Abstract
Cancer metastasis is the major cause of cancer-related deaths and accounts for poor therapeutic outcomes. A metastatic cascade is a series of complicated biological processes. N6-methyladenosine (m6A) is the most abundant and conserved epitranscriptomic modification in eukaryotic cells, which has great impacts on RNA production and metabolism, including RNA splicing, processing, degradation and translation. Accumulating evidence demonstrates that m6A plays a critical role in regulating cancer metastasis. However, there is a lack of studies that review the recent advances of m6A in cancer metastasis. Here, we systematically retrieved the functions and mechanisms of how the m6A axis regulates metastasis, and especially summarized the organ-specific liver, lung and brain metastasis mediated by m6A in various cancers. Moreover, we discussed the potential application of m6A modification in cancer diagnosis and therapy, as well as the present limitations and future perspectives of m6A in cancer metastasis. This review provides a comprehensive knowledge on the m6A-mediated regulation of gene expression, which is helpful to extensively understand the complexity of cancer metastasis from a new epitranscriptomic point of view and shed light on the developing novel strategies to anti-metastasis based on m6A alteration.
Keywords: Cancer metastasis, m6A, Epitranscriptomic modification, RNA metabolism, Organ-specific metastasis
Introduction
Cancer has ranked as the second leading cause of death worldwide, which has become a global burden and threat to human health. Cancer metastasis is the principal cause of high mortality rate, and approximately over 90% of cancer patients die of metastasis. Metastasis is a complex biological process including epithelial–mesenchymal transition (EMT), angiogenesis, intravasation and extravasation, and ultimately metastatic outgrowth. Despite great advances in cancer biology, our current knowledge on cancer metastasis is still poor. At present, there is still a lack of available measures to monitor early metastasis and target cancer metastasis for effective therapy. Therefore, it is necessary to enrich our understanding of the progression of cancer metastasis.
Accumulating evidence demonstrates that cancer cells undergo a series of complicated genetic, epigenetic and epitranscriptomic alterations during the process of metastasis. A variety of genomic variations, such as DNA methylation, histone modifications and chromatin remodeling, have been extensively studied in cancer development. With the advances of next-generation sequencing, RNA modifications, such as N6-methyladenosine (m6A), N1-methyladenosine (m1A), 3-methylcytosine (m3C), 5-methylcytosine (m5C), N1-methylguanosine (m1G), 7-methylguanosine (m7G), and N4-acetylcytidine (ac4C), have come to prominence and become a hotspot field in cancer (Barbieri and Kouzarides 2020; Roundtree et al. 2017). These highly decorated RNAs with different modifiers are an efficient pathway to regulate gene expression and execute biological functions. Among these chemical RNA modifications, N6-methyladenosine (m6A) is the most abundant and conserved epitranscriptomic alteration in eukaryotic cells, which plays a critical role in cancer metastasis.
In this review, we first systematically summarized the mechanisms of how m6A regulates metastasis in different cancers. Moreover, we also highlighted the limits and perspectives of m6A-related researches and discussed the potential application of the m6A axis in cancer diagnosis and therapy.
Cancer Metastasis Is a Complicated Process
Metastasis is the major cause of lethality for cancer patients. Despite the great advances in cancer biology, when and how cancer metastasis occurs remain largely unknown. Cancer metastasis is an extremely complicated process with multiple factors participating in it and multiple pathways being regulated. First, depolarization is triggered and cancer cells undergo EMT, acquiring invasive properties. Next, the cells successively promote angiogenesis for intravasation to the blood vessels. After successfully surviving the attacks in the circulation, the cells go through extravasation, reside on distant organs, and eventually form metastatic lesions (Gao et al. 2019; Obenauf and Massagué 2015; Suhail et al. 2019).
Cancer metastasis is not a random procedure. Clinically, the organ specificity of metastasis has been recognized early. For example, breast cancers have a propensity to metastasize to bone, small cell lung cancers are prone to brain metastasis, and colon cancers prefer to metastasize in the liver. Along this line, metastasis is highly purposeful and selective (Peinado et al. 2017). In 1889, Paget proposed the “seed-and-soil” hypothesis, which suggested that cancer cells (seeds) can live and grow only when they fall on congenial soil (Paget 1889). Based on this observation, numerous researchers have conducted more in-depth studies on the mechanism of organ-specific metastasis, and proposed that metastasis requires the coordination of cancer cells and the microenvironment. During the process, cancer cells not only gradually adapt to the new microenvironment, but also modify the environment via complex interaction through cytokines, metabolites or certain phenotypical features (Bos et al. 2009; Hoshino et al. 2015; Jin et al. 2020b; Kaplan et al. 2005). Previous analyses have indicated that the subtypes with different histological or molecular characteristics tend to colonize at different locations (Fumagalli et al. 2020; Laughney et al. 2020). The dynamic bidirectional process may be the essence of organ-specific metastasis. In addition to the famous “seed-and-soil theory”, Kaplan et al. first proposed the “pre-metastatic niches” hypothesis in 2005, which suggested that primary tumors induce the formation of pre-determined microenvironments in distant sites to facilitate survival and proliferation of the unreached cancer cells (Kaplan et al. 2005). For instance, the soluble factor, vascular endothelial growth factor (VEGF), is released from the primary tumor and then enters into the circulatory system. After arriving at the remote target organ, VEGF induces the inherent fibroblasts to produce fibronectin, which can mobilize and recruit VEGFR+VLA-4+ hematopoietic progenitor cells (HPCs) to the target organ. In this microenvironment, the HPCs interact with the fibroblasts and increase the expression of stromal cell-derived factor-1 (SDF-1) and matrix metallopeptidase 9 (MMP-9). SDF-1 promotes the adhesion of CXCR4+ circulating tumor cells (CTCs) at the target site, while MMP-9 is beneficial for remodeling the local microenvironment to make it more suitable for the colonization and growth of CTCs (Kaplan et al. 2005). Subsequently, with the efforts of the researches, some other tumor-derived soluble factors, membrane vesicles, exosomes, and recruited bone marrow-derived cells with functions in line with the “pre-metastatic niches” hypothesis have been successively identified and further confirmed (Liu et al. 2016b; Murgai et al. 2017; Olmeda et al. 2017; Zeng et al. 2018). The new concept is consistent with the previous “seed-and-soil” hypothesis, but it extends a more dynamic perspective of metastasis that cancer cells have not yet arrived at the target lesions. In this concept, the primary tumor sends special envoys to the specific site, catalyzing the formation of a specific “soil” (pre-metastatic niche), which determines the adhesion, colonization, and growth of CTCs and becomes the critical “speed-limiting” node for the formation of target organ metastases.
Recently, tumor dormancy, highly consistent with the “seed-and-soil” hypothesis, has been emphasized in metastasis by numerous researchers (Phan and Croucher 2020). As early as 1954, Hadfield used the term “dormancy” to describe malignant cancer cells that survive for a long time without significant proliferation (Hadfield 1954). The definition of tumor dormancy covers two scenarios: a solitary cell that enters the G0 phase to undergo cell cycle arrest; or a small cluster of cells having a constant population due to an equal rate of proliferation and apoptosis (Phan and Croucher 2020). Although the two scenarios are more or less the result of the interaction between the tumor and its microenvironment, single-cell dormancy that remains dormant or non-proliferating in unsuitable soil is more consistent with the “seed-and-soil” hypothesis. However, unlike the static perspective proposed by Paget (Paget 1889), dormant cells, after going through the incubation period, can be reactivated and proliferate to metastasize with clinical manifestations (Correia et al. 2021; Vera-Ramirez et al. 2018).
Many studies have indicated that cancer cells undergo a series of complicated genetic, epigenetic and epitranscriptomic alterations during metastasis, such as the well-known genomic variations, DNA methylation, histone modifications and chromatin remodeling (Audia and Campbell 2016; Calabrese et al. 2020; Jones et al. 2016; Klutstein et al. 2016; Koch et al. 2018). Recently, epitranscriptomic alterations, especially the m6A modification of RNA, have become a novel scientific hotspot in the cancer metastasis field.
Critical Components of the m6A Modification Machinery
There are hundreds of chemical modifications that have been identified in RNA, such as N1-methyladenosine (m1A), N6-methyladenosine (m6A), 5-methylcytosine (m5C), 3-methylcytosine (m3C), N1-methylguanosine (m1G), N7-methylguanosine (m7G), N4-acetylcytidine (ac4C) (Barbieri and Kouzarides 2020). With the improvement of high-throughput sequencing approaches, the m6A decoration has become the best characterized epitranscriptomic alteration at present (Barbieri and Kouzarides 2020).
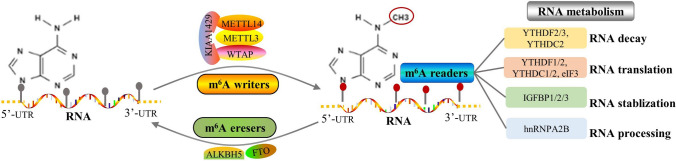
It is reported that m6A is the most abundant and conserved RNA modification in eukaryotic cells. This type of RNA modification is catalyzed by an installed m6A machinery composed of multiple methyltransferases, demethylases and m6A-binding proteins (He et al. 2019). Methyltransferases are usually called as “writers” that are responsible for methylating the N6 of adenosine. At present, the well-studied m6A writers contain methyltransferase-like 3 (METTL3), METTL14, METTL16, Wilms tumor 1-associated protein (WTAP), RBM15/15B and KIAA1429. The m6A modification is a dynamic and reversible process that can be removed by specific RNA demethylases, such as fat mass and obesity-associated protein (FTO) and alkB homolog 5 (ALKBH5). Herein, FTO and ALKBH5 are also termed as “erasers” of m6A. The functions and mechanisms of m6A modifications are usually recognized and deciphered by various m6A RNA-binding proteins called “readers”. Many protein family members have been identified as m6A “readers”, such as the members of YTH domain-containing family (YTHDFs and YTHDCs), insulin-like growth factor 2 mRNA-binding protein family (IGF2BP), eukaryotic initiation factor EIF3, heterogeneous nuclear ribonucleoproteins A2/B1 (HNRNPA2B1) and prolinerich and coiledcoilcontaining protein 2A (PRRC2A). YTH domains can directly bind to the m6A site of the RNA, and the other readers may bind to the surrounding unfolded RNA. The “writer–eraser–reader” system of m6A can determine the fate of the target RNA through regulating its transcription, processing, splicing, RNA stability and translation. The m6A regulation is described in Fig. 1.
Fig.1.
The regulatory machinery of m6A modification. The diagram showed the m6A machinery. The m6A writers mainly contain METTL3, METTL14, WTAP and KIAA1429, which are responsible for methylating at the N6 position of adenosine. The m6A eraser is composed of RNA demethylases such as FTO and ALKBH5 to remove m6A modification from target RNAs. The m6A reader is essential to recognize and decipher m6A modification, which may determine RNA fate to undergo decay, translation, RNA stabilization or RNA processing. The well-documented m6A readers include YTHDF1/2/3, YTHDC1/2/3, IGF2BP1/2/3, and hnRNPA2B1
There are some genomic alterations on m6A regulators. In hepatocellular carcinoma, we have reported that m6A regulator genes undergo a prevalent alteration of copy number variation (CNV) via analyzing the TCGA database. YTHDF3, CBLL1, IGF2BP1/3, HNRNPA2B1, KIAA1429, and YTHDF1 are found to display high frequency of CNVs in HCC (Shen et al. 2020). Wang et al. reported that HCC patients with any mutation of the m6A regulators may suffer from shorter overall survival (OS) and disease-free survival (DFS) (Wang et al. 2020d), and METTL16 or ALKBH5 deletion may predict poor OS and DFS in HCC (Wang et al. 2020d). In some other tumors, such as head and neck squamous cell carcinoma (HNSCC), uterine corpus endometrial carcinoma (UCEC), clear cell renal cell carcinoma (ccRCC) and bladder urothelial carcinoma, m6A regulators are characterized by rare somatic mutations. Although most of the writer and eraser genes tend toward loss of copy number in HCC and HNSCC, the reader genes, such as YTHDC1, YTHDC2, YTHDF3 and IGF2BP2, tend toward gain of copy number in HNSCC, UCEC and ccRCC (Wang et al. 2021b; Wang et al. 2020g; Zhou et al. 2019; Zhou et al. 2020). However, whether these genomic alterations might contribute to aberrant expression and dysfunctions of these m6A regulators needs to be further illustrated.
In contrast to the m6A, the other modifications of RNA have not been extensively studied due to the limitation of sequencing methods and the lack of specific antibodies. For instance, the m1A modification, which is mainly found in tRNA and rRNA but few in cytosolic mRNA, usually depends on TRMT10A and TRM61 complex to add methyl group. The YTH protein family is involved in recognizing the m1A modification, whereas ALKBH1 and ALKBH3 are responsible for removing the methyl group (Chen et al. 2019; Dai et al. 2018; Liu et al. 2016a; Saikia et al. 2010). The m5C is found in a wide range of RNAs, including rRNA, tRNA, mRNA, ncRNA and enhancer RNA. Recent investigation showed that the m5C, especially in tRNA, mainly functions as a structural stability regulator to promote translation accuracy (Yang et al. 2017). NSUN family members (NSUN1 to NSUN7) and DNA methyltransferase-like 2 (DNMT2) are involved in m5C methylation, and ALYREF accounts for binding and recognizing the modification, but which serves as the eraser of m5C remains largely unknown. As for the m3C modification, there are four m3C methyltransferase-like proteins (METTL2A, METTL2B, METTL6, and METTL8) and two m3C demethylases (ALKBH3 demethylating tRNAs and ALKBH1 demethylating mRNA) that have been reported (Cui et al. 2021). The m1G alteration is found mainly in eukaryotic tRNAs, and is frequently catalyzed by Trm5 and Trm10 at the position 37 and at the position 9, respectively (Jin et al. 2019b). The m7G is identified in mRNA, tRNA and rRNA, and may participate in the cap structure formation and protein translation (Barbieri and Kouzarides 2020). Alexandrov et al. found that m7G on tRNA is triggered by the METTL1-WDR4 complex, while m7G in rRNA is mediated by WBSCR22 protein (Alexandrov et al. 2002; Haag et al. 2015). The readers and erasers of m7G need to be further elucidated (Ramanathan et al. 2016). In addition to the methylation on RNAs, the ac4C modification is the first acetylation event that is found in mRNA, and successively reported in tRNAs and rRNAs. And it is catalyzed by a single enzyme NAT10. A recent study addressed that the ac4C on mRNA can promote mRNA stability and enhance the translation efficiency (Arango et al. 2018).
Accumulating evidence has demonstrated that the m6A machinery plays important roles in several physiological and pathological pathways, which have been extensively investigated in embryogenesis, neurogenesis and cancer development (He et al. 2019; Yoon et al. 2017; Zhao et al. 2017). Some of the components of the m6A machinery have promising potentials for clinical translation to become ideal diagnostic biomarkers and therapeutic targets. In this review, we mainly focus on the regulatory functions and mechanism of m6A in cancer metastasis.
The Functions and Mechanisms of m6A Modification in Metastasis of Various Cancers
M6A Modification and Hepatocellular Carcinoma Metastasis
Hepatocellular carcinoma (HCC) is one of the most common malignancies worldwide with high incidence and mortality. Metastasis accounts for the majority of deaths in HCC. The m6A modification plays an essential role in the metastasis of HCC.
The m6A writer METTL14 is downregulated in HCC, especially in the metastatic samples. METTL14 can form a complex with the microprocessor protein DGCR8 to regulate m6A-dependent miR-126 processing, which inhibits HCC metastasis (Ma et al. 2017). Another study showed that EGFR is the direct target of METTL14. METT14 can block EGFR/PI3K/AKT signaling axis to suppress EMT, metastasis and invasion of HCC (Shi et al. 2020b). KIAA1429 is another critical methyltransferase involved in m6A modifications. KIAA1429 promotes tumor growth and metastasis by downregulating GATA3 expression in HCC. Mechanistically, KIAA1429 can disassociate HuR from GATA3 pre-mRNA by inducing m6A modification at the 3' UTR, destabilizing GATA3 pre-mRNA and reducing translation. Moreover, lncRNA GATA3-As, derived from the antisense GATA3 transcript, facilitates the association between KIAA1429 and GATA3 pre-mRNA, which further enhance the pro-metastatic capacity of KIAA1429 (Lan et al. 2019). CircDLC1 is another target of KIAA1429-mediated m6A in HCC, which inhibits metastasis via abolishing the interaction between HuR and MMP1 mRNA, thus reducing MMP1 expression (Liu et al. 2021).
The m6A readers from the YTHDF protein family are widely involved in HCC metastasis. For instance, YTHDF1 binds to m6A-modified ATG2A and ATG14 mRNAs to increase their translation, thereby inducing autophagy and metastasis under hypoxia (Li et al. 2021a). A recent study reported that sublethal heat stress can elevate m6A binding near the 5’UTR of the EGFR mRNA, which facilitates the association of YTHDF1 to improve EGFR protein output. The activation of the m6A-YTHDF1-EGFR axis contributes to HCC metastasis after insufficient radiofrequency ablation treatment (Su et al. 2021). The other homolog, YTHDF2, modulates m6A binding at 5’UTR of the OCT4 mRNA to augment OCT4 translation, thus sustaining cancer stem cell properties and promoting metastasis in HCC (Zhang et al. 2020a). Wang et al. demonstrated that YTHDF3 can stabilize Zeb mRNA to induce HCC cell migration in an m6A-dependent manner (Wang et al. 2020c).
The m6A erasers also extensively participate in HCC metastasis via inducing mRNA demethylation. For example, FTO can modulate the m6A demethylation of cancer stem cell (CSC)-related genes including SOX2, KLF4 and NANOG, which increases the expressions of these genes to maintain stemness and metastasis (Bian et al. 2021). The upregulated oncoprotein AMD1 stabilizes the interaction between IQGAP1 and FTO, to prevent ubiquitination-mediated degradation of FTO, thereby enhancing HCC metastasis (Bian et al. 2021).
M6A Methylation and Colorectal Cancer Metastasis
Colorectal cancer (CRC) is the second leading cause of cancer-related deaths because of the high rate of metastasis and recurrence (Bray et al. 2018). Emerging investigations indicate that m6A modification and regulators exert pivotal roles in modulating the metastasis of CRC.
Similar to HCC, the m6A writer METTL14 is dramatically downregulated in CRC, which is associated with shorter survival. METTL14 mediates m6A methylation of SOX4 mRNA and facilitates the degradation of m6A-SOX4 mRNA in a YTHDF2-dependent manner. SOX4 has an oncogenic capacity to drive EMT and migration by activating the PI3K/AKT signaling pathway. Decreased expression of METTL14 markedly promotes invasion and migration of CRC via increasing SOX4 expression (Chen et al. 2020a). This study also found that histone demethylase KDM5C-mediated demethylation of H3K4me3 at the METTL14 promoter may account for the low transcription of METTL14 in CRC (Chen et al. 2020a). Another study reported that MeCP2 can associate with METTL14 to decrease m6A modification of the tumor suppressor KLF14 mRNA, thus reducing KLF14 expression and promoting CRC metastasis (Wang et al. 2021a). MTTL14 can mediate m6A modification of non-coding RNA, as well as coding mRNAs, in CRC progression. For example, low expression of METTL14 remarkably abolishes m6A deposition of lncRNA XIST and increases XIST expression to promote tumorigenicity and metastasis of CRC cells (Yang et al. 2020a). METTL14 can modulate the processing of miR-375 via m6A methylation, thereby increasing the expression of miR-375 targets, including YAP1 and SP1 to facilitate the metastasis of CRC (Chen et al. 2020b).
METTL3 serves as an important m6A writer that is frequently upregulated in CRC and promotes metastasis via m6A methylation (Hou et al. 2021a; Li et al. 2019). SOX2 and HMGA1 are the targets of METTL3. The m6A reader, IGF2BP2, can recognize m6A-SOX2 RNA and m6A-HMGA1 to increase their protein output, thereby driving CRC metastasis (Hou et al. 2021a; Li et al. 2019). Additionally, METTL3 can induce circ1662 expression by increasing m6A modifications in its flanking region. Circ1662 can bind to YAP1 and accelerate its nuclear accumulation to increase SMAD3 expression, thereby promoting invasion and migration of CRC (Chen et al. 2021a). Wu et al. reported that m6A-induced lncRNA RP11 mediates the dissemination of CRC cells (Wu et al. 2019). METTL13 is responsible for the m6A deposition and the demethylase ALKBH5 can reduce RP11 expression. The m6A reader hnRNPA2B1 forms a complex with lncRNA Rp11 and other target mRNAs, such as two E3 ligases Siah-1 and Fbx45 mRNAs, and subsequently reduces their translation. As a result, ZEB1 is protected from ubiquitin-mediated degradation by E3 ligases Siah-1 and Fbx45 to trigger metastasis (Wu et al. 2019).
M6A Modification and Gastric Cancer Metastasis
Gastric cancer (GC) is characterized as one of the most invasive malignancies, ranking as the third most deadly cancer worldwide (Bray et al. 2018). The regulatory roles of m6A modification in GC metastasis have attracted increasing attention.
The m6A writer METTL3 has been extensively studied in GC metastasis and progression. The oncogene ZMYM1 was identified as a target of METTL3 for m6A modification, and the m6A-ZMYM1 mRNA is recognized by the reader HuR to enhance its stability and argument translation. ZMYM1 recruits CtBP/LSD1/CoREST transcriptional complex to repress E-cadherin expression, and thus induces EMT and metastasis in GC (Yue et al. 2019). Recently, Wang et al. revealed that METTL3 was involved in tumor growth and liver metastasis of GC (Wang et al. 2020e). Mechanistically, METTL13 modulates m6A deposition of HDGF mRNA, and the reader protein IGF2BP3 enhances HDGF mRNA stability to increase the protein expression. The secreted HDGF facilitates angiogenesis, while the nuclear HDGF transcriptionally activates glycolysis by driving GLUT4 and ENO2 expression (Wang et al. 2020e). In addition to increasing oncogene expression, METTL3 decreases tumor suppressor gene expression levels. The tumor suppressor BATF2 interacts with and improves P53 protein stability to inhibit ERK phosphorylation and activation. METTL3 reduces BATF2 expression in an m6A-dependent pathway, thereby activating the ERK pathway and inducing metastasis of GC (Xie et al. 2020b). Of note, some upstream regulators are responsible for METTL3 activation in GC. For instance, P300-mediated H3K27 acetylation within the METTL13 promoter region can trigger its transcription (Wang et al. 2020e). MiR-338-5p targets METTL3 reduction to inhibit metastasis, but miR-388-5p frequently undergoes methylated silence by EED in GC progression (Zhang et al. 2021).
Besides m6A readers HuR and IGF2BP3, high-expressed reader YTHDF1 also participates in metastasis of GC and indicates poor prognosis and shorter survival time (Chen et al. 2021d). YTHDF1 can facilitate USP14 translation via the m6A-modification, thereby inducing tumor growth and metastasis (Chen et al. 2021d).
The m6A erasers ALKBH5 and FTO exert vital roles in promoting metastasis of GC. ALKBH5 can bind to and demethylate m6A modification of the lncRNA NEAT1, which increases the expression of the oncogene EZH2 to facilitate metastasis in GC (Zhang et al. 2019). A recent study reported that WNT7B reduces FTO expression to elevate TCF7L2 mRNA expression in an m6A-dependent manner, stimulating the Wnt/β-catenin signaling axis to reinforce WNT7B expression (Gao et al. 2021). The positive feedback loop WNT7B/m6A-TCF7L2/β-catenin pathway firmly promotes GC cancer progression and metastasis (Gao et al. 2021).
M6A Deposition and Pancreatic Cancer Metastasis
Pancreatic cancer is one of the most lethal cancers with a 5-year survival rate of no more than 5% (Bray et al. 2018). The m6A deposition is markedly increased in 70% of the pancreatic cancer specimens and exerts critical roles in regulating metastasis (Wang et al. 2020b).
The m6A writer METTL14 is upregulated and stimulates tumor growth and metastasis in pancreatic cancer. Further studies indicated that METTL14 mediates the m6A modification of PERP to accelerate mRNA turnover and decrease PERP protein level, thereby aggravating cancer metastasis and progression (Wang et al. 2020b). The reader YTHDC1 inhibits tumorigenesis and metastasis through the miR-30d/RUNX1/SLC2A/HK pathway in pancreatic cancer (Hou et al. 2021b). Mechanistically, the downregulation of YTHDC1 reduces the biogenesis of miR-30d in an m6A-dependent manner, and thus triggers the RUNX1-induced Warburg effect to induce tumor growth and metastasis in pancreatic cancer (Hou et al. 2021b). The m6A eraser ALKBH5 is downregulated in pancreatic cancer, which can inhibit motility via demethylating the lncRNA KCNK15-AS1 (He et al. 2018).
A recent study found that aberrant alternative splicing can regulate m6A activation in pancreatic ductal adenocarcinoma (PDAC) (Chen et al. 2021c). CLK1 kinase mediates the phosphorylation of SR-like splicing factors5250−Ser (SRSF5250−Ser), which suppresses METTL14 exon10 and Cyclin L2 exon6.3 skipping events. The aberrant splicing granted high METTL14 stronger activity to enhance m6A modifications and facilitate metastasis. Meanwhile, the aberrant Cyclin L2 splicing promotes proliferation and tumor growth in PDAC (Chen et al. 2021c).
M6A Methylation and Breast Cancer Metastasis
Breast cancer has become the second leading cause of cancer-associated mortality among women worldwide (Bray et al. 2018). The 5-year survival rate of metastatic breast cancer patients is only approximately 25%. Breast cancer prefers to metastasize to lung and brain. The roles of m6A modification in breast cancer metastasis have been extensively investigated.
In a breast cancer cell model with a high potential of lung metastasis, the m6A writer METTL3 displays increased expression, whereas the m6A eraser FTO is downregulated (Chen et al. 2021b). KRT7 was identified as the critical effector of m6A-mediated lung metastasis of breast cancer. A mechanistic study demonstrated that METTL3 induces m6A methylation at the A877 residue of KRT7-AS, which stabilizes the KRT7-AS and KRT7 mRNA duplex via IGF2BP1/HuR complexes. Additionally, the downregulation of FTO enhances the methylation of the A950 residue at the exon 6 of KRT7 to promote translation elongation by recruiting YTHDF1 and eEF1 factors toward KRT7 mRNA (Chen et al. 2021b). The cancer stem cell regulator SOX2 is the downstream effector of METTL3 in breast cancer as well (Xie et al. 2021). METTL3 induces m6A deposition of the SOX2 mRNA and increases its protein production via the IGFBP2 reader, which enhances the cancer stem cell properties and promotes invasion and migration of breast cancer (Xie et al. 2021). In contrast to the aforementioned upregulatory and oncogenic roles of METTL3, Shi et al. found that METTL3 is downregulated in breast cancer, which decreases the m6A level of COL3A1 and increases its expression, leading to metastasis of breast cancer cells (Shi et al. 2020a). These controversial results may be attributable to the highly heterogeneous characterization and different subtypes of breast cancer.
The m6A reader YTHDF3 exerts an important role in modulating the interplay of cancer cells with the brain microenvironment and ultimately leads to brain metastasis (Chang et al. 2020). Mechanistically, the upregulation of YTHDF3 promotes the translation of metastasis-related factors, including ST6GALNAC5, GJA1, and EGFR, in an m6A-dependent manner. These factors help cancer cells to break the blood–brain barrier, stimulate angiogenesis and grow in brain. The high expression of YTHDF3 in metastatic breast cancer is due to its gene copy number amplification and YTHDF3 self-regulation through m6A-dependent translation at its 5’UTR (Chang et al. 2020).
The m6A erasers ALKBH5 and FTO have impacts on the metastasis of breast cancer. The hypoxia-inducible factor triggers ALKBH5 transcription and expression. Consequently, overexpression of ALKBH5 decreases NANOG mRNA methylation at the 3'-UTR and increases NANOG protein levels, which promotes the cancer stem cell properties and aggravates breast cancer progression (Zhang et al. 2016). FTO is upregulated in breast cancer and associated with a poor prognosis. FTO induces the demethylation of tumor suppressor BNIP3 mRNA in its 3’UTR and triggers its degradation, promoting tumor growth and metastasis (Niu et al. 2019). Additionally, FTO modulates invasion and migration by inhibiting miR-181b-targeted silencing of oncogene ARL5B (Xu et al. 2020).
M6A Modification and Lung Cancer Metastasis
Lung cancer remains the leading cause of cancer-related deaths and has become a seriously global health problem (Bray et al. 2018). The functions of m6A modification have attracted more and more attention in the metastasis of lung cancer.
The m6A writer METTL3 can promote the biogenesis of precursor miR-143-3p, relying on m6A methylation, and miR-143-3p is upregulated in the brain metastasis samples of lung cancer (Wang et al. 2019a). M6A-modified miR-143-3p decreases VASH1 expression and subsequently protects VEGFA protein from VASH1-mediated proteasome degradation, and thus triggers invasion and angiogenesis, breaking the blood–brain barrier, and promoting brain metastasis of lung cancer (Wang et al. 2019a). A recent study found that METTL3 induces metastasis and chemotherapeutic resistance by facilitating YAP mRNA stability and translation in lung cancer (Jin et al. 2019a). Mechanistically, METTL3 can directly initiate m6A modification of YAP mRNA and recruit YTHDF1/3 and eIF3b to the translation complex to enhance YAP translation. In addition, METTL3 catalyzes m6A deposition of the lncRNA MALAT1 to stabilize MALAT1 mRNA. MALAT1 acts as the competing endogenous RNA to sponge miR-1914-3p, thereby increasing YAP mRNA stability. The m6A-mediated dual regulation of YAP expression contributes to metastasis and aggravates lung cancer progression (Jin et al. 2019a). Another investigation also confirmed the critical role of m6A in regulating YAP expression to affect lung cancer metastasis (Jin et al. 2020a). This study indicated that the low expression of ALKBH5 reduces m6A modification levels of YAP mRNA and decreases YAP translation and activation depending on YTHDF2. Moreover, ALKBH5 interacts with HuR to augment LAST2 expression by protecting it from miR-1914-3p-mediated degradation, which increases the phosphorylation of YAP and inhibits the activity of the YAP axis, blocking metastasis of lung cancer (Jin et al. 2020a).
The m6A reader YTHDC2 is downregulated in lung cancer and is associated with poor differentiation, lymph node metastasis and advanced TNM stage. YTHDC2 can suppress the proliferation and migration of lung cancer cells (Sun et al. 2020). Li et al. found that the m6A reader YTHDF2 is upregulated in lung adenocarcinoma tissues. YTHDF2 promotes tumorigenesis and metastasis by accelerating AXIN1 decay and consequently activating the Wnt/β-catenin signaling cascade (Li et al. 2021b).
The m6A erasers are involved in the metastasis of lung cancer. For instance, FTO can enhance NELL2 expression to trigger metastasis by declining E2F1 m6A modification levels in lung cancer (Wang et al. 2021c). Guo et al. demonstrated that ALKBH5-mediated low m6A level facilitates the maintenance of the stability of UBE2C mRNA. The oncogenic UBE2C induces metastasis by repressing autophagy (Guo et al. 2018).
M6A Modification and Urological Malignancies
The m6A axis plays critical roles in regulating metastasis of urological cancers such as prostate cancer, bladder cancer and renal cell carcinoma.
Prostate cancer has ranked the most common malignancy among men worldwide. Metastasis is the main risk factor leading to high mortality, and about 80% of metastatic prostate cancer cases may present with bone metastasis. Wen et al. found that a high m6A level of the lncRNA NEAT1 is associated with bone metastasis of prostate cancer (Wen et al. 2020). Investigation of the mechanism indicated that the lncRNA NEAT1 may function as a scaffold and bind to CYCLIN1 and CDK19 to phosphorylate Poll at Ser2 in an m6A-dependent manner. The NEAT1/CDK19/CYCLUNL1 complex could promote cancer metastasis (Wen et al. 2020). Another lncRNA PCAT6 was found to be upregulated specifically in prostate cancer specimens with bone metastasis (Lang et al. 2021). METTL3-induced m6A modification and IGF2BP3-dependent m6A recognition leads to the overexpression of PCAT6. Furthermore, PCAT6 interacts with IGF2BP3 and IGF1R mRNA to enhance IGF1R expression and facilitates bone metastasis (Lang et al. 2021). Li et al. reported that METTL3 modulates ITGB1 expression via the m6A-HuR-dependent pathway, which influences the association of ITGB1 with Collagen I to trigger bone metastasis of prostate cancer (Li et al. 2020a).
In bladder cancer, the m6A machinery, including METTL3 and YTHDF2, degrades the m6A-modified mRNAs of tumor suppressors SETD and KLF4, thereby promoting tumorigenesis and metastasis (Xie et al. 2020a). Gu et al. found that the m6A writer METTL14 is downregulated in bladder cancer tissues and tumor-initiating cells (Gu et al. 2019). Low expression of METTL14 facilitates self-renewal capacity, malignant proliferation, and metastasis through decreasing m6A modification levels of Notch1 mRNA and increasing its translation (Gu et al. 2019). The m6A eraser FTO is upregulated and modulates tumor growth and metastasis in bladder cancer (Tao et al. 2021). FTO demethylates m6A of the lncRNA MALAT1 and increases its mRNA stability via the YTHDF2 reader. MALAT1 elevates the expression level of MAL2 by sponging miR-384 to aggravate bladder cancer progression (Tao et al. 2021).
In renal cell carcinoma, the lncRNA DMDRMR binds to m6A reader IGF2BP3 to stabilize m6A-modified mRNAs including CDK4, COL6A1, LAMA5 and FN1, and increase their protein production. Therefore, DMDRMR accelerates tumor growth by increasing CDK expression and coordinates cell invasion and metastasis partially by elevating FN1 translation in an m6A-dependent manner (Gu et al. 2021). Gu et al. reported that low expression of METTL14 can decrease the m6A modification level of P2RX6 to augment P2RX6 expression and thus activate the p-ERK1/2/MMP9 signaling axis, thereby promoting renal cancer development (Gu et al. 2021).
M6A Deposition and the Metastasis of Gynecological Tumors
Cervical, ovarian and endometrial cancers are three common lethal gynecological malignancies. Metastasis is the foremost cause for the poor prognosis and mortality of patients with these cancers. The regulatory roles of m6A modification have been deeply investigated in these gynecological cancers.
Cervical cancer (CC) is the second most prevalent cancer in women worldwide. Yang et al. found that METTL3 mediates the lncRNA ZAFS1 to sponge miR-647 in an m6A-dependent manner, and this RNA–RNA interaction modulates tumor growth and metastasis in CC (Yang et al. 2020b). Another lncRNA GAS5-AS1 is markedly downregulated, which is associated with lymphatic and distant metastasis in CC (Wang et al. 2019b). Further studies showed that lncRNA GAS5-AS1 can form a ternary complex with GAS5 and the m6A eraser ALKBH5, which decreases the m6A modification levels of the tumor suppressor GAS5 and blocks GAS5 RNA decay in a YTHDF2-dependent way (Wang et al. 2019b). These m6A-induced effects confer the anti-metastatic function of lncRNA GAS5-AS1 in CC (Wang et al. 2019b). A recent study demonstrated that the overexpression of the m6A writer METTL3 is closely linked to lymphatic metastasis in CC (Wang et al. 2020f). Mechanistically, METTL3 can enrich m6A deposition of 3ʹUTR of HK2 and recruit the reader YTHDF1 to enhance HK2 expression. The m6A-mediated HK2 elevation promotes the Warburg effect and aggravates cancer progression (Wang et al. 2020f). The m6A eraser FTO is closely involved in the proliferation and migration of CC cells (Zou et al. 2019). FTO interacts with E2F1 and Myc mRNAs to accelerate the translation of these oncogenic transcripts, thereby promoting CC development (Zou et al. 2019).
Ovarian cancer has the highest mortality among gynecological tumors and has become an enormous threat to women’s health. The m6A writer METTL3 is upregulated in ovarian cancer and associated with lymph node metastasis and an advanced pathological grade (Liang et al. 2020). METTL3 activates the AKT pathway and promotes cyclin D1 expression (Liang et al. 2020). The m6A reader YTHDF1 is overexpressed in ovarian cancer, which is closely correlated with poor prognosis (Liu et al. 2020b). YTHDF1 recognizes m6A-modified EIF3C mRNA and facilitates EIF3C protein output, so as to induce tumorigenesis and metastasis (Liu et al. 2020b).
In endometrial cancer, the m6A eraser FTO mediates demethylation at the 3'-UTR of HOXB13 mRNA to enhance HOXB13 protein translation, thereby activating the Wnt signaling axis to stimulate invasion and metastasis (Zhang et al. 2020b).
M6A Modification and Metastasis in Other Cancer Types
In addition to the aforementioned cancers, the regulatory functions of the m6A axis have been found in other cancers, such as nasopharyngeal carcinoma, oral squamous cell carcinoma, osteosarcoma and thyroid cancer.
In nasopharyngeal carcinoma, m6A modification is enriched on lncRNA FAM225A to increase its RNA stability. The upregulation of FAM225A sequesters miR-590-3p and miR-1275 to elevate ITGB3 expression and activate the FAK/PI3K/AKT signaling pathway, which leads to malignant proliferation and invasion of nasopharyngeal carcinoma (Zheng et al. 2019).
In oral squamous cell carcinoma, high expression of METTL3 is closely correlated with poor prognosis (Liu et al. 2020a). METTL3 mediates the m6A modification at the 3' UTR of BMI1 mRNA and facilitates BMI1 translation via IGF2BP1, thereby promoting proliferation, self-renewal and metastasis (Liu et al. 2020a).
The m6A writer WTAP was found to promote cancer progression in osteosarcoma (Lian et al. 2018). WTAP induces m6A enrichment at the 3ʹUTR of HMBOX1 mRNA and enhances its expression, which activates the PI3K/AKT pathway to stimulate osteosarcoma growth and metastasis (Lian et al. 2018).
M6A modification plays a critical role in regulating thyroid cancer development. Ye et al. reported that the lncRNA MALAT1 increases the expression levels of the m6A reader IGF2BP2 and Myc by sponging miR-204 in an m6A-dependent manner, to stimulate migration and invasion of thyroid cancer (Ye et al. 2021). A recent study found that the m6A writer METTL3 is downregulated and markedly associates with poor prognosis in papillary thyroid carcinoma (He et al. 2021). Low expression of METTL3 activates the NF-κb pathway via abolishing the m6A modification of C-Rel and Rel A and inducing IL-18 secretion to recruit tumor-associated neutrophils, thereby aggravating cancer progression of papillary thyroid cancer (He et al. 2021).
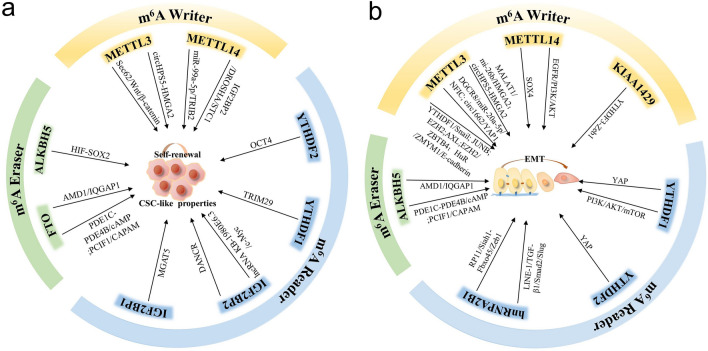
Collectively, the versatile mechanisms of m6A modification in regulating cancer metastasis are summarized in Table1. Additionally, it is well known that cancer stem cell (CSC) maintenance and EMT are two critical events in driving cancer metastasis. The m6A modification plays an important role in regulating the CSC-like properties and EMT process in various types of cancer as mentioned above. Herein, we also summarized the shared common functions of m6A regulators in sustaining CSC-like features and inducing EMT in Fig. 2.
Table 1.
The mechanisms of m6A regulators in cancer metastasis
| m6A regulators | Functions in Cancer | Target genes | Cancer type | References |
|---|---|---|---|---|
| Writer METTL14↓ | Invasion and migration | miR-126 | HCC | Ma et al. (2017) |
| METTL14↓ | Migration, invasion and EMT | EGFR | HCC | Shi et al. (2020b) |
| METTL14↓ | EMT, invasion and migration | SOX4 | CRC | Chen et al. (2020a) |
| METTL14↓ | Tumorigenicity and metastasis | lncRNA XIST | CRC | Yang et al. (2020a) |
| METTL14↓ | Metastasis | miR-375 | CRC | Chen et al. (2020b) |
| METTL14↑ | Metastasis and progression | PERP | Pancreatic cancer | Wang et al. (2020b) |
| METTL14↓ | Self-renewal capacity, malignant proliferation and metastasis | Notch1 | Bladder cancer | Gu et al. (2019) |
| METTL14 | Proliferation | P2RX6 | Renal cancer | Gu et al. (2021) |
| Writer METTL3↑ | Metastasis | SOX2 and HMGA1 | CRC | Hou et al. (2021a); Li et al. (2019) |
| METTL3↑ | Metastasis | KRT7 | Breast cancer | Chen et al. (2021b) |
| METTL3↑ | Stemness, invasion and migratory | SOX2 | Breast cancer | Xie et al. (2021) |
| METTL3↑ | Metastasis | COL3A1 | Breast cancer | Shi et al. (2020a) |
| METTL3 | Invasion and migration | circ1662 | CRC | Chen et al. (2021a) |
| METTL3↑ | EMT, progression and metastasis | ZMYM1 | GC | Yue et al. (2019) |
| METTL3↑ | Metastasis | BATF2 | GC | Xie et al. (2020b) |
| METTL3 | Metastasis | A877 residue of KRT7-AS | Breast cancer | Chen et al. (2021b) |
| METTL3↑ | Invasion, angiogenesis and metastasis | miR143-3p | Lung cancer | Wang et al. (2019a) |
| METTL3↑ | Metastasis and chemotherapeutic resistance | YAP | Lung cancer | Jin et al. (2019a, b) |
| METTL3↑ | Progression and metastasis | MALAT1 | Lung cancer | Jin et al. (2019a, b) |
| METTL3 | Metastasis | PCAT6 | Prostate cancer | Lang et al. (2021) |
| METTL3 | Metastasis | ITGB1 | Prostate cancer | Li et al. (2020a) |
| METTL3 | Tumorigenesis and metastasis | SETD and KLF4 | Bladder cancer | Xie et al. (2020a) |
| METTL3↑ | Proliferation and metastasis | lncRNA ZAFS1 | Cervical cancer | Yang et al. (2020b) |
| METTL3↑ | Progression and metastasis | HK2 | Cervical cancer | Wang et al. (2020e) |
| METTL3 | Metastasis | Cyclin D1 | Ovarian cancer | Liang et al. (2020) |
| METTL3 | Proliferation, self-renewal and metastasis | BMI1 | Oral squamous cell carcinoma | Liu et al. (2020a) |
| METTL3↓ | Progression | C-Rel and Rel A | Papillary thyroid cancer | He et al. (2021) |
| Writer METTL13 | Glycolysis and angiogenesis | HDGF | GC | Wang et al. (2020d) |
| METTL13 | Metastasis | Rp11 | CRC | Wu et al. (2019) |
| Writer KIAA1429 | Proliferation, apoptosis, invasion and migration | GATA3 | HCC | Lan et al. (2019) |
| KIAA1429 | Proliferation, invasion and metastasis | circDLC1-HuR-MMP1 axis | HCC | Liu et al. (2021) |
| Writer WTAP | Proliferation and metastasis | HMBOX1 | Osteosarcoma | Lian et al. (2018) |
| Reader hnRNPA2B1 | Metastasis | lncRNA Rp11, Siah-1 and Fbx45 | CRC | Wu et al. (2019) |
| Reader HuR | EMT and metastasis | ZMYM1 | GC | Yue et al. (2019) |
| Reader YTHDF1↑ | Autophagy and metastasis | ATG2A and ATG14 | HCC | Li et al. (2021a) |
| YTHDF1↑ | Metastasis | EGFR | HCC | Su et al. (2021) |
| YTHDF1↑ | Proliferation and metastasis | USP14 | GC | Chen et al. (2021d) |
| YTHDF1↑ | Tumorigenesis and metastasis | EIF3C | Ovarian cancer | Liu et al. (2020b) |
| YTHDF1 | Progression and metastasis | HK2 | Cervical cancer | Wang et al. (2020e) |
| Reader YTHDC1↑ | Inhibition of tumorigenesis and metastasis | miR-30d/RUNX1/SLC2A/HK pathway | Pancreatic cancer | Hou et al. (2021b) |
| Reader YTHDF2↑ | Stemness and metastasis | OCT4 | HCC | Zhang et al. (2020a) |
| YTHDF2 | Tumorigenesis and metastasis | AXIN1 | Lung cancer | Li et al. (2021b) |
| YTHDF2 | Tumorigenesis and metastasis | SETD and KLF4 | Bladder cancer | Xie et al. (2020a) |
| YTHDF2↑ | Proliferation and metastasis | MALAT1 | Bladder cancer | Tao et al. (2021) |
| Reader YTHDF3↑ | Migration | Zeb | HCC | Wang et al. (2020c) |
| YTHDF3↑ | Angiogenesis and outgrew | ST6GALNAC5, GJA1, and EGFR | Breast cancer | Chang et al. (2020) |
| Reader IGF2BP2 | Metastasis | SOX2, HMGA1 | CRC | Hou et al. (2021a); Li et al. (2019) |
| IGF2BP3 | Glycolysis and angiogenesis | HDGF | GC | Wang et al. (2020d) |
| IGF2BP3 | Metastasis | PCAT6 | Prostate cancer | Lang et al. (2021) |
| IGF2BP3↑ | Proliferation and metastasis | lncRNA DMDRMR | Renal cell carcinoma | Gu et al. (2021) |
| Eraser FTO↑ | stemness and metastasis | SOX2, KLF4 and NANOG | HCC | Bian et al. (2021) |
| FTO | Progression and metastasis | TCF7L2 | GC | Gao et al. (2021) |
| FTO↓ | Metastasis | A950 in KRT7 exon 6 | Breast cancer | Chen et al. (2021b) |
| FTO↑ | Proliferation and metastasis | BNIP3 | Breast cancer | Niu et al. (2019) |
| FTO | Invasion and migration | miR-181b | Breast cancer | Xu et al. (2020) |
| FTO | Metastasis | E2F1 | Lung cancer | Wang et al. (2021b) |
| FTO↑ | Proliferation and metastasis | MALAT1 | Bladder cancer | Tao et al. (2021) |
| FTO | Proliferation and migration | E2F1 and Myc mRNAs | Cervical cancer | Zou et al. (2019) |
| FTO | Invasion and metastasis | HOXB13 | Endometrial cancer | Zhang et al. (2020b) |
| Eraser ALKBH5 | Metastasis | lncRNA NEAT1 | GC | Zhang et al. (2019) |
| ALKBH5↓ | Motility | lncRNA KCNK15-AS1 | Pancreatic cancer | He et al. (2018) |
| ALKBH5↑ | Stemness and progression | NANOG | Breast cancer | Zhang et al. (2016) |
| ALKBH5↓ | Metastasis | YAP axis | Lung cancer | Jin et al. (2020a) |
| ALKBH5↓ | Metastasis | miR-1914-3p | Lung cancer | Jin et al. (2020a) |
| ALKBH5↓ | Repression of autophagy, metastasis | UBE2C | Lung cancer | Guo et al. (2018) |
| ALKBH5 | Anti-metastatic | lncRNA GAS5-AS1 | Cervical cancer | Wang et al. (2019b) |
| ALKBH5 | Metastasis | Rp11 | CRC | Wu et al. (2019) |
↓Indicates downregulated expression and ↑indicates upregulated expression in cancer. HCC is hepatocellular carcinoma, CRC is colorectal carcinoma and GC is gastric cancer
Fig.2.
The functions of m6A regulators in maintaining CSC-like properties and inducing EMT. The schematic diagrams showed the shared common functions of m6A-mediated CSC-like features and EMT in a and b, respectively. The m6A regulators, including writers, readers and erasers, have been extensively studied how to affect CSC and EMT, which are two critical biological events to drive cancer metastasis
M6A-Mediated Cancer Metastasis Organotropism
It is noteworthy that most cancers are prone to metastasize to a specific organ, known as “organotropism”. For instance, colorectal cancer has a high propensity to metastasize to the liver. Breast cancer preferably metastasizes to the lungs, bones and brain. Hepatocellular carcinoma is prone to lung metastasis, whereas prostate cancer frequently relapses in the bone. The specificity of the metastatic process is determined by numerous factors, including tumor-intrinsic properties, organ-specific niches, and the complicated interplay between tumor and the surrounding microenvironment. Increasing evidence demonstrates that m6A modification plays an important role in regulating metastasis organotropism in various cancer.
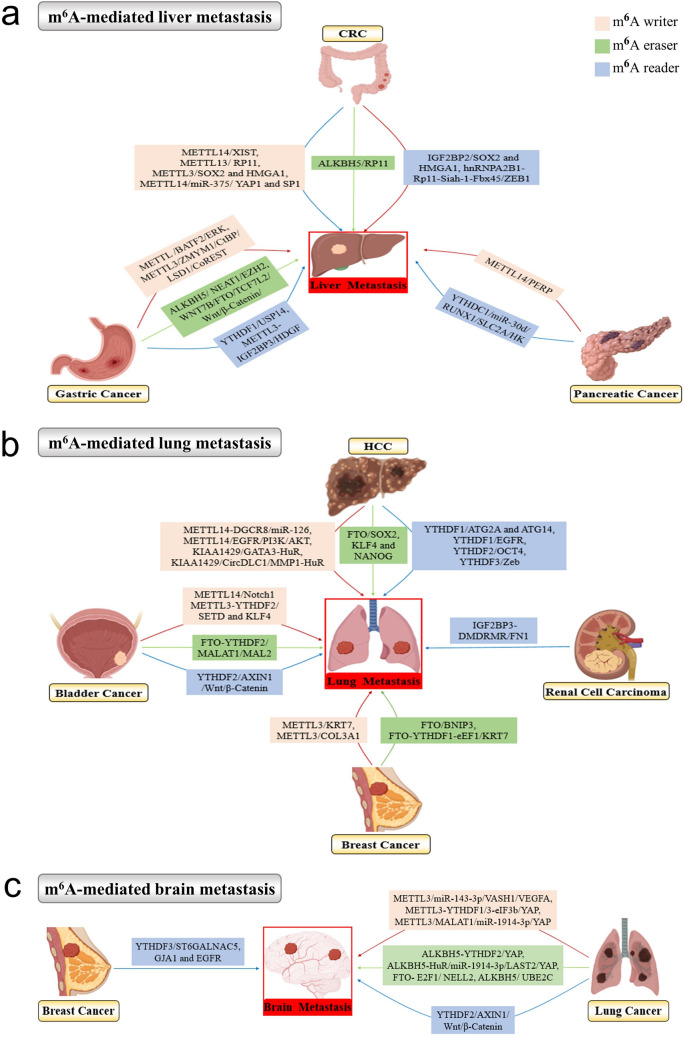
The liver is one of the most frequently distant metastatic organs in multiple cancers such as colorectal cancer, gastric cancer and pancreatic cancer. In colorectal cancer development, m6A writers, readers and erasers are extensively involved in liver-specific metastasis through the following mechanisms, including METTL14/XIST, METTL13/RP11, METTL3/SOX2 and HMGA1, METTL14/miR-375/ YAP1 and SP1, IGF2BP2/SOX2 and HMGA1, hnRNPA2B1-Rp11-Siah-1-Fbx45/ZEB1, and the ALKBH5/RP11 cascade (Chen et al. 2020b; Wu et al. 2019; Xie et al. 2021; Yang et al. 2020a). In advanced gastric cancer, several m6A pathways greatly contribute to the liver metastasis of gastric cancer including METTL3/BATF2/ERK, METTL3/ZMYM1/CtBP/LSD1/CoREST, YTHDF1/USP14, METTL3-IGF2BP3/HDGF, ALKBH5/NEAT1/EZH2, and FTO/TCF7L2/Wnt/β-catenin feedback (Chen et al. 2021d; Gao et al. 2021; Wang et al. 2020e; Xie et al. 2020b; Yue et al. 2019; Zhang et al. 2019). In pancreatic cancer, METTL14-mediated PERP m6A and YTHDC1-mediated Warburg effect promoted liver metastasis (Hou et al. 2021b; Wang et al. 2020b).
The lung is another favored metastatic site for solid tumors such as HCC, breast cancer, bladder cancer and renal cell carcinoma. There are various m6A factors to trigger lung metastasis of HCC, such as m6A writers METTL14-DGCR8/miR-126, METTL14/EGFR/PI3K/AKT, KIAA1429/GATA3-HuR, and KIAA1429/CircDLC1/ MMP1-HuR, m6A readers YTHDF1/ATG2A and ATG14, YTHDF1/EGFR, YTHDF2/OCT4, and YTHDF3/Zeb, and m6A erasers FTO/SOX2, KLF4 and NANOG (Bian et al. 2021; Lan et al. 2019; Li et al. 2021a; Ma et al. 2017; Shi et al. 2020b; Su et al. 2021; Wang et al. 2020c; Zhang et al. 2020a). In bladder cancer, these m6A-related mechanisms might account for incrased liver metastasis including METTL14/Notch1, METTL3-YTHDF2/SETD and KLF4, YTHDF2/AXIN1/Wnt/β-catenin and FTO-YTHDF2/MALAT1/MAL2 (Gu et al. 2019; Li et al. 2021b; Tao et al. 2021; Xie et al. 2020a). In breast cancer, METTL3/KRT7, METTL3/COL3A1, FTO/BNIP3, and FTO-YTHDF1-eEF1/KRT7 axis are responsible for liver metastasis (Chen et al. 2021b; Niu et al. 2019; Shi et al. 2020a). Gu et al. found that IGF2BP3-induced FN1 m6A modification contributes to liver metastasis of renal cell carcinoma (Gu et al. 2021).
Lung cancer and breast cancer exhibit great propensity for brain-specific metastasis, and m6A modification is extensively involved in the malignant behaviors. As for the brain metastasis of lung cancer, multiple m6A pathways regulate the process, such as METTL3/miR-143-3p/VASH1/VEGFA, METTL3-YTHDF1/3-eIF3b/YAP, METTL3/MALAT1/miR-1914-3p/YAP, YTHDF2/AXIN1/Wnt/β-catenin, ALKBH5-YTHDF2/YAP, ALKBH5-HuR/miR-1914-3p/LAST2/YAP, FTO-E2F1/NELL2, and ALKBH5/UBE2C (Guo et al. 2018; Jin et al. 2019a; Jin et al. 2020a; Li et al. 2021b; Wang et al. 2019a; Wang et al. 2021c). In breast cancer, YTHDF3-induced translation of m6A-enriched transcripts for ST6GALNAC5, GJA1 and EGFR promotes brain metastasis (Chang et al. 2020).
The above-mentioned m6A-mediated liver-, lung- and brain-specific metastasis in various cancers are summarized in Fig. 3. Whether m6A modification and related regulators could serve as biomarkers and targets for cancer metastasis organotropism still warrants extensive studies.
Fig.3.
The m6A-mediated organ-specific metastasis in various cancers. The schematic diagram indicated the m6A-mediated liver metastasis (a), lung metastasis (b), and brain metastasis (c) in different cancers, and the m6A writers, readers and erasers all play essential roles in the organ-specific metastatic process. The diagram of various cancer types were created with the help of BioRender.com
The Clinical Translational Potential of the m6A Axis in Cancer Diagnosis and Treatment
Given the essential functions of the m6A axis in cancer development, the clinical translation potential of m6A has attracted increasing attention. The m6A axis has become a promising target for diagnosis, prognostic prediction and therapy.
Ge et al. claimed that m6A modification has the potential to serve as an ideal biomarker in GC progression (Ge et al. 2020). It was reported that the m6A levels are significantly increased in the peripheral blood RNA in GC, compared to benign gastric disease (BGD) and healthy controls (HCs). Furthermore, m6A levels exhibit an elevated trend with the progression and metastasis of GC (Ge et al. 2020).
Our group comprehensively assessed the clinically predictive potential of m6A modification, HCC progression and therapeutic responses. According to the established m6A score system, three distinct m6A patterns were identified in HCC. The HCC cluster with a lower m6A score frequently showed metabolic hyperactivity, better prognosis and lower response rate to sorafenib treatment. In contrast, the HCC cluster with a higher m6A score usually exhibited hypoactive metabolism, poorer prognosis, and favorable response to sorafenib therapy (Shen et al. 2020). In future, these findings still need to be confirmed in a large cohort, and further efforts are required to explore the clinical translation potential of the m6A machinery in HCC metastasis.
Recently, targeting the critical enzymes of m6A modification for cancer therapy has made some inspiring progressions. For example, METTL3 contains a Rossmann fold, which binds the S-adenosyl methionine (SAM) methyl donor. STM2457 is a bioavailable inhibitor of METTL3 catalytic activity through a SAM-competitive mode, which has been developed to apply for the treatment of acute myeloid leukemia (AML) (Yankova et al. 2021). For m6A demethylases, two targeted FTO inhibitors, FB23 and FB23-2, have also been designed. FB23-2 significantly inhibits the progression of AML in cell lines and xeno-transplanted mice (Huang et al. 2019). Moreover, the two potent inhibitors of FTO showed promising anti-tumor effects in multiple types of cancers (Su et al. 2020). Another FTO-targeted molecule, Saikosaponin D (SsD), increases the global m6A RNA methylation, effectively overcoming FTO/m6A-mediated resistance to tyrosine kinase inhibitors in leukemia (Sun et al. 2021).
In view of the widespread clinical application of chemotherapy and targeted therapies in metastasis treatment, researchers have also explored the possibility of co-administration of m6A-related molecules. Depletion of methyltransferases, METTL3 and METTL14, was found to enhance the response to anti-PD-1 treatment in CRC and melanoma, by increasing cytotoxic tumor-infiltrating CD8 + T cells and elevating the secretion of IFN-γ, CXCL9, and CXCL10 in the tumor microenvironment (Wang et al. 2020a; Yang et al. 2019). Similarly, the deletion of the m6A demethylase ALKBH5 modulates the composition of tumor-infiltrating Treg and myeloid-derived suppressor cells, thereby sensitizing tumors to cancer immunotherapy (Li et al. 2020b). Despite the promising perspective of m6A in clinical translation, we should be aware that most of the results were achieved in vitro or in mice models. Large-scale clinical trials are urgently required to evaluate the effectiveness of targeting m6A for cancer therapy.
Nowadays, with the development of m6A mapping technologies, such as Mazter-Seq, it is possible to determine the precise quantity of m6A modification at specific sites of specific RNAs (Garcia-Campos et al. 2019). These new methods will greatly promote the clinical application of m6A as a novel biomarker. Additionally, the combination of m6A and target therapy or immune therapy may be a promising strategy for anti-metastasis treatment.
Limitations and Future Perspectives of m6A Modifications
Despite the great progression of m6A-related research in cancer development, there are still some limitations to be noted. First, the writers, readers and erasers of m6A are not specific to a single target, and m6A regulators have multiple substrates, including oncogenes and tumor suppressor genes. These may explain the controversial roles of a single m6A regulator that may exert opposite functions. Meanwhile, the uncertainty of m6A-targeting also increases the difficulty to develop a therapeutic strategy. Second, m6A modification may induce completely different effects on RNA metabolism. For some RNAs, m6A enrichment may facilitate RNA decay, such as EGFR, Oct4 and Sox2, but in some cases, m6A deposition may increase the stability of the RNA, such as in the case of tumor suppressor KLF14. The different fates of m6A-RNAs may develop in a contextspecific manner for different targets and different types of cancers, and the underlying mechanisms need to be clarified. Additionally, a single m6A regulator may display different expression patterns in different cancers, as well as in the same type of cancer. Taking METTL14 as an example, it is downregulated in multiple types of cancers and can inhibit cancer metastasis, but in pancreatic cancer, METTL14 is upregulated, promoting metastasis (Wang et al. 2020b). Another example is METTL3, several studies have demonstrated that METTL3 is upregulated and acts as an oncogene in breast cancer (Chen et al. 2021b; Xie et al. 2021). However, Shi et al. reported that METTL3 is downregulated and can inhibit the development of breast cancer (Shi et al. 2020a). These inconsistencies may result from different pathogenesis pathways and backgrounds of different cancer types. Moreover, the heterogeneity of cancer may lead to a discrepancy.
The m6A modification is a new frontier in the cancer field, and most of the present studies focus on it in cancer cells. As the microenvironment is essential for cancer metastasis, further study of m6A alterations and functions in the tumor microenvironment are warranted. Additionally, it is important to identify the determinative m6A site with a regulatory function, as some m6A sites may present just a constitutive event lacking of function. A particular m6A signature in some specific transcript loci may be more informative than the global m6A profiles, which would serve as better biomarkers for clinical translation in cancer. These promising perspectives of m6A deserve to be further probed in future investigations.
Conclusion
Cancer metastasis is a complicated biological event and accounts for a high mortality rate. The m6A modifications may exert versatile roles to determine the fate of RNAs, which play essential roles in regulating cancer development. Through systematically reviewing the latest progression of m6A modification in cancer metastasis, we hope to comprehensively enrich our knowledge on m6A regulation and provide some new clues to develop effective strategies to monitor and treat cancer metastasis based on m6A dysregulation.
Acknowledgements
This study was supported by the Key Program of the National Natural Science Foundation of China (81930074, 2020-2024), the Major Program of National Natural Science Foundation of China (91959203, 2020-2023) and the Natural Science Foundation of China (81672820, 2017-2020; 81672378, 2017-2020; 82173093)
Abbreviations
- m6A
N6-methyladenosine
- m1A
N1-methyladenosine
- m3C
3-Methylcytosine
- m5C
5-Methylcytosine
- m1G
N1-methylguanosine
- m7G
7-Methylguanosine
- ac4C
N4-acetylcytidine
- EMT
Epithelial-mesenchymal transition
- VEGF
Vascular endothelial growth factor
- HPCs
Hematopoietic progenitor cells
- SDF-1
Stromal cell-derived factor-1
- MMP-9
Matrix metallopeptidase 9
- CTCs
Circulating tumor cells
- METTL3
Methyltransferase-like 3
- WTAP
Wilms tumor 1-associated protein
- FTO
Obesity-associated protein
- ALKBH5
AlkB homolog 5
- IGF2BP
Insulinlike growth factor 2 mRNAbinding protein family
- HNRNPA2B1
Heterogeneous nuclear ribonucleoproteins A2/B1
- PRRC2A
Prolinerich and coiledcoilcontaining protein 2A
- CNV
Copy number variation
- OS
Overall survival
- DFS
Disease-free survival
- HNSCC
Head and neck squamous cell carcinoma
- UCEC
Uterine corpus endometrial carcinoma
- ccRCC
Clear cell renal cell carcinoma
- DNMT2
DNA methyltransferase-like 2
- HCC
Hepatocellular carcinoma
- CRC
Colorectal cancer
- GC
Gastric cancer
- PDAC
Pancreatic ductal adenocarcinoma
- CC
Cervical cancer
- CSC
Cancer stem cell
Authors' Contributions
LXQ designed the outline and directed the writing of the paper. JZP, SZX and JFX collected the related papers. JZ and HX retrieved the related studies, drafted manuscript and prepared the figures and tables. YHS revised the language and provided comments. All authors critically revised the manuscript.
Data and Material Availability
Not applicable.
Code Availability
Not applicable.
Declarations
Conflicts of Interest
No conflicts of interest to disclose.
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent to Publish
Not applicable.
Footnotes
Jing Zhao and Hao Xu have contributed equally to this work.
References
- Alexandrov A, Martzen MR, Phizicky EM. Two proteins that form a complex are required for 7-methylguanosine modification of yeast tRNA. RNA. 2002;8:1253–1266. doi: 10.1017/s1355838202024019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arango D, et al. Acetylation of cytidine in mRNA promotes translation efficiency. Cell. 2018;175:1872–1886.e1824. doi: 10.1016/j.cell.2018.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audia JE, Campbell RM. Histone Modifications and cancer cold spring. Harb Perspect Biol. 2016;8:a019521. doi: 10.1101/cshperspect.a019521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbieri I, Kouzarides T. Role of RNA modifications in cancer. Nat Rev Cancer. 2020;20:303–322. doi: 10.1038/s41568-020-0253-2. [DOI] [PubMed] [Google Scholar]
- Bian X, Shi D, Xing K, Zhou H, Lu L, Yu D, Wu W. AMD1 upregulates hepatocellular carcinoma cells stemness by FTO mediated mRNA demethylation. Clin Transl Med. 2021;11:e352. doi: 10.1002/ctm2.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos PD, et al. Genes that mediate breast cancer metastasis to the brain. Nature. 2009;459:1005–1009. doi: 10.1038/nature08021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries CA Cancer. J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- Calabrese C, et al. Genomic basis for RNA alterations in cancer. Nature. 2020;578:129–136. doi: 10.1038/s41586-020-1970-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang G, et al. YTHDF3 induces the translation of m(6)A-enriched gene transcripts to promote breast cancer brain metastasis. Cancer Cell. 2020;38:857–871.e857. doi: 10.1016/j.ccell.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, et al. Transfer RNA demethylase ALKBH3 promotes cancer progression via induction of tRNA-derived small RNAs. Nucleic Acids Res. 2019;47:2533–2545. doi: 10.1093/nar/gky1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, et al. METTL14-mediated N6-methyladenosine modification of SOX4 mRNA inhibits tumor metastasis in colorectal cancer. Mol Cancer. 2020;19:106. doi: 10.1186/s12943-020-01220-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, et al. METTL14 suppresses CRC progression via regulating N6-methyladenosine-dependent primary miR-375 processing. Mol Ther. 2020;28:599–612. doi: 10.1016/j.ymthe.2019.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Chen XY, Liang R, Yi YC, Fan HN, Chen M, Zhang J, Zhu JS. The m(6)A reader YTHDF1 facilitates the tumorigenesis and metastasis of gastric cancer via USP14 translation in an m(6)A-dependent manner. Front Cell Dev Biol. 2021;9:647702. doi: 10.3389/fcell.2021.647702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, et al. N6-methyladenosine-induced circ1662 promotes metastasis of colorectal cancer by accelerating YAP1 nuclear localization. Theranostics. 2021;11:4298–4315. doi: 10.7150/thno.51342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, et al. N(6) -methyladenosine regulates mRNA stability and translation efficiency of KRT7 to promote breast cancer lung metastasis. Cancer Res. 2021;81:2847–2860. doi: 10.1158/0008-5472.Can-20-3779. [DOI] [PubMed] [Google Scholar]
- Chen S, et al. CLK1/SRSF5 pathway induces aberrant exon skipping of METTL14 and Cyclin L2 and promotes growth and metastasis of pancreatic cancer. J Hematol Oncol. 2021;14:60. doi: 10.1186/s13045-021-01072-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia AL, et al. Hepatic stellate cells suppress NK cell-sustained breast cancer dormancy. Nature. 2021 doi: 10.1038/s41586-021-03614-z. [DOI] [PubMed] [Google Scholar]
- Cui J, Liu Q, Sendinc E, Shi Y, Gregory RI. Nucleotide resolution profiling of m3C RNA modification by HAC-seq. Nucleic Acids Res. 2021;49:e27. doi: 10.1093/nar/gkaa1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai X, Wang T, Gonzalez G, Wang Y. Identification of YTH domain-containing proteins as the readers for N1-methyladenosine in RNA. Anal Chem. 2018;90:6380–6384. doi: 10.1021/acs.analchem.8b01703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumagalli A, et al. Plasticity of Lgr5-negative cancer cells drives metastasis in colorectal cancer. Cell Stem Cell. 2020;26:569–578567. doi: 10.1016/j.stem.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Bado I, Wang H, Zhang W, Rosen JM, Zhang XH. Metastasis organotropism: redefining the congenial soil. Dev Cell. 2019;49:375–391. doi: 10.1016/j.devcel.2019.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Q, et al. A WNT7B-m(6)A-TCF7L2 positive feedback loop promotes gastric cancer progression and metastasis. Signal Transduct Target Ther. 2021;6:43. doi: 10.1038/s41392-020-00397-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Campos MA, et al. Deciphering the “m(6)A Code” via antibody-independent quantitative profiling. Cell. 2019;178:731–747.e716. doi: 10.1016/j.cell.2019.06.013. [DOI] [PubMed] [Google Scholar]
- Ge L, et al. Level of N6-methyladenosine in peripheral blood RNA: a novel predictive biomarker for gastric cancer. Clin Chem. 2020;66:342–351. doi: 10.1093/clinchem/hvz004. [DOI] [PubMed] [Google Scholar]
- Gu C, et al. Mettl14 inhibits bladder TIC self-renewal and bladder tumorigenesis through N(6)-methyladenosine of Notch1. Mol Cancer. 2019;18:168. doi: 10.1186/s12943-019-1084-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, et al. DMDRMR-mediated regulation of m(6)A-modified CDK4 by m(6)A reader IGF2BP3 drives ccRCC progression. Cancer Res. 2021;81:923–934. doi: 10.1158/0008-5472.Can-20-1619. [DOI] [PubMed] [Google Scholar]
- Guo J, et al. Deregulation of UBE2C-mediated autophagy repression aggravates NSCLC progression. Oncogenesis. 2018;7:49. doi: 10.1038/s41389-018-0054-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haag S, Kretschmer J, Bohnsack MT. WBSCR22/Merm1 is required for late nuclear pre-ribosomal RNA processing and mediates N7-methylation of G1639 in human 18S rRNA. RNA. 2015;21:180–187. doi: 10.1261/rna.047910.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadfield G. The dormant cancer cell. Br Med J. 1954;2:607–610. doi: 10.1136/bmj.2.4888.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, et al. ALKBH5 inhibits pancreatic cancer motility by decreasing long non-coding RNA KCNK15-AS1 methylation. Cell Physiol Biochem. 2018;48:838–846. doi: 10.1159/000491915. [DOI] [PubMed] [Google Scholar]
- He L, Li H, Wu A, Peng Y, Shu G, Yin G. Functions of N6-methyladenosine and its role in cancer. Mol Cancer. 2019;18:176. doi: 10.1186/s12943-019-1109-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, et al. METTL3 restrains papillary thyroid cancer progression via m(6)A/c-Rel/IL-8-mediated neutrophil infiltration. Mol Ther. 2021;29:1821–1837. doi: 10.1016/j.ymthe.2021.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino A, et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527:329–335. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou P, et al. LINC00460/DHX9/IGF2BP2 complex promotes colorectal cancer proliferation and metastasis by mediating HMGA1 mRNA stability depending on m6A modification. J Exp Clin Cancer Res. 2021;40:52. doi: 10.1186/s13046-021-01857-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y, et al. YTHDC1-mediated augmentation of miR-30d in repressing pancreatic tumorigenesis via attenuation of RUNX1-induced transcriptional activation of Warburg effect. Cell Death Differ. 2021 doi: 10.1038/s41418-021-00804-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, et al. Small-molecule targeting of oncogenic FTO demethylase in acute myeloid leukemia. Cancer Cell. 2019;35:677–691.e610. doi: 10.1016/j.ccell.2019.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin D, et al. m(6)A mRNA methylation initiated by METTL3 directly promotes YAP translation and increases YAP activity by regulating the MALAT1-miR-1914–3p-YAP axis to induce NSCLC drug resistance and metastasis. J Hematol Oncol. 2019;12:135. doi: 10.1186/s13045-019-0830-6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Jin X, et al. AtTrm5a catalyses 1-methylguanosine and 1-methylinosine formation on tRNAs and is important for vegetative and reproductive growth in Arabidopsis thaliana. Nucleic Acids Res. 2019;47:883–898. doi: 10.1093/nar/gky1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin D, et al. m(6)A demethylase ALKBH5 inhibits tumor growth and metastasis by reducing YTHDFs-mediated YAP expression and inhibiting miR-107/LATS2-mediated YAP activity in NSCLC. Mol Cancer. 2020;19:40. doi: 10.1186/s12943-020-01161-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X, et al. A metastasis map of human cancer cell lines. Nature. 2020;588:331–336. doi: 10.1038/s41586-020-2969-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PA, Issa JP, Baylin S. Targeting the cancer epigenome for therapy. Nat Rev Genet. 2016;17:630–641. doi: 10.1038/nrg.2016.93. [DOI] [PubMed] [Google Scholar]
- Kaplan RN, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klutstein M, Nejman D, Greenfield R, Cedar H. DNA methylation in cancer and aging. Cancer Res. 2016;76:3446–3450. doi: 10.1158/0008-5472.Can-15-3278. [DOI] [PubMed] [Google Scholar]
- Koch A, et al. Analysis of DNA methylation in cancer: location revisited. Nat Rev Clin Oncol. 2018;15:459–466. doi: 10.1038/s41571-018-0004-4. [DOI] [PubMed] [Google Scholar]
- Lan T, et al. KIAA1429 contributes to liver cancer progression through N6-methyladenosine-dependent post-transcriptional modification of GATA3. Mol Cancer. 2019;18:186. doi: 10.1186/s12943-019-1106-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang C, et al. m(6) A modification of lncRNA PCAT6 promotes bone metastasis in prostate cancer through IGF2BP2-mediated IGF1R mRNA stabilization. Clin Transl Med. 2021;11:e426. doi: 10.1002/ctm2.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughney AM, et al. Regenerative lineages and immune-mediated pruning in lung cancer metastasis. Nat Med. 2020;26:259–269. doi: 10.1038/s41591-019-0750-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, et al. METTL3 facilitates tumor progression via an m(6)A-IGF2BP2-dependent mechanism in colorectal carcinoma. Mol Cancer. 2019;18:112. doi: 10.1186/s12943-019-1038-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li E, Wei B, Wang X, Kang R. METTL3 enhances cell adhesion through stabilizing integrin β1 mRNA via an m6A-HuR-dependent mechanism in prostatic carcinoma. Am J Cancer Res. 2020;10:1012–1025. [PMC free article] [PubMed] [Google Scholar]
- Li N, et al. ALKBH5 regulates anti-PD-1 therapy response by modulating lactate and suppressive immune cell accumulation in tumor microenvironment. Proc Natl Acad Sci USA. 2020;117:20159–20170. doi: 10.1073/pnas.1918986117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, et al. HIF-1α-induced expression of m6A reader YTHDF1 drives hypoxia-induced autophagy and malignancy of hepatocellular carcinoma by promoting ATG2A and ATG14 translation. Signal Transduct Target Ther. 2021;6:76. doi: 10.1038/s41392-020-00453-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, et al. RNA m(6)A reader YTHDF2 facilitates lung adenocarcinoma cell proliferation and metastasis by targeting the AXIN1/Wnt/β-catenin signaling. Cell Death Dis. 2021;12:479. doi: 10.1038/s41419-021-03763-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian H, Wang QH, Zhu CB, Ma J, Jin WL. Deciphering the epitranscriptome in cancer. Trends Cancer. 2018;4:207–221. doi: 10.1016/j.trecan.2018.01.006. [DOI] [PubMed] [Google Scholar]
- Liang S, Guan H, Lin X, Li N, Geng F, Li J. METTL3 serves an oncogenic role in human ovarian cancer cells partially via the AKT signaling pathway. Oncol Lett. 2020;19:3197–3204. doi: 10.3892/ol.2020.11425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, et al. ALKBH1-mediated tRNA demethylation regulates translation. Cell. 2016;167:816–828.e816. doi: 10.1016/j.cell.2016.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, et al. Tumor exosomal RNAs promote lung pre-metastatic niche formation by activating alveolar epithelial TLR3 to recruit neutrophils. Cancer Cell. 2016;30:243–256. doi: 10.1016/j.ccell.2016.06.021. [DOI] [PubMed] [Google Scholar]
- Liu L, et al. METTL3 promotes tumorigenesis and metastasis through BMI1 m(6)A methylation in oral squamous cell carcinoma. Mol Ther. 2020;28:2177–2190. doi: 10.1016/j.ymthe.2020.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, et al. The m6A reader YTHDF1 promotes ovarian cancer progression via augmenting EIF3C translation. Nucleic Acids Res. 2020;48:3816–3831. doi: 10.1093/nar/gkaa048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, et al. Circular RNA circDLC1 inhibits MMP1-mediated liver cancer progression via interaction with HuR. Theranostics. 2021;11:1396–1411. doi: 10.7150/thno.53227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma JZ, et al. METTL14 suppresses the metastatic potential of hepatocellular carcinoma by modulating N(6) -methyladenosine-dependent primary MicroRNA processing. Hepatology. 2017;65:529–543. doi: 10.1002/hep.28885. [DOI] [PubMed] [Google Scholar]
- Murgai M, et al. KLF4-dependent perivascular cell plasticity mediates pre-metastatic niche formation and metastasis. Nat Med. 2017;23:1176–1190. doi: 10.1038/nm.4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu Y, et al. RNA N6-methyladenosine demethylase FTO promotes breast tumor progression through inhibiting BNIP3. Mol Cancer. 2019;18:46. doi: 10.1186/s12943-019-1004-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obenauf AC, Massagué J. Surviving at a distance: organ-specific metastasis trends. Cancer. 2015;1:76–91. doi: 10.1016/j.trecan.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmeda D, et al. Whole-body imaging of lymphovascular niches identifies pre-metastatic roles of midkine. Nature. 2017;546:676–680. doi: 10.1038/nature22977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paget S. The distribution of secondary growths in cancer of the breast. Lancet. 1889;133:571–573. doi: 10.1016/S0140-6736(00)49915-0. [DOI] [PubMed] [Google Scholar]
- Peinado H, et al. Pre-metastatic niches: organ-specific homes for metastases. Nat Rev Cancer. 2017;17:302–317. doi: 10.1038/nrc.2017.6. [DOI] [PubMed] [Google Scholar]
- Phan TG, Croucher PI. The dormant cancer cell life cycle. Nat Rev Cancer. 2020;20:398–411. doi: 10.1038/s41568-020-0263-0. [DOI] [PubMed] [Google Scholar]
- Ramanathan A, Robb GB, Chan SH. mRNA capping: biological functions and applications. Nucleic Acids Res. 2016;44:7511–7526. doi: 10.1093/nar/gkw551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roundtree IA, Evans ME, Pan T, He C. Dynamic RNA modifications in gene expression regulation. Cell. 2017;169:1187–1200. doi: 10.1016/j.cell.2017.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saikia M, Fu Y, Pavon-Eternod M, He C, Pan T. Genome-wide analysis of N1-methyl-adenosine modification in human tRNAs. RNA. 2010;16:1317–1327. doi: 10.1261/rna.2057810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X, et al. The m6A methylation landscape stratifies hepatocellular carcinoma into 3 subtypes with distinct metabolic characteristics. Cancer Biol Med. 2020;17:937–952. doi: 10.20892/j.issn.2095-3941.2020.0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, et al. Reduced expression of METTL3 promotes metastasis of triple-negative breast cancer by m6A methylation-mediated COL3A1 up-regulation. Front Oncol. 2020;10:1126. doi: 10.3389/fonc.2020.01126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Zhuang Y, Zhang J, Chen M, Wu S. METTL14 inhibits hepatocellular carcinoma metastasis through regulating EGFR/PI3K/AKT signaling pathway in an m6A-dependent manner. Cancer Manag Res. 2020;12:13173–13184. doi: 10.2147/cmar.S286275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su R, et al. Targeting FTO suppresses cancer stem cell maintenance and immune evasion. Cancer Cell. 2020;38:79–96.e11. doi: 10.1016/j.ccell.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su T, et al. Insufficient radiofrequency ablation promotes hepatocellular carcinoma metastasis through m(6) A mRNA methylation dependent mechanism. Hepatology. 2021 doi: 10.1002/hep.31766. [DOI] [PubMed] [Google Scholar]
- Suhail Y, Cain MP, Vanaja K, Kurywchak PA, Levchenko A, Kalluri R. Systems biology of cancer metastasis. Cell Syst. 2019;9:109–127. doi: 10.1016/j.cels.2019.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S, Han Q, Liang M, Zhang Q, Zhang J, Cao J. Downregulation of m(6) A reader YTHDC2 promotes tumor progression and predicts poor prognosis in non-small cell lung cancer. Thorac Cancer. 2020;11:3269–3279. doi: 10.1111/1759-7714.13667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun K, et al. Saikosaponin D exhibits anti-leukemic activity by targeting FTO/m(6)A signaling. Theranostics. 2021;11:5831–5846. doi: 10.7150/thno.55574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao L, et al. FTO modifies the m6A level of MALAT and promotes bladder cancer progression. Clin Transl Med. 2021;11:e310. doi: 10.1002/ctm2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vera-Ramirez L, Vodnala SK, Nini R, Hunter KW, Green JE. Autophagy promotes the survival of dormant breast cancer cells and metastatic tumour recurrence. Nat Commun. 2018;9:1944. doi: 10.1038/s41467-018-04070-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, et al. N6-methyladenosine induced miR-143–3p promotes the brain metastasis of lung cancer via regulation of VASH1. Mol Cancer. 2019;18:181. doi: 10.1186/s12943-019-1108-x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Wang X, Zhang J, Wang Y. Long noncoding RNA GAS5-AS1 suppresses growth and metastasis of cervical cancer by increasing GAS5 stability. Am J Transl Res. 2019;11:4909–4921. [PMC free article] [PubMed] [Google Scholar]
- Wang L, et al. m6 A RNA methyltransferases METTL3/14 regulate immune responses to anti-PD-1 therapy. Embo J. 2020;39:104514. doi: 10.15252/embj.2020104514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, et al. Upregulation of METTL14 mediates the elevation of PERP mRNA N(6) adenosine methylation promoting the growth and metastasis of pancreatic cancer. Mol Cancer. 2020;19:130. doi: 10.1186/s12943-020-01249-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Yang Y, Yang J, Yang J, Han S. circ_KIAA1429 accelerates hepatocellular carcinoma advancement through the mechanism of m(6)A-YTHDF3-Zeb1. Life Sci. 2020;257:118082. doi: 10.1016/j.lfs.2020.118082. [DOI] [PubMed] [Google Scholar]
- Wang P, Wang X, Zheng L, Zhuang C. Gene signatures and prognostic values of m6A regulators in hepatocellular carcinoma. Front Genet. 2020;11:540186. doi: 10.3389/fgene.2020.540186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, et al. METTL3-mediated m(6)A modification of HDGF mRNA promotes gastric cancer progression and has prognostic significance. Gut. 2020;69:1193–1205. doi: 10.1136/gutjnl-2019-319639. [DOI] [PubMed] [Google Scholar]
- Wang Q, Guo X, Li L, Gao Z, Su X, Ji M, Liu J. N(6)-methyladenosine METTL3 promotes cervical cancer tumorigenesis and Warburg effect through YTHDF1/HK2 modification. Cell Death Dis. 2020;11:911. doi: 10.1038/s41419-020-03071-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Ren F, Song Z, Wang X, Ma X. Multiomics profile and prognostic gene signature of m6A regulators in uterine corpus endometrial carcinoma. J Cancer. 2020;11:6390–6401. doi: 10.7150/jca.46386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Gan M, Chen C, Zhang Y, Kong J, Zhang H, Lai M. Methyl CpG binding protein 2 promotes colorectal cancer metastasis by regulating N(6) -methyladenosine methylation through methyltransferase-like 14. Cancer Sci. 2021 doi: 10.1111/cas.15011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, et al. Copy number variation analysis of m(6) A regulators identified METTL3 as a prognostic and immune-related biomarker in bladder cancer. Cancer Med. 2021;10:7804–7815. doi: 10.1002/cam4.3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Li M, Zhang L, Chen Y, Zhang S. m6A demethylase FTO induces NELL2 expression by inhibiting E2F1 m6A modification leading to metastasis of non-small cell lung cancer. Mol Ther Oncolytics. 2021;21:367–376. doi: 10.1016/j.omto.2021.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen S, Wei Y, Zen C, Xiong W, Niu Y, Zhao Y. Long non-coding RNA NEAT1 promotes bone metastasis of prostate cancer through N6-methyladenosine. Mol Cancer. 2020;19:171. doi: 10.1186/s12943-020-01293-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, et al. m(6)A-induced lncRNA RP11 triggers the dissemination of colorectal cancer cells via upregulation of Zeb1. Mol Cancer. 2019;18:87. doi: 10.1186/s12943-019-1014-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie H, et al. METTL3/YTHDF2 m(6) A axis promotes tumorigenesis by degrading SETD7 and KLF4 mRNAs in bladder cancer. J Cell Mol Med. 2020;24:4092–4104. doi: 10.1111/jcmm.15063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie JW, et al. m(6)A modification-mediated BATF2 acts as a tumor suppressor in gastric cancer through inhibition of ERK signaling. Mol Cancer. 2020;19:114. doi: 10.1186/s12943-020-01223-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J, Ba J, Zhang M, Wan Y, Jin Z, Yao Y. The m6A methyltransferase METTL3 promotes the stemness and malignant progression of breast cancer by mediating m6A modification on SOX2. J Buon. 2021;26:444–449. [PubMed] [Google Scholar]
- Xu Y, et al. The FTO/miR-181b-3p/ARL5B signaling pathway regulates cell migration and invasion in breast cancer. Cancer Commun (lond) 2020;40:484–500. doi: 10.1002/cac2.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, et al. 5-methylcytosine promotes mRNA export - NSUN2 as the methyltransferase and ALYREF as an m(5)C reader. Cell Res. 2017;27:606–625. doi: 10.1038/cr.2017.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, et al. m(6)A mRNA demethylase FTO regulates melanoma tumorigenicity and response to anti-PD-1 blockade. Nat Commun. 2019;10:2782. doi: 10.1038/s41467-019-10669-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, et al. METTL14 suppresses proliferation and metastasis of colorectal cancer by down-regulating oncogenic long non-coding RNA XIST. Mol Cancer. 2020;19:46. doi: 10.1186/s12943-020-1146-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Ma J, Han S, Li X, Guo H, Liu D. ZFAS1 exerts an oncogenic role via suppressing miR-647 in an m(6)A-dependent manner in cervical cancer. Onco Targets Ther. 2020;13:11795–11806. doi: 10.2147/ott.S274492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yankova E, et al. Small-molecule inhibition of METTL3 as a strategy against myeloid leukaemia. Nature. 2021;593:597–601. doi: 10.1038/s41586-021-03536-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye M, Dong S, Hou H, Zhang T, Shen M. Oncogenic role of long noncoding RNAMALAT1 in thyroid cancer progression through regulation of the miR-204/IGF2BP2/m6A-MYC signaling. Mol Ther Nucleic Acids. 2021;23:1–12. doi: 10.1016/j.omtn.2020.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon KJ, et al. Temporal control of mammalian cortical neurogenesis by m(6)A methylation. Cell. 2017;171:877–889.e817. doi: 10.1016/j.cell.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue B, Song C, Yang L, Cui R, Cheng X, Zhang Z, Zhao G. METTL3-mediated N6-methyladenosine modification is critical for epithelial-mesenchymal transition and metastasis of gastric cancer. Mol Cancer. 2019;18:142. doi: 10.1186/s12943-019-1065-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Z, et al. Cancer-derived exosomal miR-25–3p promotes pre-metastatic niche formation by inducing vascular permeability and angiogenesis. Nat Commun. 2018;9:5395. doi: 10.1038/s41467-018-07810-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, et al. Hypoxia induces the breast cancer stem cell phenotype by HIF-dependent and ALKBH5-mediated m6A-demethylation of NANOG mRNA. Proc Natl Acad Sci USA. 2016;113:E2047–2056. doi: 10.1073/pnas.1602883113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, et al. ALKBH5 promotes invasion and metastasis of gastric cancer by decreasing methylation of the lncRNA NEAT1. J Physiol Biochem. 2019;75:379–389. doi: 10.1007/s13105-019-00690-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Wan Y, Zhang Z, Jiang Y, Lang J, Cheng W. Zhu L (2020b) FTO demethylates m6A modifications in HOXB13 mRNA and promotes endometrial cancer metastasis by activating the WNT signalling pathway. RNA Biol. 2020 doi: 10.1080/154762861841458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, et al. YTHDF2 promotes the liver cancer stem cell phenotype and cancer metastasis by regulating OCT4 expression via m6A RNA methylation. Oncogene. 2020;39:4507–4518. doi: 10.1038/s41388-020-1303-7. [DOI] [PubMed] [Google Scholar]
- Zhang F, Yan Y, Cao X, Zhang J, Li Y, Guo C. Methylation of microRNA-338–5p by EED promotes METTL3-mediated translation of oncogene CDCP1 in gastric cancer. Aging. 2021;13:12224–12238. doi: 10.18632/aging.103822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao BS, Roundtree IA, He C. Post-transcriptional gene regulation by mRNA modifications. Nat Rev Mol Cell Biol. 2017;18:31–42. doi: 10.1038/nrm.2016.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng ZQ, et al. Long noncoding RNA FAM225A promotes nasopharyngeal carcinoma tumorigenesis and metastasis by acting as ceRNA to sponge miR-590–3p/miR-1275 and upregulate ITGB3. Cancer Res. 2019;79:4612–4626. doi: 10.1158/0008-5472.Can-19-0799. [DOI] [PubMed] [Google Scholar]
- Zhou J, et al. Gene signatures and prognostic values of m6A regulators in clear cell renal cell carcinoma: a retrospective study using TCGA database. Aging. 2019;11:1633–1647. doi: 10.18632/aging.101856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, et al. Analysis of genetic alteration signatures and prognostic values of m6A regulatory genes in head and neck squamous cell carcinoma. Front Oncol. 2020;10:718. doi: 10.3389/fonc.2020.00718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou D, Dong L, Li C, Yin Z, Rao S, Zhou Q. The m(6)A eraser FTO facilitates proliferation and migration of human cervical cancer cells. Cancer Cell Int. 2019;19:321. doi: 10.1186/s12935-019-1045-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.