Abstract
In the absence of interleukin-4 (IL-4), infection with Schistosoma mansoni leads to a severe fatal disease rather than the chronic survivable condition that occurs in wild-type (WT) mice. Because the sustained production of NO most closely correlates to weight loss and fatality in infected IL-4−/− mice and because gamma interferon (IFN-γ) is an important inducer of inducible NO synthase, infected IL-4−/− mice were treated with anti-IFN-γ antibodies to determine the role of IFN-γ during schistosomiasis in WT and IL-4−/− animals. When IFN-γ was neutralized, Th2 responses were enhanced and NO production was reduced in both WT and IL-4−/− mice. The decreased NO production correlated with a rescue of proliferation in splenocytes from infected IL-4−/− mice. Furthermore, the neutralization of IFN-γ in vivo improved the gross appearance of the liver and led to a reduction in granuloma size in infected IL-4−/− but not WT mice. However, the neutralization of IFN-γ in vivo did not affect the development of severe disease in infected IL-4−/− mice. These results suggest that while the increased production of IFN-γ does lead to some of the pathology observed in infected IL-4−/− mice, it is not ultimately responsible for cachexia and death.
The parasitic helminths Schistosoma spp. are endemic in many equatorial countries and cause approximately 200 million infections worldwide (5). Eggs deposited by adult worms can become trapped in the liver and induce the development of granulomatous lesions which ultimately lead to liver fibrosis and portal hypertension (5). The immune response to schistosome eggs is dominated by Th2 cytokines (interleukin-4 [IL-4], IL-5, and IL-13) (13, 27) and has been shown to be crucial to host survival, for mice deficient in IL-4 make a defective Th2 response and die during infection (4, 11). Furthermore, in humans increased soluble tumor necrosis factor (TNF) receptors (TNFRs), TNF, and gamma interferon (IFN-γ) as well as decreased IL-5 are associated with the development of severe hepatosplenic disease (23).
Morbidity in infected IL-4−/− mice closely correlates to the in vivo and in vitro levels of NO (3, 4, 19). However, mortality cannot be prevented by inhibition of NO production by aminoguanidine treatment, and moreover the absence of NO results in increased morbidity in wild-type (WT) mice, suggesting that this mediator performs an underlying protective function during infection (3). The absence of IL-12p35 or TNFR1-mediated proinflammatory pathways, which are both implicated in NO production, also do not prevent fatalities in infected IL-4−/− mice, indicating that another mediator(s) is responsible for disease development (26; E. A. Patton, A. C. La Flamme, A. S. MacDonald, A. Alcaraz, and E. J. Pearce, unpublished data).
Gamma interferon (IFN-γ) is a Th1 cytokine that can activate macrophages to produce NO and other inflammatory mediators (9). During acute infection, IL-4−/− mice have been shown to produce elevated levels of IFN-γ in the liver, spleen, and mesenteric lymph nodes (MLN) (15, 28, 29), and this increased production of IFN-γ may be responsible for morbidity. To understand the role of IFN-γ in schistosomiasis in IL-4−/− mice, IFN-γ was neutralized in vivo during infection. In the absence of IFN-γ, antigen (Ag)-specific Th2 responses were enhanced in both WT and IL-4−/− mice and NO levels were reduced. This reduction in NO restored proliferation in splenocyte cultures from IL-4−/− mice. In addition granuloma size was reduced in infected IL-4−/− mice. Nevertheless, weight loss and mortality were not prevented by anti-IFN-γ treatment, indicating that while IFN-γ does contribute to Th2 regulation and NO production, it is not alone responsible for the sequence of pathologic events that lead to death in infected IL-4−/− mice.
MATERIALS AND METHODS
Mice, parasites, and experimental infections.
Female C57BL/6 mice (6 to 12 weeks of age) were purchased from Taconic Farms (Germantown, N.Y.). Female IL-4−/− mice (C57BL/6) were bred at Cornell University and utilized at 6 to 12 weeks of age (4). For infection, mice were each exposed percutaneously to 70 Schistosoma mansoni cercariae (NIMR Puerto Rican strain). Egg burdens were assessed as previously described (6, 31). At autopsy, tissues from infected and uninfected mice were fixed in 10% buffered formalin, paraffin embedded, sectioned, and stained with hematoxylin and eosin for histological examination. Fibrosis was assessed blindly on Masson's trichrome-stained liver sections. Two independent readings by different investigators were combined to give a final score. Granuloma size was measured on liver sections using FluoView software (Olympus Optical) to calculate surface area. Cross sections of 11 granulomas, each containing one egg, were assessed per mouse.
Ag, reagents, and Ab.
Soluble egg Ag (SEA) was prepared as previously described (2). Lipopolysaccharide was purchased from Sigma (St. Louis, Mo.). Streptavidin-phycoerythrin was purchased from Jackson ImmunoResearch (West Grove, Pa.). Plate-bound anti-CD3 antibody (Ab) (PharMingen) was used at 1 μg/well. XMG-6 Rat anti-IFN-γ monoclonal Ab (MAb) was protein G purified from XMG-6 hybridoma culture supernatants. For anti-IFN-γ treatment, mice were injected intraperitoneally (i.p.) with 0.5 mg of XMG.6 or normal rat immunoglobulin G (IgG) (Sigma) in phosphate-buffered saline every three days beginning at 35 days following infection. For neutralization of IFN-γ in vitro, rat anti-IFN-γ MAb XMG.6 was added to culture wells at 10 μg/ml.
Cytokine ELISAs and NO assay.
Sandwich enzyme-linked immunosorbent assays (ELISAs) were used to measure IL-5, IL-10, and IFN-γ as previously described (8, 22, 30). Rat anti-IL-13 MAb was used for IL-13 capture, biotinylated rat anti-IL-13 MAb was used for detection, and recombinant IL-13 was used as a standard; all were purchased from R & D (Minneapolis, Minn.). NO was measured in culture supernatants using the Greiss reaction (12).
MLN and splenocyte isolation and culture.
MLN were harvested and single-cell suspensions were prepared using sterile 70-μm-pore-size cell strainers (Falcon, Franklin Lakes, N.J.) as previously described (32). MLN cells were resuspended at 5 × 106 cells/ml in complete T-cell medium containing Dulbecco's Modified Eagle medium (Sigma), 10% fetal calf serum (Hyclone), 100 U/ml penicillin plus 100 μg/ml streptomycin (GibcoBRL, Gaithersburg, Md.), 10 mM HEPES (GibcoBRL), l-glutamine (GibcoBRL), and 5 × 10−5 M 2-mercaptoethanol (Sigma). Cells (106) were cultured in 96-well flat-bottomed plates (Falcon) at 37°C with 5% CO2. Culture supernatants were harvested at 72 h for cytokine analysis. Spleens were harvested and single-cell suspensions were prepared as previously described (4). Splenocytes were resuspended to 107 cells/ml in complete T-cell medium and cultured in 96-well flat-bottom plates at 37°C with 5% CO2. Culture supernatants were harvested at 72 h for cytokine analysis. Splenocyte proliferation was assessed after 96 h of culture using 5 (and 6)-carboxyfluorescein diacetate succinimidyl ester (CFSE)-labeling as previously described (20).
Isotype-specific ELISAs.
Plasma was collected from blood drawn from mice by heart puncture and stored at −20°C. SEA-specific IgM and IgG1 were measured as previously described (20). SEA-specific IgG2a was measured with biotinylated mouse anti-mouse IgG2a (PharMingen) followed by streptavidin-peroxidase and developed with 2,2′-azinobis (3-ethylbenzthiazolinesulfonic acid) (ABTS) (Kirkegaard and Perry).
Clinical scoring.
All mice were assessed daily and scored from 0 to 3 on the deterioration of coat condition, posture, and activity (0 = normal, 1 = slight, 2 = moderate, 3 = severe). Mice were also scored from 0 to 3 on weight loss (0 = 0 to 5% weight loss, 1 = 5 to 10% weight loss, 2 = 10 to 15% weight loss, 3 = >20% weight loss). These scores were added for a final daily score. After death, mice were given a score of 15.
Statistical analysis.
Data were analyzed using Student's t test or two-way analysis of variance (ANOVA) as indicated.
RESULTS
Peak Ag-induced IFN-γ production precedes peak NO production in infected IL-4−/− mice.
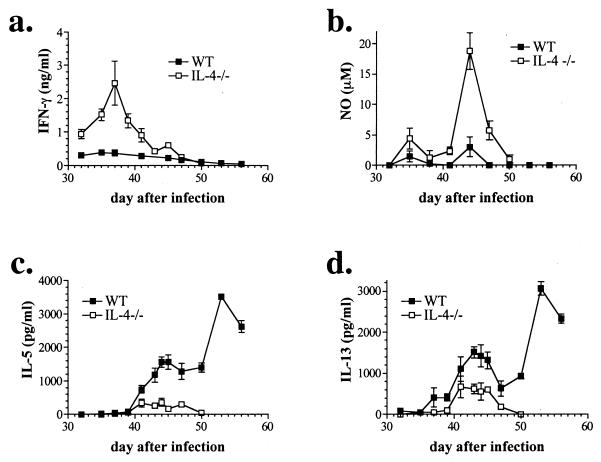
During the acute stage of schistosomiasis, which begins shortly after the start of egg deposition by adult worms, Th1 responses are downregulated and Ag-specific Th2 responses develop (13, 27). Although the initial response to injected schistosome eggs has been characterized as Th0 (32), only transiently low levels of IFN-γ can be detected in splenocyte cultures from infected WT mice, whereas in IL-4−/− mice, Ag-specific IFN-γ production is sustained over several of the days during which the host is first being exposed to parasite eggs (Fig. 1a). This production of IFN-γ precedes the peak production of NO, which like IFN-γ is induced highly in IL-4−/− mice while at relatively low levels in WT mice (Figure 1b) (28). While NO production by splenocytes from infected IL-4−/− mice decreases as the mice become sick (Fig. 1; compare time course with Fig. 6), we know from previous work that plasma levels remain elevated during this time (28), suggesting a nonsplenic source of high-output NO production. In contrast to IFN-γ, IL-5 and IL-13 production appear later and steadily increase over time in WT mice while low levels are produced only transiently in IL-4−/− mice (Figure 1c and d). No IFN-γ, NO, IL-5, or IL-13 was detected in supernatants from SEA-stimulated splenocytes from normal uninfected mice (not shown). These results suggest that the early, high production of IFN-γ in IL-4−/− mice may promote the increased output of NO and therefore contribute to the development of severe schistosomiasis in the absence of IL-4.
FIG. 1.
Peak IFN-γ production (a) precedes peak production of NO (b) by splenocytes from infected IL-4−/− mice. Splenocytes from infected WT mice produce IL-5 (c) and IL-13 (d) and little or no IFN-γ (a) and NO (b). Mice were infected with 70 cercariae, and splenocytes were isolated at the indicated times after infection and stimulated in vitro with SEA (50 μg/ml). Mediators in 72-h culture supernatants were measured by ELISA or Greiss reaction. Shown are the means and standard errors of the means (error bars) of individual mice (two to six per group) from four independent experiments. (a to d) P < 0.0001 for WT versus IL-4−/− (two-way ANOVA).
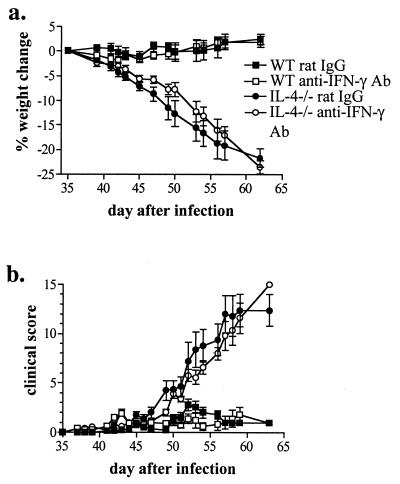
FIG. 6.
Neutralization of IFN-γ in vivo does not prevent severe schistosomiasis in IL-4−/− mice. WT and IL-4−/− mice were infected with 70 cercariae and injected with rat anti-IFN-γ Ab or control rat Ig (0.5 mg/mouse) every third day beginning 35 days after infection. Shown are the means and standard errors of the means (error bars) from individual mice from two independent experiments with a total of 16 mice per group.
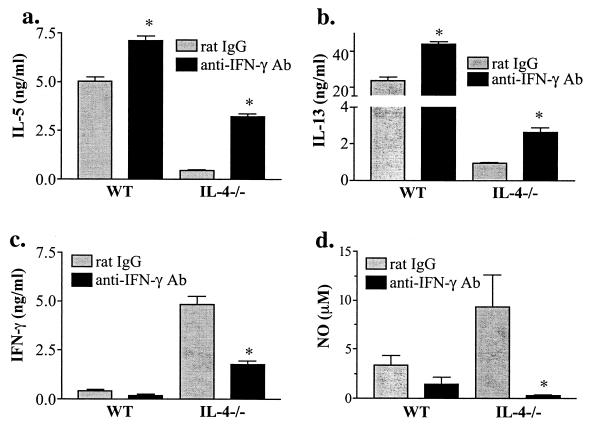
Neutralization of IFN-γ leads to enhanced production of Ag-specific Th2 cytokines and reduced production of NO in infected WT and IL-4−/− mice.
In order to determine how IFN-γ contributes to disease development in IL-4−/− mice, IFN-γ was neutralized in vivo by repeated treatment of WT and IL-4−/− mice with a neutralizing anti-IFN-γ MAb from time of onset of egg deposition. In both WT and IL-4−/− mice anti-IFN-γ treatment led to an increase in Ag-stimulated IL-5 and IL-13 production by MLN cells (Fig. 2a and b) and splenocytes (data not shown). The inhibition of IFN-γ in vivo resulted in significantly decreased Ag-stimulated production of IFN-γ in MLN cultures from IL-4−/− mice (Fig. 2c). This reduction in IFN-γ was associated with markedly reduced levels of NO in splenocyte (Fig. 2d) and MLN cultures (data not shown) from WT and IL-4−/− mice. These results indicate that during infection IFN-γ promotes NO production and downregulates Th2 cytokine production. This is the case even in infected WT mice, in which IFN-γ production is low.
FIG. 2.
Neutralization of IFN-γ in vivo and in vitro leads to enhanced Th2 (a and b) and diminished Th1 (c and d) mediator production in response to Ag stimulation. MLN cells (a to c) or splenocytes (d) were isolated from mice 7 weeks after infection with 70 cercariae and stimulated in vitro with SEA (50 μg/ml). Anti-IFN-γ Ab or control rat Ig (0.5 mg/mouse) was injected i.p. every third day and (for a, b, and d, but not for c) treatment was continued in vitro by addition of anti-IFN-γ Ab (10 μg/ml). Cytokine levels were determined by ELISA and NO levels were determined by Greiss reaction in 72-h culture supernatants. Shown are the means and standard errors of the means (error bars) of values from individual mice from one of two independent experiments. ∗, P < 0.02 rat Ig vs. anti-IFN-γ Ab.
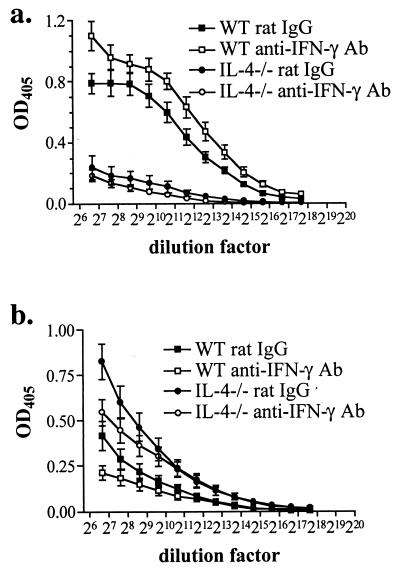
Ag-specific IgG1 production is enhanced and IgG2a production is reduced after in vivo neutralization of IFN-γ.
Because Ab isotype switching is controlled by cytokine environment, the relative levels of Ag-specific IgG1 and IgG2a were determined as an in vivo indicator of Th1 and Th2 responses in the anti-IFN-γ Ab-treated mice. IgG1, production of which is controlled by IL-4, is present at high levels in the plasma of infected WT mice and neutralization of IFN-γ in vivo resulted in enhanced production (Fig. 3a). As expected, infected IL-4−/− mice are impaired in IgG1 production, and neutralization of IFN-γ had no effect, reflecting the fact that IgG1 production is controlled solely by IL-4. The increased production of IFN-γ by infected IL-4−/− compared to WT mice results in higher levels of IgG2a, the production of which is controlled by IFN-γ (Fig. 3b). Neutralization of IFN-γ led to decreased production of IgG2a by both infected IL-4−/− and WT mice (Fig. 3b). These results indicate that during infection IFN-γ is produced in WT and IL-4−/− mice and that anti-IFN-γ Ab treatment neutralizes its effects in vivo.
FIG. 3.
In the absence of IFN-γ, Ag-specific IgG1 production is enhanced in infected WT mice (a) and IgG2a is production reduced in both infected WT and IL-4−/− mice (b). Plasma was collected from 7- to 8-week-infected mice, and IgG1 or IgG2a levels were determined by isotype-specific ELISA. Shown are the means and standard errors of the means (error bars) of individual mice (8 to 10 per group) from two identical experiments. OD405, optical density at 405 nm. P < 0.01 for rat Ig versus anti-IFN-γ Ab for WT (a and b) and IL-4−/− (b) mice (two-way ANOVA).
Splenocyte proliferation is restored in IL-4−/− mice treated with anti-IFN-γ Ab.
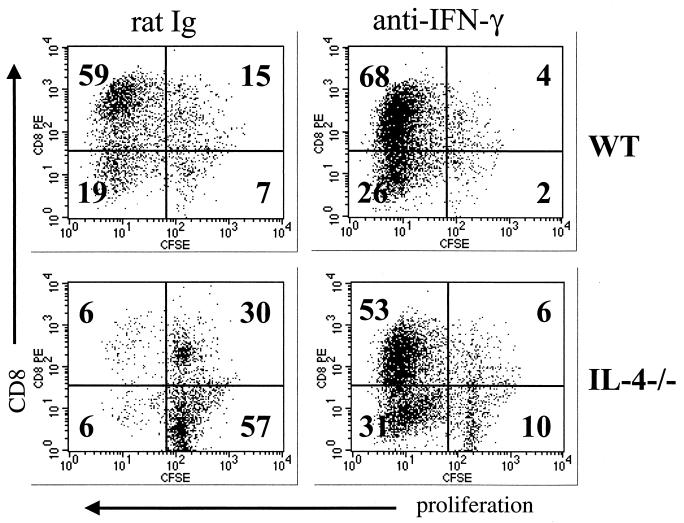
Overproduction of NO has been shown to cause impaired in vitro proliferation of lymphocytes from IL-4−/− mice infected with Schistosoma mansoni (3; E. A. Patton, A. C. La Flamme, and E. J. Pearce, submitted for publication). Because neutralization of IFN-γ led to a reduction in NO production by MLN cells and splenocytes after anti-CD3 stimulation (data not shown) as well as following Ag stimulation (Fig. 2d), the effects of IFN-γ neutralization on lymphocyte proliferation were determined. In anti-CD3-stimulated splenocyte cultures from infected WT mice, CD8 cells are the major proliferating population (Fig. 4), whereas proliferation of all cells is impaired in cultures from infected IL-4−/− mice (Fig. 4). When IFN-γ was neutralized, thus leading to reduced levels of NO, proliferation was restored in IL-4−/− cultures and increased in WT cultures (Fig. 4). In cultures from uninfected WT and IL-4−/− mice, no significant differences were observed in proliferation of lymphocytes from either strain or whether IFN-γ was neutralized (data not shown).
FIG. 4.
Neutralization of IFN-γ restores proliferation of lymphocytes from infected IL-4−/− mice. Splenocytes were isolated from mice 7 weeks after infection with 70 cercariae, labeled with CFSE, and stimulated in vitro with anti-CD3 Ab (0.5 μg/well). After 96 h, cells were stained with phycoerythrin (PE)-labeled anti-CD8 MAb and analyzed by flow cytometry. Numbers represent the percentage of cells within the quadrant, and shown are the results from one of two identical experiments. Anti-IFN-γ Ab or control rat Ig (0.5 mg/mouse) was injected i.p. every third day, and treatment was continued in vitro by addition of anti-IFN-γ Ab (10 μg/ml).
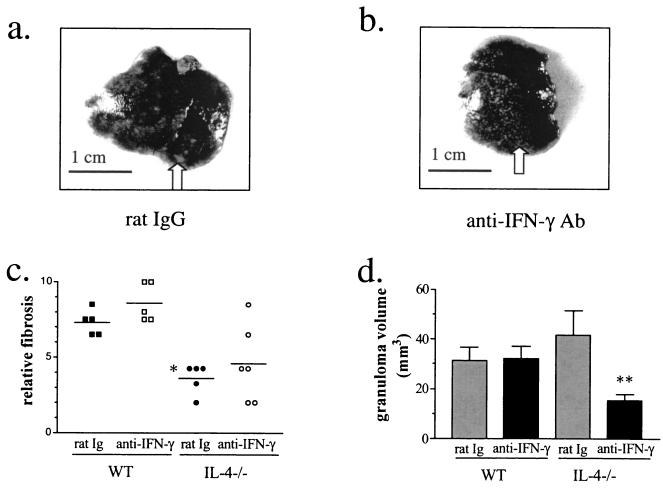
Pathologic changes in the livers of infected IL-4−/− mice are less severe when IFN-γ is neutralized.
The livers of infected IL-4−/− mice are covered in large white patches reflecting the coalescence of granulomas into larger less discrete areas of inflammation (Fig. 5a). After anti-IFN-γ treatment, however, the gross appearance of the liver improved and discrete granulomas were again discernable (Fig. 5b). This appearance was macroscopically similar to that of the livers of WT mice that had been infected for the same length of time and did not change when IFN-γ was neutralized (not shown). Fibrosis was slightly increased after anti-IFN-γ treatment, primarily in WT mice (Fig. 5c). IL-4−/− mice, as reported previously, had reduced fibrosis compared with infected WT animals (Fig. 5c) (15, 18), and there was a trend for neutralization of IFN-γ to lead to increased fibrosis. Interestingly, IFN-γ neutralization also led to a reduction in granuloma size in infected IL-4−/− mice but not in WT mice (Fig. 5d).
FIG. 5.
Pathologic changes in the liver are less severe when IFN-γ is neutralized. Gross appearance of the liver is improved after treatment of IL-4−/− mice with anti-IFN-γ MAb (b) compared to the rat Ig control (a). Arrows point to larger coalesced areas of inflammation (a) and smaller discrete areas (b). Neutralization of IFN-γ reduces the size of the granulomas in IL-4−/− but not WT mice (d) and leads to slightly increased fibrosis in WT but not IL-4−/− mice (c). WT and IL-4−/− mice were infected with 70 cercariae and injected with rat anti-IFN-γ Ab or control rat Ig (0.5 mg/mouse) every third day beginning 35 days after infection. Shown are the results from two similar experiments (five to six mice per group). Symbols: ∗, P < 0.001 (IL-4−/− versus WT); ∗∗, P < 0.03 (rat Ig versus anti-IFN-γ Ab). Error bars, standard errors of the means.
In vivo neutralization of IFN-γ does not prevent morbidity or mortality in infected IL-4−/− mice.
Despite the enhanced production of Th2 cytokines and the less-severe hepatic pathologic changes in the IL-4−/− mice after neutralization of IFN-γ, no effect was observed on the progressive weight loss associated with infection in these animals (Fig. 6a). To determine whether IFN-γ played a role in the other signs of disease development, mice were scored on activity, posture, and coat condition in addition to weight loss (19). Again, no difference was observed in the progression of severe disease in treated or untreated infected IL-4−/− mice (Fig. 6b). Moreover, no difference was found in weight change or clinical signs of disease in infected WT mice with or without anti-IFN-γ treatment (Fig. 6). Treatment of uninfected mice with anti-IFN-γ did not have any discernable gross effect (data not shown). Taken together, these data suggest that although IFN-γ may contribute to some of the pathology associated with schistosomiasis in IL-4−/− mice, it does not alone ultimately mediate severe disease.
DISCUSSION
With the onset of parasite egg deposition in schistosome-infected WT mice, Th1 responses are downregulated while Th2 responses develop and subsequently dominate (13, 27). In the absence of IL-4, Th2 response development is impaired and Ag-specific Th1 responses remain high, and this sustained proinflammatory response is believed to underlie the development of severe disease in these mice (4, 28). Other studies addressing the roles of IL-12, TNFR1, and NO in infected IL-4−/− mice revealed that neither IL-12 nor TNFR1 mediated morbidity and mortality and that NO plays an important protective role during infection (3, 26; E. A. Patton et al., unpublished data). The underlying reason for examining infection outcome in IL-4−/−/IL-12−/− mice was to assess the role of IFN-γ in disease development with the expectation that IFN-γ production would be IL-12 dependent. However, these experiments revealed that IFN-γ production in infected IL-4−/− mice is IL-12p35 independent (26) and thus left unanswered the question of the role of IFN-γ during severe disease.
In this study, we show that Ag-specific IFN-γ production precedes the production of NO in infected IL-4−/− mice and that Ab-mediated neutralization of IFN-γ in infected WT and IL-4−/− mice results in decreased NO production. This reduction in NO by IFN-γ neutralization in IL-4−/− mice restores lymphocyte proliferation, consistent with the previously described role of NO in proliferative impairment in these animals (E. A. Patton, submitted for publication). Th2 cytokine responses were enhanced in anti-IFN-γ-treated infected WT and IL-4−/− mice and Ag-specific Ab isotype analysis confirmed the enhancement of Th2 responses in vivo in WT animals. While the absence of both IL-4 and IFN-γ resulted in less-severe hepatic pathology and reduced granuloma size, anti-IFN-γ treatment only promoted an increase in fibrosis when IL-4 was present. However, despite the enhancement of Th2 responses, reduction in NO, and less-severe liver disease, neutralization of IFN-γ in vivo did not prevent the development of severe disease in IL-4−/− mice.
Previous studies which have investigated the role of IFN-γ during schistosome infection in WT mice have shown similar findings to those reported here (1, 34, 35). Several reports using IFN-γ−/− or IFN-γR−/− mice have shown that Ag-specific Th2 responses are enhanced when IFN-γ is absent (1, 34), while no differences in the size of liver granulomas are observed (1, 35). It should also be noted that one study did not find any significant differences in Ag-specific Th2 cytokine production in IFN-γ−/− mice (35). The apparent contradiction in results may stem from differences in the time at which cytokine production was assayed. It is possible that the enhancement in Th2 cytokine production occurs maximally at earlier times of infection (7 weeks) as reported here and that this difference becomes less evident at later times (8 to 16 weeks).
The increase in fibrosis which occurs in WT mice after anti-IFN-γ treatment is consistent with previous studies which have shown that immune deviation to a Th1 response in WT mice results in decreased liver fibrosis (14, 15). Here we show that neutralization of IFN-γ and the subsequent enhancement of Th2 cytokine production promotes an increase in hepatic fibrosis. A similar increase is not found after anti-IFN-γ treatment of IL-4−/− mice, but this difference most likely stems from impaired Th2 responses and associated reduction in IL-13 production. Although IL-13, which is a key profibrotic cytokine (7), is increased in anti-IFN-γ treated IL-4−/− mice, it does not reach the levels found in WT mice (Fig. 2b).
The reduction in granuloma size which occurred when both IL-4 and IFN-γ were absent during infection is similar to that observed when NO production was inhibited in IL-4−/− mice (3). In that study a similar decrease in granuloma size was reported and occurred in both WT and IL-4−/− mice (3). However, since other cytokines can be potent stimulators of NO production (e.g., tumor necrosis factor alpha) (9), it may be that in WT mice, IFN-γ, which is produced only at low levels, is not the major inducer of NO production. Therefore, neutralization of IFN-γ may not significantly reduce NO production in vivo in WT mice. In infected IL-4−/− animals, which produce elevated levels of IFN-γ, NO production may be primarily regulated by IFN-γ.
The role of IFN-γ in inducing NO-mediated pathology has been investigated by studying immune deviation in WT mice presensitized with eggs and IL-12 (14, 34). In these mice, the reduction in granuloma size and fibrosis is mediated by IFN-γ-dependent NO (14). If this pathway is disrupted (e.g., in IFN-γ−/− or inducible NO synthase-deficient mice), presensitization leads to exacerbated liver pathology (14, 34). Our studies extend these findings by showing that in the absence of a strong Th2 response, as seen in infected IL-4−/− mice, disruption in IFN-γ and therefore of NO results not in an increase but rather in a decrease in granuloma size. Together these results suggest that while Th2 cells are required for granuloma formation around schistosome eggs, it is through the action of Th1-associated responses that granuloma pathology can be controlled (33).
The enhancement of Th2 cytokine production by IFN-γ neutralization in the absence of IL-4 indicates that IL-4-independent pathways are responsible for the induction of IL-5 and IL-13 and that these mechanisms can be inhibited by IFN-γ. A recent work by Jankovic et al. (17) reported that Th2 development during schistosome infection can occur independently of Stat-6 or IL-4Rα signaling. Moreover, Ouyang et al. (25) found that Stat-6-independent GATA-3 activation can occur through autoactivation and may be responsible for Th2 development independent of IL-4. In the presence of IL-12, however, IL-4-independent IL-4 production did not occur (25). Our results suggest that IL-4-independent Th2 development does occur in schistosome-infected IL-4−/− mice and that it is controlled not through IL-12 (26) but rather by IFN-γ.
Although neutralization of IFN-γ in vivo promoted IL-4-independent Th2 development and led to less-severe liver pathology, infected IL-4−/− mice treated with anti-IFN-γ Ab suffered gross morbidity similar to untreated IL-4−/− mice, suggesting that IFN-γ is not the cause of severe disease in these animals. Previous studies have shown that cachexia and mortality in infected IL-4−/− mice are not due to IL-12p35 (26), TNFR1 signaling (E. A. Patton et al., unpublished data), or NO (3). Although NO production most closely correlates to severe disease, at low levels it is believed to have a protective effect by scavenging superoxide and other reactive oxygen intermediates (19). Our previous work has shown that treatment of infected IL-4−/− mice with antioxidants to neutralize the toxic effects of the overproduction of reactive oxygen and nitrogen intermediates prevents these mice from developing severe disease (19). The results presented here suggest that this overproduction of superoxide and other reactive intermediates is not promoted by IFN-γ. Furthermore, a recent study has implicated IL-6 in the development of severe disease in the absence of IL-4 (A. C. La Flamme, E. L. Feeney, C. R. Huxtable, and E. J. Pearce, submitted for publication). Interestingly, experimental autoimmune encephalomyelitis is another disease in which pathogenesis appears to be strongly associated with the overproduction of peroxynitrite and superoxide (16), and in the absence of IL-6, disease development is prevented (10, 21, 24). These results indicate that IL-6 may play an important proinflammatory role in promoting reactive intermediate production in several disease models. While it is clear that tumor necrosis factor alpha, IL-12, IFN-γ, and NO do not mediate fatal schistosomiasis in IL-4−/− animals, further work is needed to examine reactive intermediate production and the factors responsible for their regulation during schistosome infection.
ACKNOWLEDGMENTS
This work was supported by National Institute of Health grant R01-A1-32573 (to E.J.P.). E.J.P. is a Burroughs Wellcome Fund Scholar in Molecular Parasitology. A.C.L. is supported by National Service Award A1-10151.
Schistosome life cycle stages for this works were supplied through NIH-NIAID contract NO1-AI-55270. We thank A. S. MacDonald and M. Beall for their help and advice and B. Bauman and L. Anguish for technical assistance.
REFERENCES
- 1.Akhiani A A, Lycke N, Nilsson L A, Olling S, Ouchterlony O. Lack of interferon-gamma receptor does not influence the outcome of infection in murine schistosomiasis mansoni. Scand J Immunol. 1996;43:257–262. doi: 10.1046/j.1365-3083.1996.d01-33.x. [DOI] [PubMed] [Google Scholar]
- 2.Boros D L, Warren K S. Delayed hypersensitivity-type granuloma formation and dermal reaction induced and elicited by a soluble factor isolated from Schistosoma mansoni eggs. J Exp Med. 1970;132:488–507. doi: 10.1084/jem.132.3.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brunet L R, Beall M, Dunne D W, Pearce E J. Nitric oxide and the Th2 response combine to prevent severe hepatic damage during Schistosoma mansoni infection. J Immunol. 1999;163:4976–4984. [PubMed] [Google Scholar]
- 4.Brunet L R, Finkelman F D, Cheever A W, Kopf M A, Pearce E J. IL-4 protects against TNF-alpha-mediated cachexia and death during acute schistosomiasis. J Immunol. 1997;159:777–785. [PubMed] [Google Scholar]
- 5.Butterworth A E, Curry A J, Dunne D W, Fulford A J, Kimani G, Kariuki H C, Klumpp R, Koech D, Mbugua G, Ouma J H, et al. Immunity and morbidity in human schistosomiasis mansoni. Trop Geogr Med. 1994;46:197–208. [PubMed] [Google Scholar]
- 6.Cheever A W. Relative resistance of the eggs of human schistosomes to digestion in potassium hydroxide. Bull W H O. 1970;43:601–603. [PMC free article] [PubMed] [Google Scholar]
- 7.Chiaramonte M G, Donaldson D D, Cheever A W, Wynn T A. An IL-13 inhibitor blocks the development of hepatic fibrosis during a T-helper type 2-dominated inflammatory response. J Clin Investig. 1999;104:777–785. doi: 10.1172/JCI7325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Curry R C, Kiener P A, Spitalny G L. A sensitive immunochemical assay for biologically active MuIFN-gamma. J Immunol Methods. 1987;104:137–142. doi: 10.1016/0022-1759(87)90497-2. [DOI] [PubMed] [Google Scholar]
- 9.Ding A H, Nathan C F, Stuehr D J. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1988;141:2407–2412. [PubMed] [Google Scholar]
- 10.Eugster H P, Frei K, Kopf M, Lassmann H, Fontana A. IL-6-deficient mice resist myelin oligodendrocyte glycoprotein-induced autoimmune encephalomyelitis. Eur J Immunol. 1998;28:2178–2187. doi: 10.1002/(SICI)1521-4141(199807)28:07<2178::AID-IMMU2178>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 11.Fallon P G, Richardson E J, McKenzie G J, McKenzie A N. Schistosome infection of transgenic mice defines distinct and contrasting pathogenic roles for IL-4 and IL-13: IL-13 is a profibrotic agent. J Immunol. 2000;164:2585–2591. doi: 10.4049/jimmunol.164.5.2585. [DOI] [PubMed] [Google Scholar]
- 12.Green L C, Wagner D A, Glogowski J, Skipper P L, Wishnok J S, Tannenbaum S R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 13.Grzych J M, Pearce E, Cheever A, Caulada Z A, Caspar P, Heiny S, Lewis F, Sher A. Egg deposition is the major stimulus for the production of Th2 cytokines in murine schistosomiasis mansoni. J Immunol. 1991;146:1322–1327. [PubMed] [Google Scholar]
- 14.Hesse M, Cheever A W, Jankovic D, Wynn T A. NOS-2 mediates the protective anti-inflammatory and antifibrotic effects of the Th1-inducing adjuvant, IL-12, in a Th2 model of granulomatous disease. Am J Pathol. 2000;157:945–955. doi: 10.1016/S0002-9440(10)64607-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoffmann K F, Cheever A W, Wynn T A. IL-10 and the dangers of immune polarization: excessive type 1 and type 2 cytokine responses induce distinct forms of lethal immunopathology in murine schistosomiasis. J Immunol. 2000;164:6406–6416. doi: 10.4049/jimmunol.164.12.6406. [DOI] [PubMed] [Google Scholar]
- 16.Hooper D C, Spitsin S, Kean R B, Champion J M, Dickson G M, Chaudhry I, Koprowski H. Uric acid, a natural scavenger of peroxynitrite, in experimental allergic encephalomyelitis and multiple sclerosis. Proc Natl Acad Sci USA. 1998;95:675–680. doi: 10.1073/pnas.95.2.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jankovic D, Kullberg M C, Noben-Trauth N, Caspar P, Paul W E, Sher A. Single cell analysis reveals that IL-4 receptor/Stat6 signaling is not required for the in vivo or in vitro development of CD4+ lymphocytes with a Th2 cytokine profile. J Immunol. 2000;164:3047–3055. doi: 10.4049/jimmunol.164.6.3047. [DOI] [PubMed] [Google Scholar]
- 18.Jankovic D, Kullberg M C, Noben-Trauth N, Caspar P, Ward J M, Cheever A W, Paul W E, Sher A. Schistosome-infected IL-4 receptor knockout (KO) mice, in contrast to IL-4 KO mice, fail to develop granulomatous pathology while maintaining the same lymphokine expression profile. J Immunol. 1999;163:337–342. [PubMed] [Google Scholar]
- 19.La Flamme A C, Patton E A, Bauman B, Pearce E J. IL-4 plays a crucial role in regulating oxidative damage in the liver during schistosomiasis. J Immunol. 2001;166:1903–1911. doi: 10.4049/jimmunol.166.3.1903. [DOI] [PubMed] [Google Scholar]
- 20.La Flamme A C, Pearce E J. The absence of IL-6 does not affect Th2 cell development in vivo, but does lead to impaired proliferation, IL-2 receptor expression, and B cell responses. J Immunol. 1999;162:5829–5837. [PubMed] [Google Scholar]
- 21.Mendel I, Katz A, Kozak N, Ben-Nun A, Revel M. Interleukin-6 functions in autoimmune encephalomyelitis: a study in gene-targeted mice. Eur J Immunol. 1998;28:1727–1737. doi: 10.1002/(SICI)1521-4141(199805)28:05<1727::AID-IMMU1727>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 22.Mosmann T R, Fong T A. Specific assays for cytokine production by T cells. J Immunol Methods. 1989;116:151–158. doi: 10.1016/0022-1759(89)90198-1. [DOI] [PubMed] [Google Scholar]
- 23.Mwatha J K, Kimani G, Kamau T, Mbugua G G, Ouma J H, Mumo J, Fulford A J, Jones F M, Butterworth A E, Roberts M B, Dunne D W. High levels of TNF, soluble TNF receptors, soluble ICAM-1, and IFN-gamma, but low levels of IL-5, are associated with hepatosplenic disease in human schistosomiasis mansoni. J Immunol. 1998;160:1992–1999. [PubMed] [Google Scholar]
- 24.Okuda Y, Sakoda S, Bernard C C, Fujimura H, Saeki Y, Kishimoto T, Yanagihara T. IL-6-deficient mice are resistant to the induction of experimental autoimmune encephalomyelitis provoked by myelin oligodendrocyte glycoprotein. Int Immunol. 1998;10:703–708. doi: 10.1093/intimm/10.5.703. [DOI] [PubMed] [Google Scholar]
- 25.Ouyang W, Lohning M, Gao Z, Assenmacher M, Ranganath S, Radbruch A, Murphy K M. Stat6-independent GATA-3 autoactivation directs IL-4-independent Th2 development and commitment. Immunity. 2000;12:27–37. doi: 10.1016/s1074-7613(00)80156-9. [DOI] [PubMed] [Google Scholar]
- 26.Patton E A, Brunet L R, La Flamme A C, Pedras-Vasconcelos J, Kopf M, Pearce E J. Severe schistosomiasis in the absence of interleukin-4 (IL-4) is IL-12 independent. Infect Immun. 2001;69:589–592. doi: 10.1128/IAI.69.1.589-592.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pearce E J, Caspar P, Grzych J M, Lewis F A, Sher A. Downregulation of Th1 cytokine production accompanies induction of Th2 responses by a parasitic helminth, Schistosoma mansoni. J Exp Med. 1991;173:159–166. doi: 10.1084/jem.173.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pearce E J, Cheever A, Leonard S, Covalesky M, Fernandez-Botran R, Kohler G, Kopf M. Schistosoma mansoni in IL-4-deficient mice. Int Immunol. 1996;8:435–444. doi: 10.1093/intimm/8.4.435. [DOI] [PubMed] [Google Scholar]
- 29.Rakasz E, Blum A M, Metwali A, Elliott D E, Li J, Ballas Z K, Qadir K, Lynch R, Weinstock J V. Localization and regulation of IFN-gamma production within the granulomas of murine schistosomiasis in IL-4-deficient and control mice. J Immunol. 1998;160:4994–4999. [PubMed] [Google Scholar]
- 30.Schumacher J H, O'Garra A, Shrader B, van Kimmenade A, Bond M W, Mosmann T R, Coffman R L. The characterization of four monoclonal antibodies specific for mouse IL-5 and development of mouse and human IL-5 enzyme-linked immunosorbent. J Immunol. 1988;141:1576–1581. [PubMed] [Google Scholar]
- 31.Smithers S R, Terry R J. The infection of laboratory hosts with cercariae of Schistosoma mansoni and the recovery of the adult worms. Parasitology. 1965;55:695–700. doi: 10.1017/s0031182000086248. [DOI] [PubMed] [Google Scholar]
- 32.Vella A T, Pearce E J. Schistosoma mansoni egg-primed Th0 and Th2 cells: failure to down-regulate IFN-gamma production following in vitro culture. Scand J Immunol. 1994;39:12–18. doi: 10.1111/j.1365-3083.1994.tb03333.x. [DOI] [PubMed] [Google Scholar]
- 33.Wynn T A, Hoffmann K F. Defining a schistosomiasis vaccination strategy—is it really Th1 versus Th2? Parasitol Today. 2000;16:497–501. doi: 10.1016/s0169-4758(00)01788-9. [DOI] [PubMed] [Google Scholar]
- 34.Wynn T A, Jankovic D, Hieny S, Zioncheck K, Jardieu P, Cheever A W, Sher A. IL-12 exacerbates rather than suppresses T helper 2-dependent pathology in the absence of endogenous IFN-gamma. J Immunol. 1995;154:3999–4009. [PubMed] [Google Scholar]
- 35.Yap G, Cheever A, Caspar P, Jankovic D, Sher A. Unimpaired down-modulation of the hepatic granulomatous response in CD8 T-cell- and gamma interferon-deficient mice chronically infected with Schistosoma mansoni. Infect Immun. 1997;65:2583–2586. doi: 10.1128/iai.65.7.2583-2586.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]