprocessing. It is non-destructive and hence enables repeated NMR measurements on the same sample or the sample can be reused for other studies. Its unique ability to distinguish between various isotopomers enables tracing and quantifying metabolite fluxes in multiple pathways from a single measurement. Further, using NMR, the same metabolites can be detected through different types of atomic nuclei such as 1H, 13C, 31P or 15N, and thus NMR offers numerous options for metabolite analysis and probing metabolic pathways. These benefits of NMR far outweigh its limitations of sensitivity and resolution.
Given NMR’s benefits and in response to its challenges, efforts to advance NMR-based metabolomics have been made in terms of new technologies as well as applications to disease. Advances to date have helped to improve the resolution and sensitivity, and to detect a larger number of metabolite signals. There have been sustained efforts to achieve a quantum jump in the sensitivity through nuclear hyperpolarization. On the instrumentation side, there have been advances in technologies in both high (>1000 MHz) and low (<100 MHz) magnetic field instruments, which are suited for high resolution and/or high-throughput applications, respectively. NMR applications that focus on disease investigations have been growing exponentially too. In this review, we describe developments in the NMR based metabolomics field over the past two years, from mid-2020 to mid-2022, with an emphasis on disease related investigations. The goal, however, is not to cover all NMR-based metabolomics studies. Instead, we highlight new developments and some important applications that will provide a glimpse of the current status and future directions of the field. Several recent reviews cover different aspects of NMR in biomedicine and related areas, and are a useful source for further information.1,3,6,7
NEW METHODS TO INCREASE SENSITIVITY AND SPEED
Hyperpolarization continues to attract attention in the metabolomics field since the transient increases in nuclear spin populations boost the sensitivity enormously. Numerous methods such as dynamic nuclear polarization (DNP), spin-exchange optical pumping, and para-hydrogen methods (which include para-hydrogen-induced polarization (PHIP) and signal amplification by reversible exchange (SABRE)) are currently used to hyperpolarize nuclear spins and promise numerous applications in metabolomics.
PHIP uses a catalyt to convert singlet-spin hydrogen that has been polarized at low temperatures to observable polarization detectable by NMR using a hydrogenation reaction of unsaturated target molecules,8,9 and provides a cost effective way to achieve hyperpolarization. To date, significant progress has been made including the synthesis of new tracers, catalysts, and transfer methods. Recently, the PHIP has been extended to amino acids, which can have far reaching implications10 for metabolism studies (Figure 1). The discovery of SABRE and SABRE-SHEATH (shield enables alignment transfer to heteronuclei) as non-hydrogenation routes to achieve nuclear spin polarization has enabled high nuclear polarization of both proton and heteronuclear spins in a broad range of metabolites and other compounds and doesn’t require unsaturated target molecules.11–16 An excellent review on the application of these methods that can now selectively detect and quantify analytes down to nanomolar concentrations has been reported recently.17 Further, using this approach, the analysis of urine metabolites was recently reported,18 which avoids the need for solid phase extraction (SPE) and replaces it with a minimal sample manipulation that retains the majority of urinary metabolome. A persistent challenge, however, is that the hyperpolarization approach does not boost the signal intensity evenly across all molecules in a mixture, which makes the determination of concentrations more difficult.
Figure 1:

Parahydrogen-based hyperpolarization: Schematic view of the spin states of dihydrogen (a,b), their populations at >300 K and ca. 25 K (c), as well as the basic reactions of hydrogenative (d) and non-hydrogenative (e) hyperpolarization. The nuclear spins of dihydrogen form four different spin states: three ortho (triplet: T+, T0, T−; a), and one para state (singlet, S; b). At room temperature, all four states are approximately equally populated (c). By cooling the H2, the equilibrium is shifted toward the para state and almost pure pH2 is obtained at ca. 25 K (c). To use the NMR-invisible para order, pH2 is added either permanently to an unsaturated molecule (d,e), or brought into temporary contact through reversible exchange (f). Next, the spin order is converted spontaneously (free evolution) into observable polarization (pol) induced by RF pulses or variations of the magnetic field. The polarization may be observed on the added hydrogen atoms or transferred to other nuclei. Reprinted from Publication Parahydrogen-Induced Polarization of Amino Acids, 60(44), Pravdivtsev, A.N.; Buntkowsky, G.; Duckett, S.B.; Koptyug, I.V.; Hövener, J-B. 23496–23507 (ref 10), Copyright (2021), with permission from Elsevier.
The introduction of ultrafast NMR experiments enabled data acquisition in a single scan with a sub-second data acquisition time.19 And the combination of ultrafast experiments with hyperpolarization promises important applications in metabolomics. Recently, such experiments were demonstrated for mixtures of substrates.20 Here, TOCSY (total correlation spectroscopy) and multiple quantum 2D NMR spectra of hyperpolarized specimens were obtained based on dissolution DNP (dDNP) in which the sample is dissolved in a radical containing matrix that is hyperpolarized using microwave irradiation. Polarization transfer to nuclear spins occurs within the solid matrix, which is then quickly dissolved in a solvent, flowed to a high-resolution NMR magnet and detected.21 Generally, for this type of hyperpolarization, 13C-isotope labeled substrates are used as their longer relaxation times allow the polarization to survive the dissolution and transit times compared to 1H. However, the ability to hyperpolarize substrates at natural 13C abundance would significantly impact metabolomics research. Such an approach based on dDNP was shown for the first time for hyperpolarization of 13C at natural abundance using tomato plant extracts.22
Another approach for speeding up the NMR experiment was demonstrated by combining different NMR experiments using multiple receivers and NOAH (NMR by ordered acquisition using 1H detection) super-sequences. NOAH super-sequences allow rapid data acquisition of multiple 2D data and substantially shortens experiment time. Recently, new sensitivity-improved versions of 2D 13C-1H HSQC (heteronuclear single quantum coherence) and HSQC-TOCSY NMR experiments that can be incorporated into NOAH super sequences were presented.23 The new experiments are demonstrated for both metabolite model mixtures (Figure 2) and mouse urine. These methods promise utility for 2D NMR based metabolomics studies with large cohorts of samples.
Figure 2:
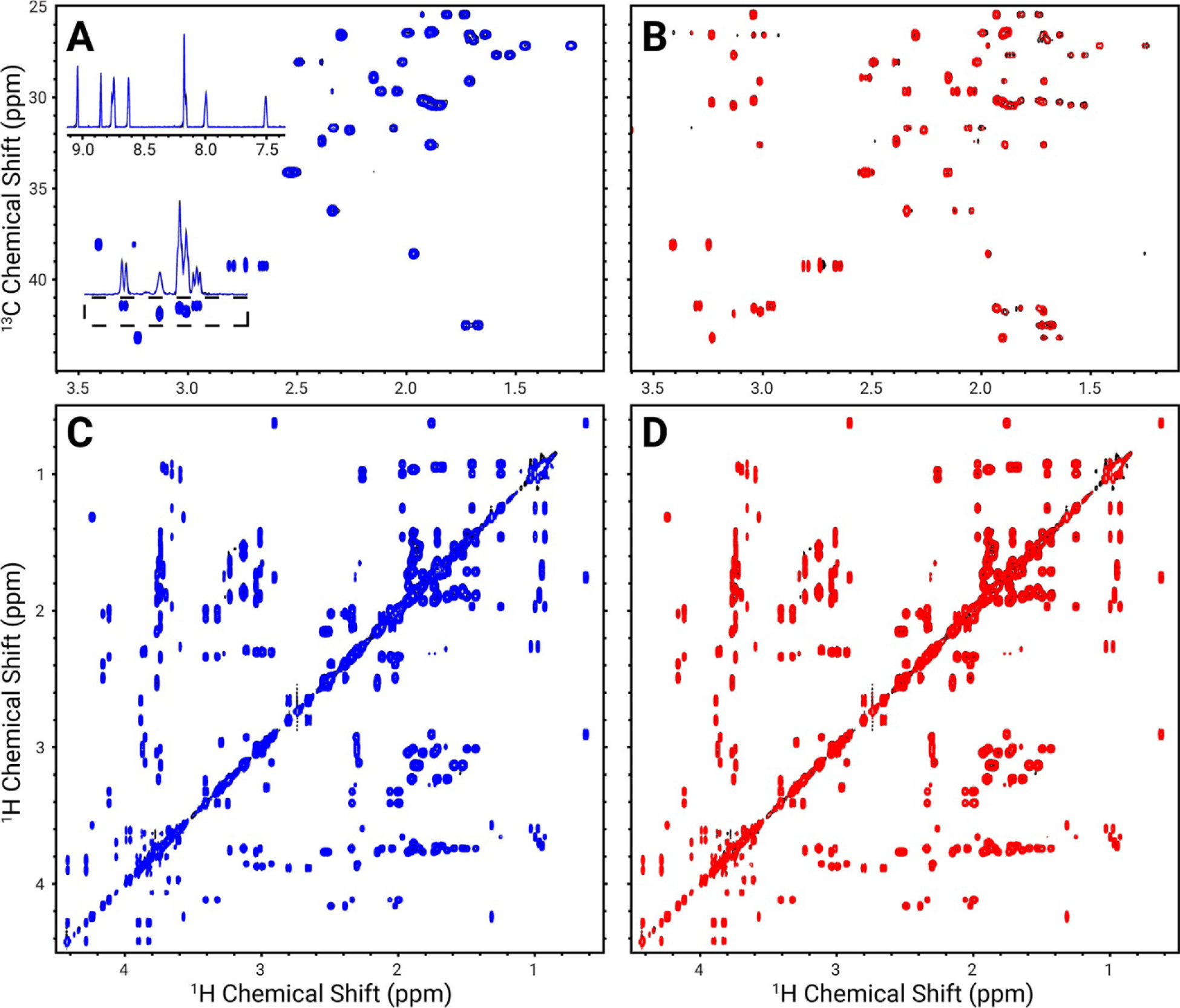
Comparison of NOAH2–COLMAR spectra with reference sequences (black) for a metabolite model mixture overlaid with spectra of the components of NOAH2–COLMAR HSQC + TOCSY (blue) and NOAH2–COLMAR HSQC–TOCSY + TOCSY (red). Spectra shown are HSQC (A), HSQC–TOCSY (B), and TOCSY (C and D). The upper inset in panel A is a projection of the aromatic region, while the lower inset shows a projection of the region in the dashed box. Reproduced from Hansen, A.L.; Kupče, E.R.; Li, D-W.; Bruschweiler-Li, L.; Wang, C.; Brüschweiler, R. 2D NMR-Based Metabolomics with HSQC/TOCSY NOAH Super sequences. Anal. Chem. 2021, 93, 15, 6112–6119 (ref. 23). Copyright 2021 American Chemical Society.
METHODS FOCUSED ON METABOLITE IDENTIFICATION
Several technological advances to date have led to the enhancement in sensitivity and resolution and thereby enabled the detection of an increased number of metabolites. However, the identification of low concentration metabolites techniques remains challenging, and thus continued efforts have focused on addressing this challenge. The automation of peak assignment continues to be of major interest and several efforts have been made in this direction recently. In one such method, metabolite identification and quantification was provided using different spectra from the same experiment. The performance of the method was evaluated using simulated and experimental data, and the method was shown to be particularly useful for low concentration peaks, which are hard to distinguish from the noise level.24 In a separate study, a set of protocols to analyze unknown metabolites was described.25 This study suggests eight different modular workflows and involves the use of tools such as Statistical Total Correlation Spectroscopy (STOCSY), Subset Optimization by Reference Matching (STORM), and Resolution-Enhanced (RED)-STORM to identify signals. Application of this protocol for routine analysis, however, is less straightforward as it can take up to a month to complete the entire set of workflows and requires a familiarity with MATLAB.
Two-dimensional (2D) NMR experiments offer improved resolution as they spread peaks across two dimensions and have been extremely useful for the identification of unknown metabolites. However, manual assignments of 2D spectra are often very laborious and time-consuming. Recently, efforts have been made to alleviate this issue based on computational and machine learning methods. For example, using computational approaches, an automated metabolite identification and assignment approach using 1H-1H the TOCSY experimental data was introduced, and was shown to assign peaks accurately.26 In another approach, a convolutional neural network (CNN) was applied to 2D 1H-13C HSQC NMR spectra.27 The neural network was trained for metabolite identification from the 2D NMR spectra and to achieve performance comparable to the conventional approaches. Machine learning offers opportunities for automated analysis of NMR spectra28 and potentially represents a new dimension to the NMR based metabolomics field. Deep neural networks (DNN), in particular, are capable of deconvoluting highly crowded NMR spectra. However, they do require careful training using large datasets to ensure accuracy.
The Human Metabolome Database (HMDB) has provided comprehensive reference information about human metabolites and their associated biological, physiological and chemical properties since 2007. It has been updated several times since then and the most recent version, HMDB 5.0, updated in 2022 has several major improvements.29 The number of metabolite entries in this version has increased from 114,100 to 217,920 compounds. Other major updates include improvements to metabolite descriptions; the addition of new structures, spectral and pathway visualization tools; inclusion of predicted NMR and MS spectral data and MS parameters; and enhancements to the HMDB’s search functions to facilitate better compound identification. This database is increasingly becoming a valuable global resource in the metabolomics field.
METABOLITE QUANTITATION
The absolute quantitation of metabolites enables direct comparison of data from different studies and different platforms, including known clinical values when analyzing blood or urine. NMR is used for metabolite quantitative analysis in a variety of sample types including intact samples, extracted samples, live organisms, cells, and even subcellular organelles such as mitochondria. To achieve reliable quantitation, incorporation of proper quality assurance (QA) and quality control (QC) protocols is important. A recent review describes how incorporation of robust QA/QC checks into the experimental design enables ex vivo analysis to extrapolate in vitro insights to in vivo.30 Another recent review highlights advances in quantitative NMR as well as limitations of current methods with emphasis on applications to metabolomics.31 The increasingly realized complexity of biological mixtures, however, continues to pose challenges for reliable analysis of a large number of metabolites, routinely. Specifically, peak overlap and peak shifting between spectra pose major challenges. However, several advanced approaches have been introduced aimed at alleviating the problem. For example, the Automated Quantification Algorithm (AQuA) was developed and its utility demonstrated for rapid and accurate absolute metabolite quantification in 1H NMR spectra.32 This improved version of a method introduced earlier monitors specific signals in spectra, and adjusts the spectral library to model interferences, automatically. Spectra from a large dataset of ethylenediaminetetraacetic acid (EDTA) plasma was tested using this program and shown to accurately quantify metabolites even in the presence of interfering signals from EDTA. In another study focused on quantification using time-series spectra, a computer program known as Ridge Tracking-based Extract (RTExtract) was developed.33 Based on this software, multiple spectral regions with overlapping and changing chemical shifts were accurately tracked in a much shorter time compared to the conventional approaches.
While methods to quantitate stable metabolites are well developed, quantitation of unstable metabolites such as coenzymes and antioxidants is generally met with many challenges. The sensitivity of these metabolites to enzyme activity and oxidation is the major problem. Their levels are sensitive to sample harvesting, metabolite extraction and analysis conditions. Recently, several factors that affect the stability were addressed and some of these unstable metabolites were shown to be reliably quantifiable.34–36 The notoriously unstable antioxidant, glutathione, however, was still outside the preview of such analysis. The active form (reduced glutathione, GSH) is notoriously unsubtle under ex vivo conditions and it spontaneously gets converted to the oxidized form (oxidized glutathione, GSSG). Hence, it is very difficult to analyze glutathione in its native form after isolation from biological mixtures. Recently, a new method, based on blocking the reactive thiol group of GSH by treating with excess N-ethylmaleimide (NEM, enabled reliable analysis of both GSH and GSSG in human blood.37 Interestingly, the excess (unreacted) NEM used for the chemical reaction can be removed from the samples during a drying step after extraction, with no need for additional processing. This is an important characteristic that enables analysis of not only the antioxidants (GSH and GSSG), but also the redox coenzymes, energy coenzymes and a large number of other blood metabolites, simultaneously, using the same one-dimensional (1D) NMR spectrum (Figure 3).
Figure 3:

1H NMR spectra of typical human whole blood extracts: (a) Blood sample (400 μL) without treatment with NEM; and (b) Blood (400 μL) sample treated with 100 μL 120 mM NEM. The insets show characteristic peaks of GSSG and GSH after derivatizing with NEM (GSH-NEM) as well as redox and energy coenzymes. In (a), virtually all the glutathione exists in the oxidized form arising from the oxidation of GSH. In (b), GSH oxidation is blocked using NEM. Note, the two characteristic triplets ~ 1.132 ppm arise from the –CH3 protons of the GSH-NEM. Triplets are separated by ~3 Hz (0.0037 ppm) and they arise from the two diastereomers of the GSH-NEM. Spectra were obtained using the ‘noesypr1d’ pulse sequence on a Bruker 800 MHz AVANCE III spectrometer equipped with a cryoprobe. NAD+: nicotinamide adenine dinucleotide, oxidized; NADH: nicotinamide adenine dinucleotide, reduced; NADP+: nicotinamide adenine dinucleotide phosphate, oxidized; NADPH: nicotinamide adenine dinucleotide phosphate, reduced: ATP: adenosine triphosphate; ADP: adenosine diphosphate; AMP: adenosine monophosphate. *Maleic acid impurity from the NEM solution; $ residual chloroform and @ residual methanol from extraction solvents. Reproduced from Nagana Gowda, G.A.; Pascua, V.; Raftery, D. Extending the Scope of 1H NMR-Based Blood Metabolomics for the Analysis of Labile Antioxidants: Reduced and Oxidized Glutathione. Anal. Chem. 2021, 93 (44), 14844–14850 (ref. 37). Copyright 2021 American Chemical Society.
Recently, two compounds, maleic acid and fumaric acid, were evaluated for their utility as a potential internal standard for absolute quantitation of metabolites in protein precipitated plasma, serum and whole blood.38 A comprehensive investigation of the two compounds showed that fumaric acid can be a robust internal standard for protein precipitated serum, plasma and whole blood; and maleic acid is suitable for plasma and serum, but it overlaps with coenzyme peaks in whole blood samples. Both fumaric acid and maleic acid provide a single peak in NMR spectrum and their peaks do not overlap with peaks from bio-specimen spectra.
LOW-FIELD BENCHTOP NMR
There has been a growing interest in the development of low field NMR for metabolomics applications.39 Low field NMR (typically < 100 MHz) has been in use since the 1960s; however, only during the past decade, fueled by the development of small and very strong permanent magnets, has the field witnessed a dramatic advancement, which has culminated in the development of benchtop NMR.40 A recent report provides an excellent overview of the topic covering instrumentation, experimental techniques and applications of benchtop NMR in comparison with conventional high field NMR.39 Their low cost, compact size, cryogen-free operation, and easy to use features are the unmatched characteristics that have garnered interest for a new facet of metabolomics applications including the potential translation of metabolomics for real-time and point-of-care testing. A number of studies have been reported that use low field benchtop NMR for applications to biofluids, including the identification and quantification of metabolites, and their validation using results from high field instruments.41–43 Recently, a study using 60 MHz benchtop NMR detected key urinary markers of type 2 diabetes and the results were validated using NMR at high field and spectrophotometry.44 The study describes the potential advantages and diagnostic/prognostic significance of benchtop NMR spectrometers for monitoring the disease based on many urinary biomarkers (≥15) at point-of-care clinical sites. In another study, benchtop NMR was applied to identify kidney disease using animal urine samples.45 The study demonstrates the ability of the method to identify fifteen distinguishing metabolites of kidney disease. However, one challenge for the analysis of NMR spectra at low field is the lack databases. Databases that currently exist for high field instruments are unsuitable for assignment of spectra at low fields. This is because the spectral patterns and the number of observed peaks change as a function of field strength. Recently, a simulation of 1H NMR spectra of metabolites was made to predict spectra of five dominant salivary metabolites from 45 to 600 MHz.46 A comparison of the simulated and experimental spectra obtained at 60 and 400 MHz was made. Prediction of singlet peaks arising from acetate, formate, methanol, and glycine was straightforward. However, prediction of complex peak patterns for propionate at low fields was not straightforward and required the use of additional fitting options. Overall, advances in low field NMR and results obtained so far support the notion that benchtop NMR can develop into a rapid metabolomics-based tool for clinical studies and potentially for eventual decision making.
MONITORING METABOLISM
Isotope Tracing for Monitoring Metabolism:
NMR experiments with stable isotope tracers generally utilize 1D 13C NMR for tracing the downstream metabolic products. The drawbacks of 1D 13C NMR are the low sensitivity and resolution. To overcome this challenge, recently, a new 2D heteronuclear NMR experiment was developed that allows quantitative analysis of isotopomers with high sensitivity and resolution.47 The experiment utilizes J-scaling and distortion-free elements to achieve high sensitivity and resolution. Application of this method enabled identification and quantitative analysis of TCA cycle activity and a novel mechanism involving the higher contribution of the pentose phosphate pathway to serine synthesis in response to a chemotherapeutic drug. In the metabolomics field, the 31P nucleus is not widely used, although it is naturally abundant and a constituent of many metabolites. Recently, to facilitate the application of 31P NMR, a protocol that allows the detection of metabolites through 31P as well as 1H, 13C, and 15N nuclei in the same sample was provided.48The protocol promises to expand the pool of NMR detected metabolites and thereby provide greater insights into metabolic pathways.
Monitoring Metabolism in Real-Time:
Metabolite profiles at steady state or at fixed time points lack information on dynamic changes associated with time-dependent metabolic activities. On the other hand, studies using live organisms, cells or even subcellular organelles promise greater mechanistic insight into altered pathways in diseases in real-time, including identification of previously unknown metabolic pathways.49–51 Such measurements are not possible with mass spectrometry as it is a destructive technique. Real time NMR-based metabolism studies in live systems are therefore gaining interest, and several recent such studies are described below.
Interest in the area of ketogenesis continues to grow with the goal of understanding its role in health and several diseases. Recently, isotope labeled tracers of two ketone bodies, acetoacetate and 3-hydroxybutyarate, were used in an in vivo study of a C57BL6 mouse model. Using 1H NMR, real-time metabolism in blood was monitored to evaluate the stability of the acetoacetate tracer during infusion and sample analysis.52 The research implemented a two-pool model using metabolic flux analysis to describe the results. The study provides insight into the rates of appearance, disposal, and exchange of the tracers.
Adenosine monophosphate activated protein kinase (AMPK) is considered a master regulator of metabolism. Recently, a real-time in-organism investigation of C. elegans was made to study the different roles of two isotypic catalytic subunits of AMPK in live worms at the whole organism level.53 Combined 13C tracer NMR and gene knockout studies identified differences in glycolysis, glucose production and tricarboxylic acid (TCA) cycle metabolism between two subunits of the kinase. The experimental model using gene knockouts for the subunits individually or together was shown to exhibit an unconventional phenotype-genotype relationship and the dominance of one of the subunits in glucose production.
Two-dimensional (2D) cell culture studies have been used for investigations of metabolism for a very long time. However, considering the limitations of the 2D cell culture, interest in three-dimensional (3D) cell culture is increasing as it is believed to represent the tissue characteristics more accurately. Spheroids are one of the widely used 3D cell models, and disease investigations using spheroids model are growing in number. NMR can give insights into time and spatially resolved metabolic changes, simultaneously. Recently, an NMR approach to study spheroids under physiological conditions in real time was designed and demonstrated for the investigation of cancer metabolism. The sensitivity of the measurements was enhanced by selecting spatial slices combined with an interleaved measurement approach. Impressively, the method detected regions of slow and fast metabolic changes in a single spheroid, simultaneously. Application to Ty82 cancer cells showed increased lactate and decreased glucose levels in the oxygen-depleted region of the spheroid.54 In another study, a micro-NMR platform was optimized to observe metabolic changes from a single spheroid in real time. Microfluidic devices containing spheroids were inserted directly into a dedicated NMR probe to obtain NMR spectra. It was shown that quantitative monitoring of time-resolved metabolism in a single spheroid consisting of 2500 cells or less could be monitored and the results demonstrated more than a factor of 2 reduction in glucose consumption and lactate production in 3D cell model when compared to 2D cells.55 Another study described monitoring of cellular metabolism and mitochondrial respiration in 3D cell culture system. It used a bioreactor system for perfusion and oxygenation and metabolic investigation using 1H NMR and, 19F-NMR simultaneously. Collagen-based 3D cell culture was used in this study, which allowed detection of the perfusion rate dependent metabolite profiles. Metabolic pathways were investigated based on inhibition of the glycolysis and mitochondrial respiration by perfusion using 2-deoxy-glucose and rotenone/oligomycin.56
The study of individual variability in response to drugs has also gained increased interest in recent years. The human gut microbiome constitutes hundreds of bacterial species, which together are thought to contain two orders of magnitude more encoding genes than the human genome itself.57 Further, the microbiome varies significantly between individuals and the variation as a function of time within the individuals is much higher than its variation between individuals.58 Such variation is attributed in part to the huge differences in microbial interactions with drugs between individuals. The mechanistic understanding of such interactions can deepen our understanding of the interaction of the gut microbiome with drugs, and may help pave the way toward development of more personalized medicine. However, studies in a living host–gut microbiome system have been challenging. Recently, development of a real-time monitoring method for metabolic interaction in vivo during the application of an anticancer drug, 5-fluorouracil (5-FU), using C. elegans and gut bacteria was reported59 (Figure 4). 19F NMR enabled investigation of 5-FU metabolism, quantitatively, and in real-time within the host utilizing human gut-microbes with variable genetics. These results not only provide experimental evidence for the role of bacteria in drug metabolism, but also for the contribution of microbiome induced differential drug catabolism to the host metabolism, which is often unobservable by conventional genetic experiments. Hence, this approach can be powerful for the investigation of metabolism for many drugs arising from the variations in host–gut microbiome interactions.
Figure 4:

Overall schematics and implementation of real-time observation of host–gut microbial interspecies interaction in anticancer drug (5-FU) metabolism. (A) C. elegans were grown in agar plates with E. coli to adult stage, harvested, washed, and then preincubated (3 h) with 5-FU in liquid media. The worms were then moved to a standard NMR tube to monitor 5-FU catabolism in the living biological system of C. elegans fed with E. coli. An inner coaxial insert filled with D2O was used for the lock signal. (B) Survival rate of C. elegans before and after 3 h NMR experiment; ns: not significant by Student’s t-test. The survival rate was measured by counting moving worms under microscopy, as shown in (C). (D) Overlay of 1D 19F-NMR spectra of the living host–gut microbiome system (OP50) showing time-dependent 5-FU catabolism and concomitant production of its catabolites after 3 h preincubation. (E) Known catabolism of 5-FU in the human system and the associated enzymes. Reproduced from Nguyen, T.T.M.; Mai, V-H.; Kim, H.S.; Kim, D.; Seo, M.; An, Y.J.; Park, S. Real-Time Monitoring of Host–Gut Microbial Interspecies Interaction in Anticancer Drug Metabolism. J. Am. Chem. Soc. 2022, 144, 19, 8529–8535 (ref. 59). Copyright 2022 American Chemical Society.
ISOTOPE TRACERS AND HYPERPOLARIZATION FOR CANCER INVESTIGATIONS
Metabolite flux analysis based on stable isotope tracers provides quantitatve informaion on the substrate utlization in biochemical pathways in health as well as disease conditions. And hyperpolarization, which significantly boosts the nuclear polarization and achieves enormous sensitivity enahancements, can enable real time monitoring of metabolism in live systems. As described below, a number of cancer investigations have exploited advances in these areas during the last two years.
Isotope Tracing and Flux Analysis:
To date, a large number of metabolic pathways, including glycolysis, pentose phosphate pathway, TCA cycle, glutaminolysis etc. have been the targets of investigations using a variety of isotope labeled substrates including both aqueous and lipid metabolites. Stable isotope tracing is a powerful approach for providing key mechanistic information and is now widely used in cancer metabolism studies. It has, so far, enabled the identification of a number of altered metabolic pathways including the high rates of glucose and/or glutamine utilizations along with many other pathway alterations present in by proliferating cancer cells.60–63
For many years, NMR-based stable isotope methods have been widely used to trace aqueous metabolites, however, its utility to trace lipids has been limited.63 The challenge for lipids analysis arises from the complexity of lipids spectra and the generally low level of isotope labeling. Hence, lipids analysis has so far been in the domain of MS. Recently, however, efforts to measure the content and isotopic enrichment of complex lipid mixtures were assessed using NMR for prostate and bladder cancer cell lines using 13C6-glucose and 13C5-glutamine tracers.64 The results demonstrated that NMR can indeed provide useful information and complement MS in the determination of metabolic fluxes through lipids.
NMR can also distinguish substrate utilization by multiple pathways in a single measurement. However, to enable quantification of such utilization, the substrate used should be such that the downstream product(s) formed through each pathway should be distinguishable by NMR. For example, an NMR study using one such a tracer, [1,2-13C2]-glucose, measured fluxes through glycolysis and PPP in HEK293 cells, which were enzymatically depleted of mitochondrial polyphosphate.65 The glucose fluxes through glycolysis and the PPP were then measured, in one step, via the 13C NMR signals resulting from [2,3-13C2]-lactate and [3-13C1]-lactate, respectively. The measurements showed that cells depleted with mitochondrial polyphosphate exhibited a significant increase in the activity of the PPP and suggest a clear effect of polyphosphate in the regulation of cellular redox balance and cellular bioenergetics and the redox status of mammalian cells. A challenge for using [1,2-13C2]-glucose as the substrate, however, is that the peak from [3-13C1]-lactate overlaps with natural abundance 13C peak. Hence, a natural abundance correction is required to obtain correct [3-13C1]-lactate concentration. Alternatively, [2,3-13C2]-glucose has been used as a substrate to measure PPP activity in a variety of conditions including fed vs fasted state, and in hepatocellular carcinoma.66 [2,3-13C2]-glucose produces [1,2-13C2]-lactate and [2,3-13C2]-lactate through the glycolysis and PPP, respectively, and their NMR peak areas can be conveniently measured by 13C NMR without the need for natural abundance correction. In some tissues, measurement of lactate 13C NMR peaks is not convenient for measuring PPP activity due to its low concentration. Recently, to overcome this obstacle, measurement of [4,5-13C2]-glutamate instead of [2,3-13C2]-lactate was shown to be convenient for measuring low PPP activity67 (Figure. 5). A comparison of results showed a strong linear correlation between [4,5-13C2]-glutamate and [2,3-13C2]-lactate, which indicates the robustness of measuring [4,5-13C2]-glutamate for low PPP activity.
Figure 5:

Schematic showing potential analytes for monitoring the pentose phosphate pathway (PPP) with [2,3-13C2]glucose. The entry of [2,3-13C2]glucose to the PPP produces [1,2-13C2]pentose after C1 decarboxylation in the oxidative phase. Carbon rearrangement of [1,2-13C2]pentose with unlabeled pentoses in the nonoxidative PPP leads to [1,2-13C2]fructose 6-phosphate (F6P). [1,2,3-13C3]F6P is also possible if 13C-labeled molecules interact through the nonoxidative phase. The number in a red bubble (13C) indicates carbon position 2 or 3 in [2,3-13C2]glucose prior to the PPP. [1,2-13C2]- and [1,2,3-13C3]F6P lead to [2,3-13C2]- and [1,2,3-13C3]pyruvate, respectively, and they are in exchange with lactate or alanine. Both [2,3-13C2]pyruvate and [1,2,3-13C3]pyruvate are converted to [1,2-13C2]acetyl-CoA after C1 decarboxylation via pyruvate dehydrogenase (PDH). The condensation of [1,2-13C2]acetyl-CoA and oxaloacetate (OAA) produces [4,5-13C2]citrate and [4,5-13C2]α-ketoglutarate through the TCA cycle. Because α-ketoglutarate is in exchange with glutamate, the detection of [4,5-13C2]glutamate is evidence of the PPP activity. [4,5-13C2]glutamate may be further converted to [4,5-13C2]glutamine or to [1,2-13C2]GABA after decarboxylation in the brain. Glycolysis of [2,3-13C2]glucose leads to [1,2-13C2]pyruvate, [1-13C1]acetyl-CoA, [5-13C1]glutamate, [5-13C1]glutamine or [1-13C1]GABA in the brain. Ala, alanine; GABA, γ-aminobutyric acid; Gln, glutamine; Glu, glutamate; α-kG, α-ketoglutarate; open circle, 12C; black circle, 13C; red circle. Reproduced from13C NMR of glutamate for monitoring the pentose phosphate pathway in myocardium, Jin, E.S.; Lee, M.H.; Malloy, C.R. NMR Biomed. Vol. 34, Issue 7, e4533 (ref 67) Copyright 2021 Wiley.
Thus far, the majority of isotope tracer studies have used cell lines and animal models, which have enabled a better understanding of metabolic pathways under controlled conditions. By contrast, studies that involve direct infusion of isotope tracers to humans are rare to date, but this trend is changing gradually.68,69 Recently, a first-in-human in vivo metabolism protocol was used to study two different types of brain tumors using NMR spectroscopy. 13C labeled 3-hydroxy butyrate was infused intravenously and its oxidation in the tumor was quantitated based on blood and resected tissue using NMR.68 Results from this study imply that the ketogenic diet may not be effective in brain tumor patients and alternatively suggest that targeting ketone body metabolism in brain tumor patients may be of therapeutic value. In a separate study, the same group, demonstrated an active pyruvate recycling mechanism in human brain tumor patients using 13C isotopomer analysis of resected tumor tissues.69 The pyruvate recycling flux from TCA cycle intermediates that were derived from the 13C-glucose tracer were quantitated. Such insights obtainable from investigations directly on humans are valuable considering that experiments using cell lines and animal models are not always predictive of outcomes in humans.70–72
Hyperpolarization:
Hyperpolarization using the DNP approach that was only briefly mentioned above, has also shown utility in the metabolomics field, mainly in the form of dissolution DNP (dDNP). dDNP polarization occurs at cryogenic temperatures, followed by rapid dissolution with hot solvent and transfer of the hyperpolarized sample to an NMR spectrometer for measurement at room temperature. dDNP NMR was applied to enhance the sensitivity for isotope labelled metabolites in four different prostate cancer cell lines. A high signal-to-noise ratio enabled interpretation of dDNP NMR spectra and the identification of 10 metabolic reactions from the downstream metabolites. Analysis of the data using a Random Forest algorithm allowed the classification of cancer cells based on differences in metabolic activity with 96.9% accuracy73 (Figure 6). This is the first study to evaluate the utility of dDNP 13C NMR for classification of cancer cell phenotypes.
Figure 6:

A) Illustration of PCA on Box-Cox transformed and normalized data. The first PC contains 34.47% of the variance in the data and second PC 26.65%. A total of 7 components could explain more than 95% of all data variance. B) Plot of the two first PC-DFA functions, illustrating the results of separation between cell lines. Colored eclipses represent 2 standard deviations of point clouds. PC3 (red) and DU145 (orange) are aggressive prostate cancer cell lines and PNT (cyan) and LNCaP (blue) are characterized as indolent. Reprinted from J. Magn. Reson., Vol. 316, Frahm, A.B.; Jensen, P. R.; Ardenkjær-Larsen, J.H.; Yigit, D.; Lerche, M.H. Stable isotope resolved metabolomics classification of prostate cancer cells using hyperpolarized NMR data, pp. 106750 (ref 73). Copyright 2020, with permission from Elsevier.
The Warburg effect is a well-known phenomenon in cancer. Glutamine addiction is one of the characteristics of the Warburg effect and is involved in mitochondrial metabolism, a major source of energy as well as nitrogen and carbon for biosynthesis.74 Inhibition of glutaminase, which enables increased glutamine intake into the TCA cycle, is therefore targeted to prevent cancer cell proliferation. Glutamine is abundant in human plasma; however, it is challenging to determine glutamine’s metabolic fate noninvasively. Recently, a platform for imaging glutamine metabolism using hyperpolarized magnetic resonance imaging was developed.75 Using this approach, glutaminolysis in pancreatic cancer could be measured spatially in vivo, and a potential biomarker for the inhibition of glutaminase was identified. The hyperpolarized glutamine probe with enhanced signals from multiple stable-isotope metabolites overcomes many challenges to assess glutamine flux and imaging of glutamine metabolism in humans.
METABOLOMICS APPLICATION TO LARGE POPULATIONS
Biomarker validation remains a major challenge in the field, and the problem is especially acute in human studies with small sample sizes, which make up the preponderance of metabolomics investigations. This is because of the overwhelming contributions from confounding factors such as age, gender, ethnicity, BMI, diet, physical activity, smoking or alcohol status and medication use. Numerous efforts, to date, have been focused on addressing the effects of such confounders on metabolomics data using novel statistical approaches.76,77 As an alternative, parallel efforts have focused on large population studies,78–80 which help to alleviate the confounding effects inherent if small studies with mismatched participant characteristics in order to derive more reliable biomarker candidates. Recently, several such large population studies using NMR-based metabolomics were reported. In one study, an association of blood metabolites with the risk of stroke was investigated using seven prospective cohorts consisting of nearly 40,000 participants that included nearly 2000 incident stroke events.81 The relationship between metabolites and stroke was assessed using Cox proportional hazards regression models. The analyses were performed considering all incident stroke events, and ischemic and hemorrhagic events, separately. The results show an association of several metabolites including amino acids, glycolysis-related metabolites, and several lipoprotein subfractions with the risk of stroke. Correlative studies such as this also have the advantage that they are not sensitive to site to site variations in levels, as long as cases and controls are obtained at all collection sites.
It is well-known that obesity is a risk factor for many types of cancer, and evidence suggests that impaired branched-chain amino acid (BCCA) metabolism occurs in obesity.82 With a hypothesis that BCCA may be associated with the development of cancer, blood plasma BCCA were investigated in >25,000 participants using NMR spectroscopy.83 The results showed that BMI was associated with a greater risk for cancer, however, total circulating BCAAs did not show any association with cancer incidence. When analyzed individually, however, leucine showed a modest association with postmenopausal breast cancer, and isoleucine with pancreatic cancer. Several large population studies were focused on the assessment of blood and urine metabolite biomarkers for the risk of major human diseases such as cancer, diabetes and cardiovascular disease (CVD). One study on > 80,000 postmenopausal women showed CVD risk association with high-animal-protein and low-fiber diets, and cancer risk association with low-carbohydrate diets, and diabetes risk association with low-fiber/low-carbohydrate diets.84 Another study using metabolite data from a similar number of postmenopausal women, showed that diet high in carbohydrate density exhibited substantially reduced risk of major chronic diseases.85
In a study with a focus on the identification of common and discordant biomarkers between two vascular diseases, peripheral artery disease (PAD) and coronary artery disease (CAD), blood metabolite profiles from five prospective cohorts from a Finnish population of >30,000 were studied.86 The study calculated associations between the diseases and >200 blood metabolic biomarkers (individual metabolites, lipid signals and important ratios) derived from NMR spectroscopy. In the 14-year follow-up, nearly 500 PAD and >2,000 CAD incidences were detected and distinct metabolite differences between the two diseases were observed. CAD exhibited an association with apolipoproteins and cholesterol, whereas PAD exhibited an association with a lower proportion of polyunsaturated fatty acids, and higher concentrations of monounsaturated fatty acids, glycolysis-related metabolites, and inflammatory protein markers (Figure 7).
Figure 7:

Consistency of metabolic biomarker associations for coronary artery disease (CAD) (2073 incident events) and peripheral artery disease (PAD) (498 incident events). The hazard ratio of each biomarker is given with 95% CIs in gray vertical and horizontal error bars. The red dashed line denotes the diagonal. Biomarkers with P<0.001 for heterogeneity between associations with PAD and CAD are marked by black color coding. Apo indicates apolipoprotein; C, cholesterol; GlycA, glycoprotein acetyls; HDL, high-density lipoprotein; IDL, intermediate-density lipoprotein; L, large; LA, linoleic acid; LDL, low-density lipoprotein; M, medium; P, particle concentration; S, small; and XL, very large. Reproduced from Metabolic Biomarker Discovery for Risk of Peripheral Artery Disease Compared with Coronary Artery Disease: Lipoprotein and Metabolite Profiling of 31 657 Individuals From 5 Prospective Cohorts, Tikkanen, E.; Jägerroos, V.; Holmes, M.V.; Sattar, N.; Ala-Korpela, M.; Jousilahti, P.; Lundqvist, A.; Perola, M.; Salomaa, V.; Würtz, P. J. Am. Heart Assoc., Vol. 10, Issue 23 (ref 86). Copyright 2021 Wiley
Profiling metabolites longitudinally enables understanding of aging and age associated metabolic changes. With this goal, recently, metabolite profiles from nearly 5,000 participants from two cohorts were investigated.87 Samples from up to three time points were analyzed for 236 metabolite/biochemical parameters using NMR and clinical biochemistry. Results showed temporal changes, many of which were distinct for men and women. Specifically, changes in glycine, phenylalanine and C-reactive protein exhibited a significant gender difference. Further, interestingly, weight gain was associated with changes in insulin, isoleucine, lipids, and C-reactive protein. The vast temporal metabolic data potentially serve as a reference material for investigation of age and weight associated metabolic changes.
Evaluation of multicenter data:
Studies involving multiple sites are extremely valuable for the development of reliable biomarkers and statistical models for clinical applications, as well as for gaining mechanistic insights into pathophysiology of diseases. To date, many potential metabolite biomarkers have failed to validate across multiple sites. To make progress, several large multicenter cohorts of samples have been made available for metabolomics studies. For example, a multicenter biobank from Europe provides access to samples and associated data for > 3 million participants.88 For better outcomes, samples need to be obtained under strict guidelines to avoid interference from pre-analytical variabilities. However, samples from biobanks often do not meet the required strict collection protocol. This is because such sample collections were generally not originally designed for metabolomics studies. Recently, to quantitatively evaluate how such multicenter samples affect metabolomics studies, blood serum and plasma samples obtained from eight biobanks from seven countries across Europe were studied using 1H NMR and statistical analyses.89 As anticipated, the study found differences in metabolite profiles arising from the deviations from the validated norms for sample collection, which further highlights the critical need to follow strict protocols to obtain meaningful outcomes from multicenter studies. Further, the results show how NMR spectroscopy guides evaluation of multicenter sample quality and helps to avoid biases in the downstream analysis.
Blood is the most widely used biological specimens since its collection is minimally invasive and is rich in information. However, whether to use serum or plasma or, even what type of plasma (EDTA, heparin, etc.) is still an unsettled question in metabolomics. To address this issue, a recent study reported a comparison of metabolomic and lipoprotein profiles in blood samples collected using different collection tubes that included citrate plasma, EDTA plasma, and serum.90 The results showed statistically significant changes for several metabolites among the three blood matrices. In addition, metabolites exhibited matrix dependent correlations. The differences, however, were less marked for lipoprotein profiles. These results highlight the importance of using the same type of tube for multicenter studies although the tube dependent changes in metabolic profiles can be minimized by using regression analysis.
EVALUATION OF ANTI-AGING NUTRITIONAL SUPPLEMENTS
The NAD (nicotinamide adenine dinucleotide) metabolites, NAD+ and NADH, mediate biochemical reactions fundamental to cellular functions in health and disease. They undergo reversible oxidation and reduction in numerous electron-transfer reactions, while the concentration ratios of the reduced and oxidized forms reflect important cellular functions including the overall redox status and regulation of ion channels, cell signaling, cell survival and death. These metabolites are known to diminish in concentration with age, which adversely affects human health. To alleviate this problem, boosting NAD levels through nutritional supplementation has been suggested. Hence, numerous clinical trials are being conducted on nutritional supplementation of precursors to NAD+ synthesis. Nicotinamide riboside (NR) is one such precursor that has been explored for nearly a decade91 and is already available in the market as a nutritional supplement. Recently, the pharmacokinetics of NR and its effect on blood NAD+ levels were evaluated.92 More recently, using in vivo 31P NMR, a first double-blinded phase I clinical trial of oral NR supplementation in Parkinson’s disease patients was made93 (Figure 8). NR recipients showed increased brain NAD levels, which was associated with altered cerebral metabolism and mild clinical improvement. Furthermore, NR supplementation was associated with improved mitochondrial, lysosomal, and proteasomal functions in blood and muscle cells, and decreased levels of inflammatory cytokines in serum and cerebrospinal fluid. NR is thought to degrade rapidly into nicotinamide and ribose, especially when ingested orally.94,95 Hence, the action of NR is thought to be due to the circulating nicotinamide, or nicotinamide and ribose. NAD+ is synthesized in mammals from nicotinamide (NAM) via the amidated salvage route, catalyzed by nicotinamide phosphoribosyl-transferase (NAMPT).96 Hence, in a different clinical trial, supplementation of a combination of nicotinamide (NAM) and D-ribose was evaluated using NMR spectroscopy.97 This randomized, triple-blinded, placebo-controlled, crossover pilot study assessed the efficacy and safety of supplementation of the precursors for boosting NAD+. The study reports significantly increased NAD+ and related metabolites in blood such as NADP+. In addition, it reports increased antioxidant levels and high energy phosphates. Blood glucose levels were also reduced without any significant change in insulin secretion, which suggests an improved insulin sensitivity and glucose tolerance. Further, it found no clinically relevant adverse effects or alterations in hematology, electrolytes, liver, and kidney markers pre- and post-supplementation.
Figure 8:

31P-MRS analysis reveals NR-mediated increase in cerebral NAD (A–C) Exemplary data from one subject. (A) Spectroscopy voxel position in the occipital cortex. Spectra were acquired for each grid position. (B) Averaged processed spectra from several occipital lobe voxels in black. The model fit of the data is shown in red. The model fit is composed of the convolution of all spectral contributions of a simulated dataset fitted to the experimental data shown in (C). The position of NAD+/NADH is indicated. (C) Phosphocreatine (PCr) was chosen as the chemical shift reference at 0 ppm. Other detected phosphometabolites are indicated. (D) Relative total NAD levels normalized to ATP-a at visit 1 and 2. Data points show individual measurements (gray) and averages (black). *p = 0.0105 (Wilcoxon test). Reproduced from The NADPARK study: A randomized phase I trial of nicotinamide riboside supplementation in Parkinson’s disease, Brakedal, B.; Dölle, C.; Riemer, F.; Ma, Y.; Nido, G.S.; Skeie, G.O.; Craven, A.R.; Schwarzlmüller, T.; Brekke, N.; Diab, J.; Sverkeli, L.; Skjeie, V.; Varhaug, K.; Tysnes, O-B.; Peng, S.; Haugarvoll, K.; Ziegler, M.; Grüner, R.; Eidelberg, D.; Tzoulis, C. Cell. Metab., Vol. 34, Issue 3 (ref 93). Copyright 2021 Wiley.
APPLICATIONS TO COVID-19.
Over the past two years NMR spectroscopy has joined the effort to investigate the effects of COVID-19. So far, the disease has affected > 500 million people with > 6 million deaths. To date, there is only limited knowledge of how the virus affects metabolism.98 As described below, a number of NMR studies have focused on identifying putative biomarkers and improving our understanding of the altered metabolism due to the infection.
Biomarker Detection:
A recent study compared serum metabolite and lipid profiles from nearly 400 patients along with 280 uninfected individuals.99 Statistical analysis of the NMR data showed a discrimination between infected and uninfected individuals, with abnormally high levels of ketone bodies in COVID-19 patients. High levels of oxidative stress markers were also observed, which is indicative of potential liver damage. Several other metabolite profiling studies support the finding that the infection is associated with altered lipid levels.100–103 Whether such findings are specific to COVID-19 and the associated disease or represent an underlying risk factor was evaluated separately.104 A comparison of results with non-COVID-19 controls indicated that COVID-19 is associated with dyslipidemia; dyslipidemia, however, these results were not observed in anti-SARS-CoV-2 antibody-positive individuals with no severe disease. This finding suggests that lipid levels may represent a confounding risk factor for COVID-19. A different study compared EDTA-plasma metabolites and lipid profiles from COVID-19 patients with post COVID-19 patients.105 COVID patients within 21 days of testing exhibited significant differences in many metabolites and lipoprotein components, compared to post COVID patients. However, interestingly, altered aqueous metabolites reverted back to normal post-COVID faster than the lipoproteins. In a separate study, it was found that for some COVID-19 patients, altered metabolite levels persist even after six months of infection whereas for some, the levels become comparable with un-infected individuals.106
Longitudinal Studies and Effect of Treatment:
Tocilizumab is an inhibitor of the pro-inflammatory interleukin-6 (IL-6) and is administered to COVID-19 patients with elevated inflammatory markers. Several studies have focused on the evaluation of COVID-19 response to tocilizumab treatment using NMR-based serum metabolomics. In one study, metabolite and lipid profiles were first compared between 30 patients and matched controls, and then the effect of tocilizumab administration was evaluated using a subset of patients.107 The results indicated that COVID-19 patients exhibited differences in metabolite and lipid profiles relative to controls and that a partial reversal takes place upon tocilizumab treatment.
The ability to monitor the disease severity and treatment outcome, accurately, is critical for patient management. Recently, a longitudinal metabolomics study combined with immune markers was made using NMR spectroscopy and high-dimensional flow cytometry using blood plasma.108 The data were analyzed to evaluate the changes based on disease severity and response to tocilizumab treatment. The results showed that many increased lipoproteins and inflammation markers correlated with COVID-19 severity. This insight from the joint use of metabolomics and immune markers promises new routes for clinical management.
A longitudinal study modeled blood plasma metabolites along with multiple plasma cytokines and chemokines from COVID-19 patients, patients with COVID-19 like symptoms, and matched controls.109 Blood plasma metabolite profiles were obtained using multiple NMR techniques (single-pulse, spin-echo, and 2D J-resolved). Single-pulse NMR data were compared with data from the in vitro diagnostic research (IVDr) method110, 111 on 112 quantitative lipoprotein profiles extracted from the raw 1D NMR data. Notably, metabolite differences among all three groups were significant. Several cytokines exhibited relationships with multiple lipoproteins and metabolites, and, for some patients, recovery with a virus-free phase was still associated with abnormal metabolite profiles. Another study from the same group compared NMR data from low sample volumes (100 μL, 3mm NMR tube) with high sample volumes (300 μL, 5 mm NMR tube) using an IVDr system and showed that 3 mm IVDr method provides quantitative lipoprotein measurements and demonstrates the utility for volume limited samples.112
Effect of Vaccination:
It is well-known that vaccination is effective for controlling the spread of COVID-19. COVID-19 vaccines work by triggering the immune system to produce antibodies that neutralize SARS-CoV-2 spike proteins. Measurements of the blood antibodies have shown different responses between COVID-19 and non-COVID-19 individuals.113,114 Further, studies have shown that mRNA vaccines elicit strong immune response in individuals who previously had COVID-19.114,115 With a focus on evaluating the associated changes in metabolite profiles in such individuals, a study of blood serum collected at six time points, before vaccination, then weekly until 7 days after the second dose, and one month after the second dose of an mRNA vaccine was made recently.116 The time dependent response to the mRNA vaccine, Pfizer-BioNTech, was evaluated for a small group of individuals with a history of COVID-19 and compared with a group that did not have a COVID-19 history. Results showed no significant differences between the two groups prior to vaccination. However, the first dose of vaccine induced significant time dependent alterations in lipoproteins, which was different for the two groups. Alterations, however, were minimal after the second vaccine dose for individuals with a history of COVID-19. These results are in accordance with an independent observation that serum antibody levels in individuals with a history of COVID-19 reach a plateau after the first dose.114 Another longitudinal study evaluated the effect of a different mRNA vaccine, BNT162b2.117 Metabolite profiles associated with antibody levels induced by the vaccine were analyzed in blood plasma by combining NMR and mass spectrometry.117 This study highlighted the role of amino acid metabolism and lipids as predictive markers of response to vaccination.
Predicting the Risk of Severe Disease:
A few studies have focused on evaluating metabolite profiles to predict the susceptibility to and outcome of the disease. An NMR study utilizing blood samples from healthy individuals from the UK Biobank, collected during 2007–2010, evaluated the susceptibility to pneumonia and COVID-19.118 Among the 105,146 individuals, severe pneumonia occurred for 2507 and severe COVID-19 was found for 652 individuals. Metabolite biomarkers were similar for the risk of severe pneumonia and severe COVID-19. A multi-biomarker score was strongly associated with severe COVID-19 as well as severe pneumonia events occurring 7–11 years after blood sampling. However, the multi-biomarker score was 4 times higher for severe pneumonia occurring during the first 2 years after blood sampling. Results also show a strong association between clinically validated biomarkers and the infectious disease score with the future onset of severe COVID-19 (Figure 9). Severe COVID-19 is associated with acute respiratory distress syndrome (ARDS), which is characterized by lung inflammation and pulmonary edema. ARDS can also occur due to other causes such as Influenza A pneumonia (IAP). With the goal of identifying biomarkers specific to COVID-19, recently, a study compared serum metabolite profiles of patients of ARDS due to COVID-19 with those of ARDS due to IAP using NMR spectroscopy.119 Metabolite profiles showed differences in amino acid, lipid, and glycolysis pathway metabolism between COVID-19 and IAP induced ARDS. Further evaluation showed that COVID-19 causes deficits in energy supply, while IAP causes marked inflammatory and oxidative stress responses. Overall, NMR-based metabolomics has proven to be quite sensitive to COVID related infections and for monitoring treatments.
Figure 9:
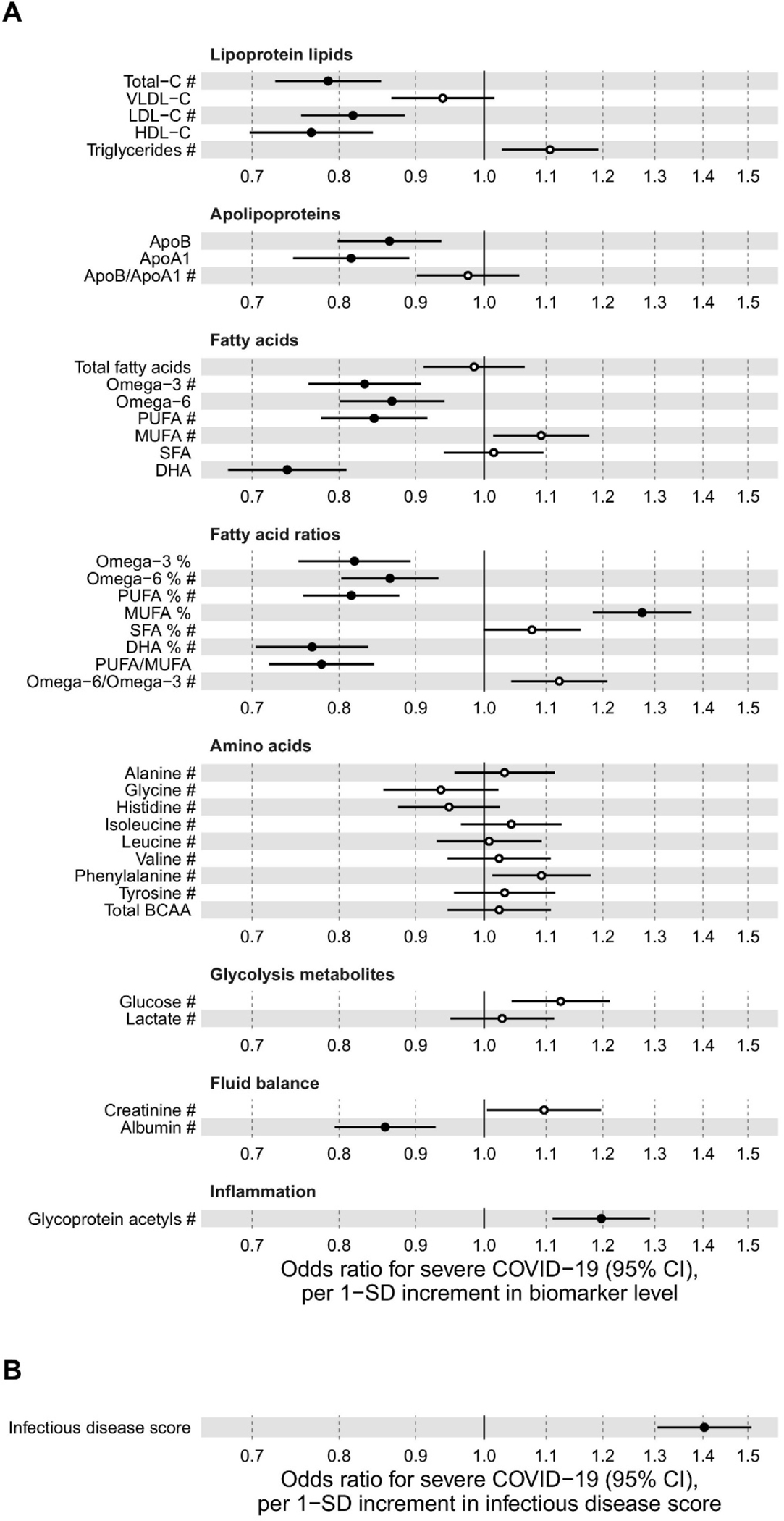
Relation of all biomarkers measured by the Nightingale Health NMR platform to risk of severe pneumonia in UK Biobank (n = 105 146; 2507 events). Odds ratios with severe pneumonia (2507 hospitalisations or deaths during a median of 8 years of follow-up) for 56 metabolic measures quantified by the Nightingale Health NMR platform. The metabolic measures cover cholesterol and triglycerides in major lipoprotein fractions, lipoprotein particle size, apolipoproteins, glycolysis related metabolites, absolute levels of fatty acids and corresponding ratio measures relative to total circulating concentration of fatty acids, amino acids, ketone bodies, and markers of fluid balance and inflammation. Statistical models are adjusted for age, sex, and assessment centre. Odds ratios are per 1-SD increment in biomarker levels. Horizontal bars denote 95% confidence intervals. Closed circles denote p-value<0.001 and open circles p-value>0.001. Abbreviations: ApoA1, apolipoprotein A1; ApoB, apolipoprotein B; BCAA: branched-chain amino acids; C, cholesterol; DHA, docosahexaenoic acid; HDL, high-density lipoprotein; LA, linoleic acid; LDL, low-density lipoprotein; LA, linoleic acid; MUFA, monounsaturated fatty acids; NA; not available; PUFA, polyunsaturated fatty acids; SFA, saturated fatty acids; TG, triglycerides; VLDL, very-low-density lipoprotein. Reproduced from Nightingale Health UK Biobank Initiative. Metabolic biomarker profiling for identification of susceptibility to severe pneumonia and COVID-19 in the general population, Julkunen, H.; Cichońska, A.; Slagboom, P.E.; Würtz, P. Elife, Vol. 10 (ref 118). Copyright 2021 Elife.
CONCLUSIONS AND FUTURE PERSPECTIVES
Multifaceted growth in NMR-based metabolomics has resulted from several technological and methodological advances, as well as a vast number of applications in the area of biomedicine. Technology and methods development have enabled improved metabolite detection and quantitation under ex vivo, in vitro as well as in vivo conditions, and provided greater mechanistic insights into health as well the pathophysiology of many diseases. Unknown metabolite identification, and accurate quantitation continue to evolve, and advances to date have significantly expanded the limit of metabolite analysis. New methods have also enabled analysis of unstable metabolites such as coenzymes and antioxidants that were hitherto unreliable or even inaccessible by NMR. Clinical trials focused on establishing the scientific basis for boosting the NAD metabolome in humans using the new methods highlight the translational utility of NMR methods. Developments in low field (<100 MHz) benchtop instruments promise simplicity, affordability, and wider access, and eventually may even be used for targeted profiling in point-of-care diagnosis applications. Methodologies for real-time metabolism studies especially using hyperpolarized NMR, are becoming more mature and their applications promise greater insights into the dynamics of metabolic changes and potentially clinically relevant biomarkers. Considering that such insights are inaccessible to conventional methods, their applications are anticipated to increase significantly in the future. Overall, the future will witness continued efforts to boost NMR sensitivity, resolution, and the speed of data acquisition and data analyses. These efforts in turn are anticipated to fuel an even larger array of important applications and greatly improve our understanding of cellular functions and systems biology, and to help to achieve breakthroughs in the prevention, early diagnosis, and treatment of human diseases.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the financial support from the NIH grants R01GM138465, R01GM131491, P30CA015704, 1P30AR074990, and P30DK035816.
Biographies
BIOGRAPHIES
G. A. Nagana Gowda is a Research Associate Professor in the Department of Anesthesiology and Pain Medicine and member of the Northwest Metabolomics Research Center at the University of Washington, Seattle where he has worked since 2012. He was a Research Scientist in the Chemistry Department, Purdue University, West Lafayette, Indiana (2006–2012). Previously, he was an Assistant Professor at the Centre of Biomedical Magnetic Resonance, Lucknow, India. He did his postdoctoral work at the State University of New York, Buffalo, USA (1996–1998), and worked at the NMR Research Centre, Indian Institute Science, Bangalore, India (1986–2001). Broadly, his research is focused on the area of metabolomics: methods development and applications with emphasis on the mechanistic insights into pathophysiology of diseases. His major efforts are focused on the development of NMR methods focused on unknown and unstable metabolite identification and quantitation in a variety biological specimens.
Daniel Raftery is the Founding Director of the Northwest Metabolomics Research Center and holds multiple academic positions at the University of Washington and Fred Hutch Cancer Research Center. He is a Medical Research and Education Endowed Professor in the Department of Anesthesiology and Pain Medicine, Adjunct Professor in the Department of Chemistry and Director of the NORC Metabolomics Sub-Core Facility, at the University of Washington, and Member, Fred Hutchinson Cancer Research Center, Seattle. Prior to 2012, he was a Professor of Chemistry at Purdue University, West Lafayette, Indiana, where he began his faculty career in 1994 in the Analytical Division. The Raftery research group focuses on the development of new methods and applications of metabolite profiling using a combination of NMR and mass spectrometry platforms which provide a broad-based approach for biomarker discovery and systems biology research.
REFERENCES
- 1.Edison AS; Colonna M; Gouveia GJ; Holderman NR; Judge MT; Shen X; Zhang S NMR: Unique Strengths That Enhance Modern Metabolomics Research. Anal Chem. 2021, 93, 478–499. [DOI] [PubMed] [Google Scholar]
- 2.Wishart DS NMR metabolomics: A look ahead. J. Magn. Reson. 2019, 306, 155–161. [DOI] [PubMed] [Google Scholar]
- 3.Nagana Gowda GA; Raftery D NMR-Based Metabolomics. Adv Exp Med Biol. 2021, 1280, 19–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nagana Gowda GA; Raftery D (eds.) NMR based metabolomics: methods and protocols. Methods in Molecular Biology, Vol. 2037. Humana Press/Springer Science, New York. 2019. [Google Scholar]
- 5.Wider G; Dreier L Measuring protein concentrations by NMR spectroscopy. J. Am. Chem. Soc. 2006, 128(8), 2571–6. [DOI] [PubMed] [Google Scholar]
- 6.Wishart DS Metabolomics for investigating physiological and pathophysiological processes. Physiol Rev. 2019, 99, 1819–1875. [DOI] [PubMed] [Google Scholar]
- 7.Vignoli A; Ghini V; Meoni G; Licari C; Takis PG; Tenori L; Turano P; Luchinat C High-Throughput Metabolomics by 1D NMR. Angew. Chem. Int. Ed. Engl. 2019, 58(4), 968–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhattacharya P; Chekmenev EY; Perman WH; Harris KC; Lin AP; Norton VA; Tan CT; Ross BD Weitekamp DP Towards hyperpolarized (13)C-succinate imaging of brain cancer, J. Magn. Reson. 2007, 186, 150–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chekmenev EY; Norton VA; Weitekamp DP; Bhattacharya P Hyperpolarized (1)H NMR employing low gamma nucleus for spin polarization storage. J. Am. Chem. Soc. 2009, 131, 3164–3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pravdivtsev AN; Buntkowsky G; Duckett SB; Koptyug IV; Hövener J -B. Parahydrogen-Induced Polarization of Amino Acids. Angew. Chem., Int. Ed. Engl. 2021, 60 (44), 23496–23507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hövener JB; Pravdivtsev AN; Kidd B; Bowers CR; Gloggler S; Kovtunov KV; Plaumann M; Katz-Brull R; Buckenmaier K; Jerschow A; Reineri F; Theis T; Shchepin RV; Wagner S; Bhattacharya P; Zacharias NM; Chekmenev EY Parahydrogen-Based Hyperpolarization for Biomedicine. Angew. Chem., Int. Ed. 2018, 57, 11140–11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adams RW; Aguilar JA; Atkinson KD; Cowley MJ; Elliott PIP; Duckett SB; Green GGR; Khazal IG; López-Serrano J; Williamson DC Reversible interactions with parahydrogen enhance NMR sensitivity by polarization transfer. Science 2009, 323, 1708–1711. [DOI] [PubMed] [Google Scholar]
- 13.Cowley MJ; Adams RW; Atkinson KD; Cockett MCR; Duckett SB; Green GGR; Lohman JAB; Kerssebaum R; Kilgour D; Mewis RE Iridium N-heterocyclic carbene complexes as efficient catalysts for magnetization transfer from parahydrogen. J. Am. Chem. Soc. 2011, 133, 6134–6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glöggler S; Muller R; Colell J; Emondts M; Dabrowski M; Blumich B; Appelt S Parahydrogen induced polarization of amino acids, peptides, and deuterium-hydrogen gas. Phys. Chem. Chem. Phys. 2011, 13, 13759–13764. [DOI] [PubMed] [Google Scholar]
- 15.Dücker EB; Kuhn LT; Munnemann K; Griesinger C Similarity of SABRE field dependence in chemically different substrates. J. Magn. Reson. 2012, 214, 159–165. [DOI] [PubMed] [Google Scholar]
- 16.Lloyd LS; Adams RW; Bernstein M; Coombes S; Duckett SB; Green GGR; Lewis RJ; Mewis RE; Sleigh CJ Utilization of SABRE-derived hyperpolarization to detect lowconcentration analytes via 1D and 2D NMR methods. J. Am. Chem. Soc. 2012, 134, 12904–12907. [DOI] [PubMed] [Google Scholar]
- 17.Fraser R; Rutjes FPJT Feiters, M.C.; Tessari, M.; Analysis of Complex Mixtures by Chemosensing NMR Using Para-Hydrogen-Induced Hyperpolarization, Acc. Chem. Res. 2022, 55(13), 1832–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ausmees K; Reimets N; Reile I 1 Parahydrogen hyperpolarization of minimally altered urine samples for sensitivity enhanced NMR metabolomics. Chem. Commun (Camb). 2022, 58(3), 463–466. [DOI] [PubMed] [Google Scholar]
- 19.Frydman L; Blazina D Ultrafast two-dimensional nuclear magnetic resonance spectroscopy of hyperpolarized solutions. Nat. Phys. 2007, 3, 415–419. [Google Scholar]
- 20.Singh K; Jacquemmoz C; Giraudeau P; Frydman L; Dumez J -N. Ultrafast 2D 1H- 1H NMR spectroscopy of DNP-hyperpolarised substrates for the analysis of mixtures. Chem. Commun (Camb). 2021, 57(65), 8035–8038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ardenkjaer-Larsen JH; Fridlund B; Gram A; Hansson G; Hansson L; Lerche MH; Servin R; Thaning M.; Golman, K. Increase in signal-to-noise ratio of > 10,000 times in liquid-state NMR. Proc. Natl. Acad. Sci. U.S.A. 2003, 100, 10158–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dey A; Charrier B; Martineau E; Deborde C; Gandriau E; Moing A; Jacob D; Eshchenko D; Schnell M; Melzi R; Kurzbach D; Ceillier M; Chappuis Q; Cousin SF; Kempf JG; Jannin S; Dumez J -N.; Giraudeau, P. Hyperpolarized NMR Metabolomics at Natural 13C Abundance. Anal. Chem. 2020, 92(22), 14867–14871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hansen AL; Kupče ER; Li D -W.; Bruschweiler-Li, L.; Wang, C.; Brüschweiler, R. 2D NMR-Based Metabolomics with HSQC/TOCSY NOAH Supersequences. Anal. Chem. 2021, 93(15), 6112–6119. [DOI] [PubMed] [Google Scholar]
- 24.Lefort G; Liaubet L; Marty-Gasset N; Canlet C; Vialaneix N; Servien R Joint Automatic Metabolite Identification and Quantification of a Set of 1 H NMR Spectr. Anal. Chem. 2021, 93(5), 2861–2870. [DOI] [PubMed] [Google Scholar]
- 25.Garcia-Perez I; Posma JM; Serrano-Contreras JI; Boulangé CL; Chan Q; Frost G; Stamler J; Elliott P; Lindon JC; Holmes E; Nicholson JK Identifying unknown metabolites using NMR-based metabolic profiling techniques. Nat. Protoc. 2020, 15(8), 2538–2567. [DOI] [PubMed] [Google Scholar]
- 26.Migdadi L; Lambert J; Telfah A; Hergenröder R; Wöhler C Automated metabolic assignment: Semi-supervised learning in metabolic analysis employing two-dimensional Nuclear Magnetic Resonance (NMR). Comput. Struct. Biotechnol. J. 2021, 19, 5047–5058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim HW; Zhang C; Cottrell GW; Gerwick WH SMART-Miner: A convolutional neural network-based metabolite identification from 1H- 13C HSQC spectra. Magn. Reson. Chem. 2021, doi: 10.1002/mrc.5240. [DOI] [PubMed] [Google Scholar]
- 28.Li D-W; Hansen AL; Bruschweiler-Li L; Yuan C; Brüschweiler R Fundamental and practical aspects of machine learning for the peak picking of biomolecular NMR spectra. J. Biomol. NMR. 2022, doi: 10.1007/s10858-022-00393-1. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wishart DS; Guo A; Oler E; Wang F; Anjum A; Peters H; Dizon R; Sayeeda Z; Tian S; Lee BL; Berjanskii M; Mah R; Yamamoto M; Jovel J; Torres-Calzada C; Hiebert-Giesbrecht M; Lui VW; Varshavi D; Varshavi D; Allen D; Arndt D; Khetarpal N; Sivakumaran A; Harford K; Sanford S; Yee K; Cao X; Budinski Z; Liigand J; Zhang L; Zheng J; Mandal R; Karu N; Dambrova M; Schiöth HB; Greiner R; Gautam V HMDB 5.0: the Human Metabolome Database for 2022. Nucleic Acids Res. 2022, 50(D1), D622–D631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maroli AS; Powers R Closing the gap between in vivo and in vitro omics: using QA/QC to strengthen ex vivo NMR metabolomics. NMR Biomed. 2021, e4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crook AA; Powers R Quantitative NMR-Based Biomedical Metabolomics: Current Status and Applications. Molecules 2020, 25(21), 5128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Röhnisch HE; Eriksson J; Tran LV; Müllner E; Sandström C; Moazzami AA Improved Automated Quantification Algorithm (AQuA) and Its Application to NMR-Based Metabolomics of EDTA-Containing Plasma. Anal. Chem. 2021, 93(25), 8729–8738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu Y; Judge MT; Arnold J; Bhandarkar SM; Edison AS RTExtract: time-series NMR spectra quantification based on 3D surface ridge tracking. Bioinformatics 2020, 36(20), 5068–5075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nagana Gowda GA; Abell L; Lee CF; Tian R; Raftery D Simultaneous Analysis of Major Coenzymes of Cellular Redox Reactions and Energy Using ex Vivo 1H NMR Spectroscopy. Anal. Chem. 2016, 88(9), 4817–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nagana Gowda GA; Raftery D Whole Blood Metabolomics by 1H NMR Spectroscopy Provides a New Opportunity to Evaluate Coenzymes of Redox Reactions, Coenzymes of Energy and Antioxidants. Anal. Chem. 2017, 89(8), 4620–4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nagana Gowda GA; Abell L; Tian R Extending the Scope of 1H NMR Spectroscopy for the Analysis of Cellular Coenzyme A and Acetyl Coenzyme A. Anal. Chem. 2019, 91(3), 2464–2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nagana Gowda GA; Pascua V; Raftery D Extending the Scope of 1H NMR-Based Blood Metabolomics for the Analysis of Labile Antioxidants: Reduced and Oxidized Glutathione. Anal. Chem. 2021, 93 (44), 14844–14850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nagana Gowda GA; Hong NN; Raftery D Evaluation of Fumaric acid and Maleic acid as Internal Standards for NMR Analysis of Protein Precipitated Plasma, Serum, and Whole Blood. Anal. Chem. 2021, 93(6), 3233–3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Castaing-Cordier T; Bouillaud D; Farjon J; Giraudeau P Recent advances in benchtop NMR and its applications. Ann. Rep. NMR Spect. 2021, 103, 191–258. [Google Scholar]
- 40.Küster SK; Danieli E; Blümich B; Casanova F High-resolution NMR spectroscopy under the fumehood. Phys. Chem. Chem. Phys. 2011, 13, 13172–13176. [DOI] [PubMed] [Google Scholar]
- 41.Percival BC; Grootveld M; Gibson M; Osman Y; Molinari M; Jafari F; Sahota T; Martin M; Casanova F; Mather ML; Edgar M; Masania J; Wilson PB Low-Field, Benchtop NMR Spectroscopy as a Potential Tool for Point-of-Care Diagnostics of Metabolic Conditions: Validation, Protocols and Computational Models. High Throughput 2019, 8(1), 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leenders J Grootveld M; Percival B; Gibson M; Casanova F; Wilson PB Benchtop Low-Frequency 60 MHz NMR Analysis of Urine: A Comparative Metabolomics Investigation. Metabolites 2020, 10(4), 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Izquierdo-Garcia JL; Comella-del-Barrio P; Campos-Olivas R; Villar-Hernández R; Prat-Aymerich C; De Souza-Galvão ML; Jiménez-Fuentes MA; Ruiz-Manzano J; Stojanovic Z; González A; Serra-Vidal M; García-García E; Muriel-Moreno B; Millet JP; Molina-Pinargote I; Casas X; Santiago J; Sabriá F; Martos C; Herzmann C; Ruiz-Cabello J; Domínguez J Discovery and validation of an NMR-based metabolomic profile in urine as TB biomarker. Sci. Rep. 2020, 10, 22317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Edgar M; Percival BC; Gibson M; Jafari F; Grootveld M Low-field benchtop NMR spectroscopy as a potential non-stationary tool for point-of-care urinary metabolite tracking in diabetic condition. Diabetes Res. Clin. Pract. 2021, 171, 108554. [DOI] [PubMed] [Google Scholar]
- 45.Finch N; Percival B; Hunter E; Blagg RJ; Blackwell E; Sagar J; Ahmad Z; Chang MW; Hunt JA; Mather ML; Tasker S; Risio LD; Wilson PB Preliminary demonstration of benchtop NMR metabolic profiling of feline urine: chronic kidney disease as a case study. BMC Res. Notes 2021, 14(1), 469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Edgar M; Kuhn S; Page G; Grootveld M Computational simulation of 1H NMR profiles of complex biofluid analyte mixtures at differential operating frequencies: Applications to low-field benchtop spectra. Magn. Reson. Chem. 2021, doi: 10.1002/mrc.5236. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 47.Cha JW; Jin X; Jo S; An YJ; Park S Metabolic mechanisms of a drug revealed by distortion-free 13C tracer analysis. Chem. Sci. 2021, 12(13), 4958–4962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bhinderwala F; Evans P; Jones K; Laws BR; Smith TG; Morton M; Powers R Phosphorus NMR and Its Application to Metabolomics. Anal. Chem. 2020, 92(14), 9536–9545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wen H; An YJ; Xu WJ; Kang KW; Park S Real-time monitoring of cancer cell metabolism and effects of an anticancer agent using 2D in-cell NMR spectroscopy. Angew. Chem. Int. Ed. Engl. 2015, 54(18), 5374–5377. [DOI] [PubMed] [Google Scholar]
- 50.Xu WJ, Wen H, Kim HS, Ko YJ, Dong SM, Park IS, Yook JI, Park S. Observation of acetyl phosphate formation in mammalian mitochondria using real-time in-organelle NMR metabolomics. Proc. Natl. Acad. Sci. U S A. 2018,115(16):4152–4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee S; Wen H; An YJ; Cha JW; Ko Y -J.; Hyberts, S.J.; Park, S. Carbon Isotopomer Analysis with Non-Unifom Sampling HSQC NMR for Cell Extract and Live Cell Metabolomics Studies. Anal. Chem. 2017, 89(2):1078–1085. [DOI] [PubMed] [Google Scholar]
- 52.Deja S; Kucejova B; Fu X; Browning JD; Young JD; Burgess S In Vivo Estimation of Ketogenesis Using Metabolic Flux Analysis-Technical Aspects and Model Interpretation. Metabolites 2021, 11(5), 279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nguyen TTM; An YJ; Cha JW; Ko Y-J; Lee H; Chung CH; Jeon S-M; Lee J; Park S. Real-Time In-Organism NMR Metabolomics Reveals Different Roles of AMP-Activated Protein Kinase Catalytic Subunits. Anal. Chem. 2020, 92(11), 7382–7387. [DOI] [PubMed] [Google Scholar]
- 54.Knitsch R; AlWahsh M; Raschke H; Lambert J; Hergenröder R In Vitro Spatio-Temporal NMR Metabolomics of Living 3D Cell Models. Anal. Chem. 2021, 93(40), 13485–13494. [DOI] [PubMed] [Google Scholar]
- 55.Patra B; Sharma M; Hale W; Utz M Time-resolved non-invasive metabolomic monitoring of a single cancer spheroid by microfluidic NMR. Sci. Rep. 2021, 11(1), 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hertig D; Maddah S; Memedovski R; Kurth S; Moreno A; Pennestri M; Felser A; Nuoffer JM; Vermathen P Live monitoring of cellular metabolism and mitochondrial respiration in 3D cell culture system using NMR spectroscopy. Analyst 2021, 146(13), 4326–4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Qin J; Li R; Raes J; Arumugam M; Burgdorf KS; Manichanh C; Nielsen T; Pons N; Levenez F; Yamada T; Mende DR; Li J; Xu J; Li S; Li D; Cao J; Wang B; Liang H; Zheng H; Xie Y; Tap J; Lepage P; Bertalan M; Batto JM; Hansen T; Le Paslier D; Linneberg A; Nielsen HB; Pelletier E; Renault P; Sicheritz-Ponten T; Turner K; Zhu H; Yu C; Li S; Jian M; Zhou Y; Li Y; Zhang X; Li S; Qin N; Yang H; Wang J; Brunak S; Doré J; Guarner F; Kristiansen K; Pedersen O; Parkhill J; Weissenbach J MetaHIT Consortium, Bork P; Ehrlich SD; Wang J. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vandeputte D; Commer LD; Tito RY; Kathagen G; Sabino J; Vermeire S; Faust K Raes J. Temporal variability in quantitative human gut microbiome profiles and implications for clinical research. Nat. Commun. 2021, 12, 6740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nguyen TTM; Mai V -H.; Kim, H.S.; Kim, D.; Seo, M.; An, Y.J.; Park, S. Real-Time Monitoring of Host–Gut Microbial Interspecies Interaction in Anticancer Drug Metabolism. J. Am. Chem. Soc. 2022, 144(19), 8529–8535. [DOI] [PubMed] [Google Scholar]
- 60.DeBerardinis RJ; Mancuso A; Daikhin E; Nissim I; Yudkoff M; Wehrli S; Thompson CB; Beyond aerobic glycolysis: Transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc. Natl. Acad. Sci. USA 2007, 104, 19345–19350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wise DR; DeBerardinis RJ; Mancuso A; Sayed N; Zhang X-Y; Pfeiffer HK; Nissim I; Daikhin E; Yudkoff M; McMahon SB; Thompson CB Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc. Natl. Acad. Sci. USA 2008, 105, 18782–18787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mashimo T; Pichumani K; Vemireddy V; Hatanpaa KJ; Singh DK; Sirasanagandla S; Nannepaga S; Piccirillo SG; Kovacs Z; Foong C; Huang Z; Barnett S; Mickey BE; DeBerardinis RJ; Tu BP; Maher EA; Bachoo RM Acetate is a bioenergetic substrate for human glioblastoma and brain metastases. Cell 2014, 159(7), 1603–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bruntz RC; Lane AN; Higashi RM; Fan T W-M. Exploring cancer metabolism using stable isotope-resolved metabolomics (SIRM). J. Biol. Chem. 2017, 292(28):11601–11609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lin P; Dai L; Crooks DR; Neckers LM; Higashi RM; Fan TW-M; Lane AN NMR Methods for Determining Lipid Turnover via Stable Isotope Resolved Metabolomics. Metabolites 2021, 11(4), 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hambardikar V; Guitart-Mampe M; Scoma ER; Urquiza P; Nagana Gowda GA; Raftery D; Collins JA; Solesio ME Enzymatic Depletion of Mitochondrial Inorganic Polyphosphate (polyP) Increases the Generation of Reactive Oxygen Species (ROS) and the Activity of the Pentose Phosphate Pathway (PPP) in Mammalian Cells. Antioxidants (Basel) 2022, 11(4), 685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee MH; Malloy CR; Corbin IR; Li J; Jin ES Assessing the pentose phosphate pathway using [2,3-13C2]-glucose. NMR Biomed. 2019, 32(6), e4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jin ES; Lee MH; Malloy CR 13C NMR of glutamate for monitoring the pentose phosphate pathway in myocardium. NMR Biomed. 2021, 34(7), e4533. [DOI] [PubMed] [Google Scholar]
- 68.Pichumani K; Ijare OB; Powell S; Baskin. Oxidation of ketone body in brain tumor patients. Proc. Intl. Soc. Mag. Reson. Med. 2022, 30, 0283. [Google Scholar]
- 69.Pichumani K; Ijare OB; Maher E; Bachoo RM; Baskin DS Quantification of pyruvate recycling in brain tumor patients. Proc. Intl. Soc. Mag. Reson. Med. 2021, 29, 0771. [Google Scholar]
- 70.Zeeberg BR, Kohn KW, Kahn A, Larionov, V.; Weinstein, J. N.; Reinhold, W.; Pommier, Y. Concordance of gene expression and functional correlation patterns across the NCI-60 cell lines and the Cancer Genome Atlas glioblastoma samples. PLoS ONE 2012, 7(7), e40062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Doncheva NT; Palasca O; Yarani R; Litman T; Anthon C; Groenen MAM; Stadler PF; Pociot F; Jensen LJ; Gorodkin J Human pathways in animal models: possibilities and limitations. Nucleic Acids Res. 2021, 49(4), 1859–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shanks N; Greek R; Greek J Are animal models predictive for humans? Philos. Ethics Humanit. Med. 2009, 4, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Frahm AB; Jensen PR; Ardenkjær-Larsen JH; Yigit D; Lerche MH Stable isotope resolved metabolomics classification of prostate cancer cells using hyperpolarized NMR data. J. Magn. Reson. 2020, 316, 106750. [DOI] [PubMed] [Google Scholar]
- 74.Wise DR; Thompson CB Glutamine addiction: a new therapeutic target in cancer. Trends. Biochem. Sci. 2010, 35(8), 427–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Eskandari R; Kim N; Mamakhanyan A; Saoi M; Zhang G; Berisaj M; Granlund KL; Poot AJ; Cross J; Thompson CB; Keshari KR Hyperpolarized [5– 13 C,4,4– 2 H 2,5– 15 N]-L-glutamine provides a means of annotating in vivo metabolic utilization of glutamine. Proc. Natl. Acad. Sci. U S A. 2022, 119(19), e2120595119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen C; Deng L; Wei S; Nagana Gowda GA; Gu H; Chiorean EG; Zaid MA; Harrison ML; Pekny JF; Loehrer PJ; Zhang D; Zhang M; Raftery D Exploring Metabolic Profile Differences between Colorectal Polyp Patients and Controls Using Seemingly Unrelated Regression. J. Proteome Res. 2015, 14(6), 2492–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen C; Nagana Gowda GA; Zhu J; Deng L; Gu H; Chiorean EG; Zaid MA; Harrison M; Zhang D; Zhang, M.; Raftery, D. Altered metabolite levels and correlations in patients with colorectal cancer and polyps detected using seemingly unrelated regression analysis. Metabolomics 2017, 13(11), 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Karaman I; Ferreira DLS; Boulange CL; Kaluarachchi MR; Herrington D; Dona AC; Castagne R; Moayyeri A; Lehne B; Loh M; et al. Workflow for Integrated Processing of Multicohort Untargeted H-1 NMR Metabolomics Data in Large-Scale Metabolic Epidemiology. J. Proteome Res. 2016, 15, 4188–4194. [DOI] [PubMed] [Google Scholar]
- 79.Wurtz P; Kangas AJ; Soininen P; Lawlor DA; Smith GD; Ala-Korpela M Quantitative Serum Nuclear Magnetic Resonance Metabolomics in Large-Scale Epidemiology: A Primer on -Omic Technologies. Am. J. Epidemiol. 2017, 186, 1084–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tynkkynen T; Wang Q; Ekholm J; Anufrieva O; Ohukainen P; Vepsäläinen J; Männikkö M; Keinänen-Kiukaanniemi S; Holmes MV; Goodwin M; Ring S; Chambers JC; Kooner J; Järvelin MR Kettunen J; Hill M; Smith GD; Ala-Korpela M Proof of concept for quantitative urine NMR metabolomics pipeline for large-scale epidemiology and genetics. Int. J. Epidemiol. B, 2019, 48(3), 978–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vojinovic D; Kalaoja M; Trompet S; Fischer K; Shipley MJ; Li S; Havulinna AS; Perola M; Salomaa V; Yang Q; Sattar N; Jousilahti P; Amin N; Satizabal CL; Taba N; Sabayan B; Vasan RS; Ikram MA; Stott DJ; Ala-Korpela M; Jukema W; Seshadri S; Kettunen J; Kivimaki M; Esko T; van Duijn CM Association of circulating metabolites in plasma or serum and risk of stroke: Meta-analysis from seven prospective cohorts. Neurology 2020, 96(8), e1110–e1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Newgard CB; An J; Bain JR; Muehlbauer MJ; Stevens RD; Lien LF; Haqq AM; Shah SH; Arlotto M; Slentz CA; Rochon J; Gallup D; Ilkayeva O; Wenner BR; Yancy WS Jr; Eisenson H; Musante G; Surwit RS; Millington DS; Butler MD Svetkey, L.P. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009, 9(4):311–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tobias DK; Hazra A; Lawler PR; Chandler PD; Chasman DI; Buring JE; Lee I; Cheng S; Manson JE; Mora S Circulating branched-chain amino acids and long-term risk of obesity-related cancers in women. Sci. Rep. 2020, 10(1), 16534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Prentice RL; Pettinger M; Zheng C; Neuhouser ML; Raftery D; Nagana Gowda GA; Huang Y; Tinker LF; Howard BV; Manson JE; Van Horn L; Wallace R; Mossavar-Rahmani Y; Johnson KC; Snetselaar L; Lampe JW Biomarkers for Components of Dietary Protein and Carbohydrate with Application to Chronic Disease Risk in Postmenopausal Women. J. Nutr. 2022, 152(4):1107–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Prentice RL; Pettinger M; Neuhouser ML; Raftery D; Zheng C; Nagana Gowda GA; Huang Y; Tinker LF; Howard BV; Manson JE; Wallace R; Mossavar-Rahmani Y; Johnson KC; Lampe JW Biomarker-Calibrated Macronutrient Intake and Chronic Disease Risk among Postmenopausal Women. J. Nutr. 2021, 151(8):2330–2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tikkanen E; Jägerroos V; Holmes MV; Sattar N; Ala-Korpela M; Jousilahti P; Lundqvist A; Perola M; Salomaa V; Würtz P Metabolic Biomarker Discovery for Risk of Peripheral Artery Disease Compared with Coronary Artery Disease: Lipoprotein and Metabolite Profiling of 31 657 Individuals From 5 Prospective Cohorts. J. Am. Heart Assoc. 2021, 10(23). e021995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mäkinen V-P; Karsikas M; Kettunen J; Lehtimäki T; Kähönen M; Viikari J; Perola M; Salomaa V; Järvelin M-R; Raitakari OT; Ala-Korpela M Longitudinal profiling of metabolic ageing trends in two population cohorts of young adults. Int. J. Epidemiol. 2022, dyac062. doi: 10.1093/ije/dyac062. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 88.Simell BA; Törnwall OM; Hämäläinen I; Wichmann H-E; Anton G; Brennan P; Bouvard L; Slimani N; Moskal A; Gunter M; Zatloukal K; Minion JT; Soini S; Mayrhofer MT; Murtagh MJ; van Ommen G-J; Johansson M; Perola M; BBMRI-LPC Consortium (FP7 GA no. 313010). Transnational access to large prospective cohorts in Europe: Current trends and unmet needs. Nat Biotechnol. 2019, 49, 98–103. [DOI] [PubMed] [Google Scholar]
- 89.Ghini V; Abuja PM; Polasek O; Kozera L; Laiho P; Anton G; Zins M; Klovins J; Metspalu A; Wichmann H -E.; Gieger, C.; Luchinat, C.; Zatloukal, K.; Turano, P. Impact of the pre-examination phase on multicenter metabolomic studies. Nat. Biotechnol. 2022, 68:37–47. [DOI] [PubMed] [Google Scholar]
- 90.Vignoli A; Tenori L; Morsiani C; Turano P; Capri M; Luchinat C Serum or Plasma (and Which Plasma), That Is the Question. J. Proteome Res. 2022, 21(4), 1061–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Trammell SAJ; Schmidt MS; Weidemann BJ; Redpath P; Jaksch F; Dellinger RW; Li Z; Abel ED; Migaud ME; Brenner C Nicotinamide riboside is uniquely and orally bioavailable in mice and humans. Nat. Commun. 2016, 7, 12948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Airhart SE; Shireman LM; Risler LJ; Anderson GD; Nagana Gowda GA; Raftery D; Tian R; Shen DD; O’Brien KD An open-label, non-randomized study of the pharmacokinetics of the nutritional supplement nicotinamide riboside (NR) and its effects on blood NAD+ levels in healthy volunteers. PLoS One. 2017, 12(12), e0186459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Brakedal B; Dölle C; Riemer F; Ma Y; Nido GS; Skeie GO; Craven AR; Schwarzlmüller T; Brekke N; Diab J; Sverkeli L; Skjeie V; Varhaug K; Tysnes O -B.; Peng, S.; Haugarvoll, K.; Ziegler, M.; Grüner, R.; Eidelberg, D.; Tzoulis, C. The NADPARK study: A randomized phase I trial of nicotinamide riboside supplementation in Parkinson’s disease. Cell. Metab. 2022, 34(3), 396–407. [DOI] [PubMed] [Google Scholar]
- 94.Liu L; Su X; Quinn WJ III; Hui S; Krukenberg K; Frederick DW; Redpath P; Zhan L; Chellappa K; White E; et al. Quantitative analysis of NAD synthesis-breakdown fluxes. Cell Metab. 2018, 27, 1067–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Campbell MT; Jones DS; Andrews GP; Li S Understanding the physicochemical properties and degradation kinetics of nicotinamide riboside, a promising vitamin B3 nutritional supplement. Food Nutr. Res. 2019, 63, 3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Imai S-I Nicotinamide Phosphoribosyltransferase (Nampt): A Link Between NAD Biology, Metabolism, and Diseases. Curr. Pharm. Des. 2009, 15, 20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Xue Y; Shamp T; Nagana Gowda GA; Crabtree M; Bagchi D; Raftery D A Combination of Nicotinamide and D-Ribose (RiaGev) Is Safe and Effective to Increase NAD+ Metabolome in Healthy Middle-Aged Adults: A Randomized, Triple-Blind, Placebo-Controlled, Cross-Over Pilot Clinical Trial, Nutrients 2022, 14, 2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pablos I; Machado Y; de Jesus HCR,; Mohamud Y; Kappelhoff R; Lindskog C; Vlok M; Bell PA; Butler GS; Grin PM; Cao QT; Nguyen JP; Solis N; Abbina S; Rut W; Vederas JC; Szekely L; Szakos A; Drag M; Kizhakkedathu JN; Mossman K; Hirota JA; Jan E; Luo H; Banerjee A; Overall CM. Mechanistic insights into COVID-19 by global analysis of the SARS-CoV-2 3CLpro substrate degradome. Cell Rep. 2021, 37(4), 109892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bruzzone C; Bizkarguenaga M; Gil-Redondo R; Diercks T; Arana E; de Vicuña AG; Seco M; Bosch A; Palazón A; Juan IS; Laín A; Gil-Martínez J; Bernardo-Seisdedos G; Fernández-Ramos D; Lopitz-Otsoa F; Embade N; Lu S; Mato JM; Millet O SARS-CoV-2 Infection Dysregulates the Metabolomic and Lipidomic Profiles of Serum. iScience 2020, 23(10), 101645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shen B; Yi X; Sun Y; Bi X; Du J; Zhang C; Quan S; Zhang F; Sun R; Qian L; Ge W; Liu W; Liang S; Chen H; Zhang Y; Li J; Xu J; He Z; Guo T (2020). Proteomic and Metabolomic Characterization of COVID-19 Patient Sera. Cell 2020, 182, 59–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wu D; Shu T; Yang X; Song J-X; Zhang M; Yao C; Liu W; Huang M; Yu Y; Yang Q; Zhu T; Xu J; Mu J; Wang Y; Wang H; Tang T; Ren Y; Wu Y; Lin S-H; Qiu Y; Zhang D-Y; Shang Y; Zhou X. Plasma Metabolomic and Lipidomic Alterations Associated with COVID-19. Natl. Sci. Rev. 2020, 7, 1157–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bruzzone C; Bizkarguenaga M; Gil-Redondo R; Diercks T; Arana E; de Vicuña AG; Seco M; Bosch A; Palazón A; Juan IS; Laín A; Gil-Martínez J; Bernardo-Seisdedos G; Fernández-Ramos D; Lopitz-Otsoa F; Embade N,; Lu S; Mato JM; Millet O. SARS-CoV-2 Infection Dysregulates the Metabolomic and Lipidomic Profiles of Serum. iScience 2020, 23(10):101645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kimhofer T; Lodge S; Whiley L; Gray N; Loo RL; Lawler NG; Nitschke P; Bong S-H; Morrison DL; Begum S; Richards T; Yeap BB; Smith C; Smith KGC; Holmes E; Nicholson JK. Integrative Modeling of Quantitative Plasma Lipoprotein, Metabolic, and Amino Acid Data Reveals a Multiorgan Pathological Signature of SARS-CoV-2 Infection. J. Proteome Res. 2020, 19, 4442–4454. [DOI] [PubMed] [Google Scholar]
- 104.Schmelter S; Föh B; Mallagaray A; Rahmöller J; Ehlers M; Lehrian S; Kopylow VV; Künsting I; Lixenfeld AS; Martin E; Ragab M; Meyer-Saraei R; Kreutzmann F; Eitel I; Taube S; Käding N; Jantzen E; Graf T; Sina C; Günther UL Metabolic and Lipidomic Markers Differentiate COVID-19 From Non-Hospitalized and Other Intensive Care Patients. Front. Mol. Biosci. 2021, 8, 737039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ghini V; Meoni G; Pelagatti L; Celli T; Veneziani F; Petrucci F; Vannucchi V; Bertini L; Luchinat C; Landini G; Turano P Profiling metabolites and lipoproteins in COMETA, an Italian cohort of COVID-19 patients. PLoS Pathog. 2022, 18(4), e1010443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bizkarguenaga M Bruzzone C; Gil-Redondo R; SanJuan I. Martin-Ruiz I; Barriales D; Palacios A; Pasco ST; González-Valle B; Laín A; Herrera L; Azkarate A; Vesga MA; Eguizabal C; Anguita J; Embade N; Mato JM; Millet O. Uneven metabolic and lipidomic profiles in recovered COVID-19 patients as investigated by plasma NMR metabolomics. NMR Biomed. 2022, 35(2), e4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Meoni G Ghini V; Maggi L; Vignoli A; Mazzoni A; Salvati L; Capone M; Vanni A; Tenori L; Fontanari P; Lavorini F; Peris A; Bartoloni; Liotta F; Cosmi L; Luchinat C; Annunziato F; Turano P Metabolomic/lipidomic profiling of COVID-19 and individual response to tocilizumab. PLoS Pathog. 2021, 17(2), e1009243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rendeiro AF; Vorkas CK; Krumsiek J; Singh HK; Kapadia SN; Cappelli LV; Cacciapuoti MT; Inghirami G; Elemento O; Salvatore M Metabolic and Immune Markers for Precise Monitoring of COVID-19 Severity and Treatment. Front. Immunol. 2022, 12, 809937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lodge S; Nitschke P; Kimhofer T; Coudert JD; Begum S; Bong S-H; Richards T; Edgar D; Raby E; Spraul M; Schaefer H; Lindon JC; Loo RL; Holmes E; Nicholson JK. NMR Spectroscopic Windows on the Systemic Effects of SARS-CoV-2 Infection on Plasma Lipoproteins and Metabolites in Relation to Circulating Cytokines. J. Proteome Res. 2021, 5;20(2), 1382–1396. [DOI] [PubMed] [Google Scholar]
- 110.Otvos JD; Jeyarajah EJ; Bennett DW Quantification of plasma lipoproteins by proton nuclear magnetic resonance spectroscopy. Clin. Chem. 1991, 37, 377–386. [PubMed] [Google Scholar]
- 111.Jiménez B; Holmes E; Heude C; Tolson RF; Harvey N; Lodge SL; Chetwynd AJ; Cannet C; Fang F; Pearce JTM; Lewis MR; Viant MR; Lindon JC; Spraul M; Schäfer H; Nicholson JK Quantitative Lipoprotein Subclass and Low Molecular Weight Metabolite Analysis in Human Serum and Plasma by (1)H NMR Spectroscopy in a Multilaboratory Trial. Anal. Chem. 2018, 90, 11962–11971. [DOI] [PubMed] [Google Scholar]
- 112.Lodge S; Nitschke P; Loo RL; Kimhofer T; Bong S-H; Richards T; Begum S; Spraul M; Schaefer H; Lindon JC; Holmes E; Nicholson JK Low Volume in Vitro Diagnostic Proton NMR Spectroscopy of Human Blood Plasma for Lipoprotein and Metabolite Analysis: Application to SARS-CoV-2 Biomarkers. J. Proteome Res. 2021, 20(2), 1415–1423. [DOI] [PubMed] [Google Scholar]
- 113.Ebinger JE; Fert-Bober J; Printsev I; Wu M; Sun N; Prostko JC; Frias EC; Stewart JL; Eyk JEV; Braun JG; Cheng S; Sobhani K Antibody Responses to the BNT162b2 mRNA Vaccine in Individuals Previously Infected with SARS-CoV-2. Nat. Med. 2021, 27, 981–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mazzoni A; Di Lauria N; Maggi L; Salvati L; Vanni A; Capone M; Lamacchia G; Mantengoli E; Spinicci M; Zammarchi L; Kiros ST; Rocca A; Lagi F; Colao MG; Parronchi P; Scaletti C; Turco L; Liotta F; Rossolini GM; Cosmi L; Bartoloni A; Annunziato F; COVID-19 Research Group. First-dose mRNA Vaccination Is Sufficient to Reactivate Immunological Memory to SARS-CoV-2 in Subjects Who Have Recovered from COVID-19. J. Clin. Invest. 2021, 131, 149150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Levi R; Azzolini E; Pozzi C; Ubaldi L; Lagioia M; Mantovani A; Rescigno M One Dose of SARS-CoV-2 Vaccine Exponentially Increases Antibodies in Individuals Who Have Recovered from Symptomatic COVID-19. J. Clin. Invest. 2021, 131, e149154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ghini V; Maggi L; Mazzoni A; Spinicci M; Zammarchi L; Bartoloni A; Annunziato F; Turano P Serum NMR Profiling Reveals Differential Alterations in the Lipoproteome Induced by Pfizer-BioNTech Vaccine in COVID-19 Recovered Subjects and Naïve Subjects. Front. Mol. Biosci. 2022, 9, 839809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dagla I; Iliou A; Benaki D; Gikas E; Mikros E; Bagratuni T; Kastritis E; Dimopoulos MA; Terpos E; Tsarbopoulos A Plasma Metabolomic Alterations Induced by COVID-19 Vaccination Reveal Putative Biomarkers Reflecting the Immune Response. Cells 2022, 11(7), 1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Julkunen H; Cichońska A; Slagboom PE; Würtz P; Nightingale Health UK Biobank Initiative. Metabolic biomarker profiling for identification of susceptibility to severe pneumonia and COVID-19 in the general population. Elife 2021, 10, e63033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lorente JA; Nin N; Villa P; Vasco D; Miguel-Coello AB; Rodriguez I; Herrero R; Peñuelas O; Ruiz-Cabello J; Izquierdo-Garcia JL Metabolomic differences between COVID-19 and H1N1 influenza induced ARDS. Crit. Care. 2021, 25(1), 390. [DOI] [PMC free article] [PubMed] [Google Scholar]


