Abstract
Background
In recent years, authors have repeatedly reported on the significance of social support in cancer survival. Although overall the studies appear to be convincing, little is known about which types of social support promote better survival rates, and which subgroups of cancer patients are more susceptible to the benefits of it. The aim of this study was to identify, organize, and examine studies reporting on the significance of social support in cancer survival.
Methods
The PubMed, CINAHL and EBSCO databases were searched using the keywords social support/marital status, cancer, and survival/mortality. Where possible we used a meta‐analytical approach, specifically a random effect model, in order to combine the results of the hazard ratios in studies from which this information could be obtained. When interpreting clinical relevance, we used the number needed to treat (NNT).
Results
Better survival was observed in married patients when compared to unmarried (single, never‐married, divorced/separated, and widowed) in overall and cancer‐specific survival. Gender group differences showed that the association was statistically significant only in cancer‐specific survival when comparing divorced/separated male and female cancer patients (p < 0.001), thus confirming results from the previous meta‐analysis.
Conclusions
Being unmarried is associated with significantly worse overall and cancer‐specific survival. The most vulnerable group found in our study were divorced/separated men. The results of this review can motivate physicians, oncologists, and other healthcare professionals to be aware of the importance of patients' social support, especially in the identified sub‐group.
Keywords: marital status, neoplasms, social support, survival, systematic review
Being married is associated with significantly better overall survival. Being unmarried male is associated with significantly worse survival. Marital status should be taken into consideration when providing cancer care.
1. INTRODUCTION
Cancer is a disease with a global health burden; it is a leading cause of deaths worldwide. 1 While several important risk factors have been identified (e.g. tobacco use, cancer‐causing infections, high body mass index, etc.), data on how psychosocial factors impact on cancer survival is less evident. Researchers have been exploring the association between social support and cancer survival since 1980 in many naturalistic (non‐interventional) and interventional studies. A recent meta‐analysis exploring the effect of randomized‐controlled trials on cancer survival concluded that the overall effect favors groups that receive psychosocial treatment. Interestingly, the effect was higher in studies that included more unmarried patients. 2
Unmarried cancer patients are more likely to be diagnosed with an advanced stage of the disease than married patients, 3 , 4 , 5 who often have a higher socioeconomic status than unmarried ones, enabling them to have better access to healthcare. 6 They can also receive instrumental support from their spouse (e.g. assistance with transportation, paperwork, household chores) so they can fully focus on their treatment. Importantly, a partner can provide emotional support, which can mitigate the stress of cancer treatment. 7
Marital status has been found to be an independent predictive factor associated with better odds of survival in various cancer types. 8 , 9 The effect of the social support provided by a partner may be physiologically mediated through neuroendocrine, nervous, and immune interactions which are directly related to cancer. 10 For example, cancer patients who have a higher quality of social support have greater activity in natural killer (NK) cells, which are important cytotoxic cells of the immune system and can recognize and destroy cancer cells. 10 The hormone oxytocin, which is released during social interactions, may also indirectly inhibit the growth of cancer cells by inhibiting the stress response. 11
To date, two systematic reviews and meta‐analyses exist on the topic of social support and cancer survival. 12 , 13 They report a 12% decrease mortality risk in married patients and that never‐married patients had a worse survival rate than widowed or divorced/separated patients. 12 Furthermore, divorced/separated men had a 12% higher risk of cancer mortality compared to the 9% mortality rate in women, thus drawing attention to the importance of the role of gender. 13 However, the study only evaluated the association between marital status and survival by gender, excluding a general marital status—cancer survival analysis. Importantly, their literature search was carried out in 2018, and this area of research experienced significant growth after their publications.
The aim of this paper is to examine the association between marital status and different groups of unmarried cancer patients (e.g. divorced/separated, single, widowed) on overall and cancer‐specific survival. As the different groups of unmarried patients likely experience different levels of stress, the examination of survival by group can unveil new data on the link between marital status and cancer survival. We set the following objectives for the review and meta‐analysis:
To analyze the difference between overall and cancer‐specific survival according to marital status (i.e., married, unmarried, never‐married single, divorced/separated and widowed)
To examine which subgroup of cancer patients (e.g. gender, cancer stage) are associated with better overall and cancer‐specific survival.
2. METHODS
2.1. Literature search strategy
The study followed the PRISMA statement (Preferred Reporting Items for Systematic Review and Meta‐Analysis). Relevant articles were identified through the PubMed, CINAHL, and EBSCO databases between January and June 2018, and regularly updated up to April 2022 (see Table S1 for the specified search strategy for EBSCO and PubMed). Additional articles were obtained by searching through the reference lists of the included studies.
2.2. Inclusion and exclusion criteria
The review included eligible studies that: (1) were published as original articles; (2) analyzed adult cancer patients (>18 years); (3) reported a correlation between marital status and survival, such as overall survival (OS) or cancer‐specific survival (CSS); (4) provided clear data from which to directly extract hazard ratios (HR) and 95% confidence intervals (CI). Articles were excluded if: (1) they analyzed patients with childhood cancer; (2) the paper was a republished report; (3) the effect of marital status on cancer survival was not a primary outcome; (4) and they were not published in English.
2.3. Data collection and quality assessment
Three authors independently examined all the selected publications and extracted data in accordance with the following protocol. From each of the selected articles, we obtained the following data: first author; year of publication; country; type of longitudinal study (prospective/retrospective); sample size (n); recruitment; follow‐up time expressed in years; sex (the percentage of male and female subjects); age of participants (M, SD); diagnosis (type and stage of cancer); dependent variable (marital status); factors that were included in the adjusted analysis; and the conclusion concerning the association between marital status and survival (reported with hazard ratio (HR) and 95% cluster interval (CI)). Data were collated in a spreadsheet with columns denoting extracted data categories and rows denoting studies (see Table 1).
TABLE 1.
Main characteristics of selected studies (n = 67)
| Study's characteristics | Patients' demographic characteristics | Patients' clinical characteristics | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First author | Year | Country | Type of study | Sample size | Recruitment | Follow‐up (years) | Sex (%) | Age (M, SD) | Type of cancer | Stage of cancer | Dependent variable | Adjusted analysis |
| Goodwin 23 | 1987 | New Mexico | Retrospective | 27,706 | Tumor Registry | 5 | ND | ND | Various | I–IV | Marital status | Stage at diagnosis and definitive treatment. |
| Osborne 39 | 2005 | USA | Retrospective | 32,268 | SEER | 3 | Women | ND | Breast cancer | I–IV | Marital status | Age, ethnicity, SEER area, tumor size, stage, grade, estrogen receptor status, comorbidity index score, treatment variables, chemotherapy, census tract education level and census tract household income in quartiles. |
| Reyes Ortiz 57 | 2007 | USA | Retrospective | 14,630 | SEER | 5 | W (39.9), M (60.1), | 75.2 (6.9) | Melanoma | I–IV | Marital status | Age, gender, race/ethnicity, socioeconomic status, histology, site, stage at diagnosis and comorbidity. |
| Saito‐Nakaya 63 | 2008 | Japan | Prospective | 1230 |
Thoracic Oncology Division, National Cancer Center Hospital East |
5 |
W (29.7), M (70.3), |
ND | Non‐small cell lung cancer | I–IV | Marital status | age, BMI, education, PS, histology type, smoke stage, definitive treatment, and HADS‐depression adjusted. |
| Datta 28 | 2009 | USA | Retrospective | 19,982 | SEER | 5 |
W (25.8), M (74.2) |
60–80+ (ND) | Bladder cancer | II–IV | Marital status | Age, race, number of comorbidities, receipt of treatment, socioeconomic status, and teaching hospital designation, stage at diagnosis. |
| Patel 52 | 2010 | USA | Retrospective | 7997 | SEER | 5 | Women | ND | Cervical cancer | I–IV | Marital status | |
| Abdollah 26 | 2011 | USA | Retrospective | 163,697 | SEER | ND | Men | 63 | Prostate cancer | I–IV | Marital status | Age, race, socioeconomic status, tumor grade, tumor stage, lymph node stage, year of surgery |
| Baine 31 | 2011 | USA | Retrospective | 34,555 | SEER | ND |
W (48.6), M (51.4) |
69 | Pancreatic cancer | I–IV | Marital status | Gender, race, age at diagnosis, year of diagnosis, cancer‐directed surgery, radiation therapy and stage. |
| Wang 86 | 2011 | USA | Retrospective | 127,753 | SEER | 5 | W (52.5), M (47.5) | ND | Colon cancer | I–IV | Marital status | Age, cancer stage, race and surgery receipt. |
| Abern 30 | 2012 | USA | Retrospective | 20,245 | SEER | 10 | Men | 35.4 | Testis cancer | I–II | Marital status | Age, stage at diagnosis, Race, Histologic type, Year of diagnosis, Region |
| Tannenbaum 78 | 2013 | USA | Retrospective | 161,228 | The Florida Cancer Data System and Florida's Agency for Health Care Administration | 5 |
W (44.3), M (55.7) |
69.8 (11.2) | Lung cancer | I–IV | Marital status | Race/Ethnicity/SES, demographics + clinical + individual comorbidities. |
| Aizer 85 | 2013 | USA | Retrospective | 734,899 | SEER | 3.1 |
W (48.1), M (51.9) |
64.5 (13) | Various | I–IV | Marital status | Age, gender, race, income, education, residence type, stage, primary site and type of treatment. |
| Mahdi 66 | 2011 | USA | Retrospective | 49,777 | SEER | 2.2 | Women | ND | Epithelial ovarian cancer | I–IV | Marital status | Age, race, histology, stage, grade, lymphadenectomy and extent of surgery. |
| Brusselaers 22 | 2015 | Sweden | Prospective | 606 | Swedish Hospitals | 5 | M (80.4), W (19.6) | 23.9% <60, 76.1% > 60 | Esophageal cancer | I–IV | Marital status | Sex, age, tumor stage, histology, major complications, comorbidity and surgeon volume. |
| Inverso 41 | 2014 | USA | Retrospective | 51,272 | SEER | 1.6 | W (25.1), M (74.9), | 61 (12.9) | Head and neck cancer | I–IV | Marital status | Age at diagnosis, gender, race, income, level of education, residence type and definitive treatment. |
| Li 67 | 2015 | USA | Retrospective | 112,776 | SEER | 5 | W (48.6), M (51.4) | ND | Colorectal cancer | I–IV | Marital status | Age, race, grade, histotype and TNM stage. |
| Wang 65 | 2016 | USA | Retrospective | 13,370 | SEER | 1.1 | W (49.43). M (50.57) | 32.8% ≤60, 67.2% >60 | Pancreatic cancer | I–IV | Marital status | Primary site location, age, race, year of diagnosis, tumor size (cm), SEER stage |
| Zhou 36 | 2016 | USA | Retrospective | 18,815 | SEER | 5 | W (37.6), M (62.4) | ND | Gastric cancers | I–IV | Marital status | Age, race, pathological differentiation, histological type, TNM stage, surgery and radiotherapy. |
| Eskander 24 | 2016 | USA | Retrospective | 11,849 | Tumor registry | 1 | W (63.8), M (36.2) | ND | Various | I–IV | Marital status | Gender, age, insurance status, race; lung: surgery models, type of therapy and stage. |
| Shi 58 | 2016 | USA | Retrospective | 61,077 | SEER | 19 | W (78.1), M (21.9) | 47.7 (14.8) | Thyroid cancer | I–IV | Marital status | Gender, age, race, follicular vs. papillary, T3/4 vs. T1/2, N stage, distant metastasis, surgery procedure, lobectomy and adjuvant therapy. |
| Jin 68 | 2016 | USA | Retrospective | 18,196 | SEER | M = 2 (1–100) | W (36.7), M (63.3) | ND | Gastric cancer | I–IV | Marital status | Age, gender, race, tumor location, histological type, differentiated grade, stage, and year of diagnosis. |
| He 69 | 2017 | USA | Retrospective | 40,809 | SEER | ND |
W (25.4), M (74.6) |
47.1% <60, 52.9% >60 | Liver cancer | I–IV | Marital status | Gender, age, race, grade, hystotype, SEER stage, type of therapy |
| Adekolujo 40 | 2016 | USA | Retrospective | 3761 | SEER | 5 | Men | Married 64.8, Unmarried 65 | Breast cancer | I–IV | Marital status | Age, race, median household income, stage, grade, combined ER/PR status, histological type, and surgical treatment |
| Du 53 | 2017 | USA | Retrospective | 69,139 | SEER | M = 1.3 | W (24.0), M (76.0) | 67 (11.7) | Esophageal cancer | I–IV | Marital status | Age, gender, race/ethnicity, household income, histology, tumor site, SEER stages, therapy, and insurance status |
| Zhang (a) 62 | 2017 | USA | Retrospective | 16,910 | SEER | 5 |
W (44.0), M (56.0) |
26.0% <57, 74.0% >57 | Gastric cancer | I–IV | Marital status |
Site, sex, race, age, grade, histotype, TNM stage, surgery type and selection of radiotherapy |
| Miao 34 | 2017 | USA | Retrospective | 112,860 | SEER | 5 |
W (36.6), M (63.4), |
38.7% <60, 61.3% >60 | Kidney cancer | I–IV | Marital status | Sex, age, race, grade, TNM, SEER stage, type of therapy |
| Li 60 | 2017 | USA | Retrospective | 6627 | SEER | 5 |
W (70.4), M (29.6) |
24.9% <60, 75.1% >60 | Gallbladder cancer | I–IV | Marital status | Age, race, grade, histologic type, AJJC stage, SEER |
| Rubin 51 | 2017 | USA | Retrospective | 65 | Boston University Medical Center | 3 |
W (18.5), M (81.5) |
61.58 (8.94) | Human papilloma virus‐positive oropharyngeal cancer | I–IV | Marital status |
age, sex, race, insurance type, smoking status, treatment, and AJCC combined pathologic stage |
| Wang 71 | 2017 | USA | Retrospective | 62,405 | SEER | 5 |
W (38.1), M (61.9) |
46.5% <60, 53.5% >60 | Renal cancer | I–IV | Marital status | Sex, age, race, tumor size, laterality, SEER stage, grade |
| Hinyard 21 | 2017 | USA | Retrospective | 166,701 | SEER | ND | Women | 64.5 (24.1) | Breast cancer | I–IV | Marital status | Unadjusted analysis |
| Zhang (b) 35 | 2017 | USA | Retrospective | 15,598 | SEER | ND |
W (19.3), M (80.7) |
20.2% <55, 79.8% >56 | Esophageal cancer | I–IV | Marital status | Sex, race, age, histology, grade, location, TNM stage, therapy |
| Wu 79 | 2017 | USA | Retrospective | 70,006 | SEER | M = 1.3 |
W (47.0), M (53.0) |
23.3% <60, 76.7% >60 | Non‐small cell lung cancer | ND |
Marital status |
Sex, age, race, diagnosis year, median household income, grade, TNM stage, histology, surgery, radiotherapy, radiotherapy |
| Alvi 87 | 2018 | USA | Retrospective | 1188 | SEER | 10 |
W (57.5) M (42.5) |
20+ (ND) | Spinal cord tumors | ND | Marital status | Age, gender, SES, insurance status |
| Chen 88 | 2018 | USA | Retrospective | 6582 | SEER | 5 |
W (49.0) M (51.0) |
18–70+ (ND | Gastrointestinal stromal tumor | Localized, regional, distant stage | Marital status | Sex, race, age histology, stage, surgery, radiotherapy |
| Liao 44 | 2018 | China | Retrospective | 457 |
Cancer registry dataset of the Kaohsiung veteran's general hospital |
5 |
W (7.7), M (92.3) |
ND | Oral cavity cancer | I–IV | Marital status | T‐category, N Category, differentiation, neck dissection, adjuvant therapy |
| Niu 72 | 2018 | USA | Retrospective | 133,846 | SEER | 5 |
W (24.2), M (75.8) |
78.5% >60+ 21.5% <60 | Bladder urothelial carcinoma | I–IV | Marital status | Sex, age, race, primary site, pathological grading, TNM stage, surgery |
| Wu 61 | 2018 | USA | Retrospective | 4001 | SEER | 8 | Women | Median 66 | Vulvar cancer | I–IV | Marital status | Age, race, grade, tumor stage, nodal stage, M stage, surgery, radiotherapy, chemotherapy |
| Xie—a 73 | 2018 | USA | Retrospective | 43,324 | SEER | ND, ~10 |
W (42.6) M (57.4) |
ND | Astrocytoma | I–IV | Marital status | Age, sex, race, WHO grade, diagnosis year, median household income, surgery |
| Xie—b 74 | 2018 | USA | Retrospective | 30,767 | SEER | ND, ~10 |
W (42.0) M (58.0) |
ND | Glioblastoma multiforme | I–IV | Marital status | Sex, age, race, registry site, diagnosis year, education, median household income, insurance, laterality of cancer, surgery, metastasis, tumor size, SEER stage |
| Zhang 56 | 2018 | USA | Retrospective | 18,013 | SEER | 5 |
W (49.7) M (50.3) |
55.6% <60, 44.4% >60 | Soft tissue sarcoma | I–IV | Marital status | Sex, age, race, diagnosis year, pathological grade, tumor size, SEER historic stage, insurance status, surgery |
| Li 38 | 2018 | USA | Retrospective | 5196 | SEER | 5 |
W (30.7) M (69.3) |
65+ | Rectal cancer | I–IV | Marital status | Sex, age, year of diagnosis, race, stage, grade, chemotherapy, radiotherapy, and surgery type |
| Wang 81 | 2018 | USA | Retrospective | 27,498 | SEER | 5 |
W (40.4) M (59.6) |
40.8% <60, 59.2% ≥60 |
Rectal cancer | I–IV | Marital status | Sex, age, race, pathologic grade, histotype, adenocarcinoma, surgery, TNM stage |
| Liu 70 | 2019 | USA | Retrospective | 824,554 | SEER | 5 | ND | 68.6 (9.05) | Prostate cancer | ND | Marital status | Age, race, Gleason score, surgery |
| Chen 46 | 2019 | USA | Retrospective | 72, 984 | SEER | 10 |
W (54.2) M (45.8) |
18–75+ (ND) | Non‐small cell lung cancer | I–IV | Marital status | Sex, race, age, histology, tumor stage, surgery, radiotherapy |
| Dong 82 | 2019 | USA | Retrospective | 39,387 | SEER | 5 | Women | Age range 18–80+, most 50–69 (ND) | Endometrial cancer | I–IV | Marital status | Age, diagnosis year, race, histology, grade of cancer (I–IV) |
| Liu 33 | 2019 | USA | Retrospective | 1342 | SEER | 5 | Women | 51.6% =56+; 48.4% <56 | Breast cancer | I–IV | Marital status | Age, race, grade, AJCC stage, Hormone receptor, HER‐2, surgery, chemotherapy, radiotherapy |
| Luo 75 | 2019 | USA | Retrospective | 19,276 | SEER | Women | 62.98 (13.75) | Ovarian cancer | I–IV | Marital status | Race, age, histological types, diagnostic year, radiotherapy | |
| Osazuwa‐Peters 29 | 2019 | USA | Retrospective | 460 | Hospital Tumor Registry | 15 |
W (26.7) M (73.3) |
59.19 (11.33) | Head and neck cancer | Early and late | Marital status | Sex, race, age, alcohol use, insurance status, tobacco use, stage, treatment type, primary site |
| Qiu 84 | 2019 | USA | Retrospective | 2725 | SEER | 4 |
W (43.5) M (56.5) |
70.8% ≤50; 29.2 >50 | Osteosarcoma | I–IV | Marital status | Age, sex, grade, TNM stage, surgery |
| Simpson 83 | 2019 | USA | Retrospective | 71,799 | SEER | ND |
W (23.8) M (76.2) |
62.3 (12.1) | Head and neck cancer | I–IV | Marital status | Race, insurance status, stage, site, treatment, age at diagnosis, year of diagnosis, county‐level median income |
| Yan 80 | 2019 | USA | Retrospective | 1581 | SEER | 5 |
W (26.7) M (73.3) |
47.7% <60; 52.3% >60 | Hepatocellular carcinoma | I–IV | Marital status | Sex, race, age, year of diagnosis, TNM stage, Tumor size, radiotherapy, chemotherapy |
| Zhai 32 | 2019 | USA | Retrospective | 298,434 | SEER | 10 |
W (99.3) M (0.7) |
ND | Breast cancer | 0‐IV | Marital status | Age, sex, race, stage, grade, surgery, hormone receptor status |
| Rosiello 45 | 2019 | USA | Retrospective | 11,167 | SEER | 5 |
W (31.1) M (68.7) |
67.9 (ND) | Non‐metastatic urothelial bladder cancer | 0‐IV | Marital status | Age, ethnicity, SES, tumor grade, tumor stage, nodal stage, year of surgery |
| Zhang 76 | 2019 | USA | Retrospective | 31,895 | SEER | 5 |
W (34.9) M (65.1) |
55 median (18–64 IQR) | Renal cell carcinoma | I–IV | Marital status | Sex, age, race, tumor size, tumor grade, stage, surgery, insurance status, county level median household income, education, county percentage unemployment |
| Khan 27 | 2019 | USA | Retrospective | 3579 | Institutional cancer registry | 10.2 | Men | 60.4 (7.2) | Prostate cancer | I–II | Marital status | Age, race, comorbidity, log‐transformed PSA, Biopsy Gleason grade |
| Yang 43 | 2019 | USA | Retrospective | 925 | Chi‐Mei medical center Cancer registry | 5 |
W (42.5) M (57.5) |
65 (12) | Colon cancer | I–IV | Marital status | Age at diagnosis, lymph node count, stage, grade, perineural invasion, circumferential resection margin, adjuvant treatment |
| Maas 47 | 2020 | USA | Retrospective | 36,578 | Florida Cancer Data System | 6–14 years |
W (41.3) M (58.7) |
62.5 (16.2) | Melanoma | Early and late stage | Marital status | Age at diagnosis, sex, insurance status, race, ethnicity, tobacco use, histology, staging, primary site, geographic are |
| Rachidi 77 | 2020 | USA | Retrospective | 73,558 | SEER | ND |
W (45.7) M (54.3) |
60.4 (15.8) | Cutaneous melanoma | ND | Marital status | Sex, race, stage, continuous age |
| Zhou 62 | 2020 | USA | Retrospective | 3947 | SEER | ND |
M (60.9) |
<older than 50 |
Gastric neuroendocrine neoplasm |
Localized, regional, distant | Marital status | Age at diagnosis, sex, year of diagnosis, ethnicity, grade, tumor stage, size, surgery |
| Cai 54 | 2020 | USA | Retrospective | 4217 | SEER | 3.8 |
W (47.5) M (52.5) |
43.0% <60, 57.0% ≥60 |
Uveal melanoma | I–IV | Marital status | Gender, age, race, diagnosis year, SEER stage, surgery, median household income, registry site |
| Alyabsi 48 | 2021 | Saudi Arabia | Retrospective | 936 | MNG‐HA Cancer registry | 5 |
W (38.3) M (61.7) |
46.6% <59, 53.4% >60 | Colorectal cancer | Localized, regional, distant metastatic, other | Marital status | Gender, age at diagnosis, stage, pathological grading, tumor site, tumor morphology, chemotherapy status, surgery status, radiotherapy status |
| Ding 55 | 2021 | USA | Retrospective | 8834 | SEER | 5 |
W (18.9) M (81.1) |
56.7% ≤65, 43.3 ≥65 | Laryngeal cancer | I–IV | Marital status | Sex, age, grade, race, histological type, surgery, AJJC stage, radiotherapy, chemotherapy |
| Liang 49 | 2021 | USA | Retrospective | 4933 | SEER | ND |
W (19.6) M (80.4) |
38.1% <60, 61.9% ≥60 | Liver cancer | I–IV | Marital status | Age, sex, race, grade, AJCC, SEER stage |
| Xing 50 | 2021 | USA | Retrospective | 3375 | SEER | ND |
W (41.3) M (58.7) |
53.2% ≤60, 46.8 >60 |
Mycosis fungoides | Localized, regional, distant stage | Marital status | Age, sex, race, T stage |
| Ai 37 | 2021 | USA | Retrospective | 1344 | SEER | ND |
W (60.7) M (39.3) |
52.9 (15.5) | Medullary thyroid cancer | T1–T4 | Marital status | Sex, age, race, tumor stage, nodal stage, metastatic, surgery |
| Wu 64 | 2022 | USA | Retrospective | 61.928 | SEER | 5 |
W (52.4) M (47.6) |
40.7% <65, 59.3% ≥65 |
Lung adenocarcinoma | I–IV | Marital status | Sex, age, race, grade, TNM stage, surgery, radiotherapy, chemotherapy, median household income |
| Ayaz 25 | 2022 | USA | Retrospective | 1561 | SEER | 2.6 |
W (42.0) M (58.0) |
55.73 (16.33) | Various | Local, regional, distant stage | Marital status | Age, race, sex, ethnicity, tumor type, primary site, grade, summary stage, number of primary tumors, laterality, use of radiation and use of chemotherapy. |
Abbreviations: Cl, confidence interval; HR, hazard ratio; MMR, mortality rate ratio; ND, no data available; NS, non‐significant result; P, prospective l ongitudinal study design; R, retrospective longitudinal study design; RR, relative risk.
Some authors reported only overall survival or cancer specific survival, while some authors reported both. To overcome the issue of unexchangeable results, we performed separate analyses for such reported outcomes. Additionally, the authors of the included studies choose different modes of comparison of marital status. In order to systematically review the published studies, we first needed to categorize the marital status groups. In this review the following marital status groups were therefore described and compared: unmarried versus married, never married versus married, single versus married, divorced/separated versus married, and widowed versus married.
A quality assessment was carried out (performed by KK and JS) following the eight‐item Newcastle‐Ottawa scale for quality assessment of observational studies, which had been adapted for the needs of this review. Two rating categories of the scale, the items “Selection of the non‐exposed cohort” and “Demonstration that outcome of interest was not present at start of study” were not relevant for this review and were therefore excluded. The highest possible score, denoting high study quality, was seven. Studies scoring six and seven were considered of high quality; studies scoring five and four were rated as of moderate quality; and studies scoring lower than four were considered of low quality.
2.4. Statistical analyses
Although most of the results are presented descriptively, where possible we used a meta‐analytical approach in order to combine the results of the hazard ratios (HR) in those studies from which this information could be obtained. 14 The analyses were performed in the software program Review Manager 5.4 (The Nordic Cochrane Centre). We used a random effect model, the DerSimonian and Laird method, 15 as we expected a certain pattern of variability in the included studies due to different types of cancer, cancer stage, age of the participants, gender, and other factors. In each meta‐analysis, we carried out a heterogeneity analysis.
For interpreting the results of heterogeneity we followed the Cochrane Handbook for Systematic Reviews of Interventions (Version 6.0): (a) 30%–60% may represent moderate heterogeneity; (b) 50%–90%: may represent substantial heterogeneity; (c) 75%–100%: considerable heterogeneity. 16 In our study, if I 2 > 75% and p < 0.05, and if we had enough studies (n ≥ 3), we analyzed the specific subgroup, for example by gender, in order to try to explain the reasons for the heterogeneity. 17 A p‐value of 0.05 or lower was considered statistically significant.
When interpreting clinical relevance, we used the number needed to treat (NNT), which is an indicator of the clinically significant threshold. It applies to the number of patients a clinician would need to treat in order to achieve, on average, one patient with a longer survival. 18 The NNT was calculated according to the formula: NNT = (1 + HR)/(1−HR). The NNT can be compared with the often‐applied appropriate effect size measure (Cohen's d). Usually single‐digit values for NNT denote a worthwhile difference. 19 According to Cohen's guidelines, 20 a NNT of 9 is interpreted as a small effect size, a NNT of 4 as a moderate effect size and a NNT of 3 as a large effect size.
3. RESULTS
3.1. Study selection
The search strategy resulted in 2423 articles, plus 394 additional articles included through the screening of the references of the systematic reviews and original articles. After the removal of duplicates and articles that were removed for specific reasons (see Figure 1), 67 articles were left and therefore evaluated in this systematic review.
FIGURE 1.
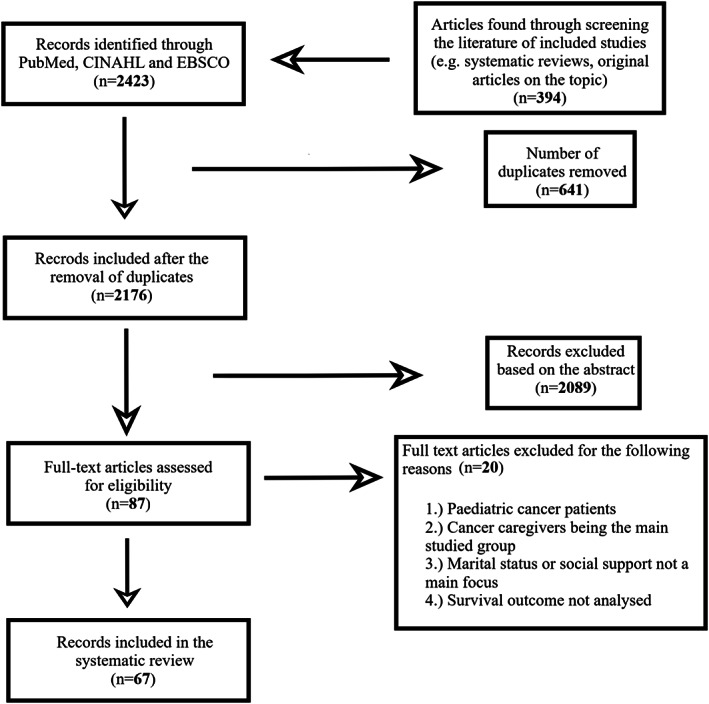
The flowchart of study selection
3.2. Characteristics of the included studies (Table 1)
The studies were published between 1987 and 2022. The median follow‐up time ranged from 1 to 19 years. Most of the included studies reported the inclusion of both sexes, but in nine studies there were only female patients and in four studies only male patients. The mean age of the participants in the eligible studies was most often 60 years, although some of the studies reported involving younger cancer patients (M < 50 years).
Of the 67 studies, four studies included patients with various cancer sites and did not report results for individual sites; five studies reported results for breast cancer; four studies separately reported results for bladder cancer, gastric cancer, and lung cancer; three studies separately included patients with head and neck cancer and prostate cancer; two separately reported results for cervical cancer, colon cancer, ovarian cancer, pancreatic cancer, gastrointestinal stromal tumor and sarcoma; and 49 studies for other cancer sites (e.g. colorectal cancer, melanoma). Fifty‐five studies included participants with cancer in stages I–IV; five separated cancer patients into “localized, regional and distant stages”; two separated cancer patients into stages 0–IV, into stages I–II and into “early and late” stages. One study separately included participants with cancer into stages II–IV, into stages “T4, N1 or M1”, into stages IB2‐IVA and into stages T1–T4. In the four remaining studies, no data on the stage of cancer were provided (see Table 1).
On assessing study quality using the Newcastle‐Ottawa scale, we found that 56 studies were deemed to be of high quality and 11 were deemed to be of moderate quality.
3.3. Adjusted analysis
All the articles except one 21 reported an adjusted analysis as their main outcome. Most commonly they adjusted the analysis for demographic characteristics such as age, gender, and race. Most articles also adjusted the analysis for tumor stage, type of therapy, tumor grade, type of surgery and other less common variables (e.g. tobacco use, household income, geographic area; see Table 1).
3.4. Analysis of the comparison between unmarried and married patients
3.4.1. Overall survival
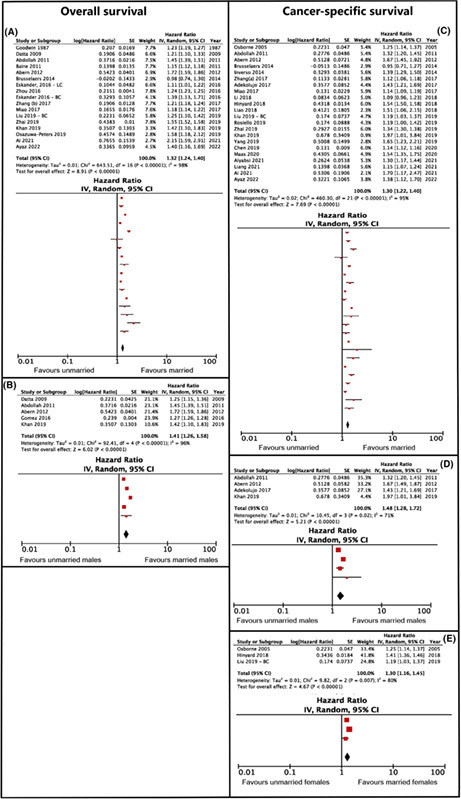
Sixteen articles reported a sub‐category which compared the overall survival of unmarried patients to married ones, of which all but one 22 reported significant difference between them. The association was found in three studies on patients with mixed types of cancer, 23 , 24 , 25 two studies on patients with prostate cancer 26 , 27 ; and one study each in patients with bladder, 28 head and neck, 29 testicular, 30 pancreatic, 31 breast, 32 , 33 kidney, 34 esophageal, 35 gastric 36 and medullary thyroid cancer. 37
The meta‐analysis showed that the total hazard ratio for unmarried versus married patients was 1.32 with a confidence interval of 1.24–1.40 (p < 0.001) and an NNT value of 7 (see Figure 2A). Due to the high heterogeneity in the results (I 2 = 98%), the studies were categorized according to gender (see Figure 2B). Following this, the total hazard ratio of unmarried versus married men amounted to 1.41 with a confidence interval of 1.26–1.58 (p < 0.001) and an NNT value of 6. However, the heterogeneity was still not decreased, meaning that there are other variables contributing to this result (I 2 = 96%). It was not possible to calculate a total hazard ratio of unmarried versus married women, due to the small number of studies that performed gender analysis (n = 2).
FIGURE 2.
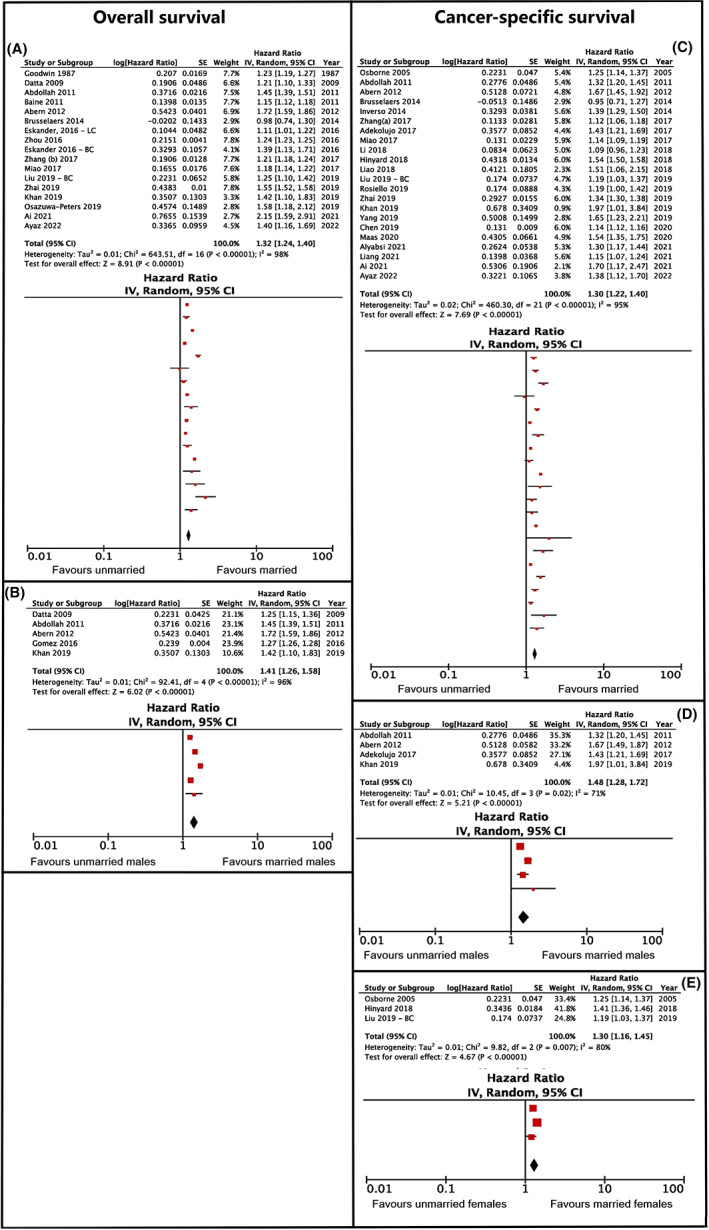
Overall and cancer‐specific survival hazard ratios comparing unmarried and married cancer patients. (A, C) Pertain to overall survival, (B, D, E) pertain to cancer‐specific survival. (A) Depicts the analysis without subanalyses by gender, (B) depicts the results of the subanalysis of overall survival for males only. (C) Shows the results of the main analysis, while (D, E) show the results of subanalyses for males and females, respectively.
3.4.2. Cancer‐specific survival
Twenty‐two studies compared cancer specific survival in unmarried and married cancer patients, of which all but two 22 , 38 reported that married patients had a higher cancer‐specific survival rate than unmarried patients. The association was found in five studies on patients with breast cancer, 21 , 32 , 33 , 39 , 40 two studies on patients with prostate cancer 26 , 27 ; and one study each in patients with testicular, 30 head and neck, 41 gastric, 42 kidney, 34 colon, 43 oral cavity, 44 non‐metastatic urothelial bladder cancer, 45 non‐small cell lung cancer, 46 melanoma, 47 medullary thyroid, 37 colorectal 48 and liver cancer, 49 and in one study with various cancer types. 25
The meta‐analysis showed that the total hazard ratio for unmarried versus married patients was 1.30, with a confidence interval of 1.22–1.40 (p < 0.001) and an NNT value of 8 (see Figure 2C). Due to the high heterogeneity in the results (I 2 = 95%), the studies were categorized according to gender (see Figure 2D,E). Following this, the total hazard ratio of unmarried versus married women rose to 1.30 with a confidence interval of 1.16–1.45 (p < 0.0001) and an NNT value of 8. The total hazard ratio of unmarried versus married men amounted to 1.48 with a confidence interval of 1.28–1.72 (p < 0.001) and an NNT value of 5. A statistical comparison between women and men showed no significant difference between the groups on cancer survival (z = 1.48; p = 0.20). The heterogeneity in both sub‐group analyses was still high (I 2 = 93%, I 2 = 71%); however, due to an insufficient number of studies (n = 3–4 in each group), heterogeneity could not be further analyzed.
3.5. Analysis of the comparison between single and married cancer patients
3.5.1. Overall survival
The sub‐category single versus married patients was compared in eight studies. All but two 50 , 51 reported that married patients had higher overall survival rates than single patients. The association was found in studies on patients with cervical, 52 esophageal, 53 uveal melanoma, 54 laryngeal cancer, 55 soft tissue sarcoma 56 and in one study with various cancer types. 25 The meta‐analysis showed that the hazard ratio was 1.19 with a confidence interval of 1.12–1.27 (p < 0.005) and an NNT value of 14 (Figure 3A). The heterogeneity was found to be substantial (I 2 = 67%); however, it was not possible to carry out further analysis.
FIGURE 3.
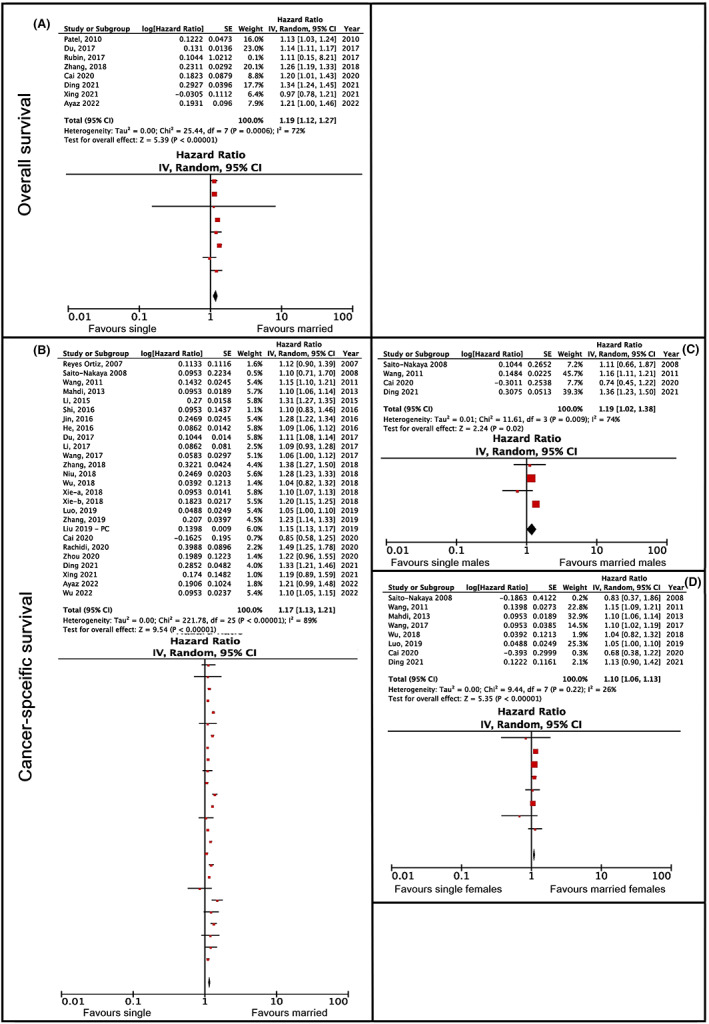
Overall and cancer‐specific survival hazard ratios comparing single and married cancer patients. (A) Pertains to overall survival, (B–D) pertain to cancer‐specific survival. (A) Shows the main analysis, without further subgroup analyses. (B) Shows the main analysis, while (C, D) represent the results of subanalyses by gender, for males and females, respectively.
3.5.2. Cancer‐specific survival
The sub‐category single versus married patients was compared in twenty‐six studies. Nine studies 25 , 50 , 54 , 57 , 58 , 59 , 60 , 61 , 62 did not find the association between the groups, while the rest reported that married patients had higher cancer‐specific survival rates than single patients. The association was found in studies on patients with non‐small cell lung cancer, 63 lung adenocarcinoma, 64 pancreatic cancer, 65 epithelial ovarian cancer, 66 colorectal cancer, 67 gastric cancer, 68 liver cancer, 69 esophageal cancer, 53 prostate cancer, 70 renal cancer, 71 bladder urothelial cancer, 72 astrocytoma, 73 ovarian cancer, 75 renal cell carcinoma, 76 cutaneous melanoma, 77 laryngeal cancer, 55 and soft tissue sarcoma. 56
The meta‐analysis showed that the hazard ratio was 1.17 with a confidence interval of 1.13–1.21 (p < 0.001) and an NNT value of 13 (Figure 3B). Due to the high heterogeneity in the results (I 2 = 89%), the studies were categorized according to gender (see Figure 3C,D). The total hazard ratio of single versus married women was 1.10, with a confidence interval of 1.06–1.13 (p < 0.001) and an NNT value of 21; the total risk of single versus married men amounted to 1.19, with a confidence interval of 1.02–1.38 (p < 0.005) and a NNT value of 12. Sex differences between single versus unmarried patients did not show any statistically significant differences between women and men (z = 0.97; p > 0.05). The heterogeneity analysis was substantial in men (I 2 > 74%), but not in women (I2 > 26%); however, due to the small number of studies (n = 3) and the lack of variables, it was not possible to carry out further sub‐analysis.
3.6. Analysis of the comparison between never‐married and married patients
3.6.1. Overall survival
The sub‐category never‐married versus married patients was compared in four studies. All the studies reported that married patients had higher overall survival rates than never‐married patients. The association was found in two studies of patients with prostate cancer, 26 , 27 and in studies with lung 78 and non‐small cell lung cancer. 79 The meta‐analysis showed that the hazard ratio was 1.22 with a confidence interval of 1.13–1.31 (p < 0.001) and an NNT value of 10 (Figure 4A). The heterogeneity was substantial (I 2 = 86%); however, due to the small number of studies, it was not possible to perform additional analyses.
FIGURE 4.
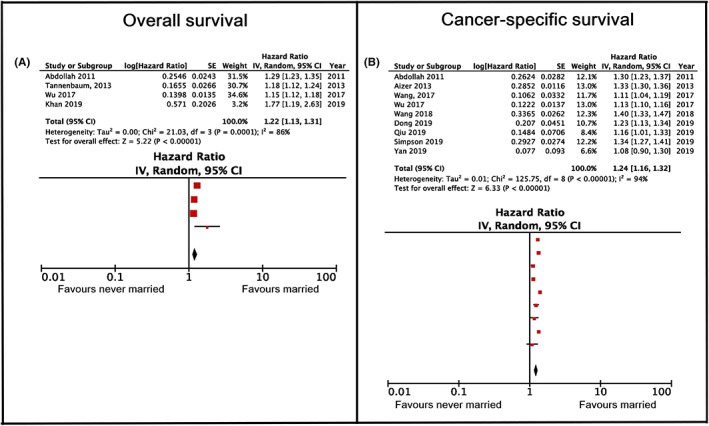
Overall and cancer‐specific survival hazard ratios comparing never married and married cancer patients. (A) Pertains to overall survival and (B) pertains to cancer‐specific survival.
3.6.2. Cancer‐specific survival
The sub‐category never‐married versus married patients was compared in nine studies. All the studies except one, 80 reported that married patients had higher cancer‐specific survival rates than never‐married patients. The association was found in studies on patients with prostate, 26 renal, 71 rectal, 81 non‐small cell lung, 79 endometrial 82 and head and neck cancer, 83 osteosarcoma 84 and in one study with patients with various cancer types. 85 The meta‐analysis showed that the hazard ratio was 1.24 with a confidence interval of 1.16–1.32 (p < 0.001) and an NNT value of 9 (Figure 4B). The heterogeneity was substantial (I 2 = 91%), but analysis with further subgroup division was not possible due to the small number of studies.
3.7. Analysis of the comparison between divorced/separated and married patients
3.7.1. Overall survival
The sub‐category of divorced/separated versus married people was compared in 12 studies. All but two 22 , 27 reported that married patients had a higher overall survival rate than divorced/separated ones. The association between marital status and better survival was found in studies on patients with cervical, 52 lung, 78 esophageal, 53 kidney, 34 vulvar, 61 breast 32 and laryngeal cancer. 55 The association was also found in studies of patients with uveal melanoma, 54 soft tissue sarcoma 56 and mycosis fungoides. 50 The meta‐analysis showed that the total hazard ratio was 1.25, with a confidence interval of 1.13–1.39 (p < 0.001) and an NNT value of 9 (Figure 5A). The heterogeneity was substantial (I 2 = 98%); however, it was not possible to perform additional analyses.
FIGURE 5.
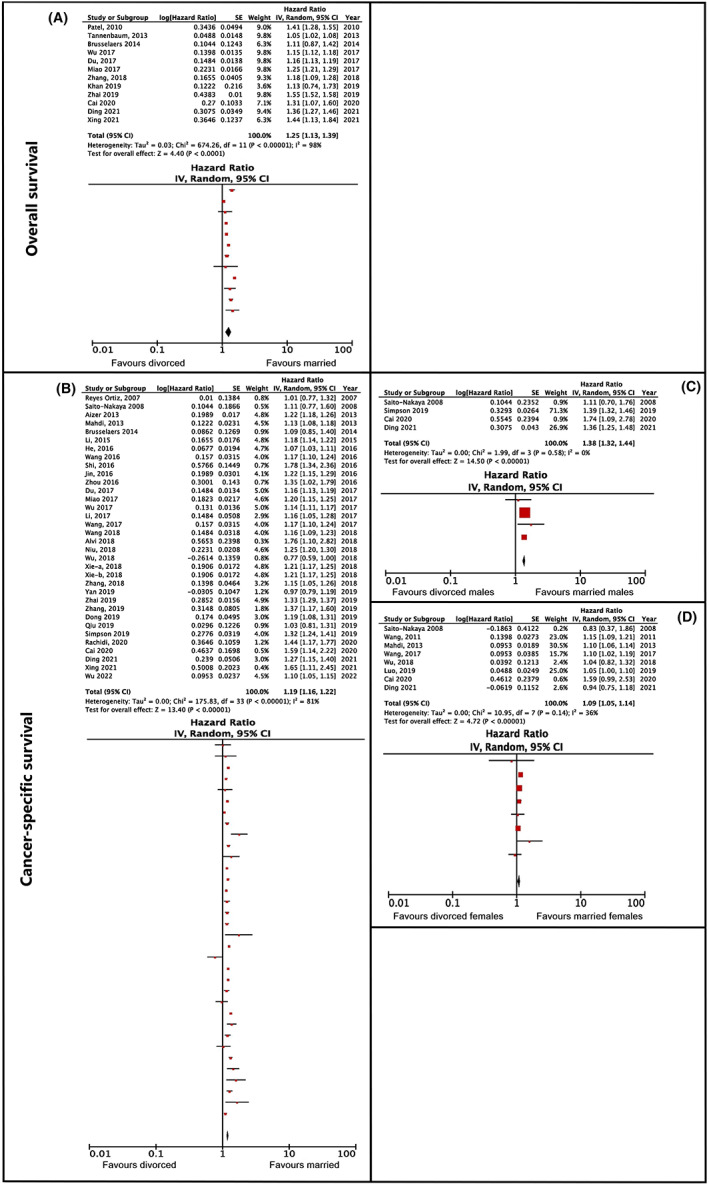
Overall and cancer‐specific survival hazard ratios comparing divorced and married cancer patients. (A) Pertains to overall survival, (B–D) pertain to cancer‐specific survival. (A) Shows the main analysis, without further subgroup analyses. (B) Shows the main analysis, while (C, D) represent the results of subanalyses by gender, for males and females, respectively.
3.7.2. Cancer‐specific survival
The sub‐category of divorced/separated versus married people was compared in 34 studies, of which six studies 22 , 57 , 61 , 63 , 80 , 84 did not find an association between the groups. Twenty‐eight reported that married patients had a higher cancer‐specific survival rate than divorced or separated patients. The association between marital status and better survival was found in two studies with patients with gastric cancer, 36 , 68 and in one study each on patients with epithelial ovarian, 66 esophageal, 53 colorectal, 67 liver, 69 renal, 71 thyroid, 58 kidney, 34 gallbladder, 60 endometrial, 82 head and neck, 83 breast, 32 rectal, 81 lung, 79 pancreatic 86 and laryngeal cancer. 55 The association was also found in one study each on patients with bladder urothelial carcinoma, 72 astrocytoma, 73 spinal cord tumours, 87 glioblastoma multiforme, 74 renal cell carcinoma, 76 cutaneous melanoma, 77 uveal melanoma, 54 laryngeal cancer, 55 lung adenocarcinoma 64 and mycosis fungoides, 50 and in one study on patients with various cancer types. 85
The meta‐analysis showed that the total hazard ratio was 1.19, with a confidence interval of 1.16–1.23 (p < 0.001) and an NNT of 12 (Figure 5B). Due to the high heterogeneity in the results (I 2 = 82%), the studies were sub‐analyzed according to gender (see Figures 3D and 5C). Following this, the total hazard ratio of divorced/separated versus married women was 1.09, with a confidence interval of 1.05–1.14 (p > 0.001) and an NNT value of 23. The total hazard ratio of men amounted to 1.38, with a confidence interval of 1.32–1.44 (p > 0.001) and an NNT value of 6. The analysis of sex differences between married versus divorced/separated found statistically significant differences between the two groups (z = 7.91; p < 0.001). The heterogeneity could be explained by gender in the sub‐group of divorced/separated versus married men (I 2 = 0%); however, in the sub‐analysis of female patients it was found to be small‐moderate (I 2 = 36%).
3.8. Analysis of the comparison between widowed and married patients
3.8.1. Overall survival
The sub‐category of widowed versus married patients appeared in 11 studies. All the studies except one 27 reported that married patients had a better overall survival than widowed patients. The association was found in studies involving patients with cervical, 52 lung, 78 kidney, 34 esophageal, 53 laryngeal, 55 and non‐small cell lung cancer. 79 The association was also found in studies of patients with uveal melanoma, 54 soft tissue sarcoma, 56 gastrointestinal stromal tumor 88 and mycosis fungoides. 50 The meta‐analysis showed that the total hazard ratio was 1.45, with a confidence interval of 1.31–1.60 (p < 0.001) and an NNT value of 5 (see Figure 6A). The heterogeneity was found to be considerable (I 2 = 98%); however, no further analysis was possible.
FIGURE 6.
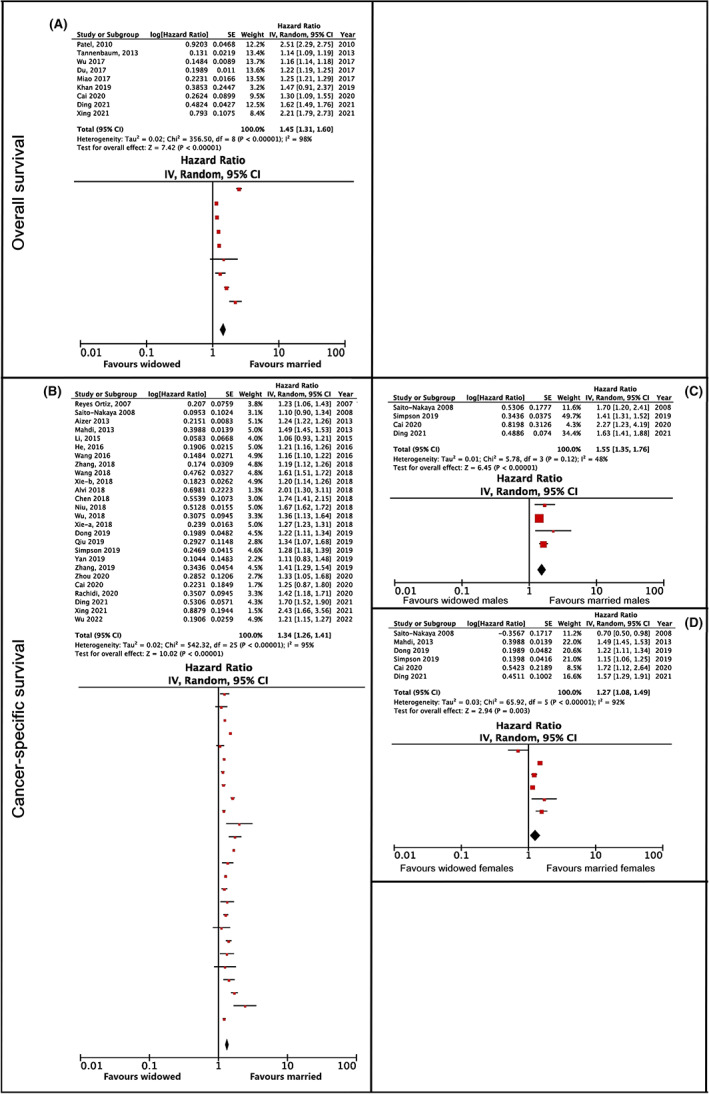
Overall and cancer‐specific survival hazard ratios comparing widowed and married cancer patients. (A) Pertains to overall survival, (B–D) pertain to cancer‐specific survival. (A) Shows the main analysis, without further subgroup analyses. (B) Shows the main analysis, while panels C and D represent the results of subanalyses by gender, for males and females, respectively.
3.8.2. Cancer‐specific survival
The category widowed versus married patients appeared in 26 studies. All the studies except two 63 , 67 reported that married patients had a better cancer‐specific survival than widowed patients. The association was found in studies involving patients with melanoma, 57 epithelial ovarian cancer, 66 liver cancer, 69 renal cancer, 86 glioblastoma multiforme, 74 spinal cord tumours, 87 gastrointestinal stromal tumor, 88 bladder urothelial carcinoma, 72 vulvar cancer, 61 astrocytoma, 73 endometrial cancer, 82 osteosarcoma, 84 head and neck cancer, 83 hepatocellular carcinoma, 80 renal cell carcinoma, 76 cutaneous melanoma, 77 gastric neuroendocrine neoplasm, 62 uveal melanoma, 54 laryngeal cancer, 55 lung adenocarcinoma, 64 mycosis fungoides 50 and rectal cancer, 81 soft tissue sarcoma 56 and in one study of patients with various types of cancer. 85
The meta‐analysis showed that the total hazard ratio was 1.34, with a confidence interval of 1.26–1.41 (p < 0.001) and an NNT value of 7 (see Figure 6B). Due to the high heterogeneity in the results (I 2 = 95%), the studies were categorized according to gender (Figure 6C,D). Following this, the total hazard ratio of widowed versus married women amounted to 1.27, with a confidence interval of 1.08–1.49 (p < 0.001) and an NNT value of 8. The total hazard ratio of widowed versus married men was 1.55, with a confidence interval of 1.35–1.76 (p < 0.001) and an NNT value of 5. Statistical comparisons between genders showed no observed difference in cancer mortality between men and women (z = 1.82; p > 0.05). The heterogeneity was still found to be considerable in the sub‐analysis of female patients (I 2 = 92%), and moderate in male patients (I 2 = 48%).
4. DISCUSSION
We systematically reviewed and performed a meta‐analysis of non‐interventional studies that explored the association between marital status and cancer‐specific and overall survival. We did not limit ourselves to the type or the stage of cancer in selecting our range of studies, so we obtained a comprehensive overview of the presented topic. Here we present data from 70 articles that reported on the association between marital status and survival.
Our meta‐analysis showed that, compared to unmarried patients, being married was significantly associated with better overall survival (NNT of 7 with a small to moderate effect, p < 0.001) and cancer‐specific survival (NNT of 8 with a small to moderate effect, p < 0.001). Additionally, we found that married patients had better overall and cancer‐specific survival when compared to single patients (an NNT of 14 with a small effect, p < 0.005, and an NNT of 13 with a small effect, p < 0.001, respectively); never‐married patients (an NNT of 10 with a small effect, p < 0.001, and an NNT of 9 with a small to moderate effect, p < 0.001, respectively); divorced/separated patients (an NNT of 9 with a small effect, p < 0.001, and an NNT of 12 with a small effect, p < 0.001, respectively); and widowed patients (an NNT of 5 with a small to moderate effect, p < 0.001 in overall and an NNT of 7 with a small to moderate effect, p < 0.001, in cancer‐specific survival). The statistics on gender group differences showed that the difference between the genders was statistically significant only between divorced/separated versus married men and women (p < 0.0001). Although the hazard ratio was higher in all the sub‐analyses for men compared to women, a statistically significant gender difference was not found in any of the other sub‐analyses.
The highest clinical significance was found when comparing married and widowed cancer patients' overall (HR 1.45, 95% Cl 1.31–1.60, p < 0.001, NNT of 5) and cancer‐specific survival (HR 1.34, 95% Cl 1.26–1.41, p < 0.001, NNT of 7) (Figure 6A,B). Besides the problems, threats and burdens associated with the illness, these patients must also face the loss of an important person, which may have a negative impact on their health. 89 Being a widow or widower is usually accompanied by high emotional stress or grief, a reduction in their social network, and at the same time the loss of the material support provided by a spouse. Widows and widowers accept chemotherapy less frequently than those who are married, and are more likely to find treatment in healthcare facilities of lower quality. 90 Studies have shown that widowed individuals have a poorer immune response: a poorer lymphocyte response in the peripheral blood regions 91 , 92 and reduced activity of natural killer cells 93 , 94 which play a key role in the identification and removal of cancer cells. 11
The second highest clinical significance was when comparing married versus unmarried patients' overall (HR 1.32, 95% CI 1.24–1.40; p > 0.001, NNT of 7) and cancer‐specific survival (HR 1.30, 95% CI 1.22–1.40; p > 0.001, NNT of 8) (Figure 2A,C). This result can be interpreted through various mechanisms which, in addition to psychosocial factors, also include economic and environmental factors. 12 Having a partner or spouse is associated with a healthier lifestyle, 95 a greater chance of discovering the disease at an earlier stage and deciding on active treatment, 96 higher financial income, 97 and better mental health. 98 Cancer is a great stressor for the person affected, so emotional support, which the spouse can offer in a specific way, can help to reduce the negative effects of stress, which can lead to better outcomes of the treatment itself. 8 The presence of a loving and caring partner is also associated with an increased release of the hormone oxytocin, which can inhibit the growth of cancer cells through indirect and direct mechanisms. 11
A smaller clinical significance (NNT >8) was found in divorced/separated patients (overall: HR 1.25, 95% CI 1.13–1.39, p < 0.001; and cancer‐specific survival: HR 1.19, 95% CI 1.16–1.23, p < 0.001; see Figure 5A,B); single patients (overall: HR 1.19, 95% CI 1.12–1.27, p < 0.005; and cancer‐specific survival: HR 1.17, 95% CI 1.13–1.21, p < 0.001; see Figure 3A,B); and never‐married patients (overall: HR 1.22, 95% CI 1.13–1.31, p < 0.001; and cancer‐specific survival: HR 1.24, 95% CI 1.16–1.32, p < 0.001; see Figure 4A,B) compared to married cancer patients. Although divorce from a spouse can be a stressful event, it is possible that patients were able to build their own social network, thus replacing the support that a partner or a spouse could have offered them. Single and never‐married patients have not been exposed to a stressful event such as separation or the death of their spouse, as experienced by the widowed patients, which could explain the lower clinical significance found in this group.
While being unmarried, single, divorced/separated, or widowed conferred a higher risk of cancer‐specific survival for men than for women, the difference between the genders was statistically significant only for the sub‐analysis of divorced/separated versus married group. This could be explained by the fact that women are more likely to encourage their spouse to have a health‐beneficial lifestyle than vice versa. 8 Married male cancer patients have reported significantly lower levels of psychological distress and higher psychological support from their spouse, which may have resulted in a better cancer prognosis. 9 It has also been suggested that men gain more social benefits from marriage, while women gain more financial benefits. 99 Women are expected to benefit more from a large social network than from marriage alone, at least according to the results of the comparison of large versus small social networks found in two studies, 100 , 101 and the finding that unmarried women have longer survival rates than unmarried men, as found in this study.
Our conclusions are somewhat different when comparing our study to the earlier two previous meta‐analyses conducted on social support and cancer survival. The first meta‐analysis published in 2010, 12 including 87 studies, reported that the worst survival rate was observed in never‐married patients compared to the married group. While our study found significant differences when comparing the never‐married versus married group, the greatest differences were found in the general unmarried group and the widowed group (as compared to the married group). The differences in the findings could be attributed to the fact that our study was conducted 12 years later when couples are less likely to decide to get married than they used to be. Being unmarried does not necessarily mean that they do not have a partner. Compared to the newer study, 13 carried out in 2018 and examining the results of 21 studies, our study confirms that divorced/separated men had a higher risk of cancer mortality compared to the mortality rate found in women. Future prospective studies should consider these findings when planning appropriate sample size, in order to be able to perform additional gender‐adjusted analyses.
Although much care was taken to limit the confounders of our analyses and literature review, some limitations are nonetheless present in this study. The first is that some of the included studies did not provide data on the age, gender, and stage of cancer of the patients, making it difficult to determine whether the observed associations were moderated by the demographic and clinical characteristics of cancer patients. Additionally, the category of unmarried patients was quite heterogeneous and, when referred to in studies without additional explanation, can be hard to interpret, as it could mean the patients were divorced, separated, never‐married, single, widowed or any combination thereof.
Despite these limitations, 63 articles represent a large portion of original research on the association between marital status and the survival of cancer patients, spanning more than three decades. Further strengths of the present systematic review are the categorization of the various subgroups of unmarried patients and the categorization of overall and cancer‐specific survival, increasing the accuracy of the conclusions made. Furthermore, where available, a separate analysis for men and women was carried out to test for possible moderator variables.
5. CONCLUSION
Our systematic review of the literature showed that being married is associated with improved overall and cancer‐specific survival. The main conclusion is that of the different subgroups of unmarried patients, the widowed are the group with the shortest survival rate, possibly reflecting diminished social contact and the effects of stress and loss on the health of patients. To elucidate the details of this association and determine the contributing factors that moderate the link between marital status and cancer survival, further research into the bodily and psychological processes following the loss of a spouse should be carried out. Additionally, the subgroup analysis by gender showed that divorced/separated men have the worst survival rate when compared to the female cancer patients. This review carries important clinical and research implications, where clinicians can benefit by being aware of the effects of marital status on cancer treatment, enabling them to more easily identify patients in need of comprehensive intervention. The research community can benefit from the findings of this review and meta‐analysis by taking into account the differences in the subgroups of unmarried patients when designing further studies, as well as the differences in the effects of marital status on men and women.
AUTHOR CONTRIBUTIONS
The contributions of the authors are as follows (as per the CRediT taxonomy); K.K.: Conceptualization, data curation, investigation, writing/original draft preparation, and writing/review and editing. S.M.: Conceptualization, data curation, formal analysis, funding acquisition, methodology, visualization, writing/original draft preparation, and writing/review and editing. J.S.: Data curation, investigation, visualization, writing/review and editing. Z.K.K.: Supervision, project administration, funding acquisition. M.D.: Funding acquisition, supervision, project administration. D.S.: Project administration, supervision, writing/review and editing. G.D. Writing/review and editing, project administration, supervision.
FUNDING INFORMATION
Spela Mirosevic and Jakob Sajovic acknowledge funding by the Slovenian Research Agency via Programs MR‐39262 and P3‐0293(B), respectively.
CONFLICT OF INTEREST
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
ETHICAL STATEMENT
Ethics approval was not required for this study.
Supporting information
Table S1
Table S2
Krajc K, Miroševič Š, Sajovic J, et al.. Marital status and survival in cancer patients: A systematic review and meta‐analysis. Cancer Med. 2023;12:1685‐1708. doi: 10.1002/cam4.5003
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Cancer [Internet] . 2022. Accessed April 29, 2022. https://www.who.int/news‐room/fact‐sheets/detail/cancer
- 2. Mirosevic S, Jo B, Kraemer HC, Ershadi M, Neri E, Spiegel D. “Not just another meta‐analysis”: sources of heterogeneity in psychosocial treatment effect on cancer survival. Cancer Med. 2019;8(1):363‐373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Thuret R, Sun M, Budaus L, et al. A population‐based analysis of the effect of marital status on overall and cancer‐specific mortality in patients with squamous cell carcinoma of the penis. Cancer Causes Control. 2013;24(1):71‐79. [DOI] [PubMed] [Google Scholar]
- 4. Carpenter WR, Howard DL, Taylor YJ, Ross LE, Wobker SE, Godley PA. Racial differences in PSA screening interval and stage at diagnosis. Cancer Causes Control. 2010;21(7):1071‐1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McLaughlin JM, Fisher JL, Paskett ED. Marital status and stage at diagnosis of cutaneous melanoma. Cancer. 2011;117(9):1984‐1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mihor A, Tomsic S, Zagar T, Lokar K, Zadnik V, Vesna ZA. Socioeconomic inequalities in cancer incidence in Europe: a comprehensive review of population‐based epidemiological studies. Radiol Oncol. 2020;54(1):1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. De Graeff A, De Leeuw JRJ, Ros WJG, Hordijk GJ, Blijham GH, Winnubst JAM. Sociodemographic factors and quality of life as prognostic indicators in head and neck cancer. Eur J Cancer. 2001;37(3):332‐339. [DOI] [PubMed] [Google Scholar]
- 8. Martínez ME, Unkart JT, Tao L, et al. Prognostic significance of marital status in breast cancer survival: a population‐based study. PLoS One. 2017;12(5):e0175515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Huang TB, Zhou GC, Dong CP, et al. Marital status independently predicts prostate cancer survival in men who underwent radical prostatectomy: an analysis of 95,846 individuals. Oncol Lett. 2018;15(4):4737‐4744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lutgendorf SK, Sood AK, Anderson B, et al. Social support, psychological distress, and natural killer cell activity in ovarian cancer. J Clin Oncol. 2005;23:7105‐7113. [DOI] [PubMed] [Google Scholar]
- 11. Uchino BN. Social support and health: a review of physiological processes potentially underlying links to disease outcomes. J Behav Med. 2006;29(4):377‐387. [DOI] [PubMed] [Google Scholar]
- 12. Pinquart M, Duberstein PR. Associations of social networks with cancer mortality: a meta‐analysis. Crit Rev Oncol Hematol. 2010;75(2):122‐137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang Y, Jiao Y, Nie J, et al. Sex differences in the association between marital status and the risk of cardiovascular, cancer, and all‐cause mortality: a systematic review and meta‐analysis of 7,881,040 individuals. Glob Heal Res Policy. 2020;5(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kastrin A. Metaanaliza in njen pomen za psihološko metodologijo [Meta‐analysis: its role in psychological methodology]. Psihol Obz / Horizons Psychol. 2008;17(3):25‐42. [Google Scholar]
- 15. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials. 1986;7(3):177‐188. [DOI] [PubMed] [Google Scholar]
- 16. Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions. 2nd ed. Chichester (UK); 2019. [Google Scholar]
- 17. Huedo‐Medina TB, Sánchez‐Meca J, Marín‐Martínez F, Botella J. Assessing heterogeneity in meta‐analysis: Q statistic or I 2 index? Psychol Methods. 2006;11(2):193‐206. [DOI] [PubMed] [Google Scholar]
- 18. Altman DG, Andersen PK. Calculating the number needed to treat for trials where the outcome is time to an event. Br Med J. 1999;319:1492‐1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Citrome L. Quantifying clinical relevance. Innov Clin Neurosci. 2014;11(5‐6):26‐30. [PMC free article] [PubMed] [Google Scholar]
- 20. Citrome L. Compelling or irrelevant? Using number needed to treat can help decide. Acta Psychiatr Scand. 2008;117:412‐419. [DOI] [PubMed] [Google Scholar]
- 21. Hinyard L, Wirth LS, Clancy JM, Schwartz T. The effect of marital status on breast cancer‐related outcomes in women under 65: a SEER database analysis. Breast. 2017;32:13‐17. [DOI] [PubMed] [Google Scholar]
- 22. Brusselaers N, Mattsson F, Johar A, et al. Marital status and survival after oesophageal cancer surgery: a population‐based nationwide cohort study in Sweden. BMJ Open. 2014;4:5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Goodwin JS, Hunt WC, Key CR, Samet JM. The effect of marital status on stage, treatment, and survival of cancer patients. Jama J Am Med Assoc. 1987;258(21):3125‐3130. [PubMed] [Google Scholar]
- 24. Eskander MF, Schapira EF, Bliss LA, et al. Keeping it in the family: the impact of marital status and next of kin on cancer treatment and survival. Am J Surg. 2016;212(4):691‐699. [DOI] [PubMed] [Google Scholar]
- 25. Ayaz T, Fredrickson S, O'mary K, Panchbhavi MA, Panchbhavi VK. Differences in cancer amputee survival based on marital status: an analysis of the surveillance, epidemiology, and end results (SEER) database. J Psychosoc Oncol. 2022;40(2):203‐214. [DOI] [PubMed] [Google Scholar]
- 26. Abdollah F, Sun M, Thuret R, et al. The effect of marital status on stage and survival of prostate cancer patients treated with radical prostatectomy: a population‐based study. Cancer Causes Control. 2011;22(8):1085‐1095. [DOI] [PubMed] [Google Scholar]
- 27. Khan S, Nepple KG, Kibel AS, et al. The association of marital status and mortality among men with early‐stage prostate cancer treated with radical prostatectomy: insight into post‐prostatectomy survival strategies. Cancer Causes Control. 2019;30(8):871‐876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dabral Datta G, Neville BA, Kawachi I, Datta NS, Earle CC. Marital status and survival following bladder cancer. J Epidemiol Community Health. 2009;63:807‐813. [DOI] [PubMed] [Google Scholar]
- 29. Osazuwa‐Peters N, Christopher KM, Cass LM, et al. What's love got to do with it? Marital status and survival of head and neck cancer. Eur J Cancer Care (Engl). 2019;28(4):e13022. [DOI] [PubMed] [Google Scholar]
- 30. Abern MR, Dude AM, Coogan CL. Marital status independently predicts testis cancer survival‐an analysis of the SEER database. Urol Oncol Semin Orig Investig. 2012;30(4):487‐493. [DOI] [PubMed] [Google Scholar]
- 31. Baine M, Sahak F, Lin C, Chakraborty S, Lyden E, Batra SK. Marital status and survival in pancreatic cancer patients: a SEER based analysis. PLoS One. 2011;6(6):e21052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhai Z, Zhang F, Zheng Y, et al. Effects of marital status on breast cancer survival by age, race, and hormone receptor status: a population‐based study. Cancer Med. 2019;8(10):4906‐4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liu Y, Wang D, Yang Z, et al. Marital status is an independent prognostic factor in inflammatory breast cancer patients: an analysis of the surveillance, epidemiology, and end results database. Breast Cancer Res Treat. 2019;178(2):379‐388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Miao T, Li Y, Sheng X, Yao D. Marital status and survival of patients with kidney cancer. Oncotarget. 2017;8(49):86157‐86167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang QW, Lin XL, Zhang CH, et al. The influence of marital status on the survival of patients with esophageal cancer: a population‐based, propensity‐matched study. Oncotarget. 2017;8(37):62261‐62273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhou R, Yan S, Li J. Influence of marital status on the survival of patients with gastric cancer. J Gastroenterol Hepatol. 2016;31(4):768‐775. [DOI] [PubMed] [Google Scholar]
- 37. Ai L, Li N, Tan HL, et al. Effects of marital status on survival of medullary thyroid cancer stratified by age. Cancer Med. 2021;10(24):8829‐8837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li Z, Wang K, Zhang X, Wen J. Marital status and survival in patients with rectal cancer. Med (United States). 2018;97(18):e0637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Osborne C, Ostir GV, Du X, Peek MK, Goodwin JS. The influence of marital status on the stage at diagnosis, treatment, and survival of older women with breast cancer. Breast Cancer Res Treat. 2005;93:41‐47. [DOI] [PubMed] [Google Scholar]
- 40. Adekolujo OS, Tadisina S, Koduru U, Gernand J, Smith SJ, Kakarala RR. Impact of marital status on tumor stage at diagnosis and on survival in male breast cancer. Am J Mens Health. 2017;11(4):1190‐1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Inverso G, Mahal BA, Aizer AA, Donoff RB, Chau NG, Haddad RI. Marital status and head and neck cancer outcomes. Cancer. 2015;121(8):1273‐1278. [DOI] [PubMed] [Google Scholar]
- 42. Zhang J, Gan L, Wu Z, Yan S, Liu X, Guo W. The influence of marital status on the stage at diagnosis, treatment, and survival of adult patients with gastric cancer: a population‐based study. Oncotarget. 2017;8(14):22385‐22405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yang CC, Cheng LC, Lin YW, et al. The impact of marital status on survival in patients with surgically treated colon cancer. Medicine (Baltimore). 2019;98(11):e14856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Liao PH, Lee CC. The influence of marital status on survival for patients aged 65 years and younger with oral cavity cancer. Auris Nasus Larynx. 2018;45(6):1227‐1232. [DOI] [PubMed] [Google Scholar]
- 45. Rosiello G, Palumbo C, Knipper S, et al. Unmarried men have worse oncologic outcomes after radical cystectomy for nonmetastatic urothelial bladder cancer. Urol Oncol Semin Orig Investig. 2020;38(3):76.e1‐76.e9. [DOI] [PubMed] [Google Scholar]
- 46. Chen Z, Yin K, Zheng D, et al. Marital status independently predicts non‐small cell lung cancer survival: a propensity‐adjusted SEER database analysis. J Cancer Res Clin Oncol. 2019;146(1):67‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Maas JA, Monreal AJ, Diaz EL, Castro G, Rodriguez De La Vega P, Varella M. Marital status and survival in patients diagnosed with melanoma. Dermatol Res Pract. 2020;2020:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Alyabsi M, Ramadan M, Algarni M, Alshammari K, Jazieh AR. The effect of marital status on stage at diagnosis and survival in Saudis diagnosed with colorectal cancer: cancer registry analysis. Sci Reports. 2021;11(1):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Liang Y, Wu X, Lu C, Xiao F. Impact of marital status on the prognosis of liver cancer patients without surgery and the critical window. Ann Palliat Med. 2021;10(3):2990‐2999. [DOI] [PubMed] [Google Scholar]
- 50. Xing LX, Zhang J, Shen H, et al. Association of marital status with stage and survival in patients with mycosis fungoides: a population‐based study. Cancer Med. 2021;10(20):7320‐7329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rubin SJ, Kirke DN, Ezzat WH, Truong MT, Salama AR, Jalisi S. Marital status as a predictor of survival in patients with human papilloma virus‐positive oropharyngeal cancer. Am J Otolaryngol ‐ Head Neck Med Surg. 2017;38(6):654‐659. [DOI] [PubMed] [Google Scholar]
- 52. Patel MK, Patel DA, Lu M, Elshaikh MA, Munkarah A, Movsas B. Impact of marital status on survival among women with invasive cervical cancer: analysis of population‐based surveillance, epidemiology, and end results data. J Low Genit Tract Dis. 2010;14(4):329‐338. [DOI] [PubMed] [Google Scholar]
- 53. Du L, Kim JJ, Chen B, Zhu S, Dai N. Marital status is associated with superior survival in patients with esophageal cancer: a surveillance, epidemiology, and end results study. Oncotarget. 2017;8(56):95965‐95972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cai W, Fan J, Shen T, Yu J. The influence of marital status on the survival of patients with uveal melanoma. J Ophthalmol. 2020;2020:7339‐7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ding Z, Yu D, Li H, Ding Y. Effects of marital status on overall and cancer‐specific survival in laryngeal cancer patients: a population‐based study. Sci Reports. 2021;11(1):1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhang S l, Wang W r, Liu Z j, Wang Z m. Marital status and survival in patients with soft tissue sarcoma: a population‐based, propensity‐matched study. Cancer Med. 2018;8(2):465‐479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Reyes Ortiz CA, Freeman JL, Kuo YF, Goodwin JS. The influence of marital status on stage at diagnosis and survival of older persons with melanoma. Journals Gerontol ‐ Ser A Biol Sci Med Sci. 2007;62(8):892‐898. [DOI] [PubMed] [Google Scholar]
- 58. Shi R l, Qu N, Lu Z w, Liao T, Gao Y, Ji Q h. The impact of marital status at diagnosis on cancer survival in patients with differentiated thyroid cancer. Cancer Med. 2016;5(8):2145‐2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Dinh KT, Aizer AA, Muralidhar V, et al. Increased vulnerability to poorer cancer‐specific outcomes following recent divorce. Am J Med. 2018;131(5):517‐523. [DOI] [PubMed] [Google Scholar]
- 60. Li X, Liu Y, Wang Y, et al. The influence of marital status on survival of gallbladder cancer patients: a population‐based study. Sci Rep. 2017;7(1):1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wu SG, Lin QJ, Li FY, Sun JY, He ZY, Zhou J. Widowed status increases the risk of death in vulvar cancer. Future Oncol. 2018;14(25):2589‐2598. [DOI] [PubMed] [Google Scholar]
- 62. Zhou YJ, Lu XF, Zheng KI, et al. Marital status, an independent predictor for survival of gastric neuroendocrine neoplasm patients: a SEER database analysis. BMC Endocr Disord. 2020;20(1):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Saito‐Nakaya K, Nakaya N, Akechi T, et al. Marital status and non‐small cell lung cancer survival: the lung cancer database project in Japan. Psychooncology. 2008;17(9):869‐876. [DOI] [PubMed] [Google Scholar]
- 64. Wu Y, Zhu PZ, Chen YQ, Chen J, Xu L, Zhang H. Relationship between marital status and survival in patients with lung adenocarcinoma: a SEER‐based study. Medicine (Baltimore). 2022;101(1):1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wang XD, Qian JJ, Bai DS, Li ZN, Jiang GQ, Yao J. Marital status independently predicts pancreatic cancer survival in patients treated with surgical resection: an analysis of the SEER database. Oncotarget. 2016;7(17):24880‐24887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Mahdi H, Kumar S, Munkarah AR, Abdalamir M, Doherty M, Swensen R. Prognostic impact of marital status on survival of women with epithelial ovarian cancer. Psychooncology. 2013;22(1):83‐88. [DOI] [PubMed] [Google Scholar]
- 67. Li Q, Gan L, Liang L, Li X, Cai S. The influence of marital status on stage at diagnosis and survival of patients with colorectal cancer. Oncotarget. 2015;6(9):7339‐7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Jin JJ, Wang W, Dai FX, et al. Marital status and survival in patients with gastric cancer. Cancer Med. 2016;5(8):1821‐1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. He XK, Lin ZH, Qian Y, Xia D, Jin P, Sun LM. Marital status and survival in patients with primary liver cancer. Oncotarget. 2017;8(39):64954‐64963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Liu Y, Xia Q, Xia J, et al. The impact of marriage on the overall survival of prostate cancer patients: a surveillance, epidemiology, and end results (SEER) analysis. Can Urol Assoc J. 2019;13(5):E135‐E139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wang H, Wang L, Kabirov I, et al. Impact of marital status on renal cancer patient survival. Oncotarget. 2017;8(41):70204‐70213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Niu Q, Lu Y, Wu Y, et al. The effect of marital status on the survival of patients with bladder urothelial carcinoma: a SEER database analysis. Med (United States). 2018;97(29):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Xie JC, Yang S, Liu XY, Zhao YX. Marital status is associated with survival of patients with astrocytoma. J Clin Neurosci. 2018;56(16412):79‐87. [DOI] [PubMed] [Google Scholar]
- 74. Xie JC, Yang S, Liu XY, Zhao YX. Effect of marital status on survival in glioblastoma multiforme by demographics, education, economic factors, and insurance status. Cancer Med. 2018;7(8):3722‐3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Luo P, Zhou JG, Jin SH, Qing MS, Ma H. Influence of marital status on overall survival in patients with ovarian serous carcinoma: finding from the surveillance epidemiology and end results (SEER) database. J Ovarian Res. 2019;12(1):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Zhang SL, Zhang ZY, Liu ZJ, et al. A real‐world study of socioeconomic factors with survival in adults aged 18–64 years with renal cell carcinoma. Future Oncol. 2019;15(21):2503‐2515. [DOI] [PubMed] [Google Scholar]
- 77. Rachidi S, Deng Z, Sullivan DY, Lipson EJ. Shorter survival and later stage at diagnosis among unmarried patients with cutaneous melanoma: a US national and tertiary care center study. J Am Acad Dermatol. 2020;83(4):1012‐1020. [DOI] [PubMed] [Google Scholar]
- 78. Tannenbaum SL, Zhao W, Koru‐Sengul T, Miao F, Lee D, Byrne MM. Marital status and its effect on lung cancer survival. Springerplus. 2013;2(1):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Wu Y, Ai Z, Xu G. Marital status and survival in patients with non‐small cell lung cancer: an analysis of 70006 patients in the SEER database. Oncotarget. 2017;8(61):103518‐103534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Yan B, Bai DS, Qian JJ, Zhang C, Jin SJ, Jiang GQ. Does marital status impact postoperative survival in patients with less differentiated hepatocellular carcinoma? A Population‐Based Study Cancer Med. 2019;8(14):6272‐6279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Wang X, Cao W, Zheng C, Hu W, Liu C. Marital status and survival in patients with rectal cancer: an analysis of the surveillance, epidemiology and end results (SEER) database. Cancer Epidemiol. 2018;54:119‐124. [DOI] [PubMed] [Google Scholar]
- 82. Dong J, Dai Q, Zhang F. The effect of marital status on endometrial cancer‐related diagnosis and prognosis: a surveillance epidemiology and end results database analysis. Future Oncol. 2019;15(34):3963‐3976. [DOI] [PubMed] [Google Scholar]
- 83. Simpson MC, Challapalli SD, Cass LM, Zahirsha ZS, Adjei Boakye E, Massa ST, Osazuwa‐Peters N Impact of gender on the association between marital status and head and neck cancer outcomes. Oral Oncol 2019;89(November 2018):48–55. [DOI] [PubMed] [Google Scholar]
- 84. Qiu S, Tao L, Zhu Y. Marital status and survival in osteosarcoma patients: an analysis of the surveillance, epidemiology, and end results (SEER) database. Med Sci Monit. 2019;25:8190‐8203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Aizer AA, Chen MH, McCarthy EP, et al. Marital status and survival in patients with cancer. J Clin Oncol. 2013;31(31):3869‐3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Wang L, Wilson SE, Steward DB, Hollenbeak CS. Marital status and colon cancer outcomes in US surveillance, epidemiology and end results registries: does marriage affect cancer survival by gender and stage? Cancer Epidemiol. 2011;35(5):417‐422. [DOI] [PubMed] [Google Scholar]
- 87. Alvi MA, Wahood W, Huang AE, Kerezoudis P, Lachance DH, Bydon M. Beyond science: effect of marital status and socioeconomic index on outcomes of spinal cord tumors: analysis from a National Cancer Registry. World Neurosurg. 2019;121:e333‐e343. [DOI] [PubMed] [Google Scholar]
- 88. Chen M, Wang X, Wei R, Wang Z. The influence of marital status on the survival of patients with operable gastrointestinal stromal tumor: a SEER‐based study. Int J Health Plann Manage. 2018;34(1):e447‐e463. [DOI] [PubMed] [Google Scholar]
- 89. Steinberg Schone B, Weinick RM. Health‐related behaviors and the benefits of marriage for elderly persons. Public Domain Gerontol. 1998;38(5):618‐627. [DOI] [PubMed] [Google Scholar]
- 90. Iwashyna TJ, Christakis NA. Marriage, widowhood, and health‐care use. Soc Sci Med. 2003;57(11):2137‐2147. [DOI] [PubMed] [Google Scholar]
- 91. Schleifer SJ, Keller SE, Camerino M, Thornton JC, Stein M. Suppression of lymphocyte stimulation following bereavement. Jama J Am Med Assoc. 1983;250(3):374‐377. [PubMed] [Google Scholar]
- 92. Bartrop RW, Lazarus L, Luckhurst E, Kiloh LG, Penny R. Depressed lymphocyte function after bereavement. Lancet. 1977;309(8016):834‐836. [DOI] [PubMed] [Google Scholar]
- 93. Irwin M, Daniels M, Smith TL, Bloom E, Weiner H. Impaired natural killer cell activity during bereavement. Brain Behav Immun. 1987;1(1):98‐104. [DOI] [PubMed] [Google Scholar]
- 94. Vitlic A, Lord JM, Carroll D, Phillips AC. Increased risk of infection in bereaved older adults: from broken heart to broken immune system. Adv Neuroimmune Biol. 2015;6(1):25‐30. [Google Scholar]
- 95. Feeney BC, Collins NL. A new look at social support: a theoretical perspective on thriving through relationships. Pers Soc Psychol Rev. 2015;19(2):113‐147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Cronin‐Fenton DP, Sharp L, Deady S, Comber H. Treatment and survival for non‐Hodgkin's lymphoma: influence of histological subtype, age, and other factors in a population‐based study (1999–2001). Eur J Cancer. 2006;42(16):2786‐2793. [DOI] [PubMed] [Google Scholar]
- 97. Blanchard CG, Albrecht TL, Ruckdeschel JC, Grant CH, Hemmick RM. The role of social support in adaptation to cancer and to survival. J Psychosoc Oncol. 1995;13(1‐2):75‐95. [Google Scholar]
- 98. Applebaum AJ, Stein EM, Lord‐Bessen J, Pessin H, Rosenfeld B, Breitbart W. Optimism, social support, and mental health outcomes in patients with advanced cancer. Psychooncology. 2014;23(3):299‐306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Kiecolt‐Glaser JK, Newton TL. Marriage and health: his and hers. Psychol Bull. 2001;127(4):472‐503. [DOI] [PubMed] [Google Scholar]
- 100. Chou AF, Stewart SL, Wild RC, Bloom JR. Social support and survival in young women with breast carcinoma. Psychooncology. 2012;21(2):125‐133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Kroenke CH, Quesenberry C, Kwan ML, Sweeney C, Castillo A, Caan BJ. Social networks, social support, and burden in relationships, and mortality after breast cancer diagnosis in the life after breast cancer epidemiology (LACE) study. Breast Cancer Res Treat. 2013;137(1):261‐271. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Table S2
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


