Summary
Drosophila Toll-9 is most closely related to mammalian Toll-like receptors; however, physiological functions of Toll-9 remain elusive. We examined the roles of Toll-9 in fly brains in aging and neurodegeneration. Toll-9 mRNA levels were increased in aged fly heads accompanied by activation of nuclear factor-kappa B (NF-kB) and stress-activated protein kinase (SAPK) signaling, and many of these changes were modulated by Toll-9 in glial cells. The loss of Toll-9 did not affect lifespan or brain integrity, whereas it exacerbated hydrogen peroxide-induced lethality. Toll-9 expression was also induced by nerve injury but did not affect acute stress response or glial engulfment activity, suggesting Toll-9 may modulate subsequent neurodegeneration. In a fly tauopathy model, Toll-9 deficiency enhanced neurodegeneration and disease-related tau phosphorylation with reduced SAPK activity, and blocking SAPK enhanced tau phosphorylation and neurodegeneration. In sum, Toll-9 is induced upon aging and nerve injury and affects neurodegeneration by modulating stress kinase signaling.
Subject areas: Biological sciences, Neuroscience, Molecular neuroscience
Graphical abstract
Highlights
-
•
Toll-9 is induced in glia upon aging and modulates NF-kB, JNK and SAPK signaling
-
•
Toll-9 is dispensable for normal aging but affects lethality by oxidative stress
-
•
Toll-9 is induced upon acute nerve injury but does not affect glial phagocytosis
-
•
Toll-9 deficiency exacerbates neurodegeneration because of reduced SAPK signaling
Biological sciences; Neuroscience; Molecular neuroscience
Introduction
Host immune responses against infection belong to either innate or adaptive immunity.1,2 Processes underlying adaptive immunity only exist in vertebrates,3,4 whereas innate immunity, a rapid and efficient defense mechanism that detects pathogen-associated molecular patterns through a set of pathogen recognition receptors,5,6 is well-conserved across animal species.7,8 In humans, ten Toll-like receptor (TLR) members (TLR1–10) function as pathogen recognition receptors against various microbial ligands, such as bacterial flagellin, lipopolysaccharide of Gram-negative bacteria, yeast zymosan, double-stranded RNA, and viral CpG-containing DNA.6,9,10,11 In Drosophila, the Toll-1 gene was originally identified for its role in specifying dorsoventral polarity during embryogenesis12,13 and was later found to play essential roles in the production of antimicrobial peptides (AMPs).14
Unlike vertebrate TLRs, the Toll-1 receptor does not directly recognize microbial motifs. Instead, bacterial peptidoglycan is recognized by peptidoglycan recognition receptor SA and triggers a sequence of proteolytic cascade events leading to the cleavage of circulating immature cytokine pro-Spätzle into an active ligand.15,16 Mature Spätzle binds to the Toll-1 receptor and activates the highly conserved nuclear factor-kappa B (NF-kB) signaling cascade17,18 to induce AMPs.19,20,21 In addition to canonical NF-kB signaling, vertebrate TLRs and Drosophila Toll-1 activate noncanonical signaling, such as mitogen-activated protein kinase (MAPK), c-Jun N-terminal kinase (JNK), and p38 stress-activated protein kinase (SAPK) pathways.22,23 For example, Drosophila Toll-1 promotes apoptosis through JNK signaling.24,25
In addition to Toll-1, the Drosophila genome contains eight additional Toll genes: Toll-2–9.6,26 However, many of these Toll receptors do not play roles in immunity but rather in neurodevelopment.27,28,29,30 Among them, Toll-9 is most closely related to mammalian TLRs at a structural level,18,31 and according to the Drosophila RNAi Screening Center 20 (DRSC) Integrative Ortholog Prediction Tool (https://www.flyrnai.org/cgi-bin/DRSC_orthologs.pl), TLR10 shows the highest similarity with fly Toll-9 among ten human TLRs. TLRs are type-I transmembrane receptors composed of an extracellular leucine-rich repeats domain, a single transmembrane domain, and an intracellular region called the Toll-interleukin receptor domain.32,33 Toll-9 is similar to vertebrate TLRs, both in its ectodomain and Toll-interleukin receptor domain. Unlike other Toll receptors, Toll-9 does not have multiple cysteine-rich motifs, which act as autoinhibitory domains unique to Drosophila Toll receptors, in its ectodomain.18,34
Several studies have shown that Toll-9 functions as a constitutively active receptor, and Toll-9 overexpression triggers basal production of AMPs, including drosomycin and cecropin, in cultured cells and transgenic flies.35,36 However, a study with Toll-9 knockout (KO) flies has shown that Toll-9 is dispensable for basal and immune-induced AMP production and for intestinal responses to oral bacterial infections.35 More recently, activation of Toll-9/NF-kB signaling has been shown to induce apoptosis to eliminate unfit cells from developing tissues during cell competition37 and to promote a special form of compensatory proliferation of surviving cells.38 However, physiological functions of Toll-9 in adult flies remain elusive.
A series of recent reports highlight critical roles of Drosophila Toll receptors in central nervous system (CNS) structural plasticity27,28 and cell death.24,25,37 In addition, mammalian TLRs are implicated in age-related neurodegenerative diseases.39,40 In this study, we systematically examined the roles of Toll-9 in the processes of aging and neurodegeneration.
Results
Toll-9 mRNA levels are increased in fly heads during aging and modulate NF-kB, JNK and SAPK signaling
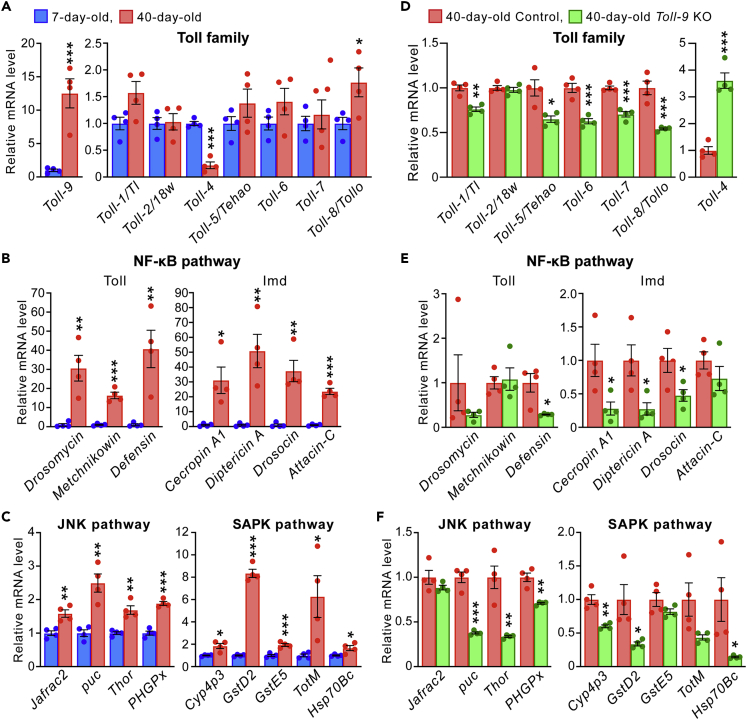
To elucidate the roles of Toll-9 in the CNS during aging, we first examined whether mRNA levels of Toll-9 and other Toll genes were altered upon aging in fly brains. Quantitative PCR (qPCR) analysis revealed that the expression of seven Toll receptors (Toll-1, -2, -4, -5, -6, -7, and -8) was readily detectable in young fly heads. Conversely, Toll-9 expression was very low, and Toll-3/MstProx expression was under the detection limit. By contrast, Toll-9 expression was strongly increased in aged fly heads (Figure 1A). In addition, Toll-8/Tollo expression was increased, whereas Toll-4 expression was decreased during aging (Figure 1A). Toll-1/TL, Toll-5/Tehao, Toll-6, and Toll-7 expression also showed a tendency to be increased; however, these changes did not reach statistical significance (Figure 1A). Next, to investigate how innate immune and stress kinase signaling is altered during aging, we examined the mRNA expression levels of genes downstream of NF-kB, JNK, and SAPK pathways. These genes were significantly upregulated in aged fly heads (Figures 1B and 1C), as reported previously.41,42
Figure 1.
Toll-9 mRNA levels are increased in fly heads during aging and modulate NF-kB, JNK and SAPK signaling
(A–C) The mRNA expression levels of Toll-related genes in young (7-day-old) and aged (40-day-old) fly heads. The mRNA expression level of Toll-9 was elevated in aged fly heads (A), and the genes downstream of NF-kB (B), JNK and SAPK (C) pathways were upregulated in aged fly heads.
(D–F) The mRNA expression levels of Toll-related genes in aged Toll-9 KO fly heads. A loss of Toll-9 affected expressions of Toll family genes (D) as well as the genes downstream of NF-kB (E), JNK and SAPK (F) pathways. The mRNA levels of Toll-related genes in fly heads were analyzed by qRT-PCR. Mean ± SEM, n = 3–4; ∗p< 0.05, ∗∗p< 0.01 and ∗∗∗p< 0.001 by Student’s t test.
As Toll-9 expression was most prominently increased during aging, we examined whether a loss of Toll-9 has any effect on the observed age-dependent changes in gene expression. Toll-9 KO flies were viable and developed normally.35 We first examined the effects of Toll-9 KO on the expression of other Toll genes in aged fly heads. Of interest, the mRNA expression levels of the Tolls that showed age-dependent increases in fly heads (Figure 1A) (Toll-1/TL, Toll-5/Tehao, Toll-6, Toll-7, and Toll-8/Tollo) were lower (Figure 1D), whereas that of Toll-4 (which showed an age-dependent decrease in aged fly heads (Figure 1A)) was higher in Toll-9 KO compared to wild-type (WT) aged fly heads (Figure 1D). These results suggest that Toll-9 affects the expression of many Toll genes, except for Toll-2, in fly heads during aging. Next, we examined whether Toll-9 KO affects genes downstream of NF-kB, JNK, and SAPK signaling. We found that mRNA expression levels of many of these genes, which showed age-dependent increases in fly heads (Figures 1B and 1C), were significantly lower in Toll-9 KO than WT aged fly heads (Figures 1E and 1F). Previous studies reported cross-regulation of the Toll and Imd pathways in innate immune response.43,44 Supporting this, a loss of Toll-9 reduced expression of AMP genes known to activated by the Imd as well as Toll signaling. These results suggest that Toll-9 modulates NF-kB, JNK and SAPK signaling during aging in fly brains.
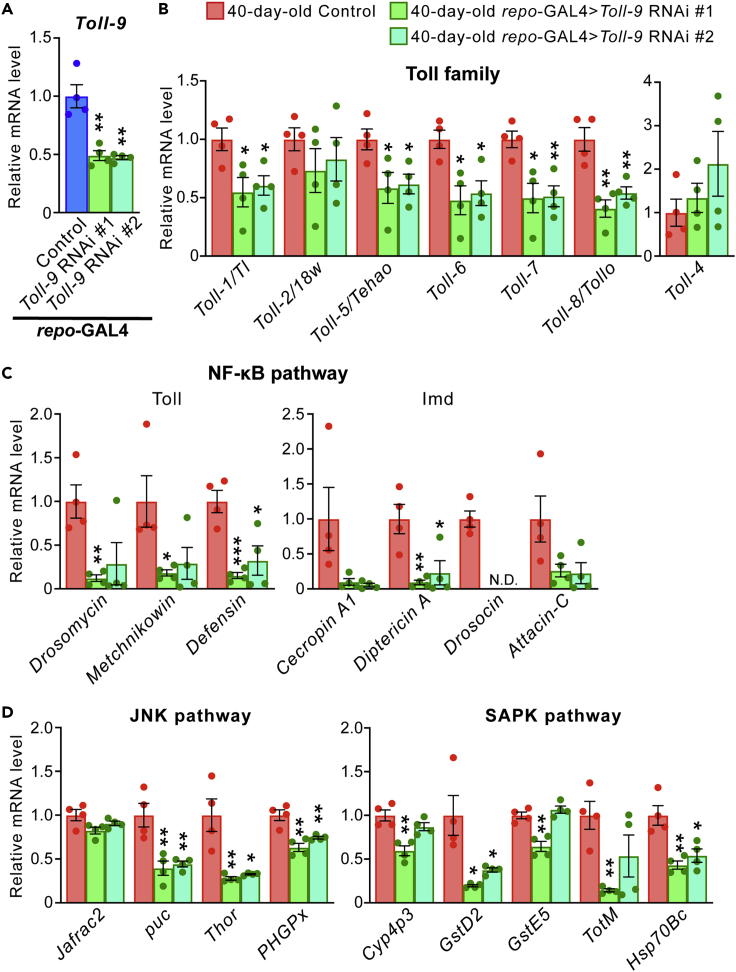
Toll-9 knockdown in glial cells attenuates age-dependent upregulation of Toll family genes and genes downstream of NF-kB, JNK and SAPK signaling
Glial cells play a central role in innate immunity via TLR-mediated signaling in the mammalian CNS.45 We first examined whether Toll-9 is expressed and functions in fly glial cells. In two independent fly lines carrying RNAi transgenes targeting different Toll-9 regions, Toll-9 knockdown in glial cells by the pan-glial repo-GAL4 driver significantly reduced Toll-9 expression (Figure 2A, compare Toll-9 RNAi #1 or Toll-9 RNAi #2 and control mCherry RNAi with same genetic background), confirming both the expression of Toll-9 in glial cells and knockdown efficiency of these RNAi lines. We next investigated whether Toll-9 knockdown in glial cells attenuates age-dependent increases in the expression of Toll genes and genes downstream of NF-kB, JNK and SAPK signaling. qPCR analysis revealed that the expression of many of these genes was significantly lower in fly heads with Toll-9 knockdown than in control aged fly heads (Figures 2B–2D). These results suggest that Toll-9 in glial cells modulates gene expression downstream of NF-kB, JNK and SAPK signaling.
Figure 2.
Toll-9 knockdown in glial cells attenuates age-dependent upregulation of Toll genes and genes downstream of NF-kB, JNK and SAPK signaling
(A) Glial knockdown of Toll-9 significantly decreased the mRNA expression level of Toll-9 in fly head. The mRNA levels of Toll-9 in heads of flies carrying the repo-GAL4 driver (Control) or carrying both the repo-GAL4 driver and UAS-Toll-9 RNAi were analyzed by qRT-PCR. Mean ± SEM, n = 4 (technical replicate); ∗∗p< 0.01 by Student’s t test versus Control.
(B–D) The mRNA expression levels of Toll-related genes in aged (40-day-old) fly heads expressing Toll-9 RNAi in glial cells. Glial knockdown of Toll-9 reduced the expression levels of Toll family genes (B) as well as the genes downstream of NF-kB (C), JNK and SAPK (D) pathways. The mRNA levels of Toll-related genes in fly heads were analyzed by qRT-PCR. Mean ± SEM, n = 3–4; ∗p< 0.05, ∗∗p< 0.01 and ∗∗∗p< 0.001 by Student’s t test versus 40-day-old Control.
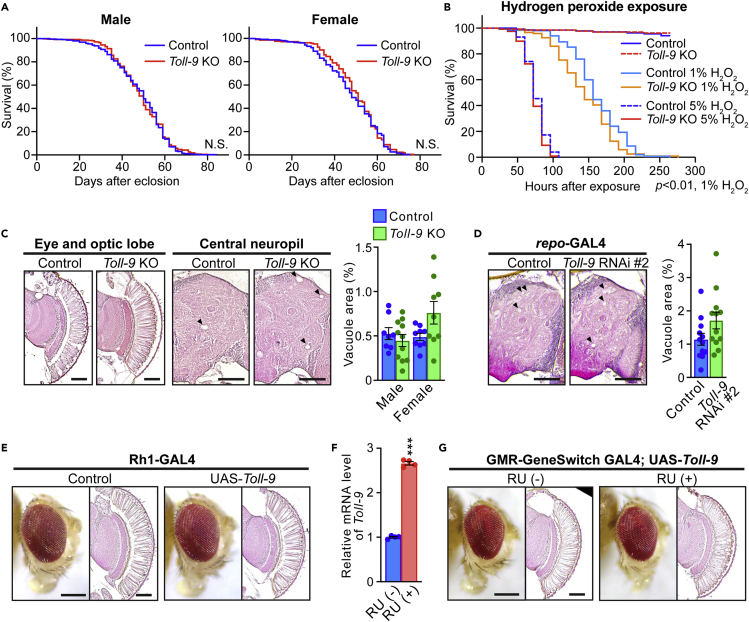
The loss of Toll-9 does not affect lifespan but slightly exacerbates hydrogen peroxide-induced lethality
A previous study reported that Toll-9 was dispensable for antibacterial responses, whereas the loss of Toll-9 shortened the lifespan of flies.35 To minimize genetic background effects,46,47 we outcrossed Toll-9 KO flies to our control w1118 background and examined the effects of Toll-9 deficiency on age-related physiological changes. There were no significant differences between the lifespans of Toll-9 KO and WT flies (Figure 3A, males and females). JNK and SAPK signaling is activated by environmental stressors, and thus we next investigated whether the loss of Toll-9 affects resistance to oxidative stress. Exposure of flies to the oxidative stressor hydrogen peroxide (H2O2) reduced the survival of WT flies (Figure 3B, compare the survival of WT flies under 1% or 5% and Control), and Toll-9 KO slightly worsened this phenotype (Figure 3B, compare the survival of Toll-9 KO and WT flies under 1% H2O2). These results suggest that the loss of Toll-9 by itself does not affect lifespan but slightly exacerbates oxidative stress-induced lethality.
Figure 3.
The loss of Toll-9 does not affect lifespan and brain integrity during normal aging, but slightly exacerbates hydrogen peroxide-induced lethality
(A) A loss of Toll-9 did not alter the lifespan in male (left panel) and female (right panel) flies (male; n = 464, control group or 383, Toll-9 KO group; female; n = 266, control group or 214, Toll-9 KO group).
(B) A loss of Toll-9 significantly reduced survival time under 1% H2O2 exposure compared with that of Control flies (n = 135, Control with 10% sucrose only, n = 131, Toll-9 KO with 10% sucrose only, n = 150, Control with 1% H2O2 exposure, n = 121, Toll-9 KO with 1% H2O2, n = 174, Control with 5% H2O2 exposure, n = 119, Toll-9 KO with 5% H2O). The lifespans or survival times after H2O2 exposure of flies were determined by Kaplan-Meier survival analysis with logrank test. p< 0.01; Control versus Toll-9 KO in 1% H2O2, not significant; Control versus Toll-9 KO in 5% H2O2.
(C–G) Neither Toll-9 loss nor overexpression affects brain integrity during normal aging.
(C) Eyes and central neuropils from paraffin-embedded brain sections with hematoxylin and eosin (HE) staining from 55-day-old control and Toll-9 KO female flies are shown (left panel). Arrowheads indicate vacuoles. A loss of Toll-9 did not cause age-dependent neurodegeneration in the central neuropil (right panel). Percentages of vacuole areas in the central neuropil from 55-day-old male and female flies were analyzed. Mean ± SEM, n = 8–11 hemispheres, not significant by Student’s t test.
(D) RNAi-mediated glial knockdown of Toll-9 did not affect age-dependent neurodegeneration. Central neuropils from 50-day-old male flies carrying the repo-GAL4 driver (Control) or carrying both the repo-GAL4 driver and UAS-Toll-9 RNAi are shown (left panel). Percentages of vacuole areas in the central neuropil from 50-day-old male flies were analyzed (right panel). Mean ± SEM, n = 12 hemispheres, not significant by Student’s t test.
(E) Post-developmental overexpression of Toll-9 by the Rh1-GAL4 driver did not cause retinal degeneration. Eyes of female flies carrying the Rh1-GAL4 driver alone (Control) or carrying both the Rh1-GAL4 driver and UAS-Toll-9 are shown.
(F–G) Overexpression of Toll-9 in adult stage was induced by RU-486 feeding combined with the GeneSwitch GMR-GAL4 driver.
(F) The mRNA expression level of Toll-9 was induced in male fly heads by RU-486 feeding for 30 days. The mRNA levels of Toll-9 in fly heads were analyzed by qRT-PCR. Mean ± SEM, n = 4 (technical replicates); ∗∗∗p< 0.001 by Student’s t test versus RU (−).
(G) Induction of Toll-9 expression in fly eyes during adult stage did not cause retinal degeneration. Eyes of male flies carrying both the GeneSwitch GMR-GAL4 driver and UAS-Toll-9 with vehicle (RU (−)) and RU-486 (RU (+)) feeding are shown. All scale bars represent 200 μm.
Neither Toll-9 loss nor overexpression affects brain integrity during normal aging
To assess whether Toll-9 plays any role in maintaining brain integrity during aging, we first investigated whether Toll-9 KO affects spontaneous neurodegeneration. Neurodegeneration in fly brains is detected as vacuoles in neuronal cell body and neuropil regions.48 The vacuole areas in several brain regions of 55-day-old WT and KO flies exhibited no statistically significant differences, although there was a tendency that neurodegeneration was slightly increased in female Toll-9 KO flies (Figure 3C). We also examined the effects of glial knockdown of Toll-9 on neurodegeneration in aged fly brains and found that there was a tendency that Toll-9 knockdown slightly worsened neurodegeneration, although the difference did not reach statistical significance (Figure 3D). Previous studies have shown that Toll-9 promotes apoptosis during development,37 and that developmental Toll-9 overexpression causes abnormal tissue development and lethality.30 Consistent with these reports, developmental overexpression of Toll-9 in eye imaginal discs by the GMR-GAL4 driver caused glazed eyes due to malformation of the internal structure of the retina (Figure S1). We next examined whether post-developmental induction of Toll-9 induces neurodegeneration in adult fly brains. To avoid hypoplasia and lethality due to developmental Toll-9 overexpression, we first used the Rh1-GAL4 driver to express Toll-9 in differentiated photoreceptor neurons.49,50 Toll-9 induction by Rh1-GAL4 did not cause developmental abnormalities or degeneration of photoreceptor neurons in flies up to 60 days old (Figure 3E). We also utilized the GMR-GAL4 driver combined with the chemically inducible GeneSwitch system to drive Toll-9 induction in the retina in adult flies. Flies carrying UAS-Toll-9 and the GeneSwitch GMR-GAL4 driver developed normally, and Toll-9 expression was induced by feeding adult flies with RU-486 (mifepristone), as measured by qPCR (Figure 3F). Toll-9 induction during the adult stage did not cause retinal degeneration in flies up to 55 days old (Figure 3G). These results suggest that Toll-9 does not affect brain integrity under normal aging conditions in flies.
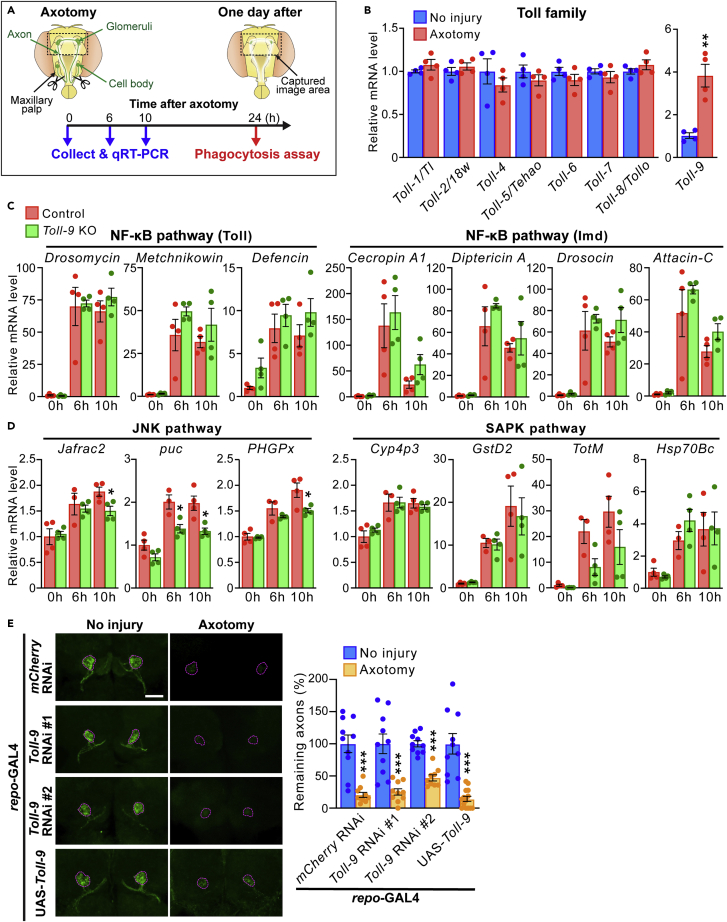
Toll-9 mRNA expression levels are increased in response to nerve injury, but a loss of Toll-9 does not either affect acute stress response or glial engulfment activity
To examine the roles of Toll-9 under neurodegenerative conditions, we investigated whether mRNA levels of Toll-9 and other Toll genes are altered in response to acute neurodegeneration. For this purpose, we utilized a well-established nerve injury model.51 Olfactory receptor neurons in the antennal lobe of the fly brain have their cell bodies at the tips of the maxillary palps and extend long axons into the antennal lobe52 (Figure 4A). Severing the maxillary palps removes the cell bodies, causes axonal degeneration in the antennal lobe, and triggers host defense responses and tissue repair programs, such as the engulfment of cellular debris by surrounding glial cells.51,53 Using this model, we compared mRNA levels of Toll-9 and other Toll-related genes in the fly brain before and 6 h after acute neurodegeneration. Toll-9 expression was specifically and significantly increased after severing the maxillary palps (Figure 4B). We also examined the mRNA levels of genes downstream of NF-kB, JNK, and SAPK signaling in fly brains 6 and 10 h after severing the maxillary palps and found that all these genes were upregulated (Figures 4C and 4D).
Figure 4.
Toll-9 mRNA expression levels are increased in response to acute nerve injury and modulate JNK signaling
(A) Schematic representation of axotomy treatment. Adult flies have a pair of the maxillary palps as an olfactory organ. The olfactory receptor neurons in the antennal lobe of fly brain have their cell bodies at the tip of the maxillary palps and extend long axons into the antennal lobe. Axotomy is induced by severing maxillary palps and 6, 10 or 24 h after axotomy, flies are subjected to qRT-PCR or phagocytosis assay.
(B) The mRNA expression levels of Toll family genes in fly heads at 0 (No injury) or 6 (Axotomy) hours after axotomy. Mean ± SEM, n = 4; ∗∗p< 0.01 by Student’s t test versus No injury.
(C–D) The mRNA expression levels of genes downstream of NF-kB (C), JNK and SAPK (D) pathways in Control and Toll-9 KO fly heads at 0, 6 and 10 h after axotomy. Mean ± SEM, n = 3–4; ∗p< 0.05 by Student’s t test versus Control.
(E) Neither knockdown nor overexpression of Toll-9 in glial cells affects glial engulfment of degenerating neurons in fly brains 24 h after axotomy. Representative z stack projections of OR85e GFP + axons (dotted line) from the groups with No injury or Axotomy of flies carrying UAS-mCherry RNAi, UAS-Toll-9 RNAi #1, UAS-Toll-9 RNAi #2, and UAS-Toll-9 under the repo-GAL4 driver are shown (left panels). Scale bar represents 25 μm. The right graph shows the percentages of GFP fluorescence normalized by the value of fluorescence intensity from the group with No injury. Mean ± SEM, n = 9–14 (mCherry RNAi: No injury n = 10, Axotomy n = 10; Toll-9 RNAi #1: No injury n = 10, Axotomy n = 10; Toll-9 RNAi #2: No injury n = 10, Axotomy n = 9; UAS-Toll-9: No injury n = 10, Axotomy n = 14); ∗∗∗p< 0.001 by Welch’s t-test versus No injury.
Next, we examined whether Toll-9 KO affects the observed gene expression changes after acute nerve injury. We found that although the induction of genes downstream of the JNK pathway was slightly attenuated in Toll-9 KO flies, mRNA levels of many genes downstream of NF-kB, JNK and SAPK signaling were similarly induced in both WT and Toll-9 KO flies (Figures 4C and 4D). These results suggest that a loss of Toll-9 does not affect acute stress response induced by nerve injury in fly brains.
Upon removal of olfactory neuron cell bodies by severing the maxillary palps, degenerating axons in the antennal lobe are efficiently engulfed by surrounding glial cells through activation of draper, a mammalian ortholog of MEGF10/11.51,54 We investigated whether inducing Toll-9 promotes glial clearance of degenerating axons. By labeling a subset of olfactory neurons with GFP, we assessed glial engulfment activity by quantifying fluorescent axonal debris in the antennal lobe 24 h after axotomy. As expected, glial engulfment activity was lost in drpr mutant flies (Figure S2), whereas degenerating axons were removed in flies with glial Toll-9 knockdown (Figure 4E, compare Toll-9 RNAi #1 or Toll-9 RNAi #2 and control mCherry RNAi) and heterozygous Toll-9 KO (Figure S2, compare Toll-9+/− and Control). Moreover, Toll-9 overexpression in glial cells did not affect the removal of GFP-labeled degenerated axons by glial cells (Figure 4E).
Taken together, these results indicate that Toll-9 expression was induced by nerve injury but did not either affect acute stress response or glial engulfment activity, suggesting that induction of Toll-9 may be involved in subsequent neurodegenerative process.
Toll-9 deficiency exacerbates disease-related tau phosphorylation and neurodegeneration accompanied by reduced SAPK activity in a fly tauopathy model
Activated TLR signaling and dysregulated kinases including JNK and SAPK have been implicated in several neurodegenerative diseases.55,56,57 To investigate whether Toll-9 KO affects neurodegeneration, we utilized a transgenic Drosophila that expresses human microtubule-associated protein tau, which is related to tauopathy.58,59 Ectopic expression of human tau under the control of the eye-specific GMR-GAL4 driver causes retinal degeneration, resulting in disorganized ommatidia structures and age-dependent and progressive degeneration of photoreceptor axons in the lamina.60,61
We found that heterozygous Toll-9 KO significantly worsened tau-induced axonal degeneration in the lamina (Figure 5A). Similarly, RNAi-mediated Toll-9 knockdown significantly worsened tau-mediated neurodegeneration, whereas Toll-9 knockdown alone did not affect eye structures (Figure 5B). As tau is hyperphosphorylated in diseased brains, and tau phosphorylation is often associated with toxicity,62 we examined whether Toll-9 deficiency increases phosphorylation levels of tau. Western blot analysis using phosphorylation-specific antibodies revealed that heterozygous Toll-9 KO significantly increased the levels of tau phosphorylated at AD-related AT8 (Ser202/Thr205/Thr208) and Thr217 sites and decreased the levels of non-phosphorylated tau at disease-related sites (Figure 5C). The levels of total tau protein, which are detected as multiple bands due to posttranslational modifications including varying degrees of phosphorylation, were not altered, suggesting that tau phosphorylation levels were increased by heterozygous Toll-9 KO (Figure 5C).
Figure 5.
Toll-9 deficiency exacerbates disease-related tau phosphorylation and neurodegeneration via reduced SAPK activity in a fly model of tauopathy
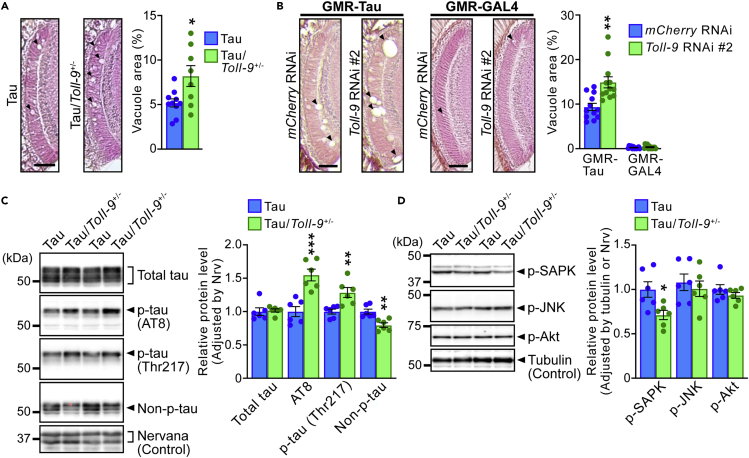
(A) The heterozygous KO of Toll-9 exaggerated axon degeneration in the lamina caused by overexpression of human tau in fly eyes. Hematoxylin and eosin (HE) staining of the paraffin-embedded head section containing the lamina from 7-day-old flies expressing human tau under control background (Tau) and heterozygous KO of Toll-9 (Tau/Toll-9+/−) are shown (left panels). Percentages of vacuole areas in the lamina (indicated by arrowheads in the images) are shown. Mean ± SEM, n = 8–10 hemispheres, ∗p< 0.05 by Student’s t test versus Tau.
(B) RNAi-mediated knockdown of Toll-9 exaggerated axon degeneration in the lamina caused by overexpression of human tau in fly eyes. The lamina of flies expressing human tau and mCherry RNAi (GMR-Tau, mCherry RNAi), flies expressing human tau and Toll-9 RNAi (GMR-Tau, Toll-9 RNAi #2), flies expressing mCherry RNAi alone (GMR-GAL4, mCherry RNAi) or flies expressing Toll-9 RNAi alone (GMR-GAL4, Toll-9 RNAi #2) are shown (left panel). Percentages of vacuole areas in the lamina are shown in right panel. Mean ± SEM, n = 12 hemispheres, ∗p< 0.05 by Student’s t test versus mCherry RNAi.
(C) Heterozygous KO of Toll-9 increased the levels of tau phosphorylated at AD-related AT8 (Ser202/Thr205/Thr208) and at Thr217 sites. In contrast, the level of non-phosphorylated tau was decreased in heterozygous KO of Toll-9.
(D) Heterozygous KO of Toll-9 significantly reduced the level of phosphorylated SAPK. Fly heads from Tau and Tau/Toll-9+/− were subjected to western blotting with anti-tau, anti-phospho tau, anti-non-phospho tau, anti-phospho SAPK (p38), anti-phospho JNK and anti-phospho Akt antibodies. Nervana, a fly ortholog of ATPase Na+/K+ transporting subunit β1, or tubulin was used as the loading control. For (C) and (D), mean ± SEM, n = 6, ∗∗p< 0.01 by Student’s t test versus Tau. Scale bars represent 100 μm.
In Drosophila, a major kinase responsible for tau phosphorylation is Shaggy, a homologue of mammalian glycogen synthase kinase (GSK) 3α/β.62,63 The activity of GSK3α/β is negatively regulated by Akt-mediated phosphorylation in vertebrates64 as well as in Drosophila,65,66 and activation of Toll signaling antagonizes insulin signaling in the fat body via Akt inhibition.67 In mammals, Akt is activated by phosphorylation at Ser473.68 We examined whether heterozygous Toll-9 KO reduced phosphorylation of Drosophila Akt at Ser505,68,69 equivalent to Ser473 of mammalian Akt, in flies expressing human tau. Western blot analysis revealed that Akt phosphorylation levels were not affected by heterozygous Toll-9 KO (Figure 5D).
As JNK and SAPK are also reported to be involved in tau phosphorylation and toxicity,70,71 we next examined whether Toll-9 deficiency alters JNK and/or SAPK activity in flies expressing tau. Western blot analysis revealed that heterozygous Toll-9 KO significantly reduced levels of phosphorylated SAPK, but not JNK (Figure 5D), suggesting that reduced SAPK activity may affect tau phosphorylation and toxicity.
Blocking SAPK function exacerbates tau phosphorylation and neurotoxicity
Previous studies have shown that SAPK plays both protective and detrimental roles in neurodegenerative processes.72,73 As our results suggest that reduced activity of fly SAPKs is associated with exacerbated neurodegeneration (Figure 5), we examined whether blocking SAPK function enhances tau phosphorylation and/or neurodegeneration in a fly tauopathy model.
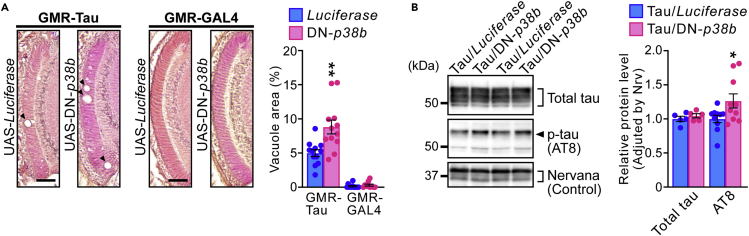
The Drosophila genome contains three SAPK-related genes (p38a, p38b, and p38c74,75), and p38b is most ubiquitously expressed in fly tissues, including the CNS, and is an ortholog of human MAPK14/p38α and MAPK11/p38β.72,74 To assess whether reduced SAPK activity exacerbates neurodegeneration, we examined the effects of inactivation of fly SAPK on tau phosphorylation and tau-mediated neurodegeneration. By utilizing a transgenic line carrying the dominant negative form of p38b (DN-p38b),76 which was generated by replacing Thr183 of the MAPKK target site with Ala, we co-expressed DN-p38b or control luciferase protein with human tau in the retina under the control of the GMR-GAL4 driver. qPCR confirmed significant increases in DN-p38b mRNA levels in fly heads (Figure S3). Flies co-expressing DN-p38b exhibited significantly enhanced tau-induced axon degeneration in the lamina compared with flies co-expressing luciferase, whereas expression of DN-p38b alone did not cause neurodegeneration (Figure 6A). In addition, western blot analysis revealed that DN-p38b co-expression significantly increased the levels of tau phosphorylated at the AD-related AT8 (Ser202/Thr205/Thr208) site (Figure 6B). The total tau protein level was not altered, suggesting that tau phosphorylation levels were increased by DN-p38b (Figure 6B). These results indicate that blocking SAPK function enhances tau phosphorylation and neurodegeneration in a Drosophila model of tauopathy, and suggest that Toll-9 deficiency exacerbates tau-mediated neurodegeneration at least in part due to reduced SAPK signaling.
Figure 6.
Blocking SAPK function by using dominant negative form of p38b exacerbates phosphorylation and neurotoxicity of tau
(A) The lamina of flies expressing human tau and control luciferase protein (GMR-Tau, UAS-Luciferase) or expressing human tau and dominant negative form of p38b (GMR-Tau, UAS-DN-p38b), flies expressing Luciferase alone (GMR-GAL4, UAS-Luciferase) or flies expressing DN-p38b alone (GMR-GAL4, UAS-DN-p38b) are shown (left panel). Percentages of vacuole areas in the lamina are shown in right panel. Mean ± SEM, n = 10–12 hemispheres, ∗∗p< 0.01 by Student’s t test versus Luciferase.
(B) Overexpression of DN-p38b significantly increased the levels of tau phosphorylated at AD-related AT8 site. Fly heads expressing Tau and control luciferase protein (Tau/Luciferase) or expressing Tau and DN-p38b (Tau/DN-p38b) were subjected to western blotting. Mean ± SEM, n = 5–10, ∗p< 0.01 by Student’s t test versus Tau/Luciferase. Scale bars represent 100 μm.
Discussion
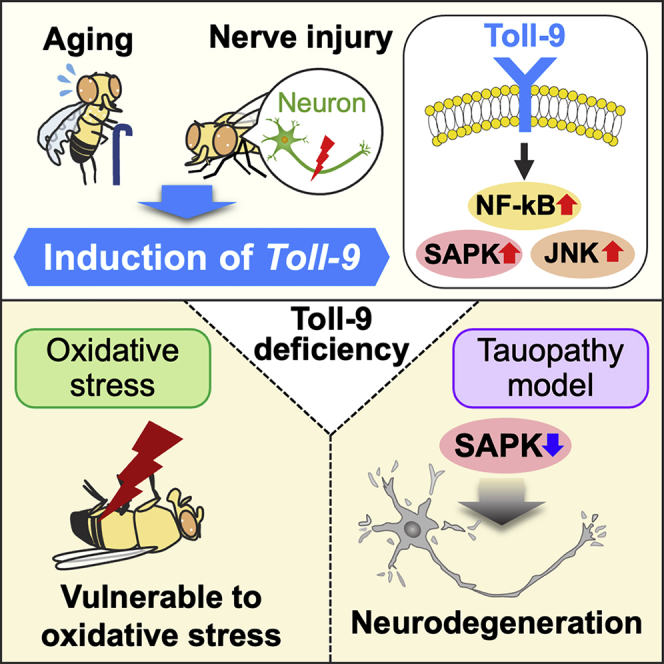
Innate immune responses and anti-stress signaling are altered during aging and in neurodegenerative diseases. TLRs are evolutionarily conserved across animal species and play a central role in host defense mechanisms by activating the NF-kB and MAPK pathways.9,11 In Drosophila, Toll-9 is most closely related to mammalian TLRs at a structural level18,31; however, the physiological functions of Toll-9 in adult flies remain elusive. In this study, we systematically examined the roles of Toll-9 in the CNS during aging and under neurodegenerative conditions. Our results suggest that Toll-9 is involved in neurodegenerative process by modulating stress kinase signaling in adult Drosophila brains.
Altered innate immunity is a general hallmark of aging and conserved across species.77,78,79 In humans, innate immunity is up- or down-regulated, and TLR signaling is generally activated due to neuroinflammation in aged brains.80 AMP gene expression levels downstream of NF-kB signaling are upregulated in aged Drosophila brains.41,42 Consistent with these reports, genes downstream of the Toll pathway, including those involved in NF-kB, JNK, and SAPK signaling, were increased in aged fly heads (Figures 1B and 1C). Among nine Toll receptors, Toll-9 expression was most prominently increased in aged fly heads, and many genes related to NF-kB, JNK and SAPK pathways were attenuated in aged flies with Toll-9 KO and glial Toll-9 knockdown (Figures 1D–1F and 2B–2D). Our data suggest that Toll-9 is induced as one of the downstream genes of innate immune and/or anti-stress signaling in aged fly brains to modulate activity of NF-kB, JNK and SAPK pathways. Of interest, human TLR10 is the best ortholog of the Drosophila Toll-9 gene, and recent reports have shown that TLR10 modulates signaling of other TLRs.81,82
A previous study reported that the loss of Toll-9 shortened the lifespan of flies, suggesting that Toll-9 plays a protective role during aging.35 In this study, we did not find significant differences in lifespan between WT and Toll-9 KO flies (Figure 3A). These inconsistent results may be because of different genetic backgrounds of the flies used in the studies: Narbonne-Reveau et al. used yw flies as a control background,35 whereas we outcrossed Toll-9 KO flies to our control w1118 background. In addition, differences in experimental conditions and environmental factors might affect the lifespan of flies. In support of this, H2O2-induced lethality was slightly exacerbated in Toll-9 KO flies (Figure 3B), suggesting that Toll-9 plays a protective role against oxidative stress.
This study also revealed that Toll-9 expression was induced in the model of acute nerve injury.51,54 In Drosophila, degenerated axons were removed by glial cells through activation of draper, a mammalian ortholog of MEGF10/11.51,54 More recently, Toll-6/dSarm signaling in glial cells was shown to promote draper-mediated phagocytic clearance of apoptotic neurons during development.83 By contrast, our data suggest that Toll-9 does not affect glial phagocytotic activity (Figures 4E and S2). In response to nerve injury, Toll-9 as well as genes downstream of NF-kB, JNK and SAPK signaling were acutely (6 and 10 h after nerve injury) induced in fly heads (Figures 4C and 4D). However, in contrast to the context of aging (Figures 1E and 1F), Toll-9 KO did not affect the induction of genes downstream of NF-kB, JNK and SAPK signaling induced by nerve injury (Figures 4C and 4D). These results suggest that basal, low expression level of Toll-9 does not play a role in acute activation of NF-kB and stress kinase signaling upon neurodegeneration. Rather, Toll-9 modulates these signaling pathways only when it is induced under stressed condition, which may explain why Toll-9 lacks an autoinhibitory domain and acts as constitutive active receptor.36
Accumulating evidence indicates that TLR signaling is actively involved in the pathogenesis of many neurodegenerative diseases, including Alzheimer’s disease (AD) and tauopathy.84,85 Several studies reported that neuroinflammation due to microglial activation exacerbates tau pathology.86,87,88,89 Induction of intra-cerebral or systemic inflammation by lipopolysaccharide, a powerful pro-inflammatory component, promotes microglial activation through TLR4, which exacerbates hyperphosphorylated tau aggregation in both rTg4510 and 3xTg-AD mouse models.90,91 In addition, TLR3 activation by double-stranded RNA increases disease-related tau phosphorylation in cultured human neuronal cells.92 By contrast, activation of TLR signaling also plays neuroprotective roles against tau pathology: chronic and mild stimulation of TLR4 by lipopolysaccharide reduces tau phosphorylation in P301S-Tg mice,93 and stimulation of TLR9 signaling with CpG oligodeoxynucleotides reduces tau pathology in 3xTg-AD mice.94 To investigate the potential role of Toll-9 in neurodegeneration, we utilized a fly tauopathy model that expresses human tau proteins,95 and found that Toll-9 loss significantly exacerbated disease-related tau phosphorylation and neurodegeneration (Figure 5). Our results contribute another line of evidence for the neuroprotective role of Toll-related signaling in tauopathy-related neurodegeneration.
As a mechanism by whichToll-9 deficiency exacerbates neurodegeneration, we demonstrated the involvement of impaired activation of SAPK signaling (Figures 5 and 6). Several studies have indicated potential links between p38 MAPK/SAPK, tau phosphorylation, and neurodegeneration.72 p38 MAPK/SAPK can directly phosphorylate tau in vitro,96 and a neuropathological study on AD patient brains has shown that p38α MAPK/SAPK colocalizes with tau,97 suggesting that p38 MAPK/SAPK may be related to tau pathology. Studies using mouse tauopathy models, including hTau and rTg4510 mice, have reported that inhibition of p38α MAPK/SAPK with selective inhibitors reduces tau pathology and cognitive deficits by suppressing inflammation.98,99 By contrast, recent studies reported that activation of p38γ MAPK/SAPK in neurons ameliorated cognitive deficits by reducing tau toxicity in mouse AD models.100,101
Our results demonstrated that Toll-9 deficiency significantly enhanced tau phosphorylation and toxicity (Figures 5A–5C) accompanied by reduced p38 MAPK/SAPK activity (Figure 5D). Moreover, inactivation of p38 MAPK/SAPK exacerbated disease-related tau phosphorylation and neurodegeneration (Figure 6), suggesting that Toll-9 deficiency promotes tau-mediated neurodegeneration due to impaired activation of p38 MAPK/SAPK signaling. However, the mechanisms by which p38 MAPK/SAPK inactivation enhances tau phosphorylation and toxicity have yet to be addressed. Tau phosphorylation at many disease-related sites is mediated by GSK3β,102,103 and a recent report revealed that p38 MAPK/SAPK can directly phosphorylate and inactivate GSK3β.104 In addition, activation of p38 MAPK/SAPK signaling upregulates the expression of several anti-stress genes, which prevent oxidative stress and protein misfolding. Impairment of these signaling may also contribute to the enhancement of tau phosphorylation and toxicity. Further study is required to unravel neuroprotective mechanisms of p38 MAPK/SAPK.
In conclusion, this study demonstrates that Toll-9 is induced upon aging and neurodegeneration to modulate stress signaling, and Toll-9 deficiency exacerbates neurodegenerative process in a fly model of tauopathy.
Limitations of the study
Although this study demonstrates that Toll-9 is induced to modulate NF-kB and stress kinase signaling in aged fly heads and suggests a potential role of Toll-9 in aging, a single knockout of Toll-9 gene does not show prominent effects on either lifespan or brain integrity during normal aging. This may be due to compensatory functions of other Toll family genes. Among them, Toll-4 is of particular interest, because mRNA expression level of Toll-4 is significantly upregulated in Toll-9 KO flies (Figure 1D). To address this possibility, generation and analysis of Toll-4 single KO and Toll-4/Toll-9 double KO flies will be important. In addition, roles of SAPK signaling in tau-mediated neurodegeneration need to be validated in mammalian systems in future study.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Mouse monoclonal anti-tau antibody (clone Tau12) | Merck Millipore | Cat# MAB2241; RRID: AB_11211810 |
| Mouse monoclonal anti-tau-1 antibody (clone PC1C6) | Merck Millipore | Cat# MAB3420; RRID: AB_94855 |
| Mouse monoclonal anti-phospho tau (Ser202/Thr205/Thr208) antibody (clone AT8) | Thermo Fisher Scientific | Cat# MN1020; RRID: AB_223647 |
| Rabbit polyclonal anti-phospho tau (Thr217) antibody | Thermo Fisher Scientific | Cat# 44–744; RRID: AB_2533741 |
| Rabbit polyclonal anti-phospho p38 MAPK (Thr180/Tyr182) antibody | Cell Signaling Technology | Cat# 9211S; RRID: AB_331641 |
| Rabbit monoclonal anti-phospho JNK (Thr183/Tyr185) antibody | Cell Signaling Technology | Cat# 4668S; RRID: AB_823588 |
| Rabbit polyclonal anti-phospho Drosophila Akt (Ser505) antibody | Cell Signaling Technology | Cat# 4054S; RRID: AB_331414 |
| Mouse monoclonal anti-Drosophila nervana protein antibody | Developmental Studies Hybridoma Bank | Cat# Nrv5F7; RRID: AB_528395 |
| Mouse monoclonal anti-α-tubulin antibody | Sigma-Aldrich | Cat# T9026; RRID: AB_477593 |
| Chicken polyclonal anti-GFP antibody | Aves Labs | Cat# GFP-1020; RRID: AB_10000240 |
| Alexa Fluor 488 AffiniPure Goat Anti-Chicken IgY (IgG) (H + L) | Jackson ImmunoResearch | Cat# 103-545-155; RRID: AB_2337390 |
| Chemicals, peptides, and recombinant proteins | ||
| Hydrogen peroxide | Fujifilm Wako Chemicals | Cat# 081-04215 |
| Sucrose | Fujifilm Wako Chemicals | Cat# 196-00015 |
| Mifepristone | Tokyo Chemical Industry | Cat# M1732 |
| Critical commercial assays | ||
| ECL Prime Western Blotting Detection Reagents | GE Healthcare | Cat# RPN2236 |
| Hematoxylin Solution, Mayer’s | Sigma-Aldrich | Cat# MHS16 |
| Eosin Y solution | Sigma-Aldrich | Cat# HT110132 |
| TRIzol Reagent | Thermo Fisher Scientific | Cat# 15596018 |
| PrimeScript RT reagent Kit with gDNA Eraser | TaKaRa Bio | Cat# RR047A |
| Thunderbird SYBR qPCR Mix | Toyobo | Cat# QPS-201 |
| SlowFade Gold | Invitrogen | Cat# S36936 |
| Experimental models: Organisms/strains | ||
| D. melanogaster: UAS-human tau | Sekiya et al., 201795 | N/A |
| D. melanogaster: Canton-S | Bloomington Drosophila Stock Center | BDSC: 64349; FlyBase: FBst0064349 |
| D. melanogaster: Toll-9 KO | Narbonne-Reveau et al., 201135 | N/A |
| D. melanogaster: UAS-Toll-9 | Yagi et al., 201030 | N/A |
| D. melanogaster: y1w67c23 | Yagi et al., 201030 | N/A |
| D. melanogaster: Or85e-mCD8::GFP | MacDonald et al., 200651 | N/A |
| D. melanogaster: repo-GAL4 (X) | Lai et al., 2006105 | N/A |
| D. melanogaster: drprΔ5 | Awasaki et al., 2006106 | N/A |
| D. melanogaster: GMR-GAL4 | Bloomington Drosophila Stock Center | BDSC: 1104; FlyBase: FBst0001104 |
| D. melanogaster: Rh1-GAL4 | Bloomington Drosophila Stock Center | BDSC: 8688; FlyBase: FBst0008688 |
| D. melanogaster: GeneSwitch GMR-GAL4 | Bloomington Drosophila Stock Center | BDSC: 6759; FlyBase: FBst0006759 |
| D. melanogaster: repo-GAL4 (III) | Bloomington Drosophila Stock Center | BDSC: 7415; FlyBase: FBst0007415 |
| D. melanogaster: UAS-mCherry RNAi | Bloomington Drosophila Stock Center | BDSC: 35785; FlyBase: FBst0035785 |
| D. melanogaster: UAS-Toll-9 RNAi #1 | Bloomington Drosophila Stock Center | BDSC: 30535; FlyBase: FBst0030535 |
| D. melanogaster: UAS-Toll-9 RNAi #2 | Bloomington Drosophila Stock Center | BDSC: 34853; FlyBase: FBst0034853 |
| D. melanogaster: UAS-DN-p38b | Bloomington Drosophila Stock Center | BDSC: 59005; FlyBase: FBst0059005 |
| D. melanogaster: attP2 | Bloomington Drosophila Stock Center | BDSC: 36303; FlyBase: FBst0036303 |
| D. melanogaster: UAS-Luciferase | This paper | N/A |
| Oligonucleotides | ||
| qRT-PCR primers for D. melanogaster, see Table in STAR Methods | This paper | N/A |
| Software and algorithms | ||
| Prism 9 | GraphPad | https://www.GraphPad.com |
| Excel | Microsoft | https://www.microsoft.com |
| ImageJ | NIH | https://imagej.nih.gov/ij/ |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by Koichi M. Iijima (iijimakm@ncgg.go.jp).
Materials availability
The materials underlying this article will be shared upon request to the lead contact.
Experimental model and subject details
Drosophila genetics
Flies were maintained in standard cornmeal media at 25°C. Transgenic fly line carrying UAS-human tau was previously described.95 Canton-S line is a kind gift from Dr. K. Akagi (Toyama University). Toll-9 KO fly is a kind gift from Dr. J. Royet (Aix-Marseille Université).35 UAS-Toll-9 and the background strain y1w67c23 line (used as a control for UAS-Toll-9) are kind gifts from Dr. Y. Yagi (Nagoya University).30 Or85e-mCD8::GFP fly is a kind gift from Dr. M.R. Freeman (Oregon Health & Science University).51 Repo-GAL4 (X) driver line and drprΔ5mutant fly are kind gifts from Dr. T. Awasaki (Kyorin University).105,106 The GMR-GAL4 (#1104), Rh1-GAL4 (#8688), GeneSwitch GMR-GAL4 (#6759), repo-GAL4 (III) (#7415), UAS-mCherry RNAi (#35785), UAS-Toll-9 RNAi #1 (#30535), UAS-Toll-9 RNAi #2 (#34853), UAS-DN-p38b (#59005) and attP2 background line (#36303) were obtained from the Bloomington Stock Center. The UAS-Luciferase transgenic flies were generated by PhiC31 integrase-mediated transgenesis systems (Best Gene Inc.). Experiments were performed using age-matched male or female flies. Genotypes and ages of all flies used in this study are described in Table S1.
Method details
Histological analysis
Preparation of paraffin sections, hematoxylin and eosin staining, and analysis of neurodegeneration were performed as described previously.60,61,107 Heads of male or female flies were fixed in 4% paraformaldehyde for 24h at 4°C and embedded in paraffin. Serial sections (6 μm thickness) through the entire heads were prepared, stained with hematoxylin and eosin (Sigma-Aldrich), and examined by bright-field microscopy. Images of the sections were captured with AxioCam 105 color (Carl Zeiss). For quantification, we focused on analyzing sections covering the central neuropil or lamina regions. We selected a section with the most severe neurodegeneration in each individual fly and the area of vacuoles was measured using ImageJ (NIH). Images of the external eye structures were captured with AxioCam 208 color (Carl Zeiss).
Lifespan analysis
Lifespan analysis was performed as described previously.60 Food vials containing 25 male and female flies were placed on their sides at 25°C under conditions of 70% humidity and a 12:12-h light:dark cycle. Food vials were changed every 2–3 days, and the number of dead flies was counted each time. At least four vials for each genotype were prepared.
Hydrogen peroxide exposure assays
As described previously,60 flies were starved with 2% agar medium for 2 h before hydrogen peroxide (H2O2) treatment. Two round and one rectangle filter papers were placed in empty vial, and 750 μL of 1% and 5% H2O2 (Fujifilm Wako Chemicals) in 10% sucrose (Fujifilm Wako Chemicals) solution was added to the filter papers. The dead flies were scored every 12 h. At least 119 male flies (7-day-old) per genotype were used to perform this experiment. For control experiment, at least 131 male flies (7-day-old) per genotype were administrated with 750 μL of 10% sucrose solution.
RU feeding
As described previously,95 flies carrying GeneSwitch GMR-GAL4 driver and UAS-Toll-9 were fed the food containing 500 μM mifepristone (Tokyo Chemical Industry) or vehicle (ethanol; final concentration 1%) from the day after eclosion. These food vials were changed every 3–4 days. The male flies were collected at 30 days and 55 days after feeding for qPCR and histological analysis, respectively.
Western blotting
Western blotting was performed as described previously.60,61 More than ten fly heads for each genotype were homogenized in Tris-Glycine SDS sample buffer, and the same amount of the lysate was loaded to each lane of 10% Tris-Glycine gels and transferred to nitrocellulose membrane. The membranes were blocked with 5% nonfat dry milk, blotted with the antibodies described below, incubated with appropriate secondary antibody, and developed using ECL Prime Western Blotting Detection Reagent (Cytiva). The membranes were also probed with anti-nervana or anti-α-tubulin and used as the loading control for other blots in each experiment. Mouse monoclonal anti-tau (clone Tau12) (Merck Millipore, Cat#. MAB2241), mouse monoclonal anti-tau-1 (clone PC1C6) (Merk Millipore, Cat#. MAB3420) used for detection of non-phospho tau, mouse monoclonal anti-phospho tau (Ser202/Thr205/Thr208) (clone AT8) (Thermo Fisher Scientific, Cat#. MN1020), rabbit polyclonal anti-phospho tau (Thr217) (Thermo Fisher Scientific, Cat#. 44–744), rabbit polyclonal anti-phospho p38 MAPK (Thr180/Tyr182) (Cell Signaling Technology, Cat#. 9211S), rabbit monoclonal anti-phospho JNK (Thr183/Tyr185) (Cell Signaling Technology, Cat#. 4668S), rabbit polyclonal anti-phospho Drosophila Akt (Ser505) (Cell Signaling Technology, Cat#. 4054S), mouse monoclonal anti-Drosophila nervana protein (Developmental Studies Hybridoma Bank, Cat#. Nrv5F7) and mouse monoclonal anti-α-tubulin (Sigma-Aldrich, Cat#. T9026) antibodies were purchased. Imaging was performed with ImageQuant LAS 4000 (GE Healthcare Life Sciences), and the signal intensity was quantified using ImageJ (NIH).
RNA extraction and quantitative real time PCR analysis
Quantitative real time PCR analysis was performed as described previously.60,61 More than 25 flies for each genotype were collected and frozen. Heads were mechanically isolated, and total RNA was extracted using TRIzol Reagent (Thermo Fisher Scientific) according to the manufacturer’s protocol with an additional centrifugation step (16,000 × g for 10 min) to remove cuticle membranes prior to the addition of chloroform. Total RNA was reverse-transcribed using PrimeScript RT-PCR kit (TaKaRa Bio), and quantitative RT-PCR was performed using Thunderbird SYBR qPCR Mix (Toyobo) on a CFX96 real time PCR detection system (Bio-Rad Laboratories). The average threshold cycle value was calculated from at least three replicates per sample. Expression of genes of interest was standardized relative to Gapdh1. Primer sequences used in this study were described in Table S2.
Axotomy treatment
Axotomy was induced by maxillary palp ablations as previously described.51,52 Maxillary nerves were severed by removing maxillary palps with forceps under CO2 anesthesia. Flies were allowed to recover in fresh food vial and maintained at 25°C. Six or 10 h after axotomy, flies were frozen for qRT-PCR analysis.
Phagocytosis assay
One day after axotomy treatment, flies were subjected to whole mount immunostaining as described previously.107 Briefly, fly brains were dissected in phosphate-buffered saline (PBS), and then fixed in 4% paraformaldehyde in PBS for 50 min. Brains were washed with 0.5% TritonX-100/PBS and blocked in 10% normal goat serum in 1% TritonX-100/PBS for 10 min. Chicken anti-GFP antibody (1:500; Aves Labs, Cat#. GFP-1020) was added and incubate for overnight at 4 °C. The next day, the brains were washed three times with 0.5% TritonX-100/PBS and incubated with anti-chicken Alexa488 secondary antibody (1:1,000; Jackson ImmunoResearch, Cat#. 103-545-155) for 2 h at room temperature. Brains were washed three times with 0.5% TritonX-100/PBS and rinsed with PBS, and then mounted on glass coverslip with antifade mounting media (SlowFadeTM Gold, Invitrogen).
Images were acquired using either a LSM780 or LSM800 confocal laser-scanning microscope (Carl Zeiss) fitted with 20× objective. For all experiments, the entire antennal lobe was imaged in 1 μm steps. Severed and non-severed brains from the same genotype were imaged on the same day with the same confocal microscope settings. Quantification of fluorescence intensity was performed on single z sections in each side of the relevant glomerulus using ImageJ (NIH). For representative images in Figures 4E and S2, z stack confocal images were reconstructed with a maximum intensity projection.
Quantification and statistical analysis
Statistics
All results were expressed as mean ± SEM. Unpaired Student’s t test (Excel, Microsoft) was used to determine statistical significance as indicated in the figure legends. Kaplan-Meier survival analyses with log-rank tests (GraphPad Prism 9, GraphPad Software) were used to determine statistical significance for lifespan analysis. Welch’s t test (GraphPad Prism 9, GraphPad Software) was used to determine statistical significance for phagocytosis assay. ∗ indicates p< 0.05, ∗∗ indicates p< 0.01 and ∗∗∗ indicates p< 0.001 throughout the manuscript.
Acknowledgments
We thank Dr. T. Aigaki at Tokyo Metropolitan University, Dr. K. Akagi at Toyama University, Dr. T. Awasaki at Kyorin University, Dr. M.R. Freeman at Oregon Health & Science University, Dr. J. Royet at Aix-Marseille Université, Dr. Y. Yagi at Nagoya University, and TRiP at Harvard Medical School (NIH/NIGMSR01-GM084947) and the Bloomington stock center for fly stocks. This study was supported by the Research Funding for Longevity Science from National Center for Geriatrics and Gerontology, Japan, Grant No. 21-13 to K.M.I. and M.S., No. 19-49 to M.S. and JSPS KAKENHI Grant No. JP16K08637, JP20K20672, JP20H03571, JP22K19763 to K.M.I., No. JP22H02963 to M.S. and No. JP20J15563 to R.Y.
Author contributions
Conceptualization, Y.S., R.Y., M.S., and K.M.I.; Investigation, Y.S., R.Y., S.C., Y.H., Y.T., R.N., K.T., and M.S.; Writing (original draft), Y.S., R.Y., M.S., and K.M.I.; Writing (review and editing), Y.S., R.Y., M.S., and K.M.I. and all authors; Supervision, M.S. and K.M.I.; Funding acquisition, R.Y., M.S., and K.M.I.
Declaration of interests
The authors declare no competing interests.
Published: February 17, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2023.105968.
Contributor Information
Michiko Sekiya, Email: mmsk@ncgg.go.jp.
Koichi M. Iijima, Email: iijimakm@ncgg.go.jp.
Supplemental information
Data and code availability
This study did not generate original code. All data produced in this study are included in this article and supplementary information. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- 1.Cooper D., Eleftherianos I. Memory and specificity in the insect immune system: current perspectives and future challenges. Front. Immunol. 2017;8:539. doi: 10.3389/fimmu.2017.00539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iwasaki A., Medzhitov R. Control of adaptive immunity by the innate immune system. Nat. Immunol. 2015;16:343–353. doi: 10.1038/ni.3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agaisse H. An adaptive immune response in Drosophila? Cell Host Microbe. 2007;1:91–93. doi: 10.1016/j.chom.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 4.Litman G.W., Rast J.P., Fugmann S.D. The origins of vertebrate adaptive immunity. Nat. Rev. Immunol. 2010;10:543–553. doi: 10.1038/nri2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin S.J.H., Cohen L.B., Wasserman S.A. Effector specificity and function in Drosophila innate immunity: getting AMPed and dropping Boms. PLoS Pathog. 2020;16:e1008480. doi: 10.1371/journal.ppat.1008480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lindsay S.A., Wasserman S.A. Conventional and non-conventional Drosophila Toll signaling. Dev. Comp. Immunol. 2014;42:16–24. doi: 10.1016/j.dci.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoffmann J.A., Kafatos F.C., Janeway C.A., Ezekowitz R.A. Phylogenetic perspectives in innate immunity. Science. 1999;284:1313–1318. doi: 10.1126/science.284.5418.1313. [DOI] [PubMed] [Google Scholar]
- 8.Hoffmann J.A., Reichhart J.M. Drosophila innate immunity: an evolutionary perspective. Nat. Immunol. 2002;3:121–126. doi: 10.1038/ni0202-121. [DOI] [PubMed] [Google Scholar]
- 9.Iwasaki A., Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 2004;5:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 10.Kawai T., Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 11.Kawasaki T., Kawai T. Toll-like receptor signaling pathways. Front. Immunol. 2014;5:461. doi: 10.3389/fimmu.2014.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anderson K.V., Bokla L., Nüsslein-Volhard C. Establishment of dorsal-ventral polarity in the Drosophila embryo: the induction of polarity by the Toll gene product. Cell. 1985;42:791–798. doi: 10.1016/0092-8674(85)90275-2. [DOI] [PubMed] [Google Scholar]
- 13.Lemaitre B., Nicolas E., Michaut L., Reichhart J.M., Hoffmann J.A. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996;86:973–983. doi: 10.1016/s0092-8674(00)80172-5. [DOI] [PubMed] [Google Scholar]
- 14.Bettencourt R., Tanji T., Yagi Y., Ip Y.T. Toll and Toll-9 in Drosophila innate immune response. J. Endotoxin Res. 2004;10:261–268. doi: 10.1179/096805104225004897. [DOI] [PubMed] [Google Scholar]
- 15.Buchon N., Poidevin M., Kwon H.M., Guillou A., Sottas V., Lee B.L., Lemaitre B. A single modular serine protease integrates signals from pattern-recognition receptors upstream of the Drosophila Toll pathway. Proc. Natl. Acad. Sci. USA. 2009;106:12442–12447. doi: 10.1073/pnas.0901924106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Michel T., Reichhart J.M., Hoffmann J.A., Royet J. Drosophila Toll is activated by Gram-positive bacteria through a circulating peptidoglycan recognition protein. Nature. 2001;414:756–759. doi: 10.1038/414756a. [DOI] [PubMed] [Google Scholar]
- 17.Belvin M.P., Anderson K.V. A conserved signaling pathway: the Drosophila toll-dorsal pathway. Annu. Rev. Cell Dev. Biol. 1996;12:393–416. doi: 10.1146/annurev.cellbio.12.1.393. [DOI] [PubMed] [Google Scholar]
- 18.Imler J.L., Zheng L. Biology of Toll receptors: lessons from insects and mammals. J. Leukoc. Biol. 2004;75:18–26. doi: 10.1189/jlb.0403160. [DOI] [PubMed] [Google Scholar]
- 19.Gangloff M., Murali A., Xiong J., Arnot C.J., Weber A.N., Sandercock A.M., Robinson C.V., Sarisky R., Holzenburg A., Kao C., Gay N.J. Structural insight into the mechanism of activation of the Toll receptor by the dimeric ligand Spatzle. J. Biol. Chem. 2008;283:14629–14635. doi: 10.1074/jbc.M800112200. [DOI] [PubMed] [Google Scholar]
- 20.Moncrieffe M.C., Grossmann J.G., Gay N.J. Assembly of oligomeric death domain complexes during Toll receptor signaling. J. Biol. Chem. 2008;283:33447–33454. doi: 10.1074/jbc.M805427200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weber A.N.R., Tauszig-Delamasure S., Hoffmann J.A., Lelièvre E., Gascan H., Ray K.P., Morse M.A., Imler J.L., Gay N.J. Binding of the Drosophila cytokine Spatzle to Toll is direct and establishes signaling. Nat. Immunol. 2003;4:794–800. doi: 10.1038/ni955. [DOI] [PubMed] [Google Scholar]
- 22.Duan T., Du Y., Xing C., Wang H.Y., Wang R.F. Toll-like receptor signaling and its role in cell-mediated immunity. Front. Immunol. 2022;13:812774. doi: 10.3389/fimmu.2022.812774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kleino A., Valanne S., Ulvila J., Kallio J., Myllymäki H., Enwald H., Stöven S., Poidevin M., Ueda R., Hultmark D., et al. Inhibitor of apoptosis 2 and TAK1-binding protein are components of the Drosophila Imd pathway. EMBO J. 2005;24:3423–3434. doi: 10.1038/sj.emboj.7600807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Z., Wu C., Ding X., Li W., Xue L. Toll signaling promotes JNK-dependent apoptosis in Drosophila. Cell Div. 2020;15:7. doi: 10.1186/s13008-020-00062-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu C., Chen C., Dai J., Zhang F., Chen Y., Li W., Pastor-Pareja J.C., Xue L. Toll pathway modulates TNF-induced JNK-dependent cell death in Drosophila. Open Biol. 2015;5:140171. doi: 10.1098/rsob.140171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tauszig S., Jouanguy E., Hoffmann J.A., Imler J.L. Toll-related receptors and the control of antimicrobial peptide expression in Drosophila. Proc. Natl. Acad. Sci. USA. 2000;97:10520–10525. doi: 10.1073/pnas.180130797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li G., Forero M.G., Wentzell J.S., Durmus I., Wolf R., Anthoney N.C., Parker M., Jiang R., Hasenauer J., Strausfeld N.J., et al. A Toll-receptor map underlies structural brain plasticity. Elife. 2020;9:e52743. doi: 10.7554/eLife.52743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li G., Hidalgo A. The toll route to structural brain plasticity. Front. Physiol. 2021;12:679766. doi: 10.3389/fphys.2021.679766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McIlroy G., Foldi I., Aurikko J., Wentzell J.S., Lim M.A., Fenton J.C., Gay N.J., Hidalgo A. Toll-6 and Toll-7 function as neurotrophin receptors in the Drosophila melanogaster CNS. Nat. Neurosci. 2013;16:1248–1256. doi: 10.1038/nn.3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yagi Y., Nishida Y., Ip Y.T. Functional analysis of Toll-related genes in Drosophila. Dev. Growth Differ. 2010;52:771–783. doi: 10.1111/j.1440-169X.2010.01213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Valanne S., Wang J.H., Rämet M. The Drosophila Toll signaling pathway. J. Immunol. 2011;186:649–656. doi: 10.4049/jimmunol.1002302. [DOI] [PubMed] [Google Scholar]
- 32.Hashimoto C., Hudson K.L., Anderson K.V. The Toll gene of Drosophila, required for dorsal-ventral embryonic polarity, appears to encode a transmembrane protein. Cell. 1988;52:269–279. doi: 10.1016/0092-8674(88)90516-8. [DOI] [PubMed] [Google Scholar]
- 33.Schneider D.S., Hudson K.L., Lin T.Y., Anderson K.V. Dominant and recessive mutations define functional domains of Toll, a transmembrane protein required for dorsal-ventral polarity in the Drosophila embryo. Genes Dev. 1991;5:797–807. doi: 10.1101/gad.5.5.797. [DOI] [PubMed] [Google Scholar]
- 34.Bilak H., Tauszig-Delamasure S., Imler J.L. Toll and toll-like receptors in Drosophila. Biochem. Soc. Trans. 2003;31:648–651. doi: 10.1042/bst0310648. [DOI] [PubMed] [Google Scholar]
- 35.Narbonne-Reveau K., Charroux B., Royet J. Lack of an antibacterial response defect in Drosophila Toll-9 mutant. PLoS One. 2011;6:e17470. doi: 10.1371/journal.pone.0017470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ooi J.Y., Yagi Y., Hu X., Ip Y.T. The Drosophila Toll-9 activates a constitutive antimicrobial defense. EMBO Rep. 2002;3:82–87. doi: 10.1093/embo-reports/kvf004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meyer S.N., Amoyel M., Bergantiños C., de la Cova C., Schertel C., Basler K., Johnston L.A. An ancient defense system eliminates unfit cells from developing tissues during cell competition. Science. 2014;346:1258236. doi: 10.1126/science.1258236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shields A., Amcheslavsky A., Brown E., Lee T.V., Nie Y., Tanji T., Ip Y.T., Bergmann A. Toll-9 interacts with Toll-1 to mediate a feedback loop during apoptosis-induced proliferation in Drosophila. Cell Rep. 2022;39:110817. doi: 10.1016/j.celrep.2022.110817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arroyo D.S., Soria J.A., Gaviglio E.A., Rodriguez-Galan M.C., Iribarren P. Toll-like receptors are key players in neurodegeneration. Int. Immunopharmacol. 2011;11:1415–1421. doi: 10.1016/j.intimp.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Okun E., Griffioen K.J., Lathia J.D., Tang S.C., Mattson M.P., Arumugam T.V. Toll-like receptors in neurodegeneration. Brain Res. Rev. 2009;59:278–292. doi: 10.1016/j.brainresrev.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Girardot F., Lasbleiz C., Monnier V., Tricoire H. Specific age related signatures in Drosophila body parts transcriptome. BMC Genomics. 2006;7:69. doi: 10.1186/1471-2164-7-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kounatidis I., Chtarbanova S., Cao Y., Hayne M., Jayanth D., Ganetzky B., Ligoxygakis P. NF-κB immunity in the brain determines fly lifespan in healthy aging and age-related neurodegeneration. Cell Rep. 2017;19:836–848. doi: 10.1016/j.celrep.2017.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Akhouayri I., Turc C., Royet J., Charroux B. Toll-8/Tollo negatively regulates antimicrobial response in the Drosophila respiratory epithelium. PLoS Pathog. 2011;7:e1002319. doi: 10.1371/journal.ppat.1002319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tanji T., Hu X., Weber A.N.R., Ip Y.T. Toll and IMD pathways synergistically activate an innate immune response in Drosophila melanogaster. Mol. Cell Biol. 2007;27:4578–4588. doi: 10.1128/MCB.01814-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carpentier P.A., Duncan D.S., Miller S.D. Glial toll-like receptor signaling in central nervous system infection and autoimmunity. Brain Behav. Immun. 2008;22:140–147. doi: 10.1016/j.bbi.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.He Y., Jasper H. Studying aging in Drosophila. Methods. 2014;68:129–133. doi: 10.1016/j.ymeth.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spencer C.C., Howell C.E., Wright A.R., Promislow D.E.L. Testing an 'aging gene' in long-lived drosophila strains: increased longevity depends on sex and genetic background. Aging Cell. 2003;2:123–130. doi: 10.1046/j.1474-9728.2003.00044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kretzschmar D., Hasan G., Sharma S., Heisenberg M., Benzer S. The swiss cheese mutant causes glial hyperwrapping and brain degeneration in Drosophila. J. Neurosci. 1997;17:7425–7432. doi: 10.1523/JNEUROSCI.17-19-07425.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Escobedo S.E., Shah A., Easton A.N., Hall H., Weake V.M. Characterizing a gene expression toolkit for eye- and photoreceptor-specific expression in Drosophila. Fly. 2021;15:73–88. doi: 10.1080/19336934.2021.1915683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mollereau B., Wernet M.F., Beaufils P., Killian D., Pichaud F., Kühnlein R., Desplan C. A green fluorescent protein enhancer trap screen in Drosophila photoreceptor cells. Mech. Dev. 2000;93:151–160. doi: 10.1016/s0925-4773(00)00287-2. [DOI] [PubMed] [Google Scholar]
- 51.MacDonald J.M., Beach M.G., Porpiglia E., Sheehan A.E., Watts R.J., Freeman M.R. The Drosophila cell corpse engulfment receptor Draper mediates glial clearance of severed axons. Neuron. 2006;50:869–881. doi: 10.1016/j.neuron.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 52.Vosshall L.B., Wong A.M., Axel R. An olfactory sensory map in the fly brain. Cell. 2000;102:147–159. doi: 10.1016/s0092-8674(00)00021-0. [DOI] [PubMed] [Google Scholar]
- 53.Doherty J., Logan M.A., Taşdemir O.E., Freeman M.R. Ensheathing glia function as phagocytes in the adult Drosophila brain. J. Neurosci. 2009;29:4768–4781. doi: 10.1523/JNEUROSCI.5951-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Freeman M.R., Freeman M.R. The scoop on the fly brain: glial engulfment functions in Drosophila. Neuron Glia Biol. 2007;3:63–74. doi: 10.1017/S1740925X07000646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kheiri G., Dolatshahi M., Rahmani F., Rezaei N. Role of p38/MAPKs in Alzheimer's disease: implications for amyloid beta toxicity targeted therapy. Rev. Neurosci. 2018;30:9–30. doi: 10.1515/revneuro-2018-0008. [DOI] [PubMed] [Google Scholar]
- 56.Momtazmanesh S., Perry G., Rezaei N. Toll-like receptors in Alzheimer's disease. J. Neuroimmunol. 2020;348:577362. doi: 10.1016/j.jneuroim.2020.577362. [DOI] [PubMed] [Google Scholar]
- 57.Yarza R., Vela S., Solas M., Ramirez M.J. c-Jun N-terminal kinase (JNK) signaling as a therapeutic target for Alzheimer's disease. Front. Pharmacol. 2015;6:321. doi: 10.3389/fphar.2015.00321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee V.M.Y., Kenyon T.K., Trojanowski J.Q. Transgenic animal models of tauopathies. Biochim. Biophys. Acta. 2005;1739:251–259. doi: 10.1016/j.bbadis.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 59.Wittmann C.W., Wszolek M.F., Shulman J.M., Salvaterra P.M., Lewis J., Hutton M., Feany M.B. Tauopathy in Drosophila: neurodegeneration without neurofibrillary tangles. Science. 2001;293:711–714. doi: 10.1126/science.1062382. [DOI] [PubMed] [Google Scholar]
- 60.Sakakibara Y., Sekiya M., Fujisaki N., Quan X., Iijima K.M. Knockdown of wfs1, a fly homolog of Wolfram syndrome 1, in the nervous system increases susceptibility to age- and stress-induced neuronal dysfunction and degeneration in Drosophila. PLoS Genet. 2018;14:e1007196. doi: 10.1371/journal.pgen.1007196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sekiya M., Wang M., Fujisaki N., Sakakibara Y., Quan X., Ehrlich M.E., De Jager P.L., Bennett D.A., Schadt E.E., Gandy S., et al. Integrated biology approach reveals molecular and pathological interactions among Alzheimer's Abeta42, Tau, TREM2, and TYROBP in Drosophila models. Genome Med. 2018;10:26. doi: 10.1186/s13073-018-0530-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chatterjee S., Sang T.K., Lawless G.M., Jackson G.R. Dissociation of tau toxicity and phosphorylation: role of GSK-3 beta, MARK and Cdk5 in a Drosophila model. Hum. Mol. Genet. 2009;18:164–177. doi: 10.1093/hmg/ddn326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jackson G.R., Wiedau-Pazos M., Sang T.K., Wagle N., Brown C.A., Massachi S., Geschwind D.H. Human wild-type tau interacts with wingless pathway components and produces neurofibrillary pathology in Drosophila. Neuron. 2002;34:509–519. doi: 10.1016/S0896-6273(02)00706-7. [DOI] [PubMed] [Google Scholar]
- 64.Cross D.A., Alessi D.R., Cohen P., Andjelkovich M., Hemmings B.A. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 65.Dionne M.S., Pham L.N., Shirasu-Hiza M., Schneider D.S. Akt and FOXO dysregulation contribute to infection-induced wasting in Drosophila. Curr. Biol. 2006;16:1977–1985. doi: 10.1016/j.cub.2006.08.052. [DOI] [PubMed] [Google Scholar]
- 66.van Eyk C.L., O'Keefe L.V., Lawlor K.T., Samaraweera S.E., McLeod C.J., Price G.R., Venter D.J., Richards R.I. Perturbation of the Akt/Gsk3-beta signalling pathway is common to Drosophila expressing expanded untranslated CAG, CUG and AUUCU repeat RNAs. Hum. Mol. Genet. 2011;20:2783–2794. doi: 10.1093/hmg/ddr177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.DiAngelo J.R., Bland M.L., Bambina S., Cherry S., Birnbaum M.J. The immune response attenuates growth and nutrient storage in Drosophila by reducing insulin signaling. Proc. Natl. Acad. Sci. USA. 2009;106:20853–20858. doi: 10.1073/pnas.0906749106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sarbassov D.D., Guertin D.A., Ali S.M., Sabatini D.M. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 69.Yang Q., Inoki K., Kim E., Guan K.L. TSC1/TSC2 and Rheb have different effects on TORC1 and TORC2 activity. Proc. Natl. Acad. Sci. USA. 2006;103:6811–6816. doi: 10.1073/pnas.0602282103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Atzori C., Ghetti B., Piva R., Srinivasan A.N., Zolo P., Delisle M.B., Mirra S.S., Migheli A. Activation of the JNK/p38 pathway occurs in diseases characterized by tau protein pathology and is related to tau phosphorylation but not to apoptosis. J. Neuropathol. Exp. Neurol. 2001;60:1190–1197. doi: 10.1093/jnen/60.12.1190. [DOI] [PubMed] [Google Scholar]
- 71.Ploia C., Antoniou X., Sclip A., Grande V., Cardinetti D., Colombo A., Canu N., Benussi L., Ghidoni R., Forloni G., Borsello T. JNK plays a key role in tau hyperphosphorylation in Alzheimer's disease models. J. Alzheimers Dis. 2011;26:315–329. doi: 10.3233/JAD-2011-110320. [DOI] [PubMed] [Google Scholar]
- 72.Asih P.R., Prikas E., Stefanoska K., Tan A.R.P., Ahel H.I., Ittner A. Functions of p38 MAP kinases in the central nervous system. Front. Mol. Neurosci. 2020;13:570586. doi: 10.3389/fnmol.2020.570586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim E.K., Choi E.J. Pathological roles of MAPK signaling pathways in human diseases. Biochim. Biophys. Acta. 2010;1802:396–405. doi: 10.1016/j.bbadis.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 74.Chen J., Xie C., Tian L., Hong L., Wu X., Han J. Participation of the p38 pathway in Drosophila host defense against pathogenic bacteria and fungi. Proc. Natl. Acad. Sci. USA. 2010;107:20774–20779. doi: 10.1073/pnas.1009223107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Han Z.S., Enslen H., Hu X., Meng X., Wu I.H., Barrett T., Davis R.J., Ip Y.T. A conserved p38 mitogen-activated protein kinase pathway regulates Drosophila immunity gene expression. Mol. Cell Biol. 1998;18:3527–3539. doi: 10.1128/MCB.18.6.3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Adachi-Yamada T., Nakamura M., Irie K., Tomoyasu Y., Sano Y., Mori E., Goto S., Ueno N., Nishida Y., Matsumoto K. p38 mitogen-activated protein kinase can be involved in transforming growth factor beta superfamily signal transduction in Drosophila wing morphogenesis. Mol. Cell Biol. 1999;19:2322–2329. doi: 10.1128/MCB.19.3.2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.DeVeale B., Brummel T., Seroude L. Immunity and aging: the enemy within? Aging Cell. 2004;3:195–208. doi: 10.1111/j.1474-9728.2004.00106.x. [DOI] [PubMed] [Google Scholar]
- 78.Muller L., Fulop T., Pawelec G. Immunosenescence in vertebrates and invertebrates. Immun. Ageing. 2013;10:12. doi: 10.1186/1742-4933-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang R., Chen H.Z., Liu D.P. The four layers of aging. Cell Syst. 2015;1:180–186. doi: 10.1016/j.cels.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 80.Shaw A.C., Goldstein D.R., Montgomery R.R. Age-dependent dysregulation of innate immunity. Nat. Rev. Immunol. 2013;13:875–887. doi: 10.1038/nri3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jiang S., Li X., Hess N.J., Guan Y., Tapping R.I. TLR10 is a negative regulator of both MyD88-dependent and -independent TLR signaling. J. Immunol. 2016;196:3834–3841. doi: 10.4049/jimmunol.1502599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Oosting M., Cheng S.C., Bolscher J.M., Vestering-Stenger R., Plantinga T.S., Verschueren I.C., Arts P., Garritsen A., van Eenennaam H., Sturm P., et al. Human TLR10 is an anti-inflammatory pattern-recognition receptor. Proc. Natl. Acad. Sci. USA. 2014;111:E4478–E4484. doi: 10.1073/pnas.1410293111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.McLaughlin C.N., Perry-Richardson J.J., Coutinho-Budd J.C., Broihier H.T. Dying neurons utilize innate immune signaling to Prime glia for phagocytosis during development. Dev. Cell. 2019;48:506–522.e6. doi: 10.1016/j.devcel.2018.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ennerfelt H.E., Lukens J.R. The role of innate immunity in Alzheimer's disease. Immunol. Rev. 2020;297:225–246. doi: 10.1111/imr.12896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hammond T.R., Marsh S.E., Stevens B. Immune signaling in neurodegeneration. Immunity. 2019;50:955–974. doi: 10.1016/j.immuni.2019.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bhaskar K., Konerth M., Kokiko-Cochran O.N., Cardona A., Ransohoff R.M., Lamb B.T. Regulation of tau pathology by the microglial fractalkine receptor. Neuron. 2010;68:19–31. doi: 10.1016/j.neuron.2010.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kitazawa M., Cheng D., Tsukamoto M.R., Koike M.A., Wes P.D., Vasilevko V., Cribbs D.H., LaFerla F.M. Blocking IL-1 signaling rescues cognition, attenuates tau pathology, and restores neuronal beta-catenin pathway function in an Alzheimer's disease model. J. Immunol. 2011;187:6539–6549. doi: 10.4049/jimmunol.1100620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kitazawa M., Oddo S., Yamasaki T.R., Green K.N., LaFerla F.M. Lipopolysaccharide-induced inflammation exacerbates tau pathology by a cyclin-dependent kinase 5-mediated pathway in a transgenic model of Alzheimer's disease. J. Neurosci. 2005;25:8843–8853. doi: 10.1523/Jneurosci.2868-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yoshiyama Y., Higuchi M., Zhang B., Huang S.M., Iwata N., Saido T.C., Maeda J., Suhara T., Trojanowski J.Q., Lee V.M.Y. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron. 2007;53:337–351. doi: 10.1016/j.neuron.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 90.Kitazawa M., Yamasaki T.R., LaFerla F.M. Microglia as a potential bridge between the amyloid beta-peptide and tau. Ann. N. Y. Acad. Sci. 2004;1035:85–103. doi: 10.1196/annals.1332.006. [DOI] [PubMed] [Google Scholar]
- 91.Lee D.C., Rizer J., Selenica M.L., Reid P., Kraft C., Johnson A., Anderson L., Gordon M.N., Dickey C.A., Morgan D. LPS-induced inflammation exacerbates phospho-tau pathology in rTg4510 mice. Cell Transplant. 2010;19:347. doi: 10.1186/1742-2094-7-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nessa B.N., Tanaka T., Kamino K., Sadik G., Ansar A.B., Kimura R., Tanii H., Okochi M., Morihara T., Tagami S., et al. Toll-like receptor 3 mediated hyperphosphorylation of tau in human SH-SY5Y neuroblastoma cells. Psychiatry Clin. Neurosci. 2006;60:S27–S33. doi: 10.1111/j.1440-1819.2006.01526.x-i1. [DOI] [Google Scholar]
- 93.Qin Y., Liu Y., Hao W., Decker Y., Tomic I., Menger M.D., Liu C., Fassbender K. Stimulation of TLR4 attenuates Alzheimer's disease-related symptoms and pathology in tau-transgenic mice. J. Immunol. 2016;197:3281–3292. doi: 10.4049/jimmunol.1600873. [DOI] [PubMed] [Google Scholar]
- 94.Scholtzova H., Chianchiano P., Pan J., Sun Y., Goñi F., Mehta P.D., Wisniewski T. Amyloid beta and Tau Alzheimer's disease related pathology is reduced by Toll -like receptor 9 stimulation. Acta Neuropathol. Commun. 2014;2:101. doi: 10.1186/s40478-014-0101-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sekiya M., Maruko-Otake A., Hearn S., Sakakibara Y., Fujisaki N., Suzuki E., Ando K., Iijima K.M. EDEM function in ERAD protects against chronic ER proteinopathy and age-related physiological decline in Drosophila. Dev. Cell. 2017;41:652–664.e5. doi: 10.1016/j.devcel.2017.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Reynolds C.H., Nebreda A.R., Gibb G.M., Utton M.A., Anderton B.H. Reactivating kinase/p38 phosphorylates tau protein in vitro. J. Neurochem. 1997;69:191–198. doi: 10.1046/j.1471-4159.1997.69010191.x. [DOI] [PubMed] [Google Scholar]
- 97.Zhu X., Rottkamp C.A., Boux H., Takeda A., Perry G., Smith M.A. Activation of p38 kinase links tau phosphorylation, oxidative stress, and cell cycle-related events in Alzheimer disease. J. Neuropathol. Exp. Neurol. 2000;59:880–888. doi: 10.1093/jnen/59.10.880. [DOI] [PubMed] [Google Scholar]
- 98.Maphis N., Jiang S., Xu G., Kokiko-Cochran O.N., Roy S.M., Van Eldik L.J., Watterson D.M., Lamb B.T., Bhaskar K. Selective suppression of the alpha isoform of p38 MAPK rescues late-stage tau pathology. Alzheimer's Res. Ther. 2016;8:54. doi: 10.1186/s13195-016-0221-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Roy S.M., Minasov G., Arancio O., Chico L.W., Van Eldik L.J., Anderson W.F., Pelletier J.C., Watterson D.M. A selective and brain penetrant p38alphaMAPK inhibitor candidate for neurologic and neuropsychiatric disorders that attenuates neuroinflammation and cognitive dysfunction. J. Med. Chem. 2019;62:5298–5311. doi: 10.1021/acs.jmedchem.9b00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ittner A., Asih P.R., Tan A.R.P., Prikas E., Bertz J., Stefanoska K., Lin Y., Volkerling A.M., Ke Y.D., Delerue F., Ittner L.M. Reduction of advanced tau-mediated memory deficits by the MAP kinase p38gamma. Acta Neuropathol. 2020;140:279–294. doi: 10.1007/s00401-020-02191-1. [DOI] [PubMed] [Google Scholar]
- 101.Ittner A., Chua S.W., Bertz J., Volkerling A., van der Hoven J., Gladbach A., Przybyla M., Bi M., van Hummel A., Stevens C.H., et al. Site-specific phosphorylation of tau inhibits amyloid-beta toxicity in Alzheimer's mice. Science. 2016;354:904–908. doi: 10.1126/science.aah6205. [DOI] [PubMed] [Google Scholar]
- 102.Spittaels K., Van den Haute C., Van Dorpe J., Geerts H., Mercken M., Bruynseels K., Lasrado R., Vandezande K., Laenen I., Boon T., et al. Glycogen synthase kinase-3beta phosphorylates protein tau and rescues the axonopathy in the central nervous system of human four-repeat tau transgenic mice. J. Biol. Chem. 2000;275:41340–41349. doi: 10.1074/jbc.M006219200. [DOI] [PubMed] [Google Scholar]
- 103.Wang J.Z., Wu Q., Smith A., Grundke-Iqbal I., Iqbal K. Tau is phosphorylated by GSK-3 at several sites found in Alzheimer disease and its biological activity markedly inhibited only after it is prephosphorylated by A-kinase. FEBS Lett. 1998;436:28–34. doi: 10.1016/s0014-5793(98)01090-4. [DOI] [PubMed] [Google Scholar]
- 104.Thornton T.M., Pedraza-Alva G., Deng B., Wood C.D., Aronshtam A., Clements J.L., Sabio G., Davis R.J., Matthews D.E., Doble B., Rincon M. Phosphorylation by p38 MAPK as an alternative pathway for GSK3beta inactivation. Science. 2008;320:667–670. doi: 10.1126/science.1156037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lai S.L., Lee T. Genetic mosaic with dual binary transcriptional systems in Drosophila. Nat. Neurosci. 2006;9:703–709. doi: 10.1038/nn1681. [DOI] [PubMed] [Google Scholar]
- 106.Awasaki T., Tatsumi R., Takahashi K., Arai K., Nakanishi Y., Ueda R., et al. Essential role of the apoptotic cell engulfment genes draper and ced-6 in programmed axon pruning during Drosophila metamorphosis. Neuron. 2006;50(6):855–867. doi: 10.1016/j.neuron.2006.04.027. [DOI] [PubMed] [Google Scholar]
- 107.Sekiya M., Iijima K.M. Phenotypic analysis of a transgenic Drosophila model of Alzheimer's amyloid-beta toxicity. STAR Protoc. 2021;2:100501. doi: 10.1016/j.xpro.2021.100501. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study did not generate original code. All data produced in this study are included in this article and supplementary information. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.