Abstract
Background
The majority of patients with autoimmune hepatitis (AIH) achieve complete remission with established treatment regiments. In patients with intolerance or insufficient response to these drugs, the remaining options are limited and novel treatment approaches necessary. In primary biliary cholangitis (PBC), ursodeoxycholic acid (UDCA) and fibrates have improved prognosis dramatically, but there remains a proportion of patients with refractory disease.
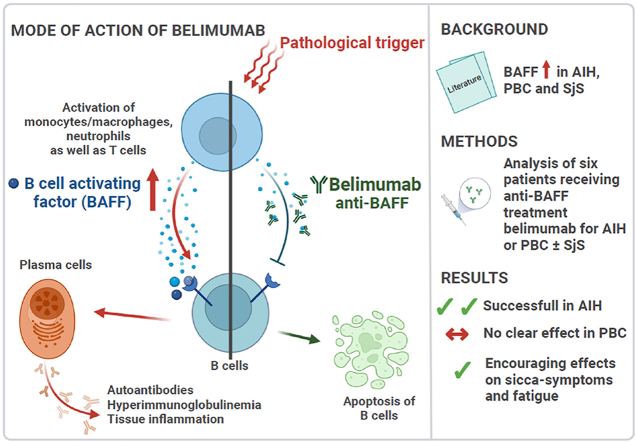
In patients with refractory AIH and/or PBC, we used a novel treatment strategy with the anti-B cell activating factor, belimumab. The first three patients had concomitant Sjögren's disease. The connecting element between all three diseases is B cell activation, including elevated levels of the B cell activating factor (BAFF). Furthermore, belimumab has been shown to be beneficial in Sjögren's disease.
Aims and methods
To retrospectively investigate treatment response in six patients with AIH or PBC with or without concomitant Sjögren's disease treated with the anti-BAFF therapy belimumab at the University Hospital in Bern, Switzerland.
Results
In all three patients with AIH, belimumab improved disease control and helped by-pass or reduce problematic side effects from corticosteroids and calcineurin inhibitors. In PBC patients (n = 3), there was no clear improvement of liver function tests, despite reduction or normalization of IgM. All patients with concomitant Sjögren's disease (n = 3) had an improvement of sicca symptoms and two out of three patients experienced an initially marked reduction in fatigue, which lessened over time.
Conclusions
Belimumab may be a promising treatment option for patients with AIH and further investigations are needed. In PBC however, response was not convincing. The effects on sicca symptoms and fatigue were encouraging.
Keywords: Autoimmune hepatitis, Primary biliary cholangitis, Autoimmune liver disease, Sjögren's disease, Belimumab
Abbreviations: AIH, autoimmune hepatitis; ALT, alanine aminotransferase; AMA, anti-mitochondrial antibodies; ANA, anti-nuclear antibodies; AZA, azathioprine; AP, alkaline phosphatase; APS, anti-phospholipid-antibody syndrome; BDN, budesonide; CI, calcineurin inhibitor; CyA, cyclosporine A; disease-modifying anti-rheumatic drugs, DMARDs; IgG, immunoglobulin G; IgM, immunoglobulin M; INR, international normalized ratio; LC 1, liver cytosol 1 antibodies; LKM-1, liver-kidney-microsomal antibodies; MMF, mycophenolate mofetil; NA, not applicable; NRH, nodular regenerative hyperplasia; MRCP, magnetic resonance cholangiopancreatography; PBC, primary biliary cholangitis; PDN, prednisolone; PLA2R, anti-phospholipase 2 receptor antibody; PSC, primary sclerosing cholangitis; RF, rheumatoid factor; SLA, soluble liver antigen antibodies; SLE, systemic lupus erythematosus; SMA, smooth-muscle cell antibodies; SS-A, SS-A (Ro) antibodies; SS-B, SS-B (La) antibodies; TNFi, tumor necrosis factor inhibitor; UDCA, ursodeoxycholic acid; ULN, upper limit of normal
Graphical abstract
Highlights
-
•
Belimumab is a B cell-activating factor inhibitor.
-
•
In three patients with autoimmune hepatitis, belimumab led to remission.
-
•
In three patients with primary biliary cholangitis, belimumab showed no clear effect on disease activity.
-
•
Effects on sicca-symptoms and fatigue were encouraging.
Lay summary
In autoimmune diseases, the body mistakenly attacks its own tissues. In autoimmune hepatitis, this leads to a destruction of liver tissue, in primary biliary cholangitis to a destruction of bile ducts and in Sjögren's disease to a response against exocrine glands. If untreated, these diseases can take a serious course. Despite successful standard treatment for some patients, there is still an urgent need for better treatment options, particularly for patients with intolerance or insufficient responses. In all three diseases the immune system is overstimulated. In this study we used belimumab, a treatment that specifically inhibits a signaling pathway that is important for the stimulation of the immune system. In autoimmune hepatitis we saw good efficacy, whereas in primary biliary cholangitis we did not see a clear response. Symptom control for fatigue and sicca symptoms was overall encouraging.
1. Introduction
Autoimmune hepatitis (AIH) is an immune-mediated inflammatory disease of the liver. While untreated disease is usually fatal, patients who respond well to therapy have an excellent prognosis [1]. The majority of patients achieve remission with established first-to- third-line treatments (azathioprine, mycophenolate mofetil and calcineurin inhibitors) [2]. In case of intolerance or insufficient response to established drugs, treatment options are limited and novel treatment approaches are urgently needed [3].
Primary biliary cholangitis (PBC) is a chronic inflammatory disease of the small biliary ducts, leading to cholestasis, fibrosis and end stage liver disease. Treatment is based on ursodeoxycholic acid (UDCA), which not only improves biochemical indices but also delays histological progression and improves survival without transplantation [4]. Second line treatments are fibrates or obeticholic acid [5]. Nevertheless, up to 30% of patients do not achieve complete remission [6].
Sjögren's disease is a chronic inflammatory autoimmune disorder characterized by lymphocytic infiltrations of the salivary glands. Extraglandular organ manifestations occur in up to 30% [7]. Ocular and oral dryness, fatigue and joint pain are the most common symptoms, with only symptomatic treatment available. In patients with systemic, extraglandular manifestations, immunosuppressive treatment regimens are used [8]. PBC and Sjögren's disease occur concomitantly in about 20% [9,10] and have many common features; clinically the fatigue and epidemiologically the female preponderance and age of disease onset around menopause. Furthermore, typical autoantibody patterns, a highly specific immune-mediated injury of the epithelium and a poor response to conventional immunosuppression [11,12].
The connecting element between AIH, PBC and Sjögren's disease is B cell activation, including elevated B cell activating factor (BAFF) levels [[13], [14], [15], [16]]. Furthermore, serum BAFF levels in AIH are related to liver inflammation and improve in response to corticosteroid therapy [17].
BAFF is secreted by T lymphocytes, monocytes and, among others, macrophages and is essential for the proliferation, differentiation and survival of B cells [17,18]. Belimumab is a human monoclonal antibody targeting BAFF, thereby reducing B cell activation [19].
In 2020 the first use of belimumab in two patients with refractory AIH was published with promising results [20]. Of note, a multicenter Phase II/III trial (NCT03217422) is ongoing, investigating the efficacy and safety of the BAFF receptor inhibitor ianalumab in patients with AIH. In Sjögren's disease efficacy and safety of belimumab has been tested in phase III trials with encouraging results [21] and recently, the European Alliance of Associations for Rheumatology included belimumab in their treatment recommendations for Sjögren's disease [22].
Herewith, we report a case series of six patients with AIH or PBC with or without co-occurring Sjögren's disease who were refractory or intolerant to standard treatment, where we used belimumab to treat the autoimmune liver diseases, based on outlined clinical and pathophysiological commonalities.
2. Patients and methods
Retrospective analysis of patients treated with belimumab for either autoimmune hepatitis or primary biliary cholangitis at the University Hospital of Bern, Switzerland. Diagnostic criteria for AIH and PBC were in accordance with criteria from the European Association for the Study of the Liver [5,23]. Diagnostic criteria for Sjögren's disease according to the European Alliance of Associations for Rheumatology recommendations [24]. Informed consent was obtained from each patient. Belimumab was administered subcutaneously 200 mg once weekly if not indicated otherwise.
3. Case presentations
3.1. Indication autoimmune hepatitis
3.1.1. Patient 1
In 2006 the 38-year old female patient was diagnosed with Sjögren's disease based on objective sicca symptoms, recurrent sialadenitis, arthralgias, fatigue, positive autoantibodies (anti-nuclear antibodies (ANA), SS-A, SS-B, rheumatoid factor), hypergammaglobulinemia and a typical salivary gland histology. Her family history was remarkable for a sister and a nephew with systemic lupus erythematosus.
In 2009 the patient presented with an acute onset of elevated liver enzymes (ALT 30xULN). SMA and actin antibodies were positive; IgG was continuously elevated in the context of Sjögren's disease. Liver biopsy showed typical features of AIH without fibrosis. After exclusion of other causes of an acute liver injury, the diagnosis AIH was established and treatment with prednisolone and azathioprine (AZA) was started [23], but AZA was discontinued after several weeks because of a severe agranulocytosis.
Between 2009 and 2017 she intermittently presented with active Sjögren's disease, which was usually treated with non-steroidal anti-inflammatory drugs or prednisolone, often in self-medication. One episode of disease activity was treated with rituximab 2 × 1g. However, she did not tolerate the drug. After rituximab AIH was in remission for several months. Later on, MMF was started due to recurrent active AIH, but not tolerated due to gastrointestinal side effects. She stopped coming to the consultations and phases of elevated liver values were treated with prednisolone (usually 20 mg/d) for several weeks by the general practitioner.
In 2019 she presented with an icteric hepatitis (ALT 30xULN, AP 2xULN and Bilirubin 111 μmol/l). Prednisolone 20 mg/d was started and upon delayed referral to our clinic, liver values were almost normal, fibroscan was 11.2 kPa and there were signs of advanced fibrosis on sonography. Antibody-testing showed positive AMA and M2 antibodies in addition to the already previously positive autoantibodies. On liver histology (under immunosuppression), there were no signs of an active AIH, but findings compatible with PBC, METAVIR F2. The diagnosis of an overlap with PBC was made. Furthermore, she had developed a renal insufficiency of unknown origin, which made the option of a calcineurin inhibitor unfavorable. Moreover, her Sjögren's disease was recurrently active, at the time with fatigue, arthralgias and sicca symptoms. After approval by the health insurance, we started belimumab intravenously 10 mg/kg on day 0, 14 and 28, and every 28 days thereafter. After three months, administration was changed to 200 mg s. c. weekly because of the COVID pandemic. Prednisolone was tapered and finally stopped six months after belimumab start. For the PBC, UDCA was begun. Liver values remained normal during the treatment with belimumab, IgG levels stayed elevated, attributed to the Sjögren's disease. IgM normalized. Fibroscan completely normalized after 6 months of treatment.
In the summer of 2021 arthralgias and fatigue worsened, most probably due to a more active Sjögren's disease accentuated by a COVID infection. The patient attributed the symptoms to belimumab and stopped all medications in the fall of 2021 against medical advice. Three months after treatment stop, PBC was active again and the patient agreed to the reintroduction of UDCA. Eleven months after belimumab cessation, she had a recurrence of the AIH.
3.1.2. Patient 2
In 2010 the 50-year old male patient presented with elevated liver enzymes with a mixed pattern, ALT 10xULN, AP 5xULN and a normal liver function. IgG was slightly elevated, IgM normal, ANA, SMA, actin-antibodies and p-ANCA were positive. Liver biopsy showed a chronic moderately active hepatitis with parenchymal damage and granulomas, interpreted as not typical but compatible with an AIH, METAVIR F2. Primary sclerosing cholangitis as well as causes for granulomatous diseases were ruled out. Diagnosis of an AIH was made and immunosuppression with prednisolone 40 mg/d followed by a combination with AZA was started. Every reduction of prednisolone below 10 mg/d led to a more active liver disease with a mixed pattern. In 2018 a follow-up liver biopsy was performed and showed no signs of an active AIH (under immunosuppression), but an almost complete ductopenia with a cholestatic phenotype of the hepatocytes, fibrosis METAVIR F2, as well as signs for nodular regenerative hyperplasia. Magnetic resonance cholangiopancreatography was repeated and showed no signs of a primary sclerosing cholangitis. Autoantibodies for PBC remained negative (AMA M2, sp100, gp210). Because of the more pronounced signs of a cholestatic autoimmune disease with vanishing bile ducts, UDCA was started, prednisolone tapered and AZA stopped, the latter also because of the nodular regenerative hyperplasia, which can be caused by AZA. Within weeks the situation worsened with severe pruritus, fatigue and a marked rise in ALT to 20xULN and AP 3xULN. Immunosuppression was restarted with prednisolone 40 mg/d and 2 × 1g MMF with a favorable response. Despite the change of treatment to MMF, prednisolone could not be reduced below 10 mg/d. Therefore, Cyclosporine (CyA) (75–100 ng/ml) was added to the treatment regimen. Despite MMF and CyA, still, prednisolone could not be reduced below 10 mg/d to achieve complete remission.
As CyA seemed to have been more efficient, we stopped MMF in 2021 and increased CyA to a trough level of 100–120 ng/ml and started belimumab 200 mg s. c. 1x/week. With this combination prednisolone could be further reduced to 5 mg/d. Doses below 5 mg led to a reactivation. At the last follow up 14 months after the introduction of belimumab, ALT and bilirubin were at 61 U/L (<50 U/L) and 19 μmol/l respectively (<17 μmol/l), whereas the other liver values were completely normal.
3.1.3. Patient 3
Elevated liver enzymes were found during a work-up of an urinary tract infection in the spring of 2020 in the 36-year old male patient. ALT was 10xULN, AP 2xULN, IgG 41.6 g/l with preserved liver function. ANA, SMA and SLA-antibodies were highly positive and the liver biopsy was typical for an AIH with pronounced fibrous collaps. Fibroscan was elevated with 26.7 kPa and there were signs of an inhomogeneous liver parenchyma on sonography. After ruling out other causes, the diagnosis of AIH was established and treatment with 40 mg prednisolone and AZA was started. However, both AZA and even the switch to MMF were only effective in combination with >20 mg prednisolone and had to be discontinued due to intolerance. Prednisolone was not well tolerated and a trial of budesonide was started, but unsuccessful. Therefore, CyA and prednisolone 40 mg/d were started which led to biochemical remission and improvement of the general condition. What became difficult for the patient, were psychological side effects of the corticosteroids with auto aggression, even in low doses of 5–10 mg/d. Prednisolone was tapered over 2 months. After three months of monotherapy with CyA (100–120 ng/ml), the AIH was again active. Corticosteroids were reintroduced at 20 mg/d and after approval by the health insurance, belimumab was started. Two months after belimumab start, prednisolone was stopped. After six months on stable remission with belimumab and CyA, CyA could be tapered (75–100 ng/ml), which improved the CyA-induced kidney insufficiency. The AIH remained in remission. Fibroscan improved to 9 kPa.
3.2. Indication primary biliary cholangitis
3.2.1. Patient 4
In 2014, elevated liver enzymes with a cholestatic pattern were seen in the asymptomatic 65-year old female patient. ALT was 4xULN, AP 10xULN. Furthermore, she had a hypercholesterinemia, an elevated IgM at 6.13 g/l (<2.3 g/l), positive AMA 1:320 (<1:80) with highly positive M2 122 Units (<20 Units). Fibroscan was slightly elevated with 8.2 kPa, sonography was normal. The liver biopsy was typical for a PBC, METAVIR F3. UDCA was started with a normalization of the transaminases and a favorable evolution of the AP going to 3xULN and IgM to 3.75 g/l. Because of the continuously active disease, bezafibrate was added. This controlled the disease for some time, but activity increased again in 2020. A follow-up liver biopsy was performed to rule out a metabolic-component of the disease, which showed active PBC with ductopenia, but no steatosis, METAVIR F3. A treatment with obeticholic acid was planned, but rejected by the health insurance. Meanwhile, the patient was diagnosed with Sjögren's disease and sicca symptoms and fatigue strongly impacted her quality of life.
Belimumab 200 mg s. c. Once weekly was started. Transaminases remained normal, and AP undulated around pre-belimumab levels (1-2xULN), IgM normalized. The fibroscan which was slightly elevated (8.6–10.1 kPa) prior to the beginning with belimumab, normalized. A follow-up biopsy after 18 months was performed and histologically, there was the same grade of inflammation, bile duct injury and fibrosis as in the previous biopsy. Because of the lack of biochemical and histological improvement, there was no further hepatological indication for the continuation of the treatment. Currently, the patient continues with the treatment because of the positive effects on her Sjögren's disease.
3.2.2. Patient 5
In 2004 the 28-year old female patient was diagnosed with a seropositive, erosive rheumatoid arthritis and in 2010 she was diagnosed with Sjögren's disease. In 2012 the patient had a subacute elevation of liver enzymes with ALT 10xULN and AP up to 9xULN. IgM was strongly elevated at 7.68 g/l (<2.3 g/l), AMA M2 were positive with 85 Units (<20 Units), while other autoantibodies remained negative. Fibroscan and sonography of the liver were normal. Liver biopsy was typical for PBC, METAVIR F1. Unfortunately, the rheumatoid arthritis was very difficult to manage and various chemical as well as biological disease-modifying anti-rheumatic drugs were applied but could not obtain remission (Table 1). Of note, these treatments did not improve liver enzymes, which stayed elevated (ALT 2-4xULN and AP 4-5xULN, undulating up to AP 7-8xULN) (see Table 2).
Table 1.
Baseline characteristics of all patients. METAVIR score is used to describe severity of fibrosis in liver histology.
| INDICATION FOR BELIMUMAB |
AUTOIMMUNE HEPATITIS |
PRIMARY BILIARY CHOLANGITIS |
||||
|---|---|---|---|---|---|---|
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | |
| DEMOGRAPHICS | ||||||
| Sex | Female | Male | Male | Female | Female | Female |
| Ethnicity | Hispanic | Caucasian | Caucasian | Caucasian | Hispanic | Caucasian |
| Age at diagnosis (years) | 41 (AIH), 51 (PBC) | 50 (AIH), 58 (PBC) | 37 | 65 | 48 | 37 |
| Year of diagnosis | 2009 (AIH), 2019 (PBC) | 2010 (AIH), 2018 (PBC) | 2020 | 2014 | 2012 | 2013 |
| Disease duration when belimumab was started (months) | 132 | 132 | 15 | 72 | 48 | 98 |
| BASELINE VALUES | ||||||
| Disease activity during disease flare before belimumab | ||||||
| ALT (<35 U/L in women, <50 U/L in men) | >1000 | 61 | 116 | 18 | 72 | 73 |
| Alkaline phosphatase (<104 U/L in women, <129 U/L in men) | 214 | 148 | 198 | 185 | 245 | 198 |
| Total bilirubin (<17 μmol) | 111 | 17 | 20 | 9 | 9 | 18 |
| Immunoglobulin G (<16 g/l) | 33.7 | 7.7 | 12.4 | 12.8 | 14.9 | 15.0 |
| Immunoglobulin M (<2.3 g/l) | 3.78 | 1.39 | 2.01 | 3.05 | 4.31 | 5.63 |
| Immunserology at diagnosis | ||||||
| ANA (<1:80) | >1:1280 | 1:1280 | >1:1280 | 1:160 | 1:320 | 1:1280 |
| SMA (<1:80) | 1:1280 | 1:80 | 1:320 | <1:80 | <1:80 | NA |
| F-Actin (<20 Units), measured in SMA positives | 144 | 34 | 128 | NA | NA | NA |
| LKM-1, (<1:80) or p450 (<20 Units) | 3 | <1:80 | 3 | 3 | 3 | NA |
| LC 1 (<10 U/ml) | 4.0 | NA | NA | 0.9 | 4.5 | NA |
| SLA (<20 Units) | 3 | 4 | 116 | 2 | 55 | NA |
| AMA (<1:80) | Disturbed by ANA | <1:80 | Disturbed by ANA | 1:320 | 1:1280 | >1:1280 |
| M2 (<20 Units) | 55 | NA | 14 | 122 | 86 | 126 |
| sp100 (<20 Units) | NA | 2 | 3 | 2 | 3 | NA |
| gp210 (<20 Units) | NA | 1 | NA | 1 | 57 | NA |
| Fibroscan at diagnosis (kPa) | NA | NA | 26.7 | 8.2 | 6.3 | 4.9 |
| Liver histology | At diagnosis 2009: periportal hepatitis rich in plasma cells, interface lesions and piecemeal-necrosis, F0 | At diagnosis 2010: chronic moderately active hepatitis with parenchymal damage and granulomas, F2 | At diagnosis 2020: chronic hepatitis including lots of plasma cells, feathery degeneration, pseudo-rosettes and fibrous collapse | At diagnosis 2014: portal inflammation with plasma cells, some florid duct lesions. Missing bile ducts in 2/13 portal fields, F2 | At diagnosis 2012: portal inflammation with plasma cells, florid duct lesions, F1 | At diagnosis 2013: singular epithelioid cell granuloma, no florid duct lesions, no inflammation, ductopenia 4/6 portal fields, F0 |
| PRESENCE OF OTHER AUTOIMMUNE DISEASES | ||||||
| Intrahepatic autoimmune diseases | Primary biliary cholangitis (PBC) | Autoimmune cholangiopathy | NA | NA | NA | NA |
| Extrahepatic autoimmune diseases | Primary Sjögren's syndrome Autoimmune thyroiditis (type Hashimoto) |
Coeliac disease Asthma bronchiale |
Autoimmune thyroiditis (type Hashimoto) | Primary Sjögren's syndrome | Seropositive, erosive rheumatoide arthritis (RA) Secondary Sjögren's syndrome Autoimmune thyroiditis (type Hashimoto) |
Asthma bronchiale |
| TREATMENT | ||||||
| Previous treatments and reason for discontinuation | Recurrent prednisolone use, AZA (severe pancytopenia), MMF (gastrointestinal symptoms), rituximab (severe hair loss) | Chronic steroid use 10–20 mg over many years (Osteoporosis); AZA (NRH, hyperkeratotic skin lesions); MMF (insufficient response); CyA ongoing but insufficient in combination with low dose steroids | AZA (insufficient response, gastrointestinal symptoms); MMF (insufficient response, hair loss, fatigue); CyA ongoing but development of kidney insufficiency plus insufficient disease control as monotherapy; PDN with psychiatric side effects even in low doses | UDCA since diagnosis; bezafibrate since 2018; Obeticholic acid not approved by the health insurance |
UDCA since 2014; 2016 obeticholic acid (pruritus); 2017 bezafibrate (elevation of liver enzymes), 2017 (only minimal response) and again since 2019 fenofibrate Treated with methotrexate, salazopyrine, cyclosporine A, leflunomid, infliximab, etanercept, adalimumab, rituximab, tozilizumab, abatacept, tofacitinib and upadacitinib for the RA with no effect on liver values |
UDCA since 2013; 2018 obeticholic acid (pruritus); since 2018 bezafibrate |
Table 2.
Characteristics when belimumab was started and stopped, if applicable, as well as results at the last follow-up.
| Timeline | START OF BELIMUMAB |
LAST FOLLOW-UP WITH BELIMUMAB |
LAST FOLLOW-UP |
|
|---|---|---|---|---|
| 01/20 | 10/21 | 11/22 | ||
| Patient1 | ALT(<35 U/L) | 18 | 23 | 259 |
| AP(<104 U/L) | 119 | 122 | 182 | |
| IgG(<16 g/l) | 32.2 | 33.5 | 34.6 | |
| IgM(<2.3 g/l) | 3.02 | 1.92 | 4.82 | |
| Fibroscan(kPa) | 11.2 | 6.2 | 6.2 | |
| Histology | Before belimumab 2019 (already under treatment with 20 mg PDN): florid bile duct lesions, F2 | |||
| Treatment | PDN 7.5 mg UDCA |
Belimumab (21 months) UDCA |
UDCA | |
| Patient 2 | Timeline | 06/21 | 08/22 | |
| ALT(<50 U/L) | 49 | 61 | ||
| AP (<129 U/L) | 152 | 74 | ||
| IgG (<16 g/l) | 8.4 | 8.4 | ||
| IgM (<2.3 g/l) | 1.43 | 1.07 | ||
| Fibroscan (kPa) | 9.0 | 5.9 | ||
| Histology | 2018 (under immunosuppression): no inflammation, cholestatic phenotype of hepatocytes, severe ductopenia (100%), nodular regenerative hyperplasia, F2 | 2021, 4 months after start of belimumab, 1 month after start of bezafibrate and stop of PDN with sudden increase of liver values: periductal mixed inflammatory infiltrate, ductopenia 3/17 portal fields, no florid duct lesions, one microgranuloma, F1 | ||
| Treatment | PDN 10 mg MMF 2 × 1g CyA 100–120 ng/ml UDCA |
PDN 5 mg CyA 100–120 ng/ml Belimumab (14 months) UDCA |
||
| Patient 3 | Timeline | 09/21 | 09/22 | |
| ALT (<50 U/L) | 30 | 27 | ||
| AP (<129 U/L) | 73 | 80 | ||
| IgG (<16 g/l) | 11.4 | 12.4 | ||
| IgM (<2.3 g/l) | 2.24 | 1.88 | ||
| Fibroscan (kPa) | 8.8 | NA | ||
| Histology | NA | NA | ||
| Treatment | PDN 10 mg CyA 100–120 ng/ml |
CyA 75–100 ng/ml Belimumab (8 months) |
||
| Patient 4 | Timeline | 10/20 | 07/22 | |
| ALT (<35 U/L) | 22 | 19 | ||
| AP (<104 U/L) | 126 | 201 | ||
| IgG (<16 g/l) | 14.3 | 11.4 | ||
| IgM (<2.3 g/l) | 2.95 | 1.97 | ||
| Fibroscan (kPa) | 10.1 | 6.1 | ||
| Histology | 2020 before belimumab: portal inflammation, focal interface activity, florid duct lesions, ductopenia 4/10 portal fields, F3 | May 2022 portal inflammation, focal interface activity, ductopenia 6/9 portal fields, F3 | ||
| Treatment | UDCA Bezafibrate |
UDCA Bezafibrate Belimumab (21 months) |
||
| Patient 5 | Timeline | 10/20 | 09/21 | 05/22 |
| ALT (<35 U/L) | 119 | 43 | 58 | |
| AP (<104 U/L) | 183 | 169 | 174 | |
| IgG (<16 g/l) | 14.1 | 9.6 | 13.3 | |
| IgM (<2.3 g/l) | 5.22 | 1.89 | 0.96 | |
| Fibroscan (kPa) | 7.0 | 8.0 | 9.2 | |
| Histology | August 2016 portal mixed infiltrate with plasma cells, one florid duct lesion, ductopenia 9/14 bile ducts, F2 | May 2022 sparse portal infiltrate, no granulomas, no florid duct lesion, ductopenia, F2 | ||
| Treatment | UDCA Fenofibrate |
UDCA Fenofibrate Belimumab (11 months) |
UDCA Fenofibrate Upadacitinib for the RA |
|
| Patient 6 | Timeline | 01/22 | 04/22 | 05/22 |
| ALT (<35 U/L) | 69 | 63 | 51 | |
| AP (<104 U/L) | 126 | 164 | 91 | |
| IgG (<16 g/l) | 15.0 | 14.7 | 14.4 | |
| IgM (<2.3 g/l) | 4.22 | 5.08 | 3.95 | |
| Fibroscan (kPa) | 5.5 | NA | NA | |
| Histology | December 2020 portal inflammation with massive plasma cells, florid bile duct lesions, ductopenia 4/10 portal fields, F2 | April 2022 portal inflammation with some interphase activity and single hepatocellular necroapoptosis, some florid duct lesions, ductopenia 9/11 portal fields, F3 | ||
| Treatment | UDCA Bezafibrate |
UDCA Bezafibrate Belimumab (4 weeks) |
UDCA Bezafibrate |
In 2016 the continuously active liver disease with an increase in fibroscan from 4.8 kPa to 7.7 kPa and signs of chronic liver disease on sonography led to a follow-up liver biopsy. The histology showed signs of an active PBC, fibrosis METAVIR F2. After approval by the health insurance, obeticholic acid was initiated, but had to be stopped shortly after starting due to pruritus. Meanwhile, fenofibrate 200 mg/d was installed. One year after start of fenofibrate, disease activity did not improve.
Overall, the patient had an insufficiently controlled rheumatoid arthritis with progressive joint destructions, active Sjögren's disease with sicca symptoms and recurrent conjunctivitis, and an active PBC with an established ductopenia and fibrosis F2. As data on belimumab in rheumatoid arthritis is rare and not convincing [25], it was a guarded decision. During the 11 months on belimumab, transaminases improved slightly from 2-3xULN to 1.5xULN, while AP stayed at 1.5–2.5xULN. IgM normalized. As suspected, the rheumatoid arthritis became more active. This led to the application of the recently approved upadacitinib, a novel JAK-inhibitor, and the cessation of belimumab. Under upadacitinib the rheumatoid arthritis was for the first time in stable remission. At the last follow-up, eight months after cessation of belimumab, liver enzymes were lower than ever before with only slightly elevated ALT and AP and normal IgM. Because fibroscan values increased to 9 kPa, a follow-up liver biopsy was performed with unchanged findings to the previous biopsy six years previously.
3.2.3. Patient 6
In 2013 the 36-year old female was evaluated for a chronic liver disease. Her family history was remarkable for the father dying from PBC. She was known for slightly elevated transaminases, gGT and positive AMA M2. AP, immunoglobulins and other liver specific autoantibodies were normal. Fibroscan and sonography were normal. A liver biopsy was performed regarding the suspected PBC with normal AP and to rule out a metabolic-associated liver disease in this overweight patient. Findings were in line with, but not diagnostic for a PBC. After ruling out other causes for a chronic liver disease, the diagnosis of a PBC was suspected and UDCA started. Initially liver values normalized except for gGT.
In 2017 PBC was more active (AP 1.5-2xULN, IgM 4.32 g/l (<2.3 g/l)). Obeticholic acid 5 mg/d was started. After a few days, the patient stopped the treatment because of an increase in pruritus, rash and general malaise. Bezafibrate was started and improved the situation, but did not lead to a remission and the follow-up was undulating. Clinically the patient was suffering from chronic fatigue that worsened after a COVID infection. In 2020 a follow-up liver biopsy was performed because of a more active disease to rule out an overlap with an AIH or a fibrate-induced toxicity. The histology showed an active PBC, METAVIR F1. Her fatigue was increasingly limiting her ability to work. At that time we had the positive experience of belimumab on fatigue in the other patients. Additionally, this patient had lots of plasma cells in the biopsy, supporting to proceed with an anti-BAFF approach. Four weeks after starting the treatment and shortly after a viral respiratory infection, the first follow-up showed markedly elevated cholestasis parameters (AP 3xULN, gGT 25xULN), ALT being 3xULN. As this was unexpected, a liver biopsy with the question of signs of drug toxicity was performed. Meanwhile, the parameters decreased already significantly despite belimumab still being effective. The biopsy again showed an active PBC, this time fibrosis F3, even though fibroscan was normal and sonography showed no signs of a chronic liver disease. Belimumab was stopped; even though causality for the flare was unknown. In the last follow up one month after the biopsy, her liver values were again back to baseline.
4. Sicca symptoms and fatigue
Three of our patients had a Sjögren's disease. All patients were suffering from objective sicca symptoms. They all experienced a subjective improvement in sicca symptoms during belimumab treatment, two patients by 20–30%, one patient became asymptomatic. Two stopped belimumab with subjective worsening thereafter.
There was an impressive improvement in quality of life after 1–2 months of treatment in two patients through subjective reduction of fatigue. This effect decreased after several months in both patients while still under treatment. The patient that is continuously under belimumab is still benefiting.
5. Safety
There were no serious adverse events and the drug was overall well tolerated. One patient noted a burning sensation during s. c. Application. Two reported fatigue for several hours after the injection, which was solved by changing the application time to the evening. One patient reported an increase of headache episodes during the time period of treatment, which improved after stopping the drug. There were no infectious complications. Three patients had a COVID-infection during treatment, all of them previously vaccinated and all three had a mild disease course.
6. Discussion
Our preliminary data supports belimumab to be an effective treatment option for patients with autoimmune hepatitis. This is in line with the experience of the two cases published by Arvaniti et al. [20] and the pathophysiological reasoning of the importance of B cells in AIH [26]. Efficacy of belimumab in AIH in regard to the indication for the switch being intolerance or insufficient response, has yet to be determined. Intolerance to previous treatments was the indication in one patient and she was in remission on belimumab monotherapy. Two patients were treated because of an insufficient response. In those, belimumab as add-on treatment had a steroid-sparing and in one patient an additional calcineurin-sparing effect, which is important because of potentially toxic side effects of these drugs, including negative effects of long-term low-dose corticosteroids [27].
Clinical experience with belimumab comes from its successful use in the treatment of systemic lupus erythematosus, for which it has been approved [28,29]. In patients with systemic lupus erythematosus, treatment response to belimumab has shown to establish after four to six months [28]. This has to be considered when using belimumab in patients with AIH and was the reason, we tapered corticosteroids over six months in the first patient. Patient 3 suffered from corticosteroids-related side effects, which is why we tapered more rapidly over two months, with no relapse.
Our preliminary data of belimumab in patients with PBC are unfortunately not convincing. Immunosuppression is known to be of limited use in PBC [30], one reasoning being the use of immunosuppression often late in the disease course [31]. As all our PBC patients had pronounced plasma cells in liver histology and biochemical signs of B cell activation (high IgM), we hoped belimumab still to be successful. Cholestasis, however, was not improved in the two PBC patients with 11 and 19 months of treatment follow up. IgM normalized in these two patients, which is expected with belimumab, as it reduces B-cell activity. Follow-up liver histology showed the same inflammatory activity as during the period of elevated IgM in both patients, meaning course of IgM and disease activity was not in parallel under belimumab treatment. Therefore, we have to be cautious in interpreting the normalization of IgM as a sign of reduced PBC activity during belimumab treatment.
The effects on symptoms of Sjögren's disease were encouraging in our patients. Subjective sicca symptoms improved persistently. These findings are similar to the objective improvement seen in the BELISS open-label phase II study on belimumab given for 12 months in patients with Sjögren's disease. In the study, improvement was found in 53.3% (8/15) of patients and was persistent during the 12 months follow-up period [21]. In our patients, subjective fatigue did improve, but more in the first 6–12 months with a decrease thereafter. As far as comparable, this was reported differently in the BELISS study, where fatigue decreased over the time course of one year [21]. In our patients, the worsening in fatigue was probably accentuated by the COVID-infection and in one of the patients by intense psychosocial stress. The positive effect of belimumab for sicca symptoms and fatigue has yet to be confirmed in a phase III study.
Our data supports the previous findings on belimumab as a safe and well tolerated drug [21,25,28,32]. Importantly, there are no liver related events or liver toxicities described [28] and there is no limitation on its use in patients with advanced liver disease. We think it is unlikely that belimumab triggered the disease flare in patient 6. Elevated liver values are not described in belimumab and there were no signs of drug toxicity on liver histology. Monoclonal antibodies can trigger hypersensitivity reactions with potential elevation of liver enzymes, but the patient did not have any other clinical signs thereof. It was more likely a disease flare, in concordance with the overall progressive PBC disease course. Furthermore, the flare subsided despite belimumab still being effective. Nevertheless, in the context of an unapproved treatment, we had to stop belimumab as a safety measure.
We chose belimumab over other B cell directed treatment strategies (e.g. rituximab, ocrelizumab), because of the favorable short- and long-term safety profile, with e.g. less infections and a better vaccination response as well as the shorter duration of drug activity, allowing flexibility in adapting the treatment [28].
Due to the purely clinical approach and the retrospective nature of the analysis, additional information on immunological markers of disease activity (e.g. measuring B cell subsets or per protocol liver histology with assessment of inflammatory pathways) is missing. Regarding sicca symptoms and fatigue, objective assessments and a placebo-controlled design should be aimed for in future studies. Furthermore, comparability between patients was not possible due to heterogeneous and complex patients’ situations.
Our experience with belimumab in AIH and PBC gives a preliminary impression of its potential use in autoimmune liver diseases. In AIH belimumab showed to be a promising treatment option and was successfully used in monotherapy, or as an add-on treatment strategy.
7. Conclusions
Our findings support previous reports that belimumab is a promising treatment option for patients with AIH and further investigations are essential. In PBC however, response was not convincing. The effects on sicca symptoms and fatigue were overall encouraging.
Financial disclosure
KM has financial support from the Swiss Liver Foundation. The funding source had no influence in the writing of the report.
Author’s contributions
KM: Participation in the diagnosis and/or management and follow-up of all patients, conception and design of the study, acquisition of data, analysis and interpretation of data, drafting the article, final approval of the submitted version. ACS, BMö, FK: Participation in the diagnosis and/or management of the patients with concomitant rheumatological diseases, revising article critically for important intellectual content, final approval of the submitted version. BM: revising article critically for important intellectual content, final approval of the submitted version. SN: Participation in the diagnosis and/or management and follow-up of all patients, conception and design of the study, interpretation of data, revising article critically for important intellectual content, final approval of the submitted version.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
MK thanks the Swiss Liver Foundation for supporting her work.
Handling editor: M.E. Gershwin
Data availability
Data will be made available on request.
References
- 1.Lohse A.W., Sebode M., Jorgensen M.H., Ytting H., Karlsen T.H., Kelly D., et al. Second-line and third-line therapy for autoimmune hepatitis: a position statement from the European reference network on hepatological diseases and the international autoimmune hepatitis group. J. Hepatol. 2020;73(6):1496–1506. doi: 10.1016/j.jhep.2020.07.023. [DOI] [PubMed] [Google Scholar]
- 2.Pape S., Snijders R., Gevers T.J.G., Chazouilleres O., Dalekos G.N., Hirschfield G.M., et al. Systematic review of response criteria and endpoints in autoimmune hepatitis by the International Autoimmune Hepatitis Group. J. Hepatol. 2022;76(4):841–849. doi: 10.1016/j.jhep.2021.12.041. [DOI] [PubMed] [Google Scholar]
- 3.Czaja A.J. Advancing biologic therapy for refractory autoimmune hepatitis. Dig Dis Sci. 2022;67(11):4979–5005. doi: 10.1007/s10620-021-07378-4. [DOI] [PubMed] [Google Scholar]
- 4.Lindor K.D., Bowlus C.L., Boyer J., Levy C., Mayo M. Primary biliary cholangitis: 2018 practice guidance from the American association for the study of liver diseases. Hepatology. 2019;69(1):394–419. doi: 10.1002/hep.30145. [DOI] [PubMed] [Google Scholar]
- 5.European Association for the Study of the Liver Electronic address eee, European Association for the Study of the L. EASL Clinical Practice Guidelines: the diagnosis and management of patients with primary biliary cholangitis. J. Hepatol. 2017;67(1):145–172. doi: 10.1016/j.jhep.2017.03.022. [DOI] [PubMed] [Google Scholar]
- 6.Grigorian A.Y., Mardini H.E., Corpechot C., Poupon R., Levy C. Fenofibrate is effective adjunctive therapy in the treatment of primary biliary cirrhosis: a meta-analysis. Clin Res Hepatol Gastroenterol. 2015;39(3):296–306. doi: 10.1016/j.clinre.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 7.Ramos-Casals M., Brito-Zeron P., Siso-Almirall A., Bosch X. Primary sjogren syndrome. BMJ. 2012;344 doi: 10.1136/bmj.e3821. [DOI] [PubMed] [Google Scholar]
- 8.Stefanski A.L., Tomiak C., Pleyer U., Dietrich T., Burmester G.R., Dorner T. The diagnosis and treatment of sjogren's syndrome. Dtsch Arztebl Int. 2017;114(20):354–361. doi: 10.3238/arztebl.2017.0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mavragani C.P., Moutsopoulos H.M. Sjogren's syndrome. Annu. Rev. Pathol. 2014;9:273–285. doi: 10.1146/annurev-pathol-012513-104728. [DOI] [PubMed] [Google Scholar]
- 10.Chalifoux S.L., Konyn P.G., Choi G., Saab S. Extrahepatic manifestations of primary biliary cholangitis. Gut Liver. 2017;11(6):771–780. doi: 10.5009/gnl16365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arvaniti P., Zachou K., Lyberopoulou A., Gatselis N.K., Brooks W.H., Dalekos G.N., et al. Epigenetic modifications in generalized autoimmune epithelitis: sjogren's syndrome and primary biliary cholangitis. Epigenomes. 2019;3(3) doi: 10.3390/epigenomes3030015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Selmi C., Gershwin M.E. Chronic autoimmune epithelitis in sjogren's syndrome and primary biliary cholangitis: a comprehensive review. Rheumatol Ther. 2017;4(2):263–279. doi: 10.1007/s40744-017-0074-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lied G.A., Berstad A. Functional and clinical aspects of the B-cell-activating factor (BAFF): a narrative review. Scand. J. Immunol. 2011;73(1):1–7. doi: 10.1111/j.1365-3083.2010.02470.x. [DOI] [PubMed] [Google Scholar]
- 14.Migita K., Abiru S., Maeda Y., Nakamura M., Komori A., Ito M., et al. Elevated serum BAFF levels in patients with autoimmune hepatitis. Hum. Immunol. 2007;68(7):586–591. doi: 10.1016/j.humimm.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 15.Migita K., Ilyassova B., Kovzel E.F., Nersesov A., Abiru S., Maeda Y., et al. Serum BAFF and APRIL levels in patients with PBC. Clin. Immunol. (San Diego, CA, U. S.) 2010;134(2):217–225. doi: 10.1016/j.clim.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 16.Lavie F., Miceli-Richard C., Quillard J., Roux S., Leclerc P., Mariette X. Expression of BAFF (BLyS) in T cells infiltrating labial salivary glands from patients with Sjogren's syndrome. J. Pathol. 2004;202(4):496–502. doi: 10.1002/path.1533. [DOI] [PubMed] [Google Scholar]
- 17.Nishikawa H., Enomoto H., Iwata Y., Kishino K., Shimono Y., Hasegawa K., et al. B-cell activating factor belonging to the tumor necrosis factor family and interferon-gamma-inducible protein-10 in autoimmune hepatitis. Medicine (Baltim.) 2016;95(12) doi: 10.1097/MD.0000000000003194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Z., Davidson A. BAFF inhibition: a new class of drugs for the treatment of autoimmunity. Exp. Cell Res. 2011;317(9):1270–1277. doi: 10.1016/j.yexcr.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pontarini E., Fabris M., Quartuccio L., Cappeletti M., Calcaterra F., Roberto A., et al. Treatment with belimumab restores B cell subsets and their expression of B cell activating factor receptor in patients with primary Sjogren's syndrome. Rheumatology. 2015;54(8):1429–1434. doi: 10.1093/rheumatology/kev005. [DOI] [PubMed] [Google Scholar]
- 20.Arvaniti P., Giannoulis G., Gabeta S., Zachou K., Koukoulis G.K., Dalekos G.N. Belimumab is a promising third-line treatment option for refractory autoimmune hepatitis. JHEP Rep. 2020;2(4) doi: 10.1016/j.jhepr.2020.100123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Vita S., Quartuccio L., Seror R., Salvin S., Ravaud P., Fabris M., et al. Efficacy and safety of belimumab given for 12 months in primary Sjogren's syndrome: the BELISS open-label phase II study. Rheumatology. 2015;54(12):2249–2256. doi: 10.1093/rheumatology/kev257. [DOI] [PubMed] [Google Scholar]
- 22.Ramos-Casals M., Brito-Zeron P., Bombardieri S., Bootsma H., De Vita S., Dorner T., et al. EULAR recommendations for the management of Sjogren's syndrome with topical and systemic therapies. Ann. Rheum. Dis. 2020;79(1):3–18. doi: 10.1136/annrheumdis-2019-216114. [DOI] [PubMed] [Google Scholar]
- 23.European association for the study of the L. EASL clinical practice guidelines: autoimmune hepatitis. J. Hepatol. 2015;63(4):971–1004. doi: 10.1016/j.jhep.2015.06.030. [DOI] [PubMed] [Google Scholar]
- 24.Shiboski C.H., Shiboski S.C., Seror R., Criswell L.A., Labetoulle M., Lietman T.M., et al. American College of Rheumatology/European League against Rheumatism classification criteria for primary Sjogren's syndrome: a consensus and data-driven methodology involving three international patient cohorts. Ann. Rheum. Dis. 2016;76(1):9–16. doi: 10.1136/annrheumdis-2016-210571. 2017. [DOI] [PubMed] [Google Scholar]
- 25.Kaegi C., Steiner U.C., Wuest B., Crowley C., Boyman O. Systematic review of safety and efficacy of belimumab in treating immune-mediated disorders. Allergy. 2021;76(9):2673–2683. doi: 10.1111/all.14704. 2021. [DOI] [PubMed] [Google Scholar]
- 26.Weiler-Normann C., Lohse A.W. Autoimmune hepatitis: from immunopathogenesis to diagnostic and therapeutic innovation. Curr. Opin. Gastroenterol. 2021;37(2):86–90. doi: 10.1097/MOG.0000000000000701. 2021. [DOI] [PubMed] [Google Scholar]
- 27.van den Brand F.F., van der Veen K.S., Lissenberg-Witte B.I., de Boer Y.S., van Hoek B., Drenth J.P.H., et al. Adverse events related to low dose corticosteroids in autoimmune hepatitis. Aliment. Pharmacol. Ther. 2019;50(10):1120–1126. doi: 10.1111/apt.15528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wallace D.J., Ginzler E.M., Merrill J.T., Furie R.A., Stohl W., Chatham W.W., et al. Safety and efficacy of belimumab plus standard therapy for up to thirteen years in patients with systemic lupus erythematosus. Arthritis Rheumatol. 2019;71(7):1125–1134. doi: 10.1002/art.40861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blair H.A., Duggan S.T. Belimumab: a review in systemic lupus erythematosus. Drugs. 2018;78(3):355–366. doi: 10.1007/s40265-018-0872-z. [DOI] [PubMed] [Google Scholar]
- 30.Molinaro A., Marschall H.U. Why doesn't primary biliary cholangitis respond to immunosuppressive medications? Curr. Hepat. Rep. 2017;16(2):119–123. doi: 10.1007/s11901-017-0345-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Terziroli Beretta-Piccoli B., Mieli-Vergani G., Vergani D., Vierling J.M., Adams D., Alpini G., et al. The challenges of primary biliary cholangitis: what is new and what needs to be done. J. Autoimmun. 2019;105 doi: 10.1016/j.jaut.2019.102328. [DOI] [PubMed] [Google Scholar]
- 32.Wise L.M., Stohl W. The safety of belimumab for the treatment of systemic lupus erythematosus. Expet Opin. Drug Saf. 2019;18(12):1133–1144. doi: 10.1080/14740338.2019.1685978. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.