Abstract
Age and gender are the important factors for brain metabolic declines in both normal aging and neurodegeneration, and the confounding effects may influence early and differential diagnosis of neurodegenerative diseases based on the [18F]fluorodeoxyglucose positron emission tomography ([18F]FDG PET). We aimed to explore the potential of the adjustment of age- and gender-related confounding factors on [18F]FDG PET images in differentiation of Parkinson’s disease (PD), multiple system atrophy (MSA) and progressive supra-nuclear palsy (PSP). Eight hundred and seventy-seven clinically definitely diagnosed Parkinsonian patients from a benchmark Huashan Parkinsonian PET imaging database were included. An age- and gender-adjusted Z (AGAZ) score was established based on the gender-specific longitudinal metabolic changes on healthy subjects. AGAZ scores and standardized uptake value ratio (SUVR) values were quantified at regional-level and support vector machine-based error-correcting output codes method was applied for classification. Additional references of the classifications based on metabolic pattern scores were included. The feature-based AGAZ score showed the best performance in classification (accuracy for PD, MSA, PSP: 93.1%, 96.3%, 94.8%). In both genders, the AGAZ score consistently achieved the best efficiency, and the improvements compared to the conventional SUVR value for PD, MSA, and PSP mainly laid in specificity (Male: 5.7%; Female: 11.1%), sensitivity (Male: 7.2%; Female: 7.3%), and sensitivity (Male: 7.3%; Female: 17.2%). Female patients benefited more from the adjustment on [18F]FDG PET in MSA and PSP groups (absolute net reclassification index, p < 0.001). Collectively, the adjustment of age- and gender-related confounding factors may improve the differential diagnosis of Parkinsonism. Particularly, the diagnosis of female Parkinsonian population has the best improvement from this correction.
Supplementary Information
The online version contains supplementary material available at 10.1007/s43657-022-00079-6.
Keywords: Age- and gender-adjustment, [18F]FDG PET, Differential diagnosis, Parkinsonism
Introduction
The overlapping and similar clinical symptoms in Parkinson’s disease (PD) and atypical parkinsonian syndromes, including multiple system atrophy (MSA), and progressive supra-nuclear palsy (PSP), are challenging for the differential diagnosis. Studies during the past decades have showed that [18F]fluorodeoxyglucose positron emission tomography ([18F]FDG PET) is a clinically useful tool for accurate disease distinction and severity assessment in parkinsonism (Meles et al. 2017). As the most used radiotracer in practice, [18F]FDG serves as a marker of neuronal function by assessing regional cerebral glucose metabolism, where elevated uptake indicates synaptic activity and decreased uptake represents neural dysfunction (Kato et al. 2016; Meles et al. 2017; Meyer et al. 2017; Nobili et al. 2018). The disease-specific changes and the relatively high availability make [18F]FDG PET an essential part in the diagnostic work-up of patients with neurodegenerative disorders (Kato et al. 2016; Meles et al. 2017; Meyer et al. 2017; Nobili et al. 2018).
Of note, cerebral glucose metabolic changes are composed of disease-related abnormalities and age-associated changes. Previous [18F]FDG PET studies found that the glucose metabolic activity was decreased with advancing age in many brain regions, including parkinsonism-related areas like frontal, occipital and parietal cortices, striatum, and thalamus (Knopman et al. 2014; Yoshizawa et al. 2014; Kakimoto et al. 2016; Ishibashi et al. 2018; van Aalst et al. 2022), and the decline rates varied (2–10% per decade) (Petit-Taboue et al1998; Greve et al. 2014; Bonte et al. 2017). Though age-associated changes were mainly discussed in the context of cognitive impairments like Alzheimer’s disease (AD) (Brugnolo et al. 2014; Ewers et al. 2014; Li et al. 2022), their impact on movement disorders cannot be ignored. As the onset of Parkinsonism spans over a broad range of age, the mixed age-related changes may affect the accuracy of an early PET-based diagnosis.
Besides, gender is considered to have an effect on the aging-related brain changes in magnetic resonance imaging (MRI) (Good et al. 2001; Cosgrove et al. 2007; Takahashi et al. 2011) and [18F]FDG PET (Cosgrove et al. 2007; Yoshizawa et al. 2014; Kakimoto et al. 2016) studies though the findings are controversial. Serving as an important variability for disease susceptibility and trajectory in neurodegeneration (Baba et al. 2006; Gillies et al. 2014; Ferretti et al. 2018), differences in brain metabolism between genders have been reported albeit the majority have focused on AD (Mosconi et al. 2017; Cavedo et al. 2018). In PD, MRI studies found that males suffered greater brain atrophy (Tremblay et al. 2020) and disrupted connectivity (De Micco et al. 2019; Tremblay et al. 2020) than disease duration and severity-matched females. Therefore, the effect of gender should also be considered when attempting to eliminate the influence of age-related metabolic changes on the [18F]FDG PET-based parkinsonism differentiation.
Herein, we hypothesized that an adjustment for the gender-specific age-related changes could improve an [18F]FDG PET-based differential diagnosis in Parkinsonian. We first applied an adjustment for the age-related changes on [18F]FDG PET images based on our previously identified gender-specific longitudinal templates of age-related changes using exponential model (Zhang et al. 2017), and then compared its [Age- and Gender-adjusted Z score (AGAZ score)] disease discrimination efficiency in patients with PD, MSA and PSP with conventional semi-quantification method [standardized uptake value ratio (SUVR)] and principal component analysis method [the disease-related metabolic patterns: PD-related pattern (PDRP) (Wu et al. 2013); MSA-related pattern (MSARP) (Shen et al. 2020); PSP-related pattern (PSPRP) (Ge et al. 2018)]. Second, we compared the difference in the degree of improvement between males and females, as well as different ages.
Materials and Methods
Subjects
The investigated subjects were the same as in our previous work from Huashan Parkinsonian PET Imaging (HPPI) Database (Wu et al. 2022). Only the patients with clinical definite diagnoses (cohort I, n = 547) and the ones with confirmative diagnoses by follow-up (cohort II, n = 330) were enrolled (Supplementary Table 1).
The institutional review board from Huashan Hospital approved this study, and all subjects signed a written informed consent.
Imaging Acquisition and Preprocessing
As described before (Wu et al. 2022), patients underwent brain [18F]FDG PET after an injection of 185 MBq [18F]FDG during 60–70 min with Biograph 64 HD positron emission tomography/computed tomography (PET/CT) (Siemens, Germany).
The PET images were preprocessed using Statistical Parametric Mapping (SPM) 12 toolbox (http://www.fil.ion.ucl.ac.uk/spm/software/spm12/) implemented in MATLAB (MathWorks, Natick, MA, USA). All PET images were spatially normalized into Montreal Neurological Institute standardized space using SPM PET template and then smoothed using a Gaussian kernel with a full width at a half maximum of 10 mm. The global brain region was regarded as reference region to count normalization. All images were parcellated into 119 anatomical regions according to the Automated Anatomical Labelling (AAL) atlas (116 regions of interest (ROIs) from AAL atlas, plus midbrain, pons, and medulla) (Li et al. 2022).
Quantification of AGAZ Score, Conventional SUVR Value and Pattern Score
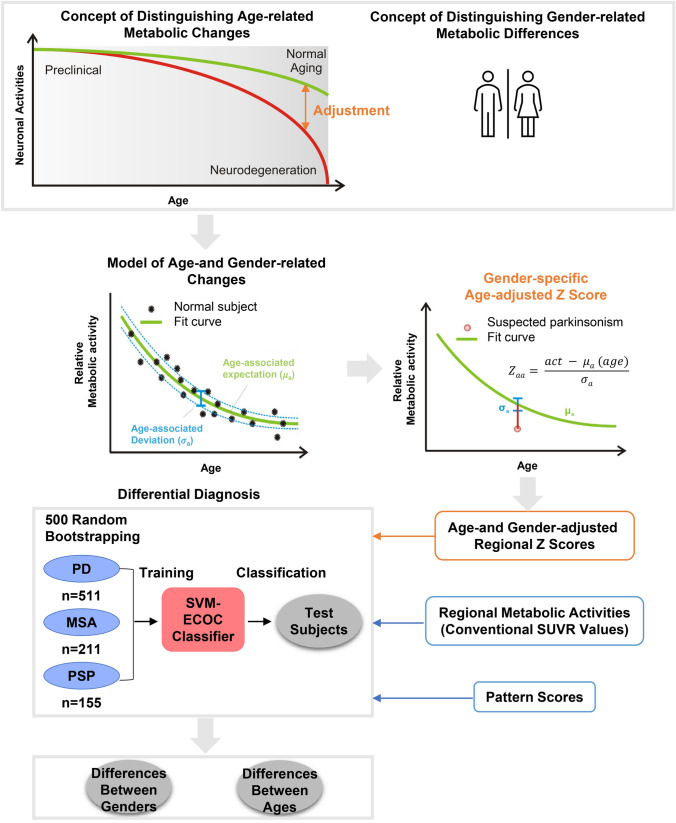
Longitudinal templates of gender-specific age-related changes were applied to adjust for the age-related metabolic changes. [18F]FDG PET scans from the normal cohorts (128 males and 127 females) were analyzed using exponential model to generate longitudinal age-related metabolism change templates for each gender as describe previously (Zhang et al. 2017). AGAZ score of each ROI was calculated by subtracting the age- and gender-associated activity from the measured activity and then normalized with standard deviation of the longitudinal template (Fig. 1). Conventional SUVRs were calculated in the same ROIs.
Fig. 1.
The study flowchart. PD Parkinson’s disease; MSA multiple system atrophy; PSP progressive supra-nuclear palsy; SUVR standardized uptake value ratio; SVM-ECOC support vector machine-based error-correcting output codes
Scores of PDRP, MSARP and PSPRP were obtained via a Scaled Subprofile Modelling/Principal Component Analysis toolbox in ScAnVP ((http://www.feinstein- neuroscience.org) and Z-transformed as previously described (Wu et al. 2013; Ge et al. 2018; Shen et al. 2020).
A total of 37 ROIs were selected as features to train the classify model for AGAZ scores and conventional SUVR values. These regions, covering the typical glucose abnormality in Parkinsonism, including bilateral precentral gyrus, supplementary motor area, middle occipital, superior parietal, caudate, putamen, pallidum, thalamus, cerebellum, medulla, pons, and midbrain, were considered to be appropriate for Parkinsonism analyses (Meles et al. 2017).
Statistical Analysis
To test the classification performance, the entirety was divided randomly into the training and test datasets as the ratio (4:1) with 100 iterations. All measurements (AGAZ score, SUVR value, pattern score) were regarded as predictors to perform multi-classification using support vector machine-based error-correcting output codes method (Chmielnicki and Stąkapor 2011). Accuracy, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated, and two-sample T test was used for comparison between AGAZ score and conventional SUVR value, AGAZ score, and pattern score, respectively.
To explore the different improvements between genders, the absolute net reclassification index (NRI) was applied to compare the predictive performance of different measurements (Alba et al. 2017). Results were considered significant at p < 0.05.
Results
Participant Characteristics
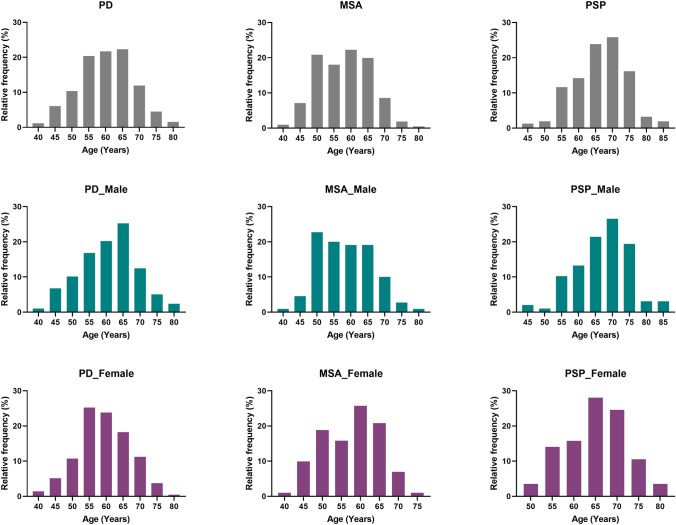
Five hundred and eleven PD, 211 MSA, and 155 PSP patients were enrolled, of whom 41.8%, 47.9% and 36.8% were females, respectively (Table 1). Age of the enrolled PD, MSA and PSP patients ranged from 41 to 82, 42 to 79, 44 to 84 years old, respectively (Fig. 2).
Table 1.
The demographical information
| n | Age (years) | Symptom duration (months) | UPDRS III | H &Y Scale | ||
|---|---|---|---|---|---|---|
|
PD (n = 511) |
Male | 299 | 60.7 ± 8.5 | 40.4 ± 40.9 | 25.3 ± 13.1 | 2.0 ± 0.9 |
| Female | 214 | 59.3 ± 7.6 | 46.2 ± 48.3 | 25.1 ± 14.2 | 2.1 ± 1.0 | |
|
MSA (n = 211) |
Male | 110 | 58.4 ± 7.5 | 23.4 ± 14.6 | 26.1 ± 12.7 | 2.9 ± 0.8 |
| Female | 101 | 57.5 ± 7.5 | 26.9 ± 21.1 | 34.5 ± 15.0 | 3.2 ± 0.9 | |
|
PSP (n = 155) |
Male | 98 | 67.2 ± 7.8 | 34.5 ± 21.2 | 27.5 ± 11.4 | 3.0 ± 0.8 |
| Female | 57 | 65.1 ± 7.0 | 33.9 ± 20.6 | 30.8 ± 14.5 | 3.4 ± 0.8 |
PD Parkinson’s disease; MSA multiple system atrophy; PSP progressive supra-nuclear palsy; UPDRS III Unified Parkinson’s Disease Rating Scale, part III; H&Y Scale Hoehn and Yahr Scale
Fig. 2.
The distribution of the subjects according to the ages. Each bin covers the age difference of 5 years. PD Parkinson’s disease; MSA multiple system atrophy; PSP progressive supra-nuclear palsy
Performance of the Three Measurements in the Training and Test Datasets
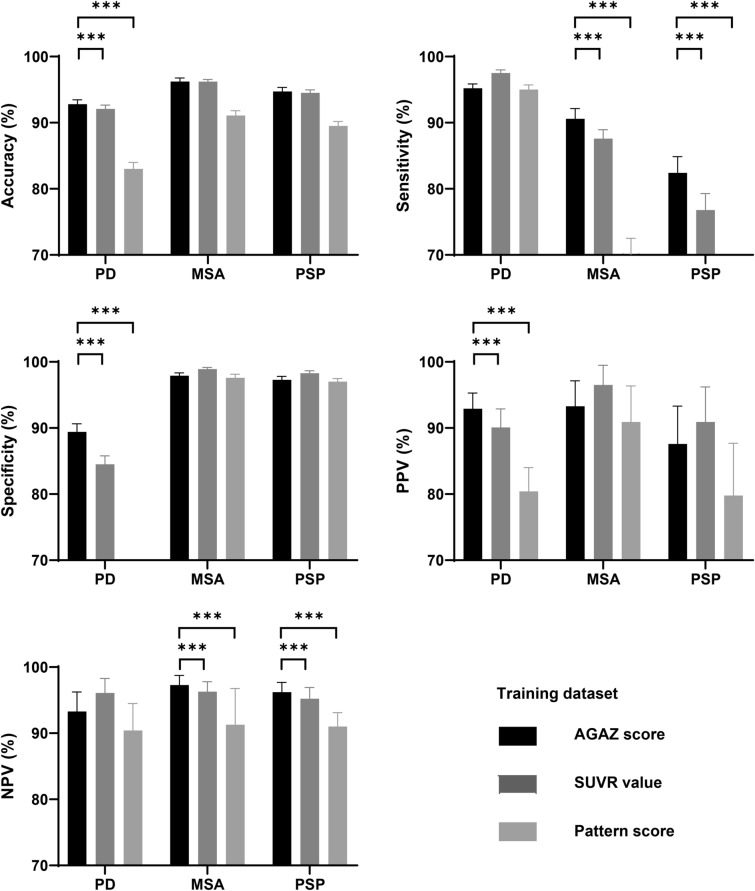
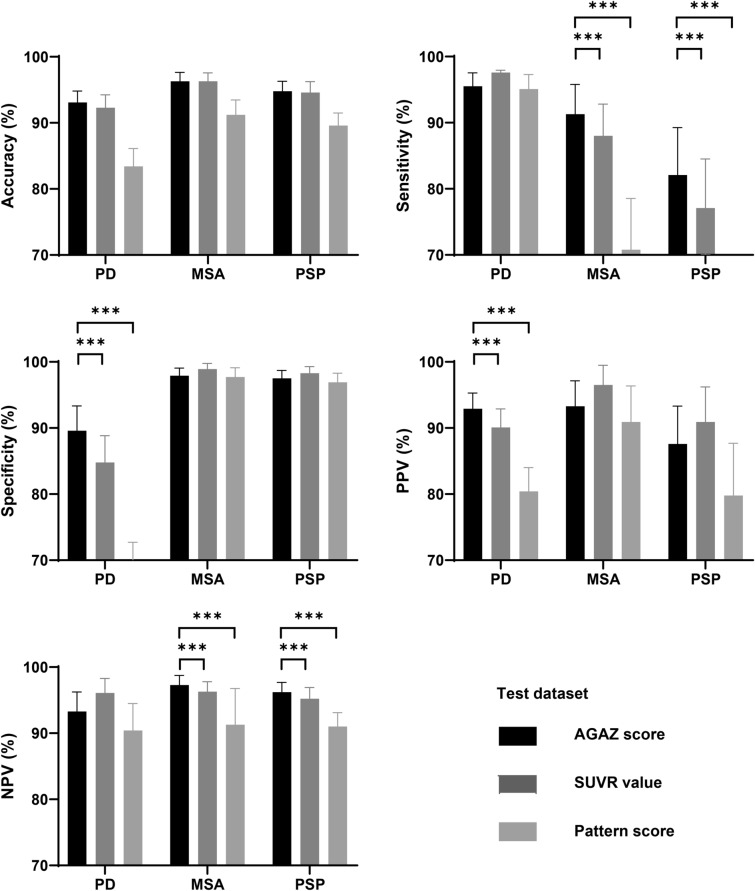
In both datasets, the feature-based conventional SUVR value achieved high accuracy in classification (92.1–96.3%), and the AGAZ score (92.8–96.3%) showed a slight improvement on top of the conventional SUVR value, while pattern score (83.0–91.2%) had the less favorable performance.
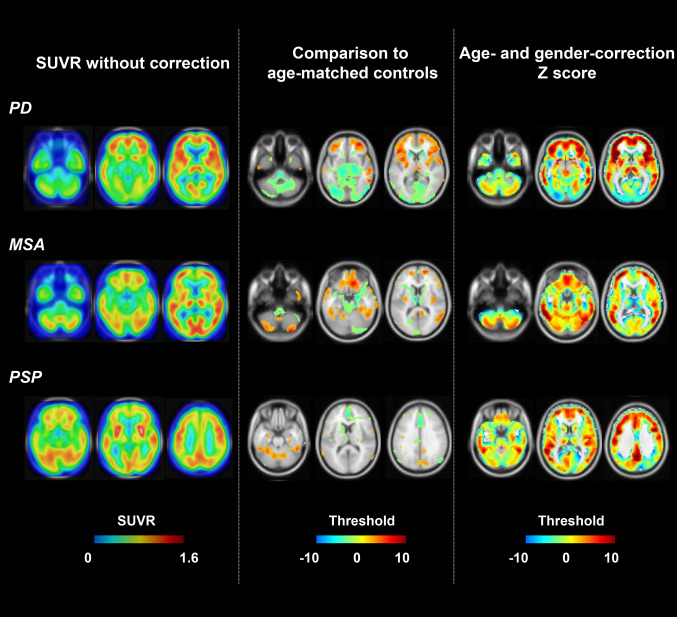
In the training dataset, compared to the classification based on conventional SUVR values and pattern scores, AGAZ scores produced a significantly higher accuracy, specificity and PPV in PD, a significantly higher sensitivity and NPV in MSA, and a significantly higher sensitivity and NPV in PSP (p < 0.05; Fig. 3). Similar improvements were observed in the test dataset (Fig. 4). Fig. 5 shows examples of patients for each disease with wrong classification by conventional SUVR value but correct identification by AGAZ score. In both displayed PD and MSA patients, by comparing to a group of age-matched healthy controls (p < 0.05, voxel-level), extensive hypometabolism was found in midbrain, strongly suggesting a diagnosis of PSP. Nevertheless, by applying age- and gender-correction, no significant decreased metabolic activity was seen in midbrain, and the disease-specific abnormalities (obvious hypermetabolism in putamen and thalamus for PD, hypometabolism in putamen for MSA) were clearer. In the showed PSP patient, the disease characterized by hypometabolism in the midbrain and caudate nucleus was more pronounced in the AGAZ map, although the decreased activity in the medial prefrontal cortex was more readily observed compared to the age-matched healthy group at voxel level.
Fig. 3.
The classification performance of different measurements within training dataset. The sensitivity of the pattern score for diagnosing MSA was around 70% (70.1 ± 2.42), and the specificity of the pattern score for diagnosing PD was less than 70% (65.7 ± 2.23) and were therefore not shown as bars in the figure. PD Parkinson’s disease; MSA multiple system atrophy; PSP progressive supra-nuclear palsy; AGAZ age- and gender-adjusted Z; SUVR standardized uptake value ratio; PPV positive predictive value; NPV negative predictive value. ***p < 0.001, two-sample T test
Fig. 4.
The classification performance of different measurements within test dataset. The specificity of the pattern score for diagnosing PD was less than 70% (66.6 ± 6.12) and was therefore not shown as a bar in the figure. PD Parkinson’s disease; MSA multiple system atrophy; PSP progressive supra-nuclear palsy; AGAZ age- and gender-adjusted Z; SUVR standardized uptake value ratio; PPV positive predictive value; NPV negative predictive value. ***p < 0.001, two-sample T test
Fig. 5.
Examples of patients for each disease with wrong classification by conventional SUVR value but correct identification by AGAZ score. The left column represented the SUVR images, the middle column showed the abnormal T-maps compared to a group of age-matched healthy controls (p < 0.05, uncorrected), and the right column presented the AGAZ score maps. The top row was the images of a patient with PD, the middle row was the images of a patient with MSA, and the bottom row was the images of a patient with PSP. All images were displayed by overlaying on the standard avg152T1 image in the Montreal Neurological Institute space, and a transparency of 30% was applied to the SUVR images. PD Parkinson’s disease; MSA multiple system atrophy; PSP progressive supra-nuclear palsy; SUVR standardized uptake value ratio; AGAZ age- and gender-adjusted Z
Performance of the Three Measurements by Genders
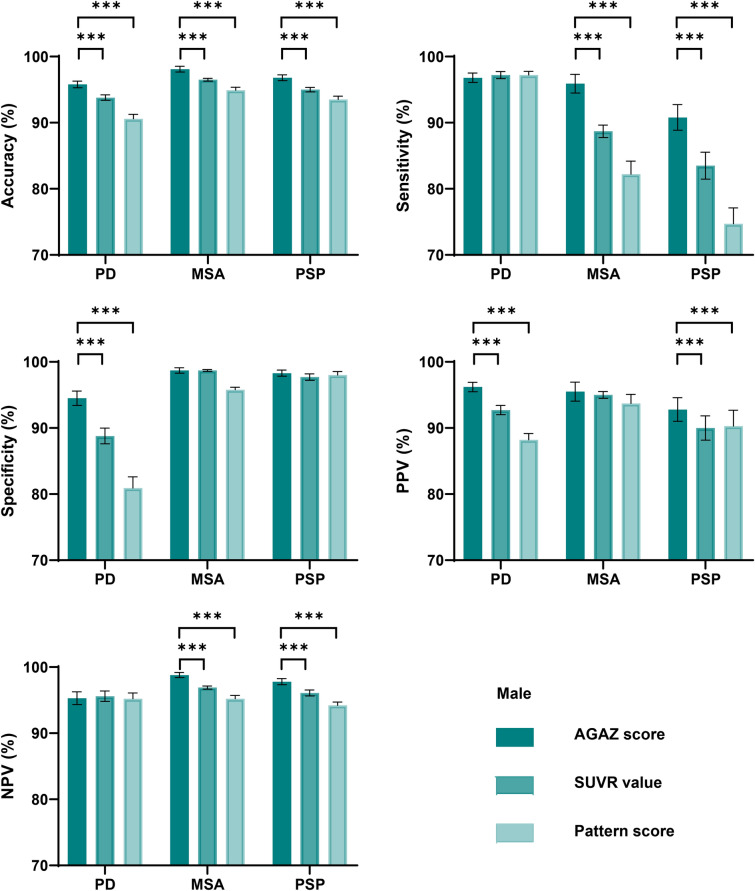
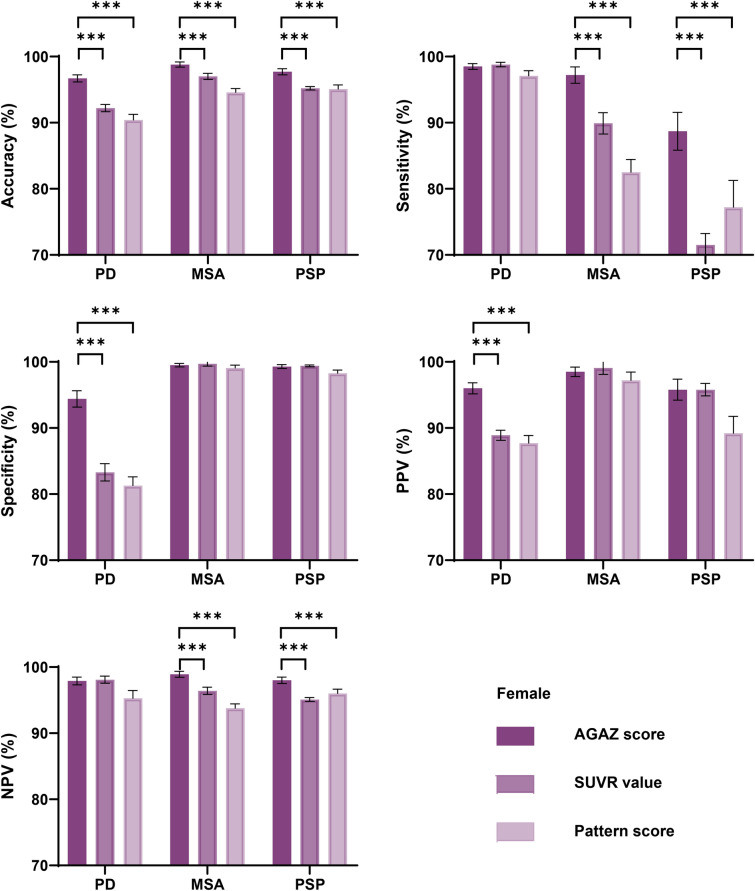
Consistently, the AGAZ scores harbored the best performance in classification when tested separately in males and females. The AGAZ scores outperformed conventional SUVR values and pattern scores in differentiation: a higher accuracy, specificity and PPV in PD, a higher accuracy, sensitivity and NPV in MSA, and a higher accuracy, sensitivity and NPV in PSP (p < 0.05; Figs. 6 and 7). For PD, the most pronounced improvement for disease classification was in specificity, and for MSA and PSP, it laid in sensitivity.
Fig. 6.
The classification performance of different measurements within males. PD Parkinson’s disease; MSA multiple system atrophy; PSP progressive supra-nuclear palsy; AGAZ age- and gender-adjusted Z; SUVR standardized uptake value ratio; PPV positive predictive value; NPV negative predictive value; NRI net reclassification index. ***p < 0.001, two-sample T test
Fig. 7.
The classification performance of different measurements within females. PD Parkinson’s disease; MSA multiple system atrophy; PSP progressive supra-nuclear palsy; AGAZ age- and gender-adjusted Z; SUVR standardized uptake value ratio; PPV positive predictive value; NPV negative predictive value. ***p < 0.001, two-sample T test
Specifically, in males with PD, the AGAZ scores showed a 2.0%, 5.7%, 3.5% improvement in accuracy, specificity, and PPV than the conventional SUVR value, and the corresponding ameliorations in the females were 4.5%, 11.1%, and 7.1%. In males with MSA, the AGAZ scores showed a 1.6%, 7.2%, 1.9% improvement in accuracy, sensitivity and NPV than the conventional SUVR value, and the corresponding ameliorations in the females were 1.8%, 7.3%, and 2.5%. For PSP, in male patients, the improvements of AGAZ scores were most significant with 1.8%, 7.3%, 2.8%, 1.7% in accuracy, sensitivity, PPV and NPV comparing to the conventional SUVR values, and the corresponding ameliorations in females were 2.5%, 17.2%, 0 and 3.1%.
Further comparing the effect of AGAZ score between genders by NRI showed that the improvements of AGAZ score compared to SUVR value were more pronounced in females with MSA (females vs males: 10.7% ± 1.7% vs 5.3% ± 1.5%, p < 0.001) and PSP (females vs males: 17.0% ± 3.2% vs 7.8% ± 2.6%, p < 0.001) than males, while no difference was seen in PD (females vs males: 7.0% ± 1.9% vs 7.2% ± 1.7%, p = 0.350; Fig. 8).
Fig. 8.
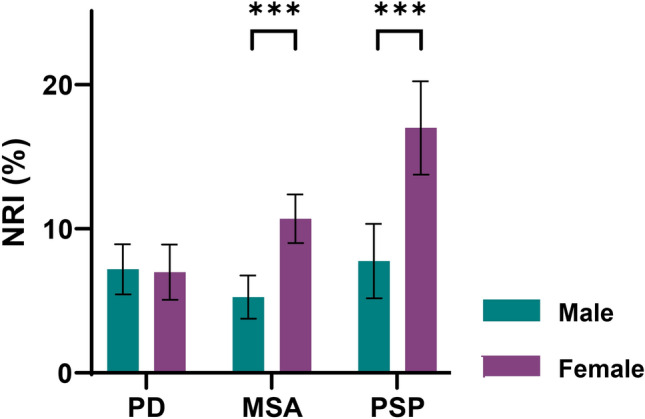
Gender differences in disease classification improvement by AGAZ score compared to SUVR value. PD Parkinson’s disease; MSA multiple system atrophy; PSP progressive supra-nuclear palsy; AGAZ age- and gender-adjusted Z; NRI net reclassification index. ***p < 0.001, two-sample T test
Performance of the Three Measurements by Ages
Each disease group was equally divided into three subgroups based on ages. In PD and MSA, the improvement of correction compared to conventional SUVR was most pronounced in the old group, while in PSP, the improvement was best in the young group (p < 0.001, Supplementary Table 2, Table 2).
Table 2.
Age differences in disease classification improvement by AGAZ score compared to SUVR value
| NRI (%) | Young | Middle | Old | p value |
|---|---|---|---|---|
| PD | 6.14 ± 2.03 | 7.35 ± 2.05 | 9.13 ± 1.61 | < 0.001 |
| MSA | 5.71 ± 1.29 | 4.74 ± 3.04 | 10.2 ± 1.97 | < 0.001 |
| PD | 13.6 ± 4.1 | 11.0 ± 2.93 | 9.34 ± 3.01 | < 0.001 |
One-way ANOVA test was performed
PD Parkinson’s disease; MSA multiple system atrophy; PSP progressive supra-nuclear palsy; AGAZ age- and gender-adjusted Z; SUVR standardized uptake value ratio; NRI net reclassification index
Discussion
We tested the gender- and age-associated effect on the differentiation power of the highly available PET tracer—[18F]FDG in parkinsonism and found the adjustment for gender-specific and age-associated metabolic changes could improve the classification performance. Furthermore, the more pronounced improvements in females suggested the potential of AGAZ score to deepen the awareness of this partially overlooked Parkinsonism population.
As an inevitable and irreversible process, aging has become one of the prominent social issues and neurodegenerative diseases account for a non-negligible part of age-related disorders (Hou et al. 2019). Changes in the brain of normal aging have been observed in various aspects of functional and structural perspectives. Given that few or no effective treatments for aging-related neurodegenerative diseases are available, the accurate diagnosis remains crucial for therapy development. In AD, previous studies reported that the structural MRI-based diagnosis could lead to a misclassification of younger AD patients and older controls when regarded the age-related changes and disease-associated changes equally (Franke et al. 2010; Dukart et al. 2011). It is believed that age-related changes may have a greater impact on image-based diagnosis in patients outside the common onset ages because changes associated with later-life disease are subtle at very young patients, and age-related brain decline is severe in very old patients, which is leading to overestimation of those disease-free areas (Dukart et al. 2011). Hence, age correction methods have been proposed for both MRI (Dukart et al. 2011; Li et al. 2021) and [18F]FDG-based (Jiang et al. 2018) diagnosis of dementia. The onset age for parkinsonism is generally around 55–65 years old, and some early onset cases may show symptoms in the early 20s, while some patients show symptoms at very old ages (i.e., late 80s). Although the utility of [18F]FDG PET in parkinsonism’s classification is well identified (Tang et al. 2010; Meles et al. 2017; Meyer et al. 2017; Wu et al. 2022), whether age correction could improve its performance has been less explored. In this study, we used our previously established gender-specific longitudinal templates of age-related metabolic change in healthy controls for the correction (Zhang et al. 2017), because in addition to age, gender was also found to play an important role in age- and disease-associated brain changes (Yoshizawa et al. 2014; Kakimoto et al. 2016; Mosconi et al. 2017; De Micco et al. 2019). As expected, the conventional SUVR values without correction showed an impressive performance for differentiation, benefiting from the feature selection for disease-prone cerebral regions and the relatively large sample size for model training. Meanwhile, pattern scores seemed to be less favorable which might because of the limited sample size for the initial pattern identification. When applying further adjustment on top of SUVR, though the improvements were not large, which might be that the baseline accuracy is quite high, and the improvements were still meaningful.
Interestingly, such improvements were more pronounced in females than males. It is known that gender plays an important role not only in brain development and aging (Kaczkurkin et al. 2019), but also in the occurrence and development of neurodegeneration (Baba et al. 2006; Gillies et al. 2014; Ferretti et al. 2018). For example, compared with the male brain, the female brain was found to have a persistently younger metabolic brain age than their chronological age during aging (Goyal et al. 2019). In PD, except as biological sex-related differences in epidemiological and clinical features, distinctive pathophysiology involving dopaminergic neurodegeneration, neuro-inflammation, and oxidative stress highlights the diferences of sex-related brain abnormalities (Cerri et al. 2019). Although the gender difference is relatively less studied in MSA and PSP, considering the more preserved structure (Tremblay et al. 2020) and function (Haaxma et al., 2007; De Micco et al. 2019) found in female PD patients by MRI and dopaminergic PET studies, it is reasonable to speculate the less damaged brain might hinder the early diagnosis on [18F]FDG PET, which might help explain why adjusting the age-associated metabolic changes contribute more to the classification of females. As gender difference was mainly observed in MSA and PSP in our study, future studies investigating possible gender distinction on brain structural and functional levels within atypical Parkinsonism would be of interest. Given the growing concern about such between-sex differences on brain degeneration, the gender-specific AGAZ score may have implications for implementing prevention, diagnosis, and treatment strategies in the context of precision medicine, as well as deepening the awareness of the female parkinsonism population, who are currently partially overlooked (Cerri et al. 2019).
Dividing all patients into young, middle, and old groups, we further explored the difference of age- and gender-correction effect by ages. While improvements were observed in all subgroups, they were slightly pronounced in the old group with PD and MSA, and in the young group with PSP. A possible explanation for this discrepancy might be that the age of onset of PSP was generally older than PD and MSA (Boxer et al. 2017), and our dataset did not cover enough young or old patients.
Several limitations needed to be mentioned. First, though routine MRI images were available to excluding patients with structural brain abnormalities, partial volume correction and spatial normalization based on individual MRI were not possible. As brain atrophy plays an important role in aging, further studies with partial volume correction are needed for validation (Bonte et al. 2017). Second, although the current dataset from HPPI contained a relatively large number of patients, the cases meeting the definition of young-onset were limited. Future research on young-onset patient groups is warranted. Third, the current template for adjustment is based on limited number of normal controls, and the inclusion of more controls may further improve the performance of AGAZ score.
Conclusion
This study proposes a new strategy for [18F]FDG PET-based parkinsonism differentiation by adjusting age- and gender-associated confounding metabolic changes to assess disease-related metabolism alone. The results show that the proposed AGAZ score improves the differential diagnosis of Parkinsonism, especially in female patients with MSA and PSP. We believe that the AGAZ score will facilitate the early and differential diagnosis of Parkinsonism, as well as the development of gender-oriented targeted interventions, and inspire the optimization of image-based diagnostic methods in other neurodegenerative diseases. Further efforts are needed to develop a user-friendly software to transfer the current tool into clinical settings, which could facilitate the interpretation of [18F]FDG PET in the differential diagnosis of parkinsonism, especially in settings without experienced readers.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank the patients and family members who participated in the research.
Authors’ Contributions
CZ, FL, and KS conceived and designed the clinical study; MW and KS conceived and developed the algorithms. MW conducted the computational experiments. JL, PW, and HZ collected the imaging data and inspected images together with IY, SZ, JJ, SF, MS, AR, and SCH. JW, FL, JL, and PW collected clinical data and defined the standards of subject inclusion and evaluations. MW, JL, and KS contributed to the analysis of the data. JL, MW, KS, CZ, and FL wrote the manuscript with the advice, input and proof of other co-authors.
Funding
This work was supported by National Natural Science Foundation of China (81671239, 81361120393, 82171252, 81701250, 81401135, 81971641, 91949118, 81771372, 82021002), the Ministry of Science and Technology of China (2016YFC1306504), Shanghai Municipal Science and Technology Major Project (2017SHZDZX01, 2018SHZDZX03) and ZJ Lab, Shanghai Aging and Maternal and Child Health Research Special Project (2020YJZX0111), Clinical Research Plan of Shanghai Hospital Development Center (SHDC2020CR1038B), Science and Technology Innovation 2030 Major Projects (2022ZD0211600), Youth Medical Talents—Medical Imaging Practitioner Program by Shanghai Municipal Health Commission and Shanghai Medical and Health Development Foundation (SHWRS(2020)_087), the Swiss National Science Foundation (188350), and Jacques & Gloria Gossweiler Foundation and Siemens Healthineers.
Availability of Data and Material
All data included in this study will be available to the scientific community upon completion of the non-disclosure agreement (NDA) with the corresponding authors according to international data protection regulations.
Declarations
Conflict of interest
Axel Rominger and Kuangyu Shi received research support from Novartis and Siemens Healthineers. Other authors report no commercial interests or potential conflicts of interest.
Ethics Approval
This study obtained ethics permission from the Institutional Review Board of Huashan Hospital. All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee, and the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent to Participate
The written informed consent was obtained from subjects included in this study.
Footnotes
Jiaying Lu and Min Wang equally contributed as first authors.
Fengtao Liu and Chuantao Zuo equally contributed as corresponding authors.
Contributor Information
Fengtao Liu, Email: liufengtao@fudan.edu.cn.
Chuantao Zuo, Email: zuochuantao@fudan.edu.cn.
References
- Alba AC, Agoritsas T, Walsh M, Hanna S, Iorio A, Devereaux PJ, et al. Discrimination and calibration of clinical prediction models: Users’ guides to the medical literature. JAMA. 2017;318(14):1377–1384. doi: 10.1001/jama.2017.12126. [DOI] [PubMed] [Google Scholar]
- Baba Y, Putzke JD, Whaley NR, Wszolek ZK, Uitti RJ. Progressive supranuclear palsy: phenotypic sex differences in a clinical cohort. Mov Disord. 2006;21(5):689–692. doi: 10.1002/mds.20769. [DOI] [PubMed] [Google Scholar]
- Bonte S, Vandemaele P, Verleden S, Audenaert K, Deblaere K, Goethals I, et al. Healthy brain ageing assessed with 18F-FDG PET and age-dependent recovery factors after partial volume effect correction. Eur J Nucl Med Mol Imaging. 2017;44(5):838–849. doi: 10.1007/s00259-016-3569-0. [DOI] [PubMed] [Google Scholar]
- Boxer AL, Yu JT, Golbe LI, Litvan I, Lang AE, Höglinger GU. Advances in progressive supranuclear palsy: new diagnostic criteria, biomarkers, and therapeutic approaches. Lancet Neurol. 2017;16(7):552–563. doi: 10.1016/S1474-4422(17)30157-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugnolo A, Morbelli S, Arnaldi D, De Carli F, Accardo J, Bossert I, et al. Metabolic correlates of rey auditory verbal learning test in elderly subjects with memory complaints. J Alzheimer’s Dis. 2014;39(1):103–113. doi: 10.3233/JAD-121684. [DOI] [PubMed] [Google Scholar]
- Cavedo E, Chiesa PA, Houot M, Ferretti MT, Grothe MJ, Teipel SJ, et al. Sex differences in functional and molecular neuroimaging biomarkers of Alzheimer’s disease in cognitively normal older adults with subjective memory complaints. Alzheimer’s Dement. 2018;14(9):1204–1215. doi: 10.1016/j.jalz.2018.05.014. [DOI] [PubMed] [Google Scholar]
- Cerri S, Mus L, Blandini F. Parkinson’s disease in women and men: what’s the difference? J Parkinsons Dis. 2019;9(3):501–515. doi: 10.3233/JPD-191683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chmielnicki W, Stąkapor K (2011) A New Approach to Multi-class SVM-Based Classification Using Error Correcting Output Codes. In: Burduk, R., Kurzyński, M., Woźniak, M., Żołnierek, A. (eds) Computer Recognition Systems 4. Advances in Intelligent and Soft Computing, vol 95. Springer, Berlin, Heidelberg, pp 499–506. 10.1007/978-3-642-20320-6_52
- Cosgrove KP, Mazure CM, Staley JK. Evolving knowledge of sex differences in brain structure, function, and chemistry. Biol Psychiatry. 2007;62(8):847–855. doi: 10.1016/j.biopsych.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Micco R, Esposito F, di Nardo F, Caiazzo G, Siciliano M, Russo A, et al. Sex-related pattern of intrinsic brain connectivity in drug-naïve Parkinson’s disease patients. Mov Disord. 2019;34(7):997–1005. doi: 10.1002/mds.27725. [DOI] [PubMed] [Google Scholar]
- Dukart J, Schroeter ML, Mueller K. Age Correction in dementia—matching to a healthy brain. PLoS ONE. 2011;6(7):e22193. doi: 10.1371/journal.pone.0022193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewers M, Brendel M, Rizk-Jackson A, Rominger A, Bartenstein P, Schuff N, et al. Reduced FDG-PET brain metabolism and executive function predict clinical progression in elderly healthy subjects. NeuroImage Clin. 2014;4:45–52. doi: 10.1016/j.nicl.2013.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferretti MT, Iulita MF, Cavedo E, Chiesa PA, Dimech AS, Chadha AS, et al. Sex differences in Alzheimer disease—the gateway to precision medicine. Nat Rev Neurol. 2018;14(8):457–469. doi: 10.1038/s41582-018-0032-9. [DOI] [PubMed] [Google Scholar]
- Franke K, Ziegler G, Klöppel S, Gaser C. Estimating the age of healthy subjects from T1-weighted MRI scans using kernel methods: exploring the influence of various parameters. Neuroimage. 2010;50(3):883–892. doi: 10.1016/j.neuroimage.2010.01.005. [DOI] [PubMed] [Google Scholar]
- Ge J, Wu J, Peng S, Wu P, Wang J, Zhang H, et al. Reproducible network and regional topographies of abnormal glucose metabolism associated with progressive supranuclear palsy: multivariate and univariate analyses in American and Chinese patient cohorts. Hum Brain Mapp. 2018;39(7):2842–2858. doi: 10.1002/hbm.24044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies GE, Pienaar IS, Vohra S, Qamhawi Z. Sex differences in Parkinson’s disease. Front Neuroendocrinol. 2014;35(3):370–384. doi: 10.1016/j.yfrne.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RNA, Friston KJ, Frackowiak RSJ. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14(1 Pt 1):21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Goyal MS, Blazey TM, Su Y, Couture LE, Durbin TJ, Bateman RJ, et al. Persistent metabolic youth in the aging female brain. Proc Natl Acad Sci USA. 2019;116(8):3251–3255. doi: 10.1073/pnas.1815917116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve DN, Svarer C, Fisher PM, Feng L, Hansen AE, Baare W, et al. Cortical surface-based analysis reduces bias and variance in kinetic modeling of brain PET data. Neuroimage. 2014;92:225–236. doi: 10.1016/j.neuroimage.2013.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haaxma CA, Bloem BR, Borm GF, Oyen WJG, Leenders KL, Eshuis S, et al. Gender differences in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2007;78(8):819–824. doi: 10.1136/jnnp.2006.103788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y, Dan X, Babbar M, Wei Y, Hasselbalch SG, Croteau DL, et al. Ageing as a risk factor for neurodegenerative disease. Nat Rev Neurol. 2019;15(10):565–581. doi: 10.1038/s41582-019-0244-7. [DOI] [PubMed] [Google Scholar]
- Ishibashi K, Onishi A, Fujiwara Y, Oda K, Ishiwata K, Ishii K. Longitudinal effects of aging on 18F-FDG distribution in cognitively normal elderly individuals. Sci Rep. 2018;8(1):11557. doi: 10.1038/s41598-018-29937-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Sun Y, Zhou H, Li S, Huang Z, Wu P, et al. Study of the influence of age in 18F-FDG PET images using a data-driven approach and its evaluation in Alzheimer’s disease. Contrast Media Mol Imaging. 2018;2018:3786083. doi: 10.1155/2018/3786083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczkurkin AN, Raznahan A, Satterthwaite TD. Sex differences in the developing brain: insights from multimodal neuroimaging. Neuropsychopharmacology. 2019;44(1):71–85. doi: 10.1038/s41386-018-0111-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakimoto A, Ito S, Okada H, Nishizawa S, Minoshima S, Ouchi Y. Age-related sex-specific changes in brain metabolism and morphology. J Nucl Med. 2016;57(2):221–225. doi: 10.2967/jnumed.115.166439. [DOI] [PubMed] [Google Scholar]
- Kato T, Inui Y, Nakamura A, Ito K. Brain fluorodeoxyglucose (FDG) PET in dementia. Ageing Res Rev. 2016;30:73–84. doi: 10.1016/j.arr.2016.02.003. [DOI] [PubMed] [Google Scholar]
- Knopman DS, Jack CR, Wiste HJ, Lundt ES, Weigand SD, Vemuri P, et al. 18F-fluorodeoxyglucose positron emission tomography, aging, and apolipoprotein E genotype in cognitively normal persons. Neurobiol Aging. 2014;35(9):2096–2106. doi: 10.1016/j.neurobiolaging.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Jang I, Riphagen J, Almaktoum R, Yochim KM, Ances BM, et al. Identifying individuals with Alzheimer’s disease-like brains based on structural imaging in the Human Connectome Project Aging cohort. Hum Brain Mapp. 2021;42(17):5535–5546. doi: 10.1002/hbm.25626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li TR, Dong QY, Jiang XY, Kang GX, Li X, Xie YY, et al. Exploring brain glucose metabolic patterns in cognitively normal adults at risk of Alzheimer’s disease: a cross-validation study with Chinese and ADNI cohorts. NeuroImage Clin. 2022;33:102900. doi: 10.1016/j.nicl.2021.102900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meles SK, Teune LK, De Jong BM, Dierckx RA, Leenders KL. Metabolic imaging in Parkinson disease. J Nucl Med. 2017;58(1):23–28. doi: 10.2967/jnumed.116.183152. [DOI] [PubMed] [Google Scholar]
- Meyer PT, Frings L, Rücker G, Hellwig S. 18F-FDG PET in Parkinsonism: differential diagnosis and evaluation of cognitive impairment. J Nucl Med. 2017;58(12):1888–1898. doi: 10.2967/jnumed.116.186403. [DOI] [PubMed] [Google Scholar]
- Mosconi L, Berti V, Quinn C, McHugh P, Petrongolo G, Varsavsky I, et al. Sex differences in Alzheimer risk: brain imaging of endocrine vs chronologic aging. Neurology. 2017;89(13):1382–1390. doi: 10.1212/WNL.0000000000004425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobili F, Arbizu J, Bouwman F, Drzezga A, Agosta F, Nestor P, et al. European Association of Nuclear Medicine and European Academy of Neurology recommendations for the use of brain 18 F-fluorodeoxyglucose positron emission tomography in neurodegenerative cognitive impairment and dementia: Delphi consensus. Eur J Neurol. 2018;25(10):1201–1217. doi: 10.1111/ene.13728. [DOI] [PubMed] [Google Scholar]
- Petit-Taboue MC, Landeau B, Desson JF, Dary M, Baron JC. Effects of healthy aging on the regional cerebral metabolic rate of glucose assessed with statistical parametric mapping. Neuroimage. 1998;7(3):176–184. doi: 10.1006/nimg.1997.0318. [DOI] [PubMed] [Google Scholar]
- Shen B, Wei S, Ge J, Peng S, Liu F, Li L, et al. Reproducible metabolic topographies associated with multiple system atrophy: network and regional analyses in Chinese and American patient cohorts. NeuroImage Clin. 2020;28:102416. doi: 10.1016/j.nicl.2020.102416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi R, Ishii K, Kakigi T, Yokoyama K. Gender and age differences in normal adult human brain: voxel-based morphometric study. Hum Brain Mapp. 2011;32(7):1050–1058. doi: 10.1002/hbm.21088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang CC, Poston KL, Eckert T, Feigin A, Frucht S, Gudesblatt M, et al. Differential diagnosis of parkinsonism: a metabolic imaging study using pattern analysis. Lancet Neurol. 2010;9(2):149–158. doi: 10.1016/S1474-4422(10)70002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay C, Abbasi N, Zeighami Y, Yau Y, Dadar M, Rahayel S, et al. Sex effects on brain structure in de novo Parkinson’s disease: a multimodal neuroimaging study. Brain. 2020;143(10):3052–3066. doi: 10.1093/brain/awaa234. [DOI] [PubMed] [Google Scholar]
- van Aalst J, Devrome M, Van Weehaeghe D, Rezaei A, Radwan A, Schramm G, et al. Regional glucose metabolic decreases with ageing are associated with microstructural white matter changes: a simultaneous PET/MR study. Eur J Nucl Med Mol Imaging. 2022;49(2):664–680. doi: 10.1007/s00259-021-05518-6. [DOI] [PubMed] [Google Scholar]
- Wu P, Wang J, Peng S, Ma Y, Zhang H, Guan Y, et al. Metabolic brain network in the Chinese patients with Parkinson’s disease based on 18F-FDG PET imaging. Parkinsonism Relat Disord. 2013;19(6):622–627. doi: 10.1016/j.parkreldis.2013.02.013. [DOI] [PubMed] [Google Scholar]
- Wu P, Zhao Y, Wu J, Brendel M, Lu J, Ge J, et al. Differential diagnosis of parkinsonism based on deep metabolic imaging indices. J Nucl Med. 2022 doi: 10.2967/jnumed.121.263029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizawa H, Gazes Y, Stern Y, Miyata Y, Uchiyama S. Characterizing the normative profile of 18F-FDG PET brain imaging: sex difference, aging effect, and cognitive reserve. Psychiatry Res. 2014;221(1):78–85. doi: 10.1016/j.pscychresns.2013.10.009. [DOI] [PubMed] [Google Scholar]
- Zhang H, Wu P, Ziegler SI, Guan Y, Wang Y, Ge J, et al. Data-driven identification of intensity normalization region based on longitudinal coherency of 18F-FDG metabolism in the healthy brain. Neuroimage. 2017;146:589–599. doi: 10.1016/j.neuroimage.2016.09.031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data included in this study will be available to the scientific community upon completion of the non-disclosure agreement (NDA) with the corresponding authors according to international data protection regulations.