Abstract
Epoxyeicosatrienoic acids (EETs) have pleiotropic endogenous cardiovascular protective effects and can be hydrolyzed to the corresponding dihydroxyeicosatrienoic acids by soluble epoxide hydrolase (sEH). Heart failure with preserved ejection fraction (HFpEF) has shown an increased prevalence and worse prognosis over the decades. However, the role of sEH activity in HFpEF remains unclear. We enrolled 500 patients with HFpEF and 500 healthy controls between February 2010 and March 2016. Eight types of sEH-related eicosanoids were measured according to target metabolomics, and their correlation with clinical endpoints was also analyzed. The primary endpoint was cardiac mortality, and the secondary endpoint was a composite of cardiac events, including heart failure (HF) readmission, cardiogenic hospitalization, and all-cause mortality. Furthermore, the effect of sEH inhibitors on cardiac diastolic function in HFpEF was investigated in vivo and in vitro. Patients with HFpEF showed significantly enhanced EET degradation by the sEH enzyme compared with healthy controls. More importantly, sEH activity was positively correlated with cardiac mortality in patients with HFpEF, especially in older patients with arrhythmia. A consistent result was obtained in the multiple adjusted models. Decreased sEH activity by the sEH inhibitor showed a significant effective effect on the improvement of cardiac diastolic function by ameliorating lipid disorders in cardiomyocytes of HFpEF mouse model. This study demonstrated that increased sEH activity was associated with cardiac mortality in patients with HFpEF and suggested that sEH inhibition could be a promising therapeutic strategy to improve diastolic cardiac function. Clinical trial identifier: NCT03461107 (https://clinicaltrials.gov)
Supplementary Information
The online version contains supplementary material available at 10.1007/s43657-022-00069-8.
Keywords: Soluble epoxide hydrolase, Heart failure with preserved ejection fraction, Eicosanoids, Epoxyeicosatrienoic acids
Introduction
Heart failure (HF) with preserved ejection fraction (HFpEF) represents more than 50% of prevalent HF cases in the community, especially in patients older than 65 years (Dunlay et al. 2017). The five-year survival rate of patients with HFpEF is only 35%, which is clinically worse than that of many cancers (Owan et al. 2006). Due to the aging population and detrimental lifestyle changes, HFpEF-related hospitalization and mortality have increased dramatically over the decades (Lam et al. 2011; Ponikowski et al. 2016a). Despite the considerable progresses in pharmacological and device management in recent years, most approved therapies for HF with reduced ejection fraction (HFrEF) have been demonstrated to be ineffective for curing HFpEF (Upadhya and Kitzman 2020), and substantial amounts of health care resources are still required for patients with HFpEF (Riegel et al. 2009). Individualized and effective treatments are urgently needed for treating patients with HFpEF.
Epoxyeicosatrienoic acids (EETs) are a series of eicosanoids, derived from arachidonic acid (AA) by cytochrome P450 (CYP450) enzymes, which are preferentially expressed in cardiomyocytes and endothelial cells (Askari et al. 2013; Imig 2012). They are rapidly hydrolyzed to the corresponding dihydroxyeicosatrienoic acids (DHETs) by soluble epoxide hydrolase (sEH) (Buczynski et al. 2009). EETs play a central role as endogenous regulators of the cardiovascular system, and showed protective effects in HF according ameliorated cardiac hypertrophy (Wang et al. 2014), anti-inflammation, and vasodilation (He et al. 2017, 2015; Imig 2019; Roman 2002). Once EETs are hydrolyzed into corresponding DHETs by sEH, their cardioprotective activities are reduced (Qiu et al. 2011). An alternative pharmaceutical approach to sEH inhibitors would be to employ EET mimics or agonists which are resistant to the sEH in vivo (Yu et al. 2000). Nowadays, sEH inhibition has become an effective experimental approach to investigate the biological roles of EETs (Sun et al. 2021). The sEH is also identified as a susceptible gene for HF and is expected to be a therapeutic target for patients with HF (Lai and Chen 2021; Monti et al. 2008; Romashko et al. 2016). Therefore, stable levels of EETs by sEH antagonist might be a therapeutic strategy for HFpEF.
However, the metabolic context of the sEH-related eicosanoid system in the plasma of patients with HFpEF remains unclear. In this study, we used liquid chromatography–tandem mass spectrometry (LC–MS/MS)-based lipidomic approach to investigate the eicosanoid profile related to the sEH enzyme in the plasma of patients with HFpEF, and we further investigated the detailed clinical significance of sEH activity in patients with HFpEF. In addition, antagonists were used to explore the therapeutic potential of the sEH enzyme in the HFpEF mouse model.
Materials and Methods
Study Design and Participants
Eligible patients with HFpEF were consecutively enrolled between February 2010 and March 2016 in Tongji Hospital. A total of 500 patients with HFpEF were recruited in this study (age range 20–85 years), who were diagnosed with HFpEF according to the European Society of Cardiology guidelines (Dickstein et al. 2008; Ponikowski et al. 2016a), based on a combination of symptoms, signs, echocardiographic results within three months before enrollment, and N-terminal B-type natriuretic peptide (NT-proBNP) concentrations. HF patients with left ventricular ejection fraction (LVEF) ≥ 50%, elevated levels of NT-proBNP (> 125 pg/mL), and diastolic dysfunction (or relevant structural heart disease) were assigned to the HFpEF group. Patients were excluded if they had been previously diagnosed with a reduced LVEF of < 40%. Other exclusion criteria were tumors, cancer, significant liver disease, and pregnancy. Moreover, 500 healthy control subjects (age range 19–85 years) were recruited after health examination. They were not receiving any medications and had no significant systemic diseases (hypertension, diabetes mellitus, or ischemic heart disease).
Clinical variables were measured following standardized protocols, including echocardiography (left ventricular [LV] and left atrial [LA] dimension, LVEF), NT-proBNP, systolic/diastolic blood pressure (SBP/DBP), total cholesterol, triglycerides, high-density lipoprotein (HDL) cholesterol, and low-density lipoprotein (LDL) cholesterol levels.
The study protocols were approved by the Ethics Committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology. The investigation conformed to the principles outlined in the Declaration of Helsinki. Written informed consents were obtained from all the participants.
Sample Preparation and Analysis for Eicosanoid Metabolomics
Blood samples were collected in tubes containing sodium Ethylenediaminetetraacetic acid in a fasting state and then centrifuged to separate the plasma (200 µL for each) from the whole blood within the same working day. After that, butylated hydroxytoluene (0.01 mol/L) was added to the plasma samples and stored at − 80 °C until extraction. The participant records were anonymized and de-identified before the analysis. Stored plasma samples were extracted, and eicosanoids were profiled using highly specific LC–MS-based methods as described previously (Zhang et al. 2015). In short, a Waters ACQUITY UPLC equipped with an ACQUITY UPLC BEH C18 Column, 130A, 1.7 µm, 2.1 mm × 50 mm, was used as the liquid chromatography system. The injection volume was 10 μL, solvent A was water, and solvent B was acetonitrile, with a column temperature of 25 °C and mobile-phase flow rate of 0.6 mL/min. Eight eicosanoids related to the sEH enzyme were profiled by multiple reaction monitoring scans in negative mode. Peak finding, integration, and concentration calculation were performed using the MutiQuant 3.0.3 software.
Study Endpoints
Patients in this study were contacted every six months and were followed up for five years after enrollment. The primary endpoint was cardiac mortality, indicating the death of cardiac origin unless there were obvious non-cardiac causes. The secondary endpoint was a composite of cardiac events, including HF readmission, cardiogenic hospitalization, and all-cause mortality. Of these, HF readmission was identified as readmitted due to symptoms and signs corresponding to the HF clinical guidelines (Ponikowski et al. 2016b). Cardiogenic hospitalization was considered as hospitalization for any cardiovascular reason. All event reports were extracted from clinical records, death certificates, and any other relevant documentation. The clinical endpoints were systematically classified and adjudicated by two independent physicians.
Animals
The animal experiments were conducted following protocols approved by the Ethics Committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology. The sEH expression levels vary among species, and mice showed much higher sEH levels than that of other species. In addition, male db/db mouse model has been reported to recapitulate the functional and histological features of human HFpEF (Alex et al. 2018). Eight-week-old male db/db mice weighing between 40 and 60 g mice generated in C57BLKS/J background were established as the animal model to investigate the role of sEH inhibitors in vivo. The control littermates (Model Animal Research Center of Nanjing University, Nanjing, China) were employed as the control group (wild-type, wt). Echocardiography was performed to measure cardiac function at the age of 16 weeks with a normal diet (20 kcal% protein, 70 kcal% carbohydrate and 10 kcal% fat, purchased from Beijing Huafukang Bioscience Co., Ltd., Beijing, China). The 12-(3-Adamantan-1-yl-ureido) dodecanoic acid (AUDA, 3 mg/kg/day, positive control, could attenuate cardiac injury in diabetic rats) (Morisseau et al. 2002; Sun et al. 2021) and TPN12419 (TPN, 1 mg/kg/day, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai) diluted in water were administered by gavage for eight weeks (Fig. 4a). All animals were killed at the age of 24 weeks, and organ samples were collected, frozen in liquid nitrogen, and stored at − 80 °C for further experiments. The investigation conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1985). Detailed methods are provided in the Supplementary Material (Methods).
Fig. 4.
Improved diastolic cardiac function and reduced HF markers in HFpEF model were mediated by TPN, better than AUDA. a Schematic diagram of animal experiments in vivo. b, c sEH inhibitors (TPN and AUDA) reduce blood glucose levels and increase body weight. d–g Echocardiographic and hemodynamic parameters were detected for HFpEF mouse model. h TPN reversed cardiac hypertrophic markers in cardiac tissue of HFpEF mouse model. i Sirius red, j H&E, and k DHE staining were performed to detect cardiac fibrosis, hypertrophy, and ROS level in mice. The image corresponding scale bar was 100 μm, 50 μm, and 100 μm, correspondingly. Compared to control group: &&p < 0.01, &&&p < 0.001. Compared to db/db group: *p < 0.05, **p < 0.01, ***p < 0.001. Compared to db/db + AUDA group: #p < 0.05. The results are means ± SEM
Statistical Analysis
The clinical baseline characteristics of the participants enrolled in two cohorts are presented as the median (interquartile range) for continuous variables and count (percentage) for categorical variables. Concentrations of eicosanoids are presented as the mean ± standard error of mean (SEM). Statistical analyses were performed at an overall significance level of 0.05, and all comparisons were two-sided. The Student’s t test or Mann–Whitney test was performed for intergroup comparisons when appropriate. The Chi-square test was used to evaluate the frequencies of categorical clinical variables. Binary logistic regression was performed to identify the relationship between eicosanoids and HF prevalence. Odds ratios and 95% confidence intervals (CIs) were also calculated. The Kaplan–Meier analysis was used to estimate the time-to-event data, and p values were evaluated based on the log-rank test. Cox proportional hazard models were used to identify the prognostic value of eicosanoids adjusted for covariates associated with the clinical endpoint, including age, sex, smoking, drinking, hypertension, diabetes, dyslipidemia, peripheral atherosclerosis, stroke, arrhythmia, and coronary artery disease. The hazard ratios (HRs) and 95% CIs were calculated. All analyses were performed using SPSS (version 22.0; IBM Corp., Armonk, NY, USA).
Results
Baseline Characteristics
Plasma samples from all 1,000 participants were collected. After eicosanoid profiling using LC–MS/MS and deletion of the outlier samples (Rodionova and Pomerantsev 2020), 976 individuals (including 482 patients with HFpEF and 494 healthy controls) were included in the further analysis (Fig. 1). The baseline characteristics and clinical parameters are shown in Table 1. Consistent with the inclusion criteria, patients with HFpEF had dramatically higher levels of BNP, larger dimensions of LV and LA, and thicker LV walls than the control subjects. Patients with HFpEF showed higher levels in most clinical (including SBP and DBP) and laboratory parameters (including blood glucose, serum creatinine, alanine aminotransferase, aspartate aminotransferase, C-reactive protein, and homocysteine) compared with the controls. A higher proportion of male patients were also found in the HFpEF group than that in the healthy control group. Hypertension, ischemic heart disease, and diabetes mellitus were the most common comorbidities in patients with HFpEF. Besides, almost all subjects were Han nationality (99.9%) from China, only one patient of HFpEF group was Mongolian Nationality. No race/ethnicity-based differences were observed. Consistent with disease prevalence, 379 (78.6%) and 267 (55.4%) patients received treatments with anti-hypertensive and antiplatelet drugs, respectively.
Fig. 1.
Flow diagram of the study. Eligible HF patients were continuously included from Feb. 2010 to Mar. 2016 for further analysis
Table 1.
Characteristics of the patients included in the study
| Control (n=494) | HFpEF(n=482) | p value | |
|---|---|---|---|
| Age, year | 57 (48–68) | 65 (57–73) | <0.001 |
| Male, n (%) | 223 (45.1) | 290 (60.2) | <0.001 |
| Echocardiography | |||
| LVEF, % | 65 (62–69) | 60 (55–65) | <0.001 |
| LVEDD, mm | 45 (43–48) | 50 (45–55) | <0.001 |
| LAESD, mm | 31 (29–34) | 40 (37–44) | <0.001 |
| LV septum thickness, mm | 9 (8–10) | 10 (9–12) | <0.001 |
| Blood pressure, mmHg | |||
| SBP | 124 (114–135) | 133 (119–151) | 0.047 |
| DBP | 78 (70–82) | 79 (69–88) | 0.007 |
| Heart rate, beats/min | 72 (66–80) | 76 (64–86) | 0.484 |
| Comorbidity | |||
| Diabetes mellitus | 0 (0) | 153 (31.7) | – |
| Hypertension | 0 (0) | 427 (88.6) | – |
| Hyperlipidemia | 0 (0) | 111 (23.0) | – |
| Arrhythmia | 0 (0) | 140 (29.1) | – |
| Stroke | 0 (0) | 61 (12.7) | – |
| Peripheral vascular AS | 0 (0) | 15 (3.1) | – |
| Family history | 0 (0) | 54 (11.3) | – |
| Ischemic | 0 (0) | 238 (49.5) | – |
| Laboratory data | |||
| NT-proBNP, pg/mL | 39.5 (24–59) | 1,027 (406–2626) | <0.001 |
| Log (NT-proBNP) | 1.60 (1.38–1.77) | 3.01 (2.61–3.42) | <0.001 |
| Total cholesterol, mmol/L | 4.38 (3.78–5.01) | 3.74 (3.17–4.34) | 0.509 |
| Total triglyceride, mmol/L | 1.19 (0.86–1.58) | 1.16 (0.80–1.84) | 0.078 |
| LDL-C, mmol/L | 2.46 (2.00–2.89) | 2.12 (1.70–2.62) | <0.001 |
| HDL-C, mmol/L | 1.27 (1.05–1.51) | 0.95 (0.79–1.15) | <0.001 |
| Blood glucose, mmol/L | 5.12 (4.79–5.59) | 5.92 (5.06–7.17) | <0.001 |
| Serum creatinine, μmol/L | 67.0 (57.0–78.0) | 82.0 (68.0–108.0) | <0.001 |
| ALT, U/L | 16.0 (12.0–21.0) | 18.0 (13.0–27.0) | 0.001 |
| AST, U/L | 20.0 (17.0–23.0) | 21.0 (17.0–30.0) | 0.004 |
| hsCRP, mg/L | 0.60 (0.30–1.23) | 3.35 (1.30–9.73) | <0.001 |
| Hcy, μmol/L | 13.39 (10.63–17.80) | 14.72 (11.57–20.00) | 0.084 |
| Medical treatment | |||
| ACE inhibitor | 0 (0) | 214 (44.4) | – |
| Beta-blocker | 0 (0) | 227 (47.1) | – |
| Diuretic | 0 (0) | 128 (26.6) | – |
| Spironolactone | 0 (0) | 131 (27.2) | – |
| Calcium antagonist | 0 (0) | 101 (21.0) | – |
| Oral anticoagulant | 0 (0) | 41 (8.5) | – |
| Digitalis | 0 (0) | 45 (9.3) | – |
| Aspirin | 0 (0) | 249 (51.7) | – |
| Other antiplatelet | 0 (0) | 160 (33.2) | – |
| Lipid-lowering | 0 (0) | 290 (60.2) | – |
| Antiarrhythmic | 0 (0) | 57 (11.8) | – |
Values are median (interquartile range) or n (%) as appropriate
LVEF left ventricular ejection fraction, LVEDD left ventricular end diastolic diameter, LV left ventricular, LA left atrium end systolic diameter, AS atherosclerosis, NT-proBNP N-terminal B-type natriuretic peptide, HDL-C high-density lipoprotein cholesterol, LDL-C low-density lipoprotein cholesterol, ALT alanine aminotransferase, AST aspartate aminotransferase, CRP C-reactive protein, Hcy homocysteine
Eicosanoid Profile in the Plasma of Patients with HFpEF
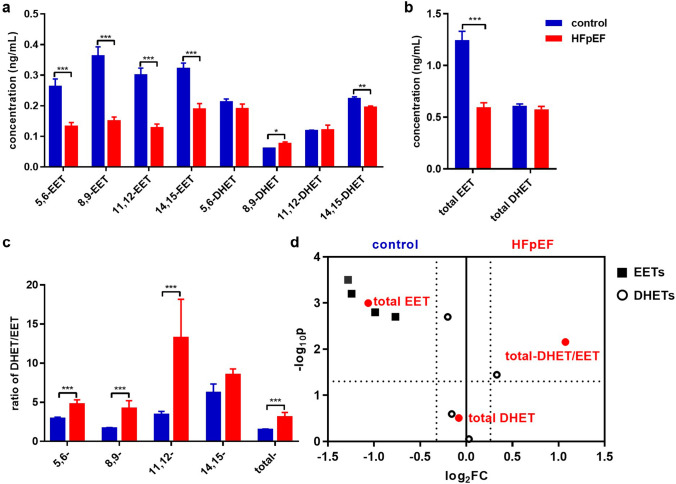
To explore the roles of eicosanoids related to the sEH enzyme, we investigated the plasma eicosanoid profile using LC–MS/MS method. Patients with HFpEF showed significantly lower levels of EETs than that of the control subjects, while total DHET levels were comparable between patients with HFpEF and the healthy subjects (Fig. 2a, b). The DHET/EET ratios were used to evaluate sEH activity (Ding et al. 2017). The DHET/EET ratios, especially total- DHET/EET (ratio of [total DHET]/[total EET]), were markedly higher in patients with HFpEF than that of the controls (Fig. 2c, d). These results suggest that sEH activity is significantly increased, indicating that the degradation of EETs was markedly enhanced via the sEH pathway in patients with HFpEF.
Fig. 2.
Soluble epoxide hydrolase (sEH) activity was elevated in patients with HFpEF. The concentration of EETs (a) and DHETs (b) in plasma of control subjects and HF patients determined by LC–MS/MS. c The relative ratio of DHET/EET was used to represent sEH activity. d Volcano plot showed the eicosanoid profile difference between HFpEF and the control group. The results are shown as means ± SEM, and p values were calculated using the Mann–Whitney test between the HFpEF group and healthy control. *p < 0.05, **p < 0.01, ***p < 0.001
Sex and age differences in eicosanoids levels could be an important underlying mechanism for the prevalence in cardiovascular diseases between male and female patients of all ages (Gerges and El-Kadi 2021). We performed binary logistic regression analysis to further identify the relationship between eicosanoids and HFpEF. After adjusting for sex and age, EETs showed a negative correlation, while DHET showed a positive correlation with HFpEF prevalence. Later, the DHET/EET ratio was included in a separate regression model and showed a positive correlation with HFpEF prevalence (Supplementary Table 1). Furthermore, a similar correlation pattern was observed for total EET, total DHET, and total DHET/EET. These results revealed increased sEH activity in patients with HFpEF, even after the adjustment of sex and age.
Furthermore, propensity score matching (PSM) was also applied to verify the clinical intergroup differences during eicosanoid profile analysis (1:1, age and sex). Similar trends of EETs and DHETs were also found in patients with HFpEF compared with the control subjects (Supplementary Table 2). The DHET/EET ratio still showed a significantly positive correlation with HFpEF prevalence (Supplementary Fig. 1a). Taken together, these results indicate higher sEH activity in plasma of patients with HFpEF of varying etiologies. As BNP has the strongest predictive power for HFpEF (Natriuretic Peptides Studies Collaboration et al. 2016), a univariate correlation analysis was also performed to determine the diagnostic power of eicosanoids. However, eicosanoids showed a weak correlation with clinical risk factors for HFpEF (the correlation coefficient was less than 0.2, Supplementary Fig. 1b), and the receiver-operating characteristic curve also showed weaker diagnostic power of eicosanoids than the well known clinical parameters (Supplementary Fig. 1c), which might suggest greater effects of sEH activity on HFpEF treatment than diagnosis.
Eicosanoid Profile in the Plasma of Patients with HFpEF of Varying Etiologies
The differences in eicosanoid profiles in HF of varying etiologies were further analyzed according to the clinical diagnosis criteria (Ponikowski et al. 2016a), and it is based on the diagnosis results of at least two senior clinicians, including ischemic, hypertrophic cardiomyopathy, dilated cardiomyopathy (DCM), hypertension, diabetes, and other related HFpEF. As shown in Supplementary Fig. 2a–c, significantly decreased EETs were observed in all types of HFpEF except DCM, while increased DHETs were only observed in patients with DCM-related HFpEF. The total sEH activity was calculated according to the ratio of total DHETs to total EETs. Interestingly, dramatically increased sEH activity was found in HFpEF of varying etiologies, especially in patients with diabetes-related HFpEF (Supplementary Fig. 2d), indicating different metabolic characteristics of the various types of HFpEF.
Prognostic Value of Eicosanoids in Patients with HF
We obtained information about the cardiac events in patients with HFpEF after 60 months of follow-up (Fig. 1). Univariate analysis of eicosanoids and clinical endpoints was performed using the Log-rank test (Table 2 and Supplementary Table 3). Previous studies have verified that endogenic EETs, especially 14,15-EET, contribute to vasodilation in various organs, including the heart (Imig and Hammock 2009; Sun et al. 2021). Interestingly, during the analysis of the prognostic value of sEH-related eicosanoids in patients with HFpEF, we observed that 14,15-DHET (hydrolyzed from 14,15-EET by the sEH enzyme) had a significant positive correlation with cardiac mortality (Log-rank = 4.887, p = 0.027) (Fig. 3a). Furthermore, higher 14,15-DHET levels were associated with an increased incidence of cardiac mortality (unadjusted HR 1.733; 95% CI 1.056–2.842; p = 0.029). After adjusting for confounders, the risk of cardiac mortality remained consistently significant in the 14,15-DHET higher group (adjusted HR 1.681; 95% CI 1.012–2.794; p = 0.045) (Table 2). In addition, the ratio of 14,15-DHET/EET also showed significantly higher levels in the 14,15-DHET high group than in the low group (Fig. 3b), which was additional proof of higher sEH activity in the 14,15-DHET high group. A subgroup analysis revealed that older patients (> 60 years) with arrhythmia showed a significantly positive correlation between high levels of 14,15-DHET and cardiac mortality (Fig. 3c). No significant correlation was observed between the secondary endpoints and eicosanoids in patients with HFpEF (Supplementary Table 3). These results indicated that enhanced degradation of EETs by the sEH enzyme positively correlated with cardiac mortality in patients with HFpEF, suggesting sEH activity as an indicator of worse prognosis, especially in older patients with HFpEF complicated with arrhythmia.
Table 2.
Unadjusted/multivariable adjusted HRs between eicosanoids and cardiac mortality in HFpEF patients during follow-up
| Cardiac mortality | Lower group | Higher group | Log-rank | p value | Univariable analysis | Multivariable analysis | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | |||||
| 5,6-EET | 43 (12.0%) | 20 (16.1%) | 2.648 | 0.104 | 1.552 | 0.909–2.650 | 0.107 | 1.494 | 0.865–2.579 | 0.150 |
| 8,9-EET | 46 (12.6%) | 17 (14.4%) | 0.155 | 0.694 | 1.118 | 0.640–1.952 | 0.695 | 1.109 | 0.625–1.967 | 0.723 |
| 11,12-EET | 47 (12.6%) | 16 (14.5%) | 0.048 | 0.827 | 1.065 | 0.603–1.881 | 0.828 | 0.993 | 0.561–1.771 | 0.993 |
| 14,15-EET | 48 (13.0%) | 15 (13.3%) | 0.124 | 0.725 | 0.901 | 0.504–1.612 | 0.726 | 0.876 | 0.483–1.590 | 0.664 |
| 5,6-DHET | 44 (12.8%) | 19 (13.7%) | 0.230 | 0.632 | 1.000 | 0.584–1.713 | 0.999 | 0.922 | 0.528–1.609 | 0.774 |
| 8,9-DHET | 42 (12.1%) | 21 (15.7%) | 0.274 | 0.600 | 1.150 | 0.680–1.944 | 0.602 | 1.134 | 0.653–1.967 | 0.655 |
| 11,12-DHET | 42 (11.9%) | 21 (16.3%) | 1.108 | 0.293 | 1.323 | 0.783–2.235 | 0.296 | 1.359 | 0.798–2.315 | 0.258 |
| 14,15-DHET | 30 (10.2%) | 33 (17.5%) | 4.887 | 0.027 | 1.733 | 1.056–2.842 | 0.029 | 1.681 | 1.012–2.794 | 0.045 |
| 5,6-DHET/EET | 46 (12.7%) | 17 (14.0%) | 0.002 | 0.969 | 0.989 | 0.566–1.727 | 0.969 | 0.988 | 0.561–1.740 | 0.967 |
| 8,9-DHET/EET | 46 (12.8%) | 17 (13.7%) | 0.007 | 0.935 | 0.977 | 0.559–1.707 | 0.935 | 0.940 | 0.533–1.656 | 0.830 |
| 11,12-DHET/EET | 46 (12.3%) | 17 (15.9%) | 0.936 | 0.333 | 1.314 | 0.753–2.293 | 0.336 | 1.435 | 0.813–2.534 | 0.213 |
| 14,15-DHET/EET | 51 (13.0%) | 12 (13.5%) | 0.040 | 0.841 | 1.067 | 0.568–2.001 | 0.841 | 1.195 | 0.629–2.270 | 0.587 |
| Total EET | 45 (12.4%) | 18 (15.1%) | 0.401 | 0.527 | 1.193 | 0.690–2.063 | 0.528 | 1.214 | 0.693-2.126 | 0.498 |
| Total DHET | 41 (12.1%) | 22 (15.5%) | 0.437 | 0.508 | 1.190 | 0.709–1.999 | 0.510 | 1.113 | 0.651–1.901 | 0.696 |
| Total DHET/total EET | 53 (12.8%) | 10 (14.5%) | 0.118 | 0.731 | 1.126 | 0.572–2.215 | 0.732 | 1.313 | 0.656-2.630 | 0.442 |
The event rate was estimated by the Kaplan–Meier method. The multivariable Cox regression was used to adjust potential confounders (including age, sex, smoking, drinking, hypertension, diabetes, dyslipidemia, peripheral atherosclerosis, stroke, arrhythmia, and coronary artery disease)
HR, hazard ratio, CI, confidence interval. Correlation is significant at the 0.05 level (2-tailed)
EET epoxyeicosatrienoic acid, DHET dihydroxyeicosatrienoic acid
Fig. 3.
The cumulative incidence of mortality in patients with HFpEF. a The cumulative incidence of mortality in patients with HFpEF with high and low levels of 14,15-DHET. High and low groups were separated from the mean value of each factor, and p values were calculated using the log-rank test. b The ratio of 14,15-DHET/EET was used to present the sEH activity between 14,15-DHET high and low groups. Mann–Whitney test was performed for intergroup comparisons. ***p < 0.001. c Adjusted HRs are shown for the composite primary endpoint and the individual endpoint of death from a cardiovascular cause for patients in 14,15-DHET high and low groups. To assess the impact of age, sex, and other factors, we also examined outcomes for patients stratified according to the subgroups indicated. The black dots and lines represent the HRs and 95% CIs, and p values were calculated by Cox proportional hazard models
TPN, a Novel sEH Inhibitor, Attenuated Cardiac Dysfunction by Reversing Cardiac Dyslipidemia
As the sEH enzyme showed therapeutic potential for HFpEF, we also investigated the protective effect of sEH inhibitors on the cardiac function of HFpEF in vivo and in vitro. Previous studies have developed a variety of potent sEH inhibitors by chemical synthesis or isolation from natural sources, and only a few of them are underway in clinical trials to treat HF (Sun et al. 2021). However, no studies have focused on the effect of sEH inhibition on HFpEF. In addition, patients with diabetic-HFpEF showed the highest DHET/EET ratio in our study, and the diabetic cardiomyopathy mouse model was used to investigate the in vivo effect of the sEH inhibitor in HFpEF. The db/db mouse at the age of 24 weeks exhibited higher body weight and increased plasma glucose levels, while comparable LVEF levels and decreased diastolic function were also found when compared with the wt group (Fig. 4a–g), meeting the diagnostic characteristics of HFpEF. More importantly, HFpEF mice showed significantly increased sEH activity compared with wt mice (Supplementary Fig. 3).
We then synthesized and screened a series of sEH inhibitors in the HFpEF mouse model and observed that sEH inhibition by TPN and AUDA showed a cardioprotective effect compared with the negative control group (wt), including recovered metabolic dysfunction (Fig. 4b, c) and diastolic cardiac function (Fig. 4e), and reduced BNP and Myosin Heavy Chain 7 (MYH7) levels (Fig. 4h). Besides, AUDA induced no significant changes in cardiac function in wt group (Supplementary Fig. 5). The sEH inhibition also induced protective morphological changes in the heart tissue of the HFpEF animal model, including decreased cardiac hypertrophy, fibrosis, and oxidative stress levels in vivo (Fig. 4i–k). In addition, sEH inhibition showed reduced neutral lipid deposition in diabetic hearts by Oil Red O staining (Fig. 5a) and decreased triglyceride, total cholesterol and LDL levels in the plasma of TPN and AUDA compared with the db/db group (Supplementary Fig. 4). More importantly, TPN had significantly better protective effects than that of AUDA in all the above aspects. These results suggested a protective effect of the sEH inhibitor on the diastolic function of the HFpEF mouse model, especially TPN.
Fig. 5.
TPN presented a better ability in lipid accumulation than AUDA in vivo, associated with modulating FFAs uptake and transport. a Oil red O staining. b The transcription level of factors related to lipid metabolism. c–e Representative images and quantification analysis of protein expression corresponding to the above key factors. Compared to control group: &&p < 0.01, &&&p < 0.001. Compared to db/db group: *p < 0.05, **p < 0.01, ***p < 0.001. Compared to db/db + AUDA group: #p < 0.05. The results are means ± SEM
Furthermore, we investigated the potential mechanism of TPN in HFpEF. TPN mainly decreased genes related to fatty acid uptake and transport (Cpt1b, Cd36, Lpl), and it was associated with glucose metabolism (Pdk4) at the transcription (Fig. 5b) and post-transcription levels (Fig. 5c–e). In addition, even if it is not statistically significant, there were increased trend of sEH activity in HFpEF patients with dyslipidemia than those without dyslipidemia (Supplementary Fig. 5). These results demonstrated that TPN mediated a better cardiac diastolic function mainly by reversing cardiac dyslipidemia in cardiomyocytes of HFpEF mouse model.
Discussion
To the best of our knowledge, this is the first study to investigate the profile of eicosanoids related to the sEH enzyme in the plasma of patients with HFpEF using targeted metabolomics, and we further investigated the prognostic value of the sEH activity in patients with HFpEF. We found an increasing trend in the sEH activity of patients with HFpEF. More importantly, cardiac mortality correlated with enhanced degradation of EETs by sEH enzyme in patients with HFpEF. The sEH inhibitor showed an excellent cardioprotective effect, especially the novel sEH inhibitor (TPN), which could also be a promising therapeutic strategy for HFpEF in future clinical settings.
It was previously reported that nearly half of all patients with HF could be classified as HFpEF type, which has different clinical characteristics from HFrEF (de Boer et al. 2018; Emdin et al. 2020; Gerber et al. 2015; Polsinelli and Shah 2017). Currently, the most globally accepted treatment strategies for HFrEF have been demonstrated as ineffective for HFpEF (Toth and Gauthier 2021). There are no generally accepted clinical therapies for treating patients with HFpEF (Kim and Park 2021; Methawasin et al. 2016). Tailoring HFpEF treatment has also been recommended clinically for years and is required urgently (Ferreira et al. 2017; Uijl et al. 2021). Our work suggests that an alternative therapeutic strategy might be the sEH inhibitor.
The sEH is identified as a susceptible gene for HF and is expected to be a therapeutic target for patients with HF (Lai and Chen 2021; Monti et al. 2008; Romashko et al. 2016). Previous study showed an elevated level of sEH in the aortic intima of both spontaneously hypertensive rats and Ang II-infused Wistar rats (Ai et al. 2007). Hypertension is reported as one of the most common risk factors for HFpEF. Control of hypertension may be the most important prevention strategy for HFpEF (Upadhya and Kitzman 2020). In the current study, we found increased sEH activity had an obvious correlation with clinical outcomes in patients with HFpEF, but the diagnostic power of eicosanoids is weaker than that of clinical parameters well known in the clinic. These results suggested placing greater emphasis on the potential role of sEH activity in future treatment strategies for patients with HFpEF.
EETs are metabolized by CYP450 enzyme from AA and then rapidly metabolized or inactivated into DHETs according to the sEH enzyme (Buczynski et al. 2009; Panigrahy et al. 2010). EETs have been reported as the major anti-inflammatory eicosanoids, especially in the cardiovascular system (Sun et al. 2021). Previous studies from our group also demonstrated the protective effects of EETs in cardiomyocytes, including reversion of cardiac dysfunction, remodeling, and fibrosis (He et al. 2017, 2015; Wang et al. 2016). A growing body of studies has reported that many cardiovascular and metabolic diseases are related to the expression of the sEH level (Sun et al. 2021). HFpEF primarily developed based on the impairment of the left ventricular microvasculature by hyperglycemia, hypertension, and inflammation in diabetic patients (D’Amario et al. 2019). Previous study also showed protective effect of EETs on diabetic cardiomyopathy (Alaeddine et al. 2021). Besides, sodium-glucose co-transporter 2 inhibitors have also been shown to improve health status in HFpEF patients (Abraham et al. 2021; Ejiri et al. 2020; Spertus et al. 2022). However, the levels of EETs and DHETs under physiological conditions or the pathophysiology function had not been elucidated in patients with HFpEF by now. Our study especially focused on the patients with HFpEF, and we found the increased activity of the sEH enzyme in patients with HFpEF, suggesting that sEH is related to worse clinical outcomes.
14,15-EET accounted for more than 63% of the regioisomeric EETs derived from AA catalyzed by CYP450 enzymes. Once synthesized, 14,15-EET could be hydrated into 14,15-DHETs by sEH in vivo (Sudhahar et al. 2010). Previous study also reported the overexpression of CYP enzyme was associated with the elevated concentration of 14,15-DHET in mice (Wang et al. 2014). The sEH knock-out mice had decreased plasma levels of 8,9-, 11,12-, and 14,15-DHET by 38%, 44%, and 67% (Lai and Chen 2021). Our study indicated the significant effect of 14,15-DHET on the prognosis of HFpEF patients. Besides, consistent with existing research results, decreased 14,15-EET and increased 14,15-DHET levels in plasma of HFpEF mouse model were found in our study. 14,15-DHET might be regarded as a metabolism biomarker for CYP-sEH pathway in the future.
The majority of eicosanoids have short half-lives (Buczynski et al. 2009). The change of sEH activity can affect metabolite levels persistently with long-term effects, and it may act as a potential therapeutic target for metabolic disorders (He et al. 2016; Lai and Chen 2021). The modification of established metabolic phenotype included metabolic enzyme activity changes, which also played important role in cardiac remodeling of failing heart (Gibb and Hill 2018). Therefore, sEH inhibition could effectively maintain the endogenous level of EETs, and the development of sEH inhibitors has been a hot research topic since the beginning of this century, especially in the area of cardiovascular and metabolic diseases (Dos Santos and Fleming 2020; Imig 2018; Romashko et al. 2016; Sun et al. 2021; Swardfager et al. 2018). Seubert et al. (2006) have demonstrated that increasing EET levels protected against LV dysfunction after ischemic injury in a mouse model. Extensive research has demonstrated the therapeutic potential of sEH inhibitors for combating detrimental cardiac remodeling and HF (Merabet et al. 2012; Swardfager et al. 2018). By far, sEH inhibitors have been reported from synthetic and natural sources from many research groups (Sun et al. 2021; Swardfager et al. 2018). Alaeddine et al. (2021) also clarified the protective effect of the sEH inhibitor (AUDA) in a diabetic cardiopathy-related HF rat model. However, no studies have reported the protective effects of sEH inhibition in HFpEF.
The sEH activity inhibition reversed the impaired diastolic dysfunction in the HFpEF mouse model in our study, indicating the protective role of sEH inhibitors in HFpEF. Unfortunately, well-documented sEH inhibitors have been characterized by poor metabolic stability, relatively high melting point, and poor solubility in water, which hinders their further pharmacological use in clinical settings (Hwang et al. 2007, 2013; Pecic et al. 2018; Sun et al. 2021; Tripathi et al. 2018; Wagner et al. 2017). TPN showed excellent cardioprotective effect in HFpEF mouse model, including a protective effect on cardiac diastolic function, reversed cardiac hypertrophy, and fibrosis. Free fatty acids (FFAs) are the primary energy substrates for the heart. Increased levels of FFAs can accelerate the pathogenesis of HFpEF. Excess circulating and cellular FFAs trigger toxic intermediates, which considerably contributed to lipotoxicity (Zhu et al. 2018). Cardiac remodeling is one of the most common pathological causes of HF, which induces myocardial dysfunction, and contributes to the morbidity and mortality of patients with HF (He et al. 2016). The novel sEH activity inhibitor, TPN, effectively reversed lipid deposition, cardiac hypertrophy, and fibrosis. HFpEF patients with dyslipidemia had increased trend of sEH activity than those without dyslipidemia in our study, indicating that lipid deposition might be a way for the effect of TPN on sEH enzyme to reverse cardiac function in HFpEF patients. Further studies are also needed to clarify the detailed mechanisms of TPN for treating HFpEF.
There are some limitations should be noticed. The subjects in our study were consecutively enrolled, so there were noticeable clinical differences among the groups. The differences in clinical parameters could be consistent with the epidemiological characteristics of HFpEF (Meta-analysis Global Group in Chronic Heart Failure (MAGGIC) 2012; Lloyd-Jones et al. 2002; Ponikowski et al. 2016b), reflecting the randomness and reliability of population selection in our study. The clinical characteristics were also adjusted for in the regression analysis. Furthermore, PSM matching (1:1, age, and sex) was used to analyze the difference in eicosanoid concentrations between the HFpEF and control groups. Although a randomized control study would have the highest power to evaluate intergroup differences, this observational study still showed valuable clinical insights and provided generalizability in practice.
Conclusions
This study demonstrated that increased sEH activity was related to cardiac mortality in patients with HFpEF, especially in older patients complicated with arrhythmia. Our study also showed treatment potential of sEH inhibitors for future clinical application in HFpEF. However, additional studies are needed to verify the detailed mechanisms of sEH in patients with HFpEF of varying etiologies and clinical characteristics.
Supplementary Information
Below is the link to the electronic supplementary material.
Abbreviations
- AA
Arachidonic acid
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- BHT
Butylated hydroxytoluene
- CIs
Confidence intervals
- CRP
C-reactive protein
- CYP450
Cytochrome P450
- DCM
Dilated cardiomyopathy
- DHETs
Dihydroxyeicosatrienoic acids
- EDTA
Ethylenediaminetetraacetic acid
- EETs
Epoxyeicosatrienoic acids
- FFAs
Free fatty acids
- HCM
Hypertrophic cardiomyopathy
- Hcy
Homocysteine
- HDL
High-density lipoprotein
- HF
Heart failure
- HFpEF
Heart failure with preserved ejection fraction
- HFrEF
HF with reduced ejection fraction
- HR
Hazard ratios
- LA
Left atrial
- LC–MS/MS
Liquid chromatography–tandem mass spectrometry
- LDL
Low-density lipoprotein
- LV
Left ventricular
- LVEF
Ejection fraction of left ventricle
- NT-proBNP
N-terminal B-type natriuretic peptide
- ORs
Odds ratios
- PSM
Propensity score matching
- SBP/DBP
Systolic/diastolic blood pressure
- sEH
Soluble epoxide hydrolase
Authors’ Contributions
LP, CC, XZ, and DW designed the experiments and interpreted the results of the manuscript. ZS, CZ and ZW performed the animal experiments. LP, XZ, LN, and CL analyzed the data. LP, ZS, XZ, and DW prepared the figures and tables. LP, ZS, CZ, KA, YW, CC, YY, YZ, HJ, XJ, and JS revised the manuscript. All the authors approved the final article.
Funding
This work was supported by grants from the National Natural Science Foundation of China (81790624 [to D.W.W.], 81900342 [to L. P.] and 81790621 [to Y. Z.]).
Data Availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of Interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Ethical Approval
The study protocols were approved by the Ethics Committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology.
Consent to Participate
Written informed consent was obtained from all the participants.
Consent for Publication
All the participants approved to publish.
Footnotes
The original online version of this article was revised: The original version of this article unfortunately contained a mistake in the corresponding authorship. Dr Chen Chen, is not properly marked in the published article although being listed as one of corresponding authors.
Liyuan Peng and Ziping Song contributed equally to this work.
Change history
11/29/2022
A Correction to this paper has been published: 10.1007/s43657-022-00088-5
Contributor Information
Chen Chen, Email: chenchen@tjh.tjmu.edu.cn.
Xu Zhang, Email: xuzhang@tmu.edu.cn.
Dao Wen Wang, Email: dwwang@tjh.tjmu.edu.cn.
References
- Abraham WT, Lindenfeld J, Ponikowski P, Agostoni P, Butler J, Desai AS, Filippatos G, Gniot J, Fu M, Gullestad L, Howlett JG, Nicholls SJ, Redon J, Schenkenberger I, Silva-Cardoso J, Störk S, Krzysztof Wranicz J, Savarese G, Brueckmann M, Jamal W, Nordaby M, Peil B, Ritter I, Ustyugova A, Zeller C, Salsali A, Anker SD. Effect of empagliflozin on exercise ability and symptoms in heart failure patients with reduced and preserved ejection fraction, with and without type 2 diabetes. Eur Heart J. 2021;42(6):700–710. doi: 10.1093/eurheartj/ehaa943. [DOI] [PubMed] [Google Scholar]
- Ai D, Fu Y, Guo D, Tanaka H, Wang N, Tang C, Hammock BD, Shyy JY, Zhu Y. Angiotensin II up-regulates soluble epoxide hydrolase in vascular endothelium in vitro and in vivo. Proc Natl Acad Sci USA. 2007;104(21):9018–9023. doi: 10.1073/pnas.0703229104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alaeddine LM, Harb F, Hamza M, Dia B, Mogharbil N, Azar NS, Noureldein MH, El Khoury M, Sabra R, Eid AA. Pharmacological regulation of cytochrome P450 metabolites of arachidonic acid attenuates cardiac injury in diabetic rats. Translat Res. 2021;235:85–101. doi: 10.1016/j.trsl.2021.03.010. [DOI] [PubMed] [Google Scholar]
- Alex L, Russo I, Holoborodko V, Frangogiannis NG. Characterization of a mouse model of obesity-related fibrotic cardiomyopathy that recapitulates features of human heart failure with preserved ejection fraction. Am J Physiol Heart Circ Physiol. 2018;315(4):H934–h949. doi: 10.1152/ajpheart.00238.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askari A, Thomson SJ, Edin ML, Zeldin DC, Bishop-Bailey D. Roles of the epoxygenase CYP2J2 in the endothelium. Prostaglandins Other Lipid Mediat. 2013;107:56–63. doi: 10.1016/j.prostaglandins.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buczynski MW, Dumlao DS, Dennis EA. Thematic Review Series: Proteomics. An integrated omics analysis of eicosanoid biology. J Lipid Res. 2009;50(6):1015–1038. doi: 10.1194/jlr.R900004-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Amario D, Migliaro S, Borovac JA, Restivo A, Vergallo R, Galli M, Leone AM, Montone RA, Niccoli G, Aspromonte N, Crea F. Microvascular dysfunction in heart failure with preserved ejection fraction. Front Physiol. 2019;10:1347. doi: 10.3389/fphys.2019.01347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer RA, Nayor M, deFilippi CR, Enserro D, Bhambhani V, Kizer JR, Blaha MJ, Brouwers FP, Cushman M, Lima JAC, Bahrami H, van der Harst P, Wang TJ, Gansevoort RT, Fox CS, Gaggin HK, Kop WJ, Liu K, Vasan RS, Psaty BM, Lee DS, Hillege HL, Bartz TM, Benjamin EJ, Chan C, Allison M, Gardin JM, Januzzi JL, Jr, Shah SJ, Levy D, Herrington DM, Larson MG, van Gilst WH, Gottdiener JS, Bertoni AG, Ho JE. Association of cardiovascular biomarkers with incident heart failure with preserved and reduced ejection fraction. JAMA Cardiol. 2018;3(3):215–224. doi: 10.1001/jamacardio.2017.4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickstein K, Cohen-Solal A, Filippatos G, McMurray JJ, Ponikowski P, Poole-Wilson PA, Strömberg A, van Veldhuisen DJ, Atar D, Hoes AW, Keren A, Mebazaa A, Nieminen M, Priori SG, Swedberg K. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM) Eur Heart J. 2008;29(19):2388–2442. doi: 10.1093/eurheartj/ehn309. [DOI] [PubMed] [Google Scholar]
- Ding Y, Li Y, Zhang X, He J, Lu D, Fang X, Wang Y, Wang J, Zhang Y, Qiao X, Gan L-M, Chen C, Zhu Y. Soluble epoxide hydrolase activation by S-nitrosation contributes to cardiac ischemia-reperfusion injury. J Mol Cell Cardiol. 2017;110:70–79. doi: 10.1016/j.yjmcc.2017.07.006. [DOI] [PubMed] [Google Scholar]
- Dos Santos LRB, Fleming I. Role of cytochrome P450-derived, polyunsaturated fatty acid mediators in diabetes and the metabolic syndrome. Prostaglandins Other Lipid Mediat. 2020;148:106407. doi: 10.1016/j.prostaglandins.2019.106407. [DOI] [PubMed] [Google Scholar]
- Dunlay SM, Roger VL, Redfield MM. Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2017;14(10):591–602. doi: 10.1038/nrcardio.2017.65. [DOI] [PubMed] [Google Scholar]
- Ejiri K, Miyoshi T, Kihara H, Hata Y, Nagano T, Takaishi A, Toda H, Nanba S, Nakamura Y, Akagi S, Sakuragi S, Minagawa T, Kawai Y, Nishii N, Fuke S, Yoshikawa M, Nakamura K, Ito H. Effect of luseogliflozin on heart failure with preserved ejection fraction in patients with diabetes Mellitus. J Am Heart Assoc. 2020;9(16):e015103. doi: 10.1161/jaha.119.015103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emdin M, Aimo A, Castiglione V, Vergaro G, Georgiopoulos G, Saccaro LF, Lombardi CM, Passino C, Cerbai E, Metra M, Senni M. Targeting cyclic guanosine monophosphate to treat heart failure: JACC review topic of the week. J Am Coll Cardiol. 2020;76(15):1795–1807. doi: 10.1016/j.jacc.2020.08.031. [DOI] [PubMed] [Google Scholar]
- Ferreira JP, Mentz RJ, Pizard A, Pitt B, Zannad F. Tailoring mineralocorticoid receptor antagonist therapy in heart failure patients: are we moving towards a personalized approach? Eur J Heart Fail. 2017;19(8):974–986. doi: 10.1002/ejhf.814. [DOI] [PubMed] [Google Scholar]
- Gerber Y, Weston SA, Redfield MM, Chamberlain AM, Manemann SM, Jiang R, Killian JM, Roger VL. A contemporary appraisal of the heart failure epidemic in Olmsted County, Minnesota, 2000 to 2010. JAMA Intern Med. 2015;175(6):996–1004. doi: 10.1001/jamainternmed.2015.0924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerges SH, El-Kadi AOS. Sex differences in eicosanoid formation and metabolism: a possible mediator of sex discrepancies in cardiovascular diseases. Pharmacol Therapeutics. 2021 doi: 10.1016/j.pharmthera.2021.108046. [DOI] [PubMed] [Google Scholar]
- Gibb AA, Hill BG. Metabolic coordination of physiological and pathological cardiac remodeling. Circ Res. 2018;123(1):107–128. doi: 10.1161/circresaha.118.312017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z, Zhang X, Chen C, Wen Z, Hoopes SL, Zeldin DC, Wang DW. Cardiomyocyte-specific expression of CYP2J2 prevents development of cardiac remodelling induced by angiotensin II. Cardiovasc Res. 2015;105(3):304–317. doi: 10.1093/cvr/cvv018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Wang C, Zhu Y, Ai DJJ. Soluble epoxide hydrolase: a potential target for metabolic diseases. J Diabetes. 2016;8(3):305–313. doi: 10.1111/1753-0407.12358. [DOI] [PubMed] [Google Scholar]
- He Z, Yang Y, Wen Z, Chen C, Xu X, Zhu Y, Wang Y, Wang DW. CYP2J2 metabolites, epoxyeicosatrienoic acids, attenuate Ang II-induced cardiac fibrotic response by targeting Galpha12/13. J Lipid Res. 2017;58(7):1338–1353. doi: 10.1194/jlr.M074229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang SH, Tsai HJ, Liu JY, Morisseau C, Hammock BD. Orally bioavailable potent soluble epoxide hydrolase inhibitors. J Med Chem. 2007;50(16):3825–3840. doi: 10.1021/jm070270t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang SH, Wecksler AT, Zhang G, Morisseau C, Nguyen LV, Fu SH, Hammock BD. Synthesis and biological evaluation of sorafenib- and regorafenib-like sEH inhibitors. Bioorg Med Chem Lett. 2013;23(13):3732–3737. doi: 10.1016/j.bmcl.2013.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imig JD. Epoxides and soluble epoxide hydrolase in cardiovascular physiology. Physiol Rev. 2012;92(1):101–130. doi: 10.1152/physrev.00021.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imig JD. Prospective for cytochrome P450 epoxygenase cardiovascular and renal therapeutics. Pharmacol Ther. 2018;192:1–19. doi: 10.1016/j.pharmthera.2018.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imig JD. Epoxyeicosanoids in hypertension. Physiol Res. 2019;68(5):695–704. doi: 10.33549/physiolres.934291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imig JD, Hammock BD. Soluble epoxide hydrolase as a therapeutic target for cardiovascular diseases. Nat Rev Drug Discovery. 2009;8(10):794–805. doi: 10.1038/nrd2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MN, Park SM. Current status of pharmacologic and nonpharmacologic therapy in heart failure with preserved ejection fraction. Heart Fail Clin. 2021;17(3):463–482. doi: 10.1016/j.hfc.2021.02.008. [DOI] [PubMed] [Google Scholar]
- Lai J, Chen C. The role of epoxyeicosatrienoic acids in Cardiac remodeling. Front Physiol. 2021;12:642470. doi: 10.3389/fphys.2021.642470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam CS, Donal E, Kraigher-Krainer E, Vasan RS. Epidemiology and clinical course of heart failure with preserved ejection fraction. Eur J Heart Fail. 2011;13(1):18–28. doi: 10.1093/eurjhf/hfq121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Jones DM, Larson MG, Leip EP, Beiser A, D'Agostino RB, Kannel WB, Murabito JM, Vasan RS, Benjamin EJ, Levy D. Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation. 2002;106(24):3068–3072. doi: 10.1161/01.cir.0000039105.49749.6f. [DOI] [PubMed] [Google Scholar]
- Meta-analysis Global Group in Chronic Heart Failure (MAGGIC) The survival of patients with heart failure with preserved or reduced left ventricular ejection fraction: an individual patient data meta-analysis. Eur Heart J. 2012;33(14):1750–1757. doi: 10.1093/eurheartj/ehr254. [DOI] [PubMed] [Google Scholar]
- Merabet N, Bellien J, Glevarec E, Nicol L, Lucas D, Remy-Jouet I, Bounoure F, Dreano Y, Wecker D, Thuillez C, Mulder P. Soluble epoxide hydrolase inhibition improves myocardial perfusion and function in experimental heart failure. J Mol Cell Cardiol. 2012;52(3):660–666. doi: 10.1016/j.yjmcc.2011.11.015. [DOI] [PubMed] [Google Scholar]
- Methawasin M, Strom JG, Slater RE, Fernandez V, Saripalli C, Granzier H. Experimentally increasing the compliance of titin through RNA binding Motif-20 (RBM20) inhibition improves diastolic function in a mouse model of heart failure with preserved ejection fraction. Circulation. 2016;134(15):1085–1099. doi: 10.1161/circulationaha.116.023003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti J, Fischer J, Paskas S, Heinig M, Schulz H, Gösele C, Heuser A, Fischer R, Schmidt C, Schirdewan A, Gross V, Hummel O, Maatz H, Patone G, Saar K, Vingron M, Weldon SM, Lindpaintner K, Hammock BD, Rohde K, Dietz R, Cook SA, Schunck W-H, Luft FC, Hubner N. Soluble epoxide hydrolase is a susceptibility factor for heart failure in a rat model of human disease. Nat Genet. 2008;40(5):529–537. doi: 10.1038/ng.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morisseau C, Goodrow MH, Newman JW, Wheelock CE, Dowdy DL, Hammock BD. Structural refinement of inhibitors of urea-based soluble epoxide hydrolases. Biochem Pharmacol. 2002;63(9):1599–1608. doi: 10.1016/s0006-2952(02)00952-8. [DOI] [PubMed] [Google Scholar]
- Natriuretic Peptides Studies C, Willeit P, Kaptoge S, Welsh P, Butterworth A, Chowdhury R, Spackman S, Pennells L, Gao P, Burgess S, Freitag D, Sweeting M, Wood A, Cook N, Judd S, Trompet S, Nambi V, Olsen M, Everett B, Kee F, Ärnlöv J, Salomaa V, Levy D, Kauhanen J, Laukkanen J, Kavousi M, Ninomiya T, Casas J-P, Daniels L, Lind L, Kistorp C, Rosenberg J, Mueller T, Rubattu S, Panagiotakos D, Franco O, de Lemos J, Luchner A, Kizer J, Kiechl S, Salonen J, Goya Wannamethee S, de Boer R, Nordestgaard B, Andersson J, Jørgensen T, Melander O, Ballantyne C, DeFilippi C, Ridker P, Cushman M, Rosamond W, Thompson S, Gudnason V, Sattar N, Danesh J, Di Angelantonio E. Natriuretic peptides and integrated risk assessment for cardiovascular disease: an individual-participant-data meta-analysis. Lancet Diabetes Endocrinol. 2016;4(10):840–849. doi: 10.1016/S2213-8587(16)30196-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355(3):251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- Panigrahy D, Kaipainen A, Greene ER, Huang S. Cytochrome P450-derived eicosanoids: the neglected pathway in cancer. Cancer Metastasis Rev. 2010;29(4):723–735. doi: 10.1007/s10555-010-9264-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecic S, Zeki AA, Xu X, Jin GY, Zhang S, Kodani S, Halim M, Morisseau C, Hammock BD, Deng SX. Novel piperidine-derived amide sEH inhibitors as mediators of lipid metabolism with improved stability. Prostaglandins Other Lipid Mediat. 2018;136:90–95. doi: 10.1016/j.prostaglandins.2018.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polsinelli VB, Shah SJ. Advances in the pharmacotherapy of chronic heart failure with preserved ejection fraction: an ideal opportunity for precision medicine. Expert Opin Pharmacother. 2017;18(4):399–409. doi: 10.1080/14656566.2017.1288717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Failure. 2016;18(8):891–975. doi: 10.1002/ejhf.592. [DOI] [PubMed] [Google Scholar]
- Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, Gonzalez-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- Qiu H, Li N, Liu JY, Harris TR, Hammock BD, Chiamvimonvat N. Soluble epoxide hydrolase inhibitors and heart failure. Cardiovasc Ther. 2011;29(2):99–111. doi: 10.1111/j.1755-5922.2010.00150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riegel B, Moser DK, Anker SD, Appel LJ, Dunbar SB, Grady KL, Gurvitz MZ, Havranek EP, Lee CS, Lindenfeld J, Peterson PN, Pressler SJ, Schocken DD, Whellan DJ. State of the science: promoting self-care in persons with heart failure: a scientific statement from the American Heart Association. Circulation. 2009;120(12):1141–1163. doi: 10.1161/circulationaha.109.192628. [DOI] [PubMed] [Google Scholar]
- Rodionova OY, Pomerantsev AL. Detection of outliers in projection-based modeling. Anal Chem. 2020;92(3):2656–2664. doi: 10.1021/acs.analchem.9b04611. [DOI] [PubMed] [Google Scholar]
- Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev. 2002;82(1):131–185. doi: 10.1152/physrev.00021.2001. [DOI] [PubMed] [Google Scholar]
- Romashko M, Schragenheim J, Abraham NG, McClung JA. Epoxyeicosatrienoic acid as therapy for diabetic and ischemic cardiomyopathy. Trends Pharmacol Sci. 2016;37(11):945–962. doi: 10.1016/j.tips.2016.08.001. [DOI] [PubMed] [Google Scholar]
- Seubert JM, Sinal CJ, Graves J, DeGraff LM, Bradbury JA, Lee CR, Goralski K, Carey MA, Luria A, Newman JW, Hammock BD, Falck JR, Roberts H, Rockman HA, Murphy E, Zeldin DC. Role of soluble epoxide hydrolase in postischemic recovery of heart contractile function. Circ Res. 2006;99(4):442–450. doi: 10.1161/01.Res.0000237390.92932.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spertus JA, Birmingham MC, Nassif M, Damaraju CV, Abbate A, Butler J, Lanfear DE, Lingvay I, Kosiborod MN, Januzzi JL. The SGLT2 inhibitor canagliflozin in heart failure: the CHIEF-HF remote, patient-centered randomized trial. Nat Med. 2022;28(4):809–813. doi: 10.1038/s41591-022-01703-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhahar V, Shaw S, Imig JD. Epoxyeicosatrienoic acid analogs and vascular function. Curr Med Chem. 2010;17(12):1181–1190. doi: 10.2174/092986710790827843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun CP, Zhang XY, Morisseau C, Hwang SH, Zhang ZJ, Hammock BD, Ma XC. Discovery of soluble epoxide hydrolase inhibitors from chemical synthesis and natural products. J Med Chem. 2021;64(1):184–215. doi: 10.1021/acs.jmedchem.0c01507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swardfager W, Hennebelle M, Yu D, Hammock BD, Levitt AJ, Hashimoto K, Taha AY. Metabolic/inflammatory/vascular comorbidity in psychiatric disorders; soluble epoxide hydrolase (sEH) as a possible new target. Neurosci Biobehav Rev. 2018;87:56–66. doi: 10.1016/j.neubiorev.2018.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth PP, Gauthier D. Heart failure with preserved ejection fraction: strategies for disease management and emerging therapeutic approaches. Postgrad Med. 2021;133(2):125–139. doi: 10.1080/00325481.2020.1842620. [DOI] [PubMed] [Google Scholar]
- Tripathi N, Paliwal S, Sharma S, Verma K, Gururani R, Tiwari A, Verma A, Chauhan M, Singh A, Kumar D, Pant A. Discovery of novel soluble epoxide hydrolase inhibitors as potent vasodilators. Sci Rep. 2018;8(1):14604. doi: 10.1038/s41598-018-32449-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uijl A, Savarese G, Vaartjes I, Dahlström U, Brugts JJ, Linssen GCM, van Empel V, Brunner-La Rocca HP, Asselbergs FW, Lund LH, Hoes AW, Koudstaal S. Identification of distinct phenotypic clusters in heart failure with preserved ejection fraction. Eur J Heart Fail. 2021 doi: 10.1002/ejhf.2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhya B, Kitzman DW. Heart failure with preserved ejection fraction: new approaches to diagnosis and management. Clin Cardiol. 2020;43(2):145–155. doi: 10.1002/clc.23321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner KM, McReynolds CB, Schmidt WK, Hammock BD. Soluble epoxide hydrolase as a therapeutic target for pain, inflammatory and neurodegenerative diseases. Pharmacol Ther. 2017;180:62–76. doi: 10.1016/j.pharmthera.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Ni L, Yang L, Duan Q, Chen C, Edin ML, Zeldin DC, Wang DW. CYP2J2-derived epoxyeicosatrienoic acids suppress endoplasmic reticulum stress in heart failure. Mol Pharmacol. 2014;85(1):105–115. doi: 10.1124/mol.113.087122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Zeng H, Wen Z, Chen C, Wang DW. CYP2J2 and its metabolites (epoxyeicosatrienoic acids) attenuate cardiac hypertrophy by activating AMPKα2 and enhancing nuclear translocation of Akt1. Aging Cell. 2016;15(5):940–952. doi: 10.1111/acel.12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z, Xu F, Huse LM, Morisseau C, Draper AJ, Newman JW, Parker C, Graham L, Engler MM, Hammock BD, Zeldin DC, Kroetz DL. Soluble epoxide hydrolase regulates hydrolysis of vasoactive epoxyeicosatrienoic acids. Circ Res. 2000;87(11):992–998. doi: 10.1161/01.res.87.11.992. [DOI] [PubMed] [Google Scholar]
- Zhang X, Yang N, Ai D, Zhu Y. Systematic metabolomic analysis of eicosanoids after omega-3 polyunsaturated fatty acid supplementation by a highly specific liquid chromatography-tandem mass spectrometry-based method. J Proteome Res. 2015;14(4):1843–1853. doi: 10.1021/pr501200u. [DOI] [PubMed] [Google Scholar]
- Zhu N, Jiang W, Wang Y, Wu Y, Chen H, Zhao X. Plasma levels of free fatty acid differ in patients with left ventricular preserved, mid-range, and reduced ejection fraction. BMC Cardiovasc Disord. 2018;18(1):104. doi: 10.1186/s12872-018-0850-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during the current study are available from the corresponding author on reasonable request.