Highlights
-
•
Salvage radiotherapy (SRT) for post-CART progression led to excellent in-field response.
-
•
PFS was better among those with locoregional disease at progression.
-
•
Post-SRT distant failure was common, highlighting the need for novel systemic agents.
Keywords: CART therapy, Radiotherapy, Salvage therapy, Non-Hodgkin lymphoma, Relapsed/refractory
Abstract
Background and purpose
CD19-targeting chimeric antigen receptor T-cell (CART) therapy is a promising treatment for relapsed/refractory non-Hodgkin lymphoma, but most patients experience post-CART progression. We describe our institutional experience of salvage radiotherapy (SRT) in this setting.
Materials and methods
Of 94 patients who received CART therapy from 2018 to 2020, 21 received SRT for post-CART progression. Patients were divided into two groups: locoregional disease (n = 9 [43 %], all disease encompassable within an RT field) and advanced disease (n = 12 [57 %]). Patterns of failure, progression-free survival (PFS), overall survival (OS), and toxicity were assessed.
Results
Median time from CART infusion to SRT was 4.0 months (range, 0.6–11.5 months). In the locoregional disease group, 8/9 patients (89 %) were treated with comprehensive SRT to a median dose of 37.5 Gy in a median of 15 fractions. In the advanced disease group, all patients (n = 12) were treated with focal SRT to a median dose of 20.8 Gy in a median of 5 fractions. Median follow-up post-SRT was 15.2 months. In-field response was observed in 8/9 (89 %) in the locoregional disease and 8/9 (89 %) evaluable patients in the advanced disease groups. 17/18 evaluable patients (94 %) patients experienced post-SRT progression, all with a distant component. Median OS was 7.4 months; 21 months for locoregional disease versus 2.4 months for advanced disease (p = 0.0002). Median PFS was 1.1 month, and similarly poor regardless of group. No grade ≥ 3 toxicities occurred.
Conclusions
SRT post-CART therapy appears safe with encouraging in-field response but high rates of out-of-field progression, even for those presenting with locoregional disease, highlighting the need for integration of novel systemic agents.
Introduction
CD19-targeting chimeric antigen receptor T-cell (CART) therapy has emerged as the standard of care for relapsed/refractory non-Hodgkin lymphoma (r/r NHL) progressing after 1–2 lines of prior therapy. CART therapy has demonstrated objective response rates of 52 %-83 % and complete response rates of 40 %–58 %. However, sustained efficacy is limited, with 1-year progression-free survival (PFS) estimates of 33 %–44 % [1], [2], [3], [4]. Most patients experience post-CART therapy progression, and prognosis is particularly poor for these patients [5]. Therefore, there is a critical need to develop effective salvage therapies, especially as the number of patients treated with commercial CART therapy increases.
Salvage radiotherapy (SRT) is potentially an important strategy for post‐CART therapy progression. SRT has been used in other settings for chemoresistant r/r NHL and may improve outcomes when used prior to high-dose therapy and autologous stem-cell transplant [6], [7]. Additionally, there is the potential for synergy with novel systemic agents or indirect immunomodulation through re-invigoration of stalled CART therapy responses [8], [9], [10], [11]. Preclinical studies suggest that low dose radiotherapy (RT) conditioning sensitizes antigen-negative tumor cells to CART-mediated cytotoxicity [12]. Moreover, RT has complementary immune-modulatory activity through tumor associated antigens cross-priming with anti-tumor CD8 + T-cell elicitation, ultimately inducing off-target or “abscopal” effects directed against tumor sites that have not been irradiated [13].
Despite the promise of SRT, current data is limited to one series of 14 patients [14]. Herein, we describe our institutional experience with SRT post-CART therapy progression.
Material and methods
We retrospectively reviewed the records of 94 patients with r/r NHL treated at the University of Pennsylvania with one of two CART therapies, tisagenlecleucel (tisa-cel) or axicabtagene ciloleucel (axi-cel), between May 2018 and June 2020. Of these patients, 60 recurred (64 %), of which 21 received SRT with or without systemic therapy, 33 received salvage systemic therapy alone, and 6 patients received no salvage therapy.
This study focuses on the 21 patients who received SRT. Dose, fractionation, and technique were dependent on the clinical scenario and reflective of multidisciplinary discussion. Patients were divided into two groups for analysis, based on disease extent at time of progression: 1) locoregional, defined as all disease encompassable within a radiation field, and 2) advanced. SRT was defined as either comprehensive, in which all sites of active disease were targeted, or focal.
Follow-up imaging (either PET/CT or CT scans) was performed within 3 months post-SRT and regularly (every 2–4 months) thereafter. In-field response was defined as a complete or partial response according to Deauville (PET/CT) or RECIST (CT) criteria. Overall response was determined according to the Lugano criteria [15]. Patterns of failure were classified with respect to both the SRT fields (local, marginal and/or distant) and prior sites of disease (pre-CART therapy site, post-CART therapy but pre-SRT site, and/or new site). Marginal failure was defined as occurring within 2 cm of the RT field.
Statistical analysis
Overall survival (OS) and progression-free survival (PFS) from the first day of SRT were calculated using the Kaplan-Meier method, and differences between the locoregional and advanced disease groups were assessed with the log-rank test. For OS analysis, patients were also stratified by the second-line age-adjusted International Prognostic Index (sAA-IPI) [16], assessed prior to the start of SRT. Univariable Cox regression was used to assess associations between various covariates of interest (age, best CART therapy response, disease extent at progression, sAA-IPI, and duration from CART therapy infusion to progression) and OS. Acute toxicity was graded with CTCAEv5. All statistical tests were 2-sided and p < 0.05 was considered statistically significant. Analyses were performed using SAS OnDemand for Academics.
Results
Baseline characteristics
Table 1 details baseline characteristics of the 21 patients who underwent SRT and includes a breakdown by disease extent (locoregional [n = 9, 43 %] versus advanced [n = 12, 57 %]). Among all patients, 14 (67 %) had diffuse large B-cell lymphoma (DLBCL) and 7 (33 %) had transformed follicular lymphoma. Median age was 64 years. Patients received a median of 2 lines of systemic therapy pre-CART therapy (range, 2 – 4). Most patients (n = 16, 76 %) received tisa-cel; the rest (n = 5, 24 %) received axi-cel. Best response to CART therapy consisted of complete response in 2 patients (10 %), partial response in 3 (14 %), stable disease in 2 (10 %), and progressive disease in 14 (67 %).
Table 1.
Baseline characteristics.
| Characteristic | Entire cohort (n = 21) n (%) |
Locoregional Disease (n = 9) n (%) | Advanced Disease (n = 12) n (%) |
|---|---|---|---|
| Age at CART infusion, y (median [range]) | 64 (37–77) | 62 (45–70) | 65 (37–77) |
| Female | 8 (38 %) | 5 (56 %) | 3 (25 %) |
| Pathology | |||
| DLBCL | 14 (67 %) | 5 (56 %) | 9 (75 %) |
| Transformed follicular lymphoma | 7 (33 %) | 4 (44 %) | 3 (25 %) |
| Cell of origin | |||
| Germinal center B-cell | 12 (57 %) | 7 (78 %) | 5 (42 %) |
| Activated B-cell | 5 (24 %) | 2 (22 %) | 3 (25 %) |
| Unknown | 4 (19 %) | 0 | 4 (33 %) |
| Pre-CART treatment | |||
| Lines of systemic therapy (median [range]) | 2 (2–4) | 2 (2–4) | 2.5 (2–4) |
| Prior autologous/allogeneic transplant | 5 (24 %) | 4 (44 %) | 1 (8 %) |
| Prior radiotherapy (including bridging) | 8 (38 %) | 4 (44 %) | 4 (33 %) |
| Conditioning regimen | |||
| Bendamustine | 16 (76 %) | 7 (78 %) | 9 (75 %) |
| Cyclophosphamide and fludarabine (Cy/Flu) | 5 (24 %) | 2 (22 %) | 3 (25 %) |
| Bridging therapy | |||
| Systemic therapy | 14 (67 %) | 5 (56 %) | 9 (75 %) |
| Radiotherapy | 4 (19 %) | 2 (22 %) | 2 (17 %) |
| None | 3 (14 %) | 2 (22 %) | 1 (8 %) |
| CART construct | |||
| Tisagenlecleucel (tisa-cel) | 16 (76 %) | 7 (78 %) | 9 (75 %) |
| Axicabtagene ciloleucel (axi-cel) | 5 (24 %) | 2 (22 %) | 3 (25 %) |
| Best CART response | |||
| Complete response | 2 (10 %) | 2 (22 %) | 0 |
| Partial response | 3 (14 %) | 1 (11 %) | 2 (17 %) |
| Stable disease | 2 (10 %) | 1 (11 %) | 1 (8 %) |
| Progressive disease | 14 (67 %) | 5 (56 %) | 9 (75 %) |
| Post-CART Progression | |||
| Time from infusion to progression, mo (median [range]) |
2.9 (0.4–9.5) | 3.1 (1.1–9.5) | 2.8 (0.4–3.4) |
| Progression location | |||
| Only at prior (pre-CART) site(s) | 3 (14 %) | 1 (11 %) | 2 (17 %) |
| Only at new site(s) | 7 (33 %) | 4 (44 %) | 3 (25 %) |
| Both prior and new sites | 11 (52 %) | 4 (44 %) | 7 (58 %) |
| Biopsy proven | 14 (67 %) | 8 (89 %) | 6 (50 %) |
CART = CD19-targeting chimeric antigen receptor T-cell; DLBCL = diffuse large B-cell lymphoma; SRT = salvage radiotherapy.
Median time from CART infusion to progression was 2.9 months (range, 0.4 – 9.5 months). Three patients (14 %) progressed at only prior (pre-CART) site(s) of disease, 7 (33 %) at only new site(s), and 11 (52 %) at both prior and new sites. Eight patients (38 %) received prior RT, including 4 (19 %) who received bridging RT. There were no in-field failures among those who received bridging RT.
SRT characteristics
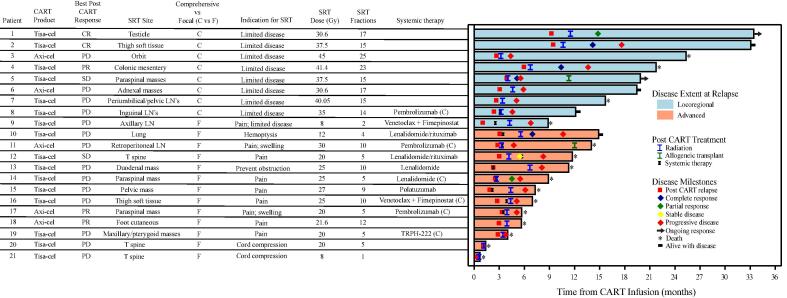
Table 2 describes SRT details. Fig. 1 depicts a swimmer plot of each patient’s disease course with respect to CART infusion date, with a focus on SRT details and outcomes. Median time from CART infusion to SRT was 4 months (range, 0.6 – 11.5 months). All patients in the locoregional group had low risk or low-intermediate risk sAA-IPI, whereas 11/12 patients (92 %) in the advanced group had high-intermediate risk or high risk sAA-IPI. Within the locoregional disease group, 8/9 patients (89 %) were treated with comprehensive SRT to a median dose of 37.5 Gy (range 8–45 Gy) in a median of 15 fractions (range 2–25 fractions). Within the advanced disease group, all were treated with focal SRT to a median dose of 20.8 Gy (range 8–30 Gy) in a median of 5 fractions (range 1–12 fractions). Concurrent systemic therapy was administered to 1/9 patient (11 %) in the locoregional disease group 5/12 patients (42 %) in the advanced disease group.
Table 2.
Salvage radiotherapy details.
| Characteristic | Entire cohort (n = 21) n (%) |
Locoregional Disease (n = 9) n (%) |
Advanced Disease (n = 12) n (%) |
|---|---|---|---|
| Time from infusion to SRT, mo (median [range]) |
4 (0.6–11.5) | 4.2 (3.2–11.5) | 3.9 (0.6–6.7) |
| sAA-IPI at SRT | |||
| Low risk | 4 (19 %) | 4 (44 %) | 0 |
| Low-intermediate risk | 6 (29 %) | 5 (56 %) | 1 (8 %) |
| High-intermediate risk | 6 (29 %) | 0 | 6 (50 %) |
| High risk | 5 (24 %) | 0 | 5 (42 %) |
| Coverage | |||
| Comprehensive | 8 (38 %) | 8 (89 %) | 0 |
| Focal | 13 (62 %) | 1 (11 %) | 12 (100 %) |
| Target | |||
| Nodal | 3 (14 %) | 2 (22 %) | 1 (8 %) |
| Extranodal | 14 (67 %) | 5 (56 %) | 9 (75 %) |
| Mixed | 4 (19 %) | 2 (22 %) | 2 (17 %) |
| Lesion max diameter, cm (median [range])* | 4.1 (0.8–12.4) | 4.2 (0.8–8.1) | 4 (2.7–12.4)* |
| Technique | |||
| 3-dimensional conformal radiotherapy | 14 (67 %) | 4 (44 %) | 10 (83 %) |
| Intensity modulated radiotherapy | 5 (24 %) | 4 (44 %) | 1 (8 %) |
| Electrons | 1 (5 %) | 0 | 1 (8 %) |
| Proton therapy | 1 (5 %) | 1 (11 %) | 0 |
| Radiation dose, Gy (median [range]) | 25 (8–45) | 37.5 (8–45) | 20.8 (8–30) |
| Number of fractions (median [range]) | 10 (1–25) | 15 (2–25) | 5 (1–12) |
| Overlap with prior radiation fields | 4 (19 %) | 2 (22 %) | 2 (17 %) |
| Concurrent systemic therapy | 6 (29 %) | 1 (11 %) | 5 (42 %) |
| Pembrolizumab | 3 (50 %) | 1 (11 %) | 2 (17 %) |
| Lenalidomide | 1 (17 %) | 0 | 1 (8 %) |
| Venetoclax + Fimepinostat | 1 (17 %) | 0 | 1 (8 %) |
| TRPH-222 | 1 (17 %) | 0 | 1 (8 %) |
SRT = salvage radiotherapy; sAA-IPI = second-line age-adjusted International Prognostic Index.
*Data unavailable for 5 patients.
Fig. 1.
Swimmer plot, with day 0 representing the date of CART infusion. “Systemic therapy” denotes the first systemic therapy given prior to further progression. SRT = salvage radiotherapy; Tisa-cel = Tisagenlecleucel; axi-cel = Axicabtagene ciloleucel; CR = complete response; PR = partial response; SD = stable disease; PD = progressive disease; LN = lymph node; T = thoracic; (C) = systemic therapy given concurrently with SRT.
Of the 14 total patients who progressed at pre-CART therapy site(s) of disease, 11 received salvage RT to a lesion present pre-CART; these pre-CART lesion characteristics are shown in Table A1. Based on pre-CART therapy PET/CT scan review, 6/10 (60 %) evaluable lesions were ≥ 5 cm, 8/11 (80 %) had an extranodal component, and 10/10 (100 %) had SUVmax ≥ 10.
Post-SRT response and patterns of failure
Post-SRT, 18 patients had restaging scans (either PET/CT or CT), performed at a median of 32 days (range, 2 – 88 days) after the end of SRT. Three of 12 patients (25 %) in the advanced disease group died from clinical progression prior to response assessment. Table 3 details response and patterns of failure among the 18 patients with restaging scans. Most patients (n = 16, 89 %) experienced in-field response, including 8/9 (89 %) in the locoregional and 8/9 (89 %) in the advanced disease groups. Best overall response consisted of complete response in 4 patients (22 %), partial response in 2 (11 %), stable disease in 1 (6 %), and progressive disease in 11 (61 %). Of the 7 patients who were initially without progressive disease, 6 (86 %) subsequently experienced progressive disease (Fig. 1).
Table 3.
Response and patterns of failure post salvage radiotherapy.
| Entire cohort (n = 18*) n (%) |
Locoregional Disease (n = 9) n (%) |
Advanced Disease (n = 9*) n (%) |
|
|---|---|---|---|
| In-field response | 16 (89 %) | 8 (89 %) | 8 (89 %) |
| Best overall response | |||
| Complete response | 4 (22 %) | 3 (33 %) | 1 (11 %) |
| Partial response | 2 (11 %) | 1 (11 %) | 1 (11 %) |
| Stable disease | 1 (6 %) | 0 (0 %) | 1 (11 %) |
| Progressive disease | 11 (61 %) | 5 (56 %) | 6 (67 %) |
| Failure pattern (with respect to radiation fields) | |||
| Local only | 0 (0 %) | 0 (0 %) | 0 (0 %) |
| Marginal only | 0 (0 %) | 0 (0 %) | 0 (0 %) |
| Distant only | 11 (61 %) | 5 (56 %) | 6 (67 %) |
| Marginal + Distant | 5 (28 %) | 2 (22 %) | 3 (33 %) |
| Local + Distant | 1 (6 %) | 1 (11 %) | 0 (0 %) |
| Failure location (with respect to prior disease) | |||
| Pre-CART site(s) | 8 (44 %) | 3 (33 %) | 5 (56 %) |
| Post-CART, pre-SRT site(s) | 9 (50 %) | 1 (11 %) | 8 (89 %) |
| New site(s) | 14 (78 %) | 7 (78 %) | 7 (78 %) |
*Excluded 3 patients without evaluable imaging post-SRT.
SRT = salvage radiotherapy; CART = CD19-targeting chimeric antigen receptor T-cell.
Disease failure after SRT occurred in 17/18 patients (94 %). There were no isolated local or marginal failures; all failures had a distant component. There was only one local failure (in combination with distant failure), and this occurred in the locoregional disease group. The majority (n = 14, 78 %) failed at a new site with respect to prior disease.
One patient in the locoregional disease group (patient 1 in Fig. 1) remains alive and free of disease 21.9 months after SRT (33.4 months after CART infusion). This patient experienced a testicular relapse 9.3 months after CART infusion and received surgery followed by adjuvant RT, without additional systemic therapy.
Twelve patients received additional lines of systemic therapy and seven patients received additional RT post-SRT failure. One patient (patient 5 in Fig. 1) who experienced distant and marginal failure subsequently experienced a complete response to polatuzumab/rituximab, 2 additional courses of RT, and hyper-CVAD, underwent allogeneic transplant, and remains free of disease 15.9 months after initial SRT (19.9 months after CART infusion).
OS and PFS
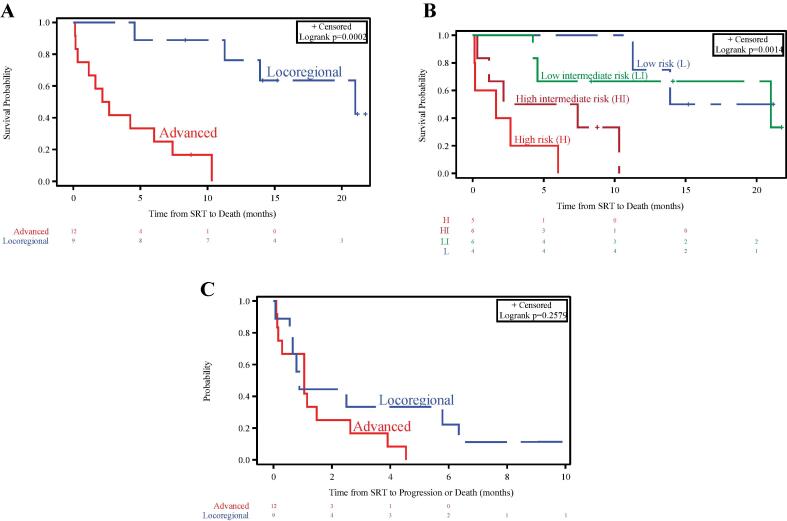
Median follow-up post-SRT was 15.2 months (95 % CI, 8.3 – 21.8 months). Median OS was 7.4 months (95 % CI, 2.2 months – 21 months). Those with locoregional disease experienced better OS than those with advanced disease (median, 21 months vs 2.4 months, p = 0.0002; Fig. 2A). sAA-IPI, which correlated with disease extent (based on Table 2), also appeared prognostic of OS (Fig. 2B). Median PFS was 1.1 month (95 % CI, 0.6 month – 2.5 months), and was relatively poor regardless of disease group (Fig. 2C). Apart from disease extent and sAA-IPI, a longer interval from CART infusion to progression was associated with better OS (Table 4).
Fig. 2.
Overall survival stratified by: (A) disease extent at time of post-CART progression, and (B) second-line age-adjusted International Prognostic Index. (C) Progression-free survival stratified by disease extent at time of post-CART progression.
Table 4.
Univariable analysis of overall survival.
| Variable | HR (95 % CI) | p value |
|---|---|---|
| Age (years) | 1.04 (0.97–1.1) | 0.28 |
| Best CART response (CR/PR/SD vs PD) | 0.46 (0.14–1.49) | 0.20 |
| Disease extent at progression (advanced vs locoregional) | 20 (2.5–162.6) | 0.005 |
| sAA-IPI (per stratum) | 3.06 (1.5–6.1) | 0.001 |
| Duration from CART infusion to progression (days) | 0.98 (0.96–0.997) | 0.021 |
HR = hazard ratio; CI = confidence interval; CART = CD19-targeting chimeric antigen receptor T-cell; CR = complete response; PR = partial response; SD = stable disease; PD = progressive disease; sAA-IPI = second-line age-adjusted International Prognostic Index.
To understand the prognosis after SRT versus other salvage therapies, an exploratory survival analysis was performed among the 60 patients in the larger cohort who experienced post-CART progression. For this analysis, OS was measured from the date of post-CART progression. OS appeared best for the SRT locoregional group, followed by the systemic therapy alone group, SRT advanced group, and no therapy group (Fig. A1).
Acute toxicity
Four patients (19 %) experienced Grade 2 toxicity post-SRT, including fatigue, nausea, constipation, and decreased joint range of motion. There were no grade ≥ 3 toxicities.
Discussion
In this series of SRT post-CART, we found encouraging in-field response rates (89 %) with no grade ≥ 3 toxicities, but high rates of out-of-field progression (94 %). Two groups of patients were identified: those with locoregional disease for whom comprehensive “definitive” SRT was typically given, and those with advanced disease for whom focal “palliative” SRT was given for various symptoms. Those with locoregional disease had better OS but no difference in PFS, highlighting the need for integration of novel systemic agents for both groups. Two patients in the locoregional disease group remain alive and free of disease post-SRT (21.9 and 15.9 months, respectively); one who never progressed following SRT (patient 1), and another who progressed shortly after SRT, but responded to further systemic therapy (polatuzumab/rituximab) and RT and underwent allogeneic transplant thereafter (patient 5).
There is a paucity of data on SRT post-CRT; to our knowledge, the only other published series was reported by Imber et al [14]. They reviewed 14 patients, 6 with localized disease and 8 with advanced disease post-CART progression. Patients with localized (versus advanced) disease who received SRT had both improved OS and freedom from subsequent relapse, with 3/6 (50 %) successfully bridged to allogeneic transplant and 5/6 (83 %) with an ongoing response at last follow-up. In contrast to their approach, we typically did not employ SRT with the intent of bridging to transplant. Additionally, 11/14 patients (79 %) in their series had an initial response (CR or PR) to CART therapy, whereas only 5/21 (24 %) in our series had an initial response to CART therapy, suggesting poorer baseline prognoses and lower expected response to salvage therapy in our cohort [17]. Indeed, the two patients with the longest OS both had initial CR to CART therapy (Fig. 1). Nevertheless, we also found that sAA-IPI was prognostic of OS post-CART progression, which has been shown in other relapsed/refractory settings [16], [18]. A unique finding of our study was that a longer interval from CART infusion to progression was associated with better OS, likely reflecting more indolent biology and probably independent of choice of salvage therapy.
Eleven of 21 patients (52 %) received SRT to lesions that were present pre-CART therapy. Patterns of failure analyses suggest that most progressions (86 %-88 %) post-CART therapy involve pre-existing lesions [19], [20]. Figura et al. identified pre-CART lesion characteristics that are at high risk for local failure post-CART, including maximum diameter ≥ 5 cm, extranodal component, and max SUV ≥ 10 [20]. In our series, all 11 lesions that were present pre-CART exhibited at least one of these characteristics and 9/10 evaluable lesions (90 %) exhibited at least two such characteristics (Table A1). This provides a strong rationale to test integration of local therapies for high-risk lesions to prevent CART therapy failures, potentially with bridging RT [8], [21], [22], [23].
SRT dose/fractionation schemes were heterogenous but were typically more protracted for those with locoregional disease. In the locoregional disease group, 8/9 (89 %) received ≥ 14 fractions, whereas in the advanced disease group, 11/12 received ≤ 10 fractions. Given the excellent in-field response rates observed and high rates of out-of-field progression, future work could consider testing shorter SRT courses for locoregionally confined disease. Furthermore, given that many allogeneic transplants contain total body irradiation (TBI), it is imperative to carefully consider RT dose/fractionation regimens for either bridging or salvage RT since an ablative TBI dose may not be possible after bridging or salvage RT depending on the dose and location.
An important rationale for integration of RT and CART is the potential for immunomodulation, minimizing the escape of antigen-null tumors by inducing epitope spreading and engaging an endogenous immune response against other tumor-associated antigens [24]. RT has complementary immune-modulatory activity through induction of increased major histocompatibility complex (MHC)-1 expression. In support of this notion, several reports have underscored the existence of doses and regimens that activate different damage signaling programs that profoundly impact responses to therapy by eliciting immunologically active tumor cell death and thereby promoting immune modulatory effects. These effects have been attributed to multiple Danger Associated Molecular Patterns pathways (DAMPs), including the activation of the RIG-I-like receptors (RLRs), cGMP-cAMP Synthetase (cGAS), Stimulator of Interferon Genes (STING) pathway with type I Interferon response, and tumor associated antigens cross-priming with anti-tumor CD8+ T-cell elicitation, ultimately inducing off-target or, as they are better known, “abscopal” effects directed against tumor sites that have not been irradiated [25], [26], [27], [28].
Qu et al. reported on one patient who relapsed after CART therapy, received RT, and subsequently achieved a CR with an associated increase in the number of CART copies, suggesting potential re-invigorating of a stalled CART response [9]. Smith et al. reported a case of synergistic abscopal-like response with B cell maturation antigen CART therapy plus RT in refractory myeloma, whereby palliative RT plus steroids for spinal cord compression resulted in a robust T cell repertoire expansion, cytokine-release syndrome-like symptoms, and robust out-of-field response that could not be attributed to either RT or CART alone [10]. In our study, we did not find evidence of abscopal-like effects among the patients with advanced disease.
There are several limitations to our study, including retrospective design, small sample size, possible referral bias (ie, only some patients were referred for SRT), lack of translational correlatives to assess immunomodulatory effects, and heterogeneity in use of systemic agents. The locoregional disease group appeared enriched for patients with less aggressive features, and the better OS for the SRT locoregional group (versus SRT advanced group or systemic therapy alone group) likely reflects the biology of the disease rather than the efficacy of SRT. Nevertheless, we add to the limited literature of SRT post-CART therapy progression and demonstrate that SRT can lead to effective local responses with minimal toxicity. Overall, we anticipate a growing need to develop effective salvage therapies with increasing adoption of CART therapy for r/rNHL.
Conclusions
Salvage RT post-CART therapy appears safe with encouraging in-field response but high rates of out-of-field progression. Those with locoregional (versus advanced) disease had better OS but no difference in PFS, highlighting the need for integration of novel systemic agents for both groups.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ctro.2023.100587.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Abramson J.S., Palomba M.L., Gordon L.I., Lunning M.A., Wang M., Arnason J., et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396(10254):839–852. doi: 10.1016/S0140-6736(20)31366-0. [DOI] [PubMed] [Google Scholar]
- 2.Schuster S.J., Bishop M.R., Tam C.S., Waller E.K., Borchmann P., McGuirk J.P., et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N Engl J Med. 2019;380(1):45–56. doi: 10.1056/NEJMoa1804980. [DOI] [PubMed] [Google Scholar]
- 3.Locke F.L., Ghobadi A., Jacobson C.A., Miklos D.B., Lekakis L.J., Oluwole O.O., et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1–2 trial. Lancet Oncol. 2019;20(1):31–42. doi: 10.1016/S1470-2045(18)30864-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neelapu S.S., Locke F.L., Bartlett N.L., Lekakis L.J., Miklos D.B., Jacobson C.A., et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N Engl J Med. 2017;377(26):2531–2544. doi: 10.1056/NEJMoa1707447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chow V.A., Gopal A.K., Maloney D.G., Turtle C.J., Smith S.D., Ujjani C.S., et al. Outcomes of patients with large B-cell lymphomas and progressive disease following CD19-specific CAR T-cell therapy. Am J Hematol. 2019;94(8) doi: 10.1002/ajh.25505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ng A.K., Yahalom J., Goda J.S., Constine L.S., Pinnix C.C., Kelsey C.R., et al. Role of Radiation Therapy in Patients With Relapsed/Refractory Diffuse Large B-Cell Lymphoma: Guidelines from the International Lymphoma Radiation Oncology Group. Int J Radiat Oncol Biol Phys. 2018;100(3):652–669. doi: 10.1016/j.ijrobp.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 7.Hoppe B.S., Moskowitz C.H., Filippa D.A., Moskowitz C.S., Kewalramani T., Zelenetz A.D., et al. Involved-Field Radiotherapy Before High-Dose Therapy and Autologous Stem-Cell Rescue in Diffuse Large-Cell Lymphoma: Long-Term Disease Control and Toxicity. J Clin Oncol. 2008;26(11):1858–1864. doi: 10.1200/JCO.2007.15.4773. [DOI] [PubMed] [Google Scholar]
- 8.Fang P.Q., Gunther J.R., Wu S.Y., Dabaja B.S., Nastoupil L.J., Ahmed S., et al. Radiation and CAR T-cell Therapy in Lymphoma: Future Frontiers and Potential Opportunities for Synergy. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.648655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qu C., Ping N., Kang L., Liu H., Qin S., Wu Q., et al. Radiation Priming Chimeric Antigen Receptor T-Cell Therapy in Relapsed/Refractory Diffuse Large B-Cell Lymphoma With High Tumor Burden. J Immunother. 2020;43(1):32–37. doi: 10.1097/CJI.0000000000000284. [DOI] [PubMed] [Google Scholar]
- 10.Smith E.L., Mailankody S., Staehr M., Wang X., Senechal B., Purdon T.J., et al. BCMA-Targeted CAR T-cell Therapy plus Radiotherapy for the Treatment of Refractory Myeloma Reveals Potential Synergy. Cancer Immunol. Res. 2019;7(7):1047–1053. doi: 10.1158/2326-6066.CIR-18-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buchwald Z.S., Wynne J., Nasti T.H., Zhu S., Mourad W.F., Yan W., et al. Radiation, Immune Checkpoint Blockade and the Abscopal Effect: A Critical Review on Timing, Dose and Fractionation. Front Oncol. 2018;8 doi: 10.3389/fonc.2018.00612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeSelm C., Palomba M.L., Yahalom J., Hamieh M., Eyquem J., Rajasekhar V.K., et al. Low-Dose Radiation Conditioning Enables CAR T Cells to Mitigate Antigen Escape. Mol Ther. 2018;26(11):2542–2552. doi: 10.1016/j.ymthe.2018.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodriguez-Ruiz M.E., Rodriguez I., Garasa S., Barbes B., Solorzano J.L., Perez-Gracia J.L., et al. Abscopal Effects of Radiotherapy Are Enhanced by Combined Immunostimulatory mAbs and Are Dependent on CD8 T Cells and Crosspriming. Cancer Res. 2016;76(20):5994–6005. doi: 10.1158/0008-5472.CAN-16-0549. [DOI] [PubMed] [Google Scholar]
- 14.Imber B.S., Sadelain M., DeSelm C., Batlevi C., Brentjens R.J., Dahi P.B., et al. Early experience using salvage radiotherapy for relapsed/refractory non-Hodgkin lymphomas after CD19 chimeric antigen receptor (CAR) T cell therapy. Br J Haematol. 2020;190(1):45–51. doi: 10.1111/bjh.16541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheson B.D., Fisher R.I., Barrington S.F., Cavalli F., Schwartz L.H., Zucca E., et al. Recommendations for Initial Evaluation, Staging, and Response Assessment of Hodgkin and Non-Hodgkin Lymphoma: The Lugano Classification. J Clin Oncol. 2014;32(27):3059–3067. doi: 10.1200/JCO.2013.54.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamlin P.A., Zelenetz A.D., Kewalramani T., Qin J., Satagopan J.M., Verbel D., et al. Age-adjusted International Prognostic Index predicts autologous stem cell transplantation outcome for patients with relapsed or primary refractory diffuse large B-cell lymphoma. Blood. 2003;102(6):1989–1996. doi: 10.1182/blood-2002-12-3837. [DOI] [PubMed] [Google Scholar]
- 17.Sigmund A.M., Denlinger N., Huang Y., Bond D., Voorhees T., Bajwa A., et al. Assessment of Salvage Regimens Post-Chimeric Antigen Receptor T Cell Therapy for Patients with Diffuse Large B Cell Lymphoma. Transplantation and Cellular. Therapy. 2022;28(6):342.e1–342.e5. doi: 10.1016/j.jtct.2022.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perales M.-A., Jenq R., Goldberg J.D., Wilton A.S., Lee S.S.E., Castro-Malaspina H.R., et al. Second-line age-adjusted International Prognostic Index in patients with advanced non-Hodgkin lymphoma after T-cell depleted allogeneic hematopoietic SCT. Bone Marrow Transplant. 2010;45(9):1408–1416. doi: 10.1038/bmt.2009.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saifi O., Breen W.G., Lester S.C., Rule W.G., Stish B., Rosenthal A., et al. Does bridging radiation therapy affect the pattern of failure after CAR T-cell therapy in non-Hodgkin lymphoma? Radiother Oncol. 2022;166:171–179. doi: 10.1016/j.radonc.2021.11.031. [DOI] [PubMed] [Google Scholar]
- 20.Figura N.B., Robinson T.J., Sim A.J., Wang X., Cao B., Chavez J.C., et al. Patterns and Predictors of Failure in Recurrent or Refractory Large B-Cell Lymphomas following Chimeric Antigen Receptor (CAR) T-Cell Therapy. Int J Radiat Oncol Biol Phys. 2021 doi: 10.1016/j.ijrobp.2021.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wright C.M., LaRiviere M.J., Baron J.A., Uche C., Xiao Y., Arscott W.T., et al. Bridging Radiation Therapy Before Commercial Chimeric Antigen Receptor T-Cell Therapy for Relapsed or Refractory Aggressive B-Cell Lymphoma. Int J Radiat Oncol Biol Phys. 2020;108(1):178–188. doi: 10.1016/j.ijrobp.2020.05.014. [DOI] [PubMed] [Google Scholar]
- 22.Pinnix C.C., Gunther J.R., Dabaja B.S., Strati P., Fang P., Hawkins M.C., et al. Bridging therapy prior to axicabtagene ciloleucel for relapsed/refractory large B-cell lymphoma. Blood Adv. 2020;4(13):2871–2883. doi: 10.1182/bloodadvances.2020001837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sim A.J., Jain M.D., Figura N.B., Chavez J.C., Shah B.D., Khimani F., et al. Radiation Therapy as a Bridging Strategy for CAR T Cell Therapy With Axicabtagene Ciloleucel in Diffuse Large B-Cell Lymphoma. Int J Radiat Oncol Biol Phys. 2019;105(5):1012–1021. doi: 10.1016/j.ijrobp.2019.05.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seyedin S.N., Schoenhals J.E., Lee D.A., Cortez M.A., Wang X., Niknam S., et al. Strategies for combining immunotherapy with radiation for anticancer therapy. Immunotherapy. 2015;7(9):967–980. doi: 10.2217/imt.15.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lugade A.A., Moran J.P., Gerber S.A., Rose R.C., Frelinger J.G., Lord E.M. Local Radiation Therapy of B16 Melanoma Tumors Increases the Generation of Tumor Antigen-Specific Effector Cells That Traffic to the Tumor. J Immunol. 2005;174(12):7516–7523. doi: 10.4049/jimmunol.174.12.7516. [DOI] [PubMed] [Google Scholar]
- 26.Reits E.A., Hodge J.W., Herberts C.A., Groothuis T.A., Chakraborty M., Wansley E.K., et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med. 2006;203(5):1259–1271. doi: 10.1084/jem.20052494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee Y., Auh S.L., Wang Y., Burnette B., Wang Y., Meng Y., et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood. 2009;114(3):589–595. doi: 10.1182/blood-2009-02-206870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burnette B.C., Liang H., Lee Y., Chlewicki L., Khodarev N.N., Weichselbaum R.R., et al. The Efficacy of Radiotherapy Relies upon Induction of Type I Interferon-Dependent Innate and Adaptive Immunity. Cancer Res. 2011;71(7):2488–2496. doi: 10.1158/0008-5472.CAN-10-2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.