Abstract
Background
Prospectively and systematically collected long-term real-world clinical data on ustekinumab (anti-interleukin-12/23) are still scarce.
Aims
To assess the long-term effectiveness of ustekinumab in patients with active Crohn’s disease (CD).
Methods
This is a prospective multicenter study of adult patients with CD initiating ustekinumab according to recommended doses at 20 Swedish hospitals. The primary outcome was clinical remission (Harvey–Bradshaw Index (HBI) ≤ 4 points) at weeks 52 and 104. Secondary outcomes included clinical response (≥ 3-point-decrease in HBI among patients with initial HBI ≥ 5 points), treatment retention, and biomarkers (C-reactive protein (CRP), hemoglobin, fecal-calprotectin) at weeks 52 and 104 compared to baseline. We also reported Health-related Quality of Life (HRQoL) measures.
Results
Of 114 included patients, 107 (94%) had previously failed ≥ 1 and 58 (51%) ≥ 2 anti-tumor necrosis factor agents. Forty (35%) had failed anti-integrin agents. Ustekinumab retention rates at weeks 52 and 104 were 70% (n = 80/114) and 61% (n = 69/114), respectively. Clinical response was seen in 36% (n = 25/69) and 29% (n = 20/69) of the patients, and remission was achieved in 32% (n = 31/96) and 29% (n = 28/96) at weeks 52 and 104, respectively. Median HBI and CRP levels decreased significantly at both timepoints as compared to baseline. Significant improvements were also observed in HRQoL. Adverse events were reported in 11% (n = 13/114) of the patients, including five cases of severe adverse events. No malignancies were observed.
Conclusions
In this nationwide prospective real-world 104-week-follow-up study of adult patients with active CD, ustekinumab was associated with long-term clinical effectiveness and improvement in HRQoL measures when used in routine clinical care.
Keywords: Crohn’s disease, Inflammatory bowel disease, Observational study, Ustekinumab
Introduction
Crohn's disease (CD) is a chronic inflammatory bowel disease (IBD) affecting the entire gastrointestinal tract [1]. More than 20,000 individuals are affected by CD in Sweden [2]. The disease is often associated with reduced health-related quality of life (HRQoL), loss of work productivity, and increased morbidity and mortality [2–4]. In case of failure or intolerance to conventional treatment, including immunomodulators, anti-tumor necrosis factor (aTNF) agents have become the mainstay of medical therapy in CD [5, 6]. However, a sizeable number of patients are non-responders or experience secondary loss of response or intolerance to aTNF treatment [7–11]. Remaining treatment options include surgery and more recently biological agents such as ustekinumab.
After approval by the European Medicines Agency in 2016, ustekinumab has emerged as an option for the treatment of adult patients with moderate to severe CD disease who have had an inadequate response, lost response to, or were intolerant to either conventional therapy or aTNF, or have medical contraindications to such therapies. The efficacy of ustekinumab to maintain long-term (≥ 52 weeks) disease remission in patients with CD has only been described in a few observational studies [12–17] and a long-term extension of the randomized UNITI I and II studies (IM-UNITI long-term extension (LTE)) [18, 19]. The pivotal trials included both patients with prior aTNF failure and aTNF naïve patients, but does not accurately reflect a real-world clinical setting where patients with CD consist of a far more heterogeneous group of patients. In some previous observational studies [20], ustekinumab was administered with various dosing patterns, not adhering to label and routine clinical care. Other limitations of previous studies include a retrospective design and only regional coverage. We are only aware of one long-term study (> 52 weeks follow-up) on the effectiveness of ustekinumab entirely based on prospectively collected data in a nationwide patient population [13]. Hence, there is a need for data from nationwide real-world patient cohorts, with a homogenous treatment regimen, representing both regional and university hospitals.
We have previously assessed the short-term clinical effectiveness of ustekinumab in Crohn's disease [21]. The aim of the current study was to examine the long-term clinical effectiveness through a nationwide observational study, where real-world data were prospectively and systematically collected, assessing outcomes at 52 and 104 weeks after treatment initiation applying an intention-to-treat-like approach.
Methods
Study Design and Setting
This was a nationwide prospective multicenter non-interventional observational study of patients initiated on ustekinumab according to routine clinical care in Sweden. Treating physicians at gastroenterology departments in 20 Swedish hospitals (9 university and 11 regional hospitals (Appendix)) independently initiated ustekinumab treatment between 23 January 2017 and 22 November 2018 and assessed clinical and biochemical response during follow-up according to national treatment guidelines.
Study Participants
All included patients had a physician-confirmed international classification of disease (ICD) diagnosis of CD (ICD-10: K50 all sub-classifications) with active disease based on clinical activity, inflammatory markers, endoscopic findings, or steroid dependence, as defined by the treating physician. Patients were naïve to, had an inadequate response, lost response to, were intolerant to either conventional therapy or aTNF, or had medical contraindications to such therapies. All patients started first treatment with ustekinumab at age ≥ 18 years. The included patients had to be first-time users of ustekinumab, initiated ustekinumab within the last 2 weeks before study inclusion, or started treatment < 12 months ago if sufficiently detailed patient data were documented in the Swedish National Quality Register for inflammatory bowel disease (SWIBREG)[22] within ± 2 weeks of treatment initiation. SWIBREG includes comprehensive data on disease phenotype, previous and current treatment (including biologics), surgery, and HRQoL. Exclusion criteria were previous exposure or contraindication to ustekinumab, concurrent participation in other clinical studies, and planned pregnancy during the study period. Baseline- and clinical follow-up data were reported using a study-specific electronic case report form (eCRF) linked to the SWIBREG.
Outcomes and Definitions
The primary objective was to determine the proportion of patients with clinical remission at weeks 52 and 104 (week 16 reported elsewhere [21]). Clinical remission was defined as a Harvey–Bradshaw Index (HBI) [23] ≤ 4 points. Secondary objectives included clinical response at weeks 52 and 104, defined as a reduction in HBI score ≥ 3 points among patients with HBI ≥ 5 points at baseline, and remission at weeks 52 and 104 among remitters at week 16. Other objectives included treatment retention rates and HRQoL measures, such as Short Health Scale (SHS)[24] and EuroQual 5-Dimensions 5-Levels (EQ-5D-5L) [25]. We also assessed the association between ustekinumab and inflammatory biomarkers, C-reactive protein (CRP), hemoglobin (Hb), and fecal (f)-calprotectin, comparing levels at baseline and weeks 52 and 104. Follow-up time was defined as time from date of first ustekinumab intravenous induction dose until end of follow-up or date of discontinuation of treatment, whichever came first.
The SHS captures four self-reported dimensions of HRQoL in IBD patients, including bowel symptom burden, social function/activity, worry, and general well-being. Each item is scored from 0 to 5, ranging from no problem (0) to worst imaginable state (5) [24].
The EQ-5D-5L includes five self-reported generic dimensions of HRQoL, mobility, self-care/hygiene, usual/daily activities, pain/discomfort, and anxiety/depression [25]. It also includes a visual analogue scale (VAS) to assess the current health state, ranging from 0 to 100, representing the worst (0) to the best possible state (100). The responses to each dimension were converted into a compound index value where 1.0 represents best possible overall wellbeing.
Ustekinumab
Ustekinumab induction was administered intravenously according to the summary of product characteristics, recommending induction dose 6 mg/kg in steps of 130 mg (≤ 55 kg: 260 mg; > 55 to ≤ 85 kg: 390 mg; > 85 kg: 520 mg). A 90 mg subcutaneous injection was administered 8 weeks after the intravenous dose followed by a 90 mg subcutaneous injection maintenance therapy administered every 8 or 12 weeks. However, in this real-world setting, maintenance dosing intervals were decided independently by the treating physician.
Data Collection
From SWIBREG [22], we collected baseline data, including demographics and clinical characteristics such as year of diagnosis, age at diagnosis, history of previous CD-related bowel surgery, extraintestinal manifestations, disease location and behavior according to the Montreal Classification of disease [26], and clinically relevant medication for CD (including previous biologics with reasons for discontinuation). Data on clinical and biochemical disease activity and HRQoL measures were recorded at baseline and at follow-up visits 16, 52 (± 4 weeks) and 104 (−8/ + 12) weeks after initiation of ustekinumab treatment. We applied strict time-windows for collected clinical, biochemical, and HRQoL data, only including such data in the analyses if reported within ± 2 weeks of the physician's follow-up visits. Endoscopy data were considered valid if reported within ± 4 weeks. In patients who stopped UST treatment, reason for discontinuation of treatment was recorded. We recorded all adverse events related to the treatment with ustekinumab, including infections and malignancies. In addition, all adverse events were reported by the local investigator to Janssen-Cilag AB and to the Swedish Medical Products Agency.
Statistical Analysis
We reported descriptive findings as median and interquartile range (IQR) for continuous variables and as proportions for categorical variables for the biochemical and clinical outcomes. Comparisons of clinical disease activity and inflammatory biomarkers between baseline and follow-up were restricted to patients who had data recorded at both timepoints. Paired HBI, CRP, Hb and f-calprotectin, EQ-5D-5L, and SHS values were analyzed by the Wilcoxon matched-pair signed-rank test. To calculate the EQ-5D-5L index, we applied the standardized valuation study protocol version 2.0 provided by the EuroQol group using STATA software [27]. Ustekinumab drug survival rates were presented in a plot using the Kaplan–Meier curves. We applied an intention-to-treat-like approach, where missing data and discontinuation, regardless of the reason for discontinuation, were classified as treatment failure. Univariable and multivariable logistic regression was used to estimate baseline predictors of remission at weeks 52 and 104. The selection of included covariates was based on potential biological association with our main outcome: remission at weeks 52 and 104. All statistical tests were two-tailed, and p-values of < 0.05 were considered statistically significant. Data management and statistical analyses were performed using Microsoft Excel (MS Office 2018), SAS version 9 (SAS Inc., Cary, NC), and STATA software version 15.1 (StataCorp, College Station, TX).
Results
Patient Characteristics
A total of 114 patients, representing 20 different hospitals with a geographical coverage of almost all of Sweden, were included. Patient characteristics at baseline are presented in.
Table 1. Nearly all patients (94%, n = 107/114) had failed at least one biological drug, and 51% (n = 58) had failed ≥ 2 biologics. Some 35% had failed anti-integrin antibody agents (n = 40). At baseline; 23% (n = 26) and 18% (n = 21) had concomitant treatment with immunomodulators and corticosteroids, respectively. Almost one third (32%, n = 37) had a history of previous bowel surgery related to CD. At baseline, 72% (n = 69/96, missing: n = 18/114) had a HBI ≥ 5 with a median HBI of 6 (IQR 4–11).
Table 1.
Baseline patient characteristics and phenotype according to the Montreal Classification of Crohn's disease patients treated with ustekinumab
| Characteristics | N (%) * | N—total |
|---|---|---|
| Median age at baseline, years (IQR) | 40 (31–54) | 114 |
| Sex, female | 54 (47) | 114 |
| BMI–Mean ± SD | 25.4 ± 5.7 | 110 |
| Smoking status | ||
| Current | 12 (11) | 114 |
| Previous | 40 (35) | |
| Never | 62 (54) | |
| Age at diagnosis | ||
| ≤ 16 years (A1) | 17 (15) | 114 |
| 17–40 years (A2) | 75 (66) | |
| < 40 years (A3) | 22 (19) | |
| Location | ||
| Ileum (L1) | 24 (21) | 114 |
| Colon (L2) | 35 (31) | |
| Ileocolonic (L3) | 55 (48) | |
| Behavior | ||
| Inflammatory (B1) | 51 (45) | 114 |
| Stricturing (B2) | 50 (44) | |
| Penetrating (B3) | 13 (11) | |
| Perianal disease (B1p –B3p) | 18 (16) | |
| Any extra intestinal manifestation | 20 (18) | 114 |
| Previous surgical resection | 37 (32) | 114 |
| Previous biological treatment | ||
| ≥ 1 | 107 (94) | 112 |
| ≥ 2 | 58 (51) | |
| ≥ 3 | 24 (21) | |
| Reason for termination of last biological agent | ||
| Lack of or loss of response | 83 (78) | 107 |
| Intolerance | 21 (20) | |
| Other reason | 3 (3) | |
| Concomitant medication at baseline | ||
| Corticosteroids | 21 (18) | 114 |
| Immunomodulators | 26 (23) | |
| HBI Median, (IQR)/Mean ± SD | 6 (4–11)/7.8 ± 5.1 | 96 |
| Clinical disease activity | ||
| Remission (HBI < 5) | 27 (28) | 96 |
| Mild (HBI 5‐7) | 31 (32) | |
| Moderate (HBI 8‐16) | 31 (32) | |
| Severe (HBI ≥ 17) | 7 (7) | |
|
Median f-calprotectin, µg/g (IQR) ≥ 250 µg/g, n (%) |
292 (163–1143) 21 (57) |
37 |
|
Median CRP, mg/l (IQR)/Mean ± SD ≥ 3 mg/l, n (%) |
7 (4–15)/15 ± 20 91 (93) |
98 |
| Median Hb, g/l (IQR)/Mean (SD) | 135 (125–146)/135(16) | 99 |
CRP = C-reactive protein; f-calprotectin = fecal calprotectin; Hb = hemoglobin; HBI = Harvey–Bradshaw Index; IQR = interquartile range; SD = standard deviation
*Number (%) where not otherwise stated
Treatment Persistence
A total of 45 patients (39%, n = 45/114) discontinued ustekinumab before week 104 after a median treatment duration of 28 weeks (IQR 18–44). The 52- and 104-week drug survival rates were 80/114 (70%) and 69/114 (61%) (Figs. 1 and 2). Reasons for termination of ustekinumab were lack of response (27%, n = 31), lost to follow-up (4%, n = 5), adverse events (3%, n = 3), withdrawal of consent (3%, n = 3), pregnancy (2%, n = 2), and death (1%, n = 1). The included 114 patients were on ustekinumab treatment for a total of 169 patient years.
Fig. 1.
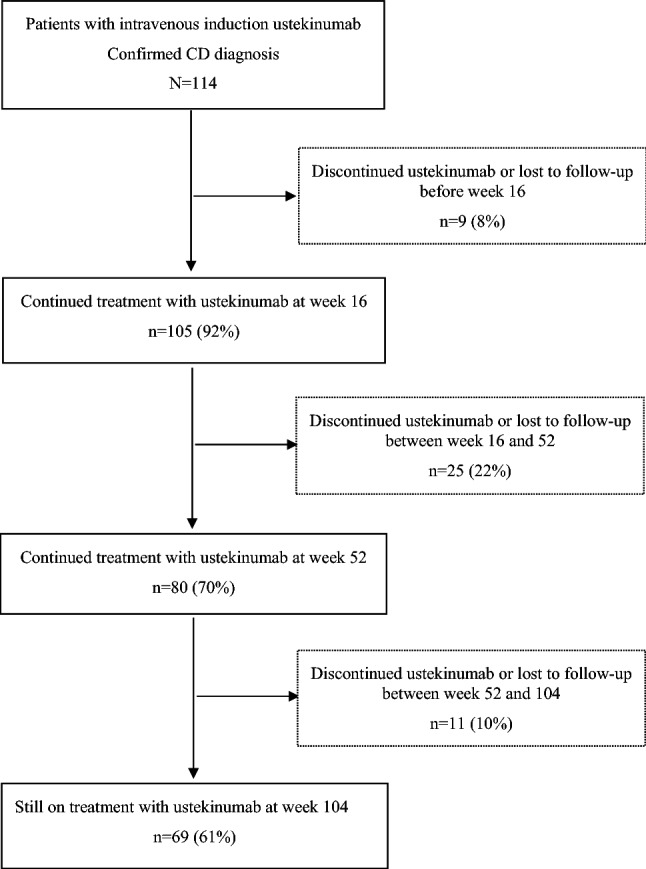
Flowchart of ustekinumab treatment in the PROSE study population. In total, 114 patients with confirmed Crohn’s disease (CD) diagnosis were included. Treatment retention at weeks 16, 52 and 104 was seen in 105 (92%), 80 (70%) and 69 (61%) patients, respectively. Of these, 9 (8%) patients discontinued or were lost to follow-up (lack of response, n = 6; pregnancy, n = 1; lost to follow-up, n = 2) before week 16. Between weeks 16 and 52, a total of 25 (22%) patients discontinued, were lost to follow-up or withdrew consent (lack of response, n = 18; adverse event, n = 3; lost to follow-up, n = 1; withdrawal of consent, n = 3). Between weeks 52 and 104, 11 (10%) patients discontinued, were lost to follow-up or died (lack of response, n = 7; pregnancy, n = 1; death, n = 1; lost to follow-up, n = 2)
Fig. 2.
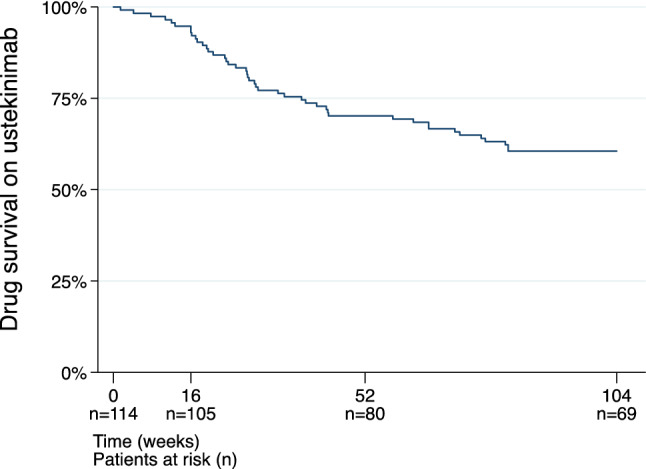
Ustekinumab drug survival Kaplan–Meier probability plot of ustekinumab drug survival during follow-up (104 weeks)
Clinical Outcomes
Of 96 (84%) patients with reported HBI scores at baseline, 56 (58%) patients also had valid HBI scores at week 52. An additional 24 (25%) patients were still on ustekinumab treatment but lacked information on HBI within the pre-specified time window. The median week 52 HBI score compared to baseline decreased from 6 (IQR 4–11) to 4 (IQR 2–6) (p < 0.001) (Table 2). The proportion of patients in clinical remission (HBI ≤ 4 points) at week 52 was 32% (n = 31/96) (Table 3, Fig. 3). Clinical response (HBI reduction in ≥ 3 points) was achieved in 36% (n = 25/69) of patients with HBI score ≥ 5 points at baseline. Clinical remission or response was achieved in 44% (n = 42/96). Of week 16 remitters, 52% (n = 13/25) were still in clinical remission at week 52.
Table 2.
Clinical and biochemical values at baseline and weeks 16, 52, and 104 among patients with Crohn’s disease treated with ustekinumab
| Baseline | Week 16 | Week 52 | Week 104 | Week 16 | Week 52 | Week 104 | |
|---|---|---|---|---|---|---|---|
| Median (IQR) | Median change vs. baseline* (IQR); p-value | ||||||
| HBI |
6 (4–11) (n = 96) |
5 (4–7) (n = 80) |
4 (2–6) (n = 66) |
3 (1–5) (n = 49) |
− 1 (− 3–1); .003 (n = 70) |
− 2 (− 4– − 1); < .001 (n = 56) |
− 2 (− 5–0); < .001 (n = 43) |
|
CRP mg/l |
7 (4–15) (n = 98) |
5 (4–7) (n = 75) |
4 (4–6) (n = 65) |
4 (4–6) (n = 48) |
− 1 (− 9–0); .006 (n = 65) |
− 1 (− 10–0); < .001 (n = 54) |
− 1 (− 7–0); < .001 (n = 39) |
| F-calprotectin µg/g |
292 (163–1143) (n = 37) |
204 (134–367) (n = 46) |
191 (58–527) (n = 52) |
139 (58–441) (n = 50) |
− 65 (− 754–19); .02** (n = 18) |
− 86 (− 548–113); .1** (n = 15) |
− 14 (− 112–318); .4** (n = 15) |
|
Hb g/l |
135 (125–146) (n = 99) |
138 (128–149) (n = 75) |
140 (130–146) (n = 66) |
134 (127–147) (n = 49) |
0 (− 4–7); .3 (n = 66) |
2 (− 4–10); .07 (n = 55) |
− 2 (− 8–6); .8 (n = 41) |
HBI = Harvey–Bradshaw Index; CRP = C-reactive protein; f-calprotectin = fecal calprotectin; Hb = hemoglobin; IQR, inter-quartile range; n = number of patients with available data within ± 2 weeks of baseline, weeks 16, 52, and 104 visits to the physician
*Includes patients with recorded data at baseline ± 2 weeks and at weeks 16, 52, and 104 ± 2 weeks; ** calculated as ln(f-calprotectin)
Table 3.
Harvey–Bradshaw Index, clinical remission, and response at baseline, weeks 16, 52, and 104
| Baseline* N = 114 (%) |
Week 16** N = 105 (%) |
Week 52** N = 80 (%) |
Week 104** N = 69 (%) |
||
|---|---|---|---|---|---|
| Mild–severe clinical activity (HBI ≥ 5) | n/N (%) | 69/96 (72) | 45/70 (71) | 25/56 (45) | 15/43 (35) |
| Clinical remission (HBI ≤ 4) | n/N (%) | 27/96 (28) | 25/70 (36) | 31/56 (55) | 28/43 (65) |
| Maintained clinical remission# | n/N (%) | – | – | 13/56 (23) | 11/43 (26) |
| Clinical response¶ | n/N (%) | – | 23/50 (29) | 25/37 (68) | 20/29 (69) |
| Clinical response or remission§ | n/N (%) | – | 38/70 (54) | 42/56 (75) | 31/43 (72) |
*Includes patients with recorded HBI scores or inflammatory markers recorded within ± 2 weeks of baseline; **includes patients with recorded HBI scores or inflammatory markers within ± 2 weeks at baseline, weeks 16, 52, and 104; # remission at week 16 and still in remission at weeks 52 or 104; ¶ HBI ≥ 5 at baseline and response at weeks 16, 52, or 104; § HBI ≥ 5 at baseline and response or remission at weeks 16, 52, or 104
HBI = Harvey–Bradshaw Index
Fig. 3.
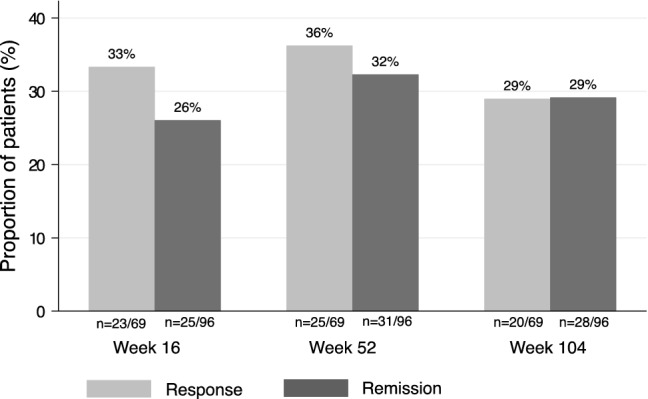
Clinical response and remission. Proportion of patients (%) with Harvey–Bradshaw Index (HBI) score ≥ 5 at baseline (n = 69) and response (≥ 3-point-decrease in HBI) at weeks 16, 52 and 104. Proportion of patients (%) in clinical remission (HBI score ≤ 4 points) at weeks 16, 52 and 104 among patients with HBI scores at baseline (n = 96)
Of the 96 patients with reported HBI scores at baseline, 43 (45%) patients also had HBI scores at week 104. Another 26 patients still on ustekinumab lacked HBI scores within the pre-specified time window. Compared to baseline, the median HBI score decreased from 6 (IQR 4–11) to 3 (IQR 1–5) (p < 0.001) (Table 2). Clinical remission at week 104 was achieved in 29% (n = 28/96). Clinical response was seen in 29% (n = 20/69) of patients with HBI score ≥ 5 points at baseline. Clinical remission or response was seen in 32% (n = 31/96) (Table 3, Fig. 3). Of patients in remission at week 16, 44% (n = 11/25) remained in clinical remission at week 104. Among patients treated with corticosteroids at baseline, 21% (n = 4/19) and 21% (n = 4/19) were in remission at weeks 52 and 104, respectively.
Biochemical Outcomes
For patients still on ustekinumab at weeks 52 (70%, n = 80/114) and 104 (61%, n = 69/114), the median CRP (mg/l) levels compared to baseline decreased from 7 (IQR 4–15) to 4 (IQR 4–6) (p < 0.001, n = 54, missing: n = 26/80) and 4 (IQR 4–6) (p < 0.001, n = 39, missing: n = 30/69), respectively. Median Hb (g/l) levels seemed to increase from 135 (IQR 125–146) at baseline to 140 (IQR 130–146) (p = 0.07, n = 55, missing: n = 25/80) at week 52 and decreased to 134 (IQR 127–147) (p = 0.8, n = 41, missing: n = 28/69) at week 104. Levels of f-calprotectin (µg/g) seemed to decrease from 292 (IQR 163–1143) to 191 (IQR 58–527) (p = 0.1, n = 15, missing: n = 65/80) and 139 (IQR 58–441) (p = 0.4, n = 15, missing: n = 45/69) at the corresponding time points.
Dosing Interval
After receiving intravenous induction, 110 (96%) received at least one 90 mg subcutaneous dose of ustekinumab 8 weeks after the induction dose. Thereafter, 89 (85%, n = 89/105) patients continued treatment with a 12-week dosing interval, 14 (13%, n = 14/105) with an 8-week interval and 2 (2%, n = 2/105) received intensified dosing intervals of 4 and 5 weeks, respectively. Dosage of ustekinumab was decided by the treating physician and was not regulated by the protocol.
There were no significant differences in the mean drug survival time between patients with a maintenance interval of 12 compared to 8 weeks (p = 0.63) at week 16. Between weeks 16 and 52, 8 (8%, 8/105) patients underwent dose escalation to 8- (n = 6) or 4- (n = 2) week intervals, and between weeks 52 and 104, another 4 (5%, 4/80) patients had their dosing intervals shortened to 8 (n = 2), 6 (n = 1) and 4 (n = 1) weeks, respectively. The proportions of patients achieving remission at weeks 52 and 104 did not significantly differ between the group with a 12-week interval and those with an interval of 8 weeks or less at week 16 follow-up (w52: p = 0.28; w104: p = 0.18). None of the patients (n = 3) discontinuing due to adverse events had shortened dosing intervals during maintenance treatment.
Health-Related Quality of Life Outcomes
Assessment of HRQoL measures showed statistically significant improvement in all separate dimension of SHS and in the EQ-5D-5L index value compared to baseline in patients still on ustekinumab at each follow-up timepoint. (Appendix, Table 5) The median change of total SHS score compared to baseline decreased by −2 (IQR −4–0) (p < 0.001), −3 (IQR −6 to −1) (p < 0.001) and −4.5 (IQR −8 to −1) (p < 0.001) points at weeks 16 (n = 86, missing: n = 19/105), 52 (n = 64, missing: n = 16/80) and 104 (n = 46, missing: n = 23/69), respectively. In parallel, the median of EQ-5D-5L index value increased by 0.03 (IQR −0.01–0.10) (p < 0.001), 0.03 (IQR 0–0.11) (p < 0.001), and 0.12 (IQR 0.09–0.21) (p < 0.001) at weeks 16 (n = 81, missing: n = 24/105), 52 (n = 61, missing: n = 19/80), and 104 (n = 47, missing: n = 22/69).
Predictors of Remission
In a univariable analysis of predictors for week-52 and 104 remission, HBI score at baseline was statistically significant, with higher HBI scores at baseline being associated with a lower probability of remission at both timepoints (Odds ratio (OR) = 0.74; 95%CI = 0.62–0.89 and OR = 0.8; 95%CI = 0.66–0.97, respectively) (Appendix, Table 6) The presence of any extraintestinal manifestation at baseline was associated with a lower probability of remission at week 52 (OR = 0.14; 95%CI = 0.03–0.79) but not at week 104 (OR = 0.12; 95%CI = 0.01–1.34), although the point estimates were similar. In a multivariable analysis, including HBI score at baseline and extraintestinal manifestations, only HBI score remained significantly associated with remission status at weeks 52 (OR = 0.64; 95%CI = 0.49–0.84) and 104 (OR = 0.72; 95%CI = 0.54–0.96).
Ustekinumab Safety Profile
In total, 13 patients reported one or more adverse events during follow-up, accounting for a total of 23 adverse events. (Table 4) The most common adverse events were pain and skin rashes. Four patients experienced infections requiring antibiotic treatment. Serious adverse events (hospitalization or death) were seen in five patients. Of these, one patient had a ruptured appendix, one gastroenteritis confirmed by fecal tests, one acute cholecystitis, and two died during follow-up. Of the two latter patients, a middle-aged male, died at week 71 of treatment due to an opportunistic infection (pneumocystis jirovecii). He was on combination therapy with ustekinumab and methotrexate. The other deceased, also a middle-aged male, died from a cardiac arrest 8 months after discontinuing (due to lack of response) treatment with ustekinumab. No incident malignancies were recorded.
Table 4.
Reported adverse events during follow-up in Crohn's disease patients treated with ustekinumab
| Adverse events by severity* | |
|---|---|
| n = (n per 100 patient-years) | |
| Mild | 16 (9.5) |
| Axillary abscess# | 1 |
| Cough | 1 |
| Diarrhea | 1 |
| Difficulty breathing | 1 |
| Fatigue | 2 |
| Gastroenteritis (SAE) | 1 |
| Iritis | 1 |
| Lipoma | 1 |
| Nausea | 1 |
| Pain in limb | 1 |
| Skin rash | 2 |
| Skin rash scaly | 1 |
| Tonsillitis | 1 |
| Watering eyes | 1 |
| Moderate | 3 (1.8) |
| Arthralgia | 1 |
| Headache | 1 |
| Spondylitis (flare) | 1 |
| Severe | 4 (2.4) |
| Acute cholecystitis # (SAE) | 1 |
| Death # (SAE) | 2¶ |
| Ruptured appendix # (SAE) | 1 |
SAE = serious adverse event, i.e., death (n = 2) or adverse event leading to hospitalization (n = 3)
*As assessed by the treating physician; # requiring treatment with antibiotics (n = 4); ¶ of these, one patient was still on ustekinumab treatment, and one died 8 months after discontinuing treatment (due to lack of response)
Discussion
In this nationwide prospective observational multicenter study, we report patient characteristics, clinical, and inflammatory biomarker response, and remission rates in a cohort of patients with active Crohn’s disease initiated on ustekinumab according to real-world clinical practice. We included a total of 114 patients. More than 50% of the patients had previously been exposed to ≥ 2 biologics, and 37% had a history of bowel resection. At weeks 52 and 104, 70% and 61% were still on treatment with ustekinumab, respectively.
We observed clinical remission in 32% and 29% of included patients at weeks 52 and 104, respectively. The corresponding clinical response rates were 36% and 29%. Week 52 and 104 response or remission was observed in 44% and 32%, respectively. We saw significant improvement in HRQoL measures during follow-up compared to baseline.
Our findings can be compared with those of the maintenance-extended registration trials IM- UNITI LTE where remission and response were achieved in 68% and 78% at week 92, respectively [18, 19]. The differences to our week 104 remission (29%) and response rates (29%) can be explained by different patient populations. We used wide inclusion criteria reflecting clinical practice, while the IM-UNITI LTE [18] study was restricted to patients with clinical response at week 8 in the UNITI-1 and 2 studies, possibly leading to higher response rates in the UNITI studies. Furthermore, inherent limitations of randomized controlled trials such as the inclusion of selected and homogenous groups of patients make comparisons difficult. In a real-world clinical setting, the patient population is more heterogeneous and treatment patterns vary depending on individual patient characteristics and decisions by treating physicians. In the IM-UNITI LTE, only 44% of patients had a previous aTNF treatment [18].
In our study, 94% (n = 107/114) had failed at least one biological drug and as many as 35% (n = 40) of the patients had previous exposure to treatment with anti-integrin antibody agent.
To our knowledge, three retrospective [14–16] and one prospective [13] observational studies have described the effectiveness and safety of ustekinumab beyond 52-week follow-up. A German retrospective study followed ten of 93 (11%) included patients up until 88 weeks, reporting response in 8.6% (n = 8) of these and remission in 5.4% (n = 5) [14]. A French retrospective study (n = 88) reported failure-free treatment persistence based on absence of surgical procedures and withdrawal due to loss of response in 66% (n = 58) [16]. Comparisons with these retrospective studies must be done with caution due to differences in characteristics, study design, and varying outcome measures.
A Dutch prospective multicenter study by Straatmijer et al.[13] reported response rates of 35% and 27%, and remission rates of 44% and 37% at weeks 52 and 104, respectively. Our rates are lower which could indicate differences in overall disease severity in our patient population. However, similar number of patients had received treatment with at least one prior biologics (94% in our study vs. 99%) as well as anti-integrin antibody agent exposure (35 vs. 43%). The positioning of ustekinumab in Sweden at the time when this study was conducted may have contributed to a clinical practice where ustekinumab was often used as last line biological treatment at time of inclusion in this study, indicating refractory and severe disease in our patient population.
Compared to the study by Straatmijer et al., we report a higher drug survival (70% and 61% compared to 64% and 55% of patients still on ustekinumab at weeks 52 and 104, respectively). These differences may be partly explained by differences in the positioning of ustekinumab in Swedish and Dutch clinical practice, where patients in Sweden tend to stay on ustekinumab treatment longer due to lack of remaining alternative treatment options.
Based on two well acknowledged indices for capturing subjective HRQoL in IBD patients, we found significantly increased HRQoL throughout the study period. Our results confirm the findings of Sands et al.[28] based on HRQoL data from the IM-UNITI where ustekinumab induced both short- and long-term improvement in HRQoL compared to placebo. Further studies on the long-term HRQoL effectiveness of ustekinumab are warranted.
We reported comparably few adverse events (n = 23) in 13 patients during follow-up. However, we recorded five serious adverse events (3.0 per hundred patient-years), including two cases of death. Of these, one patient died while still on ustekinumab, and another died 8 months after discontinuing treatment (due to lack of response). No incident malignancies were reported. As a comparison, 18.8 serious adverse events per hundred patient-years and three deaths (n = 3/567) were reported in the IM-UNITI LTE study through 96-week follow-up [18].
In a multivariable analysis of predictors of remission, only HBI score at baseline was statistically significant, with higher score at baseline associated with a lower probability of weeks 52 and 104 remission. Due to limited follow-up data, we were likely to lack necessary statistical powered to fully explore possible predictors of remission.
Our study has several strengths, including the nationwide inclusion of patients from 20 Swedish regional and university hospitals based on the physician’s independent decision to initiate treatment with ustekinumab according to clinical practice enabling us to capture the real-world clinical effectiveness of ustekinumab. To our knowledge, only one long-term prospective study of ustekinumab has included patients with CD representing different hospital types in different clinical settings [13]. The quality of included data was enhanced by a highly standardized data collection through a study specific eCRF. We applied strict criteria for all clinical (HBI, SHS and EQ-5D-5L) and biochemical outcome measures and only included data reported within tight time-windows (± 2 weeks) of scheduled physician's follow-up visits in the analyses. This approach strengthens the link between reported clinical and biochemical data, hence increasing the accuracy of our analyses of these outcome measures. Such strict approach is lacking in some of the previous observational studies on ustekinumab. Furthermore, induction and maintenance dosing adhered to the label and national treatment guidelines. In the current study, all patients therefore received one intravenous induction dose followed by subcutaneous doses. However, individual physicians diverged from recommended 8- or 12-week dosing intervals in some patients. Finally, as one of few observational studies[29] we also reported HRQoL measures using indices previously validated for assessment of HRQoL in patients with CD.
This study has some limitations related to the observational study design, such as a lack of compulsory assessment of HBI, inflammatory markers and endoscopic activity, and also reporting bias by the individual physician. These limitations affected the number of patients with reported clinical and inflammatory biomarker data within the time windows for the data to be included in the analyses, rendering us lower statistical power. However, we found statistically significant clinical response and remission rates at all follow-up timepoints, also supported by significant reductions in CRP concentrations. We treated missing data as treatment failures in the analyses of response and remission, which may lead to an underestimation of the true remission and response rates since those with missing values did not differ in basic patient characteristics from those with reported data. Furthermore, we lacked enough reported endoscopy data to evaluate objective measures of response and remission. Only 32 patients had endoscopy data reported within the predefined time window (± 4 weeks of physician's visit) at baseline and even less during follow-up. The pandemic may possibly have affected the endoscopy rates negatively and may also have contributed to the low number of patients with data on f-calprotectin available at follow-up. Endoscopic remission is an important endpoint in clinical studies of the effectiveness of biologics. An Italian real-world study of ustekinumab in patients with CD reported clinical response and remission rates after 52 weeks of 51% and 35%, respectively [30]. However, the endoscopic response (45%) and mucosal healing (35%) rates were lower. These findings indicate a discrepancy between response and remission rates based on disease activity indices and endoscopic outcomes not captured by our data.
When comparing studies, it is important to acknowledge existing differences in presentation and location of disease of included patients. CD is a heterogeneous disease entity, consisting of distinctly different phenotypes [31, 32]. Therefore, categorization of IBD into CD and ulcerative colitis (UC) may not be appropriate for providing guidance on biological treatment alternatives. Previous treatment episodes may also influence the response to a specific biologic. An expansion of apoptosis-resistant intestinal TNFR2 + IL23R + T cells has previously been linked to resistance to anti-TNF therapy in Crohn’s disease [33]. Whether these molecular changes influence the response to anti-IL23 treatment in clinical practice is largely unknown. In our study, we found no statistically significant association between disease location and remission rates. Results of previous observational studies are inconsistent and no clear conclusions on the relation between disease location or previous treatments and treatment outcomes can be made. Hence, there is a need for further insights in the mechanisms of aTNF failure and knowledge about the effectiveness of ustekinumab and other biologics in different subgroups and phenotypes of CD.
In conclusion, our results give further support for ustekinumab as a treatment of moderate to severe CD associated with long-term clinical effectiveness and improvement in HRQoL.
In a real-world setting.
Acknowledgments
Ida Gustavsson, Kristin Klarström-Engström, Mariam Lashkariani
Abbreviations
- aTNF
Anti-tumor necrosis factor
- CD
Crohn’s disease
- CRP
C-Reactive protein
- EQ-5D-5L
EuroQual 5-dimensions 5-Levels
- f-calprotectin
Fecal-calprotectin
- Hb
Hemoglobin
- HBI
Harvey–Bradshaw Index
- HRQoL
Health-related quality of life
- IBD
Inflammatory bowel disease
- ICD
International classification of disease
- SHS
Short health scale
- SWIBREG
Swedish inflammatory bowel disease registry
Appendix
List of Swedish Hospitals Including Patients in the PROSE Study
Blekinge Hospital, Karlskrona.
Danderyd Hospital, Stockholm.
Kalmar County Hospital, Kalmar.
Karolinska University Hospital, Stockholm.
Linköping University Hospital, Linköping.
NÄL Hospital Trollhättan, Trollhättan.
Ryhov County Hospital, Jönköping.
Sahlgrenska University Hospital, Göteborg.
Skaraborgs Hospital Lidköping, Lidköping.
Skåne University Hospital, Lund.
Skåne University Hospital, Malmö.
Stockholm Gastro Center, Stockholm.
Capio St Göran Hospital, Stockholm.
Sunderby Hospital, Luleå.
Sundsvall Regional Hospital, Sundsvall.
Södra Älvsborg Hospital, Borås.
University Hospital of Umeå, Umeå.
Uppsala University Hospital, Uppsala.
Västerås Central Hospital, Västerås.
Örebro University Hospital, Örebro.
Table 5.
Health-related quality of life in Crohn’s disease patients treated with ustekinumab
| Baseline Median (IQR) |
Week 16 Median (IQR); p-value |
Week 52 Median (IQR); p-value |
Week 104 Median (IQR); p-value |
|
|---|---|---|---|---|
| SHS | n = 105/114 | n = 93/105 | n = 69/80 | n = 52/69 |
| Bowel symptoms | 2 (2–3) | 2 (1–2); < .001 | 1 (1–2); < .001 | 1 (0–1); < .001 |
| Activities of daily living | 2 (2–3) | 1 (1–2); < .001 | 1 (0–2); < .001 | 1 (0–1); < .001 |
| Worry | 2 (2–3) | 2 (1–2); < .001 | 1 (1–2); < .001 | 1 (1–1); < .001 |
| General wellbeing | 2 (2–3) | 2 (1–2); < .001 | 2 (1–2); < .001 | 1 (1–2); < .001 |
| Sum SHS | 9 (7–12) | 6 (4–9); < .001 | 5 (3–8); < .001 | 4 (2–5); < .001 |
| EQ-5D-5L | n = 103/114 | n = 89/105 | n = 69/80 | n = 54/69 |
| Mobility | 1 (1–1) | 1 (1–1); .88 | 1 (1–1); .69 | 1 (1–1); .31 |
| Self-care | 1 (1–1) | 1 (1–1); .14 | 1 (1–1); .41 | 1 (1–1); .08 |
| Usual activities | 1 (1–3) | 1 (1–2); .03 | 1 (1–2); .01 | 1 (1–1) < 0.001 |
| Pain/discomfort | 3 (2–3) | 2 (1–3); < .001 | 2 (1–3); < .001 | 1 (1–2) < 0.001 |
| Anxiety/depression | 2 (1–3) | 2 (1–2); .21 | 2 (1–2); .04 | 2 (1–2); .23 |
| Visual analogue scale | 70 (45–80) | 75 (65–80); < .001 | 80 (70–90); < .001 | 85 (80–90); < .001 |
| EQ-5D-5L index value | 0.86 (0.77–0.92) | 0.91 (0.83–0.94); < .001 | 0.91 (0.86–0.97); < .001 | 0.97 (0.91–1); < .001 |
EQ-5D-5L = EuroQual 5-Dimensions, 5-Levels; IQR = interquartile range; SHS = Short Health Scale
Median and p-values at weeks 16, 52 and 104 compared to baseline
Table 6.
Univariable and multivariable analyses of predictors of clinical remission.
| Week 52 OR (95%CI); p-value* |
Week 104 OR (95%CI); p-value* |
Week 52 OR (95%CI); p-value* |
Week 104 OR (95%CI); p-value* |
|
|---|---|---|---|---|
| Sex Female (ref. male) | 0.58 (0.20–1.69); .32 | 0.71 (0.19–2.61); .61 | ||
| HBI score at baseline | 0.74 (0.62–0.89); < .001 | 0.80 (0.66–0.97); .02 | 0.64 (0.49–0.84); .002 | 0.72 (0.54–0.96); .02 |
| Age at diagnosis** | ||||
| A1 | Ref | Ref | ||
| A2 | 7.00 (0.72–68.15); .09 | 0.67 (0.06–7.18); .74 | ||
| A3 | 1.60 (0.10–24.70); .74 | 0.33 (0.02–5.33); .44 | ||
| At inclusion | 1.01 (0.98–1.05); .72 | 0.98 (0.94–1.03); .46 | ||
| Location** | ||||
| Ileal, L1 | Ref | Ref | ||
| Colonic. L2 | 1.60 (0.30–8.49); .58 | 1.00 (0.16–6.25) 1.00 | ||
| Ileocolonic, L3 | 0.49 (0.14–1.70); .26 | 0.88 (0.20–3.85); .86 | ||
| Behavior** | ||||
| Inflammatory, B1 | Ref | Ref | ||
| Stricturing, B2 | 0.60 (0.16–2.31); .46 | 0.37 (0.06–2.19); .27 | ||
| Penetrating, B3 | 0.56 (0.10–3.25); .51 | 0.20 (0.02–2.39); .20 | ||
| Perianal, p | 0.69 (0.13–3.84); .68 | 0.15 (0.02–1.26); .08 | ||
| EIM at baseline | 0.14 (0.03–0.79); .03 | 0.12 (0.01–1.34); .09 | 0.22 (0.02–2.12); .19 | 0.08 (0.004–1.56); .10 |
| Previous surgery | 0.50 (0.17–1.45); .20 | 0.27 (0.07–1.07); .06 | ||
| Medication at baseline | ||||
| Corticosteroids | 0.47 (0.12–1.89); .29 | 0.67 (0.13–3.47); .63 | ||
| Immunomodulators | 0.51 (0.15–1.74); .28 | 0.43 (0.10–1.84); .26 | ||
| Termination of last biological drug | ||||
| aTNF or anti-integrin naïve | Ref | Ref | ||
| Lack of response | 0.65 (0.05–7.69); .73 | 2.36 (0.14–41.27); .56 | ||
| Adverse drug event or other reason | 0.38 (0.02–6.35); .50 | 0.33 (0.01–11.94); .55 | ||
aTNF = anti-tumor necrosis factor; EIM = extra-intestinal manifestation; HBI = Harvey-Bradshaw Index
* p-values of < 0.05 were considered statistically significant and are given in italics
**According to the Montreal Classification of Disease
Author’s contribution
JFL, JH, and FH took part in study concept and design. AF, JFL, JH, HS, CS, AW, DA involved in acquisition of data. AF and MC involved in statistical analyses. AF, JFL, and JH took part in first draft of the manuscript. JH, JFL, AF, OO, PM, HS, CS, AW, DA, FH involved in critical revision of the manuscript for important intellectual content. FH took part in funding. JFL involved in study supervision.
Funding
Open access funding provided by Karolinska Institute. This work was supported by Janssen Cilag AB, Sweden.
Availability of data and materials
The data underlying this article were collected through an electronic case report form linked to the Swedish Inflammatory Bowel Disease Registry (SWIBREG). Data will only be shared on request to the corresponding author and if permission is granted by SWIBREG.
Declarations
Conflict of interest
PM served as speaker and/or advisory board member for AbbVie, Ferring, Janssen, Pfizer, Takeda, Baxter, and Tillotts Pharma. HS served as speaker and/or advisory board member for AbbVie, Ferring, Janssen, Pfizer, Takeda, Gilead, and Tillotts Pharma. DA served as speaker and/or advisory board member for AbbVie, Janssen, and Takeda. FH is an employee of Janssen Cilag AB, Sweden. OO has been PI on projects at Karolinska Institutet, partly financed by investigator-initiated grants from Janssen and Ferring, and Karolinska Institutet has received fees for lectures and participation on advisory boards from Janssen, Ferring, Takeda, and Pfizer. OO also reports a grant from Pfizer in the context of a national safety monitoring program. JH served as speaker and/or advisory board member for AbbVie, Celgene, Celltrion, Dr. Falk Pharma and the Falk Foundation, Ferring, Hospira, Janssen, MEDA, Medivir, MSD, Olink Proteomics, Pfizer, Prometheus Laboratories, Sandoz/Novartis, Shire, Takeda, Thermo Fisher Scientific, Tillotts Pharma, Vifor Pharma, UCB and received grant support from Janssen, MSD, and Takeda.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. All study participants signed an informed consent authorizing the use of data collected in this study. This project was approved by the Regional Ethical Review Board in Linköping, Sweden (2017–290-31).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jonas Halfvarson and Jonas F. Ludvigsson have contributed equally to this work.
Contributor Information
Anders Forss, Email: anders.forss@ki.se.
Mark Clements, Email: mark.clements@ki.se.
Pär Myrelid, Email: par.myrelid@liu.se.
Hans Strid, Email: hans.strid@vgregion.se.
Charlotte Söderman, Email: charlotte.soderman@capiostgoran.se.
Agnieszka Wagner, Email: agnieszka.wagner@regionblekinge.se.
David Andersson, Email: david.c.andersson@regionstockholm.se.
Fredrik Hjelm, Email: fhjelm@its.jnj.com.
Ola Olén, Email: ola.olen@ki.se.
Jonas Halfvarson, Email: jonas.halfvarson@regionorebrolan.se.
Jonas F. Ludvigsson, Email: jonasludvigsson@yahoo.com
References
- 1.Baumgart DC, Sandborn WJ. Crohn's disease. Lancet. 2012;380:1590–1605. doi: 10.1016/S0140-6736(12)60026-9. [DOI] [PubMed] [Google Scholar]
- 2.Olen O, Erichsen R, Sachs MC, et al. Colorectal cancer in Crohn's disease: a Scandinavian population-based cohort study. Lancet Gastroenterol. Hepatol. 2020;5:475–484. doi: 10.1016/S2468-1253(20)30005-4. [DOI] [PubMed] [Google Scholar]
- 3.Olen O, Askling J, Sachs MC, et al. Mortality in adult-onset and elderly-onset IBD: a nationwide register-based cohort study 1964–2014. Gut. 2020;69:453–461. doi: 10.1136/gutjnl-2018-317572. [DOI] [PubMed] [Google Scholar]
- 4.Khalili H, Everhov AH, Halfvarson J, et al. Healthcare use, work loss and total costs in incident and prevalent Crohn's disease and ulcerative colitis: results from a nationwide study in Sweden. Aliment Pharmacol. Ther. 2020;52:655–668. doi: 10.1111/apt.15889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Everhov AH, Halfvarson J, Myrelid P, et al. Incidence and treatment of patients diagnosed with inflammatory bowel diseases at 60 years or older in Sweden. Gastroenterology. 2018;154:518–528. doi: 10.1053/j.gastro.2017.10.034. [DOI] [PubMed] [Google Scholar]
- 6.Zhulina Y, Udumyan R, Henriksson I, Tysk C, Montgomery S, Halfvarson J. Temporal trends in non- stricturing and non-penetrating behaviour at diagnosis of Crohn's disease in Örebro, Sweden: a population-based retrospective study. J. Crohns Colitis. 2014;8:1653. doi: 10.1016/j.crohns.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 7.Ben-Horin S, Kopylov U, Chowers Y. Optimizing anti-TNF treatments in inflammatory bowel disease. Autoimmun. Rev. 2014;13:24–30. doi: 10.1016/j.autrev.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 8.Allez M, Karmiris K, Louis E, et al. Report of the ECCO pathogenesis workshop on anti-TNF therapy failures in inflammatory bowel diseases: definitions, frequency and pharmacological aspects. J. Crohns Colitis. 2010;4:355–366. doi: 10.1016/j.crohns.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 9.Papamichael K, Gils A, Rutgeerts P, et al. Role for therapeutic drug monitoring during induction therapy with TNF antagonists in IBD: evolution in the definition and management of primary nonresponse. Inflamm. Bowel Dis. 2015;21:182–197. doi: 10.1097/MIB.0000000000000202. [DOI] [PubMed] [Google Scholar]
- 10.Ding NS, Hart A, De Cruz P. Systematic review: predicting and optimising response to anti-TNF therapy in Crohn's disease - algorithm for practical management. Aliment Pharmacol. Ther. 2016;43:30–51. doi: 10.1111/apt.13445. [DOI] [PubMed] [Google Scholar]
- 11.Gisbert JP, Panes J. Loss of response and requirement of infliximab dose intensification in Crohn's disease: a review. Am. J. Gastroenterol. 2009;104:760–767. doi: 10.1038/ajg.2008.88. [DOI] [PubMed] [Google Scholar]
- 12.Liefferinckx C, Verstockt B, Gils A, et al. Long-term clinical effectiveness of ustekinumab in patients with Crohn's disease who failed biological therapies: a national cohort study. J. Crohns Colitis. 2019;13:1401–1409. doi: 10.1093/ecco-jcc/jjz080. [DOI] [PubMed] [Google Scholar]
- 13.Straatmijer T, Biemans VBC, Hoentjen F, et al. Ustekinumab for crohn's disease: two-year results of the initiative on crohn and colitis (ICC) registry, a nationwide prospective observational cohort study. J. Crohns Colitis. 2021;15:1920–1930. doi: 10.1093/ecco-jcc/jjab081. [DOI] [PubMed] [Google Scholar]
- 14.Kubesch A, Rueter L, Farrag K, et al. Short and long-term effectiveness of ustekinumab in patients with crohn's disease: real-world data from a German IBD cohort. J. Clin. Med. 2019;8:2140. doi: 10.3390/jcm8122140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greenup AJ, Rosenfeld G, Bressler B. Ustekinumab use in Crohn's disease: a Canadian tertiary care centre experience. Scand J. Gastroenterol. 2017;52:1354–1359. doi: 10.1080/00365521.2017.1373847. [DOI] [PubMed] [Google Scholar]
- 16.Wils P, Bouhnik Y, Michetti P, et al. Long-term efficacy and safety of ustekinumab in 122 refractory Crohn's disease patients: a multicentre experience. Aliment Pharmacol. Ther. 2018;47:588–595. doi: 10.1111/apt.14487. [DOI] [PubMed] [Google Scholar]
- 17.Iborra M, Beltran B, Fernandez-Clotet A, et al. Real-world long-term effectiveness of ustekinumab in Crohn's disease: results from the ENEIDA registry. Aliment Pharmacol. Ther. 2020;52:1017–1030. doi: 10.1111/apt.15958. [DOI] [PubMed] [Google Scholar]
- 18.Sandborn WJ, Rutgeerts P, Gasink C, et al. Long-term efficacy and safety of ustekinumab for Crohn's disease through the second year of therapy. Aliment Pharmacol. Ther. 2018;48:65–77. doi: 10.1111/apt.14794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanauer SB, Sandborn WJ, Feagan BG, et al. IM-UNITI: 3 Year Efficacy, Safety, and Immunogenicity of Ustekinumab Treatment of Crohn's Disease. J. Crohns Colitis. 2019;14:23–32. doi: 10.1093/ecco-jcc/jjz110. [DOI] [PubMed] [Google Scholar]
- 20.Ma C, Fedorak RN, Kaplan GG, et al. Long-term maintenance of clinical, endoscopic, and radiographic response to Ustekinumab in moderate-to-severe crohn's disease: real-world experience from a multicenter cohort study. Inflamm. Bowel Dis. 2017;23:833–839. doi: 10.1097/MIB.0000000000001074. [DOI] [PubMed] [Google Scholar]
- 21.Forss A, Clements M, Myrelid P, et al. Prospective observational study on Stelara (ustekinumab) assessing effectiveness in Crohn's disease (PROSE): a 16-week follow-up. Scand. J. Gastroenterol. 2021;56:680–686. doi: 10.1080/00365521.2021.1906946. [DOI] [PubMed] [Google Scholar]
- 22.Ludvigsson JF, Andersson M, Bengtsson J, et al. Swedish inflammatory bowel disease register (SWIBREG) - a nationwide quality register. Scand. J. Gastro. 2019;54:1089–1101. doi: 10.1080/00365521.2019.1660799. [DOI] [PubMed] [Google Scholar]
- 23.Harvey RF, Bradshaw JM. A simple index of Crohn's-disease activity. Lancet. 1980;1:514. doi: 10.1016/S0140-6736(80)92767-1. [DOI] [PubMed] [Google Scholar]
- 24.Stjernman H, Granno C, Jarnerot G, et al. Short health scale: a valid, reliable, and responsive instrument for subjective health assessment in Crohn's disease. Inflamm. Bowel Dis. 2008;14:47–52. doi: 10.1002/ibd.20255. [DOI] [PubMed] [Google Scholar]
- 25.EuroQol Group EuroQol—a new facility for the management of health-related quality of life. Health Policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 26.Satsangi J, Silverberg MS, Vermeire S, et al. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006;55:749–753. doi: 10.1136/gut.2005.082909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ludwig K, Graf von der Schulenburg JM, Greiner W. German value set for the EQ-5D-5L. PharmacoEconomics. 2018;36:663–674. doi: 10.1007/s40273-018-0615-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sands BE, Han C, Gasink C, et al. The effects of Ustekinumab on health-related quality of life in patients with moderate to severe Crohn's disease. J. Crohns Colitis. 2018;12:883–895. doi: 10.1093/ecco-jcc/jjy055. [DOI] [PubMed] [Google Scholar]
- 29.Marques-Cami M, Robles Alonso V, Borruel N, Herrera de Guise C, Mayorga L, Casellas F. Normalization of long-term quality of life in Crohn's disease patients receiving ustekinumab. Rev Esp Enferm Dig. 2021;113:313–317. doi: 10.17235/reed.2020.6941/2020. [DOI] [PubMed] [Google Scholar]
- 30.Miranda A, Gravina AG, Cuomo A, et al. Efficacy of ustekinumab in the treatment of patients with Crohn's disease with failure to previous conventional or biologic therapy: a prospective observational real-life study. J Physiol Pharmacol. 2021;72:537–543. doi: 10.26402/jpp.2021.4.05. [DOI] [PubMed] [Google Scholar]
- 31.Cleynen I, Boucher G, Jostins L, et al. Inherited determinants of Crohn's disease and ulcerative colitis phenotypes: a genetic association study. Lancet. 2016;387:156–167. doi: 10.1016/S0140-6736(15)00465-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andersson E, Bergemalm D, Kruse R, et al. Subphenotypes of inflammatory bowel disease are characterized by specific serum protein profiles. PLoS One. 2017;12:e0186142. doi: 10.1371/journal.pone.0186142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmitt H, Billmeier U, Dieterich W, et al. Expansion of IL-23 receptor bearing TNFR2+ T cells is associated with molecular resistance to anti-TNF therapy in Crohn's disease. Gut. 2019;68(5):814–828. doi: 10.1136/gutjnl-2017-315671. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article were collected through an electronic case report form linked to the Swedish Inflammatory Bowel Disease Registry (SWIBREG). Data will only be shared on request to the corresponding author and if permission is granted by SWIBREG.


