Abstract
Nonpeptide antigens (including glycolipids of microbial origin) can be presented to T cells by CD1 molecules expressed on monocyte-derived dendritic cells. These HLA unrestricted responses appear to play a role in host immunity against Mycobacterium tuberculosis and other pathogenic bacteria. It is known that vaccination with Mycobacterium bovis bacillus Calmette-Guérin (BCG) has limited efficacy in many clinical settings, although the reasons for its inadequacy remain unclear. Here we have investigated the influence of BCG on the induction of CD1b on human monocytes by granulocyte-macrophage colony-stimulating factor (GM-CSF), which is believed to be the principal inducer of this antigen-presenting molecule. Although BCG alone led to a slight induction of CD1b expression, this agent reduced markedly the ability of GM-CSF to induce high levels of CD1b that were typically observed in uninfected cells. Inhibition of CD1b expression in BCG-infected monocytes was apparent at both the mRNA transcript and CD1b protein levels. Down-regulation of CD1b expression by BCG was mediated, at least in part, by one or more soluble factors and could not be reversed with high concentrations of GM-CSF or a variety of other cytokines. The present results suggest that BCG could diminish the efficiency of CD1-restricted T-cell responses against nonpeptide mycobacterial antigens by reducing CD1 expression on antigen-presenting cells. These findings have potential implications for understanding the nature of the immune response elicited by BCG in humans and suggest potential strategies that could be important for the development of better vaccines for the prevention of tuberculosis.
Data from the world literature show that morbidity and mortality from mycobacterial infections are continuously increasing (3, 9, 17). This appears to be due not only to a higher transmission rate of the disease, especially in immunocompromised human immunodeficiency virus (HIV)-infected patients (2, 5), but also to the emergence of multidrug-resistant strains of Mycobacterium tuberculosis (17, 21, 25). Therefore, effective early vaccination of individuals at high risk for developing active tuberculosis has been targeted as an important approach for tuberculosis control. Vaccination against M. tuberculosis has been attempted on a large scale using Mycobacterium bovis bacillus Calmette-Guérin (BCG), a live attenuated strain. However, the results of clinical trials that enrolled an extraordinary number of cases immunized with BCG were not consistently appealing (24, 29). A recent meta-analysis of the literature showed that the vaccine significantly reduces the risk of tuberculosis by an average of only 50% (4). The reasons why BCG does not provide optimal protection are not clear, since the organism is known to share a number of major histocompatibility complex (MHC)-restricted antigens with virulent M. tuberculosis and also activates γδ T cells that may facilitate the responses of CD4+ and CD8+ responder T cells that are important in maintaining immunity to M. tuberculosis (14).
At present, the most important cell-mediated mechanism involved in protective sensitization against mycobacteria appears to rely on the classical HLA-restricted responses against bacterial peptides (35) mediated mainly by gamma interferon (IFN-γ)-producing CD4 T cells (6, 8). However, in recent years growing interest has been elicited in an arm of the immune system involving T-cell reactivity directed against lipid or glycolipid antigens presented by CD1 molecules (27). The CD1 molecules are expressed most prominently on antigen-presenting cells of the myeloid lineage, including dendritic cells derived from circulating monocytes. Adherent blood mononuclear cells can be activated by granulocyte-macrophage colony-stimulating factor (GM-CSF) to express group I CD1 (i.e., CD1a, CD1b, CD1c) proteins (16, 27, 38). These molecules are the products of the CD1A, -B, and -C genes and are known to be involved in the presentation of nonpeptide microbial antigens (27, 33). Among the CD1-restricted antigens, of primary interest are lipoarabinomannan (ManLAM), phosphatidylinositol mannosides, mycolic acids, and glycosylated mycolates, all of which are abundant constituents of the cell wall of the mycobacterial species (33).
A fraction of the T cells that respond to mycobacterial lipids and glycolipids presented by CD1 molecules comes from the CD4− CD8− phenotypic subset of CD3+ αβ T-cell receptor (TCR) T cells. These cells, sometimes referred to as double negative αβ T lymphocytes (26), proliferate and generate cytotoxic clones following interaction with mycobacterial glycolipids, presented by CD1+ monocyte-derived dendritic cells that develop from blood monocytes preactivated with GM-CSF, alone or in combination with interleukin-4 (IL-4) (26). More recently, CD8+ TCR αβ T-cell clones with similar properties have also been demonstrated (37), and a recent report has described CD4+ TCR αβ T cells that react with mycobacterial antigens presented by CD1 molecules (34). Thus, responder cells that potentially play a role in CD1-restricted responses to nonpeptide antigens have been demonstrated among all of the major phenotypic subsets of T cells, as presently defined (27)
There is a general consensus that the known CD1b presented antigens, such as ManLAM(s) and mycolic acids, isolated from BCG (43) and M. tuberculosis (10, 15) share the same basic structures. It follows that sensitization with BCG is expected to generate CD1-restricted T cells that could be cytotoxic for M. tuberculosis and possibly for other pathogenic mycobacteria and for dendritic cells and macrophages containing viable bacilli. However, Stenger et al. found that infection with virulent M. tuberculosis severely depresses the expression of CD1b in GM-CSF-activated monocytes (36). These observations prompted us to explore whether BCG itself could play a negative role in the CD1-dependent immune system similar to that described for M. tuberculosis. In particular, we focused on the question of whether the process of CD1b induction by GM-CSF could be inhibited by BCG infection rather than the expression of CD1b protein already present in adherent mononuclear cells preactivated by the cytokine. Our results indicated that BCG affects adversely the GM-CSF-dependent induction of all group I CD1 molecules. In contrast, we found that the microorganism does not seem to produce similar effects in preactivated, CD1b+ adherent cells, in which the vaccine produced only a modest downregulation of this molecule. These findings raise the question of whether BCG vaccination could produce self-limiting cellular and/or humoral signals with respect to CD1-dependent immunity, which could be a potential factor contributing to its limited efficacy in tuberculosis prevention.
MATERIALS AND METHODS
Reagents and antibodies.
All chemical reagents, if not otherwise specified, were obtained from Sigma Chemical Co. (St. Louis, Mo.). Recombinant human GM-CSF and IL-4 were obtained from Sandoz (Milan, Italy) and Genzyme (Cambridge, Mass.), respectively. Recombinant human IL-10 was obtained from R&D Systems (Minneapolis, Minn.).
Mouse monoclonal antibody (MAb) (immunoglobulin G2b [IgG2b], clone 23738.111) able to neutralize IL-10 was obtained from R&D Systems (catalog no. MAB217). Rat MAb (IgG2a, clone 3F9) able to block IL-10 receptor was obtained from BD PharMingen (catalog no. 556012). Fluorescein isothiocyanate (FITC)-conjugated anti-CD1 MAbs used in this study were OKT6 (anti-CD1a, IgG1; American Type Culture Collection, Manassas, Va.), F10/2A3.1 (anti-CD1c, IgG1; S. Porcelli, unpublished data), and SN13 (IgG1κ, K5-1B8 clone; Ancell, Bayport, Minn.). FITC-conjugated MAb recognizing CD14 (IgG2a, UCHM1 clone) was obtained from Ancell. FITC-conjugated mouse IgG1 or IgG2a (Becton Dickinson, Oxnard, Calif.) was used as a negative control. Purified anti-HLA-ABC MAb (IgG2a, W6/32 hybridoma) was obtained from Dako (Dakopatts, Copenhagen, Denmark), and FITC-conjugated anti-HLA-DR MAb (IgG2a, L243 hybridoma) was obtained from Becton Dickinson.
Rabbit polyclonal antiserum recognizing the denatured CD1b protein for Western blot analysis was obtained as previously described (11).
Cell lines and culture conditions.
The CD1b-transfected C1R.b6 lymphoblastoid cell line was generated by stably transfecting human lymphoblastoid C1R cells with pSRα-NEO vector containing CD1b cDNA, as previously described (1). The control, CD1b-negative C1R/MOCK subline was obtained following transfection of C1R cells with a mock plasmid. Lymphoblastoid cells were grown in suspension in RPMI 1640 medium (GIBCO, Paisley, Scotland) supplemented with 10% fetal calf serum (GIBCO), 10 mM HEPES (Flow Laboratories, McLean, Va.), and 2 mM l-glutamine (Flow) and subcultured two or three times weekly.
Preparation and in vitro culture of human AMNC.
Peripheral blood mononuclear cells were separated from heparinized whole blood obtained from healthy donors on a Ficoll-Hypaque (Pharmacia, Uppsala, Sweden) gradient, washed twice in RPMI 1640 medium, and resuspended in RPMI 1640 medium supplemented with 10% fetal calf serum, 10 mM HEPES, 2 mM l-glutamine, 0.8 mM nonessential amino acids (GIBCO), 0.4 mM essential amino acids, and 50 μM 2-mercaptoethanol (hereafter referred to as complete medium [CM]). Adherent mononuclear cells (AMNC) were isolated by plastic adherence at 37°C for 2 h, followed by extensive washing with RPMI 1640 at 37°C. In the majority of cases, more than 70% of AMNC were positive for CD14 on day 0, whereas marked down-regulation of this antigen occurred progressively in the following days of culture (data not shown). On the contrary, CD1b was essentially absent in all AMNC preparations on day 0. To activate AMNC, when not otherwise stated, GM-CSF was used at a concentration of 200 IU/ml and left in the medium for the entire period of cell culture.
Cultivation of BCG and infection of AMNC.
BCG (ATCC 27291) was grown in Middlebrook 7H10 agar (Difco Laboratories, Detroit, Mich.) at 37°C under a humidified 5% CO2 atmosphere for 2 weeks. Bacterial suspensions were prepared by dispersing colonies with glass beads in RPMI 1640. The tubes were vortexed for 1 min and allowed to stand for 30 min to allow larger particles to settle. The upper supernatant was stored at −40°C until use. CFU were counted by the standard viable count technique on Middlebrook 7H10 agar plates.
Infection of AMNC was carried out by coculturing adherent target cells, seeded in 25- or 75-cm2 flasks (Corning Costar Co., Cambridge, Mass.) in 5 ml (2 × 106 AMNC/flask) or 15 ml (6 × 106 AMNC/flask) of CM, respectively, with BCG organisms at different BCG CFU/AMNC ratios (i.e., multiplicity of infection [MOI]) at 37°C in a 5% CO2 atmosphere for 4 h, followed by thorough washing with CM at 37°C. Thereafter, control or infected AMNC were incubated with CM alone or activated with GM-CSF (200 IU/ml) or GM-CSF plus IL-4 (200 IU/ml), and expression of CD1 molecules was tested from day 3 of culture onward, as indicated in each experiment.
The Kinyoun method (20) was used to stain intracellular BCG in AMNC dispensed in multiwell slides used for immunofluorescence microscopy analysis.
Flow cytometry analysis.
Cultured cells were washed twice in phosphate-buffered saline (PBS) supplemented with 0.1% bovine serum albumin and 0.02% sodium azide (PBS-A). One million cells were resuspended in 50 μl of the same medium containing the appropriate mouse immunoglobulin. Samples were incubated at 4°C for 30 min and then washed in PBS-A. Pellets were resuspended in 1% formaldehyde (Merck KGaA, Darmstadt, Germany) to inactivate BCG organisms and left at 4°C for 30 min. The labeled samples were then washed with PBS-A, resuspended in the same medium, and analyzed with a FACScan flow cytometer (Becton Dickinson). Data were collected on 104 viable cells as determined by forward and side angle light scatter. Data analysis was performed by using Lysis II software (Becton Dickinson).
Northern blot analysis.
Total cellular RNA was extracted using the TriPure isolation reagent (Boehringer Mannheim, Indianapolis, Ind.). RNAs were fractionated by electrophoresis on a formaldehyde-containing 1.2% agarose gel, transferred to a nylon membrane (Gene Screen Plus; NEN Research Products, Boston, Mass.), and hybridized at 68°C for 1 h with a 32P-labeled probe using the QuickHyb hybridization solution (Stratagene, Cambridge, United Kingdom). CD1b-specific transcripts were detected as previously described using as a probe a 266-bp cDNA fragment corresponding to the second exon of the CD1B gene, which encodes the first extracellular domain of the mature CD1b protein, designated α1 (11, 19, 26). The glyceraldehyde-3-phosphate dehydrogenase (GAPDH) probe, a 0.9-kb EcoRI fragment of the human GAPDH gene, was a generous gift from R. Dalla Favera (Department of Pathology, Columbia University, New York, N.Y.). Washing of the blots was performed according to the manufacturer's instructions. The blotted membrane was exposed to X-ray film at −80°C (Kodak, Rochester, N.Y.).
RT-PCR analysis.
cDNA was synthesized by incubating 1.5 μg of total RNA with 0.5 U of avian myeloblastosis virus reverse transcriptase (RT) and 0.2 μg of oligo(dT) primer at 42°C for 1 h using the cDNA Cycle kit from Invitrogen (Carlsbad, Calif.). The PCR was performed by adding cDNA samples to a solution (total volume, 50 μl) containing 1× PCR buffer (10 mM Tris-HCl [pH 8.3], 50 mM KCl, 1.5 mM MgCl2, 0.01% gelatin) and 200 μM (each) dCTP, dATP, dGTP, and dTTP. Ten picomoles each of two synthetic oligonucleotide primers (Biogen, Rome, Italy) was added to the mixture, and amplification was performed using Taq DNA polymerase (1.25 U) (Boehringer Mannheim) for 28 cycles in a DNA thermal cycler (Perkin Elmer Cetus, Norwalk, Conn.). Each cycle consisted of denaturation at 95°C for 45 s, annealing at 57°C (CD1b) or 53°C (GAPDH) for 1.30 min, and extension at 72°C for 2 min.
The oligonucleotide primer pairs used for CD1b amplification were 5′-CCTTCCAGGGGCCGACCTCCTTT-3′ and 5′-CATGGGATATTCTGATATGACCG-3′. These primers allow amplification of a 940-bp DNA fragment, spanning from exon 2 to the exon 5-6 boundary of the CD1b sequence (19). The primers used for GAPDH (5′-TGGTATCGTGGAAGGACTCATGAC-3′ and 5′-ATGCCAGTGAGCTTCCCGTTCAGC-3′) amplify a 190-bp product.
Nucleotide sequence analysis.
For nucleotide sequence analysis, the amplified products derived from RT-PCR were subjected to electrophoresis and purified using the QIAquick gel extraction kit (Qiagen, Hilden, Germany). The nucleotide sequence of the purified PCR products was determined using the dye terminator cycle sequencing kit with Amplitaq DNA polymerase (Perkin Elmer, Foster City, Calif.) and run on an Applied Biosystem PRISM 377 DNA sequencer.
Western blot analysis.
Cells were washed extensively with PBS. The cell pellet was suspended in 5 volumes of lysis buffer (25 mM HEPES [pH 7.5], 2.5 mM MgCl2, 2.5 mM EGTA, 50 mM 2-mercaptoethanol, 200 μg of leupeptin per ml, 5 μg of aprotinin per ml, 1 mM phenylmethylsulfonyl fluoride, 400 μg of soybean trypsin inhibitor per ml), sonicated at 4°C for 5 s, and centrifuged at 100,000 × g at 4°C for 1 h. The supernatant was collected and designated the cytosol fraction. The pellet was resuspended in lysis buffer containing 1% Triton X-100, sonicated for 5 s, and centrifuged at 15,000 × g at 4°C in a microcentrifuge for 10 min. The supernatant was collected and defined as the membrane fraction.
Membrane and cytosol fractions were separated in sodium dodecyl sulfate–12% (wt/vol) polyacrylamide gels as described by Laemmli (18) and transferred to nitrocellulose filters, according to the method described by Towbin et al. (42), using a Bio-Rad (Hercules, Calif.) electrophoretic mini blotting apparatus.
Filters were incubated with 3% (wt/vol) nonfat dry milk (Bio-Rad) in Tris-HCl (20 mM; pH 7.5) plus 0.9% NaCl (TBS) for 1 h and then with rabbit anti-CD1b serum diluted 1:2,000 in TBS containing 0.05% Tween 20 (TBST) for 30 min. Thereafter, the membranes were washed twice with TBST and incubated with alkaline phosphatase-coupled secondary antibody diluted 1:7,500 in TBST for 1 h. The bands were visualized using the Protoblot (Promega Biotec, Madison, Wis.) reagents according to the procedures provided by the manufacturer.
RESULTS
Influence of BCG on the induction of group I CD1 molecules by GM-CSF on AMNC.
A series of preliminary experiments was conducted to obtain quantitative information on the effect of different levels of BCG exposure of AMNC on GM-CSF-induced CD1b expression. AMNC were infected with BCG at MOIs ranging from 0.25 to 10 and incubated with GM-CSF alone. On day 3, flow cytometry analysis of CD1b expression showed that BCG at all concentrations used antagonized the effect of GM-CSF on CD1b induction. When BCG was used at a high MOI (i.e., greater than 5), the expression of CD1b declined markedly and reached almost undetectable values at an MOI of 10 (data not shown). Although a certain degree of donor-dependent variability was apparent, induction of CD1b (in terms of percentage of CD1b-positive cells) by GM-CSF was found to be consistently inhibited (by 35 to 75%) by infection of AMNC at an MOI of 1 in more than 10 different AMNC samples studied.
Time-course studies were performed by exposing AMNC to graded amounts of BCG (from 0.25 to 1 MOI), followed by GM-CSF. Flow cytometry analysis of CD1b antigen was performed on days 3, 5, and 7 of culture. The results of one of two comparable experiments (Fig. 1) show a reasonably good concentration-inhibition relationship between BCG amount and CD1b expression on day 3, as confirmed by the regression line analysis illustrated in the legend to Fig. 1. A similar inhibitory effect of BCG was maintained up to 7 days in culture, as evidenced by the 50% inhibitory concentration (IC50) values relative to MOI calculated on days 5 and 7 (see legend of Fig. 1). It must be pointed out that persistence of intracellular bacilli was found in approximately 50% of infected AMNC in all experiments up to 7 days after BCG infection (data not shown).
FIG. 1.
Cytofluorimetric analysis relative to time-course of CD1b expression of AMNC activated with 200 IU of GM-CSF (GM) per ml on day 0 and exposed to BCG at different MOIs, i.e., GM-B(0.25), MOI of 0.25; GM-B(0.50), MOI of 0.5; GM-B(1.00), MOI of 1. On day 3, at an MOI of 2.0, the percentage of CD1b+ cells was 15.17 (data not shown in the figure). Regression line analysis was performed in order to calculate the MOI of BCG able to produce 50% inhibition of CD1b expression (IC50), with the fitness of the line illustrating the linear relationship between the extent of MOI and CD1b reduction (expressed on the basis of the Pearson coefficient [PC]) and the 95% confidence limits (CL) of the calculated IC50 values. The results of this analysis, expressed in terms of IC50 (CL; PC), were as follows: day 3, 1.37 (2.4 to 0.8; 0.9858); day 5, 1.46 (1.33 to 1.60; 0.9897); day 7, 1.78 (0.92 to 2.63; 0.591).
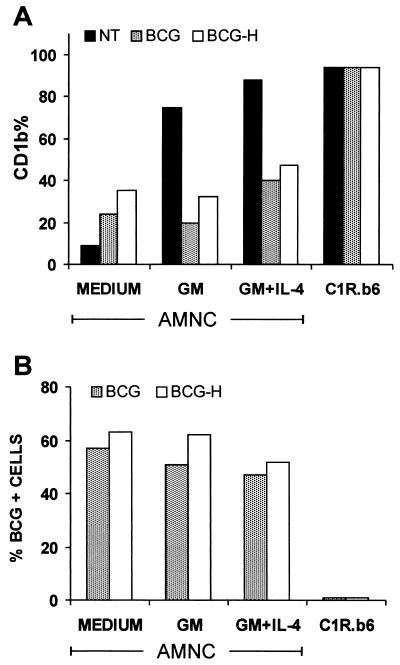
Preliminary studies performed in our laboratory showed that chemical inactivation of BCG with rifampin does not prevent the inhibitory effect of the microorganism on CD1b expression (28). Therefore, further experiments were carried out using either viable or heat-killed BCG (BCG-H) organisms. AMNC were exposed to BCG or to BCG-H, washed, and cultured without further treatment or activated with GM-CSF alone or with GM-CSF plus IL-4. Parallel experiments were conducted with CD1b-transfected C1R.b6 cells that were untreated or exposed to BCG or to BCG-H, without GM-CSF or IL-4. On day 3 of culture, the percentage of CD1b-positive cells was evaluated by flow cytometry, and the percentage of cells carrying one or more intracellular bacilli was established microscopically. The results of one of two independent experiments with similar data are illustrated in Fig. 2, demonstrating that limited induction of CD1b expression was produced by exposure of AMNC to BCG or to BCG-H without GM-CSF. However, both BCG and BCG-H limited the induction of CD1b by GM-CSF alone or by GM-CSF plus IL-4. BCG and BCG-H were taken up to a similar extent by AMNC, regardless of the culture conditions used for activation of the cells. In contrast, BCG and BCG-H were not taken up by the C1R.b6 B lymphoblastoid cell line, and no decrease in the expression of CD1b was observed on these cells following exposure to BCG (Fig. 2).
FIG. 2.
Effect of BCG (at an MOI of 1 for 4 h on day 0), either viable (BCG) or heat inactivated (BCG-H; treated at 80°C for 1 h), on CD1b expression of nonactivated or cytokine-activated AMNC or of CD1b-transfected C1R.b6 lymphoblastoid line. On day 0, AMNC were activated with 200 IU of GM-CSF alone (GM) per ml or with the same concentration of GM-CSF associated with 200 IU of IL-4 per ml (GM+IL-4). Since treatment with IL-4 without GM-CSF did not induce substantial amounts of CD1b molecules on AMNC surfaces, the data relative to the effect of IL-4 alone have been omitted. (A) Percent positivity of AMNC or C1R.b6 cells for CD1b membrane molecules tested on day 3 of culture. Data shown indicate the mean fluorescence values. (B) Percentages of AMNC or C1R.b6 cells showing the presence of one or more BCG organisms in the cytoplasm (see Materials and Methods) on day 3 of culture. On day 0, the proportion of AMNC showing intracellular bacilli after the initial 4 h of incubation with viable BCG or BCG-H was 26 and 33%, respectively. On the same day, no more than 3% of C1R.b6 cells showed the presence of BCG or BCG-H organisms.
The specificity of BCG-induced impairment of CD1b expression was assayed by testing whether BCG could modify the level of HLA antigens. The results of a representative experiment, confirmed by two additional experiments, are illustrated in Table 1. The mycobacterium was able to down-regulate the induction of CD1b expression by either GM-CSF alone or by GM-CSF plus IL-4 but did not inhibit the constitutive or inducible expression or up-regulation of class I (i.e., HLA-A, -B, -C) and HLA-DR antigens. Actually, BCG infection tended to increase the levels of MHC class I and II monomorphic antigens.
TABLE 1.
Effect of BCG infection on CD1b, HLA class I, and HLA-DR expression of AMNC that were untreated or activated by GM-CSF alone or GM-CSF plus IL-4
| Treatmenta | Expression of:
|
|||||
|---|---|---|---|---|---|---|
| CD1b
|
HLA class I
|
HLA-DR
|
||||
| % Positive cells | MFVb | % Positive cells | MFV | % Positive cells | MFV | |
| None | 12 | 16 | 83 | 740 | 67 | 144 |
| GM-CSF | 42 | 161 | 88 | 490 | 67 | 353 |
| GM-CSF plus IL-4 | 60 | 238 | 81 | 562 | 75 | 452 |
| BCG | 22 | 38 | 85 | 900 | 82 | 680 |
| BCG plus GM-CSF | 23 | 50 | 84 | 914 | 81 | 835 |
| BCG plus GM-CSF plus IL-4 | 26 | 66 | 84 | 1002 | 77 | 860 |
GM-CSF, 200 IU/ml; IL-4, 200 IU/ml; BCG, MOI of 2 in a total of 5 ml/flask (25 cm2) on day 0; cytofluorimetric analysis of CD1b, HLA class I, and HLA-DR membrane expression was performed on day 3 of culture.
MFV, mean fluorescence values.
Four experiments were performed to test whether effects similar to those detected on CD1b expression could also be seen for two other group I CD1 molecules, CD1a and CD1c. In this case, nonpretreated or BCG-pretreated AMNC were cultured for 3 days with or without GM-CSF, followed by flow cytometry analysis. These experiments revealed significant reduction of CD1a and CD1c expression in response to BCG infection, as shown in the representative experiment summarized in Table 2.
TABLE 2.
Effect of BCG infection on CD1a and CD1c molecule expression of AMNC that were untreated or activated by GM-CSF
| Treatmenta | Expression of:
|
|||
|---|---|---|---|---|
| CD1a
|
CD1c
|
|||
| % Positive cells | MFVb | % Positive cells | MFV | |
| None | 10 | 18 | 7 | 17 |
| GM-CSF | 58 | 141 | 62 | 62 |
| BCG | 34 | 125 | 45 | 49 |
| BCG plus GM-CSF | 33 | 118 | 30 | 36 |
GM-CSF, 200 IU/ml; BCG, MOI of 2 in a total of 5 ml/flask (25 cm2) on day 0; cytofluorimetric analysis of membrane expression was performed on day 3 of culture.
MFV, mean fluorescence values.
Additional studies were performed to explore whether BCG could influence CD1b expression by CD1b-positive AMNC that had been previously activated by GM-CSF. The results (Table 3) showed that a 4-h exposure of AMNC preactivated by GM-CSF or by GM-CSF plus IL-4 (on day 0) to bacilli at an MOI of 1 or 2.5 on day 3 was followed by a modest down-regulation of the antigen-presenting molecule on day 5 (in the case of GM-CSF-pretreated AMNC only) and on day 7. Similar results were obtained in two additional experiments not reported in the table.
TABLE 3.
Effect of BCG infection (on day 3) on CD1b expression of AMNC that were untreated or activated by GM-CSF alone or GM-CSF plus IL-4 on day 0
| Treatment on day 0a | Additional treatment on day 3b | Expression of CD1b on:
|
|||
|---|---|---|---|---|---|
| Day 5
|
Day 7
|
||||
| % Positive cells | MFVc | % Positive cells | MFV | ||
| GM-CSF | None | 62 | 242 | 58 | 175 |
| GM-CSF + IL-4 | None | 60 | 135 | 43 | 124 |
| GM-CSF | BCG-1 | 48 | 96 | 42 | 115 |
| GM-CSF + IL-4 | BCG-1 | 66 | 157 | 38 | 64 |
| GM-CSF + IL-4 | BCG-2 | 68 | 152 | 35 | 60 |
GM-CSF, 200 IU/ml; IL-4, 200 IU/ml in a total of 5 ml/flask (25 cm2) on day 0.
BCG-1 (at an MOI of 1) and BCG-2 (at an MOI of 2.5) were added on day 3 and left in coculture for 4 h, followed by accurate washing with CM at 37°C. More than 50% inhibition of CD1b expression (in terms of percent CD1b-positive cells and of MFV) was found in the case of BCG added to GM-CSF or to GM-CSF plus IL-4 at an MOI of 1 on day 0 (data not shown).
MFV, mean fluorescence values.
Influence of BCG on the level of CD1b-specific transcripts.
To investigate whether reduced expression of CD1b protein might be the consequence of decreased mRNA levels, Northern blot analysis was performed on total RNA extracted from AMNC that were either unstimulated or stimulated with GM-CSF for 72 h with or without prior BCG infection. Hybridization with the CD1b probe revealed CD1b transcripts only in GM-CSF-activated AMNC, either uninfected or BCG infected (Fig. 3A), whereas hybridization of the same blot with the GAPDH probe allowed detection of the corresponding transcript in all samples (Fig. 3B). The hybridization signals were quantified by densitometric scanning of the autoradiograms and CD1b expression was normalized in relation to GAPDH. This demonstrated that BCG-infected AMNC had lower levels of CD1b transcripts with respect to uninfected and GM-CSF-activated AMNC (approximately 35% reduction).
FIG. 3.
Northern blot analysis of CD1b-specific transcript (A) or GAPDH (B) in AMNC exposed to BCG infection. Total RNA was extracted on day 3 of culture of AMNC treated with BCG (MOI of 1 for 4 h on day 0) in the absence or presence of GM-CSF (200 IU/ml from day 0). Lane 1, nonstimulated AMNC (20 μg); lane 2, GM-CSF-activated AMNC (15 μg); lane 3, GM-CSF-activated AMNC exposed to BCG (15 μg). Control of the integrity and amount of RNA loaded in each lane was performed by ethidium bromide staining of the gel (data not shown). The hybridization signals were quantified by densitometric scanning of the autoradiograms (imaging densitometer GS-670; Bio-Rad) and normalized in relation to GAPDH. The ratio between normalized optical densities of GM-CSF-activated AMNC infected with BCG and GM-CSF-activated AMNC was 0.65.
Down-regulation of CD1b transcripts was further confirmed by RT-PCR analysis of RNA samples, as illustrated in Fig. 4A. No amplification of CD1b-specific transcripts was observed in nonstimulated AMNC. In contrast, amplification of the GAPDH cDNA was obtained in all samples (Fig. 4B). In addition to a 940-bp fragment corresponding to the nucleotide length expected from amplification of the CD1b sequence spanning between the beginning of exon 2 and the exon 5-6 boundary (19), RT-PCR using CD1b-specific primers also detected a slightly shorter and less abundant product (Fig. 4A, lanes 2 and 3). Analysis of the nucleotide sequence revealed that this fragment lacked 165 nucleotides compared to the 940-bp amplified product. The fragment derives from an alternatively spliced CD1b transcript in which a cryptic donor splice site within the α3 domain sequence (nucleotides 115 and 116 of exon 4) is joined to the splice acceptor site of the transmembrane-cytoplasmic domain (exon 5) (data not shown). This alternative mRNA splicing was previously predicted to result in a transcript encoding a truncated and likely inactive form of the protein (44).
FIG. 4.
RT-PCR analysis of CD1b-specific transcript (A) or GAPDH (B) in AMNC exposed to BCG infection. Lane 1, nonstimulated AMNC; lane 2, GM-CSF-activated AMNC; lane 3, GM-CSF-activated AMNC infected with BCG. The molecular size marker (M) used for CD1b analysis was a 1-kb DNA ladder (Gibco-BRL), whereas for GAPDH, the marker used was pBR322 plasmid DNA digested with Msp1 (Gibco-BRL).
Western blot analysis of the influence of BCG on induction of the CD1b protein.
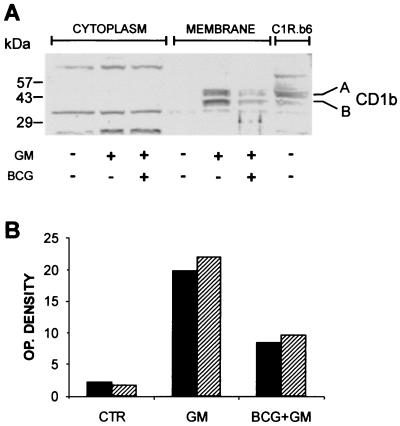
Western blot analysis of cytosol and cell membrane preparations was performed using the rabbit polyclonal antiserum that recognizes the denatured form of CD1b protein (11). The results of one of two similar independent experiments (Fig. 5A) showed two bands that were detectable in the membrane extract of either C1R.b6 cells and AMNC activated by GM-CSF. The upper band showed a molecular weight of about 45 kDa, corresponding to the expected size of the mature form of CD1b (i.e., with multiple sialic acid additions). The lower band (an approximately 40-kDa molecule) most likely corresponded to a less glycosylated (i.e., immature) form of CD1b. These bands were almost undetectable in the membrane extract of nonstimulated AMNC and were absent in that of C1R/MOCK cells (data not shown). Exposure of AMNC to BCG prior to treatment with GM-CSF reduced significantly the GM-CSF-dependent induction of CD1b expression, as confirmed by densitometric analysis illustrated in Fig. 5B. No bands corresponding to the expected molecular weights of CD1b were found in the cytosolic fraction of AMNC.
FIG. 5.
Western blot analysis of CD1b protein in AMNC exposed to BCG (at an MOI of 1 for 4 h) and GM-CSF (GM; 200 IU/ml) on day 0 and tested on day 3. (A) The 45-kDa protein (band A) corresponds to the expected size of the mature form of CD1b molecule; the protein of approximately 40 kDa (band B) could correspond to the less-glycosylated (i.e., immature) form of CD1b. Membrane fractions obtained from C1R.b6 cells were used as a positive control. Numbers on the left represent molecular size (in kilodaltons) standards. (B) Immunoblots were scanned by densitometer and optical (OP.) density values are expressed as arbitrary units. Filled columns, band A; dashed columns, band B.
A soluble factor(s) is involved in the inhibition of CD1b induction by BCG.
A transwell system consisting of an upper and lower well separated by a permeable 0.40-μm-pore-size membrane was used to investigate whether soluble inhibitory factors could be released from BCG-infected cells. The experimental design adopted in these experiments, illustrated in Table 4, was validated by the observation that GM-CSF added to the upper well was able to activate AMNC of the lower well (group 1 versus group 11), as expected for a soluble, diffusible factor. BCG infection of AMNC in either the upper or lower wells antagonized the induction of CD1b by GM-CSF on AMNC cultured in the lower wells (groups 4 and 10). This indicated that a soluble factor produced by BCG-infected AMNC was able to pass through the membrane separating the upper and lower wells and mediate the inhibition of CD1b induction on uninfected AMNC (group 10). Interestingly, when BCG was placed in the upper well without AMNC, no inhibition but rather an increase of GM-CSF-induced CD1b expression was detected in AMNC of the lower well (group 2 versus group 9). This indicated that the inhibitory factor was most likely not produced by live extracellular BCG organisms but was released following infection of AMNC.
TABLE 4.
Induction of CD1b molecule by GM-CSF is reduced by a soluble factor(s) released by AMNC exposed to BCGa
| Group | Contents of well
|
Expression of CD1b (day 3)b
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Lower well (flat bottom) (2 ml/well, day 0)
|
Upper well (membrane bottom) (2 ml/well, day 0)
|
|||||||||
| AMNC/well | BCG (MOI) | GM-CSFc (IU/ml) | AMNC/well | BCG (MOI) | GM-CSF (IU/ml) | % Positive cells | MFV | % variation
|
||
| % Positive cells | MFV | |||||||||
| 1 | 106 | None | None | None | None | None | 2 | 6 | ||
| 2 | 106 | None | 400 | None | None | None | 27.3 | 49 | 0 | 0 |
| 3 | 106 | 1 | None | None | None | None | 12.7 | 20 | ||
| 4 | 106 | 1 | 400 | None | None | None | 8.2 | 11 | −70 | −77.6 |
| 5 | 106 | None | None | 106 | None | None | 3.5 | 5 | ||
| 6 | 106 | None | 400 | 106 | None | None | 23.5 | 38 | −13.9 | −22.4 |
| 7 | 106 | None | None | None | 1 | None | 2.0 | 1 | ||
| 8 | 106 | None | None | 106 | 1 | None | 7.8 | 10 | ||
| 9 | 106 | None | 400 | None | 1 | None | 39.0 | 48 | +42.8 | −2.0 |
| 10 | 106 | None | 400 | 106 | 1 | None | 17.6 | 19 | −35.5 | −61.2 |
| 11 | 106 | None | None | None | None | 400 | 34.1 | 64 | +24.9 | +30.6 |
Data refer to a representative experiment repeated three times with similar results. Upper wells are separated by lower wells by the 0.40-μm-pore-size transwell membrane that allows free exchange of soluble material but not of cells or bacteria. BCG at an MOI of 1 was added to AMNC (approximately 1 million AMNC/well) in the lower wells, and in selected groups, in the upper wells, left for 4 h, and washed. Thereafter, cells were incubated with or without GM-CSF for an additional 3 days, and expression of CD1b was evaluated on day 3 by flow cytometry analysis.
Percentages of CD1b-positive cells and mean fluorescence values (MFV) refer to AMNC seeded in lower wells. Percent variations of CD1b-positive cells and MFV refer to values of control GM-CSF-activated AMNC seeded in lower wells (i.e., group 2).
Since previous observations showed that GM-CSF activates CD1b less efficiently in these wells than in 25-cm2 flasks, 400 instead of 200 IU/ml of GM-CSF was used.
Attempts to revert BCG-mediated impairment of CD1b induction using high concentrations of GM-CSF or GM-CSF combined with various cytokines.
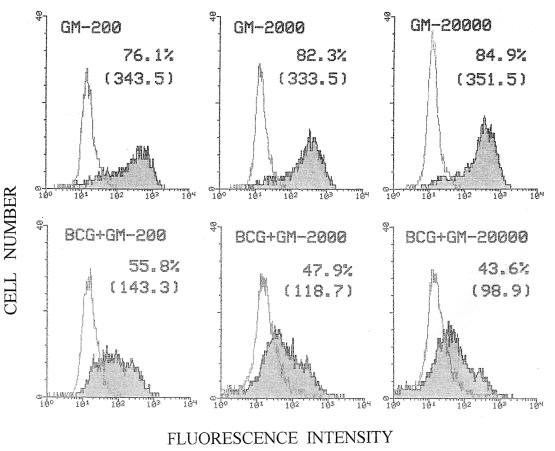
Previous studies performed in our laboratory showed that induction of CD1b expression by GM-CSF in AMNC reaches a plateau at a concentration of 200 IU/ml (unpublished data). Although higher concentrations of the cytokine did not substantially increase antigen levels, attempts were made in the present study to revert BCG-induced down-regulation of CD1b expression using high amounts of GM-CSF. This approach stemmed from the consideration that the effect of BCG might be to make infected cells partially refractory to this cytokine. Therefore, AMNC were exposed to BCG (MOI of 1) and activated with “standard” (200 IU/ml), high (2,000 IU/ml), or extremely high (20,000 IU/ml) concentrations of GM-CSF. The results of two comparable experiments (Fig. 6 and data not shown) showed that these treatments failed to significantly overcome the negative effect of BCG on CD1b expression. It was also demonstrated that treatment with GM-CSF plus IL-4 can induce CD1b expression higher than that obtainable with GM-CSF alone (references 26 and 38 and the present report). However, exposure of AMNC to GM-CSF associated with IL-4 or with other cytokines (i.e., IL-6, IL-15, IFN-α, IFN-γ) did not revert the inhibitory effects of BCG on CD1b expression (Table 1; Fig. 2; data not shown).
FIG. 6.
Cytofluorimetric analysis relative to CD1b expression of AMNC. Target cells, noninfected or infected with BCG (at an MOI of 1 for 4 h), were activated with GM-CSF using conventional (200 IU/ml) (GM-200), high (2,000 IU/ml) (GM-2000), or extremely high (20,000 IU/ml) (GM-20000) concentrations of the cytokine in the attempt to reverse CD1b down-regulation induced by BCG infection.
DISCUSSION
The worldwide spread of tubercular infections, especially in malnourished and/or immunocompromised subjects, and the rapid increase of drug-resistant mycobacteria strains (2, 25) encourage investigation on immunological approaches to control these diseases. Several lines of evidence are in favor of a relationship between CD1-dependent immune reactivity and tuberculosis in humans (7, 30, 32, 33, 37, 39). Therefore, biological and pharmacological agents able to influence the expression of CD1 molecules on AMNC could have a significant impact on resistance against tuberculosis and related diseases. In the present study, we have shown that pretreatment of AMNC with BCG inhibited the induction of high levels of CD1 molecules by GM-CSF, which is believed to be a key inducer of the expression of this family of antigen-presenting molecules on myeloid lineage cells. This effect, which is detectable at both the protein and mRNA levels, was mediated at least in part by a soluble factor(s) released by target cells following interaction with either live or heat-killed BCG.
Circulating monocytes give rise to CD1-positive dendritic cells upon activation with selected cytokines, i.e., GM-CSF (26) or IL-3 in combination with IL-4 (41). These cytokines can be released during inflammatory processes and sensitization procedures, including exposure to BCG, which activates sequential production of Th1 and Th2 cytokines (31). Group I CD1 (i.e., CD1a, CD1b, and CD1c [26]) molecules present glycolipids (33), and in particular ManLAM of mycobacterial origin, through the hydrophobic interactions between ManLAM fatty acids and nonpolar amino acids of the CD1 groove to the antigen-binding site of the αβ TCR of T cells (23). Although M. tuberculosis-reactive CD1-restricted T cells have been isolated from healthy donors (30), in other cases CD1-dependent anti-M. tuberculosis activity was detected in patients preexposed to mycobacterial infections, including leprosy patients (34), or in HIV-positive patients who may have been sensitized by increased exposure to opportunistic mycobacteria (13). Moreover, CD1c-restricted T cells recognizing mannosyl-β1-phosphodolichols of M. tuberculosis origin have been found in the peripheral blood of human subjects infected with M. tuberculosis, but not in the blood of healthy controls (22).
Previous investigations revealed that CD1 expression induced by GM-CSF in AMNC can be up-regulated by rifampin, an antitubercular antibiotic (12, 38), or down-regulated by the Th2 cytokine IL-10 (40) and by infection with virulent M. tuberculosis (36). The present study showed that BCG negatively influences the GM-CSF-mediated induction phase of CD1 expression in AMNC at the relatively low MOI of 1. It is reasonable to suggest that a similar biological situation could occur in the initial steps of BCG vaccination in vivo. Actually, no data are available on the precise role played by the CD1 system in the long-term protection against mycobacterial infections in vaccinated subjects. It can be hypothesized that CD1-restricted responses could act in concert with classical MHC-restricted T-cell responses directed against peptides of mycobacterial origin (35). If this is the case, the present results could offer novel insights into the overall pattern of host-BCG interaction involved in the immune responses against M. tuberculosis. Moreover, the observation that the inhibitory effects mediated by BCG show measurable differences among the healthy donors tested could account, at least in part, for the well-known variability of the protective action of this vaccine.
Stenger et al. (36) reported that virulent M. tuberculosis is able to severely down-regulate transcription of CD1 genes and expression of the corresponding products on the membranes of GM-CSF-activated AMNC. Their experimental model was designed to analyze the effect of mycobacteria on AMNC preactivated by GM-CSF plus IL-4 and already expressing CD1 molecules. In this case the model was properly tailored to recreate events occurring in an established infection, in which cytokine-mediated activation of AMNC would be likely to have already occurred during the initial stages of development of the infection. In contrast, in the experimental design of our study, BCG has been applied in a manner more consistent with its role as a vaccine rather than as an infectious agent causing a systemic disease. Therefore, the analysis of the influence of the bacterium on the CD1 system was performed during the induction phase of cytokine-mediated CD1 generation. No experiments with virulent M. tuberculosis have been conducted using this model. It follows that the results obtained with BCG cannot be compared directly with those previously described for virulent M. tuberculosis. However, when CD1-positive AMNC preactivated with GM-CSF were exposed to BCG, only limited down-regulation of the antigen-presenting molecule was detected (Table 2), suggesting a significant difference between the effects of BCG and virulent M. tuberculosis in this regard.
The present study showed that BCG, like M. tuberculosis, reduces CD1b mRNA levels, suggesting that it interferes with CD1B gene transcription or accelerates degradation of its transcripts. The results illustrated in Fig. 4B demonstrate that preexposure of AMNC to BCG consistently reduces the levels of two mRNA transcripts of CD1B origin, namely the main final product and an alternatively spliced mRNA. Alternative splicing of mRNA has already been described for CD1A and CD1C gene transcripts, revealing rather complex and tissue-specific mRNA splicing patterns (44). However, no data are available from the literature concerning splicing pattern of mRNA transcribed from CD1B gene in GM-CSF-activated human AMNC. In any case, it has been suggested that the less abundant alternatively spliced form of mRNA, shown in Fig. 4B, would encode a truncated and likely inactive CD1b protein (S. Porcelli, unpublished data).
BCG-mediated reduction of CD1B gene transcript levels was followed by a consistent decrease of CD1b on the cell membrane, as evidenced by flow cytometry and Western blot analysis. It seems likely that the molecular mechanism involved in the prevention of GM-CSF-dependent up-regulation of the protein is at least in part based on the reduced availability of CD1B gene transcripts, although it remains possible that additional posttranscriptional mechanisms could also be involved. Moreover, since both principal and alternatively spliced mRNA transcripts are lower in BCG-infected GM-CSF-activated AMNC, it is not likely that alternative splicing mechanisms would play a significant role in the effect of BCG on CD1 expression.
The results of the present investigation point out that one or more soluble factors are involved in the effect of BCG on CD1b expression. Previous studies showed that pretreatment of AMNC with IL-10 markedly down-regulated CD1 expression in human adherent cells exposed to GM-CSF (40). Since BCG is able to induce production of IL-10 in adherent cells (31), this cytokine appeared to be a good candidate to play a role in the mechanism of action of BCG. However, treatment of AMNC with neutralizing MAbs directed against IL-10 or against IL-10 receptor prior to (30 min) and during (4 h) exposure of target cells to BCG did not prevent the adverse effects of the microorganism on the induction of GM-CSF-dependent CD1b expression. On the contrary, both MAbs consistently abrogated the inhibition produced by IL-10 on the induction of CD1b by GM-CSF. In a representative experiment, treatment with GM-CSF alone induced 60% of CD1b-positive AMNC on day 3 of culture. Addition of IL-10 (10 ng/ml) reduced the percentage of CD1b+ cells to 43%, whereas treatment with IL-10 plus both types of MAbs restored the full response of AMNC to GM-CSF. Moreover, it must be pointed out that a concentration of IL-10 of 10 ng/ml is usually higher than that obtainable after a 3-day coculture of AMNC and BCG under our experimental conditions (data not shown). Nevertheless, it cannot be ruled out that IL-10 would contribute to the overall effect of the mycobacterium, sharing this role with other unrecognized factors.
Several attempts have been made to overcome the detrimental influence of BCG on CD1 induction by GM-CSF. Actually, a limited increment of AMNC-associated CD1b expression can be provoked by the organism itself in the absence of added GM-CSF, as shown in Table 1. This could be explained by direct influence of the organism on monocytes or by BCG-induced release of cytokines (e.g., GM-CSF or IL-3 [31]) able to increase CD1 expression (26, 41). Thus, infection with this microorganism appears to be followed by induction of a threshold CD1 level that cannot be increased even by extremely high amounts of GM-CSF exogenously added to infected cultures (see Fig. 6). In addition, the synergistic effect of IL-4 on CD1b induction was also blocked by BCG infection.
In conclusion, the present study reveals for the first time that BCG is a potent negative modulator of GM-CSF-induced expression of group I CD1 molecules. This suggests that BCG could adversely influence nonpeptide antigen presentation to lymphocyte subsets involved in resistance against tuberculosis during the induction phase of the sensitization process. Our results call attention to one potential limitation of live attenuated mycobacteria such as BCG as vaccine strains. Given that these organisms have coevolved with the vertebrate immune system, it appears likely that they have incorporated numerous mechanisms to subvert the immune response of the hosts that they infect and parasitize. However, identification of specific immune modulating activities of mycobacterial vaccine strains will potentially allow their genetic manipulation to inactivate the genes that control their ability to prevent effective immunization. In the case of BCG, modification of the bacillus to abrogate its ability to down-regulate group I CD1 molecules represents a potential strategy by which the efficacy of this vaccine for prevention of tuberculosis might be significantly improved.
ACKNOWLEDGMENTS
This work was supported in part by a grant of “Progetto AIDS” of the National Institutes of Health, Rome, Italy (Istituto Superiore di Sanità; contract # 50C.1, 2000), and in part by a grant from the Ministry of the University and Research of the Italian Government (MURST; Research Unit Graziani, 1999). S.A.P. was supported by grants from the NIH (AI45888) and from the Irene Diamond Foundation.
We thank G. Girolomoni for fruitful discussion and suggestions.
REFERENCES
- 1.Behar S M, Porcelli S A, Beckman E M, Brenner M B. A pathway of costimulation that prevents anergy in CD28-T cells: B7-independent costimulation of CD1-restricted T cells. J Exp Med. 1995;182:2007–2018. doi: 10.1084/jem.182.6.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benator D A, Gordin F M. Nontuberculous mycobacteria in patients with human immunodeficiency virus infection. Semin Respir Infect. 1996;11:285–300. [PubMed] [Google Scholar]
- 3.Bloom B R, Murray C J L. Tuberculosis: commentary on a reemergent killer. Science. 1992;257:1055–1064. doi: 10.1126/science.257.5073.1055. [DOI] [PubMed] [Google Scholar]
- 4.Brewer T F. Preventing tuberculosis with bacillus Calmette-Guerin vaccine: a meta-analysis of the literature. Clin Infect Dis Suppl. 2000;3:S64–S67. doi: 10.1086/314072. [DOI] [PubMed] [Google Scholar]
- 5.Chin D P, Hopewell P C. Mycobacterial complications of HIV infection. Clin Chest Med. 1996;17:697–711. doi: 10.1016/s0272-5231(05)70340-3. [DOI] [PubMed] [Google Scholar]
- 6.Cooper A M, Dalton D K, Stewart T A, Griffin J P, Russell D G, Orme I M. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J Exp Med. 1993;178:2243–2247. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dieli F, Sireci G, Di Sano C, Romano A, Titone L, Di Carlo P, Ivanyi J, Fourniè J J, Salerno A. Ligand-specific αβ and γδ T cell responses in childhood tuberculosis. J Infect Dis. 2000;181:294–301. doi: 10.1086/315180. [DOI] [PubMed] [Google Scholar]
- 8.Flynn J L, Chan J, Triebold K J, Dalton D K, Stewart T A, Bloom B R. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178:2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friedland J S. TB still a global killer. Nature. 1997;387:226. doi: 10.1038/387226a0. [DOI] [PubMed] [Google Scholar]
- 10.Gilleron M, Bala L, Brando T, Vercellone A, Puzo G. Mycobacterium tuberculosis H37Rv parietal and cellular lipoarabinomannans. Characterization of the acyl- and glyco-forms. J Biol Chem. 2000;275:677–684. doi: 10.1074/jbc.275.1.677. [DOI] [PubMed] [Google Scholar]
- 11.Giuliani A, Tentori L, Pepponi R, Porcelli S A, Aquino A, Orlando R, Sugita M, Brenner M B, Bonmassar E, Graziani G. Cytokine-induced expression of CD1b molecules by peripheral blood monocytes: influence of 3′-azido-3′-deoxythymidine. Pharm Res. 1997;35:135–140. doi: 10.1006/phrs.1997.0130. [DOI] [PubMed] [Google Scholar]
- 12.Giuliani A, Porcelli S A, Tentori L, Graziani G, Testorelli C, Prete S P, Bussini S, Cappelletti D, Brenner M B, Bonmassar E, Aquino A. Effect of rifampin on CD1b expression and double-negative T cell responses against mycobacteria-derived glycolipid agents. Life Sci. 1998;63:985–994. doi: 10.1016/s0024-3205(98)00360-9. [DOI] [PubMed] [Google Scholar]
- 13.Gong J, Stenger S, Zack J A, Jones B E, Bristol G C, Modlin R L, Morrissey P J, Barnes P F. Isolation of Mycobacterium-reactive CD1-restricted T cells from patients with human immunodeficiency virus infection. J Clin Investig. 1998;101:383–389. doi: 10.1172/JCI318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoft D F, Brown R M, Roodman S T. Bacille Calmette-Guerin vaccination enhances human γδ T cell responsiveness to mycobacteria suggestive of a memory-like phenotype. J Immunol. 1998;161:1045–1054. [PubMed] [Google Scholar]
- 15.Hunter S W, Brennan P J. Evidence for the presence of a phosphatidylinositol anchor on the lipoarabinomannan and lipomannan of Mycobacterium tuberculosis. J Biol Chem. 1990;265:9272–9279. [PubMed] [Google Scholar]
- 16.Kasinrerk W, Baumruker T, Majdic O, Knapp W, Stockinger H. CD1 molecule expression on human monocytes induced by granulocyte-macrophage colony-stimulating factor. J Immunol. 1993;150:579–584. [PubMed] [Google Scholar]
- 17.Kent J H. The epidemiology of multidrug-resistant tuberculosis in the United States. Med Clin N Am. 1993;77:1391–1409. doi: 10.1016/s0025-7125(16)30200-0. [DOI] [PubMed] [Google Scholar]
- 18.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 19.Martin L H, Calabi F, Lefebvre F A, Bilsland C A G, Anmilstein C. Structure and expression of the human thymocyte antigens CD1a, CD1b, and CD1c. Proc Natl Acad Sci USA. 1987;84:9189–9193. doi: 10.1073/pnas.84.24.9189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Master R N. Mycobacteriology. In: Isenberg H D, editor. Clinical microbiology procedure handbook. Vol. 1. Washington, D.C.: American Society for Microbiology; 1992. pp. 3.1–3.16. [Google Scholar]
- 21.Mazzei L, Croce G F, Zarzana A D, Biagioli B, Sposato B, Pulcinelli A. Drug-resistance of Mycobacterium tuberculosis in time. Eur Rev Med Pharmacol Sci. 1998;2:21–24. [PubMed] [Google Scholar]
- 22.Moody D B, Ulrichs T, Muhlecker W, Young D C, Gurcha S S, Grant E, Rosat J P, Brenner M B, Costello C E, Besra G S, Porcelli S A. CD1c-mediated T cell recognition of isoprenoid glycolipids in Mycobacterial infections. Nature. 2000;404:884–888. doi: 10.1038/35009119. [DOI] [PubMed] [Google Scholar]
- 23.Nigou J, Gilleron M, Puzo G. Lipoarabinomannans: characterization of the multiacylated forms of the phosphatidyl-myo-inositol anchor by NMR spectroscopy. Biochem J. 1999;337:453–460. [PMC free article] [PubMed] [Google Scholar]
- 24.Orme I M. Prospects for new vaccines against tuberculosis. Trends Microbiol. 1995;3:401–404. doi: 10.1016/s0966-842x(00)88987-8. [DOI] [PubMed] [Google Scholar]
- 25.Parsons L M, Driscoll J R, Taber H W, Salfinger M. Drug resistance in tuberculosis. Infect Dis Clin N Am. 1997;11:905–928. doi: 10.1016/s0891-5520(05)70397-4. [DOI] [PubMed] [Google Scholar]
- 26.Porcelli S A. The CD1 family: a third lineage of antigen-presenting molecules. Adv Immunol. 1995;59:1–98. doi: 10.1016/s0065-2776(08)60629-x. [DOI] [PubMed] [Google Scholar]
- 27.Porcelli S A, Modlin R L. The CD1 system: antigen-presenting molecules for T cell recognition of lipids and glycolipids. Annu Rev Immunol. 1999;17:297–329. doi: 10.1146/annurev.immunol.17.1.297. [DOI] [PubMed] [Google Scholar]
- 28.Prete S P, Giuliani A, Iona E, Fattorini L, Orefici G, Francese O, Bonmassar E, Graziani G. Bacillus Calmette-Guerin down-regulates CD1b induction by granulocyte-macrophage colony stimulating factor in human peripheral blood monocytes. J Chemother. 2001;13:52–58. doi: 10.1179/joc.2001.13.1.52. [DOI] [PubMed] [Google Scholar]
- 29.Roche P W, Triccas J A, Winter N. BCG vaccination against tuberculosis: past disappointments and future hopes. Trends Microbiol. 1995;3:397–401. doi: 10.1016/s0966-842x(00)88986-6. [DOI] [PubMed] [Google Scholar]
- 30.Rosat J P, Grant E P, Beckman E M, Dasher C C, Sieling P A, Frederique D, Modlin R L, Porcelli S A, Furlong S T, Brenner M B. CD1-restricted microbial lipid antigen-specific recognition found in the CD8+ alpha beta T cell pool. J Immunol. 1999;162:366–371. [PubMed] [Google Scholar]
- 31.Sander B, Skansen-Saphir U, Damm O, Hakansson L, Andersson J, Andersson U. Sequential production of Th1 and Th2 cytokines in response to live bacillus Calmette-Guerin. Immunology. 1995;86:512–518. [PMC free article] [PubMed] [Google Scholar]
- 32.Schaible U E, Kaufmann S H. CD1 and CD1-restricted T cells in infections with intracellular bacteria. Trends Microbiol. 2000;8:419–425. doi: 10.1016/s0966-842x(00)01829-1. [DOI] [PubMed] [Google Scholar]
- 33.Schaible U E, Hagens C, Fischer K, Collins H L, Kaufmann S H. Intersection of group I CD1 molecules and mycobacteria in different intracellular compartments of dendritic cells. J Immunol. 2000;164:4843–4852. doi: 10.4049/jimmunol.164.9.4843. [DOI] [PubMed] [Google Scholar]
- 34.Sieling P A, Ochoa M T, Jullieu D, Leslie D S, Sabet S, Rasat J P, Burdick A E, Rea T H, Brenner M B, Porcelli S A, Modlin R L. Evidence for human CD4+ T cells in the CD1-restricted repertoire: derivation of mycobacteria-reactive T cells from leprosy lesions. J Immunol. 2000;164:4790–4796. doi: 10.4049/jimmunol.164.9.4790. [DOI] [PubMed] [Google Scholar]
- 35.Stenger S, Modlin R L. T cell mediated immunity to Mycobacterium tuberculosis. Curr Opin Microbiol. 1999;2:89–93. doi: 10.1016/s1369-5274(99)80015-0. [DOI] [PubMed] [Google Scholar]
- 36.Stenger S, Niazi K R, Modlin R L. Down-regulation of CD1 on antigen-presenting cells by infection with Mycobacterium tuberculosis. J Immunol. 1998;161:3582–3588. [PubMed] [Google Scholar]
- 37.Stenger S, Mazzacaro R J, Uyemera K, Cho S, Barnes P F, Rosat J P, Sette A, Brenner M B, Porcelli S A, Bloom B R, Modlin R L. Differential effects of cytolytic T cell subsets on intracellular infection. Science. 1997;276:1684–1687. doi: 10.1126/science.276.5319.1684. [DOI] [PubMed] [Google Scholar]
- 38.Tentori L, Graziani G, Porcelli S A, Sagita M, Brenner M B, Madaio R, Bonmassar E, Giuliani A, Aquino A. Rifampin increases cytokine-induced expression of the CD1b molecule in human peripheral blood monocytes. Antimicrob Agents Chemother. 1998;42:550–554. doi: 10.1128/aac.42.3.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thomssen H, Ivanyi J, Espitia C, Arya A, Londei M. Human CD4− CD8− alpha beta+ T-cell receptor T cells recognize different mycobacterial strains in the context of CD1b. Immunology. 1995;85:33–40. [PMC free article] [PubMed] [Google Scholar]
- 40.Thomssen H, Kahan M, Londei M. Differential effects of interleukin-10 on the expression of HLA class II and CD1 molecules induced by granulocyte/macrophage colony-stimulating factor/interleukin-4. Eur J Immunol. 1995;25:2465–2470. doi: 10.1002/eji.1830250909. [DOI] [PubMed] [Google Scholar]
- 41.Thomssen H, Kahan M, Londei M. IL-3 in combination with IL-4, induces the expression of functional CD1 molecules on monocytes. Cytokine. 1996;8:476–481. doi: 10.1006/cyto.1996.0064. [DOI] [PubMed] [Google Scholar]
- 42.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of protein from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Venisse A, Berjeaud J M, Chaurand P, Gilleron M, Puzo G. Structural features of lipoarabinomannan from Mycobacterium bovis BCG. Determination of molecular mass by laser desorption mass spectrometry. J Biol Chem. 1993;268:12401–12411. [PubMed] [Google Scholar]
- 44.Woolfson A, Milstein C. Alternative splicing generates secretory isoforms of human CD1. Proc Natl Acad Sci USA. 1994;91:6683–6687. doi: 10.1073/pnas.91.14.6683. [DOI] [PMC free article] [PubMed] [Google Scholar]