Abstract
Cryptosporidiosis, giardiasis, and blastocystosis are among the most important parasitic diseases common between humans and cats. In addition, there are concerns about the possible transmission of zoonotic parasites from infected cats to humans. Hence, we investigated the molecular epidemiology of Cryptosporidium spp., Giardia duodenalis, and Blastocystis sp. in stray and household cats and cat owners. Our study was performed on 132, 33, and 33 fecal samples of stray and household cats, as well as cat owners in Tehran, Iran. Cryptosporidium spp. was identified using a nested PCR targeting the small subunit ribosomal RNA gene (SSU rRNA) and sequencing the internal amplified fragments. Furthermore, to perform multilocus genotyping of G. duodenalis, the ß-giardin (bg), glutamate dehydrogenase (gdh), and triosephosphate isomerase (tpi) genes were amplified to assess the DNA of G. duodenalis in the fecal samples of cats and cat owners. In addition, Blastocystis was detected by targeting the SSU rRNA gene, and the subtypes of Blastocystis were determined via the sequencing of amplicons. Cryptosporidium felis and Cryptosporidium canis were detected in seven stray cats (5.3%) and one household cat (3%). The bg gene of G. duodenalis was amplified and successfully sequenced in two (1.5%) stray cats and revealed assemblages F and B of G. duodenalis. Sequencing and phylogenic analysis of SSU rRNA gene nucleotide sequences of Blastocystis detected ST5 and ST10 in stray cats (1.5%), ST1 in household cats (9.1%), and ST1, ST2, ST3, and ST7 in owners (30.3%). The low prevalence of Cryptosporidium, Giardia and Blastocystis in cats and the presence of species/assemblages/subtypes with limited zoonotic potential indicate that cats had a minor role in their owners' infection in the investigated population. However, the presence of zoonotic protozoa in cats suggests the necessity of special attention to high-risk individuals during close contact with cats. Therefore, it is recommended that veterinarians, physicians, and urban managers plan to prevent, control, or treat these parasites to help the urban community live healthily alongside cats.
Subject terms: Microbiology, Molecular biology
Introduction
Zoonosis is any disease or infection naturally transmissible from vertebrate animals to humans1. Some of the most critical zoonosis infectious diseases are parasitic diseases transmitted to humans from companion and pet animals1. As the most popular pet, cats (Felis catus) have a close relationship with human societies. Even stray cats, which receive no standard veterinary care, freely pass in our yards and share public places with us, so they probably have a crucial role in transmitting parasitic diseases to humans. Cryptosporidiosis, giardiasis, and blastocystosis are among the most important parasitic diseases common between humans and cats2.
Giardia and Cryptosporidium can cause gastrointestinal disorders globally in many mammalian hosts with a wide range of clinical symptoms from self-limiting and asymptomatic to acute and life-threatening forms. The cysts/oocysts of these two enteric protozoan parasites are shedding in the hosts' feces. Accordingly, the main route of infection is fecal–oral transmission through contaminated food, water, or direct contact with infected humans or animals3, 4. Therefore, animals have an essential role in transmitting these parasites and the epidemiology of cryptosporidiosis and giardiasis4.
Cryptosporidium, an important apicomplexan parasite, comprises 44 species and more than 120 genotypes. Up to now, at least 19 species and four genotypes of Cryptosporidium have been reported in humans. However, most human cryptosporidiosis results from infection with C. hominis, C. parvum, C. meleagridis, C. canis, C. felis, and C. ubiquitum3. Cats are usually infected with C. felis with varying infection rates from 0 to 30% throughout the world, whereas in most reporting, infection rates were lower than 10%5. In addition, the zoonotic transmission of C. felis has been reported in numerous studies through close contact with cats in immunocompromised and immunocompetent humans5.
Giardia duodenalis infects a wide range of mammals, including humans. It is estimated that more than 280 million human giardiasis cases occur annually worldwide6. Hence, genotyping of G. duodenalis is a valuable tool for epidemiological studies, which are mainly performed using sequence analysis of PCR products from β-giardin (bg), triosephosphate isomerase (tpi), and glutamate dehydrogenase (gdh) genes. Over the last few decades, molecular studies have shown that G. duodenalis has eight genetically distinguishable assemblages (A-H) identified in mammalian hosts. Assemblages A and B have a broad range of human and animal hosts. Human infection mainly occurs with assemblages A and B, whereas assemblages C to H are more host-adapted, except for some assemblages C, D, E, and F in humans6, 7. The G. duodenalis infection of cats has been reported from 1.3 to 27.3%, mainly with the feline-specific assemblages F, with fewer reports of assemblages A, C, B, and D6.
Blastocystis is a common intestinal single-celled stramenopile protist with a vast range of hosts, from humans to insects8. Human Blastocystis infection has been reported from 0.5 to 100%9 globally and from 5 to 50% in Asia10. However, the pathogenicity and the public health importance of Blastocystis remain controversial, as it has been highly represented in asymptomatic healthy individuals as well as in a variety of acute or chronic gastrointestinal patients11, 12. Blastocystis is a polymorphic microscopic organism with high genetic diversity, especially across the SSU rRNA gene, which is the base of the classification of this genus to subtypes. The Blastocystis genus is currently classified into 28 subtypes (STs), which are ST1–ST17, ST21, ST23–ST29, and ST30–ST329, 11–14. Although ST1–10, ST12, ST14, and ST16 have been reported from humans, more than 90% of Blastocystis STs found in humans are ST1–ST3 globally and ST4 mainly in Europe, whereas STs 5–9 sporadically, and STs 10, 12, 14, and 16 rarely have been reported from humans. However, all subtypes have been identified in animals except ST9, which has been reported up to now just in humans9, 12. Therefore, most Blastocystis STs have low host specificity, making zoonotic transmission possible10. The prevalence of Blastocystis infection in cats has been vastly reported from 0.0 to 100%. The reported subtypes in cats are ST1, ST3, ST4, ST10, and ST1410, 15.
Cats are mainly infected with intestinal parasites such as Cryptosporidium spp., G. duodenalis, and Blastocystis sp. Due to the close contact between humans and cats, there are concerns about the possible transmission of the zoonotic species/assemblages/subtypes of these parasites from infected cats to humans. Although the information on the regional prevalence of cat intestinal parasites is vital, few studies have been conducted on their prevalence and relation to human contamination in Iran. This information is necessary for cooperation between local veterinarians and public health authorities to develop effective strategies for treating and controlling parasites and educating pet owners16. Hence, this study was performed to identify and determine the molecular epidemiology of these zoonotic parasites in stray/household cats and cat owners of Tehran, the capital of Iran.
Materials and methods
Ethical approval
All cat owners who participated in this study gave informed written consent. The protocols of this study were reviewed by the Ethics Committee of Iran University of Medical Sciences and approved under the code IR.IUMS.FMD.REC 1396.31834. All methods were performed in accordance with the animal and human research guidelines and regulations from the Iranian Ministry of Health, Treatment, and Medical Education.
Sample collection and preparation
The DNA of fecal samples of 132 and 33 stray and household cats previously collected17 were included in this study, along with 33 fecal samples collected from cat owners from January to September 2019, simultaneously collecting the fecal samples of their cats in Tehran (35.6892° N, 51.3890° E), Iran. In addition, demographic data concerning the age, sex, breed, weight, and residence area of cats, as well as the age, sex, and residence area of cat owners, were recorded. Cat owners reported no particular clinical symptoms at the sampling time in themselves or their cats. First, the sucrose flotation procedure was performed on 33 human fecal samples for concentrating oocysts/cysts of Cryptosporidium/Giardia17, 18. Then, the DNA of samples was extracted using the QIAamp DNA Mini Kit following the manufacturer’s instructions. The extracted DNA was stored at − 20 °C until further molecular analysis.
Molecular identification, sequencing, and phylogenetic analysis
Molecular identification and characterization of Cryptosporidium spp. were performed by evaluating Cryptosporidium DNA in the fecal samples using a nested PCR targeting a 611-bp fragment of the small subunit ribosomal RNA (SSU rRNA) gene by specific primers designed by Silva et al.19 (Table 1). The species of Cryptosporidium isolates were determined by sequencing the internal amplified fragments.
Table 1.
Genetic markers, Primers, and PCR amplification schemes for detecting and characterizing Cryptosporidium spp., Giardia duodenalis, and Blastocystis sp. in the fecal samples of cats and cat owners in Tehran, Iran.
| Species | Gene | Primer nucleotide Sequences (5′–3′) | Amplicon size (bp) | Refs. | Cycling conditions | Refs. |
|---|---|---|---|---|---|---|
| Cryptosporidium | SSU rRNA |
F: ACCTATCAGCTTTAGACGGTAGGGTAT R: TTCTCATAAGGTGCTGAAGGAGTAAGG F: ACAGGGAGGTAGTGACAAGAAATAACA R: AAGGAGTAAGGAACAACCTCCA |
611 | 19 |
PCR 1: 45 s/94 °C, 45 s/56 °C, 45 s/72 °C, 39 cycles PCR2: 45 s/94 °C, 45 s/58 °C, 30 s/70 °C, 35 cycles |
This study |
| G. duodenalis | bg |
F: AAGCCCGACGACCTCACCCGCAGTGC R: GAGGCCGCCCTGGATCTTCGAGACGAC F: GAACGAACGAGATCGAGGTCCG R: CTCGACGAGCTTCGTGTT |
511 | 20 |
PCR 1: 30 s/95 °C, 30 s/65 °C, 60 s/72 °C, 35 cycles PCR 2: 30 s/95 °C, 30 s/55 °C, 60 s/72 °C, 35 cycles |
23 |
| G. duodenalis | gdh |
F: TCAACGTYAAYCGYGGYTTCCGT R: GTTRTCCTTGCACATCTCC F: CAGTACAACTCYGCTCTCGG R: GTTRTCCTTGCACATCTCC |
430 | 21 |
PCR 1: 2 min/94 °C, 60 s/61 °C, 2 min/68 °C, 1 cycle; 30 s/94 °C, 20 s/61 °C, 20 s/68 °C, 30 cycles PCR 2: 2 min/94 °C, 60 s/60 °C, 2 min/65 °C, 1 cycle; 30 s/94 °C, 20 s/60 °C, 20 s/65 °C, 15 cycles |
18 |
| G. duodenalis | tpi |
F: AAATIATGCCTGCTCGTCG R: CAAACCTTITCCGCAAACC F: CCCTTCATCGGIGGTAACTT R: GTGGCCACCACICCCGTGCC |
530 | 22 |
PCR 1: 45 s/94 °C, 45 s/50 °C, 60 s/72 °C, 35 cycles PCR 2: 45 s/94 °C, 45 s/58 °C, 60 s/72 °C, 35 cycles |
23 |
| Blastocystis | SSU rRNA |
F: GGAGGTAGTGACAATAAATC R: TAAGACTACGAGGGTATCTA |
550–585 | 24 | 60 s/94 °C, 45 s/56 °C, 45 s/72 °C, 35 cycles | 24 |
Molecular identification and multilocus genotyping of G. duodenalis were performed by amplification of the ß-giardin (bg), glutamate dehydrogenase (gdh), and triosephosphate isomerase (tpi) genes to assess the DNA of G. duodenalis in the fecal samples of cats and cat owners. For this purpose, a 511-bp fragment of the bg gene20, a 432-bp fragment of the gdh gene21, and a 530-bp fragment of the tpi gene22 were amplified by a nested PCR, a semi-nested PCR, and a nested PCR, respectively, according to previous procedures18, 23 (Table 1). The assemblages, sub-assemblages, and genotypes of G. duodenalis isolates were identified by sequencing each marker's internal amplified fragments.
Molecular identification and characterization of Blastocystis were accomplished by a PCR method targeting a 550- to a 585-bp nucleotide fragment of the SSU RNA gene on the genomic DNA extracted directly from fecal samples24. In addition, the subtypes of isolates were identified via the sequencing of amplicons.
All PCRs were performed in 25 µL of the amplification reaction mixture using primer pairs and conditions listed in Table 1. The amplification reaction mixture consisted of 12.5 µL of 2 × Taq DNA Polymerase Master Mix RED (Amplicon III, Denmark, Copenhagen, cat. no. 180301), 2.5 µL of primer pair mix (0.4 µM of each primer in the primary and secondary PCR reactions for detection of Cryptosporidium spp.; 0.2 μM for tpi and bg loci or 0.5 µM for gdh locus in the PCR reactions for detection of G. duodenalis, or 0.5 µM for detection of Blastocystis), and 2 μM of template DNA of each sample in the single PCR reaction or the primary PCR reaction of nested or semi-nested PCR reaction and 2 μM of PCR products in the secondary PCR reaction of nested or semi-nested PCR. Amplicons were analyzed on 1.5% (w/v) agarose gel. In the case of positive samples, the PCR or the secondary PCR reaction of nested or semi-nested PCR was repeated in 50 µL of the amplification reaction mixture with each parasite’s corresponding primer pairs. After electrophoresis on 1% agarose gels, the resulting amplicons were purified with the MinElute Gel Extraction Kit (Qiagen, Hilden, Germany) for sequencing in both directions using forward and reverse primers (Macrogen Inc., Seoul, South Korea). The results of each parasite sample's corresponding forward and reverse sequences were read and verified by the Chromas software (Technelysium Pty Ltd., Queensland, Australia) and subsequently aligned and assembled using MEGA X software (www.megasoftware.net). The species of Cryptosporidium, the assemblage, sub-assemblage, and genotype of G. duodenalis, as well as the subtype of Blastocystis sp., were identified by comparing the homology of the final nucleotide sequences with corresponding sequences retrieved from the GenBank database (http://blast.ncbi.nlm.nih.gov). The achieved nucleotide sequences were deposited in the GenBank under accession numbers LC700089–LC700096 for the SSU rRNA gene of C. felis and C. cains, LC700097–LC700098 for the bg gene of G. duodenalis, and LC700104–LC700118 for the SSU rRNA gene of Blastocystis. The phylogenetic analysis was accomplished in MEGA X (www.megasoftware.net) using the maximum likelihood (ML) algorithm with evolutionary distances calculated by the Kimura-2 parameter (K2) model and a bootstrap value of 1000 to estimate the consistency of clusters.
Statistical analyses
Statistical analysis was conducted using SPSS 24.0 Statistical Software (SPSS Inc., Chicago, IL, USA). Chi-square tests estimated potential associations between qualitative variables with a 95% confidence interval (CIs), and the p value of < 0.05 were considered significantly different.
Results
Prevalence of Cryptosporidium, Giardia, and Blastocystis among stray/household cats and their owners
The prevalence of Cryptosporidium spp. was 5.3% (7/132; 95% CI 2.6–10.5), 3.0% (1/33; 95% CI 0.5–15.3), and 0.0% (0/33; 95% CI 0.0–10.4) in stray cats, household cats, and cat owners, respectively. The SSU rRNA gene nucleotide of Cryptosporidium spp. was not found in the feces of cat owners. Statistical differences were not observed between the prevalence of Cryptosporidium spp. in stray cats, household cats, and cat owners. The prevalence of Cryptosporidium spp. infection based on age, sex, breed, weight, and the urban region in stray and household cats is shown in Tables 2 and 3. Cryptosporidium spp. infection was not statistically related to age, breed, weight, and urban region of stray or household cats. However, the prevalence of Cryptosporidium spp. in stray cats was affected by sex (p = 0.028), as it was in female cats (16.7%, 6/60; 95% CI 4.7–20.1) higher than in male cats (1.4%, 1/72; 95% CI 0.2–7.5). The only Cryptosporidium-infected household cat was a two-year male Persian cat with a 3.8 kg weight.
Table 2.
The demographics and the prevalence of Cryptosporidium spp., Giardia duodenalis, and Blastocystis sp. in the stray cats of Tehran, Iran.
| Factors | Samples % (N) |
Cryptosporidium spp. | Giardia duodenalis | Blastocystis sp. | |||
|---|---|---|---|---|---|---|---|
| % (N) | p | % (N) | p | % (N) | p | ||
| Age | |||||||
| < 1 year | 47.0% (62) | 3.2% (2) | 0.316 | 1.6% (1) | 0.931 | 1.6% (1) | 0.467 |
| > 1 year | 53.0% (70) | 7.1% (5) | 1.4% (1) | 1.4% (1) | |||
| Sex | |||||||
| Male | 54.5% (72) | 1.4% (1) | 0.028 | 1.4% (1) | 0.896 | 2.8% (2) | 0.193 |
| Female | 45.5% (60) | 10.0% (6) | 1.7% (1) | 0.0% (0) | |||
| Breed | |||||||
| DSH | 72.7% (96) | 6.2% (6) | 0.719 | 2.1% (2) | 0.683 | 0.0% (0) | 0.054 |
| DLH | 25.8% (34) | 2.9% (1) | 0.0% (0) | 5.9% (2) | |||
| Persian | 1.5% (2) | 0.0% (0) | 0.0% (0) | 0.0% (0) | |||
| Weight | |||||||
| < 2 kg | 47.0% (62) | 4.8% (3) | 0.470 | 1.6% (1) | 0.854 | 1.6% (1) | 0.854 |
| 2–4 | 40.2% (53) | 7.5% (4) | 0.8% (1) | 1.9% (1) | |||
| > 4 kg | 12.8% (17) | 0.0% (0) | 0.0% (0) | 0.0% (0) | |||
| Urban region | |||||||
| North | 28.8% (38) | 5.3% (2) | 0.794 | 0.0% (0) | 0.430 | 0.0% (0) | 0.008 |
| Center | 18.9% (25) | 4.0% (1) | 0.0% (0) | 0.0% (0) | |||
| South | 12.9% (17) | 11.8% (2) | 5.9% (1) | 0.0% (0) | |||
| West | 26.5% (35) | 2.9% (1) | 2.9% (1) | 0.0% (0) | |||
| East | 12.9% (17) | 5.9% (1) | 0.0% (0) | 11.8% (2) | |||
| Total | 100.0% (132) | 5.3% (7) | 1.5% (2) | 1.5% (2) | |||
Table 3.
The demographics and the prevalence of Cryptosporidium spp. and Blastocystis sp. in the household cats of Tehran, Iran.
| Factors | Samples % (N) |
Cryptosporidium spp. | Blastocystis sp. | ||
|---|---|---|---|---|---|
| % (N) | p | % (N) | p | ||
| Age | |||||
| < 1 year | 15.2% (5) | 0.0% (0) | 0.688 | 20.0% (1) | 0.357 |
| >1 year | 84.8% (28) | 3.6% (1) | 7.1% (2) | ||
| Sex | |||||
| Male | 51.5% (17) | 5.9% (1) | 0.325 | 5.9% (1) | 0.509 |
| Female | 48.5% (16) | 0.0% (0) | 12.5% (2) | ||
| Breed | |||||
| DSH | 33.3% (11) | 0.0% (0) | 0.684 | 9.1% (1) | 0.291 |
| DLH | 9.1% (3) | 0.0% (0) | 33.3% (1) | ||
| Persian | 57.6% (19) | 5.3% (1) | 5.3% (1) | ||
| Weight | |||||
| < 2 kg | 8.2% (6) | 0.0% (0) | 0.684 | 16.7% (1) | 0.649 |
| 2–4 | 57.6% (19) | 5.3% (1) | 11.1% (1) | ||
| > 4 kg | 24.2% (8) | 0.0% (0) | 12.5% (1) | ||
| Urban region | |||||
| North | 21.2% (7) | 0.0% (0) | 0.811 | 0.0% (0) | 0.024 |
| Center | 24.2% (8) | 0.0% (0) | 12.5% (1) | ||
| South | 3.0% (1) | 0.0% (0) | 100% (1) | ||
| West | 39.4% (13) | 7.7% (1) | 7.7% (1) | ||
| East | 12.1% (4) | 0.0% (0) | 0.0% (0) | ||
| Total | 100.0% (33) | 3.0% (1) | 9.1% (3) | ||
The prevalence of G. duodenalis was 1.5% (2/132; 95% CI 0.4–5.4), 0.0% (0/33; 95% CI 0.0–10.4), and 0.0% (0/33; 95% CI 0.0–10.4) in stray cats, household cats, and cat owners, respectively. The DNA of G. duodenalis was not found in the feces of household cats or cat owners. No statistical differences were observed in the prevalence of G. duodenalis infection in stray cats, household cats, and cat owners, as well as between any demographic variables and infection with G. duodenalis in stray cats. The association between G. duodenalis infection and stray cats' demographic variables is revealed in Table 2.
The prevalence of Blastocystis sp. was 1.5% (2/132; 95% CI 0.4–5.4), 9.1% (3/33; 95% CI 3.1–23.6), and 30.3% (10/33; 95% CI 17.4–47.3) in stray cats, household cats, and cat owners, respectively. No statistical differences were found between the prevalence of Blastocystis sp. infection in stray and household cats, as well as between household cats and cat owners. However, the prevalence of Blastocystis sp. infection in cat owners was statistically higher than in stray cats. The prevalence of Blastocystis sp. infection associated with demographic variables of stray cats, household cats, and cat owners are shown in Tables 2, 3, and 4, respectively. There was no statistically significant relationship between age, sex, breed, weight, and infection with Blastocystis in stray and household cats. The Chi-Square statistical analysis showed a significant relationship between the prevalence of Blastocystis sp. and the urban region in the stray cats (p = 0.008) and household cats (p = 0.024). The highest prevalence of Blastocystis infection was detected in stray cats (2/17; 11.8%; 95% CI 3.3–34.3) and household cats (1/1; 100.0%; 95% CI 20.7–100.0) living in the east and south of Tehran, respectively. However, there was no statistically significant relationship between any demographic variables and infection with Blastocystis in the cat owners.
Table 4.
The demographics and the prevalence of Blastocystis sp. in the cat owners of Tehran, Iran.
| Factors | Samples % (N) |
Blastocystis sp. | |
|---|---|---|---|
| % (N) | p | ||
| Age | |||
| 20–29 | 27.3% (9) | 11.1% (1) | 0.051 |
| 30–39 | 33.3% (11) | 45.5% (5) | |
| 40–49 | 18.2% (6) | 0.0% (0) | |
| > 50 | 21.2% (7) | 57.1% (4) | |
| Sex | |||
| Male | 57.6% (19) | 36.9% (7) | 0.341 |
| Female | 42.4% (14) | 21.4% (3) | |
| Urban region | |||
| North | 21.2% (7) | 14.3% (1) | 0.354 |
| Center | 24.2% (8) | 37.5% (3) | |
| South | 3.0% (1) | 100.0% (1) | |
| West | 39.4% (13) | 23.1% (3) | |
| East | 12.1% (4) | 50.0% (2) | |
| Total | 100.0% (33) | 30.3% (10) | |
Totally, molecular analysis revealed infection with Cryptosporidium spp., G. duodenalis, or Blastocystis sp. occurred in 11 of 132 (8.3%; 95% CI: 4.7–14.3%) stray cats, although Cryptosporidium spp. or Blastocystis sp. was detected in 4 of 33 (12.1%; 95% CI: 4.3–27.3%) household cats. However, mixed infections of these parasites were not observed in any of the stray/household cats or their owners.
Cryptosporidium species/genotype in stray and household cats
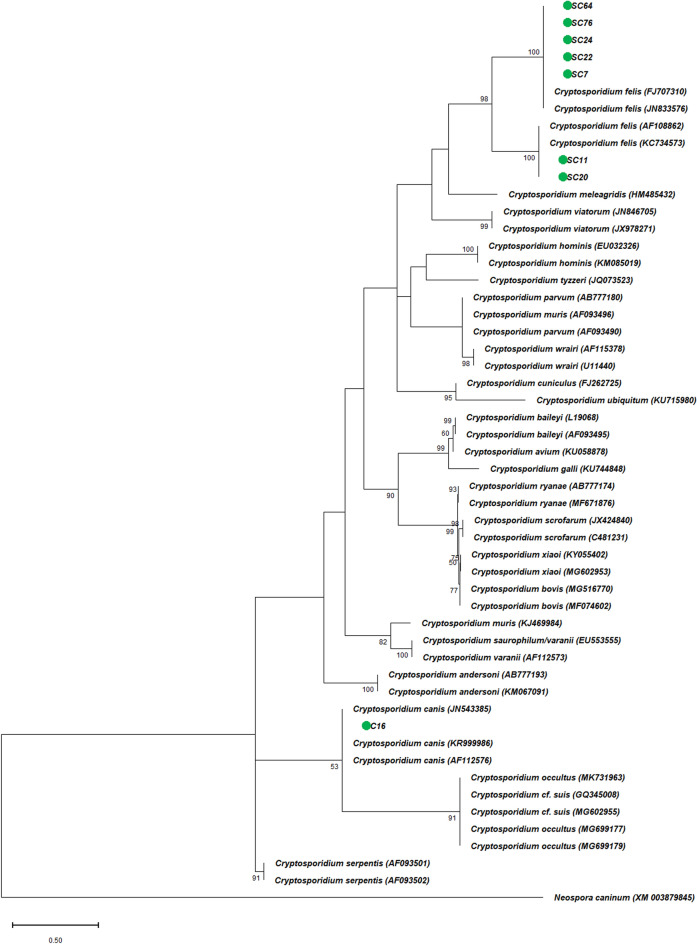
Sequencing of the SSU rRNA gene identified two Cryptosporidium species among the eight Cryptosporidium-positive samples, including seven C. felis in stray cats and one C. canis in household cats. In addition, the multiple alignments of the SSU rRNA gene nucleotide fragment sequences of seven C. felis samples with the deposited sequences retrieved from GenBank showed two nucleotide patterns. Five C. felis isolates had 100% homology with GenBank sequence accession numbers FJ707310 and JN833576, and the other two isolates revealed 100% sequence identity to GenBank sequence accession numbers AF108862 and KC734573 (Supplementary Table 1). Sequencing analysis of the only C. canis isolate showed 100% homology with nucleotide deposited sequence in GenBank (AF112576, KR999986, and JN543385). Furthermore, the phylogenetic trees of the SSU rRNA gene nucleotide fragment sequences of the Cryptosporidium parasites isolated in this study compared with nucleotide sequences of Cryptosporidium species retrieved from GenBank confirmed the sequencing analysis observations (Fig. 1).
Figure 1.
The phylogram of Cryptosporidium spp. was inferred based on the nucleotide sequences of SSU rRNA gene. The evolutionary relationship of Cryptosporidium spp. was constructed by the Maximum Likelihood method and Kimura 2-parameter model, based on the nucleotide sequences of SSU rRNA gene of C. felis and C. canis isolated from stray cats [SC] and household cats [C] in this study (green circles) compared with nucleotide sequences of Cryptosporidium species retrieved from GenBank, with Neospora caninum (XM_003879845) as outgroup. Bootstrap values obtained from 1000 replicates are indicated on branches in percentage; only bootstrap values > 50% are displayed. Evolutionary analyses were conducted in MEGA X.
Giardia duodenalis assemblages and genotypes in stray cats
The PCR amplification of the bg, gdh, and tpi genes showed two positive samples of G. duodenalis in stray cats, which were detected only at the bg locus. No DNA amplification was performed at the gdh or tpi loci. The sequencing analysis of two bg-positive samples revealed two assemblages, B (50.0%) and F (50.0%).
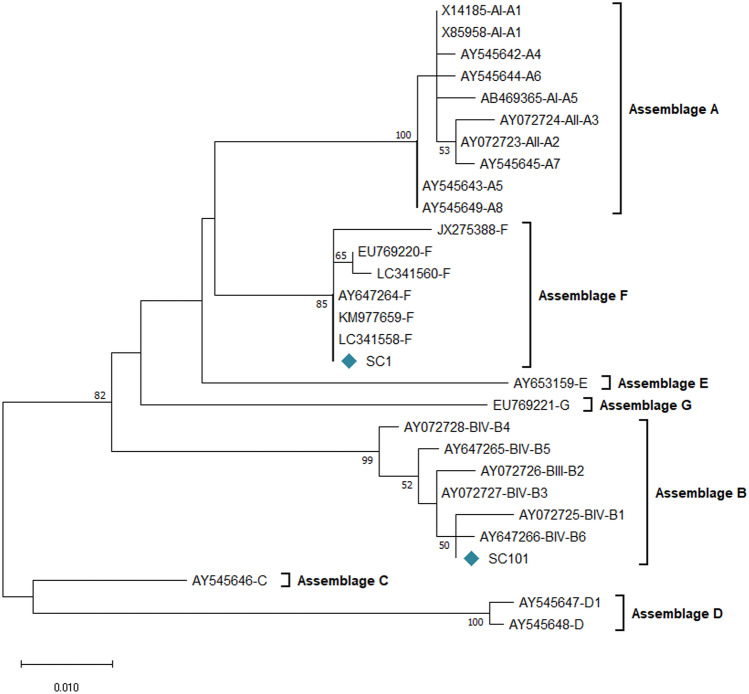
Sequence multiple alignment analysis of the bg gene with reference sequences classified the assemblage B isolate at the BIV sub-assemblage. In addition, it revealed some single nucleotide polymorphisms (SNPs), resulting in a novel genotype. The comparative analysis, with the reference sequences of B1–B6 genotypes, indicated the occurrence of two SNPs where a thymidine substituted a cytosine at the position of 185 (T185C) or 210 (T210C) compared with genotype B6 (AY647266), or B3 (AY072727), respectively (Supplementary Table 2). However, no SNPs were detected in the assemblage F isolate compared with the reference sequence (AY647264) representing F assemblage. The bg gene phylogenetic analysis classified assemblage F isolate (SC1) in one cluster with assemblage F demonstrating 100% homology with reference sequences (AY647264, KM977659, LC341558), and assemblage B isolate (SC101) in one group with sub-assemblage BIV, among genotype B3 and B6 sequence references (Fig. 2).
Figure 2.
The phylogram of Giardia duodenalis was inferred based on the nucleotide sequences of the β-giardin (bg) gene. The evolutionary relationship of G. duodenalis was constructed by the Maximum Likelihood method and Kimura 2-parameter model, based on the nucleotide sequences of the bg gene of G. duodenalis isolated from stray cats [SC] in this study (light sea green rhombus) compared with nucleotide sequences of known assemblages retrieved from GenBank. Bootstrap values obtained from 1000 replicates are indicated on branches in percentage; only bootstrap values > 50% are displayed. Evolutionary analyses were conducted in MEGA X.
Blastocystis subtypes and intra-subtype variability in stray cats, household cats, and cat owners
Sequencing and phylogenetic analysis of the amplified fragment of the SSU rRNA gene recognized six subtypes of Blastocystis, including ST5 and ST10 in stray cats, ST1 in household cats, and ST1, ST2, ST3, and ST7 in cat owners. The multiple alignments of the SSU rRNA gene nucleotide fragment sequences of five ST1 isolates with the deposited sequences retrieved from GenBank showed one nucleotide pattern, which had three SNPs with GenBank sequence accession number U51151, where an adenine substituted guanine at the position of 172 (A172G), and thymidine substituted cytosine at the position of 195 (T195C) and 247 (T247C). In contrast, this ST1 nucleotide pattern revealed one SNP at the position of 260 (C260G) compared with MK801411 (Supplementary Table 3). Sequencing analysis of two ST2 isolates had one pattern with one SNP (T15A) compared with EU445491. Four ST3 isolates from humans revealed two different patterns. One isolate showed 100% homology with nucleotide deposited sequences in GenBank (AB107965 and EU445496), and the other three isolates revealed one SNP (G164A) compared with nucleotide sequences retrieved from GenBank (AB107963 and KC294170). Sequencing analysis of the only ST5 isolate from the stray cat showed one SNP compared with nucleotide sequences isolated from pig AB107964 (G385A) and cattle AB107966 (A185T). Two ST7 isolates of humans revealed two different patterns, with the highest nucleotide diversity (30–31 SNPs) compared with nucleotide sequences retrieved from GenBank (AF408427 and AB070991). Finally, the only ST10 isolate from the stray cat showed 12 SNPs compared with the nucleotide sequence isolated from the camel (KC148207). The phylogenetic analysis of the SSU rRNA gene nucleotide fragment sequences of the Blastocystis subtypes isolated in this study compared with nucleotide sequences of Blastocystis subtypes retrieved from GenBank confirmed the sequencing analysis observations (Fig. 3).
Figure 3.

The phylogram of Blastocystis subtypes was inferred based on the nucleotide sequences of SSU rRNA gene. The evolutionary relationship of Blastocystis subtypes was constructed by the Maximum Likelihood method and Kimura 2-parameter model, based on the nucleotide sequences of SSU rRNA gene of Blastocystis isolated from stray cats [SC], household cats [C], and cat owners [H] in this study (blue circles) compared with nucleotide sequences of Blastocystis subtypes retrieved from GenBank, with Proteromonas lacertae (U37108) as outgroup. Bootstrap values obtained from 1000 replicates are indicated on branches in percentage; only bootstrap values > 50% are displayed. Evolutionary analyses were conducted in MEGA X.
Discussion
In the present study, molecular analyses were performed to identify species, assemblages, or subtypes of Cryptosporidium spp., G. duodenalis, and Blastocystis sp. in stray/household cats and their owners in Tehran to acquire sufficient information about the prevalence of these intestinal parasites in stray/household cats and to assess the potential role of household cats in the transmission of these zoonotic parasitic infections to their owners.
Consistent with the global prevalence of Cryptosporidium infection (4.2%) reported in cats by molecular diagnostic methods25, we found Cryptosporidium spp. infection in 4.8% (8/165; 95% CI 2.6–10.5) of the investigated cats. Although the prevalence of Cryptosporidium spp. in the investigated stray cats (5.3%) was in the ranges reported in China (5.6–5.8%)26, it is lower than previous similar studies done in Australia (13.4%)27 and the Czech Republic (7.4%)2. In contrast, it is higher than in Italy (0%)28 and South Korea (0.6%)29. However, the prevalence of Cryptosporidium spp. in household cats (3%) was lower than in molecular studies done in Australia (7.1%)27 and Japan (12.7%)30. In comparison, it is higher than in Italy (0%)28, China (0.6%)26, the Czech Republic (0.8%)2, Japan (2%)31, and the others study in Iran (0.7%)32. These discrepancies might result from the study populations, geographical distribution, different identification methods, and various levels of living conditions. As reported before27, high Cryptosporidium infection in the cats living in a refuge center might be related to their close contact and keeping conditions. The investigation of possible factors associated with Cryptosporidium infection revealed that it was more frequent in stray female cats (p = 0.028), which contradicted previous reports2, 29. There is no evidence that sex has a role in increasing the chance of infection with Cryptosporidium species. Therefore, more studies with a larger sample size are necessary to evaluate this relationship. Furthermore, the Cryptosporidium spp. infection was not detected in the cat owners, which could be attributed to good veterinary care and, considering hygiene principles, correspondingly no infection in their cats, except for one case. There are limited studies on the Cryptosporidium infection in household cats and their owners. There are merely two reports, one concomitant infection of a Swedish woman and her cat with C. felis and another detection of C. felis infection in a cat and its owner33, 34. Consistent with previous findings2, 26, 27, 29, C. felis was the only species identified in the Cryptosporidium-positive isolates from stray cats, whereas C. canis was distinguished in the sole infected household cat. Based on our knowledge, this is the first report of finding C. canis in household cats. This two-year male Persian cat was adopted at three months old from a pet shop, and about one month before sampling was in pet boarding for a week due to his owner's travel, where he might have been infected with C. canis. Further studies on the prevalence of this species in cats seem necessary. Although C. felis is the main species infecting cats, it is one of the six prevalent species infecting humans after C. hominis and C. parvum, which are responsible for 95% of human cryptosporidiosis3, 4. The zoonotic potential of feline and canine cryptosporidiosis concerns veterinarians and physicians worldwide due to the close contact between humans and companion animals3, 5, 34. However, the low infection rate of household cats (3%) and no infection found in the cat owners suggest a limited role of cats in the human cryptosporidiosis in the studied population. Nevertheless, special attention is necessary to the zoonotic potential of these species, especially in children and immunocompromised individuals who have close contact with cats.
The prevalence of G. duodenalis infection in stray cats (1.5%) was lower than in South Korea (3.8%), the Czech Republic (7.4%), and Italy (10.9%)2, 28, 29, whereas it was higher than in China (0%)35. However, we did not detect any G. duodenalis infection in household cats by amplifying bg, tpi, and gdh loci, which was less than in Shiraz, Iran (1.3%), China (1.2%), Italy (4%), the Czech Republic (5%), and Denmark (10.5%)2, 26, 28, 32, 36. The different ranges of G. duodenalis infection reported in the cats might reflect the various loci and molecular methods applied for the detection of DNA of this parasite. As seen in our study, the tpi and gdh loci could not detect the DNA of two positive samples detected by the bg locus, which is consistent with the different amplification rates of these three genetic loci37. Moreover, geographical distribution and studied populations affecting the living conditions have the leading role in the prevalence of G. duodenalis in cats6. Accordingly, consistent with the previous reports, we found higher infection rates in stray cats than in household cats2, 27. Furthermore, the assemblage F of G. duodenalis is mainly detected in cats, followed by assemblage A6. However, assemblage B has been reported in a few studies from China, Europe, and Australia21, 37, 38. Here, we found the zoonotic assemblage B for the first time in Iran and the feline-specific assemblage F in stray cats. Consistent with other reports from the world6, the further sequence alignment and phylogenetic analysis of two isolates showed that assemblage F had no SNP, while assemblage B revealed some SNPs. Identifying only two G. duodenalis infection cases in stray cats and no infection in the household cats or their owners, suggesting cats had a minimal potential role in human giardiasis in the studied population.
The epidemiology of Blastocystis in cats is controversial, like other biological aspects of this parasite. The molecular prevalence of Blastocystis in cats has been reported from 0.6 to 100%, whereas some studies reported no presence of Blastocystis in Carnivora, including cats8, 15. We found Blastocystis infection in 3% (5/165; 95% CI 1.3–6.9) of the investigated cats. The prevalence of Blastocystis in stray cats (1.5%) was lower than in previous studies in Fars Province, Iran (17.7%)39, Malaysia (20.0%)40, the USA (11.6%)41, and Turkey (3.6%)42 and higher than in South Korea (0.6%)29. At the same time, the prevalence of Blastocystis in household cats (9.1%) was lower than in molecular studies in Australia (100%)43 and Turkey (100.0%)44 and higher than in China (0.6%)45, the USA (0%)41, Thailand46, and Spain (0%)47. Although there have been some studies on the presence of Blastocystis in cats, this data is not enough to conclude a reason for explaining so much variety in the distribution of this parasite in different studies. Albeit, it is likely that the prevalence of Blastocystis attributed to the cats’ standard of care and hygiene. This relation has been revealed in a study in which Blastocystis infection was only reported in the shelter cats (11.6%), while owned cats were not infected41. Although in our study, the Blastocystis infection in household cats was more prevalent than in stray cats, which suggested that owned cats might get infected via close contact with their owner. Consistent with the prevalence of Blastocystis infection reported in Asia (5% to 50%) and healthy general populations of Iran (3.3% to 30.1%) by molecular diagnostic methods10, we found Blastocystis infection in 30.3% of the cat owners. The variety in the prevalence of Blastocystis from 0.5 to 100% across the world is considered related to inadequate hygiene and sanitation that increased the chance of potential anthroponotic and zoonotic transmission via the fecal–oral route contamination9. However, many studies have suggested that Blastocystis is one of the gastrointestinal microbiota of healthy individuals9, 11, 12, 48. Therefore, the latter hypothesis is more plausible about the human samples in our study. Furthermore, the phylogenetic analysis documented the ST5 and ST10 in stray cats, which are mainly reported from pigs and cattle worldwide, with less zoonotic importance10, 15, 49. Although, in the only published molecular study performed on stray cats in Fars Province, Iran, five Blastocystis subtypes were determined, ST1, ST3, ST4, ST10, and ST14, with the more zoonotic significance39. Moreover, the Blastocystis subtypes of ST1, ST3, and ST10 in the USA41, ST1 in Malaysia40, and ST4 in South Korea29 and Turkey42 have been reported from the shelter or stray cats. While the phylogenetic analysis distinguished a zoonotic Blastocystis subtype, ST1, in the three household cats, which is the subtype also reported in household cats in China45 and Australia43, another zoonotic Blastocystis subtype, ST3, was distinguished in three household cats in Turkey44. In addition, we identified the Blastocystis subtypes of ST1, ST2, ST3, and ST7, with a predominance of ST3, in cat owners, which is consistent with human-reported subtypes in Iran and Asia10. In only one case, both household cats and their owner were infected with Blastocystis ST1, suggesting a possible common source of infection. However, due to the low prevalence rates and nonspecific STs presented in stray or household cats, it seems cats were not the main potential reservoirs for transmitting human Blastocystis infection.
Conclusion
The low prevalence of Cryptosporidium (4.8%) and Giardia (1.2%) in cats and the presence of species and assemblages with low zoonotic potential, as well as no infections found in cat owners, limit the role of cats in the human cryptosporidiosis or giardiasis in the investigated population. Comparing the prevalence of Blastocystis in cats (3%) and their owners (30.3%), in addition to nonspecific STs detected in stray cats, shows a minor role of cats in human infection with Blastocystis. However, the presence of zoonotic protozoa in cats needs special attention from cat enthusiasts, especially children and immunocompromised individuals. Therefore, it is recommended that veterinarians, physicians, and urban managers plan to prevent, control, or treat these parasites to help the urban community live healthily alongside these companion animals.
Supplementary Information
Acknowledgements
We thank the cat owners who participate in this study, as well as the veterinarians and the staff of pet hospitals and veterinary clinics, for helping to sample stray cats. This study is a part of the Ph.D. dissertation of Dr. Poorya Karimi.
Abbreviations
- bg
ß-giardin
- bp
Base pair
- CI
Confidence interval
- DLH
Domestic long hair
- DSH
Domestic short hair
- gdh
Glutamate dehydrogenase
- K2
Kimura 2-parameter
- ML
Maximum likelihood
- SNPs
Single nucleotide polymorphisms
- SSU rRNA gene
Small subunit ribosomal ribonucleic acid gene
- ST
Subtype
- tpi
Triosephosphate isomerase
Author contributions
P.K. conceptualization, methodology, validation, formal analysis, investigation, resources, data curation, and writing the original draft. S.S. methodology, verification, formal analysis, investigation, resources, data curation. A.R.M. validation, resources, review and editing of the final manuscript. E.R. conceptualization, methodology, validation, formal analysis, resources, data curation, writing the original draft, review and editing of the final manuscript, visualization, supervision, project administration, and funding acquisition. All authors reviewed the manuscript.
Funding
This study was financially supported by the Iran University of Medical Sciences under grant number 96-03-30-31834 to E.R.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files. The sequences were submitted to DDBJ/EMBL/GenBank databases (https://www.ncbi.nlm.nih.gov/nuccore/) under accession numbers LC700089–LC700098 and LC700104–LC700118.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-28768-w.
References
- 1.Baneth G, Thamsborg SM, Otranto D, Guillot J, Blaga R, Deplazes P, et al. Major parasitic zoonoses associated with dogs and cats in Europe. J. Comp. Pathol. 2016;155(1 Suppl 1):S54–74. doi: 10.1016/j.jcpa.2015.10.179. [DOI] [PubMed] [Google Scholar]
- 2.Kvac M, Hofmannova L, Ortega Y, Holubova N, Horcickova M, Kicia M, et al. Stray cats are more frequently infected with zoonotic protists than pet cats. Folia Parasitol. (Praha). 2017;64:034. doi: 10.14411/fp.2017.034. [DOI] [PubMed] [Google Scholar]
- 3.Ryan U, Zahedi A, Feng Y, Xiao L. An update on zoonotic Cryptosporidium species and genotypes in humans. Animals. 2021;11:3307. doi: 10.3390/ani11113307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ryan UM, Feng Y, Fayer R, Xiao L. Taxonomy and molecular epidemiology of Cryptosporidium and Giardia—A 50 year perspective (1971–2021) Int. J. Parasitol. 2021;51(13):1099–1119. doi: 10.1016/j.ijpara.2021.08.007. [DOI] [PubMed] [Google Scholar]
- 5.Li J, Ryan U, Guo Y, Feng Y, Xiao L. Advances in molecular epidemiology of cryptosporidiosis in dogs and cats. Int. J. Parasitol. 2021;51(10):787–795. doi: 10.1016/j.ijpara.2021.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Cai W, Ryan U, Xiao L, Feng Y. Zoonotic giardiasis: An update. Parasitol. Res. 2021;120(12):4199–4218. doi: 10.1007/s00436-021-07325-2. [DOI] [PubMed] [Google Scholar]
- 7.Pipiková J, Papajová I, Majláthová V, Šoltys J, Bystrianska J, Schusterová I, et al. First report on Giardia duodenalis assemblage F in Slovakian children living in poor environmental conditions. J. Microbiol. Immunol. Infect. 2020;53(1):148–156. doi: 10.1016/j.jmii.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 8.Rauff-Adedotun AA, MohdZain SN, FarahHaziqah MT. Current status of Blastocystis sp. in animals from Southeast Asia: A review. Parasitol. Res. 2020;119(11):3559–3570. doi: 10.1007/s00436-020-06828-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Popruk S, Adao DEV, Rivera WL. Epidemiology and subtype distribution of Blastocystis in humans: A review. Infect. Genet. Evol. 2021;95:105085. doi: 10.1016/j.meegid.2021.105085. [DOI] [PubMed] [Google Scholar]
- 10.Rauff-Adedotun AA, Meor Termizi FH, Shaari N, Lee IL. The coexistence of Blastocystis spp. in humans, animals and environmental sources from 2010–2021 in Asia. Biology. 2021;10(10):990. doi: 10.3390/biology10100990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maloney JG, Santin M. Mind the gap: New full-length sequences of Blastocystis subtypes generated via Oxford nanopore minion sequencing allow for comparisons between full-length and partial sequences of the small subunit of the ribosomal RNA gene. Microorganisms. 2021;9(5):997. doi: 10.3390/microorganisms9050997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stensvold CR, Tan KSW, Clark CG. Blastocystis. Trends Parasitol. 2020;36(3):315–316. doi: 10.1016/j.pt.2019.12.008. [DOI] [PubMed] [Google Scholar]
- 13.Alfellani MA, Taner-Mulla D, Jacob AS, Imeede CA, Yoshikawa H, Stensvold CR, et al. Genetic diversity of Blastocystis in livestock and zoo animals. Protist. 2013;164(4):497–509. doi: 10.1016/j.protis.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 14.Maloney JG, Jang Y, Molokin A, George NS, Santin M. Wide genetic diversity of Blastocystis in white-tailed deer (Odocoileus virginianus) from Maryland, USA. Microorganisms. 2021;9(6):1343. doi: 10.3390/microorganisms9061343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hublin JSY, Maloney JG, Santin M. Blastocystis in domesticated and wild mammals and birds. Res. Vet. Sci. 2021;135:260–282. doi: 10.1016/j.rvsc.2020.09.031. [DOI] [PubMed] [Google Scholar]
- 16.Palmer CS, Thompson RC, Traub RJ, Rees R, Robertson ID. National study of the gastrointestinal parasites of dogs and cats in Australia. Vet. Parasitol. 2008;151(2–4):181–190. doi: 10.1016/j.vetpar.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 17.Karimi P, Shafaghi-Sisi S, Meamar AR, Nasiri G, Razmjou E. Prevalence and molecular characterization of Toxoplasma gondii and Toxocara cati among stray and household cats and cat owners in Tehran, Iran. Front. Vet. Sci. 2022 doi: 10.3389/fvets.2022.927185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hashemi-Hafshejani S, Meamar AR, Moradi M, Hemmati N, Solaymani-Mohammadi S, Razmjou E. Multilocus sequence typing of Giardia duodenalis genotypes circulating in humans in a major metropolitan area. Front. Med. 2022 doi: 10.3389/fmed.2022.976956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silva SOS, Richtzenhain LJ, Barros IN, Gomes AMMC, Silva AV, Kozerski ND, et al. A new set of primers directed to 18S rRNA gene for molecular identification of Cryptosporidium spp. and their performance in the detection and differentiation of oocysts shed by synanthropic rodents. Exp. Parasitol. 2013;135(3):551–557. doi: 10.1016/j.exppara.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 20.Cacciò SM, De Giacomo M, Pozio E. Sequence analysis of the β-giardin gene and development of a polymerase chain reaction–restriction fragment length polymorphism assay to genotype Giardia duodenalis cysts from human faecal samples. Int. J. Parasitol. 2002;32(8):1023–1030. doi: 10.1016/S0020-7519(02)00068-1. [DOI] [PubMed] [Google Scholar]
- 21.Read CM, Monis PT, Andrew Thompson RC. Discrimination of all genotypes of Giardia duodenalis at the glutamate dehydrogenase locus using PCR-RFLP. Infect. Genet. Evol. 2004;4(2):125–130. doi: 10.1016/j.meegid.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 22.Sulaiman IM, Fayer R, Bern C, Gilman RH, Trout JM, Schantz PM, et al. Triosephosphate isomerase gene characterization and potential zoonotic transmission of Giardia duodenalis. Emerg. Infect. Dis. 2003;9(11):1444–1452. doi: 10.3201/eid0911.030084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huey CS, Mahdy MAK, Al-Mekhlafi HM, Nasr NA, Lim YAL, Mahmud R, et al. Multilocus genotyping of Giardia duodenalis in Malaysia. Infect. Genet. Evol. 2013;17:269–276. doi: 10.1016/j.meegid.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 24.Stensvold CR, Arendrup MC, Jespersgaard C, Molbak K, Nielsen HV. Detecting Blastocystis using parasitologic and DNA-based methods: a comparative study. Diagn. Microbiol. Infect. Dis. 2007;59(3):303–307. doi: 10.1016/j.diagmicrobio.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 25.Meng XZ, Li MY, Lyu C, Qin YF, Zhao ZY, Yang XB, et al. The global prevalence and risk factors of Cryptosporidium infection among cats during 1988–2021: A systematic review and meta-analysis. Microb. Pathog. 2021;158:105096. doi: 10.1016/j.micpath.2021.105096. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y-G, Zou Y, Yu Z-Z, Chen D, Gui B-Z, Yang J-F, et al. Molecular investigation of zoonotic intestinal protozoa in pet dogs and cats in Yunnan Province, Southwestern China. Pathogens. 2021;10(9):1107. doi: 10.3390/pathogens10091107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang R, Ying JL, Monis P, Ryan U. Molecular characterisation of Cryptosporidium and Giardia in cats (Felis catus) in Western Australia. Exp. Parasitol. 2015;155:13–18. doi: 10.1016/j.exppara.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 28.Paoletti B, Otranto D, Weigl S, Giangaspero A, Di Cesare A, Traversa D. Prevalence and genetic characterization of Giardia and Cryptosporidium in cats from Italy. Res. Vet. Sci. 2011;91(3):397–399. doi: 10.1016/j.rvsc.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 29.Kwak D, Seo M-G. Genetic analysis of zoonotic gastrointestinal protozoa and Microsporidia in shelter cats in South Korea. Pathogens. 2020;9(11):894. doi: 10.3390/pathogens9110894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoshiuchi R, Matsubayashi M, Kimata I, Furuya M, Tani H, Sasai K. Survey and molecular characterization of Cryptosporidium and Giardia spp. in owned companion animal, dogs and cats, in Japan. Vet. Parasitol. 2010;174(3–4):313–316. doi: 10.1016/j.vetpar.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 31.Ito Y, Itoh N, Iijima Y, Kimura Y. Molecular prevalence of Cryptosporidium species among household cats and pet shop kittens in Japan. JFMS Open Rep. 2017 doi: 10.1177/2055116917730719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Homayouni MM, Razavi SM, Shaddel M, Asadpour M. Prevalence and molecular characterization of Cryptosporidium spp. and Giardia intestinalis in household dogs and cats from Shiraz, Southwestern Iran. Vet. Ital. 2019;55(4):311–318. doi: 10.12834/VetIt.1710.9049.3. [DOI] [PubMed] [Google Scholar]
- 33.Beser J, Toresson L, Eitrem R, Troell K, Winiecka-Krusnell J, Lebbad M. Possible zoonotic transmission of Cryptosporidium felis in a household. Infect. Ecol. Epidemiol. 2015;5(1):28463. doi: 10.3402/iee.v5.28463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rojas-Lopez L, Elwin K, Chalmers RM, Enemark HL, Beser J, Troell K. Development of a gp60-subtyping method for Cryptosporidium felis. Parasit. Vectors. 2020;13(1):39. doi: 10.1186/s13071-020-3906-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li J, Dan X, Zhu K, Li N, Guo Y, Zheng Z, et al. Genetic characterization of Cryptosporidium spp. and Giardia duodenalis in dogs and cats in Guangdong, China. Parasit. Vectors. 2019;12(1):571. doi: 10.1186/s13071-019-3822-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sotiriadou I, Pantchev N, Gassmann D, Karanis P. Molecular identification of Giardia and Cryptosporidium from dogs and cats. Parasite. 2013;20:8. doi: 10.1051/parasite/2013008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feng Y, Xiao L. Zoonotic potential and molecular epidemiology of Giardia species and giardiasis. Clin. Microbiol. Rev. 2011;24(1):110–140. doi: 10.1128/cmr.00033-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu H, Jin Y, Wu W, Li P, Wang L, Li N, et al. Genotypes of Cryptosporidium spp., Enterocytozoon bieneusi and Giardia duodenalis in dogs and cats in Shanghai, China. Parasit. Vectors. 2016;9(1):121. doi: 10.1186/s13071-016-1409-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mohammadpour I, Bozorg-Ghalati F, Gazzonis AL, Manfredi MT, Motazedian MH, Mohammadpour N. First molecular subtyping and phylogeny of Blastocystis sp. isolated from domestic and synanthropic animals (dogs, cats and brown rats) in southern Iran. Parasit. Vectors. 2020;13(1):365. doi: 10.1186/s13071-020-04225-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Farah Haziqah MT, Chandrawathani P, Douadi B, Suresh K, Wilson JJ, Mohd Khalid MKN, et al. Impact of pH on the viability and morphology of Blastocystis isolates. Trop. Biomed. 2018;35(2):501–510. [PubMed] [Google Scholar]
- 41.Ruaux CG, Stang BV. Prevalence of Blastocystis in shelter-resident and client-owned companion animals in the US Pacific Northwest. PLoS ONE. 2014;9(9):e107496. doi: 10.1371/journal.pone.0107496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Can H, Köseoğlu AE, Erkunt Alak S, Güvendi M, Ün C, Karakavuk M, et al. Molecular prevalence and subtyping of Blastocystis sp. isolates in stray cats of İzmir, Turkey: First report of "ST4 allele 42" in cats. Pol. J. Vet. Sci. 2021;24(2):217–223. doi: 10.24425/pjvs.2021.137656. [DOI] [PubMed] [Google Scholar]
- 43.Nagel R, Cuttell L, Stensvold CR, Mills PC, Bielefeldt-Ohmann H, Traub RJ. Blastocystis subtypes in symptomatic and asymptomatic family members and pets and response to therapy. Intern. Med. J. 2012;42(11):1187–1195. doi: 10.1111/j.1445-5994.2011.02626.x. [DOI] [PubMed] [Google Scholar]
- 44.Eroglu F, Koltas IS. Evaluation of the transmission mode of B. hominis by using PCR method. Parasitol. Res. 2010;107(4):841–845. doi: 10.1007/s00436-010-1937-4. [DOI] [PubMed] [Google Scholar]
- 45.Li W, Liu X, Gu Y, Liu J, Luo J. Prevalence of Cryptosporidium, Giardia, Blastocystis, and trichomonads in domestic cats in East China. J. Vet. Med. Sci. 2019 doi: 10.1292/jvms.19-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Udonsom R, Prasertbun R, Mahittikorn A, Mori H, Changbunjong T, Komalamisra C, et al. Blastocystis infection and subtype distribution in humans, cattle, goats, and pigs in central and western Thailand. Infect. Genet. Evol. 2018;65:107–111. doi: 10.1016/j.meegid.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 47.Paulos S, Köster PC, de Lucio A, Hernández-de-Mingo M, Cardona GA, Fernández-Crespo JC, et al. Occurrence and subtype distribution of Blastocystis sp. in humans, dogs and cats sharing household in northern Spain and assessment of zoonotic transmission risk. Zoonoses Public Health. 2018;65(8):993–1002. doi: 10.1111/zph.12522. [DOI] [PubMed] [Google Scholar]
- 48.Tito RY, Chaffron S, Caenepeel C, Lima-Mendez G, Wang J, Vieira-Silva S, et al. Population-level analysis of Blastocystis subtype prevalence and variation in the human gut microbiota. Gut. 2019;68(7):1180–1189. doi: 10.1136/gutjnl-2018-316106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stensvold CR, Clark CG. Current status of Blastocystis: A personal view. Parasitol. Int. 2016;65(6 Pt B):763–771. doi: 10.1016/j.parint.2016.05.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its supplementary information files. The sequences were submitted to DDBJ/EMBL/GenBank databases (https://www.ncbi.nlm.nih.gov/nuccore/) under accession numbers LC700089–LC700098 and LC700104–LC700118.