Abstract
Purpose
Breast cancer-related lymphedema (BCRL) represents a significant concern for patients following breast cancer treatment, and assessment for BCRL represents a key component of survivorship efforts. Growing data has demonstrated the benefits of early detection and treatment of BCRL. Traditional diagnostic modalities are less able to detect reversible subclinical BCRL while newer techniques such as bioimpedance spectroscopy (BIS) have shown the ability to detect subclinical BCRL, allowing for early intervention and low rates of chronic BCRL with level I evidence. We present updated clinical practice guidelines for BIS utilization to assess for BCRL.
Methods and Results
Review of the literature identified a randomized controlled trial and other published data which form the basis for the recommendations made. The final results of the PREVENT trial, with 3-year follow-up, demonstrated an absolute reduction of 11.3% and relative reduction of 59% in chronic BCRL (through utilization of compression garment therapy) with BIS as compared to tape measurement. This is in keeping with real-world data demonstrating the effectiveness of BIS in a prospective surveillance model. For optimal outcomes patients should receive an initial pre-treatment measurement and subsequently be followed at a minimum quarterly for first 3 years then biannually for years 4–5, then annually as appropriate, consistent with previous guidelines; the target for intervention has been changed from a change in L-Dex of 10 to 6.5. The lack of pre-operative measure does not preclude inclusion in the prospective surveillance model of care.
Conclusion
The updated clinical practice guidelines present a standardized approach for a prospective model of care using BIS for BCRL assessment and supported by evidence from a randomized controlled trial as well as real-world data.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10549-022-06850-7.
Keywords: Breast cancer, Lymphedema, Bioimpedance spectroscopy, L-Dex, Clinical guidelines
Introduction
Breast cancer-related lymphedema (BCRL) represents a major potential sequela of breast cancer treatment and is a source of significant morbidity (limited limb mobility, infections) as well as quality of life detriment for patients, while also increasing costs for patients, payors, and the healthcare system [1, 2]. The risk of developing BCRL is based on the extent of locoregional therapy (axillary management, radiation therapy), systemic therapy (taxane chemotherapy, specifically docetaxel) as well as patient specific factors such as elevated BMI (> 30 kg/m2), with an incidence range of less than 10% for sentinel node biopsy alone to up to 50% for axillary dissection and adjuvant radiation therapy including regional nodal irradiation and systemic therapy [1, 3, 4]. Acknowledging the incidence and impact of BCRL, major international organizations including the American Cancer Society, American Society of Clinical Oncology (ASCO), the American Physical Therapy Association (APTA), the British Lymphology Society, the Australasian Lymphology Association, and the National Comprehensive Cancer Network (NCCN) have incorporated BCRL assessment and management as part of breast cancer survivorship and post-treatment surveillance guidelines (Table 1) [5–10].
Table 1.
Clinical recommendations regarding breast cancer-related lymphedema
| Organization | Recommendation |
|---|---|
|
National Comprehensive Cancer Network Breast Cancer (2022) [7] |
• “Lymphedema is a potential side effect after the treatment of axillary lymph node surgery resulting from damage to the lymphatic system. Early detection/diagnosis of lymphedema is key for optimal management. Consider pretreatment measurement of both arms as a baseline for patients with risk factors for lymphedema.” • “Educate, monitor, and refer for lymphedema management” |
|
National Comprehensive Cancer Network Survivorship (2021) [8] |
• “Lymphedema is a potential side effect after the treatment of cancer resulting from damage to the lymphatic system. Approximately three in four cases of lymphedema are diagnosed within three years of treatment; however, it can develop anytime in the life of the survivor. Depending on stage of diagnosis lymphoedema can be an acute or chronic condition.” • “Pretreatment limb measurement of both sides should be performed as a baseline for survivors with treatment-related or individual risk-factors, preferably by a trained lymphedema specialist” • “Early detection/diagnosis is key for optimal lymphedema management because stages 0 and 1 are reversible, whereas stages 2 and 3 are less responsive to treatment” |
| American Society of Clinical Oncology/American Cancer Society [5] |
• “Counsel survivors on how to prevent/reduce the risk of lymphedema…” • “refer patients with clinical symptoms or swelling suggestive of lymphedema…” |
| American Physical Therapy Association (APTA) [6] | • Bioimpedance analysis (BIA) should be used to detect subclinical/early-stage lymphedema |
| British Lymphology Society [9] | • Those ‘at risk’ should be given information about what this means by a health care professional backed up with information leaflets (provided by the Lymphoedema Support Network (LSN) www.lymphoedema.org) or local leaflets. For individuals with cancer, the information should be provided before cancer treatment begins. A contact number of a key worker should be provided so a prompt referral can be made to a lymphoedema service if required. Ideally the key worker would be able to provide initial advice about managing lymphoedema symptoms and manage anxiety and expectations |
| Australasian Lymphology Association [10] |
• Based on the currently available evidence, at this time, the ALA recommends that: • All patients be pre-operatively assessed using circumference (volume) measurements and/or bioimpedance spectroscopy • These measurements should be provided to the patient for ongoing monitoring where available/convenient • All patients should receive information about the possibility of developing lymphoedema as well as the early signs and symptoms and the known risk factors • Patients who are deemed to be at high risk for the development of breast cancer-related lymphedema should be monitored more regularly during the first year, and then at regular intervals for one more year • Patients who are deemed to be at lower risk for the development of breast cancer-related lymphoedema should be provided with information about who to contact if they have concerns about lymphoedema |
Despite the growing acknowledgment of BCRL and its impact, standardized recommendations for BCRL surveillance with respect to diagnostic techniques, surveillance schedules, and intervention criteria for patients diagnosed with BCRL are not widely available. With respect to diagnostic techniques, growing data support early detection with subclinical BCRL allowing for early intervention [11]. Traditional BCRL diagnostic techniques such as tape measure have limited ability to detect subclinical BCRL, with newer techniques such as bioimpedance spectroscopy (BIS), 3D digital volumetry, ICG lymphography, and perometry able to detect subclinical BCRL [12, 13]. This ability to detect subclinical disease allows for early intervention with non-invasive strategies such as compression garments [13, 14]. This concept was validated in a prospective study from Stout-Gergich et al., which found that patients followed with perometry and treated with early intervention (compression garments) had reduced arm volumes and the need for further BCRL treatment [13, 14]. Similar findings have been seen with respect to BIS; data from the University of Pittsburgh, which incorporated BIS surveillance, found a reduction in clinical BCRL as compared to historical controls, which has also been confirmed in other series evaluating BIS [15–22]. With regards to surveillance schedules, current guidelines do not provide consistent surveillance schedules [5–10]. With regards to intervention criteria, criteria differ by diagnostic technique and within each technique there are differences, though modern trials are increasingly standardizing intervention criteria for arm volume assessments [23].
We have previously published clinical practice guidelines for utilizing BIS to assess for BCRL, which provided guidelines for BIS technique, patient population (all breast patients with targeted high risk population including those undergoing mastectomy, axillary dissection, greater than 6 sentinel nodes, regional nodal irradiation, or taxane chemotherapy), surveillance and screening schedules (prior to locoregional/neoadjuvant therapy, quarterly for 3 years, decrease year 4 and beyond as appropriate), and intervention criteria (L-Dex change of greater than 10) based on data available at the time [24]; however, since its publication, advances in BIS technology and additional data including the recent publication of a randomized controlled trial evaluating the technique as compared to tape measure have become available. For example, the original guidelines called for a change of greater than 10 in the L-Dex score to trigger intervention [12, 15, 24–27]. However, growing data supported a lower threshold for increased sensitivity to detect subclinical BCRL when going from three standard deviations (L-Dex change of 10) to two standard deviations (L-Dex change of 6.5) [12, 27–31]. Given such emerging data, we present updated clinical practice guidelines.
Results
Rationale for early detection and intervention
As noted above, the rationale for BCRL surveillance programs is data from previous studies that demonstrated a reduction in chronic BCRL with early intervention, coupled with current data showing subclinical BCRL detection and early non-invasive intervention reduced rates of chronic BCRL [32, 33]. Recently, a randomized trial evaluating BIS and early intervention was published; the PREVENT trial compared BCRL surveillance with BIS versus tape measure, with early intervention triggered by either evaluation method. Final results demonstrated that surveillance with BIS coupled with early intervention was associated with an 11.3% absolute reduction in complex decongestive physiotherapy (CDP), which per the study was defined as a surrogate for chronic BCRL [34]. Given the randomized nature, size of the study, and long-term follow-up, this recent trial provides level I evidence to support prospective surveillance with BIS in conjunction with early intervention to reduce chronic BCRL, something that is not available with other BCRL diagnostic techniques. Table 2 presents outcomes with prospective BCRL surveillance using BIS [15, 22, 35, 36].
Table 2.
Outcomes with prospective BCRL surveillance with bioimpedance spectroscopy
| Study type | Number of patients | Screening frequency | Follow-up | Outcomes | |
|---|---|---|---|---|---|
| PREVENT [34] | Randomized | 879 | Baseline, 3, 6, 12, 15, 18, 21, 24, 30, and 36 months | 33 months |
As compared to tape measure, BIS had reduced rates of CDP (19.2% vs. 7.9%) |
| University of Kansas Cancer Center [35] | Single-Institution | 146 | Baseline, 3, 6, 9, 1-,18, 24, 36, 48 month | 21 months | Rate of persistent BCRL 6% |
| University of Pittsburgh [15] | Single-Institution | 186 | Baseline, every 3–6 months X 5 years | 20 months (average) | Rate of clinical lymphedema 4.4% vs. 36.4% with control group |
| Macquarie University [16] | Single-Institution | 753 | Baseline or within 90 days of surgery | Reduced clinical lymphedema with BIS surveillance (14% vs. 39%), reduced severe lymphedema (Stage II-III, 4% vs. 24%) | |
| Nashville Breast Center [17] | Single-Institution | 505/93 (high risk) | – | 24 months | High risk cohort (n = 93)- 11% required CDP, 3% at last follow-up had unresolved lymphedema |
| Breast Care Specialists [22] | Single-Institution | 206 | Pre-operative and post-operative measurements | 26 months | 23% elevated L-Dex score; no patients required CDP |
Clinical practice guidelines for bioimpedance spectroscopy in the management of breast cancer-related lymphedema
The updated clinical practice guidelines (Table 3) are based on the methods and data from the recent randomized trial and other real-world evidence [15–17, 23, 34, 35]. They are the consensus opinion of the authors based on clinical experience as well as a review of the literature.
Table 3.
Clinical practice guidelines summary
| Recommendation | |
|---|---|
| Who to screen? |
Patients meeting at least one of the following criteria: 1. Axillary Management: Axillary Lymph Node Dissection or Sentinel Lymph Node Biopsy with > 6 nodes removed 2. Regional Node Irradiation 3. Taxane based chemotherapy 4. BMI > 30 kg/m2 5. Mastectomy in the setting of invasive breast cancer |
| How often should patients have L-Dex measurements |
Baseline Years 1–3: Quarterly Years 4–5: Semi-annually Years 6 +: Annually as clinically indicated |
| When to initiate BCRL treatment? |
Change of L-Dex Score > 6.5 over baseline Compression garment × 4 weeks |
| Management Post Early-intervention |
Measure following completion of intervention If remains abnormal, refer for complex decongestive physiotherapy For those returning to normal following intervention, follow quarterly for at least 3 years post-treatment |
Patient selection
Patient selection for BCRL surveillance should account for treatment and patient characteristics; while all patients are eligible to undergo BCRL surveillance, targeting those patients at highest risk to develop BCRL would improve the utility of surveillance. With respect to surveillance consideration, BIS should be considered for patients undergoing any surgical lymph node evaluation (sentinel lymph node biopsy alone, targeted axillary excision or axillary lymph node dissection), receiving regional node irradiation, and/or receiving taxane based chemotherapy; of note, while mastectomy was included as a criteria for the recent randomized trial and previous guidelines, patients undergoing mastectomy for prophylaxis or DCIS may not require BCRL surveillance, while those undergoing mastectomy for invasive malignancy should be considered [1, 3, 4, 24, 25, 37]. Additionally, patient characteristics that would suggest a role for surveillance include an elevated BMI (> 30 kg/m2) as well as rurality, which has been identified as a BCRL risk factor [1, 4, 34].
BIS technique
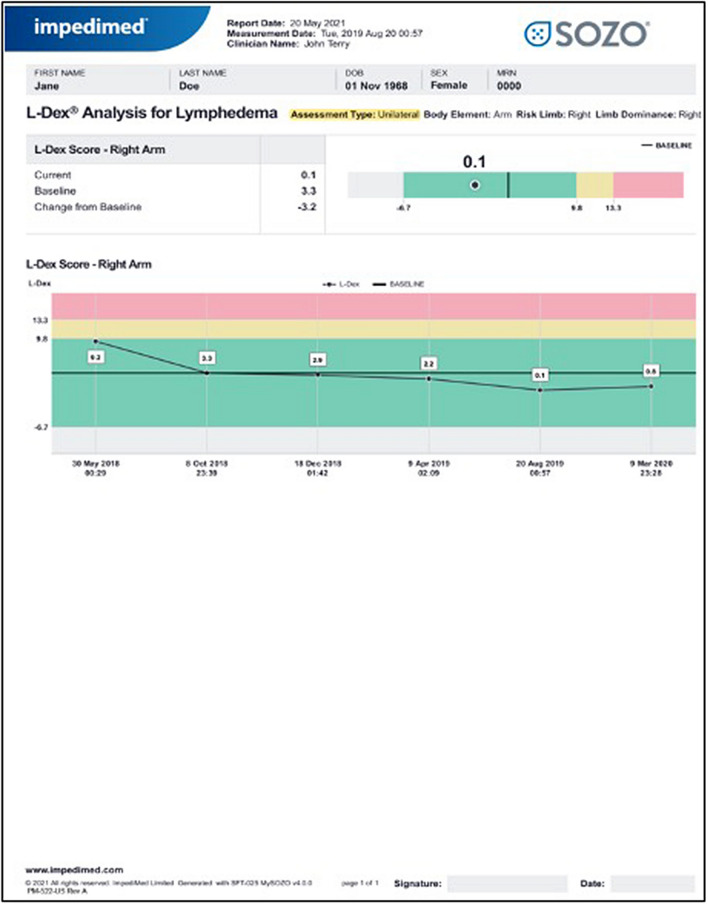
Previously, BIS guidelines based on the utilization the L-Dex U400 device were published; while BIS is the measurement, the L-Dex score has been utilized clinically and forms the basis for much of the data using BIS for BCRL assessment [24]. However, more recently, the SOZO device has been released simplifying BIS measurements, reducing test time, removing the need and cost associated with single use gel backed electrodes and eliminating the requirement for a dedicated procedure room to perform the test [38]. The SOZO device has been validated against the U400 and found to be substantially equivalent to the U400 by the FDA [38, 39]. The results are instantly displayed in numeric and graphical format enabling real-time review by the clinician (Fig. 1). Measurements should be performed by a trained health professional and may include a medical assistant or registered nurse, depending on clinic set up. Interpretation of results and initiation of treatment is commonly performed by a physician in the United States.
Fig. 1.
Reading from SOZO device
Baseline measurement
Ideally, a pre-treatment baseline should be obtained to enable the earliest opportunity to intervene, but this may not be achievable for all patients. The lack of pre-treatment baseline measure does not preclude participation in a prospective BCRL surveillance program, consistent with previous guidelines as well as studies that have shown that early detection as part of a prospective surveillance program can be achieved in patients without pre-treatment baseline measurements when using bioimpedance [16, 24]. A baseline can be set for patients who present post-treatment with no clinical signs of lymphedema and an L-Dex score in the normal range and then followed for an increase of 6.5 of more (2 standard deviations) to initiate intervention [15, 16].
Screening frequency
Determining the appropriate screening frequency with BIS is essential in implementing a BCRL surveillance program. Based on the recent randomized trial, L-Dex measurements should be taken at baseline (prior to locoregional or neoadjuvant therapy), 4–6 weeks after surgery (prior to initiation of radiation), and then subsequently at a minimum quarterly for the first 3 years post-treatment when the risk of developing lymphedema is the greatest [25, 40]. For years 4 and 5, assessments can be reduced to biannually then annually as appropriate [24]. These are consistent with previous guidelines and the recent randomized trial [24, 25]. While there has been some suggestion that shorter durations of surveillance may be appropriate, recent data has shown continued increases in L-Dex scores beyond 15 months suggesting the need for continued surveillance [1, 41]. At this time, there is no prospective data with long-term follow-up comparing alternative screening intervals.
Intervention initiation
An important question facing clinicians utilizing BIS has been when to initiate early intervention with a compression garment; importantly, the goal of prospective BCRL surveillance is to risk stratify and identify those patients who can benefit from compression garment utilization rather than prescribing to all patients. When patients demonstrate an L-Dex increase of greater than 6.5, they should be prescribed a compression sleeve/sleeve and gauntlet delivering 20–30 mm of pressure for 4 weeks, 12 h per day; such an approach has been associated with reduced rates of clinical BCRL as compared traditional surveillance [16, 25]. Importantly, the compression garment and gauntlet should be appropriately fitted to avoid ill-fitting garments. Of note, despite concerns for increased triggers and unnecessary interventions with BIS surveillance, there were fewer triggers in the BIS group than the tape measure group seen in the recent randomized trial, highlighting the sensitivity and specificity of BIS, while allaying fears of overtreatment with BIS surveillance [34]. Following treatment with compression garment, repeat assessment should be performed and for those patients with persistent elevated score, referral for CDT should be considered. For those returning to normal, follow-up measurements should be performed quarterly for up to 3 years following breast cancer treatment. Of note, the recent RCT allowed patients who triggered an intervention to keep their garment and use as needed as part of the protocol, though this was only for patients who had already triggered.
Discussion
BCRL represents one of the most feared complications for breast cancer survivors, impacting their quality of life as well as forcing patients to address the associated morbidities and costly, time-intensive interventions [42]. Given the increasing number of breast cancer survivors, assessing for BCRL as part of a prospective standardized survivorship plan is essential given that data supports the role of early detection and intervention in preventing chronic BCRL; for example, a recent meta-analysis found that prospective surveillance drastically reduced the rates of chronic BCRL [43]. To date, data support the utilization of BIS as part of a prospective model of care in which patients are followed closely at routine intervals that can result in early identification of lymphedema and improved patient outcomes [15, 35, 36]. Importantly, this approach is distinct from the treat all patients at risk of BCRL approach: a recent randomized study (which utilized BIS), found that compression for all patients undergoing ALND reduced rates of BCRL; however, this required all patients to undergo therapy rather than tailoring the need to for therapy to those demonstrating sub-clinical BCRL which may have an impact on psychosocial functioning, while also having short follow-up [44].
The present clinical guidelines present clinicians with a standardized evidence based approach to BCRL surveillance with follow-up time points as well as intervention criteria and methodology based on level I evidence. Importantly, the guidelines support that while the key to a successful BCRL prevention program is early identification and subsequent intervention, the absence of a pre-treatment baseline does not prohibit participation in prospective surveillance. The first visit can be considered baseline in patients who display no clinical signs of lymphedema, despite being post-treatment, allowing for BCRL surveillance for all at risk patients [15, 16]. Finally, it's important to recognize that these guidelines are for L-Dex utilization in prospective surveillance and do not address other measurement methods.
Implementing a prospective BCRL surveillance program will require multi-disciplinary collaboration with multiple models available. At some centers, BIS measurements are taken on all breast cancer patients in a multi-disciplinary clinic, with physicians of any discipline able to assess and initiate intervention with prescription/referral for compression garment; alternatively, some centers may use a similar model for intervention but limit assessments to patients with risk factors, which can be flagged in a chart/medical record. Alternatively, some practices may have a single discipline manage BCRL surveillance including breast surgeons, oncologists, or survivorship clinics; in such a model, the discipline can measure and prescribe/refer for intervention with measurements at each visit. Importantly, BIS is user friendly and any trained member of the health-care team can perform the actual measurement in a fraction of the time needed to perform tape measurements, with improved chronic BCRL outcomes with BIS.
A major concern with implementing BCRL surveillance using BIS are the associated costs. However, while acknowledging costs associated with prospective BCRL surveillance, it is important to recognize that the costs associated with chronic BCRL can include higher rates of hospitalizations as compared to breast cancer patients who do not develop BCRL as well as the costs of managing chronic BCRL [2, 45]. As such, studies have demonstrated the cost-effectiveness and value of prospective BCRL surveillance. Stout et al. evaluated the cost of prospective surveillance as compared to traditional care finding the cost to manage early BCRL was $636 per patient per year as compared to $3,125 with late-stage BCRL, offering the potential for a substantial cost savings [46]. For programs implementing BIS surveillance, a cost analysis based on the recent RCT demonstrated a substantial cost savings, regardless of program size, when implementing BIS prospective surveillance as compared to tape measure; use of BIS reduced costs by $356-$770 per patient and when accounting for potential hospitalizations by more than $16,000 at 1 year [47]. Together, these data support that prospective BCRL surveillance is a value-oriented approach, reducing chronic BCRL and the costs associated. Additionally, data from the recent RCT support that as compared to tape measure, BIS is cost effective, by reducing false positives, and tailoring early intervention to those patients at risk for chronic BCRL.
Conclusion
Bioimpedance spectroscopy represents a standard diagnostic approach to assess for breast cancer-related lymphedema, allowing for early detection and treatment. BIS should be used as part of routine clinical care starting with measurement prior to treatment; however, BCRL surveillance can be utilized without a pre-treatment assessment. The updated clinical practice guidelines are supported by evidence from a randomized controlled trial and other real-world data.
Supplementary Information
Below is the link to the electronic supplementary material.
Funding
Study was not supported by pharmaceutical company or other funding
Data Availability
Enquiries about data availability should be directed to the authors.
Declarations
Conflict of interest
Chirag Shah- Scientific Consultant ImpediMed, Consultant PreludeX, Consultant Evicore, Consultant Videra Surgical. Frank Vicini- CMO ImpediMed. Stephanie Valente- Oncology Advisory Board/Consultant for ImpediMed, AxoGen, Merit and Pacira. Pat Whitworth- Partner TME LLC, Consultant Impedimed. Graham Schwarz- Consultant Impedimed, Quest Medical Imaging. Megan Kruse declares that she has no conflicts of interest. Manpreet Kohli- Oncology Advisory Board, Impedimed. Kirstyn Brownson- Oncology Advisory Board, Impedimed. Laura Lawson- Oncology Advisory Board, Impedimed. Beth Dupree declares that she has no conflicts of interest.
Ethics approval
This article does not contain any studies with human participants performed by any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Shah C, Vicini FA. Breast cancer-related arm lymphedema: incidence rates, diagnostic techniques, optimal management and risk reduction strategies. Int J Radiat Oncol Biol Phys. 2011;81(4):907–914. doi: 10.1016/j.ijrobp.2011.05.043. [DOI] [PubMed] [Google Scholar]
- 2.Shih YC, Xu Y, Cormier JN, et al. Incidence, treatment costs, and complications of lymphedema after breast cancer among women of working age: a 2-year follow-up study. J Clin Oncol. 2009 doi: 10.1200/JCO.2008.18.3517. [DOI] [PubMed] [Google Scholar]
- 3.Lee MJ, Beith J, Ward L, Kilbreath S. Lymphedema following taxane-based chemotherapy in women with early breast cancer. Lymphat Res Biol. 2014;12(4):282–288. doi: 10.1089/lrb.2014.0030. [DOI] [PubMed] [Google Scholar]
- 4.Jung S-Y, Shin KH, Kim M, et al. Treatment factors affecting breast cancer-related lymphedema after systemic chemotherapy and radiotherapy in stage II/III breast cancer patients. Breast Cancer Res Treat. 2014;148(1):91–98. doi: 10.1007/s10549-014-3137-x. [DOI] [PubMed] [Google Scholar]
- 5.Runowicz CD, Leach CR, Henry NL, et al. American Cancer Society/American Society of Clinical Oncology Breast Cancer Survivorship Care Guideline. CA Cancer J Clin. 2016;66(1):43–73. doi: 10.3322/caac.21319. [DOI] [PubMed] [Google Scholar]
- 6.Levenhagen K, Davies C, Perdomo M, Ryans K, Gilchrist L. Diagnosis of upper quadrant lymphedema secondary to cancer: clinical practice guideline from the oncology section of the American Physical Therapy Association. Phys Ther. 2017;97:1–17. doi: 10.1093/ptj/pzx050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.NCCN. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Breast Cancer V.2.2022. 2022.
- 8.NCCN. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Survivorship V.3.2021. 2021.
- 9.British Lymphology Society, Lymph Facts - What information, advice and support should be provided for those at risk of lymphoedema? Available at: https://www.thebls.com/documents-library. Accessed 2 Aug 2022.
- 10.Australasian Lymphology Association, Position Paper – Early detection of breast cancer-related lymphoedema Available at: https://www.lymphoedema.org.au/about-lymphoedema/position-statements/. Accessed 2 Aug 2022.
- 11.Shah C, Badiyan S, Khwaja S, et al. Breast cancer related lymphedema: a review of recent developments. Androl Gynecol. 2013 doi: 10.4172/2327-4360.1000102. [DOI] [Google Scholar]
- 12.Cornish BH, Chapman M, Hirst C, et al. Early diagnosis of lymphedema using multiple frequency bioimpedance. Lymphology. 2001;34(1):2–11. [PubMed] [Google Scholar]
- 13.Stout Gergich NL, Pfalzer LA, McGarvey C, Springer B, Gerber LH, Soballe P. Preoperative assessment enables the early diagnosis and successful treatment of lymphedema. Cancer. 2008;112(12):2809–2819. doi: 10.1002/cncr.23494. [DOI] [PubMed] [Google Scholar]
- 14.Stout NL, Pfalzer L, Levy E, McGarvey C, Gerber L, Springer B, Soballe P. Five year preliminary outcomes of a prospective surveillance model to reduce upper extremity morbidity related to breast cancer treatment. Can Res. 2011;71(24):1s–653s. [Google Scholar]
- 15.Soran A, Ozmen T, McGuire KP, et al. The importance of detection of subclinical lymphedema for the prevention of breast cancer-related clinical lymphedema after axillary lymph node dissection; a prospective observational study. Lymphat Res Biol. 2014;12(4):289–294. doi: 10.1089/lrb.2014.0035. [DOI] [PubMed] [Google Scholar]
- 16.Koelmeyer LA, Borotkanics RJ, Alcorso J, et al. Early surveillance is associated with less incidence and severity of breast cancer-related lymphedema compared with a traditional referral model of care. Cancer. 2018;125(6):854–862. doi: 10.1002/cncr.31873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Whitworth PW, Shah C, Vicini F, Cooper A. Preventing breast cancer-related lymphedema in high-risk patients: the impact of a structured surveillance protocol using bioimpedance spectroscopy. Front Oncol. 2018;8:197. doi: 10.3389/fonc.2018.00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vicini F, Shah C, Whitworth P, Walker M, Shi J. Correlation of bioimpedance spectroscopy with risk factors for the development of breast cancer-related lymphedema. Lymphat Res Biol. 2018;16(6):533–537. doi: 10.1089/lrb.2017.0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shah C, Vicini F, Beitsch P, et al. The use of bioimpedance spectroscopy to monitor therapeutic intervention in patients treated for breast cancer related lymphedema. Lymphology. 2013;46(4):184–192. [PubMed] [Google Scholar]
- 20.Ward LC. Bioelectrical impedance analysis: proven utility in lymphedema risk assessment and therapeutic monitoring. Lymphat Res Biol. 2006;4(1):51–56. doi: 10.1089/lrb.2006.4.51. [DOI] [PubMed] [Google Scholar]
- 21.Ward LC, Bunce IH, Cornish BH, Mirolo BR, Thomas BJ, Jones LC. Multi-frequency bioelectrical impedance augments the diagnosis and management of lymphoedema in post-mastectomy patients. Eur J Clin Invest. 1992;22(11):751–754. doi: 10.1111/j.1365-2362.1992.tb01440.x. [DOI] [PubMed] [Google Scholar]
- 22.Kaufman DI, Shah C, Vicini FA, Rizzi M. Utilization of bioimpedance spectroscopy in the prevention of chronic breast cancer-related lymphedema. Breast Cancer Res Treat. 2017;166(3):809–815. doi: 10.1007/s10549-017-4451-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hypofractionated radiation therapy after mastectomy in preventing recurrence in patients with Stage IIA-IIA Breast Cancer. Available at: https://clinicaltrials.gov/ct2/show/NCT03414970. Accessed 2 Aug 2022.
- 24.Shah C, Vicini FA, Arthur D. Bioimpedance spectroscopy for breast cancer related lymphedema assessment: clinical practice guidelines. Breast journal. 2016;22(6):645–650. doi: 10.1111/tbj.12647. [DOI] [PubMed] [Google Scholar]
- 25.Ridner SH, Dietrich MS, Cowher MS, et al. A Randomized trial evaluating bioimpedance spectroscopy versus tape measurement for the prevention of lymphedema following treatment for breast cancer: interim analysis. Ann Surg Oncol. 2019;26(10):3250–3259. doi: 10.1245/s10434-019-07344-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gaw R, Box R, Cornish B. Bioimpedance in the assessment of unilateral lymphedema of a limb: the optimal frequency. Lymphat Res Biol. 2011;9(2):93–99. doi: 10.1089/lrb.2010.0020. [DOI] [PubMed] [Google Scholar]
- 27.Ward L, Dylke E, Czerniec S, Isenring E, Kilbreath S. Confirmation of the reference impedance ratios used for assessment of breast cancer-related lymphedema by bioelectrical impedance spectroscopy. Lymphat Res Biol. 2011;9(1):47–51. doi: 10.1089/lrb.2010.0014. [DOI] [PubMed] [Google Scholar]
- 28.Ridner SH, Dietrich MS, Spotanski K, et al. A prospective study of L-Dex values in breast cancer patients pretreatment and through 12 months postoperatively. Lymphat Res Biol. 2018;16(5):435–441. doi: 10.1089/lrb.2017.0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lahtinen T, Seppala J, Viren T, Johansson K. Experimental and analytical comparisons of tissue dielectric constant (TDC) and bioimpedance spectroscopy (BIS) in assessment of early arm lymphedema in breast cancer patients after axillary surgery and radiotherapy. Lymphat Res Biol. 2015;13(3):176–185. doi: 10.1089/lrb.2015.0019. [DOI] [PubMed] [Google Scholar]
- 30.Dylke ES, Schembri GP, Bailey DL, et al. Diagnosis of upper limb lymphedema: development of an evidence-based approach. Acta Oncol. 2016;55(12):1477–1483. doi: 10.1080/0284186X.2016.1191668. [DOI] [PubMed] [Google Scholar]
- 31.Fu MR, Cleland CM, Guth AA, et al. L-dex ratio in detecting breast cancer-related lymphedema: reliability, sensitivity, and specificity. Lymphology. 2013;46(2):85–96. [PMC free article] [PubMed] [Google Scholar]
- 32.Torres Lacomba M, Yuste Sanchez MJ, Zapico Goni A, et al. Effectiveness of early physiotherapy to prevent lymphoedema after surgery for breast cancer: randomised, single blinded, clinical trial. BMJ. 2010;340:b5396. doi: 10.1136/bmj.b5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Box RC, Reul-Hirche HM, Bullock-Saxton JE, Furnival CM. Physiotherapy after breast cancer surgery: results of a randomised controlled study to minimise lymphoedema. Breast Cancer Res Treat. 2002;75(1):51–64. doi: 10.1023/A:1016591121762. [DOI] [PubMed] [Google Scholar]
- 34.Ridner SH, Dietrich MS, Boyages J, et al. A comparison of bioimpedance spectroscopy or tape measure triggered compression intervention in chronic breast cancer lymphedema prevention. Lymph Res Biol. 2022 doi: 10.1089/lrb.2021.0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kilgore LJ, Korentager SS, Hangge AN, et al. Reducing breast cancer-related lymphedema (BCRL) through prospective surveillance monitoring using bioimpedance spectroscopy (BIS) and patient directed self-interventions. Ann Surg Oncol. 2018;25(10):2948–2952. doi: 10.1245/s10434-018-6601-8. [DOI] [PubMed] [Google Scholar]
- 36.Koelmeyer L, Gaitatzis K, Ridner SH, et al. Implementing a prospective surveillance and early intervention model of care for breast cancer–related lymphedema into clinical practice: application of the RE-AIM framework. Supportive Care Cancer. 2021;29(2):1081–1089. doi: 10.1007/s00520-020-05597-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lawenda BD, Mondry TE, Johnstone PA. Lymphedema: a primer on the identification and management of a chronic condition in oncologic treatment. CA Cancer J Clin. 2009;59(1):8–24. doi: 10.3322/caac.20001. [DOI] [PubMed] [Google Scholar]
- 38.Koelmeyer LA, Ward LC, Dean C, Boyages J. Body positional effects on bioimpedance spectroscopy measurements for lymphedema assessment of the arm. Lymphat Res Biol. 2020;18(5):464–473. doi: 10.1089/lrb.2019.0067. [DOI] [PubMed] [Google Scholar]
- 39.FDA. K180126 - SOZO - L-Dex Monitor, Extracellular Fluid, Lymphedema, Extremity. Silver Spring MD: US Food and Drug Administration; 2008.
- 40.McDuff SGR, Mina AI, Brunelle CL, et al. Timing of lymphedema following treatment for breast cancer: when are patients most at-risk? Int J Radiat Oncol Biol Phys. 2018 doi: 10.1016/j.ijrobp.2018.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ridner SH, Shah C, Boyages J, et al. L-Dex, arm volume, and symptom trajectories 24 months after breast cancer surgery. Cancer Med. 2020;9:5164–5173. doi: 10.1002/cam4.3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McLaughlin SA. Lymphedema: separating fact from fiction. Oncology. 2012;26(3):242–249. [PubMed] [Google Scholar]
- 43.Rafn BS, Christensen J, Larsen A, et al. Prospective surveillance for breast cancer- related arm lymphedema: a systematic review and meta-analysis. J Clin Oncol. 2002;40(9):1009–1026. doi: 10.1200/JCO.21.01681. [DOI] [PubMed] [Google Scholar]
- 44.Paramandandam VS, Dylke E, Clark GM, et al. Prophylactic use of compression sleeves reduces the incidence of arm swelling in women at high risk of breast cancer-related lymphedema: a randomized controlled trial. J Clin Oncol. 2022 doi: 10.1200/JCO.21.02567. [DOI] [PubMed] [Google Scholar]
- 45.Basta MN, Fox JP, Kanchwala SK, et al. Complicated breast cancer-related lymphedema: evaluating health care resource utilization and associated costs of management. Am J Surg. 2016;211(1):133–141. doi: 10.1016/j.amjsurg.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 46.Stout NL, Pfalzer LA, Springer B, et al. Breast cancer-related lymphedema: comparing direct costs of a prospective surveillance model and a traditional model of care. Phys Ther. 2012;92(1):152–163. doi: 10.2522/ptj.20100167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shah C. Bioimpedance spectroscopy in the detection of breast cancer-related lymphedema: an ounce of prevention. Breast J. 2019;25(6):1323–1325. doi: 10.1111/tbj.13618. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Enquiries about data availability should be directed to the authors.