Abstract
Background
Aging is a multifactorial degenerative process that can be modulated by fasting through activation of the Fork-head transcription factor of the O class 3 (FOXO3), which plays an important role in increasing lifespans. However, the effects of different fasting durations on the expression of FOXO3 in the liver has not yet been reported.
Objective
This study analyzed the effects of different fasting durations on the FOXO3 expression and its pathway by measuring sirtuin1 (SIRT1), insulin-like growth factor-1 (IGF-1), and superoxide dismutase (SOD) activity in the liver.
Methods
New Zealand white rabbits were used to mimic the effects of fasting on humans. The rabbits were divided into the control, intermittent fasting (IF), and prolonged fasting (PF) groups. Both fasting groups were interspersed with the non-fasting phase for 8 h. This treatment was conducted for 6 days. On Day 7, all the rabbits were sacrificed, and their livers were taken to measure the FOXO3 and SIRT1 mRNA expressions, the IGF-1 protein level, and the SOD activity level. ANOVA, multiple comparison, and Pearson's correlation were performed for statistical analysis.
Results
The FOXO3 and SIRT1 mRNA expressions were significantly higher in the IF group than in the control group. The FOXO3 expression was also 2.5 times higher in the IF group than in the PF group. There was a positive correlation between the FOXO3 and SIRT1 mRNA expressions. The IGF-1 protein level was significantly lower in the IF and PF groups than in the control group. The SOD-specific activity level was significantly higher in the IF group than in the control and PF groups.
Conclusions
Intermittent fasting significantly increased the FOXO3 and SIRT1 mRNA expressions and the SOD activity level in the livers of the rabbits and significantly decreased the circulating and hepatic IGF-1. Therefore, intermittent fasting may give a protective intervention effect towards aging.
Keywords: FOXO3, IGF-1, SIRT1, SOD, Liver, Fasting
1. Introduction
Aging is a complex process resulting from the accumulation of various factors, consisting of destruction in molecular, cellular, and organ levels, which consequently leads to loss of function and increased vulnerability towards diseases, ultimately causing death [1]. The number of older people (people aged 60 or above) in the population continues to increase globally, and by 2050, is estimated to be more than 1.5 billion. Within the same period, the oldest group (people aged 80 or above) is predicted to triple in number. The increase in the aged population will definitely bring many challenges. For instance, an increase in lifespan is not always beneficial if not accompanied by an equal increase in health span, as the additional years of life will only be filled with diseases and disability. Moreover, there is a wide variability in the health status of older individuals; for instance, the mental and physical capacities of some 80-year-olds are similar to those of a 20-year-old, while those of other 80-year-olds decline at much earlier ages [2]. Unfortunately, the complex processes and reasons behind this variability are not yet fully understood.
Despite the complicated process of aging, it has been found that genetic and dietary changes can substantially increase the longevity of organisms [3]. First, with regard to genetics, many mutations that increase longevity affect stress-response or nutrient-sensor genes. One of these genes that have been extensively investigated and have shown a promising association with longevity is the fork-head transcription factor of the O class (FOXO), which is activated by upstream signals such as Sirtuin1 (SIRT1) when the organism faces low nutrient availability, such as during starvation [4,5]. FOXOs are crucial as they regulate several target genes that are involved in energy metabolism. In other words, they regulate metabolic balance. For instance, when we are starved, our resulting low insulin level activates the FOXOs in our liver to increase our glucose levels via gluconeogenesis and glycogenolysis [6].
Many studies have found that FOXOs also have important roles in various cellular processes. As transcription factors, they can either activate or inhibit downstream target genes, which eventually induce different cellular processes. One of the FOXOs subfamily, FOXO3, can respond to stimuli such as stress from metabolism, oxidation, reduction in insulin and growth factor number. These processes would further create response of anti-aging, by facilitating important processes such as stem cell homeostasis, differentiation, cell apoptosis regulation, and reactive oxygen species (ROS) destruction through activation of endogenous antioxidants such as superoxide dismutase (SOD) [6,7]. These processes collectively protect organisms from many diseases. Thus, it can be concluded that FOXO3 gene expression prevents aging by regulating various cellular processes [7,8].
In terms of dietary changes, nutrient and stress sensors can mediate lifespan extensions by allowing the organism to adapt to different physiological and environmental signals. This leads to the selective investment of energy into various protective systems, which minimize damage that can reduce fitness. Caloric restriction and fasting have been proven to be the optimal signals that can extend lifespan and promote stress resistance effects that have been shown to be conserved throughout the course of the evolution of low-level organisms such as yeast all the way to higher organisms such as primates [3]. Fasting generally induces significant reduction of the level of circulating insulin-like growth factor-1 (IGF-1), whose signaling pathways, through the protein kinase B (PKB or Akt) pathway, negatively regulate FOXO transcription factors [3]. A better understanding of the relationship between FOXO3 gene expression and dietary restriction in the form of fasting, both of which can boost an organism's longevity, would help determine a specific target for therapy. In addition, deeper insight into this area will allow us to further modulate FOXO3 gene expression through dietary modification and to propose the optimal dietary restriction or form of fasting to slow down the decline in function and the onset of disease due to aging. Thus, this study expounded how different durations of fasting affect FOXO3 expression and its pathway in the liver by implementing intermittent fasting (i.e., fasting for 16 h) and prolonged fasting (i.e., fasting for 40 h).
In this study, New Zealand white (NZW) rabbits were used to mimic the effects of fasting that are generally represented in humans and not in small laboratory rodents (i.e., mice and rats). Previous studies were more focused on low-level organisms [9], which limited such studies as these organisms are less capable of representing or simulating the effects of fasting on humans. The liver was chosen as the sample because it has shown a significant response to fasting to maintain homeostasis [10]. Moreover, aging decreases the liver function, and the elderly are more susceptible to liver disease [11]. In addition, the effects of different fasting durations on FOXO3 expression in the liver have yet to be explored. The aim of this study was to analyze FOXO3 expression and its pathway by measuring the SIRT1 and IGF-1 expressions as FOXO3's upstream signals, and the SOD activity as FOXO3's downstream target in the livers of rabbits whose diets have been restricted through intermittent fasting (for 16 h) and prolonged fasting (for 40 h). Therefore, this study is proposed to be the basis of FOXO3 gene modulation and fasting in humans to achieve healthy aging.
2. Methods
This research was an experimental study conducted on 15 NZW male rabbits with weights between 1800 and 2500 g. They were divided into three groups of five. The most suitable rabbit age that can represent human age, the calculation was performed by setting the life expectancy of NZW rabbits as about 8–12 years in general. The disparity between the developmental stages of humans, and rabbits is also being acknowledge in which life phase of NZW rabbits are being considered to be able accurately correlates with the life phase in human. Age of the animals was 4 months which is about 12 years in human age. NZW rabbits are in the pubertal phase during this age, and one human year is equivalent to 13.04 days for NZW rabbits. This calculation was achieved by understanding that NZW rabbits attain their puberty around P150, thus it can be calculated as 11.5 years × 365 days equal to 4198 days. Further, 4198 days were divided by 150 which equals 27.98 human days equal to 1 rabbit day. Lastly, 365 days divided by 27.98 human days equal to 13.04 NZW rabbits day that equal to 1 human year. It is the shortest equivalent value for a rabbit's lifespan in its life phase [12].
The rabbits were maintained in a natural light and dark cycle at 25 ± 2 °C. Each group was given a different treatment. The control group was normally fed ad libitum with a commercial pellet diet and given free access to water for six days. The IF group was not fed for 16 h (from 16:00 to 08:00 of the next day) for the same six days, interspersed by the non fasting phase for 8 h in diurnal time hours. Finally, the PF group was not fed for 40 h (starting on 16:00–16:00 for the first 24 h and continued until 08:00 in the second 24 h), with a similar 8 h non fasting phase interspersed in diurnal time hours. During the 8-h period when the rabbits could eat, the food that was given to them was 5% of their body weight, which was their daily normal intake [13]. The rabbits’ body weights were measured every day until Day 7, when they were anesthetized using 35 mg of ketamine per kg of body weight and 5 mg of intramuscular xylazine per kg of body weight to obtain their liver as the sample. At the end of the treatments, plasma glucose was detected in all the groups using the GLUC-PAP kit (Randox®). All the procedures were approved by the Ethics Committee of the Faculty of Medicine of Universitas Indonesia, with ethical approval number KET-249/UN2.F1/ETIK/PPM.00.02/2020.
2.1. RNA isolation for FOXO3 and SIRT1 mRNA expressions
From each of the liver samples of the three groups of rabbits, an RNA sample was isolated using an RNA isolation kit (Quick-RNA Miniprep Plus, Zymo®), according to the manufacturer's procedure. The isolated RNA of each liver sample was quantified using the Varioskan™ Flash Multimode Reader. Then, a Thermo Scientific μDrop Plate was wiped with lens tissue dampened with distilled water, followed by lens tissue dampened with 70% ethanol, to remove any contaminants, after which 2 μl of the purified RNA of each sample was pipetted onto the plate. Then, the concentrations (in ng/μl) and purities (A260/A280 and A260/A230) of RNA were measured, following which the quantified RNA samples were diluted in nuclease-free water to obtain the final concentration of 50 ng/μl.
2.2. FOXO3 and SIRT1 mRNA expressions via quantitative polymerase chain reaction
The mRNA sample (100 ng) was amplified using a quantitative polymerase chain reaction (qPCR) machine (Applied Biosystems 7500 Fast Instrument, USA) using a SensiFAST SYBR No-ROX One-Step Kit (Bioline®). First, the RNA was converted to complementary DNA (cDNA) by the reverse transcriptase enzyme for 10 min at 42 °C. Second, the reverse transcriptase was inactivated by setting the temperature at 95 °C for 5 min. Third, the cDNA was amplified through 40 cycles of PCR using the primers listed in Table 1. The primers were designed using BLAST primer tools, with 18S rRNA as the reference gene. The mRNA expressions of FOXO3 and SIRT1 were determined using the Livak (2−ΔΔCt) method and written in the form of a ratio (a relative expression). The Livak formula was used to analyze the expression levels of FOXO3 and SIRT1 in the control, IF, and PF groups. The Livak method is one of the most common methods of analyzing changes in gene expression relative to the real-time qPCR result [14].
Table 1.
List of primers.
| No. | Gene | Sequence | Product Size |
|---|---|---|---|
| 1. |
FOXO3 XM_008263339 |
F: TTGGAAGAACTCCATCCGACA R: CCACGGCTCTTGGTGTACTT |
180 bp |
| 2. |
SIRT1 XM_002718460 |
F: GAGCTGGGGTGTCTGTTTCA R: ACTTGAAGAATGGCCGAGGA |
153 bp |
| 3.. |
18S rRNA NR_033238.1 |
F: AAACGGCTACCACATCCAAG R: CCTCCAATGGATCCTCGTTA |
155 bp |
Note:
ΔΔCT = ΔCT(test) - ΔCT(calibrator)
ΔCT(test) = CT(target, test) - CT(reference, test)
ΔCT(calibrator) = CT(target, calibrator) - CT(reference, calibrator)
Targets = FOXO3 and SIRT1 genes.
Test = IF and PF groups.
Calibrator = control group.
Reference = 18S rRNA gene (reference gene)
2.3. Analysis of the FOXO3 Protein Expression via Enzyme-Linked Immunosorbent Assay (ELISA)
The FOXO3 protein was determined using the Rabbit Forkhead Box Protein O3 (FOXO3) ELISA Kit (MyBioSource®). This kit uses the Double Antibody Sandwich Technique, which depends on the tested antigen characteristics with more than two valences. Moreover, the kit can simultaneously identify both detection antibodies and coated antibodies. One hundred μL of rabbit FOXO3 standards with different concentrations or samples were added into the corresponding wells and incubated for 90 min at 37 °C. One hundred microliters of biotinylated rabbit FOXO3 antibody was added into each of the wells after the washing process and incubated at 37 °C for 60 min. Then, the plate was washed three times, after which 100 μL of the enzyme-conjugate liquid was put into each well and incubated for 30 min at 37 °C. This was followed by the addition of 100 μL of color reagent liquid after the plate was 5X washed. The occurrence of the chromogenic reaction was controlled within 30 min inside the dark incubator at 37 °C. The optical density was read at 450 nm within 10 min after 50 μL of the stop solution was added.
2.4. Analysis of the IGF-1 protein expression via ELISA
IGF-1 protein was detected in plasma in the forms of circulating IGF-1 and IGF-1 in the liver. It was determined using a rabbit IGF-1 ELISA Kit (Cusabio, USA®). The rabbit IGF-1 standard with different concentrations or samples was added into the corresponding wells (with each well filled to 100 μL) and incubated for 2 h at 37 °C. One hundred microliters of biotinylated rabbit IGF-1 antibody was added into each of the wells after the washing process and incubated at 37 °C for 60 min. Then, the plate was washed three times, and then 100 μL of the enzyme-conjugate liquid was put into each well and incubated for 60 min at 37 °C. The addition of 90 μL of color reagent liquid was performed to each well after the 5X washing process. The occurrence of the chromogenic reaction was controlled within 15 min inside the dark incubator at 37 °C. The optical density was read at 450 nm within 10 min after 50 μL of the stop solution was added.
2.5. Specific activity of the superoxide dismutase (SOD) enzyme
The SOD activity was determined using a Ransod kit (RANDOX,®, United Kingdom). This method uses xanthine along with xanthine oxidase to produce superoxide radicals that will further react with 2-(4-iodophenyl)-3-(4-nitrophenol)-5-phenyltetrazolium chloride (INT) to produce red formazan dye. Thus, the SOD activity can be measured through the degree of inhibition of this reaction. A 50% inhibition of the rate of INT reduction in this assay was equal to one unit of active SOD. Thirty microliters of the samples were put in the cuvette, after which 1000 μL of mixed substrate was added, mixed, and followed by the addition of 150 μL of xanthine oxidase. The standards were prepared using the same protocol. The absorbance was read at 505 nm 2 times after the first 30 s and 3 min later. The specific activity of the SOD enzyme was determined by dividing the SOD activity by the protein level [15].
2.6. Statistical analysis
The results are presented in mean ± standard deviation (SD) values. Significance differences between the groups were analyzed using one-way analysis of variance (ANOVA) and Least Significant Difference (LSD) post hoc multiple comparison. The parametric distribution assumption was tested first using the Shapiro–Wilk test for the test of homogeneity of variances. Correlation analysis was performed using the Pearson correlation test. The data were said to be significant when the p value was <0.05.
3. Results
3.1. Characteristics of rabbits
-
a.
Body Weight Measurement
The changes in the body weights of the rabbits in the three experiment groups throughout the experiment period were measured. However, there was no significant difference in the body weights day to day within and among the groups, as shown in Table 2.
-
b.
Plasma Glucose Levels
Table 2.
Body weight of the rabbits.
| Body Weight (g ± SD) |
p-value | |||||||
|---|---|---|---|---|---|---|---|---|
| Experiment Group | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | |
| Control (n = 5) | 1912 ± 430.9 | 1984 ± 461.3 | 2058 ± 481.5 | 2024 ± 490.2 | 2124 ± 557.2 | 2170 ± 521.4 | 2160 ± 511.1 | >0.05 |
| IF (n = 5) | 2210 ± 281.9 | 2228 ± 305.7 | 2138 ± 414.3 | 2192 ± 301.7 | 2316 ± 340.9 | 2304 ± 349.6 | 2288 ± 375.9 | >0.05 |
| PF (n = 5) | 1910 ± 329 | 1968 ± 303.9 | 1858 ± 318.9 | 1914 ± 270.2 | 1878 ± 302.2 | 2018 ± 278.6 | 1966 ± 264.8 | >0.05 |
| P | >0.05 | >0.05 | >0.05 | >0.05 | >0.05 | >0.05 | >0.05 | |
Note: From the one-way analysis of variance (ANOVA) test. IF = intermittent fasting; PF = prolonged fasting.
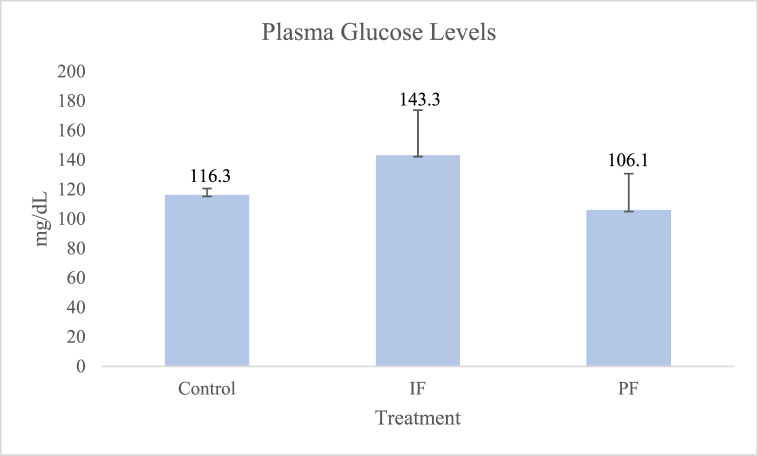
There was no significant difference between the plasma glucose levels of the three groups, as shown in Fig. 1 (control group, 116.3 ± 4.4 mg/dL; IF group, 143.3 ± 30.56 mg/dL; PF group, 106.1 ± 24.6 mg/dL).
-
c.
Levels of FOXO3 and SIRT1 mRNA Expressions
Fig. 1.
The plasma glucose levels of the three groups. There was no significant difference (p > 0.05) between the groups [based on one-way analysis of variance (ANOVA)]. IF = intermittent fasting; PF = prolonged fasting.
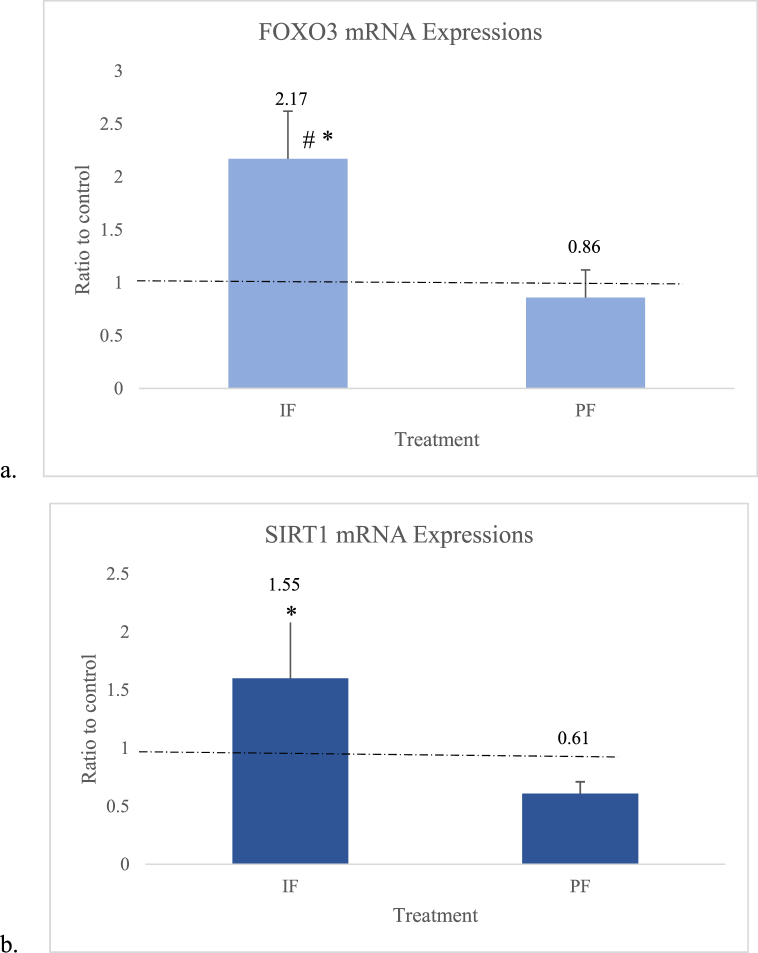
Our research showed that a significant increase in the FOXO3 mRNA expression in the IF group by approximately 2.17 times (p < 0.01) compared to the control group (Fig. 2a). The FOXO3 mRNA expression in the PF group was lower, 0.86 times (Fig. 2a), in comparison with the control group. The SIRT1 mRNA expression in the IF group increased by 1.55 times (p > 0.05) compared to the control group and significantly increased by 2.55 times (p < 0.01) compared to the PF group. On the other hand, the SIRT1 mRNA expression of the PF group significantly decreased by 0.61 times compared to the control group (Fig. 2b). There was a significant positive correlation (R = 0.641, p = 0.010) between the FOXO3 and SIRT1 mRNA expressions.
-
d.
FOXO3 Protein Expressions
Fig. 2.
a. FOXO3 mRNA expressions. b. SIRT1 mRNA expressions. #: significantly different between the IF and control groups, *: significantly different between the IF and PF groups. IF = intermittent fasting, PF = prolonged fasting. For the FOXO3 mRNA expressions: analysis of variance (ANOVA) and Least Significant Difference (LSD post hoc multiple comparisons. For the SIRT1 mRNA expressions: Kruskal–Wallis and LSD post hoc multiple comparisons. Each group consisted of five New Zealand white rabbits.
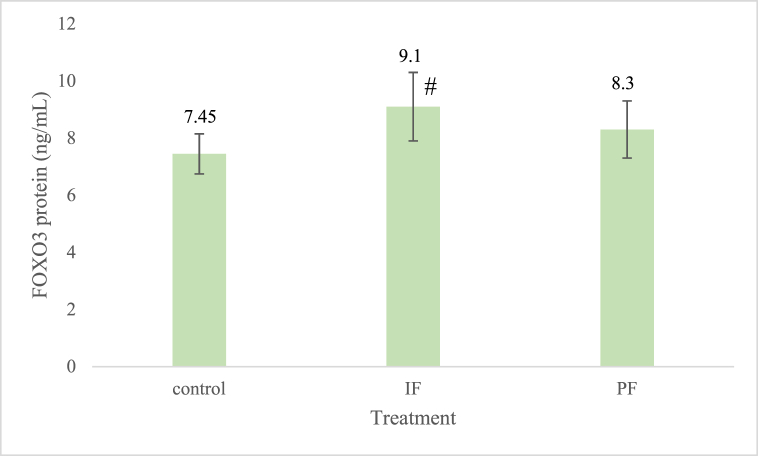
The FOXO3 protein level in the control group was 7.45 ± 0.7 ng/mL, whereas in the IF group, it was 9.1 ± 1.2 ng/mL and in the PF group, 8.3 ± 1 ng/mL, as shown in Fig. 3. The FOXO3 protein expressions were determined to ensure that the increases in the mRNA levels corresponded with the increases in the protein levels. There was a significantly positive correlation (R = 0.533, p = 0.041) between the FOXO3 mRNA and protein levels. Moreover, the FOXO3 protein was significantly correlated with the SIRT1 mRNA (R = 0.530, p = 0.042).
-
e.
Plasma and Hepatic IGF-1 Proteins
Fig. 3.
FOXO3 protein expressions in the three groups. The FOXO3 protein expression in the IF group was significantly higher (p = 0.025) than that in the control group. #: significantly different between the IF and control groups. IF = intermittent fasting, PF = prolonged fasting. Based on analysis of variance (ANOVA) and Least Significant Difference (LSD) post hoc multiple comparisons. There were five New Zealand white rabbits in each group.
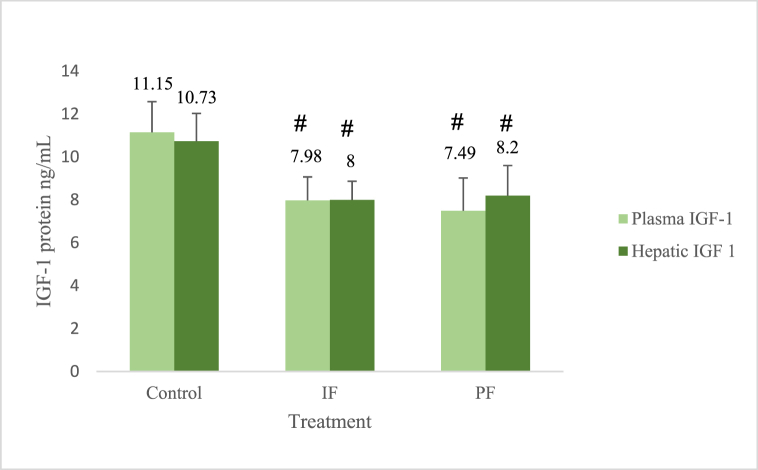
Both the plasma IGF-1 protein, which represent the circulating IGF-1, and the hepatic IGF-1 protein, were significantly lower in the IF and PF groups than in the control group, as shown in Fig. 4. The plasma and hepatic IGF-1 proteins in the control group were 11.15 and 10.73 ng/mL; in the IF were 7.98 and 8 ng/mL, and in the PF were 7.49 and 8.2 ng/mL, respectively.
-
f.
Specific Activity of the Superoxide Dismutase Enzyme
Fig. 4.
IGF-1 protein levels. The plasma IGF-1 levels were significantly lower both in the IF group (p = 0.010) and in the PF group (p = 0.004) than in the control group. The hepatic IGF-1 levels were significantly lower both in the IF group (p = 0.012) and in the PF group (p = 0.018) than in the control group. #: significantly different between the IF or PF and control groups. Based on the analysis of variance (ANOVA) and Least Significant Difference (LSD) post hoc multiple comparisons. Five New Zealand white rabbits were in each group.
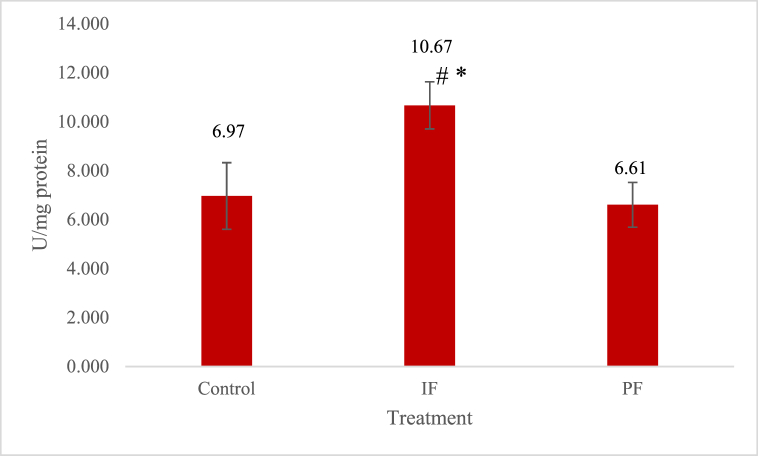
The specific activity of superoxide dismutase (SOD) in the IF group (10.67 ± 0.963 U/mg protein) was significantly higher than in the control group (6.97 ± 1.36 U/mg protein) and in the PF group (6.61 ± 0.913 U/mg protein), as shown in Fig. 5. There was no significant difference between the control and PF groups. This specific SOD activity was significantly correlated with the FOXO3 mRNA (R = 0.583, p = 0.023).
Fig. 5.
Specific activities of the SOD enzymes in the liver tissues of the three groups. The SOD activity in the IF group was significantly higher (p < 0.05) than that in the control and PF groups. Note: significantly different between the IF and control groups. *: significantly different between the IF and PF groups. IF = intermittent fasting, PF = prolonged fasting. Based on analysis of variance (ANOVA) and Least Significant Difference (LSD) post hoc multiple comparisons. There were five New Zealand white rabbits in each group.
4. Discussion
The body weights of the rabbits changed only slightly, fluctuating at around 50–60 g per day. Kawamura et al. [16] also found fluctuations of around 15–70 g per day in the body weights of male NZW rabbits aged 5–7 months. The daily fluctuations in the body weights of the rabbits is a common physiological phenomenon [17]. The short duration of the fasting (six days) in this experiment might explain the non-occurrence of weight loss in both the IF and PF groups. There was no significant decrease in the plasma glucose level in the fasting group compared to the control group. An insignificant decrease in the plasma glucose level was detected in the PF group. The plasma glucose level could have remained normal in all the groups due to the gluconeogenesis process, which is regulated by the FOXO3 expression. Gluconeogenesis is the sole energy source of many tissues during fasting [8].
A vast body of evidence is surfacing that proves that IF lowers oxidative stress and inflammation. This trend was the result of the discovery that metabolic diseases such as diabetes start with oxidative stress and chronic inflammation. Various studies have attempted to discover the impact of IF, such as of Ramadan intermittent fasting (RIF), on the expressions of antioxidant genes and genes correlated with cellular metabolism, such as SIRT1. The research found that RIF could increase the genetic expressions of various genes, including of metabolic regulatory, anti-inflammatory, and antioxidant genes. Therefore, it was suggested that RIF might have a protective effect against adverse metabolism-related derangements and oxidative stress [18]. Another study supported this statement after finding that RIF lowered visceral adiposity and body weight, which reduced the risk factor of ROS accumulation [19]. This was further confirmed by the finding from a systematic review and meta-analysis that diurnal IF during Ramadan had a protective effect against an increase in oxidative stress and inflammatory markers. Thus, it can suppress low-grade systemic oxidative stress and inflammation and prevent subsequent adverse health effects in humans [20].
Consistent with previous research, our experiment showed that the FOXO3 expression both at the mRNA and protein levels increased more significantly in the IF group (i.e., with 16 h of fasting) than in the control group and the PF group. These data implied that IF in NZW rabbits had higher liver FOXO3 expressions than in the two other groups. This finding is similar to that of Imae et al. [9], who examined the mRNA concentration in rat liver in response to internal and external triggers such as hormonal and nutritional factors. The rat group was subjected to fasting, after which significant increases were seen in the levels of its FOXO1 mRNA (by 1.5 times), FOXO4 (by 1.6 times), and FOXO3 (by 1.4 times). After the rats were fed again for 3 h, the levels of their induced FOXO1 and FOXO3 mRNA expressions went back to the control levels, but not of their FOXO4 mRNA expressions [9]. Furuyama et al. [21] also investigated the expression levels of mammalian daf-16 gene homologs, including of the mRNAs of the FOXO family genes examined in the rat skeletal muscle, which were found to have increased during fasting.
Higher FOXO3 expression has a protective effect on the liver against the aging process. The main anti-aging mechanism of FOXOs is their being transcription factors, which are crucial for stem cell function in both adults and embryos [22]. In adults, FOXOs are needed for stem cell functionality in multiple tissues. FOXO3 plays an important role in aging by maintaining stem cell homeostasis such as hematopoietic, neural, and muscle cell homeostasis. The correlation of stem cells with FOXO suggests the hypothesis that FOXO functions and regulations are pivotal in maintaining stem cell properties. Thus, it is proposed that the main contribution of FOXO3 to longevity is its ability to modulate stem cells [22]. For instance, FOXO3 plays a role in satellite cells as the stem cells of skeletal muscle. In the event of cellular damage or stressful conditions, FOXO3 proteins are expressed to induce Notch signaling activation to increase the rate of stem cell self-renewal. In normal conditions, they maintain the satellite cell pool so that it could actively replicate while remaining in its undifferentiated state [23]. However, in pathological conditions, overexpression of FOXO3 is associated with the development and progression of several diseases, such as Alzheimer's disease [24] and cancer [[25], [26], [27]]. The pathogenesis of Alzheimer's disease involves activation of the FOXO signaling pathway, which induces neuronal cell death [24]. Moreover, activation of FOXO3 stimulates chemotherapy drug resistance in glioblastoma multiforme [25] and hepatocellular carcinoma [26,27]. Therefore, the protective effect of FOXO3 mainly plays a role in normal cells.
In 1990, Straus et al. [28] bridged the knowledge between fasting (nutritional) and growth factors (hormonal) that may affect the aging process. Their research further investigated the effect of fasting on the level of IGF-1 expression in animals based on its mRNA levels. Animal growth is fundamentally regulated by the intricate interplay between nutritional, hormonal, and even genetic variables. The hormones that are largely known to have important functions in regulating the somatic growth of cells are IGF-I and -II. Straus et al. measured the mRNA levels of IGF-I in six-week-old male control rats that were fed ad libitum and also fasted for 24, 48, or 72 h. The mRNA concentrations of several IGF-I species (8.0, 4.0, 1.7, and 1.0 kilobases) were reduced in the animals that fasted and further rebounded after 24 h of refeeding, even though they were not able to reach the initial control levels [28]. It was discovered that the insulin/IGF-1 cascade was involved in inhibiting the activity of the FOXO3 gene, the gene most widely known to be correlated to human longevity due to its role in the molecular signaling hub and chromatin conformation. Thus, decreasing the IGF-I level positively affects the organism by increasing its FOXO3 expression [29]. Our study showed that the circulating and hepatic IGF-1 levels more significantly decreased in the IF group than in the control group. Therefore, the increase in FOXO3 in the IF group was probably due to the decrease in IGF-1.
Activation of the FOXO3 expression was regulated by various types of post-translational modification (PTM). Modulation of FOXO3 expression and subsequent controlled target genes greatly depends on the process of FOXO3 translocation control between the cytoplasm and the nucleus, which can be done by utilizing the phosphorylation process with the help of various kinases. Nuclear FOXO3, which undergoes the serial process of PTMs, has the main function of modulating FOXO3 transcriptional activity by altering the binding specificity of the promoter and changing the binding affinity of DNA [30]. In the nucleus itself, FOXO3, which remains, will be further acetylated utilizing p300 and CREB-binding protein (CBP), or it can also go through a vice versa mechanism in which it is deacetylated by SIRT1 [4]. Fasting results in low-calorie intake, which triggers the activation of sirtuins [31] and eventually activates FOXOs. Our results showed that SIRT1 mRNA expression was higher in the IF group and was positively correlated with FOXO3 expression. SIRT1 deacetylates FOXOs, inducing the expression of genes correlated with mitochondrial biogenesis and stress resistance adaptation [32].
Increased activation of SIRT1 gene expression in IF mediates the beneficial metabolic switch in various organs. During fasting, there is a period of starvation in which there is no dietary intake, meaning it induces low cellular energy status, which increases the NAD level+ as well as the NAD+/NADH ratio. Considering that NAD+ is an absolute requirement for SIRT1 enzymatic reaction, the increase in this cofactor increases the activation of SIRT1. However, Madkour et al. [18] found that relative mRNA expression of SIRT1 insignificantly decreased by around 10.4% in 56 obese and overweight subjects (22 women and 34 men) who underwent Ramadan IF. The insignificant decline in the SIRT1 expression in such research was related to the insignificant change in the total energy and the lipid dietary intake, accompanied by abundant simple carbohydrate intake during the non-fasting time at night during the Ramadan period. The low expression of SIRT1 in that research was in line with insignificant changes in insulin and insulin resistance (determined via HOMA-IR) as markers of glucose homeostasis. Hence, the activity of SIRT1 is said to reflect the metabolic state of cells [33]. When SIRT1 increases, the deacetylation of Peroxisome proliferator-activated receptor-gamma coactivator (PGC)-1a increases, and it is activated. As PGC-1a regulates numerous genes involved in metabolism processes such as thermogenesis, biogenesis of mitochondria, and gluconeogenesis, its activation leads to an increase in mitochondrial activity and glucose metabolism [34]. Several studies with SIRT1 knockout mice further revealed the importance of SIRT1 in mediating response, as they lack the characteristic changes in IGF-1 and longevity [35,36]. Therefore, previous studies have shown that deprivation of food during fasting can lead to protective and pro-longevity effects on different organisms, but the specific response to fasting differs from species to species [37].
Activation of FOXO3 expression by IF plays an important role in maintaining redox balance and ROS detoxification [38]. FOXO3 expression regulates SOD [38], an endogenous antioxidant that converts superoxide radicals to less reactive hydrogen peroxide (H2O2) [39]. Our results proved that the specific activity of the SOD enzyme in the IF group was significantly higher than in the control and PF groups. Preoperative fasting has been studied in mice to protect against renal ischemia–reperfusion injury, which is closely related to the production of ROS [40,41]. Ischemia-reperfusion cause oxidative stress, as can be seen in the increased levels of 4-Hydroxynonenal (4-HNE) and protein carbonyls in the kidney and the decreased activity of glutathione S-transferase, SOD, and glutathione peroxidase [42]. Mitchell et al. [41] proposed that the mRNA of specific antioxidant proteins involved in ischemia-reperfusion injury are increased during fasting, which suggests the protective effect of fasting against oxidative stress caused by reperfusion.
In contrast to the IF group, in the PF group, there was no significant change in the FOXO3 expression, SIRT1 mRNA expression, and SOD specific activity compared to the control, but the expressions were shown to have decreased compared to the IF group. It is possible that in PF, there is a reduction in the protein synthesis and overall proteolytic rates. Price et al. [43] demonstrated the effect of long-term calorie restriction on hepatic tissue in mice. Other studies postulated that chronic dietary restriction or PF itself has a negative effect on longevity, as already shown in several other studies on various types of organisms, such as rodents [44,45]. The significant decrease in the IGF-1 levels in the PF group compared to the control group was unable to increase the FOXO3 expression. Therefore, we can assume that other pathways besides IGF-1 may be involved in regulating FOXO3 expression during PF conditions, but that pathway is outside the scope of this study.
This study was limited by a relatively short period of treatment, which prevented full description of the effects of different fasting regimens with much longer durations. Similar experiments with longer durations could show if the effect could be sustained in the long term. Furthermore, in this experiment, the food intake and fecal production were not assessed, which made it unclear whether the metabolic changes in the NZW rabbits were due to the fasting regimen or the overall change or reduction in the total caloric and macronutrient intake. In addition, this study used a minimal sample for each group (five rabbits) and did not use older rabbits. This was because we wanted to analyze the effect of fasting on the expression of FOXO3 as an anti-aging molecule at a young age to promote such intervention as stimulating anti-aging molecules from a young age, which is probably beneficial for achieving a healthy condition in old age. However, comparison of various age groups is needed to analyze the response of different ages in this research scheme. We also recommend that future researchers discover the other factors that may contribute to the expression rate of the FOXO3 gene, especially in the fasting condition. In addition, further research should measure the protein levels of FOXO3, SIRT1, and IGF-1 using western blotting, immunochemistry, or immunoblot to validate the ELISA results in this study. Moreover, the exact and clear molecular pathway between fasting and FOXO3 gene expression has not yet been fully discovered. This discovery would surely bring physicians one step closer to fully implementing fasting as a new alternative mode of therapy for humans with aging-related diseases. Exploration of FOXO3 expression and other pathways that impact cellular aging, such as PGC1a, phosphoenolpyruvate carboxykinase (PEPCK), and glucose 6 phosphatase, in various tissues is also important in analyzing the effectiveness of fasting. Despite of several limitations, it was a first study which explore the effect of different fasting duration on the FOXO3 expression as anti-aging biomarker and its pathway in the liver.
5. Conclusion
IF significantly increased the FOXO3 expression, SIRT1 mRNA expression, and specific activity of the SOD enzyme in the liver, and significantly decreased the circulating and hepatic IGF-1 levels. The increase in the FOXO3 expression was mediated by the decrease in the IGF-I levels during fasting. Therefore, IF may prevent aging. On the contrary, PF did not significantly change the FOXO3 expression nor its pathway compared to the control, and insignificantly increased the FOXO3 expression compared to IF.
Author contribution statement
Novi Silvia Hardiany: Conceived and designed the experiment; Performed the experiment; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Muhammad Alifian Remifta Putra & Raya Makarim Penantian: Performed the experiment; Analyzed and interpreted the data; Wrote the paper.
Radiana Dhewayani Antarianto: Conceived and designed the experiment; Contributed reagents, materials, analysis tools or data.
Funding statement
DR Novi Silvia Hardiany was supported by Directorate Research & Community Engagement Universitas Indonesia [NKB-1510/UN2.RST/HKP.05.00/2020].
Data availability statement
Data will be made available on request.
Declaration of interest’s statement
The authors declare no competing interests.
References
- 1.Mc Auley M.T., Guimera A.M., Hodgson D., Mcdonald N., Mooney K.M., Morgan A.E., Proctor C.J. Modelling the molecular mechanisms of aging. Biosci. Rep. 2017;37(1) doi: 10.1042/BSR20160177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.United Nations, Department of Economic and Social Affairs Population Division . 2020. World Population Ageing 2020 Highlights. [Google Scholar]
- 3.Fontana L., Partridge L., Longo V.D. Extending healthy life span--from yeast to humans. Science. 2010;328(5976):321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giannakou M.E., Partridge L. The interaction between FOXO and SIRT1: tipping the balance towards survival. Trends Cell Biol. 2004;14(8):408–412. doi: 10.1016/j.tcb.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 5.Kenyon C.J. The genetics of ageing. Nature. 2010;464(7288):504–512. doi: 10.1038/nature08980. [DOI] [PubMed] [Google Scholar]
- 6.Morris B.J., Willcox D.C., Donlon T.A., Willcox B.J. FOXO3: a major gene for human longevity-a mini-review. Gerontology. 2015;61(6):515–525. doi: 10.1159/000375235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martins R., Lithgow G.J., Link W. Long live FOXO: unraveling the role of FOXO proteins in aging and longevity. Aging Cell. 2016;15(2):196–207. doi: 10.1111/acel.12427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang Y., Yan F., Feng Z., Lazarovici C., Zheng W. Signaling network of forkhead family of transcription factors (FOXO) in dietary restriction. Cells. 2020;9(100):1–16. doi: 10.3390/cells9010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Imae M., Fu Z., Yoshida A., Noguchi T., Kato H. Nutritional and hormonal factors control the gene expression of FoxOs, the mammalian homologues of DAF-16. J. Mol. Endocrinol. 2003;30:253–262. doi: 10.1677/jme.0.0300253. [DOI] [PubMed] [Google Scholar]
- 10.Geisler C.E., Hepler C., Higgins M.R., Renquist B.J. Hepatic adaptation to maintain metabolic homeostasis in response to fasting and refeeding in mice. Nutr. Metab. 2016;13(62):1–13. doi: 10.1186/s12986-016-0122-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim H., Kisseleva T., Brenner D.A. Aging and liver disease. Curr. Opin. Gastroenterol. 2015;31(3):184–191. doi: 10.1097/MOG.0000000000000176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sengupta P., Dutta S. Mapping the age of laboratory rabbit strains to human. Int. J. Prev. Med. 2019;10:1–8. doi: 10.4103/ijpvm.IJPVM_530_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yanni A.E. Laboratory rabbit and high-cholesterol diet: what is taken for granted may not be so simple. Lab. Anim. 2014;48(4):349–350. doi: 10.1177/0023677214544184. [DOI] [PubMed] [Google Scholar]
- 14.Livak K.J., Schmittgen T.D. Analysis of relatice gene expression data using real- time quantitative PCR and the 2-ΔΔCT Method. Methods. 2001;25:402–405. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 15.Hardiany N.S., Sadikin M., Siregar N.C., Wanandi S.I. The suppression of manganese superoxide dismutase decreased the survival of human glioblastoma multiforme T98G cells. Med. J. Indones. 2017 May 16;26(1):19–25. doi: 10.13181/mji.v26i1.1511. [DOI] [Google Scholar]
- 16.Kawamura S., Yamazoe H., Hosokawa Y. Diurnal gain and nocturnal reduction of body weight in young adults rabbits: the reverse of the circadian rhythm observed in rats and mice. J. Toxicol. Cur. Res. 2020;4:16. doi: 10.24966/TCR-3735/100016. [DOI] [Google Scholar]
- 17.Tsutsumi Y., Shinohara T., Hachinohe Y. Some physiological aspect in body weight in rabbits. J. Fac. Agric. Hokkaido Univ. 1967;55(2):111–132. [Google Scholar]
- 18.Madkour M.I., T El-Serafi A., Jahrami H.A., Sherif N.M., Hassan R.E., Awadallah S., Faris M.A.E. Ramadan diurnal intermittent fasting modulates SOD2, TFAM, Nrf2, and sirtuins (SIRT1, SIRT3) gene expressions in subjects with overweight and obesity. Diabetes Res. Clin. Pract. 2019 Sep;155 doi: 10.1016/j.diabres.2019.107801. [DOI] [PubMed] [Google Scholar]
- 19.Faris M.A.E., Madkour M.I., Obaideen A.K., Dalah E.Z., Hasan H.A., Radwan H., Jahrami H.A., Hamdy O., Mohammad M.G. Effect of Ramadan diurnal fasting on visceral adiposity and serum adipokines in overweight and obese individuals. Diabetes Res. Clin. Pract. 2019;153:166–175. doi: 10.1016/j.diabres.2019.05.023. [DOI] [PubMed] [Google Scholar]
- 20.Faris A.E., Jahrami H.A., Alsibai J., Obaideen A.A. Impact of Ramadan diurnal intermittent fasting on the metabolic syndrome components in healthy, non-athletic Muslim people aged over 15 years: a systematic review and meta-analysis. Br. J. Nutr. 2020;123(1):1–22. doi: 10.1017/S000711451900254X. [DOI] [PubMed] [Google Scholar]
- 21.Furuyama T., Yamashita H., Kitayama K., Higami Y., Shimokawa I., Mori N. Effects of aging and caloric restriction on the gene expression of Foxo1, 3, and 4 (FKHR, FKHRL1, and AFX) in the rat skeletal muscles. Microsc. Res. Tech. 2002;59:331–334. doi: 10.1002/jemt.10213. [DOI] [PubMed] [Google Scholar]
- 22.McClelland Descalzo D.L., Satoorian T.S., Walker L.M., Sparks N.R., Pulyanina P.Y., Zur Nieden N.I. Glucose-induced oxidative stress reduces proliferation in embryonic stem cells via FOXO3A/beta-catenin-dependent transcription of p21(cip1) Stem Cell Rep. 2016;7(1):55–68. doi: 10.1016/j.stemcr.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stefanetti R.J., Voisin S., Russell A., Lamon S. Recent advances in understanding the role of FOXO3. F1000Res. 2018 Aug 31;7:F1000. doi: 10.12688/f1000research.15258.1. Faculty Rev-1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kang K., Bai J., Zhong S., Zhang R., Zhang X., Xu Y., Zhao M., Zhao C., Zhou Z. Down-regulation of insulin like growth factor 1 involved in Alzheimer's disease via MAPK, Ras, and FoxO signaling pathways. Oxid. Med. Cell. Longev. 2022;2022 doi: 10.1155/2022/8169981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu K., Zhang Z., Pei H., Wang H., Li L., Xia Q. FoxO3a induces temozolomide resistance in glioblastoma cells via the regulation of β-catenin nuclear accumulation. Oncol. Rep. 2017;37(4):2391–2397. doi: 10.3892/or.2017.5459. [DOI] [PubMed] [Google Scholar]
- 26.Liang C., Dong Z., Cai X., Shen J., Xu Y., Zhang M., et al. Hypoxia induces sorafenib resistance mediated by autophagy via activating FOXO3a in hepatocellular carcinoma. Cell Death Dis. 2020 Nov 29;11(11):1017. doi: 10.1038/s41419-020-03233-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fondevila F., Méndez-Blanco C., Fernández-Palanca P., Payo-Serafín T., van Pelt J., Verslype C., et al. Autophagy-Related chemoprotection against sorafenib in human hepatocarcinoma: role of FOXO3 upregulation and modulation by regorafenib. Int. J. Mol. Sci. 2021 Oct 29;22(21) doi: 10.3390/ijms222111770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Straus D.S., Takemoto C.D. Effect of fasting on insulin-like growth factor-I (IGF-I) and growth hormone receptor mRNA levels and IGF-I gene transcription in rat liver. Mol. Endocrinol. 1990;4(1):91–100. doi: 10.1210/mend-4-1-91. [DOI] [PubMed] [Google Scholar]
- 29.Lee C., Longo V.D. Fasting vs dietary restriction in cellular protection and cancer treatment: from model organisms to patients. Oncogene. 2011;30:3305–3316. doi: 10.1038/onc.2011.91. [DOI] [PubMed] [Google Scholar]
- 30.Daitoku H., Sakamaki J., Fukamizu A. Regulation of FoxO transcription factors by acetylation and protein-protein interactions. Biochim. Biophys. Acta. 2011;1813(11):1954–1960. doi: 10.1016/j.bbamcr.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 31.Zullo A., Simone E., Grimaldi M., Gagliardi M., Zullo Lmatrazzo M.R., et al. Effect of nutrient deprivation on the expression and the epigenetic signature of sirtuin genes. Nutr. Metabol. Cardiovasc. Dis. 2018;28:418–424. doi: 10.1016/j.numecd.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 32.Brunet A., Sweeney L.B., Sturgill J.F., Chua K.F., Greer P.L., Lin Y., et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- 33.Nakagawa T., Guarente L. Sirtuins at a glance. J. Cell Sci. 2011;124(6):833–838. doi: 10.1242/jcs.081067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Y., He J., Liao M., Hu M., Li W., Ouyang H., et al. An overview of sirtuins as potential therapeutic target: structure, function and modulators. Eur. J. Med. Chem. 2018;161:48–77. doi: 10.1016/j.ejmech.2018.10.028. [DOI] [PubMed] [Google Scholar]
- 35.Greer E.L., Brunet A. Different dietary restriction regimens extend lifespan by both independent and overlapping genetic pathways in C. elegans. Aging Cell. 2009;8(2):113–127. doi: 10.1111/j.1474-9726.2009.00459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu Y., Yan Y., Gius D.R., Vassilopoulos A. Metabolic regulation of Sirtuins upon fasting and the implication for cancer. Curr. Opin. Oncol. 2013;25(6):630–636. doi: 10.1097/01.cco.0000432527.49984.a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kapahi P., Kaeberlein M., Hansen M. Dietary restriction and lifespan: lessons from invertebrate models. Ageing Res. Rev. 2017;39:3–14. doi: 10.1016/j.arr.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klotz L.O., Ramos C.S., Arroyo I.P., Urbanek P., Steinbrenner H., Monsalve M. Redox regulation of FoxO transcription factors. Redox Biol. 2015;6:51–72. doi: 10.1016/j.redox.2015.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fukai T., Fukai M.U. Superoxide dismutases: role in redox signaling, vascular function, and diseases. Antioxidants Redox Signal. 2011;15(6):1583–1606. doi: 10.1089/ars.2011.3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jongbloed F., de Bruin R.W.F., Pennings J.L.A., Payán-Gómez C., van den Engel S., van Oostrom C.T., et al. Preoperative fasting protects against renal ischemia-reperfusion injury in aged and overweight mice. Ashton N., editor. PLoS One. 2014;9(6) doi: 10.1371/journal.pone.0100853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mitchell J.R., Verweij M., Brand K., van de Ven M., Goemaere N., van den Engel S., et al. Short-term dietary restriction and fasting precondition against ischemia reperfusion injury in mice. Aging Cell. 2010;9(1):40–53. doi: 10.1111/j.1474-9726.2009.00532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rojas-Morales P., León-Contreras J.C., Aparicio-Trejo O.E., Reyes-Ocampo J.G., Medina-Campos O.N., Jiménez-Osorio A.S., et al. Fasting reduces oxidative stress, mitochondrial dysfunction and fibrosis induced by renal ischemia-reperfusion injury. Free Radic. Biol. Med. 2019;135:60–67. doi: 10.1016/j.freeradbiomed.2019.02.018. [DOI] [PubMed] [Google Scholar]
- 43.Price J.C., Khambatta C.F., Li K.W., Bruss M.D., Shankaran M., Dalidd M., et al. The effect of long term calorie restriction on in vivo hepatic proteostatis: a novel combination of dynamic and quantitative proteomics. Mol. Cell. Proteomics. 2012;11(12):1801–1814. doi: 10.1074/mcp.M112.021204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Longo V.D., Mattson M.P. Fasting: molecular mechanisms and clinical applications. Cell Metabol. 2014;19(2):181–192. doi: 10.1016/j.cmet.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cheng C.W., Adams G.B., Perin L., Wei M., Zhou X., Lam B.S., et al. Prolonged fasting reduces IGF-1/PKA to promote hematopoietic-stem-cell-based regeneration and reverse immunosuppression. Cell Stem Cell. 2014;14(6):810–823. doi: 10.1016/j.stem.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.