Abstract
Bruton’s tyrosine kinase (BTK) inhibition is one of the treatment standards for patients with relapsed/refractory Waldenström’s macroglobulinemia (WM) and for patients with WM who are unsuitable for immunochemotherapy (ICT). It offers deep and durable responses with a manageable safety profile that is generally favorable compared with ICT regimens. However, the limitations of the first approved BTK inhibitor (BTKi), ibrutinib, include reduced efficacy in patients lacking the characteristic WM mutation (MYD88L265P) and toxicities related to off-target activity. The risk of atrial fibrillation (AF) and other cardiovascular side effects are a notable feature of ibrutinib therapy. Several next-generation covalent BTKis with greater selectivity for BTK are at various stages of development. In November 2021, zanubrutinib became the first of these agents to be approved by the European Medicines Agency for the treatment of WM. Head-to-head trial data indicate that it has comparable efficacy to ibrutinib for patients with WM overall, although it may be more effective in patients with CXCR4 mutations or wild-type MYD88. In the clinical trial setting, its greater selectivity translates into a reduced risk of cardiovascular side effects, including AF. Acalabrutinib, which is pre-approval in WM, appears to offer similar advantages over ibrutinib in terms of its safety profile. Beyond the next-generation covalent BTKis, non-covalent BTKis are an emerging class with the potential to provide a therapeutic option for patients who relapse on covalent BTKis. In the future, BTKis may be increasingly utilized within combination regimens. Several ongoing trials in WM are investigating the potential for BTKi use in combination with established and novel targeted agents.
Subject terms: B-cell lymphoma, Targeted therapies
Introduction
Waldenström’s macroglobulinemia (WM) is a rare indolent B-cell lymphoma characterized primarily by bone marrow infiltration by lymphoplasmacytic cells and IgM monoclonal gammopathy [1]. It comprises approximately 1–2% of all cases of non-Hodgkin’s lymphoma in Europe and the USA, with an incidence of ~4 cases per 1 million person-years [2–6]. Prevalence is higher in males than in females and in Caucasians versus other races, and increases with age, with most WM patients aged ≥65 years at the time of diagnosis [4, 5, 7]. It is incurable, with a median survival of 10–12 years [8], and many patients die from causes unrelated to the disease [9].
The emergence of more effective treatments over the last two decades has resulted in improved outcomes for patients [10]. Rituximab-based immunochemotherapy (ICT) regimens were the mainstay of treatment for first-line and relapsed/refractory (R/R) WM [11], but Bruton’s tyrosine kinase inhibitors (BTKi), such as ibrutinib, are now standard for R/R disease (Fig. 1) [12]. Ibrutinib offers deep and durable responses with an acceptable toxicity profile. However, it has limitations, including potentially serious side effects attributed to significant off-target activity [13]. Next-generation BTKis have been developed with the aim of achieving greater target occupancy and selectivity, and this review will provide a practical overview of these agents. The greatest differentiation between the BTKis may be in their safety profiles, and this article will outline the most common and problematic side effects, including ibrutinib-associated AF. We will also consider future developments in the treatment of WM that may overcome some of the limitations of BTKi monotherapy.
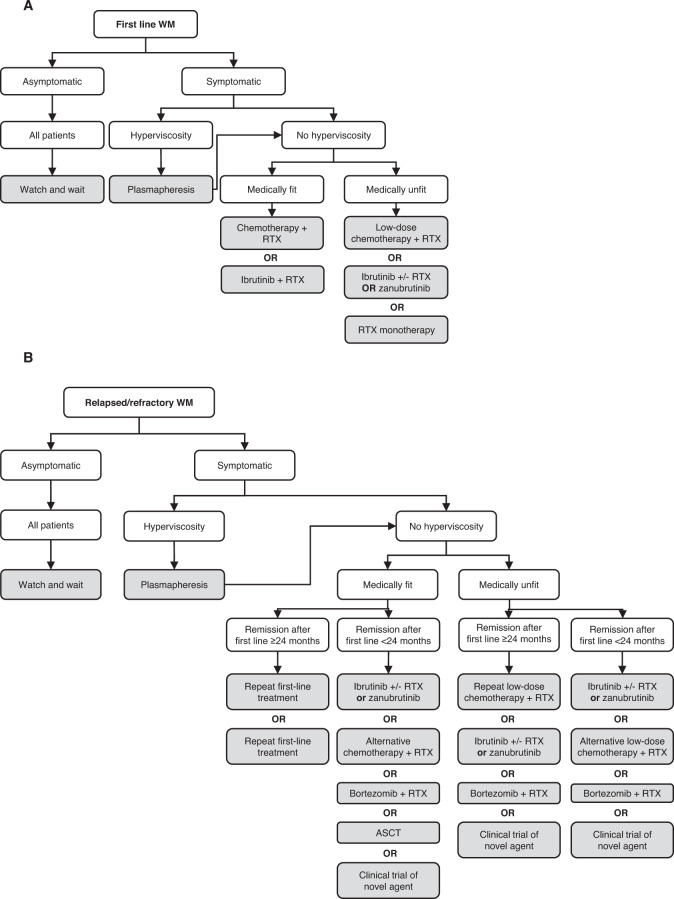
Fig. 1. Treatment algorithms for WM.
Figure adapted from DGHO-onkopedia guidelines [12]. RTX rituximab.
BTK and WM biology
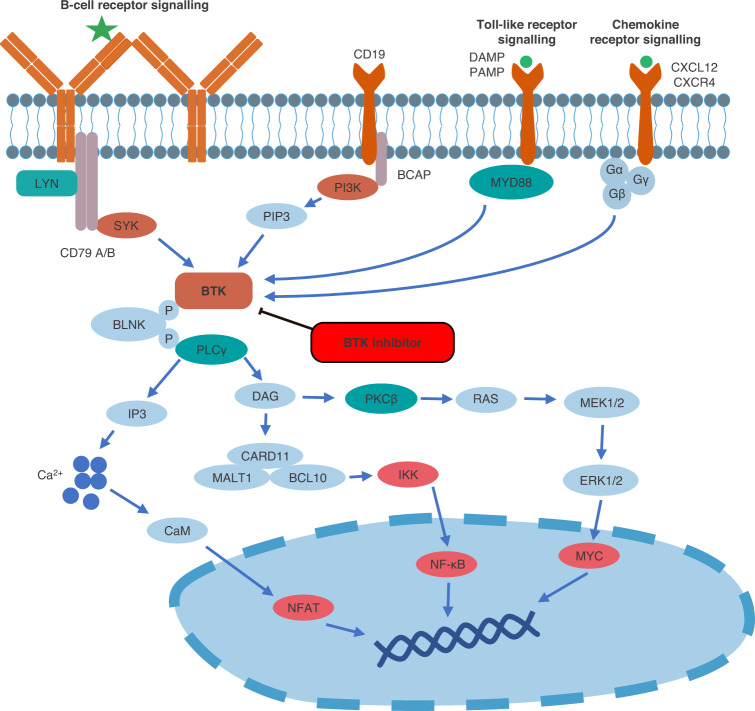
BTK belongs to the TEC family of non-receptor kinases, which comprises five kinases (BMX, BTK, ITK, TEC, and TXK), which all have important roles in immunity [14]. BTK, ITK, and TXK appear to be selectively expressed in hematopoietic cells, whereas BMX and TEC are expressed more widely. TEC, for example, is best characterized for its role in T cell development, but is also expressed in the liver, heart, kidney, and ovary [15]. BTK is expressed in B cells and other hematopoietic cell types, including T cells and mast cells [14–17]. Its role is best characterized in B cells, where it acts downstream of the B-cell receptor and within multiple other pathways that are essential for maturation, proliferation, and survival (Fig. 2) [14]. The importance of BTK to B-cell function is highlighted by the severe immunodeficiency disorder X-linked agammaglobulinemia, which is characterized by a lack of mature B cells in the periphery and greatly decreased or absent immunoglobulins. The disease is caused by loss-of-function mutations in BTK [18, 19] and provides the rationale for targeting BTK in B-cell malignancies. Of note, besides the described loss-of-function mutations in the BTK gene, neither inherited, nor somatic BTK driver mutations are known.
Fig. 2. BTK signaling pathways in B cells.
BTK has a crucial role in signaling pathways that regulate various aspects of B-cell behavior, including survival and proliferation. Adapted from [96].
Pertinent to the biology of WM, BTK is involved in toll-like receptor signaling, which also involves the adaptor protein MYD88. The activating MYD88L265P mutation, which is present in >90% of patients with WM, causes MYD88 to spontaneously assemble protein complexes that trigger pro-survival signaling along multiple pathways [20]. Activating mutations in the chemokine receptor gene CXCR4 are also common in patients with WM (~40%) and almost always occur with MYD88 mutations [20]. CXCR4 is a ubiquitously expressed G protein-coupled receptor that acts as a conventional chemokine receptor. Its natural ligand is CXCL12, and CXCL12-CXCR4 binding leads to activation of an array of signaling pathways involved in cell proliferation and migration. CXCR4 gene mutations in patients with WM impair CXCR4 desensitization and internalization, which results in prolonged signaling upon binding of CXCL12. This leads to enhanced AKT and subsequent MAPK1/2 signaling, which results in sustained survival signals [21].
Mutational analysis of MYD88 from bone marrow samples is standard in WM diagnosis. Screening is usually performed initially by allele-specific or reverse transcription PCR for the hallmark L265P mutation with Sanger sequencing used to check for other MYD88 mutations when L265P is not present [22]. CXCR4 is not analyzed routinely within clinical practice because of its weaker diagnostic value and the challenges associated with a complex mutational landscape and subclonality [20]. When CXCR4 analysis is performed, Sanger or next-generation sequencing on CD19 + enriched bone marrow samples should be used. As will be discussed in more detail in later sections, MYD88 and CXCR4 status are relevant for prognosis and may be relevant to treatment selection. In particular, the minority of patients with the MYD88WT genotype have a more aggressive disease than patients with mutated MYD88 and respond poorly to ibrutinib monotherapy [20].
BTKi efficacy
The targeting of BTK in B-cell malignancies by ibrutinib has met the expectations engendered by preclinical data and led to the development of several next-generation BTKis. Here, we review the clinical trial data reported for current and emerging BTKis and look beyond the summary efficacy data to understand what differentiates them.
Ibrutinib
The first-generation BTKi ibrutinib was approved in 2015 for the treatment of WM in Europe [23]. The efficacy of ibrutinib monotherapy in previously treated patients with WM was demonstrated in a pivotal phase II trial (NCT01614821) (Table 1), which reported an overall response rate (ORR) and a major response rate (MRR) of 90.5% and 79.4%, respectively, at a median follow-up of 59 months [24, 25]. The 5-year progression-free survival (PFS) rate was 54% for all patients, and overall survival (OS) at 5 years was 87% [24]. This study established ibrutinib as a highly active treatment in pretreated patients, but there were differences in response rates between patients according to their MYD88 and CXCR4 mutational status. Almost all MYD88MUT/CXCR4WT patients had a major response to ibrutinib (97.2%) and almost half had a very good partial response (VGPR; 47.2%). Patients with the MYD88MUT/CXCR4MUT genotype had poorer responses (MRR: 68.2%; VGPR: 9.1%), but the poorest responses were reported for the four patients with MYD88WT/CXCR4WT status, none of whom achieved a major response [24].
Table 1.
BTKi monotherapy outcomes from phase II/III trials.
| BTKi | Publication | Description (phase, patients) | Outcomes | Safety |
|---|---|---|---|---|
| Ibrutinib | Treon et al. [24, 25] | Phase II, R/R (N = 63) |
Median FU 59 months: ORR: 90.5% MRR: 79.4% PFS: 2 years, 69.1% OS: 2 years, 95.2% OS: 5 years, 87% Median time to response: 4 weeks |
59 months median FU: Grade ≥3 AEs: neutropenia (15.9%), thrombocytopenia (11.1%), and pneumonia (3.2%). AF: all grades 12.7%. |
| Dimopoulos et al. [29] | Phase III, rituximab-refractory (N = 31) |
Median FU 18.1 months: ORR: 90% MRR: 71% PFS: 18 months, 86% OS: 18 months, 97% |
Common Grade ≥3 AEs: neutropenia (13%); hypertension (10%); and anemia, thrombocytopenia, and diarrhea (all 6%). | |
| Castillo et al. [30] | Phase II, TN (N = 30) |
Median FU 50 months: ORR: 100% MRR: 87% VGPR: 30% PFS: 4-year, 76% OS: 4-year, 100% |
Grade 2–4 AEs: Fatigue (33%); upper respiratory tract infection (30%); hematoma (27%); atrial fibrillation and urinary tract infection (both 20%); hypertension, lower tract respiratory infection, and rash (all 17%) |
|
| Zanubrutinib | Trotman et al. [95] | Phase I/II, TN (n = 24) and R/R (n = 53) |
Median FU 30 months: ORR: 95.9% MRR: 82.2% VGPR + CR: 45.2% PFS: 3-year, 81% OS: 3-year, 85% |
The most common all-grade AEs: upper respiratory tract infection (51.9%), contusion (32.5%), and cough (22.1%). Grade ≥3 AEs: neutropenia 15.6%; anemia 9.1%; and basal cell carcinoma and cellulitis (both 5.2%). |
| Zanubrutinib versus ibrutinib | Tam et al. [32], ASPEN trial | Phase III, R/R (n = 164) and TN (n = 37) |
Median FU 19.4 months: Zanubrutinib ORR: 94% MRR: 77% VGPR: 28% PFS: 18 months, 85% OS: 18 months, 97% Ibrutinib ORR: 93% MRR: 78% VGPR: 19% PFS: 18 months, 84% OS: 18 months, 93% |
AEs (zanubrutinib versus ibrutinib): All-grade neutropenia: 13% vs 19% Pneumonia: 2% vs 12% AF: 2% vs 15% |
| Zanubrutinib | Dimopoulos et al. [33], ASPEN trial extension | Phase III, MYD88WT (23 R/R and 5 TN) |
Median FU 17.9 months: ORR: 81% MRR: 50% VGPR: 27% |
AEs similar to those reported by Tam et al [32] in the head-to-head arm of the ASPEN trial |
| Acalabrutinib | Owen et al. [34] | Phase II, R/R (n = 92) and TN (n = 14) |
Median FU 27.4 months: R/R ORR: 93% TN ORR: 93% |
Grade 3–4 AEs >5%: neutropenia (16%) and pneumonia (7%). Grade 3–4 AF: 1%. Grade 3–4 bleeding: 3%. |
| Tirabrutinib | Sekiguchi et al. [35] | Phase II, TN (n = 19) and R/R (n = 9) |
Median FU 6.5–8.3 months: MRR: 88.9% ORR: 96.3% |
Common all-grade AEs: rash (44.4%), neutropenia (25.9%), and leukopenia (22.2%). Grade ≥3 AEs: neutropenia (11.1%), lymphopenia (11.1%), and leukopenia (7.4%). All bleeding events were Grade 1; no AF or hypertension. |
| Pirtobrutinib | Mato et al. [37] | Phase I/II, R/R B-cell malignancies (N = 323); WM (n = 19) |
In WM patients (n = 19): ORR: 68% MRR: 47% |
In all patients (N = 323): Grade ≥3 neutropenia: 10%; all grade AF: 1%. |
AF atrial fibrillation, FU follow-up, MRR major response rate, ORR overall response rate, PFS progression-free survival, R/R relapsed/refractory, TN treatment-naïve, VGPR very good partial response.
The phase III iNNOVATE trial (NCT02165397) compared the combination of ibrutinib plus the anti-CD20 monoclonal rituximab versus rituximab monotherapy in patients with WM. The combination resulted in significantly higher response rates compared with rituximab monotherapy, regardless of genotype. Notably, at a median follow-up of 50 months, patients with the MYD88WT/CXCR4WT genotype achieved robust responses with ibrutinib plus rituximab, with an ORR of 82% and an MRR of 73%, including 27% VGPRs. An ORR of 100% was reported for patients with the MYD88L265P/CXCR4WHIM genotype, which included 77% MRRs and 23% VGPRs. The results suggest that ibrutinib plus rituximab may benefit patients with the MYD88WT genotype who respond poorly to ibrutinib monotherapy [26, 27]. It is important to state that the iNNOVATE trial did not include an ibrutinib monotherapy arm and only small numbers of patients were represented for some genotypes, such as MYD88WT [26, 27]. However, particularly for patients with CXCR4 mutations, ibrutinib plus rituximab is an attractive treatment that may overcome delayed and suboptimal treatment responses that have been reported with ibrutinib monotherapy [24].
ICT regimens based on rituximab are the mainstay of first-line treatment in WM, so it is essential to assess the effectiveness of ibrutinib post rituximab failure [28]. A substudy of the iNNOVATE trial in rituximab-refractory patients showed that ibrutinib monotherapy was associated with an MRR of 71%, a PFS rate of 86%, and an estimated 18-month OS rate of 97% at a median follow-up of 18.1 months [29]. These outcomes were notable given that the substudy population had a median of four previous lines of therapy [29].
Ibrutinib’s activity in the first-line setting was demonstrated in an open-label trial (NCT02604511) of 30 treatment-naïve (TN) patients with WM, all of whom had the MYD88L265P mutation [30]. At 50 months of follow-up, MRR and VGPR rates were 87% and 30%, respectively, and median PFS was not reached. VGPR rates were numerically, but not significantly, lower for patients with CXCR4 mutations (14% vs 44%; P = 0.09). The 4-year PFS rates reflected this trend (59% vs 92%; P = 0.06) [30].
Zanubrutinib
The covalent BTKi zanubrutinib was approved by the European Medicines Agency in November 2021 for the treatment of adult patients with WM who have received at least one prior therapy, or in first-line treatment for patients unsuitable for ICT [31]. In the phase III ASPEN trial (NCT03053440) comparing zanubrutinib with ibrutinib in MYD88L265P patients, rates of overall and deep responses were comparable between both molecules (Table 1) [32]. There were no significant differences for zanubrutinib versus ibrutinib in ORR (94% vs 93%), MRR (77% vs 78%), PFS (85% vs 84%), or OS (97% vs 93%) at 18 months [32]. The trial did not meet its primary endpoint of more complete responses (CRs) or VGPRs with zanubrutinib versus ibrutinib. However, there was a trend toward deeper responses in favor of zanubrutinib (VGPR: 28% vs 19%; P = 0.09) [32].
Median time to a major response for both arms was 2.8 months, and median time to VGPR was very similar for zanubrutinib versus ibrutinib in R/R patients (4.7 vs 5.1 months) [32]. Interestingly, TN patients treated with zanubrutinib had a much faster median time to VGPR than the ibrutinib-treated patients (5.6 vs 22.1 months). TN patient numbers were low (19 zanubrutinib and 18 ibrutinib) [32], but these data suggest a possible advantage for the use of zanubrutinib compared with ibrutinib in the first-line setting.
The proportion of patients in the ASPEN study with the CXCR4WHIM mutation detected by Sanger sequencing in the primary analysis was significantly lower (9%) than expected. A post hoc analysis employing next-generation sequencing of bone marrow samples from 95% of patients detected CXCR4WHIM in 28% of all patients, which comprised 34% of patients in the zanubrutinib arm and 22% in the ibrutinib arm. The VGPR rate for zanubrutinib-treated patients with CXCR4WHIM was lower than for patients with CXCR4WT (29% vs 34%). Zanubrutinib-treated patients with CXCR4WHIM had a higher VGPR rate than those treated with ibrutinib (29% vs 21%) [32].
Given the poor responses reported for ibrutinib in patients with the MYD88WT genotype, all 26 patients with MYD88WT status were assigned to a separate arm of the ASPEN trial to receive open-label zanubrutinib. Importantly, the study employed a two-step process to detect MYD88 mutations with a PCR-based method for detection of L265P followed by next-generation Sanger sequencing of MYD88 in patients negative for L265P. Compared with previously reported outcomes with ibrutinib, MYD88WT patients responded well to zanubrutinib, with 50% of patients achieving a major response and 27% a VGPR at 18 months [33]. Only cautious conclusions can be made based on cross-trial comparisons and the molecular basis of any disparity in the responses of this patient subset to zanubrutinib versus ibrutinib is unclear. However, the ASPEN study authors noted that zanubrutinib achieves full versus partial occupancy of BTK in blood and lymph nodes over 24 hours versus ibrutinib [33]. This may provide a greater potential for deep and sustained remissions in patients with more aggressive disease.
To date, the ASPEN study is the only clinical trial to directly compare two different BTKis in WM. It demonstrated the high efficacy and safety of zanubrutinib and ibrutinib in the treatment of WM. However, no patients in either arm of the trial achieved a CR [32, 33], which highlights the fact that eradication of WM remains a challenge with BTKi monotherapy.
Acalabrutinib
Acalabrutinib is another covalent BTKi that has undergone clinical assessment in WM, but it is not currently approved for the treatment of WM in Europe. A single-arm, phase II study (NCT02180724) of acalabrutinib reported a 24-month PFS rate of 90% for TN patients and 82% for R/R patients. At a median duration of follow-up of 27.4 months, ORRs were 93% for TN and R/R patients [34]. VGPRs were reported for 9% of R/R patients and no TN patients, and there were no CRs. The limitations of cross-trial comparisons apply, but the VGPR rates reported for acalabrutinib are lower than the rates reported in the ASPEN study for zanubrutinib (TN: 26%; R/R: 29%) or ibrutinib (TN: 17%; R/R: 20%) after a similar follow-up period (median: 19.4 months) [32].
Patients with MYD88WT status treated with acalabrutinib appear to have poorer outcomes than MYD88MUT patients, with ORRs of 79% vs 94%, respectively. No VGPRs or CRs were reported for these patients [34].
Other next-generation BTKis
Data for other BTKis in WM are very limited (see Table 1). Outcomes from a small, multicenter, phase II study (JapicCTI-173646) of the covalent BTKi tirabrutinib in TN and R/R WM patients (MRR: 88.9%; ORR: 96.3%) were similar to outcomes with other BTKis. However, the study numbers were too small to draw conclusions about the impact of genotype [35].
Non-covalent, reversible inhibitors of BTK have the potential for greater target specificity than covalent BTKis and could be a valuable option for patients resistant to covalent BTKis [36]. Pirtobrutinib (formerly known as LOXO-305) was reported to have good efficacy in the phase I/II BRUIN study (NCT03740529) in patients with R/R B-cell malignancies. An ORR of 68% was reported for 19 patients with R/R WM, with partial responses in 50% of patients (no CRs or VGPRs). In 13 patients who had previously received a covalent BTKi, the ORR was 69%, with partial responses in 38% of patients [37].
Ibrutinib tolerability and patient management
Ibrutinib is associated with a range of side effects that are usually manageable but do result in significant rates of discontinuations. In a retrospective analysis of real-world outcomes for 80 patients with WM, 17 patients (21%) had discontinued ibrutinib because of treatment-related toxicities at a median follow-up of 19 months [38]. AF and elevated liver enzymes (n = 3 patients for both; 18%) were the most common side effects leading to discontinuation. Ibrutinib-induced hepatotoxicity is a rare but potentially serious side effect, and close monitoring of liver function is justified; elevated transaminases may be the first sign of hepatotoxicity [39]. Other reasons for discontinuation included uncontrolled infection (2 patients; 12%), fatigue with petechial rash (1 patient; 6%), and blistering rash (1 patient; 6%) [38].
For outcomes with ibrutinib treatment in larger patient populations, it is necessary to consider studies of patients with other B-cell malignancies. A retrospective study from Mato et al. reported outcomes and toxicities for 616 patients with chronic lymphocytic leukemia / small lymphocytic lymphoma (CLL/SLL) in the USA treated with ibrutinib as monotherapy (86%) or within combination regimens (14%) [40]. At a median follow-up of 17 months (range: 1–60 months) 21% of patients had discontinued ibrutinib because of toxicity. In first-line therapy, arthralgia (41.6%), AF (25%), and rash (16.7%) were the leading causes of discontinuation. In subsequent lines of therapy, AF (12.3%), infection (10.7%), pneumonitis (9.9%), bleeding (9%), and diarrhea (6.6%) were the most cited reasons for discontinuation [40]. The rate of discontinuations subsided from the date of ibrutinib initiation, with >75% of total discontinuations occurring within 1 year and >95% within 2 years. Rash was typically the earliest AE to result in discontinuation (median: 3.5 months), followed by pneumonitis (4.5 months), arthralgia (5 months), infection (6 months), atrial fibrillation (7 months), diarrhea (7.5 months), and bleeding (median: 8 months) [40].
Arthralgia/myalgia associated with ibrutinib is usually mild or moderate in severity and most common in the first few months following treatment, although it can occur late in the treatment course [41, 42]. These events are usually tolerable and may resolve without intervention, but in some cases may significantly reduce a patient’s quality of life and become intolerable [41, 42]. Stephens and Byrd recommend that patients with CLL/SLL and intolerable arthralgia are treated with acetaminophen or short pulses of prednisone therapy while continuing ibrutinib at the full dose [42]. Where possible, the use of platelet-inhibiting agents, such as ibuprofen, should be avoided because of the bleeding risk associated with ibrutinib, but they may be introduced if acetaminophen and steroid therapy is not effective. As a last resort, Stephens and Byrd recommend holding ibrutinib for 1 week before restarting at one dose level lower (e.g. 420 mg to 280 mg) [42]. This broadly concurs with the results of Rhodes et al., who found in a retrospective study that dose holds were more effective than dose reductions in patients with CLL/SLL treated with ibrutinib who required management of arthralgia/myalgia [41].
Rash is another common side effect of ibrutinib that is usually low grade. Pruritic rash may be managed with topical corticosteroid therapy and oral antihistamines. In rare cases, dose holds may be considered, but dose reductions and discontinuations are rarely necessary [43]. Diarrhea is another side effect that is frequently reported with ibrutinib, but is rarely severe and usually resolves without intervention [42].
Cytopenias are another common side effect of ibrutinib. Ibrutinib-associated neutropenia is common in WM treatment and may be associated with an increased risk of infections. Neutropenia associated with ibrutinib treatment is more easily reversed than some ICT-associated neutropenia in WM. Fludarabine, for example, causes prolonged and potentially irreversible cytopenias that are associated with a significantly increased risk of infection [44, 45]. In the case of neutropenia caused by ibrutinib, granulocyte colony-stimulating factor support may be used to rescue Grade ≥3 neutropenia. Ibrutinib dose reductions are not usually necessary but may be considered for recurrent neutropenia.
Conversely, ibrutinib may cause B cell lymphocytosis upon initiation, which is usually transient but may be prolonged in some patients. This is thought to be a class effect caused by disruption to B-cell homing mechanisms, which results in distribution of malignant B cells into the peripheral blood. Although lymphocytosis is usually asymptomatic and does not require management [43], it is important to not mistake lymphocytosis with disease progression in patients treated with ibrutinib or other BTKis.
Ibrutinib and cardiovascular toxicity
Perhaps the most challenging aspect of treating patients with ibrutinib is the associated cardiovascular adverse drug reactions, of which AF is the most common. Mining the international pharmacovigilance database VigiBase, Salem et al. found that supraventricular arrhythmias (SVAs), of which 93.8% were AF, were 23.1 times overreported with ibrutinib compared with all other drugs within VigiBase [13]. AF rates are usually reported at 5–10% for patients treated with ibrutinib in clinical trials [46], but the true incidence is likely to be higher. With systematic cardiological screening, Baptiste et al. reported an AF rate of 38% at 24 months in patients with B-cell malignancies treated with ibrutinib [47].
AF is usually manageable, but it is likely to require lifelong management [48] and is associated with an increased risk of mortality (Fig. 3) [13]. A major risk associated with AF is stroke [49] and patients with AF who are treated with ibrutinib should be evaluated for the need for anticoagulants to reduce this risk [50], potentially using the CHA2DS2-VASc assessment [51]. There is no consensus on the preferred oral anticoagulant for patients who have AF being treated with ibrutinib, although warfarin or other vitamin K antagonists were associated with significant bleeding rates when used in combination with ibrutinib in seminal clinical trials [52, 53]. Low-molecular-weight heparin or factor Xa inhibitors may be considered.
Fig. 3. Death in patients with cardiovascular adverse-drug reactions with ibrutinib reported to VigiBase from its creation in November 1967 to February 2018.
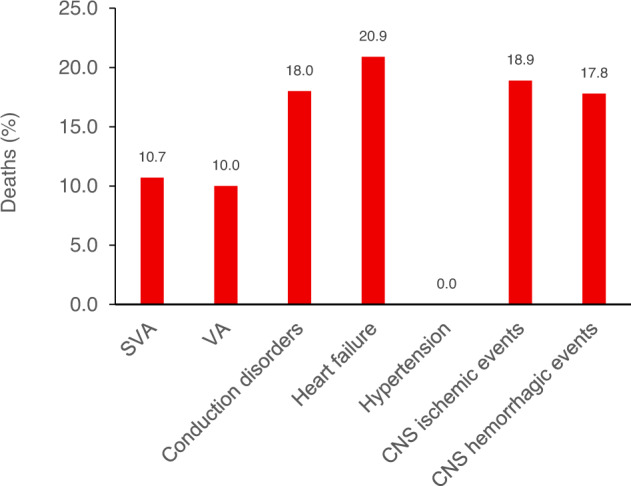
Adapted from [13]. CNS central nervous system, SVA supraventricular arrhythmia, VA ventricular arrhythmia.
Tachycardia leading to heart failure is the other major risk associated with ibrutinib-associated AF. Lenient rate control with beta-blockers is favored over rhythm control [13] because continuing treatment with ibrutinib may limit a patient’s ability to maintain sinus rhythm after cardioversion [46]. Electrical or chemical cardioversion should be considered only in patients who are still symptomatic after rate control and who can tolerate anticoagulation, because there is an increased risk of thromboembolic events after cardioversion [46].
Ventricular arrhythmia (VA) is a related potential complication of ibrutinib therapy that is less common than AF but was reported to be 4.7 times more likely with the use of ibrutinib compared with all other drugs within VigiBase [13]. VA is the most common cause of sudden cardiac death [54], and the benefits of ibrutinib therapy should be carefully weighed against the risk of worsening VA in patients who have a history of premature ventricular contractions and hyperexcitability.
A risk of hypertension should also be considered and requires long-term vigilance. Unlike AF and VA, which usually occur in the first few months after treatment initiation, hypertension is associated with an increasing risk over time. The RESONATE study followed patients with R/R CLL/SLL treated with ibrutinib for up to 6 years and reported a prevalence of Grade ≥3 hypertension of 4% in years 0–1 and 11% in years 5–6. With a median treatment duration of 41 months, any grade hypertension occurred in 21% of patients [55]. The real-world prevalence of hypertension in patients treated with ibrutinib may be significantly higher than this figure. A retrospective study that followed 562 lymphoma patients treated with ibrutinib over a 17-year period in a real-world setting reported that 71.6% experienced new hypertension [56].
Ibrutinib and bleeding risk
Bleeding events are common in patients treated with ibrutinib, with most being low grade and occurring within 6 months of initiation [57]. Ibrutinib-associated bleeding may exacerbate disease-specific risk factors for patients with WM. A significant proportion of patients are thrombocytopenic and/or anemic [58] and hyperviscosity caused by high levels of circulating IgM can lead to tearing of small blood vessels, particularly in the nose, gums, or retina [59]. Patients with WM may also develop acquired von Willebrand disease and this risk also appears to be higher in patients with high serum IgM levels [60].
Patients treated with ibrutinib may also require anticoagulants to decrease the risk of stroke associated with AF, and these patients should be monitored particularly carefully, especially in the first few months after ibrutinib initiation. The benefits of other medications associated with an increased bleeding risk, such as aspirin and nonsteroidal anti-inflammatory drugs, should be weighed against the risks. In the case of aspirin, it is recommended that aspirin is stopped for patients with a low or moderate cardiovascular risk, and that those at high risk continue with a dose of ≤81 mg/day [57]. Fish oil and vitamin E supplements are also associated with severe bleeding events and should be avoided [57].
Because of the bleeding risk, it is recommended that ibrutinib is paused perioperatively [57]. The anti-platelet effects of ibrutinib appear to be reversed within a week of discontinuation [61, 62], so pausing ibrutinib for one week prior to, and up to 2–3 days after, surgery is reasonable. Platelet transfusion can reduce the risk of bleeding during unplanned surgeries [57, 61]. The elapsed time since the last dose of ibrutinib should be considered because this will affect the efficacy of a transfusion. Ibrutinib has a half-life of 4–13 hours [23], and transfusions given very soon after the last ibrutinib dose are likely to be less effective in reversing the hemostatic defect.
Ibrutinib withdrawal
Planned surgery is the most common reason to pause ibrutinib, but it may also be paused because of toxicities, drug interactions, and patient decision [63]. A retrospective single-center study reported ibrutinib-withdrawal symptoms in 19% of patients with WM undergoing ibrutinib pause, with fever, body aches, night sweats, and arthralgias being the most common. One-third of these patients had symptoms associated with progressive disease and two-thirds had symptoms in the absence of progressive disease [63]. Responses are usually regained quickly following ibrutinib resumption [64], but interruptions may be associated with a shorter PFS and should be minimized where possible [63]. Patients experiencing withdrawal may benefit from a short course of prednisone (10 mg twice daily) during the interruption period [63].
Zanubrutinib tolerability and patient management
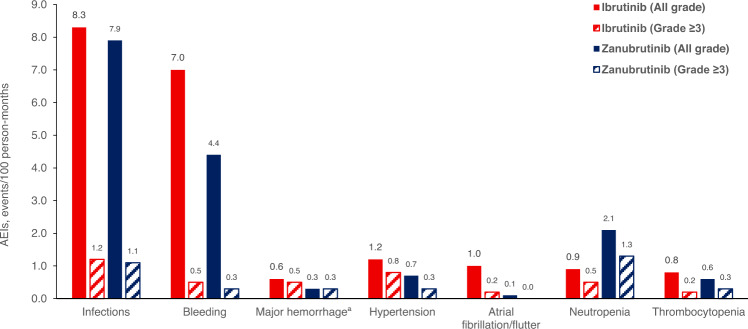
In the phase III, head-to-head ASPEN trial, fewer patients treated with zanubrutinib versus ibrutinib required dose reductions (14% vs 29%) and treatment discontinuation (4% vs 9%). The most common AEs with zanubrutinib were neutropenia (all grades: 29%; Grade ≥3: 20%), upper respiratory infection (all grades: 24%; Grade ≥3: 0%), and diarrhea (all grades: 21%; Grade ≥3: 3%) [32]. The rate of neutropenia for zanubrutinib (all grades: 29%; Grade ≥3: 20%) was more than twice the rate for ibrutinib (all grades: 13%; Grade ≥3: 8%). Febrile neutropenia was also reported in the zanubrutinib arm (all grades: 4%; Grade ≥3: 4%), but not in the ibrutinib arm. Interestingly, the increased rate of neutropenia did not translate into an increased risk of infections with zanubrutinib. Infection events per 100 person-months were almost identical for zanubrutinib and ibrutinib for all-grade (7.9 vs 8.3) and Grade ≥3 infections (1.1 vs 1.2) (Fig. 4) [32]. Incidence of pneumonia was actually higher among patients treated with ibrutinib (all grades: 12%; Grade ≥3: 7%) than in patients treated with zanubrutinib (all grades: 2%; Grade ≥3: 1%). However, more neutropenic patients received granulocyte colony-stimulating factor support in the zanubrutinib arm than in the ibrutinib arm (47% vs 31%) [32], and this intervention should be considered in patients with Grade ≥3 neutropenia.
Fig. 4. BTK inhibitor class adverse event rates in the ASPEN Phase III trial.
All grade and Grade 3 events of interest reported with ibrutinib and zanubrutinib monotherapy in the ASPEN Phase III trial. Adapted from [32]. aMajor hemorrhage was defined as serious or grade ≥3 bleeding at any site, or central nervous system bleeding of any grade. AESI adverse event of special interest.
Preclinical data suggest that ibrutinib, but not zanubrutinib, induces platelet receptor shedding and is associated with extended bleeding times and increased thrombus formation [32, 65]. In the ASPEN trial, the incidence rates of minor and major hemorrhage favored zanubrutinib compared with ibrutinib; however, the incidence rates were similar between the two molecules [32].
Longer-term follow-up is needed to confirm that a lower incidence rate of cardiovascular adverse drug reactions with zanubrutinib versus ibrutinib is reflected in real-world data. However, the increased selectivity of zanubrutinib appears to result in reduced cardiotoxicity. Rates of AF were reduced with zanubrutinib (all grades: 2%; Grade ≥3: 0%) compared with ibrutinib (all grades: 15%; Grade ≥3: 4%), as was the incidence of hypertension (all grades: 11% vs 16%, respectively; Grade ≥3: 6% vs 11%, respectively) [32].
In common with ibrutinib, most adverse events – including neutropenia, infections, and hemorrhage – occur within the first 6–18 months of exposure to zanubrutinib. A pooled safety analysis of zanubrutinib across six trials in patients with B-cell malignancies revealed second primary malignancies and hypertension as possible exceptions, with both associated with relatively flat event-rate curves [66]. Again, this is consistent with data reported for ibrutinib [56, 67]. AF showed no clear relationship to zanubrutinib exposure, but the total number of events was small [66].
Acalabrutinib tolerability and patient management
Like zanubrutinib, acalabrutinib appears to have a better safety profile than ibrutinib, with a lower incidence of AF and hypertension [34, 68]. In a phase II trial in WM, the most common Grade ≥3 AEs were neutropenia (16%) and pneumonia (7%) [34]. Grade ≥3 bleeding was rare (3%) [34], and preclinical data suggest that acalabrutinib is associated with a decreased bleeding risk compared with ibrutinib [69].
The most common all-grade AE was headache (39%) [34], and this side effect has also been reported at a high incidence in clinical trials of acalabrutinib in other B-cell malignancies [70]. Headache with acalabrutinib is usually mild or moderate in severity and resolves within the first month of treatment without the need for intervention [71]. Low-grade diarrhea is also common with acalabrutinib, reported at similar rates to headache [70].
A highly relevant property of acalabrutinib compared with the other BTKis is that its absorption is significantly reduced in patients who have taken gastric acid–reducing agents. The summary of product characteristics for acalabrutinib recommends suitable spacing for administration of H2 blockers and antacids and advises against concomitant use of proton pump inhibitors (Table 2) [72].
Table 2.
| BTKi | Pharmacokinetics | Metabolism | Interactions | ||
|---|---|---|---|---|---|
| Tmax (median) | Volume of distribution at steady state | Half-life (mean) | |||
| Acalabrutinib | 0.5–1.5 h | 34 L | 1–2 h | Primarily CYP3A; to a minor extent by glutathione conjugation and amide hydrolysis | Avoid strong CYP3A4 inhibitors or inducers; gastric acid–reducing agents may decrease the area under the curve of acalabrutinib |
| Ibrutinib | 1–2 h | 10 000 L | 4–13 h | Primarily CYP3A; to a minor extent by CYP2D6 | Avoid strong CYP3A4 inhibitors or inducers |
| Zanubrutinib | 2 h | 522 L | 2–4 h | Primarily CYP3A | Avoid strong CYP3A inducers; modify the dose of zanubrutinib with moderate or strong CYP3A inhibitors |
CYP cytochrome P450.
Tolerability of other BTKis
There are limited safety data for other BTKis. In a trial of 27 patients treated with tirabrutinib, no incidents of AF were reported. Grade ≥3 AEs included neutropenia (11.1%), lymphopenia (11.1%), and leukopenia (7.4%) [35]. The non-covalent, reversible inhibitor pirtobrutinib was reported to have low toxicity in phase I/II trials in patients with R/R B-cell malignancies; notably, there were no incidents of treatment-related AF or hemorrhage [37].
Tolerability and off-target inhibition
The next-generation BTKis have been developed with the aim of achieving greater tolerability through greater selectivity of BTK. However, the contribution of off-target inhibition to tolerability is not fully understood. Cardiovascular toxicities with ibrutinib are often attributed to off-target inhibition and the greater selectivity of next-generation BTKis potentially translates into a reduced risk of cardiovascular adverse events [73]. Various mechanisms have been proposed for ibrutinib-associated AF, with data from a mouse model indicating that ibrutinib-associated AF may be caused by off-target inhibition of C-terminal SRC kinase [48]. Bleeding with ibrutinib is likely to be caused by a combination of on-target and off-target inhibition as both BTK and TEC have a role in downstream signaling of several platelet transmembrane receptors [57].
Current role of BTKis in the treatment of WM
Ibrutinib and zanubrutinib are approved for the treatment of R/R WM and for TN patients who are unsuitable for ICT [23, 31]. In addition, ibrutinib in combination with rituximab is approved for all patients with WM regardless of medical fitness. Rituximab-based ICT regimens remain the standard of care in the first-line treatment of patients with WM. These regimens are highly effective, have a manageable toxicity profile, and are fixed-duration regimens that provide the opportunity for treatment-free periods [74]. BTKis offer a high clinical benefit as second- and subsequent lines of therapy, particularly after ICT failure. Therefore, rituximab/chemotherapy and BTK inhibition are pillars for the clinical management of WM today.
Treatment options following BTKi therapy
BTKis are the treatment standard for patients with R/R WM, but there may be a tendency for some clinicians to reserve BTKis as a therapy of last resort because of the lack of evidence for effective treatments after BTKi failure. Potential options for patients who need to discontinue certain BTKis include other BTKis, proteasome inhibitors, BCL2 inhibitors, PI3Kδ inhibitors, or cellular therapies.
Following the approval of zanubrutinib in November 2021, there is the potential to continue BTK inhibition with zanubrutinib or ibrutinib for patients with WM who discontinue the other BTKi because of toxicities. An ongoing phase II study (NCT04116437) is evaluating the use of zanubrutinib in patients with B-cell malignancies who discontinued ibrutinib or acalabrutinib because of intolerance. At a median follow-up of 4.2 months, zanubrutinib was well tolerated, with no patients needing to discontinue treatment. All efficacy-evaluable patients (n = 26) had sustained or improved responses [75].
Non-covalent BTKis, such as pirtobrutinib, have more potential as a salvage therapy for R/R patients with WM previously treated with a covalent BTKi compared with other covalent BTKis. Data from the BRUIN study of pirtobrutinib indicate that non-covalent BTKis are effective in patients who relapse on ibrutinib [37], but there are no data to indicate that the reverse is true. Considering known and probable resistance mechanisms, using non-covalent BTKis after failure of covalent agents may be appropriate in general. Non-covalent inhibitors are active against BTK with the C481S mutation, which is the most common mutation found in ibrutinib-resistant patients [76]. Conversely, mutations to a ‘gatekeeper’ residue in BTK’s ATP pocket, which are predicted to be a probable path of resistance to non-covalent inhibitors [77], also reduce the binding of covalent BTKis [78].
Other options following BTKi failure include proteasome inhibitors and PI3Kδ inhibitors. The proteasome-inhibitor bortezomib is effective as a monotherapy [79] or in combination with rituximab for patients with R/R WM [80]. The BCL2 inhibitor venetoclax is well tolerated and produces high rates of responses in R/R WM, although patients with previous exposure to BTKis may be less likely to have deep responses [81]. Idelalisib, a selective oral inhibitor of PI3Kδ, produces durable responses as a monotherapy in R/R WM [82], as does the combination of idelalisib plus the anti-CD20 monoclonal antibody obinutuzumab [83].
For eligible younger patients, autologous or allogeneic stem cell therapies are options, although these both carry significant risks of non-relapse mortality [84, 85]. Although currently largely confined to clinical trials, chimeric antigen receptor T-cell therapy may in the future be available in the clinic for eligible patients [86, 87]. Indeed, the anti-CD20-targeted autologous CAR T-cell therapy MB-106 has recently been granted orphan drug designation by the FDA. It is currently being investigated in patients with relapsed or refractory CLL and various B-cell non-Hodgkin’s lymphomas, including WM, in a multicenter phase 1/2 trial (NCT05360238) of 287 patients that is scheduled to complete in September 2026.
Bispecific antibodies that engage both B cells and T cells are another emerging therapeutic class with potential across B-cell malignancies. For WM, the CD20/CD3-targeting antibodies mosunetuzumab and glofitamab are the most advanced relevant bispecific antibodies. There are promising data for their activity in other B cell lymphomas, particularly follicular and diffuse large B-cell lymphoma, but expanded trials are required in WM [88].
Future outlook: combination therapies
Limitations of BTKi monotherapy include the need for indefinite use rather than a fixed duration of treatment and relatively low rates of VGPRs and CRs. Patients with the MYD88WT genotype also appear to have poorer outcomes compared with those with the MYD88L265P mutation, although data from the ASPEN trial substudy indicate that zanubrutinib may be effective in these patients [33]. To overcome these limitations in the future, many patients may use BTKis as part of combination regimens.
Ibrutinib inhibits some of the anti-tumor activity of anti-CD20 monoclonals through inhibition of antibody-dependent cell-mediated cytotoxicity and phagocytosis [89, 90]. Zanubrutinib and acalabrutinib have less of an inhibitory effect on the activity of anti-CD20 monoclonals [89–91] and may be more suitable for use in combination regimens with anti-CD20 monoclonals. Promising results have been reported for the combination regimens of zanubrutinib plus obinutuzumab in patients with CLL/SLL and follicular lymphoma [92] and acalabrutinib plus obinutuzumab in CLL/SLL [93].
The potential for effective fixed-duration regimens with BTKis has been demonstrated by the multicenter phase II CAPTIVATE study in CLL/SLL of ibrutinib with venetoclax. Primary results from the study suggest the combination offers the potential for treatment-free remission in patients with CLL/SLL with a fixed-duration regimen [94]. There are other active trials in WM investigating the combination of BTKis with various agents, including chemotherapeutics, monoclonal antibodies, proteasome inhibitors, and targeted inhibitors (Table 3).
Table 3.
Ongoing clinical trials of BTKi combinations for treatment of patients with WM.
| Trial | Treatments (class) | Description | Estimated primary completion date |
|---|---|---|---|
| NCT04260217 | Ibrutinib and APG-2575 (BCL2 inhibitor) | Phase Ib /II, open label | September 2022 |
| NCT03679624 | Ibrutinib and daratumumab (anti-CD38 monoclonal antibody) | Phase II, open label | October 2022 |
| NCT03620903 | Ibrutinib and bortezomib (proteasome inhibitor), and rituximab | Phase II, open label | December 2022 |
| NCT04274738 | Ibrutinib and mavorixafor (CXCR4 inhibitor) | Phase I, open label | January 2023 |
| NCT03225716 | Ibrutinib and ulocuplumab (anti-CXCR4 monoclonal antibody) | Phase I/II, open label | January 2023 |
| NCT04463953 | Zanubrutinib, ixazomib (proteasome inhibitor), and dexamethasone | Phase II, open label | May 2023 |
| NCT04273139 | Ibrutinib and venetoclax (BCL2 inhibitor) | Phase II, open label | June 2023 |
| NCT04624906 | Acalabrutinib, bendamustine, and rituximab | Phase II, open label | December 2024 |
| NCT03506373 | Ibrutinib and ixazomib (proteasome inhibitor) | Phase II, open label | May 2025 |
| NCT04263480 | Ibrutinib and carfilzomib (proteasome inhibitor) | Phase II, open label | February 2028 |
Conclusion
Clinical trial data suggest there are only limited differences in efficacy against WM between the different BTKis, although zanubrutinib and acalabrutinib may produce faster and deeper responses for patients with the mutated CXCR4 and MYD88WT genotype compared with ibrutinib. However, data from these same studies indicate that zanubrutinib and acalabrutinib have improved safety profiles in comparison with ibrutinib, including a reduction in the risk of cardiovascular side effects. Currently, the options for anti-BTK treatment of WM in Europe are ibrutinib and zanubrutinib.
BTKis are established as effective and well-tolerated treatment options for patients with WM, and next-generation BTKis provide additional advantages that strengthen the role of this class in lymphoma treatment. A remaining key challenge with BTKi therapy is the need for continuous treatment versus fixed-duration and treatment-free intervals afforded by ICT. The future of BTKi treatment in WM and other B-cell malignancies is likely to be as part of combination regimens that offer deep responses with a fixed duration of treatment.
Acknowledgements
Editorial support was provided by Luke Smith, PhD, of Porterhouse Medical, to collate and incorporate author input on the manuscript, all carried out under the authors’ direction. Editorial support was funded by BeiGene.
Author contributions
All authors contributed to the conception of the review manuscript. JES was lead author for sections of the manuscript concerning the cardiovascular toxicities associated with ibrutinib. CB, MD, and WJ were lead authors of the other sections of the manuscript. All authors critically reviewed and edited the content. At all stages, the authors had full control over the content of the manuscript, they approved the final version for submission, and take full responsibility for its content.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Data availability
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
Competing interests
CB declares honoraria from Roche, Pfizer, Janssen, Hexal, Celltrion, AbbVie, Novartis, Bayer, Morphosys, Regeneron, Beigene; and research funding from Roche, Janssen, Celltrion, AbbVie, Bayer, Amgen, and MSD. WJ declares honoraria and consultancy fees from AstraZeneca, BeiGene, and Janssen. JES declares consultancy fees and research grants from AstraZeneca, BeiGene, BMS, and Novartis. MD declares honoraria and consultancy fees from Amgen, BeiGene, Janssen, Celgene/Genesis, Sanofi, Takeda, Karyopharm, and GSK.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Owen RG, Treon SP, Al-Katib A, Fonseca R, Greipp PR, McMaster ML, et al. Clinicopathological definition of Waldenström’s macroglobulinemia: consensus panel recommendations from the Second International Workshop on Waldenström’s Macroglobulinemia. Semin Oncol. 2003;30:110–5. doi: 10.1053/sonc.2003.50082. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 3.Wang H, Chen Y, Li F, Delasalle K, Wang J, Alexanian R, et al. Temporal and geographic variations of Waldenström macroglobulinemia incidence: a large population-based study. Cancer. 2012;118:3793–3800. doi: 10.1002/cncr.26627. [DOI] [PubMed] [Google Scholar]
- 4.Groves FD, Travis LB, Devesa SS, Ries LA, Fraumeni JF., Jr Waldenström’s macroglobulinemia: incidence patterns in the United States, 1988-1994. Cancer. 1998;82:1078–81. [PubMed] [Google Scholar]
- 5.Herrinton LJ, Weiss NS. Incidence of Waldenström’s macroglobulinemia. Blood. 1993;82:3148–50. [PubMed] [Google Scholar]
- 6.Sekhar J, Sanfilippo K, Zhang Q, Trinkaus K, Vij R, Morgensztern D. Waldenström macroglobulinemia: a Surveillance, Epidemiology, and End Results database review from 1988 to 2005. Leuk Lymphoma. 2012;53:1625–6. doi: 10.3109/10428194.2012.656103. [DOI] [PubMed] [Google Scholar]
- 7.Teras LR, DeSantis CE, Cerhan JR, Morton LM, Jemal A, Flowers CR. 2016 US lymphoid malignancy statistics by World Health Organization subtypes. CA Cancer J Clin. 2016;66:443–59. doi: 10.3322/caac.21357. [DOI] [PubMed] [Google Scholar]
- 8.Castillo JJ, Olszewski AJ, Cronin AM, Hunter ZR, Treon SP. Survival trends in Waldenström macroglobulinemia: an analysis of the Surveillance, Epidemiology and End Results database. Blood. 2014;123:3999–4000. doi: 10.1182/blood-2014-05-574871. [DOI] [PubMed] [Google Scholar]
- 9.Kastritis E, Kyrtsonis MC, Morel P, Gavriatopoulou M, Hatjiharissi E, Symeonidis AS, et al. Competing risk survival analysis in patients with symptomatic Waldenström macroglobulinemia: the impact of disease unrelated mortality and of rituximab-based primary therapy. Haematologica. 2015;100:e446–449. doi: 10.3324/haematol.2015.124149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brandefors L, Melin B, Lindh J, Lundqvist K, Kimby E. Prognostic factors and primary treatment for Waldenström macroglobulinemia – a Swedish Lymphoma Registry study. Br J Haematol. 2018;183:564–77. doi: 10.1111/bjh.15558. [DOI] [PubMed] [Google Scholar]
- 11.Buske C, Sadullah S, Kastritis E, Tedeschi A, García-Sanz R, Bolkun L, et al. Treatment and outcome patterns in European patients with Waldenström’s macroglobulinaemia: a large, observational, retrospective chart review. Lancet Haematol. 2018;5:e299–e309. doi: 10.1016/S2352-3026(18)30087-5. [DOI] [PubMed] [Google Scholar]
- 12.Buske C, Heim D, Herold M, Staber PB, Dreyling M. DGHO-onkopedia guidelines: Waldenström’s disease (lymphoplasmocytic lymphoma). 2022 [cited; Available from: https://www.onkopedia.com/de/onkopedia/guidelines/morbus-waldenstroem-lymphoplasmozytisches-lymphom/@@guideline/html/index.html Accessed May 2022.]
- 13.Salem JE, Manouchehri A, Bretagne M, Lebrun-Vignes B, Groarke JD, Johnson DB, et al. Cardiovascular Toxicities Associated With Ibrutinib. J Am Coll Cardiol. 2019;74:1667–78. doi: 10.1016/j.jacc.2019.07.056. [DOI] [PubMed] [Google Scholar]
- 14.Hendriks RW, Yuvaraj S, Kil LP. Targeting Bruton’s tyrosine kinase in B cell malignancies. Nat Rev Cancer. 2014;14:219–32. doi: 10.1038/nrc3702. [DOI] [PubMed] [Google Scholar]
- 15.Yin Z, Zou Y, Wang D, Huang X, Xiong S, Cao L, et al. Regulation of the Tec family of non-receptor tyrosine kinases in cardiovascular disease. Cell Death Discov. 2022;8:119. doi: 10.1038/s41420-022-00927-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuehn HS, Radinger M, Brown JM, Ali K, Vanhaesebroeck B, Beaven MA, et al. Btk-dependent Rac activation and actin rearrangement following FcepsilonRI aggregation promotes enhanced chemotactic responses of mast cells. J Cell Sci. 2010;123:2576–85. doi: 10.1242/jcs.071043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xia S, Liu X, Cao X, Xu S. T-cell expression of Bruton’s tyrosine kinase promotes autoreactive T-cell activation and exacerbates aplastic anemia. Cell Mol Immunol. 2020;17:1042–52. doi: 10.1038/s41423-019-0270-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsukada S, Saffran DC, Rawlings DJ, Parolini O, Allen RC, Klisak I, et al. Deficient expression of a B cell cytoplasmic tyrosine kinase in human X-linked agammaglobulinemia. Cell. 1993;72:279–90. doi: 10.1016/0092-8674(93)90667-f. [DOI] [PubMed] [Google Scholar]
- 19.Vetrie D, Vorechovský I, Sideras P, Holland J, Davies A, Flinter F, et al. The gene involved in X-linked agammaglobulinaemia is a member of the src family of protein-tyrosine kinases. Nature. 1993;361:226–33. doi: 10.1038/361226a0. [DOI] [PubMed] [Google Scholar]
- 20.Treon SP, Xu L, Guerrera ML, Jimenez C, Hunter ZR, Liu X, et al. Genomic Landscape of Waldenström Macroglobulinemia and Its Impact on Treatment Strategies. J Clin Oncol. 2020;38:1198–208. doi: 10.1200/JCO.19.02314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaiser LM, Hunter ZR, Treon SP, Buske C. CXCR4 in Waldenström’s macroglobulinema: chances and challenges. Leukemia. 2021;35:333–45. doi: 10.1038/s41375-020-01102-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castillo JJ, Garcia-Sanz R, Hatjiharissi E, Kyle RA, Leleu X, McMaster M, et al. Recommendations for the diagnosis and initial evaluation of patients with Waldenström Macroglobulinaemia: A Task Force from the 8th International Workshop on Waldenström Macroglobulinaemia. Br J Haematol. 2016;175:77–86. doi: 10.1111/bjh.14196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Imbruvica 140 mg hard capsules – summary of product characteristics. Janssen-Cilag International NV; Beerse, Belgium, January 2022.
- 24.Treon SP, Meid K, Gustine J, Yang G, Xu L, Liu X, et al. Long-term follow-up of ibrutinib monotherapy in symptomatic, previously treated patients with Waldenström macroglobulinemia. J Clin Oncol. 2021;39:565–75. doi: 10.1200/JCO.20.00555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Treon SP, Tripsas CK, Meid K, Warren D, Varma G, Green R, et al. Ibrutinib in previously treated Waldenström’s macroglobulinemia. N Engl J Med. 2015;372:1430–40. doi: 10.1056/NEJMoa1501548. [DOI] [PubMed] [Google Scholar]
- 26.Dimopoulos MA, Tedeschi A, Trotman J, García-Sanz R, Macdonald D, Leblond V, et al. Phase 3 trial of ibrutinib plus rituximab in Waldenström’s macroglobulinemia. N Engl J Med. 2018;378:2399–410. doi: 10.1056/NEJMoa1802917. [DOI] [PubMed] [Google Scholar]
- 27.Buske C, Tedeschi A, Trotman J, Garcia-Sanz R, MacDonald D, Leblond V, et al. Ibrutinib plus rituximab versus placebo plus rituximab for Waldenström’s macroglobulinemia: final analysis from the randomized phase III iNNOVATE study. J Clin Oncol. 2022;40:52–62. doi: 10.1200/JCO.21.00838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kastritis E, Leblond V, Dimopoulos MA, Kimby E, Staber P, Kersten MJ, et al. Waldenström’s macroglobulinaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:iv41–iv50. doi: 10.1093/annonc/mdy146. [DOI] [PubMed] [Google Scholar]
- 29.Dimopoulos MA, Trotman J, Tedeschi A, Matous JV, Macdonald D, Tam C, et al. Ibrutinib for patients with rituximab-refractory Waldenström’s macroglobulinaemia (iNNOVATE): an open-label substudy of an international, multicentre, phase 3 trial. Lancet Oncol. 2017;18:241–50. doi: 10.1016/S1470-2045(16)30632-5. [DOI] [PubMed] [Google Scholar]
- 30.Castillo JJ, Meid K, Gustine JN, Leventoff C, White T, Flynn CA, et al. Long-term follow-up of ibrutinib monotherapy in treatment-naive patients with Waldenström macroglobulinemia. Leukemia. 2022;36:532–9. doi: 10.1038/s41375-021-01417-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brukinsa 80 mg hard capsules – summary of product characteristics. BeiGene Ireland Ltd; January 2022.
- 32.Tam CS, Opat S, D’Sa S, Jurczak W, Lee HP, Cull G, et al. A randomized phase 3 trial of zanubrutinib vs ibrutinib in symptomatic Waldenström macroglobulinemia: the ASPEN study. Blood. 2020;136:2038–50. doi: 10.1182/blood.2020006844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dimopoulos M, Sanz RG, Lee HP, Trneny M, Varettoni M, Opat S, et al. Zanubrutinib for the treatment of MYD88 wild-type Waldenström macroglobulinemia: a substudy of the phase 3 ASPEN trial. Blood Adv. 2020;4:6009–18. doi: 10.1182/bloodadvances.2020003010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Owen RG, McCarthy H, Rule S, D’Sa S, Thomas SK, Tournilhac O, et al. Acalabrutinib monotherapy in patients with Waldenström macroglobulinemia: a single-arm, multicentre, phase 2 study. Lancet Haematol. 2020;7:e112–e121. doi: 10.1016/S2352-3026(19)30210-8. [DOI] [PubMed] [Google Scholar]
- 35.Sekiguchi N, Rai S, Munakata W, Suzuki K, Handa H, Shibayama H, et al. A multicenter, open-label, phase II study of tirabrutinib (ONO/GS-4059) in patients with Waldenström’s macroglobulinemia. Cancer Sci. 2020;111:3327–37. doi: 10.1111/cas.14561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gu D, Tang H, Wu J, Li J, Miao Y. Targeting Bruton tyrosine kinase using non-covalent inhibitors in B cell malignancies. J Hematol Oncol. 2021;14:40. doi: 10.1186/s13045-021-01049-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mato AR, Shah NN, Jurczak W, Cheah CY, Pagel JM, Woyach JA, et al. Pirtobrutinib in relapsed or refractory B-cell malignancies (BRUIN): a phase 1/2 study. Lancet. 2021;397:892–901. doi: 10.1016/S0140-6736(21)00224-5. [DOI] [PubMed] [Google Scholar]
- 38.Abeykoon JP, Zanwar S, Ansell SM, Gertz MA, Kumar S, Manske M, et al. Ibrutinib monotherapy outside of clinical trial setting in Waldenström macroglobulinaemia: practice patterns, toxicities and outcomes. Br J Haematol. 2020;188:394–403. doi: 10.1111/bjh.16168. [DOI] [PubMed] [Google Scholar]
- 39.Kleijwegt FS, Roda AA, Rolvink J, Kater AP, Kersten MJ, Vos JMI. Rare but Serious: Ibrutinib Induced Liver Failure. Hemasphere. 2019;3:e307. doi: 10.1097/HS9.0000000000000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mato AR, Nabhan C, Thompson MC, Lamanna N, Brander DM, Hill B, et al. Toxicities and outcomes of 616 ibrutinib-treated patients in the United States: a real-world analysis. Haematologica. 2018;103:874–9. doi: 10.3324/haematol.2017.182907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rhodes JM, LoRe VA, 3rd, Mato AR, Chong EA, Barrientos JC, Gerson JN, et al. Ibrutinib-associated Arthralgias/Myalgias in Patients With Chronic Lymphocytic Leukemia: Incidence and Impact on Clinical Outcomes. Clin Lymphoma Myeloma Leuk. 2020;20:438–44.e1. doi: 10.1016/j.clml.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stephens DM, Byrd JC. How I manage ibrutinib intolerance and complications in patients with chronic lymphocytic leukemia. Blood. 2019;133:1298–307. doi: 10.1182/blood-2018-11-846808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.de Weerdt I, Koopmans SM, Kater AP, van Gelder M. Incidence and management of toxicity associated with ibrutinib and idelalisib: a practical approach. Haematologica. 2017;102:1629–39. doi: 10.3324/haematol.2017.164103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gill S, Carney D, Ritchie D, Wolf M, Westerman D, Prince HM, et al. The frequency, manifestations, and duration of prolonged cytopenias after first-line fludarabine combination chemotherapy. Ann Oncol. 2010;21:331–4. doi: 10.1093/annonc/mdp297. [DOI] [PubMed] [Google Scholar]
- 45.Lukenbill J, Kalaycio M. Fludarabine: a review of the clear benefits and potential harms. Leuk Res. 2013;37:986–94. doi: 10.1016/j.leukres.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 46.Ganatra S, Sharma A, Shah S, Chaudhry GM, Martin DT, Neilan TG, et al. Ibrutinib-Associated Atrial Fibrillation. JACC Clin Electrophysiol. 2018;4:1491–1500. doi: 10.1016/j.jacep.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 47.Baptiste F, Cautela J, Ancedy Y, Resseguier N, Aurran T, Farnault L, et al. High incidence of atrial fibrillation in patients treated with ibrutinib. Open Heart. 2019;6:e001049. doi: 10.1136/openhrt-2019-001049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xiao L, Salem JE, Clauss S, Hanley A, Bapat A, Hulsmans M, et al. Ibrutinib-Mediated Atrial Fibrillation Attributable to Inhibition of C-Terminal Src Kinase. Circulation. 2020;142:2443–55. doi: 10.1161/CIRCULATIONAHA.120.049210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goldman ME, Pearce LA, Hart RG, Zabalgoitia M, Asinger RW, Safford R, et al. Pathophysiologic correlates of thromboembolism in nonvalvular atrial fibrillation: I. Reduced flow velocity in the left atrial appendage (The Stroke Prevention in Atrial Fibrillation [SPAF-III] study) J Am Soc Echocardiogr. 1999;12:1080–7. doi: 10.1016/s0894-7317(99)70105-7. [DOI] [PubMed] [Google Scholar]
- 50.Castillo JJ, Palomba ML, Advani R, Treon SP. Ibrutinib in Waldenström macroglobulinemia: latest evidence and clinical experience. Ther Adv Hematol. 2016;7:179–86. doi: 10.1177/2040620716654102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC); Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42:373–498. doi: 10.1093/eurheartj/ehaa612. [DOI] [PubMed] [Google Scholar]
- 52.Wang ML, Blum KA, Martin P, Goy A, Auer R, Kahl BS, et al. Long-term follow-up of MCL patients treated with single-agent ibrutinib: updated safety and efficacy results. Blood. 2015;126:739–45. doi: 10.1182/blood-2015-03-635326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Byrd JC, Furman RR, Coutre SE, Burger JA, Blum KA, Coleman M, et al. Three-year follow-up of treatment-naïve and previously treated patients with CLL and SLL receiving single-agent ibrutinib. Blood. 2015;125:2497–506. doi: 10.1182/blood-2014-10-606038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wellens HJ, Schwartz PJ, Lindemans FW, Buxton AE, Goldberger JJ, Hohnloser SH, et al. Risk stratification for sudden cardiac death: current status and challenges for the future. Eur Heart J. 2014;35:1642–51. doi: 10.1093/eurheartj/ehu176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Munir T, Brown JR, O’Brien S, Barrientos JC, Barr PM, Reddy NM, et al. Final analysis from RESONATE: up to six years of follow-up on ibrutinib in patients with previously treated chronic lymphocytic leukemia or small lymphocytic lymphoma. Am J Hematol. 2019;94:1353–63. doi: 10.1002/ajh.25638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dickerson T, Wiczer T, Waller A, Philippon J, Porter K, Haddad D, et al. Hypertension and incident cardiovascular events following ibrutinib initiation. Blood. 2019;134:1919–28. doi: 10.1182/blood.2019000840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shatzel JJ, Olson SR, Tao DL, McCarty OJT, Danilov AV, DeLoughery TG. Ibrutinib-associated bleeding: pathogenesis, management and risk reduction strategies. J Thromb Haemost. 2017;15:835–47. doi: 10.1111/jth.13651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dimopoulos MA, Kastritis E. How I treat Waldenström macroglobulinemia. Blood. 2019;134:2022–35. doi: 10.1182/blood.2019000725. [DOI] [PubMed] [Google Scholar]
- 59.Gertz MA. Acute hyperviscosity: syndromes and management. Blood. 2018;132:1379–85. doi: 10.1182/blood-2018-06-846816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Castillo JJ, Gustine J, Meid K, Dubeau T, Severns P, Treon SP. Acquired Von Willebrand disease in Patients with Waldenström Macroglobulinemia. Blood. 2017;130:1088. [Google Scholar]
- 61.Levade M, David E, Garcia C, Laurent PA, Cadot S, Michallet AS, et al. Ibrutinib treatment affects collagen and von Willebrand factor-dependent platelet functions. Blood. 2014;124:3991–5. doi: 10.1182/blood-2014-06-583294. [DOI] [PubMed] [Google Scholar]
- 62.Kamel S, Horton L, Ysebaert L, Levade M, Burbury K, Tan S, et al. Ibrutinib inhibits collagen-mediated but not ADP-mediated platelet aggregation. Leukemia. 2015;29:783–7. doi: 10.1038/leu.2014.247. [DOI] [PubMed] [Google Scholar]
- 63.Castillo JJ, Gustine JN, Meid K, Dubeau T, Severns P, Treon SP. Ibrutinib withdrawal symptoms in patients with Waldenström macroglobulinemia. Haematologica. 2018;103:e307–e310. doi: 10.3324/haematol.2017.186908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Castillo JJ, Gustine JN, Meid K, Dubeau TE, Xu L, Yang G, et al. Impact of ibrutinib dose intensity on patient outcomes in previously treated Waldenström macroglobulinemia. Haematologica. 2018;103:e466–e468. doi: 10.3324/haematol.2018.191999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dobie G, Kuriri FA, Omar MMA, Alanazi F, Gazwani AM, Tang CPS, et al. Ibrutinib, but not zanubrutinib, induces platelet receptor shedding of GPIb-IX-V complex and integrin αIIbβ3 in mice and humans. Blood Adv. 2019;3:4298–311. doi: 10.1182/bloodadvances.2019000640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tam CS, Dimopoulos M, Garcia-Sanz R, Trotman J, Opat S, Roberts AW, et al. Pooled safety analysis of zanubrutinib monotherapy in patients with B-cell malignancies. Blood Adv. 2022;6:1296–308. doi: 10.1182/bloodadvances.2021005621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bond DA, Huang Y, Fisher JL, Ruppert AS, Owen DH, Bertino EM, et al. Second cancer incidence in CLL patients receiving BTK inhibitors. Leukemia. 2020;34:3197–205. doi: 10.1038/s41375-020-0987-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sharman JP, Banerji V, Fogliatto LM, Herishanu Y, Munir T, Walewska R, et al. ELEVATE TN: Phase 3 study of acalabrutinib combined with obinutuzumab (O) or alone vs O plus chlorambucil (Clb) in patients (Pts) with treatment-naive chronic lymphocytic leukemia (CLL) Blood. 2019;134:31. [Google Scholar]
- 69.Bye AP, Unsworth AJ, Desborough MJ, Hildyard CAT, Appleby N, Bruce D, et al. Severe platelet dysfunction in NHL patients receiving ibrutinib is absent in patients receiving acalabrutinib. Blood Adv. 2017;1:2610–23. doi: 10.1182/bloodadvances.2017011999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Abbas HA, Wierda WG. Acalabrutinib: a selective bruton tyrosine kinase inhibitor for the treatment of B-cell malignancies. Front Oncol. 2021;11:668162. doi: 10.3389/fonc.2021.668162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Awan FT, Jurczak W. Use of acalabrutinib in patients with mantle cell lymphoma. Expert Rev Hematol. 2018;11:495–502. doi: 10.1080/17474086.2018.1473030. [DOI] [PubMed] [Google Scholar]
- 72.Calquence 100 mg hard capsules – summary of product characteristics. AstraZeneca UK Limited; December 2021.
- 73.Estupinan HY, Berglof A, Zain R, Smith CIE. Comparative analysis of BTK inhibitors and mechanisms underlying adverse effects. Front Cell Dev Biol. 2021;9:630942. doi: 10.3389/fcell.2021.630942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tedeschi A, Frustaci AM. Waldenström’s macroglobulinemia front line treatment. Hemasphere. 2019;3:62–4. doi: 10.1097/HS9.0000000000000222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shadman M, Sharman JP, Levy MY, Porter R, Zafar SF, Burke JM, et al. Preliminary results of the phase 2 study of zanubrutinib in patients with previously treated B-cell malignancies intolerant to ibrutinib and/or acalabrutinib. J Clin Oncol. 2021;39:e19506. [Google Scholar]
- 76.Buhimschi AD, Armstrong HA, Toure M, Jaime-Figueroa S, Chen TL, Lehman AM, et al. Targeting the C481S ibrutinib-resistance mutation in Bruton’s tyrosine kinase using PROTAC-mediated degradation. Biochemistry. 2018;57:3564–75. doi: 10.1021/acs.biochem.8b00391. [DOI] [PubMed] [Google Scholar]
- 77.Wang S, Mondal S, Zhao C, Berishaj M, Ghanakota P, Batlevi CL, et al. Noncovalent inhibitors reveal BTK gatekeeper and auto-inhibitory residues that control its transforming activity. JCI Insight. 2019;4:e127566. doi: 10.1172/jci.insight.127566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Estupinan HY, Wang Q, Berglof A, Schaafsma GCP, Shi Y, Zhou L, et al. BTK gatekeeper residue variation combined with cysteine 481 substitution causes super-resistance to irreversible inhibitors acalabrutinib, ibrutinib and zanubrutinib. Leukemia. 2021;35:1317–29. doi: 10.1038/s41375-021-01123-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Treon SP, Hunter ZR, Matous J, Joyce RM, Mannion B, Advani R, et al. Multicenter clinical trial of bortezomib in relapsed/refractory Waldenström’s macroglobulinemia: results of WMCTG Trial 03-248. Clin Cancer Res. 2007;13:3320–5. doi: 10.1158/1078-0432.CCR-06-2511. [DOI] [PubMed] [Google Scholar]
- 80.Ghobrial IM, Hong F, Padmanabhan S, Badros A, Rourke M, Leduc R, et al. Phase II trial of weekly bortezomib in combination with rituximab in relapsed or relapsed and refractory Waldenström macroglobulinemia. J Clin Oncol. 2010;28:1422–8. doi: 10.1200/JCO.2009.25.3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Castillo JJ, Allan JN, Siddiqi T, Advani RH, Meid K, Leventoff C, et al. Venetoclax in previously treated Waldenström macroglobulinemia. J Clin Oncol. 2022;40:63–71. doi: 10.1200/JCO.21.01194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wagner-Johnston ND, Schuster SJ, de Vos S, Salles GA, Jurczak W, Rajakumaraswamy N, et al. Long-term follow-up of idelalisib monotherapy in patients with double-refractory marginal zone lymphoma or lymphoplasmacytic lymphoma/ Waldenström’s macroglobulinemia. Blood. 2019;134:4006. [Google Scholar]
- 83.Tomowiak C, Poulain S, Herbaux C, Perrot A, Mahe B, Morel P, et al. Obinutuzumab and idelalisib in symptomatic patients with relapsed/refractory Waldenström macroglobulinemia. Blood Adv. 2021;5:2438–46. doi: 10.1182/bloodadvances.2020003895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kyriakou C, Canals C, Cornelissen JJ, Socie G, Willemze R, Ifrah N, et al. Allogeneic stem-cell transplantation in patients with Waldenström macroglobulinemia: report from the Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. J Clin Oncol. 2010;28:4926–34. doi: 10.1200/JCO.2009.27.3607. [DOI] [PubMed] [Google Scholar]
- 85.Kyriakou C, Canals C, Sibon D, Cahn JY, Kazmi M, Arcese W, et al. High-dose therapy and autologous stem-cell transplantation in Waldenström macroglobulinemia: the Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. J Clin Oncol. 2010;28:2227–32. doi: 10.1200/JCO.2009.24.4905. [DOI] [PubMed] [Google Scholar]
- 86.Palomba ML, Qualls D, Monette S, Sethi S, Dogan A, Roshal M, et al. CD19-directed chimeric antigen receptor T cell therapy in Waldenström macroglobulinemia: a preclinical model and initial clinical experience. J Immunother Cancer. 2022;10:e004128. doi: 10.1136/jitc-2021-004128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bansal R, Jurcic JG, Sawas A, Mapara MY, Reshef R. Chimeric antigen receptor T cells for treatment of transformed Waldenström macroglobulinemia. Leuk Lymphoma. 2020;61:465–8. doi: 10.1080/10428194.2019.1665668. [DOI] [PubMed] [Google Scholar]
- 88.Salvaris R, Ong J, Gregory GP. Bispecific antibodies: a review of development, clinical efficacy and toxicity in B-cell lymphomas. J Pers Med. 2021;11:355. doi: 10.3390/jpm11050355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Borge M, Belén Almejún M, Podaza E, Colado A, Fernández Grecco H, Cabrejo M, et al. Ibrutinib impairs the phagocytosis of rituximab-coated leukemic cells from chronic lymphocytic leukemia patients by human macrophages. Haematologica. 2015;100:e140–2. doi: 10.3324/haematol.2014.119669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.VanDerMeid KR, Elliott MR, Baran AM, Barr PM, Chu CC, Zent CS. Cellular cytotoxicity of next-generation CD20 monoclonal antibodies. Cancer Immunol Res. 2018;6:1150–60. doi: 10.1158/2326-6066.CIR-18-0319. [DOI] [PubMed] [Google Scholar]
- 91.Flinsenberg TWH, Tromedjo CC, Hu N, Liu Y, Guo Y, Thia KYT, et al. Differential effects of BTK inhibitors ibrutinib and zanubrutinib on NK-cell effector function in patients with mantle cell lymphoma. Haematologica. 2020;105:e76–9. doi: 10.3324/haematol.2019.220590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tam CS, Quach H, Nicol A, Badoux X, Rose H, Prince HM, et al. Zanubrutinib (BGB-3111) plus obinutuzumab in patients with chronic lymphocytic leukemia and follicular lymphoma. Blood Adv. 2020;4:4802–11. doi: 10.1182/bloodadvances.2020002183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sharman JP, Egyed M, Jurczak W, Skarbnik A, Pagel JM, Flinn IW, et al. Acalabrutinib with or without obinutuzumab versus chlorambucil and obinutuzmab for treatment-naive chronic lymphocytic leukaemia (ELEVATE TN): a randomised, controlled, phase 3 trial. Lancet. 2020;395:1278–91. doi: 10.1016/S0140-6736(20)30262-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wierda WG, Allan JN, Siddiqi T, Kipps TJ, Opat S, Tedeschi A, et al. Ibrutinib plus venetoclax for first-line treatment of chronic lymphocytic leukemia: primary analysis results from the minimal residual disease cohort of the randomized phase II CAPTIVATE study. J Clin Oncol. 2021;39:3853–65. doi: 10.1200/JCO.21.00807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Trotman J, Opat S, Gottlieb D, Simpson D, Marlton P, Cull G, et al. Zanubrutinib for the treatment of patients with Waldenström macroglobulinemia: 3 years of follow-up. Blood. 2020;136:2027–37. doi: 10.1182/blood.2020006449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wen T, Wang J, Shi Y, Qian H, Liu P. Inhibitors targeting Bruton’s tyrosine kinase in cancers: drug development advances. Leukemia. 2021;35:312–32. doi: 10.1038/s41375-020-01072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.