Abstract
In order to find viable alternative protein sources for aquaculture, we evaluated the effect of partial or complete replacement of dietary soybean meal with yellow mealworm (TM) on the flesh quality of grass carp. In this study, 180 grass carp (511.85 ± 0.25 g) were fed 3 experimental diets in which 0% (CN), 30% (YM30) and 100% (YM100) dietary soybean meal was replaced by TM for 90 d. The results showed that growth performance, biological parameters and serum antioxidant capacity of grass carp were not affected by dietary TM (P > 0.05). Both muscle and whole body crude protein were obviously promoted with the increase of dietary TM (P < 0.05), and the concentration of heavy metal in muscle was not influenced (P > 0.05), indicating that food safety was not influenced by TM. Dietary TM improved muscle textural characteristics by elevating adhesiveness, springiness and chewiness in YM100 (P < 0.05). In addition, the muscle tenderness was significantly increased by declining the shear force (P < 0.05). The muscle fiber density in YM30 &YM100 and length of dark bands and sarcomeres in YM100 were obviously increased (P < 0.05). The expression of myf5, myog and myhc exhibited a significant upward trend with the increase of dietary TM (P < 0.05), which promoted fiber density, length of sarcomere and texture of grass carp muscle. According to the results of metabolomics, the arachidonate (ARA) and eicosapentaenoic acid (EPA) were notably elevated in YM30 and YM100, which indicated that the improvement of flesh quality of grass carp may contribute to the dietary TM influence on muscle lipid metabolism, especially the polyunsaturated fatty acids. In conclusion, TM can completely replace dietary soybean meal and improve the nutritional value of grass carp.
Keywords: Grass carp, Soybean meal, Yellow mealworm, Flesh quality, Metabolomics
1. Introduction
According to the FAO, the world population is expected to exceed 9 billion by 2050 (FAO, 2020), which will lead to increased demand for aquatic products. Over past decades, global per capita aquatic product consumption increased from 13.4 kg in 1986 to 20.5 kg in 2018 (FAO, 2020), and populations generally rely on aquatic products to meet dietary protein requirements. However, in order to meet consumer demand for aquatic products, the aquaculture industry has experienced heightened pressure regarding the supply of feed ingredients (Teves and Ragaza, 2016), such as fishmeal and fish oil. Terrestrial plants are often used as substitute for fishmeal, especially soybean meal.
Grass carp is the most productive freshwater fish in the world, with global production in 2018 exceeding 5,700 thousand tons (FAO, 2020). Grass carp has become an attractive protein source for consumers due to low price, high nutritive value and flavor (Yu et al., 2018). It is well known that grass carp is an herbivorous aquatic animal, and their major dietary protein source is soybean meal rather than fishmeal. However, the limited supply, continuous rise in price and unsustainability of soybean meal has challenged the expansion of the ever-growing grass carp aquaculture industry. Thus, it is extremely urgent to find a substitute for soybean meal in the grass carp diet. In recent years, insect resources have been investigated as substitutes for fishmeal or soybean meal in livestock and aquaculture (Makkar et al., 2014).
The yellow mealworm (Tenebrio molitor, TM) is an insect that is environmentally friendly, grows fast, reproduces rapidly, is sustainable and feeds on low-nutritive plant waste products such as dried fruit, vegetables and cereal residues in various combinations (da Sousa, 2020). TM, as a relatively available and cheap protein with high nutritional value, was reported to be one of the most regarded insects as a potential alternative protein (Tran et al., 2021). Studies have shown that the contents of protein, lipid and nitrogen-free extracts of TM are between 47.2% and 66.3%, 14.9% to 43.1%, and 2.8% to 3.4%, respectively (Nowak et al., 2016). In addition, TM is also rich in various vitamins, minerals, polysaccharides, chitosan, chitin and other biologically active substances (Gasco et al., 2020; Nowak et al., 2016).
In general, flesh quality of fish is dependent on its sensory characteristics (texture, tenderness, water holding capacity), nutritional value [protein, lipids, amino acids, docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA)] and safety indices (heavy metals, contaminants) (Picard et al., 2012). In fish, the flesh quality is influenced by many factors, including the characteristics of the fish (species, sex, age, etc.), environmental factors (temperature, salinity, etc.), feeding history (diet composition, feeding rate, etc.), and slaughtering method (Grigorakis, 2007). The flesh quality of farmed fish is closely related to nutrients in the diet (Grigorakis, 2007). Some previous studies have reported that alternative dietary protein resulted in differing degrees of effects on fish fillet quality by changing the chemical composition, sensory characteristics and oxidative stability of the fish fillet (D'Souza et al., 2006; de Francesco et al., 2004). In terms of TM, Iaconisi et al. (2017) reported that the water-holding capacity, hardness, cohesiveness, resilience, gumminess and adhesiveness of blackspot seabream (Pagellus bogaraveo) muscle were not influenced by dietary TM. On the contrary, it was found that the DHA and EPA content was decreased in shrimp and rainbow trout fillet with the increase of dietary of TM (Iaconisi et al., 2018; Panini et al., 2017). In addition, the crude protein and crude lipid in the whole body of mandarin fish (Siniperca scherzeri) and largemouth bass (Micropterus salmoides) were decreased with the increase of dietary TM (Gu et al., 2022). These results indicate that different levels of dietary TM have different effects on meat quality of aquatic animals.
To the best of our knowledge, there were no previous studies about the substitution of grass carp dietary soybean meal by TM. Therefore, how much dietary soybean meal of grass carp can be substituted by TM? What is the effect of dietary TM on the flesh quality of grass carp? As a consequence, the aim of this study was to investigate the effect of partial or complete replacement of dietary soybean meal with TM on the flesh quality of grass carp.
2. Materials and methods
2.1. Animal ethics statement
The experimental design and procedures of the presented research have been approved by the Committee on Ethics of Animal Experiments of Northwest A&F University.
2.2. Experimental design and diets
In order to meet the nutritional requirements of grass carp (Zheng et al., 2018), 3 experimental diets were designed to be isonitrogenous and isolipidic, with TM levels of 0%, partial (30%) and complete (100%) replacement of dietary soybean meal of grass carp. The three experimental diets were named CN, YM30 and YM100, respectively. The ingredients and proximate compositions of the diets, TM and soybean meal are presented in Table 1. The amino acid composition of the diets, TM and soybean meal are showed in Table 2. Experimental ingredients were finely ground through an 80-mesh sieve, then all ingredients weighted according to the formulation and fully mixed. Finally, pellets with a diameter of 4 mm were produced by a pellet machine (JL00SL01, Beijing Jinglai Construction Machinery Co., Ltd). All pellets were dried in an oven at 40 °C until the moisture fell below 10% and stored at −20 °C until use. The TM used in this study was provided by Guangdong Zehecheng Biotechnology Co., Ltd (Guangdong, China).
Table 1.
Formulation of experimental diets and chemical composition of soybean meal, yellow mealworm and experimental diets (%, dry matter).
| Item | Soybean meal | TM1 | Diets2 |
||
|---|---|---|---|---|---|
| CN | YM30 | YM100 | |||
| Soybean meal3 | 24.00 | 16.80 | 0.00 | ||
| Yellow mealworm4 | 0.00 | 5.03 | 16.76 | ||
| Casein5 | 18.05 | 18.05 | 18.05 | ||
| Corn starch6 | 10.00 | 10.00 | 10.00 | ||
| Flour7 | 30.00 | 30.00 | 30.00 | ||
| Soybean oil8 | 4.73 | 4.69 | 4.60 | ||
| Choline chloride9 | 0.50 | 0.50 | 0.50 | ||
| Mineral premix10 | 1.00 | 1.00 | 1.00 | ||
| Vitamin premix11 | 1.00 | 1.00 | 1.00 | ||
| Ethoxyquin12 | 0.05 | 0.05 | 0.05 | ||
| Chromic oxide13 | 0.50 | 0.50 | 0.50 | ||
| Calcium phosphate primary14 | 2.00 | 2.00 | 2.00 | ||
| Carboxyl methyl cellulose15 | 2.00 | 2.00 | 2.00 | ||
| Cellulose16 | 6.17 | 8.38 | 13.54 | ||
| Chemical composition | |||||
| Crude protein | 46.05 | 65.88 | 28.38 | 29.41 | 29.50 |
| Crude lipid | 1.14 | 2.38 | 4.87 | 4.50 | 4.79 |
| Crude ash | 7.11 | 5.10 | 4.52 | 4.72 | 4.66 |
| Moisture | 10.14 | 8.43 | 9.56 | 9.77 | 9.51 |
| As, mg/kg | 0.04 | 0.66 | 0.13 | 0.14 | 0.24 |
| Cd, mg/kg | 0.05 | 0.08 | 0.03 | 0.03 | 0.04 |
| Cu, mg/kg | 15.25 | 13.80 | 14.10 | 14.40 | 13.40 |
TM: yellow mealworm.
CN, YM30, YM100 represent diets in which yellow mealworm replaced soybean meal levels of 0%, 30% and 100%, respectively.
Soybean meal: from Qingdao Bohai Agricultural Development Co., Ltd., Qingdao, Shandong, China.
Yellow mealworm: from Guangdong Zehecheng Biotechnology Co., Ltd., Guangdong, China.
Casein: from Lanzhou Longruan Casein Co., Ltd., Lanzhou, Gansu, China.
Corn starch: from Yufeng Industrial Group Co., Ltd., Xiangtai, Heibei, China.
Flour: from Central Grain Reserve Ankang Direct Warehouse Co., Ltd., Ankang, Shanxi, China.
Soybean oil: from Yihai Kerry Arawana Holdings Co., Ltd., Shanghai, China.
Choline chloride: from Beijing Yinghuier Agriculture and Animal Husbandry Technology Co., Ltd., Beijing, China.
The mineral mix: from Beijing Yinghuier Agriculture and Animal Husbandry Technology Co., Ltd., Beijing, China. Contained (g/100 g of the total mineral): KAl(SO4)2 0.159, CaCO3 18.101, Ca(H2PO4)2 44.601, CoCl2 0.070, MgSO4 5.216, MnSO4·H2O 0.070, KCl 16.553, KI 0.014, ZnCO3 0.192, NaH2PO4 13.605, Na2SeO3 0.006, CuSO4·5H2O 0.075, ferric citrate 1.338.
The vitamin mix: from Beijing Yinghuier Agriculture and Animal Husbandry Technology Co., Ltd., Beijing, China. Contained (mg/1,000 g of diet): vitamin C, 200; thiamine, 10; riboflavin, 20; vitamin A, 3,000 IU; vitamin E, 50 IU; vitamin D3, 100; menadione, 10; pyridoxine HCl, 10; cyanocobalamin, 0.02; biotin, 1.0; calcium pantothenate, 40; folic acid, 5; niacin, 20; inositol, 400; cholinechloride, 2,000; and cellulose was used as a carrier.
Ethoxyquin: from Guangdong Nutriera Group, Guangzhou, China.
Chromic oxide: from Sinopharm Chemical Reagent Co., Ltd., China.
Calcium phosphate primary: from Sinopharm Chemical Reagent Co., Ltd., China.
Carboxyl methyl cellulose: from Wuhan Yimeite Biotechnology Co., Ltd., Wuhan, China.
Cellulose: from Shandong Liujia Pharmaceutical Excipients Co., Ltd., Jining, Shandong, China.
Table 2.
Amino acid composition of soybean meal, yellow mealworm and experimental diets (%, wet matter).
| Item | Soybean meal | TM1 | Diets2 |
||
|---|---|---|---|---|---|
| CN | YM30 | YM100 | |||
| EAA3 | |||||
| Threonine | 1.71 | 2.42 | 1.14 | 1.15 | 1.17 |
| Valine | 1.90 | 3.70 | 1.51 | 1.62 | 1.65 |
| Methionine | 0.38 | 1.19 | 0.43 | 0.44 | 0.48 |
| Phenylalanine | 2.12 | 2.79 | 1.44 | 1.39 | 1.53 |
| Isoleucine | 1.80 | 2.53 | 1.34 | 1.38 | 1.27 |
| Leucine | 3.26 | 4.77 | 2.43 | 2.46 | 2.38 |
| Lysine | 2.78 | 4.52 | 1.86 | 1.89 | 1.87 |
| Arginine | 3.07 | 3.66 | 1.84 | 1.80 | 1.55 |
| Histidine | 1.13 | 0.73 | 0.79 | 0.78 | 0.67 |
| ∑EAA4 | 18.13 | 26.28 | 12.76 | 12.89 | 12.54 |
| NEAA5 | |||||
| Aspartic acid | 4.77 | 4.65 | 2.83 | 2.73 | 2.44 |
| Serine | 2.15 | 5.14 | 1.52 | 1.60 | 1.90 |
| Glutamic acid | 8.01 | 7.28 | 6.58 | 6.27 | 5.86 |
| Glycine | 1.79 | 4.83 | 1.04 | 1.13 | 1.31 |
| Alanine | 1.82 | 2.93 | 1.15 | 1.19 | 1.19 |
| Cysteine | 0.62 | 0.58 | 0.37 | 0.35 | 0.36 |
| Tyrosine | 1.35 | 2.45 | 1.04 | 1.10 | 1.10 |
| Proline | 2.00 | 4.91 | 2.12 | 2.12 | 2.29 |
| ∑NEAA6 | 22.49 | 32.76 | 16.65 | 16.46 | 16.43 |
| ∑AA7 | 40.62 | 59.04 | 29.4 | 29.34 | 28.97 |
Values with different superscripts letter in the same row are significantly different (P < 0.05).
TM: yellow mealworm.
CN, YM30, YM100 represent diets in which yellow mealworm replaced soybean meal levels of 0%, 30% and 100%, respectively.
EAA: essential amino acid.
∑EAA: sum of essential amino acids.
NEAA: non-essential amino acid.
∑NEAA: sum of non-essential amino acids.
∑AA: sum of total amino acids.
2.3. Fish and feeding trial
Grass carp were obtained from Ankang Fisheries Experimental and Demonstration Station of Northwest A&F University, Shanxi, China. Before the experiment, all fish were reared in floating net cages (1.5 m × 1.5 m × 1.8 m, Length × Width × Height) in the center of pond (water depth: 3 m, located in Ankang Fisheries Experimental and Demonstration Station of Northwest A&F University, Shanxi, China) and fed with the commercial diet (NO:127; Crude protein: 29%; Crude lipid: 5%) with diameter 4 mm (Tongwei Co., Ltd, Sichuan, China) 3 times a day for 2 wk to make fish acclimated to the culture system. Prior to the experiment, fish fasted for 24 h. Subsequently, 180 healthy fish of similar size (average initial body weight 511.85 ± 0.25 g) were randomly distributed into 9 experimental net cages (20 fish per cage). Experimental diets were randomly assigned to triplicate cages and hand-fed to apparent visual satiation (3 times daily, 8:00, 12:00, 16:00) for 90 d. Discs with a 100 cm diameter were placed at the bottom of each cage to collect the uneaten feed according to Ni et al. (2016). The ponds were supplied with flowing water during the 90-d feeding period. Each cage was equipped with a microporous oxygenation system to maintain dissolved oxygen above 7 mg/L. During the 90-d feeding trial, the water temperature ranged from 18 to 33 °C, dissolved oxygen ranged from 7 to 11 mg/L, pH ranged from 7.8 to 9. The ammonia-N and nitrite-N were below 0.1 mg/L and 0.01 mg/L, respectively.
2.4. Sample collection and analysis
At the beginning of the feeding trial, triplicate groups of 3 fish were randomly sampled for detecting initial body composition. At the end of the feeding trial, all fish were starved for 24 h before sampling, and anesthetized by 0.1 g/L MS-222 (Sigma, St. Louis, MO, USA). The growth parameters including final body weight (FBW) and body length were measured. Six fish were prepared from each treatment (2 fish per cage) to determine whole body composition. The blood samples (4 fish per cage) were collected from the caudal vein with a syringe, and after centrifugation (1,369.55 × g, 4 °C, 15 min), serum was obtained and stored at −80 °C for analysis of antioxidant capacity. After blood sampling, the left fillets between the head and dorsal fin above the lateral line were quickly removed using a sharp scalpel, frozen immediately in liquid nitrogen and then stored at −80 °C for metabolomic analysis. Subsequently, the right muscle between the head and tail fin above the lateral line was sampled for measurement of muscle composition, amino acids and heavy metals. After that, the right muscle sample of another 4 fish per cage below the dorsal fin and lateral line was obtained, and cut into 1 cm3 cubes with a sharp scalpel for texture analysis, then the left side muscle was sampled for measuring pH, drip loss, cooking loss and histological analysis.
Proximate composition of diets, whole body and muscle were analyzed according to the methods described by AOAC (2003). Moisture, ash, crude protein and crude lipid were measured by oven drying at 105 °C to constant weight, at 550 °C for 12 h with a muffle furnace (TMF-3100, EYELA Co., Tokyo, Japan), and using the Kjeldahl method and ethyl ether extraction, respectively. The copper (Cu), cadmium (Cd) and arsenic (As) concentration of diets and muscle were determined according to the methods described by Alvarado et al. (2021). A modified detection method was used to analyse the amino acid composition of diets and fillets described by Ezaki et al. (2011). The total antioxidant capacity (T-AOC), superoxide dismutase (SOD) activity, content of malonaldehyde (MDA) and hydrogen peroxide (H2O2) of serum were measured with assay kits from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). The homogenization buffer of muscle and distilled water (1:10, g:mL) were obtained to measure the muscle pH with a digital pH meter (PHS–3C, Shanghai, China) (Cai et al., 2013). The method of analyzing muscle drip loss is described as follows. Firstly, approximately 10 g of muscle was weighed and suspended in a refrigerator at 4 °C. Then, it was weighed again after 24 h and the drip loss calculated based on the difference (Yang et al., 2022). Muscle cooking loss was measured by the modified detection method reported in (Yang et al., 2022), but in the present study, approximately 10 g of muscle was collected and weighed.
The muscle textural parameters, which included hardness, springiness, adhesiveness, cohesiveness, gumminess and chewiness, were measured using Texture Profile Analysis mode in a physical food property analyzer (TMS-pllot, FTC, USA). The shear force of muscle was measured according to the method previously described (Brinker and Reiter, 2011).
Paraffin sections of histological specimens were produced by Yangling Demonstration Area Hospital (Shaanxi, China). Obtained stained sections were photographed with a light microscope (Eclipse 50i, Nikon, Tokyo). The transmission electron microscope section of muscle sample used to measure sarcomere, bright band, dark band and H band was prepared by Chengdu Lilai Biotechnology Co., LTD (Chengdu, Sichuan), and photographed with a transmission electron microscope (JEM-1400PLUS, Japan). Finally, Image Pro Plus was used to analyse the muscle fiber diameter, fiber density, sarcomere, bright band, dark band and H band. Individual muscle fiber area (ā, μm2), muscle fiber diameter and muscle fiber density were measured based on the methodology described by Valente et al. (2016). Briefly, the individual muscle fiber area (ā, μm2) was directly measured by Image Pro Plus software and then the corresponding muscle fiber diameter (μm) was calculated by the formula: diameter (μm) = 2 × ā 0.5 × π−0.5. Muscle fiber density was measured as: total number of fibers counted over all sampled fields/the total area of the fiber counting fields (mm2).
Total RNA of muscle was extracted by homogenization in TRNzol reagent (Tiangen, Beijing, China). In order to avoid genomic DNA amplification during RT-PCR, the extracted RNA was treated with RNase-free DNase (TaKaRa, Dalian, China). Prior to RT-PCR, the integrity of RNA was examined by electrophoresis on 2% agarose gels. The PrimeScript RT reagent kit (TaKaRa, Dalian, P.R. China) was used to reverse the total RNA into cDNA. Real-time qPCR was performed using a CFX 96 Real-time PCR Detection System (Bio-Rad, Hercules, CA, USA). The final volume of the PCR reaction was 20 μL containing 0.6 μL of each Primer (10 μM), 1 μL of the diluted cDNA, 10 μL of 2 × SYBR Premix Ex TaqTMII (TaKaRa, Dalian, P.R. China) and 7.8 μL of sterilized double-distilled water. RT- PCR contained an initial activation step at 95 °C for 30 s, followed by 40 cycles of 95 °C for 15 s and 60 °C for 15 s. Quantitative PCR primer sequences and GenBank accession numbers are shown in Table 3 β-Actin mRNA was used as the internal control and remained stable throughout our study. The expression of genes were determined by the delta–delta CT method (2-△△CT) (Livak and Schmittgen, 2001).
Table 3.
Primers used for quantitative real-time PCR in this study.
| Gene | Forward primer (5′–3′) | Reverse primer (5′–3′) | Accession number |
|---|---|---|---|
| fgf6a1 | CGCATACGAGTCTTCCAT | CCTACGAGAACATCCAACA | MK050993 |
| fgf6b2 | TCCAGTCCGCTTCCGAGTA | AGATGAAACCCGATGCCTACA | MK050992 |
| myf53 | GTGCCTGTGCCTCATCTCCT | AATGCGTGGTTCACCTTCTTCA | GU290227 |
| mrf44 | TCGCTCCTGTATTGATGTTGATGA | GCTCCTGTCTCGCATTCGTT | KT899334 |
| myog5 | TTACGAAGGCGGCGATAACTT | TGGTGAGGAGACATGGACAGA | JQ793897 |
| myod6 | ATGGAGTTGTCGGATATTCCCTTC | GCGGTCAGCGTTGGTTGTT | MG544985 |
| myhc7 | GACGCTCATCACCACCAACC | TGCTCCTCACGCTGCTTCT | EU414733 |
| mstn8 | CTGACGCCAAGTTCCACATACA | CGACTCTGCTTCAAGTTCTTCTCT | KP719016 |
| β-actin | TATGTTGGTGACGAGGCTCA | GCAGCTCGTTGTAGAAGGTG | M25013 |
fgf6a: fibroblast growth factor 6a.
fgf6b: fibroblast growth factor 6b.
myf5: myogenic regulatory factor 5.
mrf4: myogenic regulatory factors 4.
myog: myogenin.
myod: myogenic determining factor.
myhc: myosin heavy chain.
mstn: myostatin.
Targeted metabolomics of muscle was carried out on 6 independent biological replicates per group by Wuhan Metware Biotechnology Co., Ltd. (Wuhan, China). The sample was thawed on ice first, then approximately 50 mg of one sample was taken from each group and cold steel balls were added to the mixture and homogenized at 30 Hz for 3 min. After this, 1 mL 70% methanol with internal standard extract was added to the homogenized centrifuge tube with a whirl of the mixture for 5 min, and then centrifuged at 16,099.2 × g at 4 °C for 10 min. Following this, 400 μL of supernatant was drawn from into the corresponding EP tube and refrigerated in −20 °C overnight, then centrifuged at 16,099.2 × g at 4 °C for 3 min, and 200 μL of supernatant was taken in the liner of the corresponding injection bottle for on-board analysis. LC-ESI-MS/MS system (UPLC, ExionLC AD, https://sciex.com.cn/; MS, QTRAP System, https://sciex.com/) was used to analyse the sample extracts. The analytical conditions were as follows: the UPLC column with a Waters ACQUITY UPLC HSS T3 C18 (1.8 μm, 2.1 × 100 mm) was used; the temperature of column, flow rate and injection volume were 40 °C, 0.4 mL/min, and 2 μL, respectively. In liquid chromatography, the mobile phase was mainly composed of water (0.1% formic acid) and acetonitrile (0.1% formic acid); the gradient program was as follows: 95:5 (vol:vol) at 0 min, 10:90 (vol:vol) at 10.0 min, 10:90 (vol:vol) at 11.0 min, 95:5 (vol:vol) at 11.1 min, 95:5 (vol:vol) at 14.0 min, respectively. The differentially expressed metabolites between groups were determined by VIP ≥1 and absolute Log2FC (fold change) ≥ 1. VIP values were extracted from OPLS-DA result. Before OPLS-DA, the data was log transformed (log2) with mean centering. A permutation test (200 permutations) was performed to avoid overfitting. KEGG Compound database (http://www.kegg.jp/kegg/compound/) and KEGG Pathway database (http://www.kegg.jp/kegg/pathway.html) were used to annotate identified metabolites and map annotated metabolites, respectively. Pathways with significantly regulated metabolites were mapped then fed into MSEA (metabolite sets enrichment analysis); their significance was determined by hypergeometric test p-values.
2.5. Statistical analysis
All data were subjected to one-way ANOVA after normality and homogeneity were checked using SPSS 26.0 (SPSS, IL, USA). After ANOVA identified whether the overall differences were significant (P < 0.05), Duncan's multiple range test was used to test the difference between treatments. The graphs in this study were created using Origin 2019 (Origin Software, CA, USA). Principal component analysis (PCA) was conducted with the statistics function prcomp within R (www.r-project.org). All data are presented as mean ± SEM.
3. Results
3.1. Growth performance and biological parameters
The growth performance and biological parameters of grass carp fed the experimental diets are shown in Table 4. After partial or complete replacement of dietary soybean meal with TM, no significant changes were found in final body weight (FBW), specific growth rate (SGR), feed conversion ratio (FCR), protein retention efficiency (PRE) and protein efficiency ratio (PER) (P > 0.05). The same trend also observed in condition factor (CF), viscerosomaic index (VSI), and hepatosomatic index (HSI) (P > 0.05).
Table 4.
Growth performance and biological parameters of grass carp fed the experimental diets (n = 3 for IBW, FBW, SGR, FCR, PRE, and PER; n = 15 for CF, VSI, and HSI).
| Item | Diets1 |
||
|---|---|---|---|
| CN | YM30 | YM100 | |
| IBW2, g | 511.75 ± 0.63 | 511.83 ± 0.88 | 511.92 ± 0.74 |
| FBW3, g | 993.52 ± 18.79 | 978.7 ± 20.43 | 1042.36 ± 63.20 |
| SGR4, %/d | 0.76 ± 0.02 | 0.74 ± 0.02 | 0.81 ± 0.07 |
| FCR5 | 1.86 ± 0.06 | 1.90 ± 0.06 | 1.92 ± 0.02 |
| PRE6, % | 38.69 ± 2.48 | 35.3 ± 1.31 | 36.82 ± 0.98 |
| PER7 | 1.75 ± 0.06 | 1.65 ± 0.05 | 1.63 ± 0.01 |
| CF8, g/cm3 | 2.41 ± 0.44 | 2.02 ± 0.11 | 2.37 ± 0.47 |
| VSI9, % | 6.23 ± 0.74 | 6.01 ± 0.45 | 6.82 ± 0.83 |
| HSI10, % | 2.22 ± 0.25 | 2.16 ± 0.28 | 2.41 ± 0.32 |
CN, YM30, YM100 represent diets in which yellow mealworm replaced soybean meal levels of 0%, 30% and 100%, respectively.
IBW (initial body weight, g).
FBW (final body weight, g).
SGR (specific growth rate, %/day) = 100 × [ln (final body weight, g) − ln (initial body weight, g)]/days.
FCR (feed conversion ratio) = (feed intake in dry matter, g)/[(final body weight, g) − (initial body weight, g)].
PRE (protein retention efficiency, %) = 100 × (retained protein, g)/(protein intake, g).
PER (protein efficiency ratio) = (wet weight gain, g)/(protein intake, g).
CF (condition factor, g/cm3) = 100 × (body weight, g)/(body length, cm3).
VSI (viscerosomaic index, %) = 100 × (viscera weight, g)/(body weight, g).
HSI (hepatosomatic index, %) = 100 × (liver weight, g)/(body weight, g).
3.2. Serum antioxidant capacity
Table 5 presents serum antioxidant capacity, where it can be seen that no marked difference was found in the T-AOC, SOD activity and content of MDA and H2O2 of serum (P > 0.05).
Table 5.
The serum antioxidant capacity of grass carp fed the experimental diets (n = 6).
| Item | Diets1 |
||
|---|---|---|---|
| CN | YM30 | YM100 | |
| T-AOC2, mmol/L | 1.35 ± 0.01 | 1.34 ± 0.01 | 1.31 ± 0.07 |
| SOD3, U/mL | 346.71 ± 8.34 | 353.56 ± 13.61 | 339.34 ± 2.77 |
| MDA4, nmol/mL | 7.31 ± 0.44 | 5.73 ± 0.82 | 6.38 ± 0.27 |
| H2O25, mmol/L | 0.11 ± 0.01 | 0.09 ± 0.01 | 0.10 ± 0.00 |
CN, YM30, YM100 represent diets in which yellow mealworm replaced soybean meal levels of 0%, 30% and 100%, respectively.
T-AOC: total antioxidant capacity.
SOD: superoxide dismutase.
MDA: malonaldehyde.
H2O2: hydrogen peroxide.
3.3. Whole body and muscle composition
As shown in Table 6, partial and complete substitution of soybean meal with TM in the diet could reduce the lipid content of grass carp. The whole-body crude lipid content in YM100 was significantly lower than that of the CN group (P < 0.05). However, muscle crude protein showed the opposite trend, being significantly higher in YM30 and YM100 than that of CN (P < 0.05). The TM in the diets was not affected by the ash content, moisture of both whole body and muscle, and whole body crude protein (P > 0.05).
Table 6.
Muscle and whole body composition of grass carp fed the experimental diets (%, wet matter; n = 3).
| Diets1 | Muscle |
Whole body |
||||||
|---|---|---|---|---|---|---|---|---|
| Ash | Crude lipid | Crude protein | Moisture | Ash | Crude lipid | Crude protein | Moisture | |
| CN | 1.34 ± 0.17 | 1.79 ± 0.14a | 20.52 ± 0.23b | 75.51 ± 0.09 | 3.59 ± 0.12 | 10.25 ± 0.33a | 16.55 ± 0.68 | 68.76 ± 0.8 |
| YM30 | 1.32 ± 0.09 | 1.67 ± 0.02a | 21.66 ± 0.36a | 75.78 ± 0.37 | 3.43 ± 0.05 | 9.75 ± 0.47ab | 16.52 ± 0.52 | 68.69 ± 1.15 |
| YM100 | 1.34 ± 0.13 | 1.27 ± 0.10b | 21.31 ± 0.13a | 75.86 ± 0.69 | 3.36 ± 0.18 | 8.88 ± 0.14b | 16.05 ± 0.64 | 70.45 ± 0.58 |
Values with different superscript letters in the same column are significantly different (P < 0.05).
CN, YM30, YM100 represent diets in which yellow mealworm replaced soybean meal levels of 0%, 30% and 100%, respectively.
3.4. Muscle amino acid composition and heavy metals
The muscle amino acid composition is shown in Table 7. Except for histidine (His), no marked difference was observed in essential amino acids (EAA), non-essential amino acids (NEAA) and total of amino acids in muscle among all treatments (P > 0.05). However, the muscle His was significantly reduced in YM100 when compared to the CN group (P < 0.05).
Table 7.
Muscle amino acid composition of grass carp fed the experimental diets (%, wet matter; n = 6).
| Item | Diets1 |
||
|---|---|---|---|
| CN | YM30 | YM100 | |
| EAA2 | |||
| Threonine | 0.90 ± 0.00 | 0.92 ± 0.01 | 0.95 ± 0.04 |
| Valine | 0.96 ± 0.02 | 1.00 ± 0.01 | 0.98 ± 0.05 |
| Methionine | 0.60 ± 0.01 | 0.61 ± 0.01 | 0.63 ± 0.02 |
| Phenylalanine | 0.86 ± 0.01 | 0.85 ± 0.02 | 0.85 ± 0.03 |
| Isoleucine | 0.91 ± 0.02 | 0.93 ± 0.01 | 0.93 ± 0.03 |
| Leucine | 1.66 ± 0.01 | 1.70 ± 0.01 | 1.70 ± 0.06 |
| Lysine | 1.99 ± 0.01 | 2.06 ± 0.05 | 2.11 ± 0.08 |
| Arginine | 1.26 ± 0.02 | 1.27 ± 0.01 | 1.26 ± 0.05 |
| Histidine | 0.78 ± 0.02a | 0.76 ± 0.02a | 0.68 ± 0.02b |
| ∑EAA3 | 9.91 ± 0.05 | 10.09 ± 0.10 | 10.09 ± 0.37 |
| NEAA4 | |||
| Aspartic acid | 2.10 ± 0.01 | 2.19 ± 0.04 | 2.18 ± 0.09 |
| Serine | 0.84 ± 0.01 | 0.86 ± 0.03 | 0.88 ± 0.04 |
| Glutamic acid | 3.19 ± 0.03 | 3.27 ± 0.08 | 3.33 ± 0.13 |
| Glycine | 0.95 ± 0.01 | 0.94 ± 0.01 | 0.95 ± 0.03 |
| Alanine | 1.17 ± 0.02 | 1.21 ± 0.03 | 1.22 ± 0.04 |
| Cysteine | 0.21 ± 0.00 | 0.21 ± 0.00 | 0.21 ± 0.00 |
| Tyrosine | 0.78 ± 0.00 | 0.79 ± 0.01 | 0.77 ± 0.03 |
| Proline | 0.77 ± 0.04 | 0.68 ± 0.04 | 0.79 ± 0.01 |
| ∑NEAA5 | 10.03 ± 0.04 | 10.13 ± 0.18 | 10.32 ± 0.36 |
| ∑AA6 | 19.93 ± 0.07 | 20.23 ± 0.29 | 20.40 ± 0.73 |
Values with different superscript letters in the same row are significantly different (P < 0.05).
CN, YM30, YM100 represent diets in which yellow mealworm replaced soybean meal levels of 0%, 30% and 100%, respectively.
EAA: essential amino acid.
∑EAA: sum of essential amino acids.
NEAA: non-essential amino acid.
∑NEAA: sum of non-essential amino acids.
∑AA: sum of total amino acids.
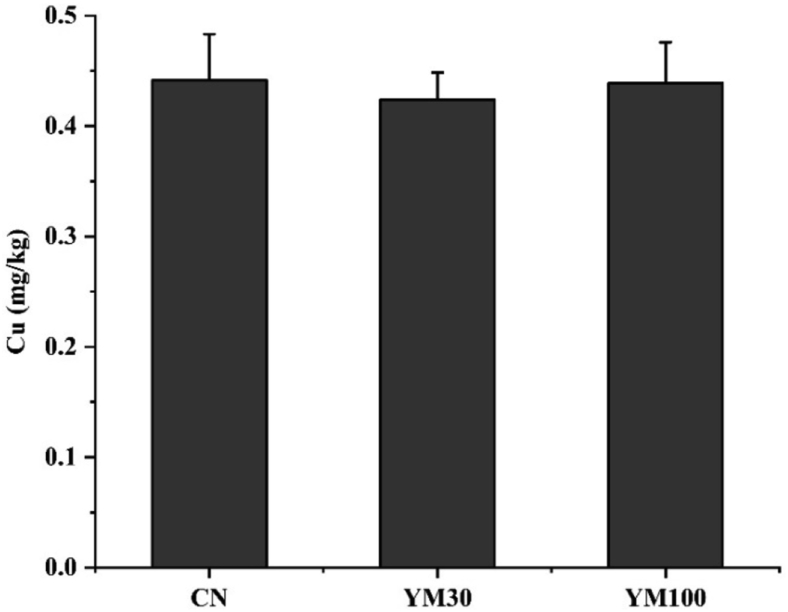
In this study, Cd and As were not detected in the muscle of grass carp fed the experimental diets, and the concentration of Cu in muscle is shown in Fig. 1. As shown in Fig. 1, the muscle Cu was not influenced by dietary TM (P > 0.05).
Fig. 1.
The muscle copper (Cu) content of grass carp fed experimental diets (n = 3). CN, YM30, YM100 represent diets in which yellow mealworm replaced soybean meal levels of 0%, 30% and 100%, respectively.
3.5. Muscle texture properties, water holding capacity and pH
Table 8 displays the texture properties, water holding capacity and pH of grass carp muscle. The pH and water holding capacity (drip loss, cooking loss) of grass carp muscle were not influenced by the dietary TM (P > 0.05). The adhesiveness and chewiness of muscle were significantly elevated in the YM100 group (P < 0.05). The muscle springiness in YM30 and YM100 were markedly higher than that of CN (P < 0.05) but muscle shear force was obviously declined with the increase of dietary TM (P < 0.05). There were no differences in hardness, cohesiveness and gumminess among all groups (P > 0.05).
Table 8.
Muscle textural characteristics, water holding capacity and pH of grass carp fed experimental diets (n = 12).
| Item | Diets1 |
||
|---|---|---|---|
| CN | YM30 | YM100 | |
| Adhesiveness, N⋅mm | 0.10 ± 0.01b | 0.14 ± 0.02ab | 0.15 ± 0.01a |
| Springiness, mm | 1.72 ± 0.02b | 2.03 ± 0.06a | 2.08 ± 0.04a |
| Shear force, N | 11.06 ± 0.61a | 9.56 ± 0.06b | 8.12 ± 0.11c |
| Chewiness, mJ | 4.51 ± 0.61b | 5.94 ± 0.44ab | 7.18 ± 0.80a |
| Hardness, N | 18.62 ± 0.79 | 20.87 ± 1.51 | 21.52 ± 2.87 |
| Cohesiveness ratio | 0.13 ± 0.01 | 0.13 ± 0.00 | 0.13 ± 0.00 |
| Gumminess, N | 2.53 ± 0.30 | 2.71 ± 0.15 | 2.95 ± 0.49 |
| Drip loss2, % | 2.88 ± 0.12 | 2.69 ± 0.04 | 2.95 ± 0.20 |
| Cooking loss3, % | 23.08 ± 0.71 | 21.19 ± 1.68 | 23.2 ± 0.92 |
| pH | 6.11 ± 0.03 | 6.15 ± 0.01 | 6.15 ± 0.03 |
Values with different superscript letters in the same row are significantly different (P < 0.05).
CN, YM30, YM100 represent diets in which yellow mealworm replaced soybean meal levels of 0%, 30% and 100%, respectively.
Drip loss (%) = 100 × [(muscle before suspended weight, g) – (muscle after being suspended weight, g)]/(muscles before suspended weight, g).
Cooking loss (%) = 100 × [(muscle before cooked Weight, g) – (muscle after cooked weight, g)]/(muscle before cooked weight, g).
3.6. Muscle morphology analysis
Fig. 2 presents the muscle transversal section (Fig. 2A), frequency distribution of muscle fiber diameter (Fig. 2B) and the muscle fiber density (Fig. 2C) of grass carp fed experimental diets. The frequency of Class 20 (20 < fiber diameter ≤ 40) fibers and fiber density of grass carp in YM30 and YM100 were obviously higher than that of CN (P < 0.05). There was no difference in fiber frequencies of classes Class 0, Class 40, Class 60, Class 80 and Class 100 among treatments (P > 0.05).
Fig. 2.
Muscle H&E staining analysis of grass carp fed experimental diets (n = 6). CN, YM30, YM100 represent diets in which yellow mealworm replaced soybean meal levels of 0%, 30% and 100%, respectively. (A) Representative images (200 × , scale bar = 100 mm). (B) Frequency distribution of muscle fiber diameter (μm). Class 0: 0 < diameter ≤ 20, class 20: 20 < diameter ≤40, class 40: 40 < diameter ≤ 60, class 60: 60 < diameter ≤ 80, class 80: 80 < diameter ≤ 100, class 100: 100 < diameter. (C) Muscle fiber density. Bars with different letters on top are significantly different (P < 0.05).
Fig. 3A shows the muscle transmission electron microscope image, and Table 9 shows the length of H-band, bright band, dark band and sarcomere of grass carp muscle. According to Table 9, no difference was observed in the length of H band and bright band (P > 0.05), but dark band and sarcomere in YM100 were significantly lengthier than that of CN and YM30 (P < 0.05).
Fig. 3.
Muscle transmission electron microscope images and relative mRNA expression of muscle growth-related genes of grass carp fed experimental diets (n = 6). CN, YM30, YM100 represent diets in which yellow mealworm replaced soybean meal levels of 0%, 30% and 100%, respectively. (A) Representative images (magnification 25,000 × , scale bar = 500 nm); H: H band; B: bright band; D: dark band; S: sarcomere; C: chondriosome. (B) Relative mRNA expression of muscle growth-related genes. fgf6a = fibroblast growth factor 6a; fgf6b = fibroblast growth factor 6b; myf5 = myogenic regulatory factor 5; mrf4 = myogenic regulatory factors 4; myog = myogenin; myod = myogenic determining factor; myhc = myosin heavy chain; mstn = myostatin. Bars with different letters on top are significantly different (P < 0.05).
Table 9.
The muscle H band, bright band, dark band and sarcomere length of grass carp fed experimental diets (n = 6).
| Item | Diets1 |
||
|---|---|---|---|
| CN | YM30 | YM100 | |
| H band, nm | 103.05 ± 1.44 | 101.97 ± 2.50 | 107.88 ± 3.19 |
| Bright band, nm | 282.95 ± 6.06 | 294.71 ± 14.67 | 338.4 ± 25.92 |
| Dark band, nm | 1,358.71 ± 23.55b | 1,363.61 ± 34.10b | 1,490.00 ± 16.38a |
| Sarcomere, nm | 1,641.65 ± 29.61b | 1,658.33 ± 29.19b | 1,828.40 ± 26.16a |
Values with different superscript letters in the same row are significantly different (P < 0.05).
CN, YM30, YM100 represent diets in which yellow mealworm replaced soybean meal levels of 0%, 30% and 100%, respectively.
3.7. Relative mRNA expression of muscle growth-related genes
The relative mRNA expression of muscle growth-related genes is shown in Fig. 3B. The expression of myf5, myog and myhc exhibited a significant upward trend with the increase of dietary TM (P < 0.05). The expression of fgf6b in YM30 was notably higher than that of both CN and YM100 (P < 0.05), but the expression of mstn was markedly declined in YM100 compared with CN and YM30 (P < 0.05). There were no significant differences in fgf6a, mrf4 and myod across all groups (P > 0.05), but there was an upward trend.
3.8. Muscle metabonomic analysis
To further investigate the molecular characteristics of partial or complete replacement dietary soybean meal with TM on the flesh quality of grass carp, we performed a widely targeted metabolomic analysis. OPLS-DA showed obvious separation between the CN and YM30 and between CN and YM100, which indicated that a VIP analysis could be performed for screening differential metabolites (Fig. 4). A total of 605 metabolites were identified. Among them, there were 151 lipids and their derivatives, 92 amino acids and their derivatives, 76 organic acids and their derivatives, 67 nucleotides and their derivatives, 40 carbohydrates and their metabolites, 37 benzene and its derivatives.
Fig. 4.
Orthogonal projection to latent structures-discrimination analysis (OPLS-DA) of between CN and YM30 or YM100 (n = 6). CN, YM30, YM100 represent diets in which yellow mealworm replaced soybean meal levels of 0%, 30% and 100%, respectively. (A) The OPLS-DA between CN and YM30. (B) The OPLS-DA between CN and YM100.
In the comparison of muscle metabolites of CN and YM30, 11 metabolites were annotated to the KEGG database. Those metabolites were significantly enriched in 9 types of metabolic pathways, and the metabolic pathways related to flesh quality were amino acid metabolism and fatty acid metabolism (Fig. 5A). In the comparison of muscle metabolites of CN vs YM30, there were 78 metabolites annotated to the KEGG database. Those metabolites were significantly enriched in 52 types of metabolic pathways, and the metabolic pathways related to flesh quality were amino acid metabolism, lipid metabolism, fatty acid metabolism and carbohydrate metabolism (Fig. 5B). The number of different metabolites of CN vs YM30, CN vs YM100 and overlapping different metabolites between CN vs YM30 and CN vs YM100 were 20, 33 and 13 (Fig. 6), respectively. The overlapping different metabolites between CN vs YM30 and CN vs YM100 are shown in Table 10. There were 12 metabolites that were up-regulated and 1 metabolite down-regulated.
Fig. 5.
Kyoto Encyclopedia of Genes and Genomes (KEGG) classification of differential metabolites between CN and YM30 or YM100 (n = 6). CN, YM30, YM100 represent diets in which yellow mealworm replaced soybean meal levels of 0%, 30% and 100%, respectively. The annotated metabolic pathways are classified according to the pathway type in KEGG. The abscissa is the number of metabolites annotated to this pathway and the ratio of the number of metabolites annotated in the corresponding pathway to the total number of annotated metabolites. (A) KEGG classification of differential metabolites between CN and YM30. (B) KEGG classification of differential metabolites between CN and YM100.
Fig. 6.
The number of different metabolites of CN vs YM30, CN vs YM100 and overlapping different metabolites between CN vs YM30 and CN vs YM100 (n = 6). CN, YM30, YM100 represent diets in which yellow mealworm replaced soybean meal levels of 0%, 30% and 100%, respectively.
Table 10.
The overlapping different metabolites between CN vs YM30 and CN vs YM100 (n = 6).
| Item | Class | Type | VIP |
Fold change |
||
|---|---|---|---|---|---|---|
| CN vs YM30 | CN vs YM100 | CN vs YM30 | CN vs YM100 | |||
| L-Tartaric acid | Organic acid and its derivatives | up | 1.39 | 1.15 | 3.02 | 3.46 |
| 5-Aminovaleric acid | Amino acid and its metabolites | up | 2.00 | 1.98 | 2.01 | 3.05 |
| D-Inositol-1,4-diphosphate | Alcohol and amines | up | 1.96 | 1.67 | 2.92 | 3.36 |
| δ-Valerolactam | Alcohol and amines | up | 3.00 | 2.46 | 65,244.07 | 32,8701.85 |
| Arachidonate (ARA) | Fatty acid | up | 1.75 | 1.70 | 2.70 | 2.75 |
| Eicosapentaenoic acid (EPA) | Fatty acid | up | 1.81 | 1.88 | 2.68 | 3.02 |
| Carnitine C5:0 isomer | Fatty acyl | up | 3.00 | 2.46 | 33,056.85 | 12,5334.81 |
| Carnitine C5:0 | Fatty acyl | up | 3.00 | 2.46 | 25,835.37 | 13,3275.19 |
| Monoglyceride (MG (22:6) isomer) | Glycerides | up | 2.13 | 1.68 | 2.37 | 2.60 |
| Monoglyceride (MG (22:6)) | Glycerides | up | 2.13 | 1.68 | 2.37 | 2.60 |
| Monoglyceride (MG (20:5) isomer) | Glycerides | up | 2.31 | 1.47 | 2.77 | 2.58 |
| Monoglyceride (MG (20:5)) | Glycerides | up | 2.31 | 1.47 | 2.77 | 2.58 |
| Trigonelline | Coenzyme and vitamins | down | 2.09 | 2.35 | 0.46 | 0.13 |
4. Discussion
4.1. Growth performance
According to the results, there were no significant differences in FBW, SGR, FCR, PRE and PER among all experimental groups. Similarly, dietary TM also had no influence on biological indices (CF, VSI, HSI) of grass carp. Several previous related studies conducted in other fish have shown that dietary TM dose not negatively affect growth performance (Belforti et al., 2015; Choi et al., 2018; Feng et al., 2019; Iaconisi et al., 2017; Panini et al., 2017; Sankian et al., 2018; Song et al., 2018; Tubin et al., 2020). However, in a study of Largemouth bass, the FBW and SGR decreased significantly with the increase of dietary TM (Gu et al., 2022). Similarly results with Gu et al. (2022) also found this in African catfish (Clarias gariepinus) (Ng et al., 2001). One study reported that when the level of dietary black soldier fly (Hermetia illucensthe) exceeded 40%, the growth performance of Eurasian Perch (Perca fluviatilis) was noticeably diminished (Stejskal et al., 2020). Moreover, when dietary fishmeal substituted by TM exceeded 25%, the growth and feed utilization of European sea bass (Dicentrarchus labrax L.) decreased significantly (Gasco et al., 2016). Furthermore, the final body and weight gain rate of large yellow croaker (Larimichthys crocea) were reduced significantly when TM levels in the diet exceeded 30% (Yuan et al., 2022). In However, in the studies by Gasco et al. (2016), Gu et al. (2022), Ng et al. (2001) and Stejskal et al. (2020), TM replaced fishmeal rather than soybean meal. Barroso et al. (2014) indicated that the amino acid profile of insects is taxon-dependent, with Diptera being close to fish meal and Coleoptera and Orthoptera being close to soybean. The yellow mealworm belongs to the order Coleoptera. This led to the high replacement of fish meal by TM, which led to a decrease in fish growth. As shown in Table 2, the amino acid profile of TM is close to soybean, and the previous findings thus confirmed in this study. As consequence, similar amino acid profiles between TM and soybean meal can explain why dietary TM did not affect the growth performance of grass carp. On the other hand, plant feedstuffs (such as soybean meal, rapeseed meal) lack certain fish meal components which are beneficial for fish growth, such as hydroxyproline and taurine (Henry et al., 2015). On the contrary, previous studies have also shown that insects, including TM, contain valuable levels of taurine and hydroxyproline (Henry et al., 2015). Therefore, based on the growth performance, TM has a greater potential to replace dietary soybean meal than fishmeal, and the inclusion of TM is more suited to herbivorous or omnivorous fish diets than carnivorous (Shafique et al., 2021).
4.2. Serum antioxidant capacity
Various antioxidant enzymes can indicate the antioxidant status of aquatic animals and serve as markers of oxidative stress (Kohen and Nyska, 2002). Over-production of reactive oxygen species (ROS) can lead to oxidative stress when aquatic animals consume harmful substances in their diet, such as oxidized fish oil (Lushchak, 2011). ROS is first converted to H2O2 catalyzed by SOD, and then GSH-Px and CAT decompose H2O2 into H2O and O2 (Zhang and Piao, 2022). The concentration of MDA is usually used as a marker of lipid peroxidation (Shafique et al., 2021). In our study, we found the concentration of serum MDA and H2O2 were not increased, and the activity of SOD and T-AOC were not influenced by dietary TM. These results above demonstrated that dietary TM did not cause oxidative stress in grass carp. Previous research confirmed our results, which pointed out that the inclusion level of TM in the diet of mandarin fish and rainbow trout did not change the serum SOD activity (Jeong et al., 2020; Sankian et al., 2018). Conversely, giant freshwater prawns (Macrobrachium rosenbergii) fed TM protein experienced a significant increase in serum SOD activity (Feng et al., 2019). However, Sánchez-Muros et al. (2016) observed a significantly decrease in hepatic SOD activity with the increase of dietary full-fat TM, with no effect on the hepatic CAT, GSH-Px, GR, and GST activities of Nile tilapia. The hepatic MDA level of mirror carp was significantly elevated when fed diets supplemented with 2.5% TM oil (Xu et al., 2020). Studies have reported that the antioxidant capacity of TM is related to chitin, chitosan and other bioactive compounds (Khoushab and Yamabhai, 2010). In our opinion, the concentration of chitin, chitosan and various bioactive compounds in defatted TM, full-fat TM and TM oil is different, which contributes to different degrees of antioxidant capacity in aquatic animals.
4.3. Approximate composition of muscle and whole body, and the level of muscle amino acids and heavy metals
The nutritional value of fish is directly reflected by the approximate body composition of fish. In this trial, dietary TM reduced both muscle and whole-body lipids, and increased the crude protein in muscle. A study on rainbow trout has shown a notable increase in the crude protein and decrease in crude lipid in fillets of fish fed diets with 25% and 50% TM compared with those in the fishmeal group (Belforti et al., 2015). In addition, previous researchers have also reported that dietary TM elevated whole body crude protein content in aquatic animals (Rema et al., 2019) and declined the whole body crude lipid content of European sea bass and rainbow trout (Mastoraki et al., 2020; Rema et al., 2019). These results were confirmed in this study. However, the whole body lipids of Litopenaeus vannamei increased with higher dietary TM, and whole body protein was not influenced by dietary TM (Panini et al., 2017). Furthermore, the whole body proximate composition of mandarin fish and muscle of blackspot sea bream were not influenced by dietary TM (Sankian et al., 2018). Nutrient deposition in fish is inseparable from digestibility of diet. In research on sea bass and rainbow trout, the apparent digestibility of crude protein in the diet was markedly increased with higher dietary TM, which resulted in more protein deposition in the body of the fish (Chemello et al., 2020; Gasco et al., 2016). However, in the study about Largemouth bass, Gu et al. (2022) found the protein apparent digestibility coefficient of the diet was firstly increased and then declined with an increase in dietary TM. According to the above mentioned research, the digestibility of dietary protein may be related to the actual TM level in the diet, which contributes to a different rate of protein deposition. On the other hand, the lipid content of fish fed with inclusion TM diets may in part be attributed to the content of chitin in TM that could interfere with lipid utilization (Khosravi et al., 2018). For instance, previous research found that a high content of dietary chitin could seriously suppress lipid digestion and absorption in fish and livestock (Khosravi et al., 2018).
Muscle flavor also may be influenced by the profile of fish muscle amino acids (Yang et al., 2022). It was found that the Ala, Leu and Lys levels were notably elevated in the muscle of gilthead seabream when fed diets with 50% of fishmeal replaced by full-fat TM, but the His and Phe levels showed the opposite trend (Iaconisi et al., 2019). Similar result was obtained in our study, where the muscle His declined linearly with the increase of dietary TM. However, the muscle Arg and His of tilapia was elevated with the increase of dietary TM (Sánchez-Muros et al., 2016). Previous studies reported that the amino acid profiles of fish generally reflect the dietary amino acid content (Shafique et al., 2021). In the present study, the His content of TM was nearly half that of soybean meal, and the experimental diet His content also declined linearly with an increasing level of replacement of soybean meal with TM (Table 2). So, the decrease in muscle His contributed to the decline of His in the diets. The nutritional composition of TM varies subject to feed, life stages, processed forms (raw/fresh, freeze-dried and fried) and rearing conditions (Bordiean et al., 2020). In the studies mentioned above, as well as different studies have shown that TM has different effects on the amino acid profile of fish. We think one of the reasons is that the TM used in different studies had a different nutritional composition.
It is especially important to examine the metal level in fish meat to ensure compliance with food safety regulations and consumer protection (Poma et al., 2017). In the present trial, the Cu content of grass carp muscle was not influenced by dietary TM, and the Cu content in CN (0.44 ± 0.04 mg/kg) was highest and was still lower than the threshold values (<50 mg/kg) stipulated by China as potential risk to human consumption (Liu et al., 2011). As and Cd have not been detected in grass carp muscle. Therefore, the grass carp fed diets with soybean meal complete substituted by TM were safe for consumers. On the contrary, when Atlantic salmon (Salmo salar) were fed diets with more insect meal (Black soldier fly) and less fish meal, this led to a marked decline in the total arsenic content of fillets (Biancarosa et al., 2019). Currently, most studies only focus on the heavy metal content of mealworms themselves (Bordiean et al., 2020), but there are few studies about the heavy metal content of aquatic animals fed diets with TM. Nonetheless, as we mentioned earlier, the nutritional composition of TM varies subject to feed, life stage, processed forms and rearing conditions. The level of heavy metals in insects may be indirectly toxic for consumers and animals. Thus, it is still necessary to evaluate the heavy metal content of aquatic animals when TM is used as aquatic feed ingredient in the future.
4.4. Muscle texture, morphology and mRNA expression of muscle growth-related genes
It is well known that fish flesh quality is closely associated with diets (García-Romero et al., 2014). In terms of textural characteristics, we found that all textural parameters, except for shear force and cohesiveness, were promoted, although no statistical differences were observed in hardness and gumminess. However, previous research has shown that there were no effects on the texture of sea bream (Iaconisi et al., 2017). Similar results from Iaconisi et al. (2017) were obtained in European seabass, which the muscle texture was not influenced by dietary TM, except for chewiness (da Sousa, 2020). Furthermore, Yuan et al. (2022) reported that dietary TM increased the muscle adhesiveness and declined the shear force of Largemouth bass, which was partially in agreement with our results, but had no influence on the other texture parameters. Different results were obtained in different studies, which may have been caused by the different protein sources (fish meal or soybean meal) being replaced by TM and different fish species. Muscle cellularity (muscle fiber diameter and density) is a key determinant of the muscle textural characteristics (Periago et al., 2005). Roy et al. (2012), Periago et al. (2005) and Johnston et al. (2000) demonstrated that muscle fiber density was positively correlated with muscle hardness, adhesiveness, springiness, chewiness and gumminess. In this study, the muscle fiber density and the frequency of Class 20 (20 < diameter ≤40) were significantly increased in the YM30 and YM100 groups, which could explain how the muscle textural characteristics were improved with increasing dietary TM by promoting muscle fiber proliferation. However, Zhang et al. (2022) showed that muscle fiber density of large yellow was not influenced when TM substituted fish meal below 45% (25.70% TM in diet). Furthermore, Yuan et al. (2022) proved that fiber density in fish fed a diet with complete TM replacement of fish meal (56.83% TM in diet) was markedly lower than fish fed with no TM. The studies above show that a high level of dietary TM may be the main reason for the decrease in muscle fiber density.
The pH value of fish muscle is used as a marker for fish fillet freshness. A high pH will result in high drip loss and is often associated with poor flesh quality (Knight et al., 2019). In other words, there is a positive correlation between pH and water-holding capacity (drip loss and cooking loss). The above viewpoint has also been well verified in our research, in which the pH, drip loss and cooking loss were not influenced by dietary TM. Similar results also obtained in rainbow trout and large yellow croaker fed diets with inclusion of TM (Iaconisi et al., 2018; Zhang et al., 2022). However, the fillet drip loss of large yellow croaker declined with the increase in level of TM substitution of fish meal (Yuan et al., 2022). According to a report from Lucero-Borja et al. (2014), consumers are willing to choose more tender flesh. In general, flesh tenderness is usually expressed as shear force, and there is a negative correlation between tenderness and shear force (Wang et al., 2022). So, the muscle tenderness of grass carp was obviously promoted by dietary TM (Table 8). Wheeler and Koohmaraie (1994) reported shear force decreased with increasing sarcomere length during rigor development of ovine longissimus muscle. Research in livestock also observed that longer sarcomere lengths result in higher tenderness (Wang et al., 2020), which is in agreement with our findings in this study. As the basic contractile unit of muscle, in our study, we suggested that sarcomere length was not only directly related to an increase in tenderness but also closely related to the improvement of muscle textural characteristics of grass carp.
Myogenic regulatory factors (MRFs) and their related regulatory genes play a key role in the regulation of proliferation and differentiation processes that affect fish muscle growth (Yang et al., 2022). Liu et al. (2020) reported that muscular lineage was determined by myf5 and myod and mrf4 and myog play a vital role in myocyte differentiation (Kassar-Duchossoy et al., 2004). In addition, fish muscle proliferation and differentiation were positively correlated with fgf6 and myhc expression, whereas mstn acted negatively effect on fish muscle growth (Megeney and Rudnicki, 1995). A previous study showed that when dietary fish meal was completely replaced by TM (20% TM in diet), the expression of myod of rainbow trout was markedly increased, but there was no difference in the expression of myf5, myog and mrf4 (Chemello et al., 2021). In addition, the muscle expression of mrf4 in large yellow croaker was firstly increased and then declined with a higher level of TM substitution for fish meal, but mstn showed the opposite trend (Yuan et al., 2022). Our study observed that the gene expressions of fgf6, myf5, myog and myhc were up-regulated by the increase in dietary TM and the gene expression of mstn was significantly depressed in YM100. These results have shown that satellite cell activity was elevated and muscle proliferation was increased (Zhang et al., 2022). These could be the internal factors that promoted fiber density, length of sarcomere, adhesiveness, springiness, chewiness and tenderness of grass carp muscle. In addition, previous research has also reported that muscle fiber proliferation was promoted by EPA (Bhullar et al., 2016; Ewaschuk et al., 2014). In this study, muscle fiber proliferation may have contributed to the increase of EPA in muscle. Apart from that, muscle texture is also closely related to protein levels, characteristics and properties (Picard et al., 2012). The increase in crude protein in muscle may also be an important reason for the improvement in muscle textural characteristics of grass carp.
4.5. Muscle metabonomic analysis
To further reveal the internal mechanisms of dietary TM on improving the muscle quality of grass carp, metabolomics was used in this study. We found that the overlapping metabolic pathways of CN vs YM30 and CN vs YM100 involved amino acid metabolism and biosynthesis of unsaturated fatty acids. In addition, we were surprised to find that the nutritional value of grass carp muscle was elevated by increased arachidonate (ARA) and EPA levels in muscle. As an important polyunsaturated fatty acid, EPA plays a crucial role in regulating normal growth and development, immune function, nervous system development and so on (Wang et al., 2020). Previous reviews have also reported that n-3 fatty acids, such as EPA and DHA, act by increasing insulin sensitivity, reducing inflammatory mediators, and promoting proliferation and differentiation, altering adipokine profiles and transcription factors to improve the muscle mass and decrease intramuscular fat (Bhullar et al., 2016; Ewaschuk et al., 2014). The above conclusions were highly similar to the results of metabonomic analysis, muscle approximate composition and muscle quality obtained in this study. Therefore, we speculate that the improvement of muscle quality of grass carp was due to the increase in polyunsaturated fatty acid (EPA) synthesis in muscle promoted by dietary TM. We will further explore the molecular mechanism of improving the muscle quality of grass carp fed diets with complete substitution of soybean meal by TM.
5. Conclusion
This study explored the effects of partial or complete replacement of dietary soybean meal with TM on growth performance, antioxidant capacity, and flesh quality of grass carp. Results showed that yellow mealworm could not only completely replace soybean meal in the diet, but also improve the flesh quality of grass carp. Those positive effects of dietary yellow mealworm on the flesh quality of grass carp may be closely related to promoted muscle EPA synthesis by yellow mealworm.
Author contributions
Handong Li: Methodology, Investigation, Data curation, Writing original draft, Formal analysis; Rongrong Xue: Investigation, Data curation; Jian Sun: Methodology, Writing - review & editing; Hong Ji: Methodology, Resources, Supervision, Project administration, Funding acquisition, Writing - review & editing.
Declaration of competing interest
We declare that we have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. Paper is containing original research and has not been submitted/published earlier in any journal and is not being considered for publication elsewhere. All authors have seen and approved the manuscript and have contributed significantly for the paper.
Acknowledgements
This research was financially supported by National Key R&D Program of China (2019YFD0900200). The authors would like to express their sincere thanks to the personnel of the team for their kind assistance.
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- Alvarado C., Cortez-Valladolid D.M., Herrera-López E.J., Godínez X., Ramírez J.M. Metal bioaccumulation by carp and catfish cultured in lake chapala, and weekly intake assessment. Appl Sci. 2021;11(13):6087. doi: 10.3390/app11136087. [DOI] [Google Scholar]
- AOAC . 17th ed. Association of Official Analytical Chemists; Arlington, VA, USA: 2003. Official methods of analysis of official analytical chemists international. [Google Scholar]
- Barroso F.G., de Haro C., Sánchez-Muros M.J., Venegas E., Martínez-Sánchez A., Pérez-Bañón C. The potential of various insect species for use as food for fish. Aquaculture. 2014;422–423:193–201. doi: 10.1016/j.aquaculture.2013.12.024. [DOI] [Google Scholar]
- Belforti M., Gai F., Lussiana C., Renna M., Malfatto V., Rotolo L., et al. Tenebrio molitor meal in rainbow trout (Oncorhynchus Mykiss) diets: effects on animal performance, nutrient digestibility and chemical composition of fillets. Ital J Anim Sci. 2015;14(4):4170. doi: 10.4081/ijas.2015.4170. [DOI] [Google Scholar]
- Bhullar A.S., Putman C.T., Mazurak V.C. Potential role of omega-3 fatty acids on the myogenic program of satellite cells. Nutr Metab Insights. 2016;9:1–10. doi: 10.4137/NMI.S27481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biancarosa I., Sele V., Belghit I., Ornsrud R., Lock E.J., Amlund H. Replacing fish meal with insect meal in the diet of atlantic salmon (Salmo salar) does not impact the amount of contaminants in the feed and it lowers accumulation of arsenic in the fillet. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2019;36:1191–1205. doi: 10.1080/19440049.2019.1619938. [DOI] [PubMed] [Google Scholar]
- Bordiean A., Krzyżaniak M., Stolarski M.J., Czachorowski S., Peni D. Will yellow mealworm become a source of safe proteins for europe? Agriculture. 2020;10(6):233. doi: 10.3390/agriculture10060233. [DOI] [Google Scholar]
- Brinker A., Reiter R. Fish meal replacement by plant protein substitution and guar gum addition in trout feed, Part I: effects on feed utilization and fish quality. Aquaculture. 2011;310:350–360. doi: 10.1016/j.aquaculture.2010.09.041. [DOI] [Google Scholar]
- Cai L.Y., Li X.P., Wu X.S., Lv Y.F., Liu X.F., Li J.R. Effect of chitosan coating enriched with ergothioneine on quality changes of Japanese sea bass (Lateolabrax japonicas) Food Bioprocess Technol. 2013;7:2281–2290. doi: 10.1007/s11947-013-1215-4. [DOI] [Google Scholar]
- Chemello G., Renna M., Caimi C., Guerreiro I., Oliva-Teles A., Enes P., et al. Partially defatted tenebrio molitor larva meal in diets for grow-out rainbow trout, oncorhynchus mykiss (Walbaum): effects on growth performance, diet digestibility and metabolic responses. Animals. 2020;10(2):229. doi: 10.3390/ani10020229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemello G., Biasato I., Gai F., Capucchio M.T., Colombino E., Schiavone A., Gasco L., Pauciullo A. Effects of tenebrio molitor larvae meal inclusion in rainbow trout feed: myogenesis-related gene expression and histomorphological features. Ital J Anim Sci. 2021;20:1211–1221. doi: 10.1080/1828051X.2021.1945959. [DOI] [Google Scholar]
- Choi I.H., Kim J.M., Kim N.J., Kim J.D., Park C., Park J.H., et al. Replacing fish meal by mealworm (Tenebrio molitor) on the growth performance and immunologic responses of white shrimp (Litopenaeus vannamei) Animal Sciences. 2018;40 doi: 10.4025/actascianimsci.v40i1.39077. [DOI] [Google Scholar]
- D'Souza N., Skonberg D.I., Stone D.A.J., Brown P.B. Effect of soybean meal-based diets on the product quality of rainbow trout fillets. J Food Sci. 2006;71:S337–S342. doi: 10.1111/j.1750-3841.2006.00018.x. [DOI] [Google Scholar]
- da Sousa A.S. Abel Salazar Institute of Biomedical Sciences of the University of Porto; 2020. Impact of Defatted mealworm larvae meal on european seabass (Dicentrarchus labrax) flesh quality. Master Degree Thesis Dissertation. [Google Scholar]
- de Francesco M., Parisi G., Médale F., Lupi P., Kaushik S.J., Poli B.M. Effect of long-term feeding with a plant protein mixture based diet on growth and body/fillet quality traits of large rainbow trout (Oncorhynchus mykiss) Aquaculture. 2004;236:413–429. doi: 10.1016/j.aquaculture.2004.01.006. [DOI] [Google Scholar]
- Ewaschuk J.B., Almasud A., Mazurak V.C. Role of n-3 fatty acids in muscle loss and myosteatosis. Appl Physiol Nutr Metabol. 2014;39:654–662. doi: 10.1139/apnm-2013-0423. [DOI] [PubMed] [Google Scholar]
- Ezaki J., Matsumoto N., Takeda-Ezaki M., Komatsu M., Takahashi K., Hiraoka Y., et al. Liver autophagy contributes to the maintenance of blood glucose and amino acid levels. Autophagy. 2011;7:727–736. doi: 10.4161/auto.7.7.15371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO (Food and Agriculture Organization of the United Nations) The state of world Fisheries and aquaculture 2020. Sustainability in action. Rome. 2020 doi: 10.4060/ca9229en. [DOI] [Google Scholar]
- Feng P.F., He J.Z., Lv M., Huang G.H., Chen X.L., Yang Q., et al. Effect of dietary Tenebrio molitor protein on growth performance and immunological parameters in Macrobrachium rosenbergii. Aquaculture. 2019;511 doi: 10.1016/j.aquaculture.2019.734247. [DOI] [Google Scholar]
- García-Romero J., Ginés R., Izquierdo M.S., Haroun R., Badilla R., Robaina L. Effect of dietary substitution of fish meal for marine crab and echinoderm meals on growth performance, ammonia excretion, skin colour, and flesh quality and oxidation of red porgy (Pagrus pagrus) Aquaculture. 2014;422:239–248. doi: 10.1016/j.aquaculture.2013.11.024. [DOI] [Google Scholar]
- Gasco L., Henry M., Piccolo G., Marono S., Gai F., Renna M., et al. Tenebrio molitor meal in diets for European sea bass (Dicentrarchus labrax L.) juveniles: growth performance, whole body composition and in vivo apparent digestibility. Anim Feed Sci Technol. 2016;220:34–45. doi: 10.1016/j.anifeedsci.2016.07.003. [DOI] [Google Scholar]
- Gasco L., Acuti G., Bani P., Dalle Zotte A., Danieli P.P., De Angelis A., et al. Insect and fish by-products as sustainable alternatives to conventional animal proteins in animal nutrition. Ital J Anim Sci. 2020;19:360–372. doi: 10.1080/1828051X.2020.1743209. [DOI] [Google Scholar]
- Grigorakis K. Compositional and organoleptic quality of farmed and wild gilthead sea bream (Sparus aurata) and sea bass (Dicentrarchus labrax) and factors affecting it: a review. Aquaculture. 2007;272:55–75. doi: 10.1016/j.aquaculture.2007.04.062. [DOI] [Google Scholar]
- Gu J.Z., Liang H.L., Ge X.P., Xia D., Pan L.K., Mi H.F. A study of the potential effect of yellow mealworm (Tenebrio molitor) substitution for fish meal on growth, immune and antioxidant capacity in juvenile largemouth bass (Micropterus salmoides) Fish Shellfish Immunol. 2022;120:214–221. doi: 10.1016/j.fsi.2021.11.024. [DOI] [PubMed] [Google Scholar]
- Henry M., Gasco L., Piccolo G., Fountoulaki E. Review on the use of insects in the diet of farmed fish: past and future. Anim Feed Sci Technol. 2015;203:1–22. doi: 10.1016/j.anifeedsci.2015.03.001. [DOI] [Google Scholar]
- Iaconisi V., Bonelli A., Pupino R., Gai F., Parisi G. Mealworm as dietary protein source for rainbow trout: body and fillet quality traits. Aquaculture. 2018;484:197–204. doi: 10.1016/j.aquaculture.2017.11.034. [DOI] [Google Scholar]
- Iaconisi V., Secci G., Sabatino G., Piccolo G., Gasco L., Papini A.M. Effect of mealworm (Tenebrio molitor L.) larvae meal on amino acid composition of gilthead sea bream (Sparus aurata L.) and rainbow trout (Oncorhynchus mykiss W.) fillets. Aquaculture. 2019;513 doi: 10.1016/j.aquaculture.2019.734403. [DOI] [Google Scholar]
- Iaconisi V., Marono S., Parisi G., Gasco L., Genovese L., Maricchiolo G. Dietary inclusion of Tenebrio molitor larvae meal: effects on growth performance and final quality treats of blackspot sea bream (Pagellus bogaraveo) Aquaculture. 2017;476:49–58. doi: 10.1016/j.aquaculture.2017.04.007. [DOI] [Google Scholar]
- Jeong S.M., Khosravi S., Mauliasari I.R., Lee S.M. Dietary inclusion of mealworm (Tenebrio molitor) meal as an alternative protein source in practical diets for rainbow trout (Oncorhynchus mykiss) fry. Fisheries and Aquatic Sciences. 2020;23(1):1–8. doi: 10.1186/s41240-020-00158-7. [DOI] [Google Scholar]
- Johnston I.A., Alderson R., Sandham C., Dingwall A., Mitchell D., Selkirk C., Springate J. Muscle fibre density in relation to the colour and texture of smoked atlantic salmon (Salmo salar L.) Aquaculture. 2000;189:335–349. doi: 10.1016/S0044-8486(00)00373-2. [DOI] [Google Scholar]
- Kassar-Duchossoy L., Gayraud-Morel B., Gomès D., Rocancourt D., Buckingham M., Shinin V., et al. Mrf4 determines skeletal muscle identity in Myf5: myod double-mutant mice. Nature. 2004;431(7007):466–471. doi: 10.1038/nature02876. [DOI] [PubMed] [Google Scholar]
- Khosravi S., Kim E., Lee Y.S., Lee S.M. Dietary inclusion of mealworm (Tenebrio molitor) meal as an alternative protein source in practical diets for juvenile rockfish (Sebastes schlegeli) Entomol Res. 2018;48:214–221. doi: 10.1111/1748-5967.12306. [DOI] [Google Scholar]
- Khoushab F., Yamabhai M. Chitin research revisited. Mar Drugs. 2010;8:1988–2012. doi: 10.3390/md8071988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight M.I., Linden N., Ponnampalam E.N., Kerr M.G., Brown W.G., Hopkins D.L., et al. Development of VISNIR predictive regression models for ultimate pH, meat tenderness (shear force) and intramuscular fat content of Australian lamb. Meat Sci. 2019;155:102–108. doi: 10.1016/j.meatsci.2019.05.009. [DOI] [PubMed] [Google Scholar]
- Kohen R., Nyska A. Oxidation of biological systems: oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol Pathol. 2002;30:620–650. doi: 10.1080/01926230290166724. [DOI] [PubMed] [Google Scholar]
- Liu F., Ni H.G., Chen F., Luo Z.X., Shen H., Liu L.P., et al. Metal accumulation in the tissues of grass carps (Ctenopharyngodon idellus) from fresh water around a copper mine in Southeast China. Environ Monit Assess. 2011;184:4289–4299. doi: 10.1007/s10661-011-2264-7. [DOI] [PubMed] [Google Scholar]
- Liu J., Deng K., Pan M., Liu G., Wu J., Yang M., Huang D., Zhang W., Mai K. Dietary carbohydrates influence muscle texture of olive flounder paralichthys olivaceus through impacting mitochondria function and metabolism of glycogen and protein. Sci Rep. 2020;10 doi: 10.1038/s41598-020-76255-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lucero-Borja J., Pouzo L.B., de la Torre M.S., Langman L., Carduza F., Corva P.M., et al. Slaughter weight, sex and age effects on beef shear force and tenderness. Livest Sci. 2014;163:140–149. doi: 10.1016/j.livsci.2014.02.003. [DOI] [Google Scholar]
- Lushchak V.I. Environmentally induced oxidative stress in aquatic animals. Aquat Toxicol. 2011;101:13–30. doi: 10.1016/j.aquatox.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Makkar H.P.S., Tran G., Heuzé V., Ankers P. State-of-the-art on use of insects as animal feed. Anim Feed Sci Technol. 2014;197:1–33. doi: 10.1016/j.anifeedsci.2014.07.008. [DOI] [Google Scholar]
- Mastoraki M., Mollá Ferrándiz P., Vardali S.C., Kontodimas D.C., Kotzamanis Y.P., Gasco L., et al. A comparative study on the effect of fish meal substitution with three different insect meals on growth, body composition and metabolism of European sea bass (Dicentrarchus labrax L.) Aquaculture. 2020;528 doi: 10.1016/j.aquaculture.2020.735511. [DOI] [Google Scholar]
- Megeney L.A., Rudnicki M.A. Determination versus differentiation and the MyoD family of transcription factors. Biochem Cell Biol. 1995;73:723–732. doi: 10.1139/o95-080. [DOI] [PubMed] [Google Scholar]
- Ng W.K., Liew F.L., Ang L.P., Wong K.W. Potential of mealworm (Tenebrio molitor) as an alternative protein source in practical diets for African catfish, Clarias gariepinus. Aquacult Res. 2001;32:273–280. doi: 10.1046/j.1355-557x.2001.00024.x. [DOI] [Google Scholar]
- Ni P.J., Jiang W.D., Wu P., Liu Y., Kuang S.Y., Tang L., et al. Dietary low or excess levels of lipids reduced growth performance, and impaired immune function and structure of head kidney, spleen and skin in young grass carp (Ctenopharyngodon idella) under the infection of Aeromonas hydrophila. Fish Shellfish Immunol. 2016;55:28–47. doi: 10.1016/j.fsi.2016.03.163. [DOI] [PubMed] [Google Scholar]
- Nowak V., Persijn D., Rittenschober D., Charrondiere U.R. Review of food composition data for edible insects. Food Chem. 2016;193:39–46. doi: 10.1016/j.foodchem.2014.10.114. [DOI] [PubMed] [Google Scholar]
- Panini R.L., Freitas L.E., Guimarães A.M., Rio C., da Silva M.F., Vieira F.N., et al. Potential use of mealworms as an alternative protein source for Pacific white shrimp: digestibility and performance. Aquaculture. 2017;473:115–120. doi: 10.1016/j.aquaculture.2017.02.008. [DOI] [Google Scholar]
- Periago M.J., Ayala M.D., López-Albors O., Abdel I., Martínez C., García-Alcázar A., et al. Muscle cellularity and flesh quality of wild and farmed sea bass. Dicentrarchus labrax L. Aquaculture. 2005;249:175–188. doi: 10.1016/j.aquaculture.2005.02.047. [DOI] [Google Scholar]
- Picard B., Lefèvre F., Lebret B. Meat and fish flesh quality improvement with proteomic applications. Animal Frontiers. 2012;2:18–25. doi: 10.2527/af.2012-0058. [DOI] [Google Scholar]
- Poma G., Cuykx M., Amato E., Calaprice C., Focant J.F., Covaci A. Evaluation of hazardous chemicals in edible insects and insect-based food intended for human consumption. Food Chem Toxicol. 2017;100:70–79. doi: 10.1016/j.fct.2016.12.006. [DOI] [PubMed] [Google Scholar]
- Rema P., Saravanan S., Armenjon B., Motte C., Dias J. Graded incorporation of defatted yellow mealworm (Tenebrio molitor) in rainbow trout (Oncorhynchus mykiss) diet improves growth performance and nutrient retention. Animals. 2019;9(4):187. doi: 10.3390/ani9040187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy B.C., Ando M., Itoh T., Tsukamasa Y. Structural and ultrastructural changes of full-cycle cultured Pacific bluefin tuna (Thunnus orientalis) muscle slices during chilled storage. J Sci Food Agric. 2012;92:1755–1764. doi: 10.1002/jsfa.5542. [DOI] [PubMed] [Google Scholar]
- Sánchez-Muros M.J., de Haro C., Sanz A., Trenzado C.E., Villareces S., Barroso F.G. Nutritional evaluation of Tenebrio molitormeal as fishmeal substitute for tilapia (Oreochromis niloticus) diet. Aquacult Nutr. 2016;22:943–955. doi: 10.1111/anu.12313. [DOI] [Google Scholar]
- Sankian Z., Khosravi S., Kim Y.O., Lee S.M. Effects of dietary inclusion of yellow mealworm (Tenebrio molitor) meal on growth performance, feed utilization, body composition, plasma biochemical indices, selected immune parameters and antioxidant enzyme activities of Mandarin fish (Siniperca scherzeri) juveniles. Aquaculture. 2018;496:79–87. doi: 10.1016/j.aquaculture.2018.07.012. [DOI] [Google Scholar]
- Shafique L., Abdel-Latif H.M., Hassan F.U., Alagawany M., Naiel M.A., Dawood M.A., et al. The feasibility of using yellow mealworms (Tenebrio molitor): towards a sustainable aquafeed industry. Animals. 2021;11(3):811. doi: 10.3390/ani11030811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S.G., Chi S.Y., Tan B.P., Liang G.L., Lu B.Q., Dong X.H., et al. Effects of fishmeal replacement by Tenebrio molitor meal on growth performance, antioxidant enzyme activities and disease resistance of the juvenile pearl gentian grouper (Epinephelus lanceolatus♂× Epinephelus fuscoguttatus ♀) Aquacult Res. 2018;49:2210–2217. doi: 10.1111/are.13677. [DOI] [Google Scholar]
- Stejskal V., Tran H.Q., Prokesova M., Gebauer T., Giang P.T., Gai F., et al. Partially defatted hermetia illucens larva meal in diet of eurasian perch (Perca fluviatilis) juveniles. Animals. 2020;10(10):1876. doi: 10.3390/ani10101876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teves J.F.C., Ragaza J.A. The quest for indigenous aquafeed ingredients: a review. Rev Aquacult. 2016;8:154–171. doi: 10.1111/raq.12089. [DOI] [Google Scholar]
- Tran H.Q., Kiljunen M., Doan H.V., Stejskal V. European perch (Perca fluviatilis) fed dietary insect meal (Tenebrio molitor): from a stable isotope perspective. Aquaculture. 2021;545 doi: 10.1016/j.aquaculture.2021.737265. [DOI] [Google Scholar]
- Tubin J.S.B., Paian D., de Oliveira Hashimoto G.S., Furtado W.E., Martins M.L., Durigon E., et al. Tenebrio molitor meal in diets for Nile tilapia juveniles reared in biofloc system. Aquaculture. 2020;519 doi: 10.1016/j.aquaculture.2019.734763. [DOI] [Google Scholar]
- Wang C.C., Liu W.B., Huang Y.Y., Wang X., Li X.F., Zhang D.D., et al. Dietary DHA affects muscle fiber development by activating AMPK/Sirt1 pathway in blunt snout bream (Megalobrama amblycephala) Aquaculture. 2020;518 doi: 10.1016/j.aquaculture.2019.734835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T.T., Feng X.C., Li L.Z., Luo J., Liu X.B., Zheng J., et al. Effects of quercetin on tenderness, apoptotic and autophagy signalling in chickens during post-mortem ageing. Food Chem. 2022;383 doi: 10.1016/j.foodchem.2022.132409. [DOI] [PubMed] [Google Scholar]
- Wheeler T.L., Koohmaraie M. Prerigor and postrigor changes in tenderness of ovine longissimus muscle. J Anim Sci. 1994;72:1232–1238. doi: 10.2527/1994.7251232x. [DOI] [PubMed] [Google Scholar]
- Xu X., Ji H., Belghit I., Sun J. Black soldier fly larvae as a better lipid source than yellow mealworm or silkworm oils for juvenile mirror carp (Cyprinus carpio var. specularis) Aquaculture. 2020;527 doi: 10.1016/j.aquaculture.2020.735453. [DOI] [Google Scholar]
- Yang X.Y., Zhi X.Y., Song Z.L., Wang G.H., Zhao X.M., Chi S.Y., et al. Flesh quality of hybrid grouper (Epinephelus fuscoguttatus ♀ × E. lanceolatus ♂) fed with hydrolyzed porcine mucosa-supplemented low fishmeal diet. Anim Nutr. 2022;8:114–124. doi: 10.1016/j.aninu.2021.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D.W., Xu Y.S., Regenstein J.M., Xia W.S., Yang F., Jiang Q.X., et al. The effects of edible chitosan-based coatings on flavor quality of raw grass carp (Ctenopharyngodon idellus) fillets during refrigerated storage. Food Chem. 2018;242:412–420. doi: 10.1016/j.foodchem.2017.09.037. [DOI] [PubMed] [Google Scholar]
- Yuan J., Wu Y., Zhang Z.Y., Tian S.J., Zhou H.H., Zhang W.B., Mai K.S. Replacement of fishmeal by yellow mealworm meal on the growth performance, feed utilisation and quality of large yellow croaker. Journal of Insects as Food and Feed. 2022:1–22. doi: 10.3920/JIFF2021.0144. [DOI] [Google Scholar]
- Zhang L.H., Piao X.S. Different dietary protein sources influence growth performance, antioxidant capacity, immunity, fecal microbiota and metabolites in weaned piglets. Anim Nutr. 2022;8:71–81. doi: 10.1016/j.aninu.2021.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z.Y., Yuan J., Tian S.J., Wu Y., Liu Y., Zhou H.H., et al. Effects of dietary vitamin E supplementation on growth, feed utilization and flesh quality of large yellow croaker Larimichthys crocea fed with different levels of dietary yellow mealworm Tenebrio molitor meal. Aquaculture. 2022;551 doi: 10.1016/j.aquaculture.2022.737954. [DOI] [Google Scholar]
- Zheng L., Feng L., Jiang W.D., Wu P., Tang L., Kuang S.Y., Zeng Y.Y., Zhou X.Q., Liu Y. Selenium deficiency impaired immune function of the immune organs in young grass carp (Ctenopharyngodon idella) Fish Shellfish Immunol. 2018;77:53–70. doi: 10.1016/j.fsi.2018.03.024. [DOI] [PubMed] [Google Scholar]