Abstract
The storage of food grains against the fungal infection has been a great challenge to the farmers, but nanotechnology provides a solution to address this problem. The application of nanotechnology for the storage of food grains replaces synthetic fungicides in agriculture. Inorganic nanoparticles such as silver and zinc oxide are well-known for their antifungal activity. Green synthesized nanoparticles show enhanced antimicrobial activity than the chemically synthesized nanoparticles. Extracts and essential oils derived from plants exhibit very good antifungal properties. The synthesized nanoparticles can be impregnated in packaging materials, which are used to store food grains. Natural materials are having advantages like non-toxicity and easier to degrade and are suitable for food safety. This overview discusses the nanomaterials-mediated protection of food materials from mycotoxin and its releases into the open environment.
Keywords: Nanomaterials, Food safety, Aflatoxin, Anti-fungus, Natural adsorbents, Hermetic storage
Introduction
Fungi are able to produce a toxic secondary metabolite known as mycotoxins and cause diseases in humans and in animals. They result in chronic dietary risk fact than synthetic contaminants, food additives or pesticide residues. Hepatotoxins, nephrotoxins, vomitoxin and neuro-musculotoxin are carcinogenic and mutagenic mycotoxins. Hence, the protection of the food grains and the food storage from the fungi as well as the metabolite mycotoxins is essential. Penicillium verrucosum commonly produces some mycotoxins and is identified as a wheat contaminant (Kotzybik et al. 2016). Bennett and Klich considered the following mycotoxins, such as aflatoxins, trichothecenes, fumonisins, alternariol, tenuazonic acid, ochratoxins, patulin and citrinin (Zain 2011). Other important mycotoxins are deoxynivalenol (DON), zearalenone (ZEN) and T2 toxin produced by Fusarium; PR toxin produced by Penicillium; and botulinum toxins. Usually, any feed contaminated by mycotoxins contains a mixture of various mycotoxins. Aspergillus fumigates, A. ochraceus, Candida albicans, Clostridium perfringens, Trichophyton mentagrophytes and T. beigelii produce mycotoxins. Fusarium, Trichothecium, Myrothecium and Stachybotrys produce trichothecenes toxins. Trichothecene mycotoxins have been classified into two categories, i.e. Type A and Type B. Examples of Type A mycotoxins include T-2 and HT-2 toxins. Deoxynivalenol and nivalenol (NIV) have been classified as Type B. Vomitoxin is also known as deoxynivalenol.
Aflatoxin is a more dangerous mycotoxin from Aspergillus parasiticus. It is seen in the food and feedstuffs and pollutes the herbages and foods. This mycotoxin plays a major role to cause liver cancer. Also, it affects the digestive tract, blood vessels, and kidneys and produces neurological disorders (Pickova et al. 2021). The Agricultural and food industries are facing many problems in the removal of mycotoxins. Mycotoxins cause the discoloration of food grains and degrade the proteins in the food grains. So, prevention is the best way instead of mycotoxins removal. Peanuts, rice, corn and cottonseed have suitable conditions for the growth of fungi. So, they are not suitable for consumption because of the contamination. High humidity and high-water activity are the reason for the production of mycotoxins. Therefore, for each commodity, it is essential to control the moisture content below a certain critical level (Bullerman et al. 1984). Grain loss during storage is the main contributor to the food crisis. For the protection of stored grains, nanotechnology can emerge as an alternative to chemical pesticides and can offer some eco-friendly approaches. More than 50% of cereal grains are lost during storage. Advanced technological solutions can be provided to protect the stored grains and to reduce the losses drastically, i.e. as low as 1–2%. The mycotoxins produced by the fungi contaminate 25–40% of the stored cereal grains. They cause dry matter, quality loss, and grain germination reduction. The grains possess an unfavourable odour with high fatty acids and less starch and sugar. The mycotoxins also cause lipid peroxidation in food grains and self-heating in food grains (Kumar and Kalita 2017). Therefore, a series of measures should be taken to introduce new innovative methods to store food grains. Figure 1 shows the SEM image and culture of the mycotoxin-producing fungi Aspergillus.
Fig. 1.
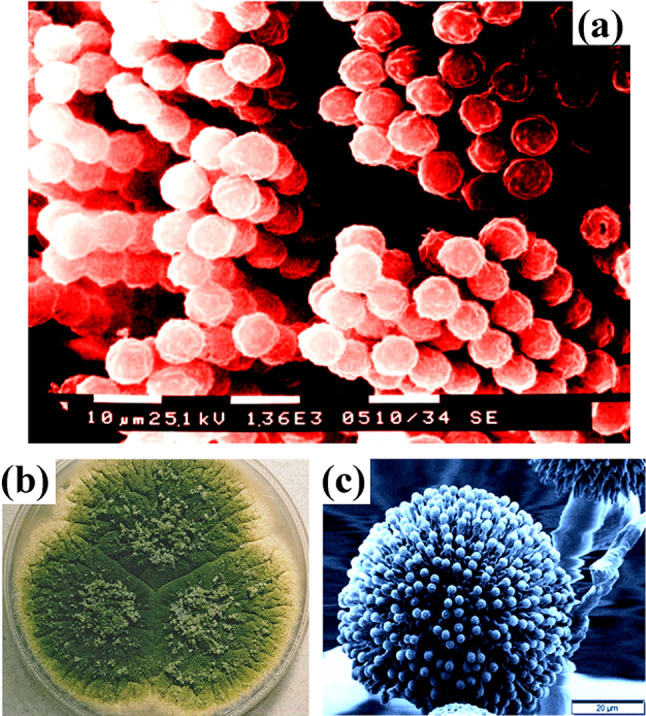
Aflatoxin (mycotoxin) producing fungi Aspergillus a SEM image depicts the conidiospores of fungi Aspergillus sp. b A. parasiticus colony grown at room temperature c SEM image of freeze-dried A. niger. a Adapted from https://phil.cdc.gov/details_linked.aspx?pid=202; https://commons.wikimedia.org/wiki/File:Aspergillus_parasiticus_UAMH3108.jpg and https://doi.org/10.5772/61720
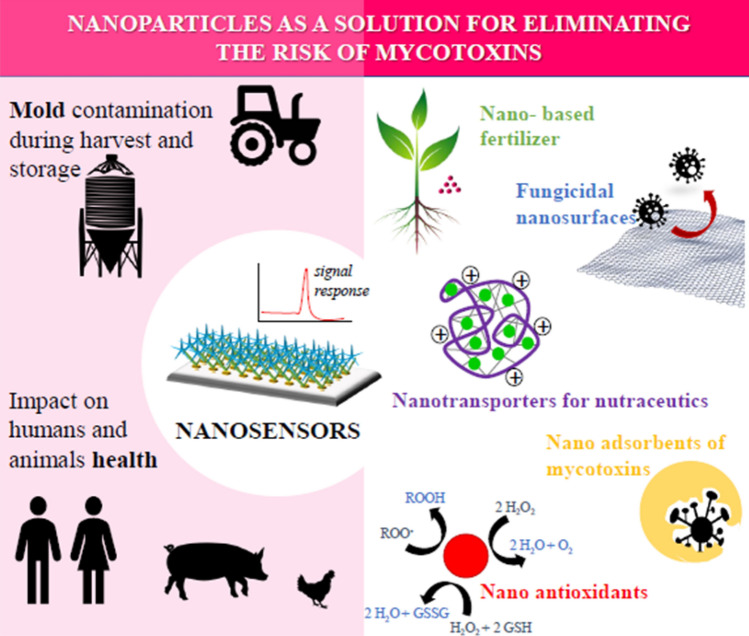
Preventive actions against mycotoxins should be performed from the agricultural field to the finished product. Mycotoxins are associated with both field moulds like Fusarium and storage spoilers like Penicillium. Continuous monitoring of the crop is also important. Wheat is very vulnerable to Fusarium infection during the flowering period (Eeckhout et al. 2013). The seeds without pests and diseases are to be used in order to grow healthy plants. This helps for the plants to fight against the mycotoxins during their growth (Jard et al. 2011). Good agricultural practices include planting crops through rotation methods, tillage, irrigation, and the less utility of chemicals in the field (Devreese et al. 2013). New nano-formulations can be prepared from plant material to produce nanofungicides. They are nontoxic to human and animal health. Nano-packaging materials are made from polymers by impregnating metallic and magnetic nanoparticles in them to improve the antifungal effect. Such packaging materials can be used for storing food grains. Packaging materials can be manufactured using nanomaterials possessing high antifungal activity. They can be impregnated in packaging materials like polypropylene bags and jute fabric materials, which are used for grain storage. Several nanoparticles have been reported as antifungal, food additive, food packaging materials and food storage materials. Plant materials are added with food grains during storage as aqueous extracts, essential oils and also in powder form to resist against mycotoxins. Natural materials like nano-curcumin and glucomannan are biocompatible and already reported as very good adsorbents of mycotoxins. They can be added to food grains during storage. Kaushik et al. reported that recently, nanotechnology increases its research focus on agriculture. Nanomaterials to remove mycotoxins were developed in the year 2009 (Kaushik et al. 2009). Already many studies have focused on the use of nanotechnology for mycotoxin elimination (Horky et al. 2018). Figure 2 shows nanotechnology for the management of mycotoxins in agriculture. This chapter deals with and reviews the involvement of the nanotechnology to protect the food storage from the toxic mycotoxins.
Fig. 2.
Schematic diagram shows the applications of the nanotechnology for detection and elimination of mycotoxins. Figure is adapted from Horky et al. (2018)
Nanotechnology in food industry to eliminate mycotoxin
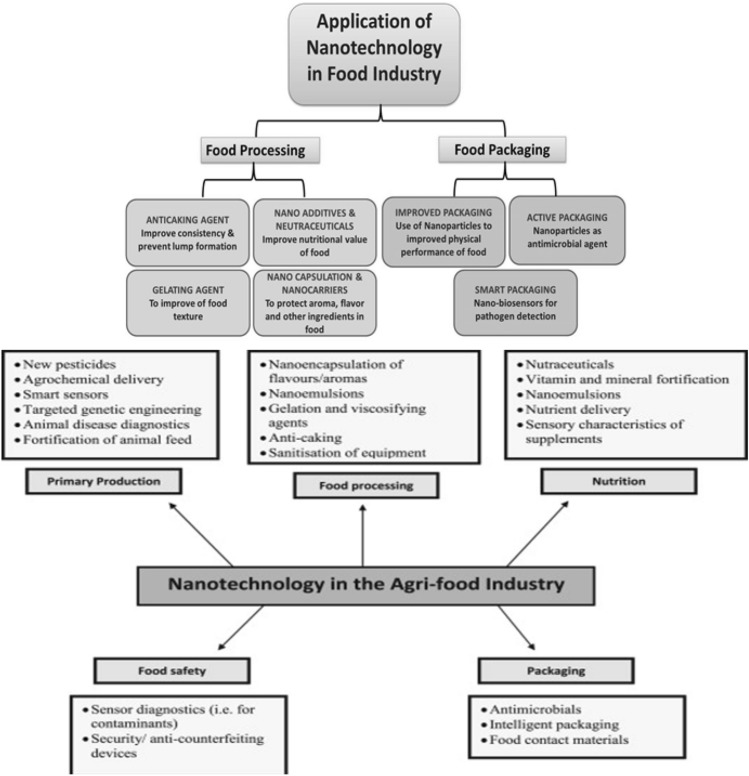
Nanotechnology improves the shelf life and safety of foods in a number of ways, and nanoparticles are added in the foods to add colours and flavours. They are also used as preservatives and to add the nutrients to the food (Fig. 3). They can also modify the rheological properties of foods. Nowadays, nanoparticles are embedded in food packaging as sensors to know about the spoilage of food. Nanotechnology can improve the shelf life of food and prevent contamination during the processing, packaging and storage of food which results in an enhanced food quality. The functional food developed using nanomaterials increases the bioavailability of food and the properties of food such as taste, and consistency can be modulated. This is achieved by modifying the particle size and surface charge of food materials. Active packaging uses nanomaterials in food protection, and the utilization of sensors in smart and intelligent helps in monitoring the quality of the stored foods (Nile et al 2020). The synthetic nanoparticles such as silver NPs, iron oxide NPs, titanium dioxide and zinc oxide NPs and natural nanoparticles such as lipid, protein and carbohydrate nanoparticles can change the behaviour of food. Nanoparticles exist in numerous natural foods. Nanoparticles will be applied as functional ingredients of food by the food industries (McClements and Xiao 2017) (Fig. 4).
Fig. 3.
Applications of nanotechnology in food industries. Upper panel; https://www.frontiersin.org/articles/10.3389/fmicb.2017.01501/full and lower panel https://www.sciencedirect.com/science/article/abs/pii/S0924224414001988, covered all information with applications
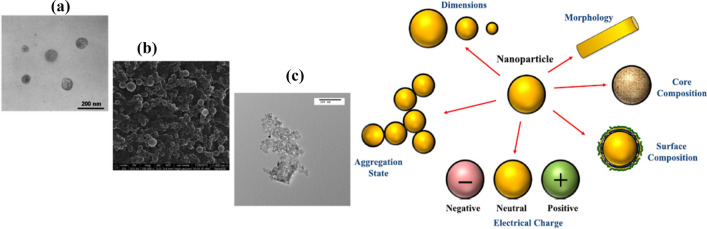
Fig. 4.
Food associated nanoparticles. Upper panel; a lipid nanoparticle; b protein nanoparticle; c Titanium dioxide nanoparticle. Lower panel—different states of food nanoparticle. It includes morphology, dimension, charge, aggregation and composition
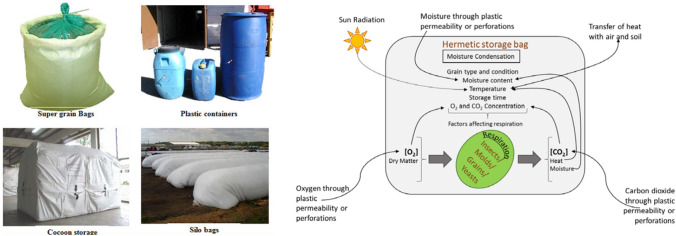
Biopolymers like chitosan were used in the packaging of food materials. In order to get high-quality films, crosslinking is done. When cross-linked with glutaraldehyde, chitosan is able to adsorb various mycotoxins such as AFB1, OTA, ZEN and FB1 to a larger extent (Zhao et al. 2015). Hermetic storage is a type of sealed storage/airtight storage (Fig. 5a). This storage method is used for storing cereals, pulses and coffee powders. In this method, the use of chemicals and pesticides are avoided to protect the food. Instead, a modified atmosphere with high carbon dioxide is incorporated in sealed waterproof bags (Bell 2002; Tefera et al. 2011). Hermetic storage seals the grain from the outside atmosphere. It prevents the uptake of moisture and oxygen from the atmosphere and protects the grain (Fig. 5b). This storage reduces the inter-granular oxygen levels from 21% to 5%. If the oxygen level is 8–9%, the insect activity will be ceased, and if it is 2%, the mortality will be achieved. Carbon nanoparticles are obtained during the preparation of food such as bread, cornflakes and biscuits. These nanoparticles are obtained during the heating of these food caramels. These particles are in the size range 4–30 nm and have an amorphous shape. The photoluminescence quantum yield (QY) value of these nanoparticles depends on the starting material. The QY value of the nanoparticles obtained from the bread, jaggery and sugar caramel is 1.2, 0.55 and 0.63, respectively. These carbon nanoparticles can be used for various biological applications (Sk et al. 2012).
Fig. 5.
Different storage by packaging methods (upper panel). Factors affect the grain and microorganism respiration in hermetic storage method (lower panel). Figures adapted from Kumar and Kalita (2017)
Antifungal activity of nanoparticles to eliminate mycotoxin
Agriculture and food industries are slowly accepting nanotechnology. One of the difficulties is the large-scale production of nanoparticles because the synthesis process requires high cost and also affects the environment. Mousavi et al. say that 90 μg/mL of Ag NPs is sufficient to inhibit aflatoxin B1. This concentration is below the cytotoxicity level of human cells. Silver nanoparticles also show an inhibitory effect against the fungi Trichophyton mentagrophytes, Trichosporon beigelii and Candida albicans (Mousavi and Pourtalebi 2015). Animal studies with swine kidney cells are also performed with silver nanoparticles. Mycotoxins, organic acids and extracellular enzymes are produced in the cells by moulds. Silver nanoparticles reduce mould cytotoxicity and the organic acid production of Aspergillus (Pietrzak et al. 2015). Nanoparticles interact with the lipids and proteins of microorganisms that results in the disruption of the cell membranes. This becomes possible even with the lower concentration of nanoparticles which is less than the cytotoxic concentration. The nanoparticles generate reactive oxygen species that cause the obstruction of proton pumps, affect the electron transport and finally cause cell death in the fungi (Jangjou et al. 2022). Nanoparticles are more effective against both fungicide-sensitive and fungicide-resistant fungi when the particle size is less than 5 nm. Fusarium graminearum causes Fusarium head blight in cereal crops that result in yield loss and mycotoxins in food. Silver nanoparticles can be applied as an alternative to fungicides for the management of the disease in the field (Ni et al. 2022). Carbon nanotubes are identified as possessing a high antifungal activity. So, it is decorated with various nanoparticles to enhance the antifungal activity. Kotzybik et al. reported that silica and silver nanoparticles exhibit antifungal activity against the fungus Penicillium verrucosum. The mechanism of action is that silver nanoparticles diffuse inside the cell through the mycelial surface and stays in the cytoplasm and work on the cell organelles (Kotzybik et al. 2016). An antifungal compound is synthesized with silver NP, zinc oxide NP and multi-walled carbon nanotubes (MWCNTs). Its potential is very good against Aspergillus fumigatus and Aspergillus ochraceus (Fosso-Kankeu et al. 2016). Similarly, TiO2 nanoparticles are decorated over the surface of carbon nanotubes. TiO2 is a photocatalytic material. So, the antifungal activity is tested under visible light against Candida albicans biofilms. The photocatalytic antifungal activity is found to be very high. When visible light is incident on the nanoparticles, electron–hole pairs are created that help with the interaction between the cells and the nanoparticles. The variations in the morphology of the cells can be observed with a scanning electron microscope (Darbari et al. 2011). A carbon nanotube is also reported as a drug delivery system to deliver antifungal medicine amphotericin B (Benincasa et al. 2011). An enhanced and lengthened antifungal activity against Candida albicans and Candida tropical is reported from graphene oxide–silver nanocomposite by directly depositing Ag nanoparticles on graphene oxide. This composite is able to release silver ions slowly, and graphene oxide is not having any antifungal activity. Carbon nanomaterials and some metal oxide nanoparticles effectively inhibit Botrytis cinerea on Rose Petals with very low concentrations of nanoparticles. So, nanomaterials fight against fungi during the growth of flowers (Hao et al. 2017). The multiwall carbon nanotubes modified with functional groups OH-, COOH- and NH2 show enhanced antifungal activities against Fusarium graminearum. These MWCNTs inhibit spore elongation and germination in fungi and may find applications in plant protection (Wang et al. 2017).
Combinations of metallic and zinc oxide nanoparticles (50:10 μg/mL) exhibit photocatalytic antifungal effects with fluorescent light. The nanoparticles also do not exhibit any cytotoxic effects and inflammatory responses (Auyeung et al. 2017). Selenium shows antioxidant activity by protecting the liver against oxidation. It also prevents apoptosis of proteins and thereby shows an enhanced immunity in aflatoxicosis-affected animals (Limaye et al. 2018). Iron oxide (Maghemite) nanoparticles are useful in citrinin removal from Monascus which is able to produce a high amount of natural dyes and cholesterol-lowering statins (Magro et al. 2016). To protect stored grains from insects, single-celled algae known as diatoms are used. They are basically natural, nontoxic silica materials based on diatomaceous earth. This material disrupts the cuticle of the insect and makes the insect to dehydrate. So, this material can be used for the protection of stored grains for a longer period of time without reapplication.
Nanoparticles as adsorbents of mycotoxins elimination
Bentonites, zeolites, activated charcoal, fibres from plant sources and diatomaceous earth are some of the recently reported effective adsorbent materials. An adsorbent should be effective at sequestering the mycotoxins, low cost and low toxicity. The adsorbents strongly bind with mycotoxins thus preventing the mixing of mycotoxins with animal blood. The polarity and the suitable position of functional groups in the nanomaterial are more important for the adsorption of mycotoxins in any feed. When the blood flows from the stomach and intestine, the liver is the first organ affected by ingested metals, drugs and other toxicants. Mycotoxin contamination results in teratogenic, carcinogenic and oestrogenic or immune-suppressive effects. To decrease their bioavailability, mycotoxin-binding agents or adsorbents are used. Using adsorbents is a good approach to detoxifying mycotoxins. The adsorbent should be stable in the digestive tract to channel away the mycotoxins via urine and faeces. Organic montmorillonite nanocomposite when used as an adsorbent shows protective effects for the mycotoxins such as aflatoxin and ochratoxin since these mycotoxins cause the hepatonephrotoxicity in rats. Aflatoxin, ochratoxin and deoxynivalenol are generally observed in naturally mycotoxin-contaminated diets. These mycotoxins severely affect chickens, Fabricius and antibody titre against NDV and IBD virus. 0.1% of esterified glucomannan offers a better protection in this case (Mohaghegh et al. 2017).
Algo-clay can be used to adsorb mycotoxins in broiler chicken. Mycotoxins are not affected during storage. They cannot be destroyed by any technological treatments because of their thermo-resistant property. This algo-clay complex can reduce the mycotoxins to a level of 2.8 ppm for aflatoxin, 2 ppm for T2/HT2 toxin or 100 ppm for fumonisin. Metal oxide nanocrystals are employed as adsorbents to destroy aflatoxins, botulinum and Clostridium perfringens toxins. This is because of more reactive atoms available on their surface. Cellulosic polymers and chitosan are very good adsorbents of aflatoxin, ochratoxin and deoxynivalenol in poultry. Molecularly imprinted polymers can be used to adsorb mycotoxins. They are reusable and ecologically friendly and can be produced in large quantities. They are having more applications in dietary therapeutic, prophylactic, food and beverage processing and manufacture and quality control. Nanoparticle adsorbents decrease the exposure to mycotoxins using some nanocomposite materials. These materials avoid the bioavailability of mycotoxins through their synergistic effects. Magnetic graphene oxide is one such example that shows multifunctional performance as an adsorbent (Abbasi Pirouz et al 2021).
Carbon materials as adsorbents of mycotoxins
Developing low-cost and nontoxic functional nanomaterials is a recent trend in nanotechnology research. Carbon nanomaterials are synthesized from plant materials by carbonizing them, i.e. by heating the plant materials to very high temperatures in the presence of an activating agent. They have wide applications in the removal of various contaminants. Chen et al. reported that carbon nanomaterials such as graphene and its derivatives, carbon nanotubes and fullerenes are the very good adsorbents of mycotoxins. Nanocarbon structures have the capacity to bind with both polar and non-polar compounds (Chen et al. 2007). Kalagatur et al. utilized the environmentally benign plant material for mycotoxin removal. They derived the activated carbon by thermochemical treatment of Jatropha curcas seed shells using ZnCl2 as an activating agent. The obtained activated carbon is used to adsorb the zearalenone mycotoxin. Activated carbon is more biocompatible for use in feed items. This property supports while using this adsorbent material to protect food items (Kalagatur et al. 2017).
Graphene oxide is used to adsorb B1, B2, G1 and G2 aflatoxins. The quantity of aflatoxins is measured using high-performance liquid chromatography (Yu et al. 2013). The esterified glucomannan and sodium calcium aluminosilicate resist the mycotoxins in mould-contaminated feed. These adsorbents can bring more economic benefits to animal industries (Che et al. 2011). Poultry birds affected by aflatoxin B1 are treated with carbon particles obtained through the carbonization of maize straw and iron oxide nanoparticles. Carbon nanomaterials produced from biomaterials like maize straw are porous with a large surface area. The effect of the adsorbent in the gastrointestinal tract is analysed. Adsorbent at a dosage of 0.3% mixed in the feed was found to be highly effective. Negative effects are not observed by the application of nanocomposite (Zafar et al. 2017). Aflatoxin B1adsorption is quickly done by using nanodiamonds. The adsorption process is completed within 2–3 min. Nanodiamonds are biocompatible that is confirmed in many in vivo animal studies (Mogilnaya et al. 2010). Clays, yeast cell walls and various antioxidants are used as adsorbents against mycotoxins. Graphene adsorbs mycotoxins more effectively. Nanotechnology follows three strategies for the elimination of mycotoxin, i.e. mould inhibition, mycotoxin adsorption and nanoparticles to eradicate the toxins. Carbon nanomaterials participate in the elimination of mycotoxin. Also, polymeric nanoparticles are utilized as substitute adsorbents or can be incorporated with other substances. Figure 6 shows the quercetin loaded on the biopolymer (chitosan) nanoparticles (Horky et al. 2018). Zearalenone, a mycotoxin, produces contamination in corn oil. Activated carbon is able to adsorb zearalenone through π–π interaction. The material removes the toxin up to 83%, and the removal rate is not decreasing even after five regeneration cycles (Hu et al.2022). Chitosan is carbonized to produce nanoparticles which are then added with rectorite to form a nanocomposite. This nanocomposite is used as an effective adsorbent for the mycotoxin zearalenone. The mycotoxin sorption ability is attributed to both the high carbon content and the high surface area of the nanocomposite. The nanocomposite can also be applied for in vivo adsorption of mycotoxins since there is no desorption even in the pH present in the intestinal fluid (Sun et al 2020). Mycotoxin management in agriculture becomes more important to protect the health of human beings. This is possible through the application of adsorbents in the food. Graphene oxide is one such adsorbent with a high adsorption ability of more than 1 mg/g for the removal of mycotoxins such as aflatoxin, zearalenone and deoxynivalenol. When analysed in crushed wheat samples, graphene oxide adsorbs Mg, Cu and Zn to a larger extent (Horky et al. 2020).
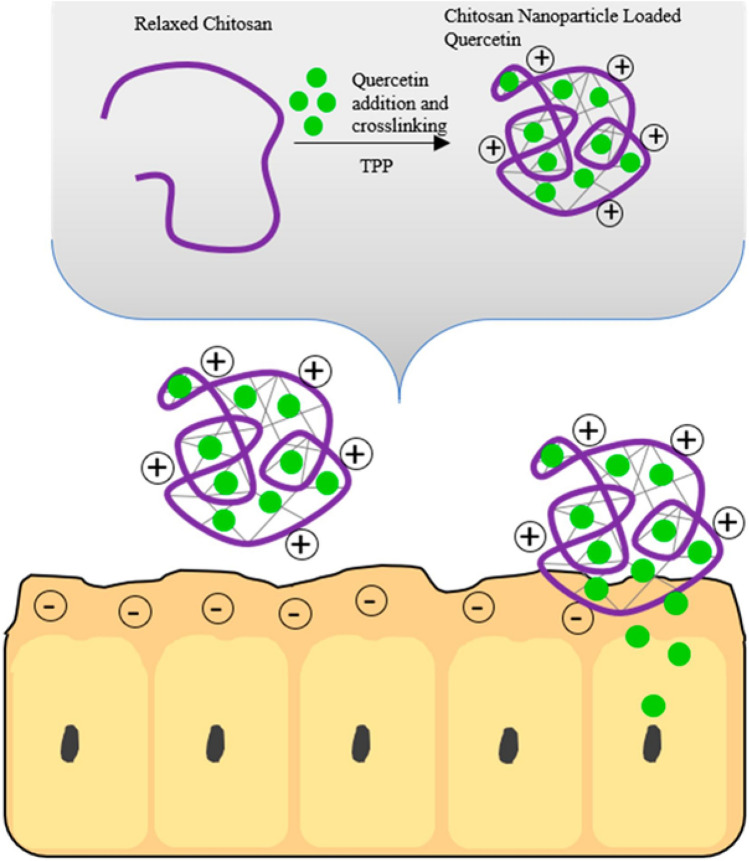
Fig. 6.
Schematic diagram shows the mechanism of action of the quercetin (Q)-loaded chitosan (CS) nanoparticle prepared by ionic gelation method. Figure is adapted from Horky et al. (2018)
Magnetic nanoparticles in mycotoxins reduction
Magnetic nanoparticles when come to nano-size exhibit the property of superparamagnetic instead of ferromagnetism. They are more special in nanotechnology because of their applications in cancer hyperthermia and as a contrast agent in MRI. They are also nowadays extensively used in water purification to adsorb the pollutants. They are synthesized with high dispersive nature using high-quality capping agents and polymers. Magnetic nanoparticles possess the advantage of selective adsorption and ease of separation of mycotoxins from food since they have magnetic susceptibility and stability. They are also more economical and biodegradable (Targuma et al 2021). Superparamagnetic nanoparticles can adsorb aflatoxins up to 89.0% more effectively using the solid-phase extraction method. In the conventional solid-phase extraction method, the adsorbent like iron powder is packed in a cartridge. But in magnetic solid-phase extraction, magnetic nanoparticles are dispersed in the sample solution. After the mycotoxins had been removed, the nanoparticles are removed by magnetic separation with the help of an external magnet. McCullum et al. reported that the detection of aflatoxin is performed using HPLC–MS/MS analysis. The instrument performs well with a very low detection limit of 0.0012 ng/mL for aflatoxins (McCullum et al. 2014).
Magnetic nanoparticles are easy to separate from aqueous mixtures. When compared to the conventional methods like filtration and centrifugation, separation using nanoparticles needs less energy. Pirouz et al. utilized magnetic graphene oxide nanocomposite to adsorb the Fusarium mycotoxins in palm kernel cake. The mycotoxins can be reduced at pH 6.2 for 5.2 h at 40.6 °C and the reduced levels are 69.57, 67.28, 57.40 and 37.17% (Pirouz et al. 2017). To enhance the activity of magnetic graphene oxide nanocomposite, the composite is grafted with the biopolymer chitosan. This biopolymer has amino and carboxyl functional groups. These groups support for the anionic binding of mycotoxins in acids. The carboxyl groups of graphene oxide and the amine groups of chitosan are responsible for effective mycotoxin detoxification (Pirouz et al. 2018).
Aluminosilicates are identified as favourable mycotoxin adsorbents mainly for aflatoxins in animal feeds because of their cation exchange capacity. Kurtbay et al. identified that ochratoxin A can be absorbed easily. OTA is adsorbed from wine by nano-clays such as bentonite, montmorillonite, and chitosan bead at pH 3.5. The amount of adsorbent may vary from 25 to 250 mg and the adsorbing time from 120 to 240 min (Kurtbay et al. 2008). In the same way, activated sodium bentonite, egg albumin, plant protein, amorphous silica, gelatin and chitosan are also tested to adsorb ochratoxin in wine. Apart from inorganic nanomaterials, the biomaterials are also used as very good adsorbents of mycotoxins. As per the report of Kandel, adsorbents from yeast, Saccharomyces cerevisiae and lactic acid bacteria, microorganisms from the rumen of cattle, the intestine of pigs and chickens and some soil bacteria show effective binding of Fusarium toxins in vitro. Adsorbents of hybrid materials with both inorganic and organic aries also studied to remove mycotoxins. Deoxynivalenol is removed in a study using activated carbon/yeast cell wall adsorbents. The experiment is performed for various pH, amount of adsorbent, amount of mycotoxin, activated carbon/yeast cell and incubation periods. The combination of activated carbon and yeast cell wall perform as effective adsorbent materials (De Souza et al. 2015).
Sensing and detection of mycotoxin
To analyse the trace amounts of mycotoxins, present in the food, biosensors are needed with high sensitivity. When nanomaterials are incorporated into biosensors, the limit of detection can be increased at low production costs (Malik et al. 2021). Nanotechnology is able to promote the food production by developing nano-biosensors for the detection of mycotoxins, pesticides and food additives. This has become possible through the introduction of new technologies like active and intelligent packaging (Pushparaj et al. 2023). Nanotechnology is applied in making the sophisticated bio-sensing techniques to detect mycotoxins. Various inorganic nanomaterials, organic nanomaterials, polymer nanocomposites, metal nanoparticles, metal oxide nanoparticles and noble metals (gold, silver and platinum) are utilized in biosensors. Metal nanoparticles have a special property like surface plasmon resonance. This property makes metal nanoparticles as highly sensitive. Badea et al., designed a simple impedimetric immunosensor to detect the mycotoxin aflatoxin B1 utilizing gold screen printed film electrodes.
Simple impedimetric immunosensor made with gold electrodes surfaces requires only less concentration of mycotoxin. Aflatoxin is a common toxic food contaminant produced by fungi. It is a poisonous carcinogen found in soil and agricultural wastes. Functionalization of the gold electrode surfaces of the designed electrochemical biosensor is essential to perform the perfect detection of aflatoxin. Antibodies and bovine serum albumin are immobilized on the gold electrode surfaces as bio-recognition elements. Figure 7 illustrates the electrochemical immunosensor designed for sensing mycotoxin (Badea et al. 2016).
Fig. 7.
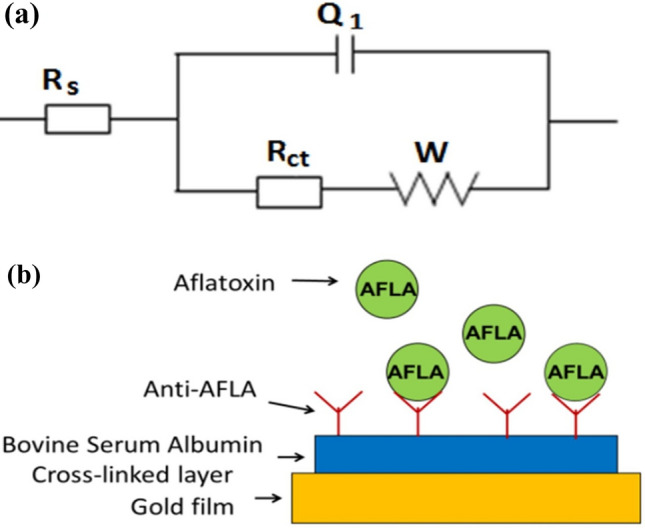
Schematic diagram illustrates the aflatoxin B1 sensing by electrochemical impedance spectroscopy using Functionalized gold film with cross-linked bovine serum albumin layer and immobilized anti-aflatoxin antibody as sensing material. Figure is adapted from Badea et al. (2016)
Natural materials for inhibition of microbial mycotoxin
Nanotechnology has much advancement in the fields of engineering, biotechnology, electronics, medicine, analytical chemistry and agriculture. However, its applications in crop protection are in the infancy stage. Natural materials such as biological nanomaterials are utilized for pest control (Kitherian 2016). Green synthesized silver nanoparticles using honey reduces the aflatoxin B1 toxin by 88% in stored maize grains (Pietrzak et al. 2015).
Nano-curcumin
Nano-curcumin from turmeric possesses a high solubility in water. It is more effective against the hepatotoxicity caused by Zearalenone (Ismaiel et al. 2015). Turmeric is reported as a traditional antioxidant and anticarcinogenic agent. It can be used to control the liver damage produced by aflatoxin. Curcumin, a compound of the turmeric, can be utilized to control the aflatoxigenic effects caused by the Aspergillus and inhibits cytochrome P450 isoenzymes (CYP2A6 isoform). Inhibition of these isoenzymes leads to reducing the creation of the toxic agents which cause aflatoxicosis (Limaye et al. 2018). Essential oils (EDs) from plants like cinnamon, clove and thyme show high efficacy against mycotoxins. They show the efficiency alone or with chitosan encapsulation. They prevent the radial growth, spore germination and mycotoxin production of the fungus such as Aspergillus and Fusarium. The major components which are responsible for this activity are eugenol, clove and 2-methyl-5-(1-methyethyl)-phenol in cinnamon and thyme. To prevent the degradation by external parameters such as light, oxygen and temperature, they are encapsulated with chitosan. An additional advantage of this encapsulation is that the material chitosan has antimicrobial and antifungal activity (Villegas-Rascón et al. 2018).
Thymol
Thymol, a phenolic compound of thyme is accepted as a food additive by FDA. Also, Environmental Protection Agency has declared that thymol has no adverse effects (Nagoor Meeran et al. 2017). Thyme is also used as food preservative in many countries. Thyme leaves also find applications in medicine (Rivera 2005). Thymol inhibits the Candida albicans through its antifungal activity (Pemmaraju et al. 2013). Nanoemulsions are used to encapsulate the natural pest-managing agents. Pulegone is such an organic compound that can be extracted from plants like Nepeta cataria (catnip), Mentha piperita and pennyroyal. It appears like an oily liquid with a smell similar to camphor. It protects the grains from the insects like rice weevil and red flour beetle. It is effective for more than one month with high (> 90%) mortality rates (Golden et al. 2018).
Plant oils
The oil extracted from the plant Chenopodium ambrosioides Linn. controls the damage of stored cereal grains and wheat grains more effectively. The extracted oil notably protects the wheat grains more effectively from Aspergillus flavus. Chenopodium leaf powder with a concentration of 0.05–6.4% (w/w) offers protection from bruchids, weevils and borers. Oils from the grains such as soybean, cottonseed, rice bran and palm kernel are also used as fumigants. These oils are utilized to protect the beans and wheat from the attacks of common insects (Kumar and Kalita 2017). Wheat grain samples stored for 180 days are tested for Aspergillus spp., Penicillium spp. and Fusarium spp. The samples are also analysed for aflatoxins, ochratoxin A, zearalenone, deoxynivalenol and fumonisins. Phosphine with concentrations equal to 3 g m−3 was able to control the growth and development of the Aspergillus flavus. Phosphine has the ability to delay the development of fungus in stored seeds with humidity content above the recommended for safe storage (Birck et al. 2006). Oregano and thyme essential oils are used to protect the stored grains as fumigants. They can be applied to the mycelia and spores of Aspergillus as well as against the natural microflora of wheat grains. The concentration requirement of oregano oil is 2.0–2.5 μL/L. This oil shows a better inhibitory effect even at a high moisture content (Paster et al. 1995). Aspergillus is also prevented by essential oils of thyme, cinnamon, marigold, spearmint, basil, alyssum and caraway with a concentration of ⩽500 ppm particularly in wheat grains (Soliman and Badeaa 2002).
Lemongrass essential oil protects the stored rice grains from the aflatoxigenic fungi Aspergillus flavus Link. Thin-layer chromatography and gas chromatography analysis results of lemongrass oil confirm that two major fungicidal constituents citral a and b are present in this essential oil. Essential oils will not produce any harmful effects and are safe (Paranagama et al. 2003). Aqueous extracts of aerial parts of Artemisia herba-alba, Cotula cinerea, Asphodelus tenuifolius, and Euphorbia guyoniana effectively inhibit Fusarium graminearum and Fusarium sporotrichioides. The presence of biochemical compounds like tannins, flavonoids, saponins, steroids and alkaloids produce the antifungal activities. These plants have the potential to control the fungi which affect wheat yields (Salhi et al. 2017). Essential oils obtained from Nutmeg, Eucalyptus, Cinnamon and Clove exhibit better against many fungi (Shirurkar and Wahegaonkar 2012). Apart from essential oils and nanomaterials, extracts of the plant sources are useful to protect the stored grains from the pathogenic fungi. Satish et al. reported that the solvent extracts (petroleum ether, benzene, chloroform, methanol and ethanol) and aqueous extracts of Acacia nilotica, Achras zapota, Datura stramonium, Emblica officinalis, Eucalyptus globules, Lawsonia inermis, Mimusops elengi, Peltophorum pterocarpum, Polyalthia longifolia, Prosopis juliflora, Punica granatum and Syzygium cumini have shown significant antifungal activity against Aspergillus species. Methanol is found to be a suitable candidate for making plant extracts. Rice weevil and red flour beetle are pests of maize that cause storage deterioration by an infestation. The crude extracts and purified fractions from leaves of papaya (Carica papaya L.), ivy gourd (Coccinia indica), bitter gourd (Momordica charantia L.), curry leaf (Murraya koenigii L.), chilli plant (Capsicum annuum L.) and brinjal (Solanum melongena L.) are tested against the infestation in the vapour and contact form. The extracts of M. koenigii, M. charantia and S. melongena reduce the exposure of Aspergillus flavus during germination period (Rani and Devanand 2011).
Conclusion and future perspectives
Mycotoxins are toxic substances given by fungi, more dangerous and pollute the storage grains during storage. The production of mycotoxin can be prevented using nanotechnology. Various nanomaterials such as carbon nanomaterials and metal nanoparticles are reported for their effective antifungal activities. Nanomaterials are also used as adsorbents to remove mycotoxins. Mycotoxins are also detected by nanomaterials by various sensing mechanisms. Natural nanoparticles such as chitosan nanoparticles and solid lipid nanoparticles are used as antifungal agents. Essential oils from plants such as lemongrass and thyme are loaded inside nanoparticles to function against various fungi. The above discussion has brought several things together and elevated the insights. As stated, the future directions will be highly occupied by the participation of nanomaterials. Currently, several nanomaterials are emerging as a tailor-made in the desired sizes and shapes. Further, the combination of different nanomaterials as the hybrids has broad potentials. With these, hybrids and the inclusion of natural anti-mycotoxins become a great combination for industrial benefits and environmental safety. To meet the global population, it is required to produce food in a safe manner from the adverse effects of microorganisms. Nanotechnology is able to provide many innovative solutions for these kinds of applications. Carbon nanomaterials are demonstrated as very good adsorbents for mycotoxins. The antifungal effect is offered by the technology of active packaging in which the active ingredients are released in a controlled manner. This type of solution from nanotechnology will improve the shelf life of food products and food grains. Also, regulations should be made to consider the toxicity of nanoparticles. Green synthesis of nanoparticles will be the right solution for the toxic effects of nanomaterials and carbon nanomaterials are also not having much toxic effects when compared to another type of nanomaterials. Many nano-based sensors are now developing that can be used in food industries. They are more useful to measure the amounts of microorganisms in the food, and some devices are available even to measure the viscosity of curries.
Acknowledgements
None
Author contributions
The authors are contributed to the preparation of the manuscript and discussion. All authors read and approved the final manuscript.
Data availability
Data available upon request to the authors.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical statement
None.
Contributor Information
Theivasanthi Thirugnanasambandan, Email: t.theivasanthi@klu.ac.in.
Subash C. B. Gopinath, Email: subash@unimap.edu.my
References
- Auyeung A, Casillas-Santana MÁ, Martínez-Castañón GA, Slavin YN, Zhao W, Asnis J, Häfeli UO, Bach H. Effective control of molds using a combination of nanoparticles. PLoS ONE. 2017;12:e0169940. doi: 10.1371/journal.pone.0169940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badea M, Floroian L, Restani P, Moga M. Simple surface functionalization strategy for immunosensing detection of aflatoxin B1. Int J Electrochem Sci. 2016;11:6719–6734. doi: 10.20964/2016.08.21. [DOI] [Google Scholar]
- Bell C. Alternatives to pesticides in stored-product IPM. Plant Sci. 2002;162:327–328. doi: 10.1016/s0168-9452(01)00560-x. [DOI] [Google Scholar]
- Benincasa M, Pacor S, Wu W, Prato M, Bianco A, Gennaro R. Antifungal activity of amphotericin B conjugated to carbon nanotubes. ACS Nano. 2011;5:199–208. doi: 10.1021/nn1023522. [DOI] [PubMed] [Google Scholar]
- Birck NMM, Lorini I, Scussel VM (2006) Fungus and mycotoxins in wheat grain at post harvest. In: 9th International working conference on stored product protection. pp 198–205
- Bullerman LB, Schroeder LL, Park KY. Formation and control of mycotoxins in food. J Food Protect. 1984;47:637–646. doi: 10.4315/0362-028X-47.8.637. [DOI] [PubMed] [Google Scholar]
- Che Z, Liu Y, Wang H, Zhu H, Hou Y, Ding B. The protective effects of different mycotoxin adsorbents against blood and liver pathological changes induced by mold-contaminated feed in broilers. Asian-Austral J Anim Sci. 2011;24:250–257. doi: 10.5713/ajas.2011.10022. [DOI] [Google Scholar]
- Chen W, Duan L, Zhu D. Adsorption of polar and nonpolar organic chemicals to carbon nanotubes. Environ Sci Technol. 2007;41:8295–8300. doi: 10.1021/es071230h. [DOI] [PubMed] [Google Scholar]
- Darbari S, Abdi Y, Haghighi F, Mohajerzadeh S, Haghighi N. Investigating the antifungal activity of TiO2 nanoparticles deposited on branched carbon nanotube arrays. J Phys D Appl Phys. 2011;44:245401. doi: 10.1088/0022-3727/44/24/245401. [DOI] [Google Scholar]
- De Souza AF, Borsato D, Lofrano AD, De Oliveira AS, Ono MA, Bordini JG, Hirozawa MT, Yabe MJS, Ono EYS. In vitro removal of deoxynivalenol by a mixture of organic and inorganic adsorbents. World Mycotoxin J. 2015;8:113–119. doi: 10.3920/WMJ2013.1666. [DOI] [Google Scholar]
- Devreese M, De Backer P, Croubels S. Different methods to counteract mycotoxin production and its impact on animal health. Vlaams Diergeneeskund Tijdschr. 2013;82:181–190. doi: 10.21825/vdt.v82i4.16695. [DOI] [Google Scholar]
- Eeckhout M, Landschoot S, Deschuyffeleer N, De Laethauwer S, Haesaert G (2013) Guidelines for prevention and control of mould growth and mycotoxin production in cereals. Mycohunt, Synagra. be/Download.ashx
- Fosso-Kankeu E, De Klerk CM, Botha TA, Waanders F, Phoku JPS (2016) The antifungal activities of multi-walled carbon nanotubes decorated with silver, copper and zinc oxide particles. In: International Conference on Advances in Science, Engineering, Technology & Natural Resources, pp 24–25
- Golden G, Quinn E, Shaaya E, Kostyukovsky M, Poverenov E. Coarse and nano emulsions for effective delivery of the natural pest control agent pulegone for stored grain protection. Pest Manage Sci. 2018;74:820–827. doi: 10.1002/ps.4787. [DOI] [PubMed] [Google Scholar]
- Hao Y, Cao X, Ma C, Zhang Z, Zhao N, Ali A, Hou T, Xiang Z, Zhuang J, Wu S, Xing B, Zhang Z, Rui Y. Potential applications and antifungal activities of engineered nanomaterials against gray mold disease agent botrytis cinerea on rose petals. Front Plant Sci. 2017;8:1332. doi: 10.3389/fpls.2017.01332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horky P, Skalickova S, Baholet D, Skladanka J. Nanoparticles as a solution for eliminating the risk of mycotoxins. Nanomaterials. 2018 doi: 10.3390/nano8090727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horky P, Venusova E, Aulichova T, Ridoskova A, Skladanka J, Skalickova S. Usability of graphene oxide as a mycotoxin binder: In vitro study. PLoS ONE. 2020;15(9):e0239479. doi: 10.1371/journal.pone.0239479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Ma C, Huang W, Guo S, Wang T, Liu J. Adsorption behavior of activated carbon for the elimination of zearalenone during bleaching process of corn oil. Grain Oil Sci Technol. 2022 doi: 10.1016/j.gaost.2022.11.002. [DOI] [Google Scholar]
- Ismaiel AAM, El-Denshary ES, El-Nekeety AA, Al-Yamani MF, Gad AS, Hassan NS, Abdel-Wahhab MA. Ameliorative effects of curcumin nanoparticles on hepatotoxicity induced by zearalenone mycotoxin. Glob J Pharmacol. 2015;9:234–245. doi: 10.5829/idosi.gjp.2015.9.3.96120. [DOI] [Google Scholar]
- Jangjou A, Zareshahrabadi Z, Abbasi M, Talaiekhozani A, Kamyab H, Chelliapan S, Vaez A, et al. Time to conquer fungal infectious diseases: employing nanoparticles as powerful and versatile antifungal nanosystems against a wide variety of fungal species. Sustainability. 2022;14(19):12942. doi: 10.3390/su141912942. [DOI] [Google Scholar]
- Jard G, Liboz T, Mathieu F, Guyonvarch A, Lebrihi A. Review of mycotoxin reduction in food and feed: from prevention in the field to detoxification by adsorption or transformation. Food Addit Contam A Chem Anal Control Expo Risk Assess. 2011 doi: 10.1080/19440049.2011.595377. [DOI] [PubMed] [Google Scholar]
- Kalagatur NK, Karthick K, Allen JA, Ghosh OSN, Chandranayaka S, Gupta VK, Krishna K, Mudili V. Application of activated carbon derived from seed shells of Jatropha curcas for decontamination of zearalenone mycotoxin. Front Pharmacol. 2017;8:760. doi: 10.3389/fphar.2017.00760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushik A, Solanki PR, Ansari AA, Ahmad S, Malhotra BD. A nanostructured cerium oxide film-based immunosensor for mycotoxin detection. Nanotechnology. 2009;20:055105. doi: 10.1088/0957-4484/20/5/055105. [DOI] [PubMed] [Google Scholar]
- Kitherian S. Nano and bio-nanoparticles for insect control. Res J Nanosci Nanotechnol. 2016;7:1–9. doi: 10.3923/rjnn.2017.1.9. [DOI] [Google Scholar]
- Kotzybik K, Gräf V, Kugler L, Stoll DA, Greiner R, Geisen R, Schmidt-Heydt M. Influence of different nanomaterials on growth and mycotoxin production of penicillium verrucosum. PLoS ONE. 2016;11:e0150855. doi: 10.1371/journal.pone.0150855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar D, Kalita P. Reducing postharvest losses during storage of grain crops to strengthen food security in developing countries. Foods. 2017;6(1):8. doi: 10.3390/foods6010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtbay HM, Bekçi Z, Merdivan M, Yurdakoç K. Reduction of ochratoxin a levels in red wine by bentonite, modified bentonites, and chitosan. J Agric Food Chem. 2008;56:2541–2545. doi: 10.1021/jf073419i. [DOI] [PubMed] [Google Scholar]
- Limaye A, Yu RC, Chou CC, Liu JR, Cheng KC. Protective and detoxifying effects conferred by dietary selenium and curcumin against AFB1-mediated toxicity in livestock: a review. Toxins. 2018 doi: 10.3390/toxins10010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magro M, Moritz DE, Bonaiuto E, Baratella D, Terzo M, Jakubec P, Malina O, Čépe K, De Aragao GMF, Zboril R, Vianello F. Citrinin mycotoxin recognition and removal by naked magnetic nanoparticles. Food Chem. 2016;203:505–512. doi: 10.1016/j.foodchem.2016.01.147. [DOI] [PubMed] [Google Scholar]
- Malik P, Gupta R, Malik V, Ameta RK. Emerging nanomaterials for improved biosensing. Meas Sens. 2021;16:100050. doi: 10.1016/j.measen.2021.100050. [DOI] [Google Scholar]
- McClements DJ, Xiao H. Is nano safe in foods? Establishing the factors impacting the gastrointestinal fate and toxicity of organic and inorganic food-grade nanoparticles. NPJ Sci Food. 2017 doi: 10.1038/s41538-017-0005-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullum C, Tchounwou P, Ding LS, Liao X, Liu YM. Extraction of aflatoxins from liquid foodstuff samples with polydopamine-coated superparamagnetic nanoparticles for HPLC-MS/MS analysis. J Agric Food Chem. 2014;62:4261–4267. doi: 10.1021/jf501659m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogilnaya OA, Puzyr AP, Baron AV, Bondar VS. Hematological parameters and the state of liver cells of rats after oral administration of aflatoxin B1 alone and together with nanodiamonds. Nanoscale Res Lett. 2010;5:908–912. doi: 10.1007/s11671-010-9571-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohaghegh A, Chamani M, Shivazad M, Sadeghi AA, Afzali N. Effect of esterified glucomannan on broilers exposed to natural mycotoxin-contaminated diets. J Appl Anim Res. 2017;45:285–291. doi: 10.1080/09712119.2016.1174122. [DOI] [Google Scholar]
- Mousavi SAA, Pourtalebi S. Inhibitory effects of silver nanoparticles on growth and aflatoxin B1 production by aspergillus parasiticus. Iran J Med Sci. 2015;40:501–506. [PMC free article] [PubMed] [Google Scholar]
- NagoorMeeran MF, Javed H, Taee HA, Azimullah S, Ojha SK. Pharmacological properties and molecular mechanisms of thymol: prospects for its therapeutic potential and pharmaceutical development. Front Pharmacol. 2017 doi: 10.3389/fphar.2017.00380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni J, Yang Z, Zhang Y, et al. Aaqueous exposure to silver nanoparticles synthesized by abalone viscera hydrolysates promotes the growth, immunity and gut health of zebrafish (Danio rerio) Front Microbiol. 2022;13:1–13. doi: 10.3389/fmicb.2022.1048216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nile SH, Baskar V, Selvaraj D, Nile A, Xiao J, Kai G. Nanotechnologies in food science: applications, recent trends, and future perspectives. Nano-Micro Lett. 2020;12(1):1–34. doi: 10.1007/s40820-020-0383-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paranagama PA, Abeysekera KHT, Abeywickrama K, Nugaliyadde L. Fungicidal and anti-aflatoxigenic effects of the essential oil of Cymbopogon citratus (DC.) Stapf. (lemongrass) against Aspergillus flavus Link. isolated from stored rice. Lett Appl Microbiol. 2003;37:86–90. doi: 10.1046/j.1472-765X.2003.01351.x. [DOI] [PubMed] [Google Scholar]
- Paster N, Menasherov M, Ravid U, Juven B. Antifungal activity of oregano and thyme essential oils applied as fumigants against fungi attacking stored grain. J Food Protect. 1995;58:81–90. doi: 10.4315/0362-028X-58.1.81. [DOI] [PubMed] [Google Scholar]
- Pemmaraju SC, Pruthi PA, Prasad R, Pruthi V. Candida albicans biofilm inhibition by synergistic action of terpenes and fluconazole. Indian J Exp Biol. 2013;51:1032–1037. [PubMed] [Google Scholar]
- Pickova D, Ostry V, Toman J, Malir F. Aflatoxins: history, significant milestones, recent data on their toxicity and ways to mitigation. Toxins. 2021 doi: 10.3390/toxins13060399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrzak K, Twaruzek M, Czyzowska A, Kosicki R, Gutarowska B. Influence of silver nanoparticles on metabolism and toxicity of moulds. Acta Biochim Pol. 2015;62:851–857. doi: 10.18388/abp.2015_1146. [DOI] [PubMed] [Google Scholar]
- Pirouz AA, Selamat J, Iqbal SZ, Mirhosseini H, Karjiban RA, Bakar FA. The use of innovative and efficient nanocomposite (magnetic graphene oxide) for the reduction on of Fusarium mycotoxins in palm kernel cake. Sci Rep. 2017;7:12453. doi: 10.1038/s41598-017-12341-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirouz AA, Karjiban RA, Bakar FA, Selamat J. A novel adsorbent magnetic graphene oxide modified with Chitosan for the simultaneous reduction of mycotoxins. Toxins. 2018;10:361. doi: 10.3390/toxins10090361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirouz AA, Selamat J, Sukor R, NoorahyaJambari N. Effective detoxification of aflatoxin B1 and ochratoxin A using magnetic graphene oxide nanocomposite: isotherm and kinetic study. Coatings. 2021;11(11):1346. doi: 10.3390/coatings11111346. [DOI] [Google Scholar]
- Pushparaj K, Meyyazhagan A, Pappuswamy M et al (2023) Occurrence, identification, and decontamination of potential mycotoxins in fruits and fruit by-products. Food Front 1–15. 10.1002/fft2.198
- Rani PU, Devanand P. Efficiency of different plant foliar extracts on grain protection and seed germination in maize. Res J Seed Sci. 2011;4:1–14. doi: 10.3923/rjss.2011.1.14. [DOI] [Google Scholar]
- Rivera D. Eucalyptus. The genus Eucalyptus. Medicinal and aromatic plants—Industrial profiles. Volume 22. Econ Bot. 2005;59:300. doi: 10.1663/0013-0001(2005)059[0300:dfabre]2.0.co;2. [DOI] [Google Scholar]
- Salhi N, Mohammed Saghir SA, Terzi V, Brahmi I, Ghedairi N, Bissati S. Antifungal activity of aqueous extracts of some dominant algerian medicinal plants. Biomed Res Int. 2017;2017:7526291. doi: 10.1155/2017/7526291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirurkar DD, Wahegaonkar NK. Antifungal activity of selected plant derived oils and some fungicides against seed borne fungi of maize. Eur J Exp Biol. 2012;2:B163–B170. [Google Scholar]
- Sk MP, Jaiswal A, Paul A, Ghosh SS, Chattopadhyay A. Presence of amorphous carbon nanoparticles in food caramels. Sci Rep. 2012;2:383. doi: 10.1038/srep00383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliman KM, Badeaa RI. Effect of oil extracted from some medicinal plants on different mycotoxigenic fungi. Food Chem Toxicol. 2002;40:1669–1675. doi: 10.1016/S0278-6915(02)00120-5. [DOI] [PubMed] [Google Scholar]
- Sun Z, Jie Xu, Wang G, Song A, Li C, Zheng S. Hydrothermal fabrication of rectorite based biocomposite modified by chitosan derived carbon nanoparticles as efficient mycotoxins adsorbents. Appl Clay Sci. 2020;184:105373. doi: 10.1016/j.clay.2019.105373. [DOI] [Google Scholar]
- Targuma S, Njobeh PB, Ndungu PG. Current applications of magnetic nanomaterials for extraction of mycotoxins, pesticides, and pharmaceuticals in food commodities. Molecules. 2021;26(14):4284. doi: 10.3390/molecules26144284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tefera T, Kanampiu F, De Groote H, Hellin J, Mugo S, Kimenju S, Beyene Y, Boddupalli PM, Shiferaw B, Banziger M. The metal silo: an effective grain storage technology for reducing post-harvest insect and pathogen losses in maize while improving smallholder farmers’ food security in developing countries. Crop Prot. 2011 doi: 10.1016/j.cropro.2010.11.015. [DOI] [Google Scholar]
- Villegas-Rascón RE, López-Meneses AK, Plascencia-Jatomea M, Cota-Arriola O, Moreno-Ibarra GM, Castillón-Campaña LG, Sánchez-Mariñez RI, Cortez-Rocha MO. Control of mycotoxigenic fungi with microcapsules of essential oils encapsulated in chitosan. Food Sci Technol (Brazil) 2018 doi: 10.1590/1678-457X.04817. [DOI] [Google Scholar]
- Wang X, Zhou Z, Chen F. Surface modification of carbon nanotubes with an enhanced antifungal activity for the control of plant fungal pathogen. Materials. 2017 doi: 10.3390/ma10121375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Li P, Zhang Q, Zhang W, Ding X, Wang X. Graphene oxide: An adsorbent for the extraction and quantification of aflatoxins in peanuts by high-performance liquid chromatography. J Chromatogr A. 2013;1318:27–34. doi: 10.1016/j.chroma.2013.10.006. [DOI] [PubMed] [Google Scholar]
- Zafar R, Khan FA, Zahoor M. In vivo amelioration of aflatoxin B1 in broiler chicks by magnetic carbon nanocomposite. Pesquisa Veterin Brasil. 2017;37:1213–1219. doi: 10.1590/s0100-736x2017001100005. [DOI] [Google Scholar]
- Zain ME. Impact of mycotoxins on humans and animals. J Saudi Chem Soc. 2011;15:129–144. doi: 10.1016/j.jscs.2010.06.006. [DOI] [Google Scholar]
- Zhao Z, Liu N, Yang L, Wang J, Song S, Nie D, Yang X, Hou J, Wu A. Cross-linked chitosan polymers as generic adsorbents for simultaneous adsorption of multiple mycotoxins. Food Control. 2015;57:362–369. doi: 10.1016/j.foodcont.2015.05.014. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available upon request to the authors.