Abstract
Introduction:
Inhibitor formation is a major complication of haemophilia for which clinical trials are planned. Despite emerging novel haemostatic agents, challenges of rare disease trials are limited subjects and lack of an organized research organization with strategic resources and partnerships.
Aim:
The charge to Working Group 1 was to establish scientific priorities and innovative implementation strategies to conduct inhibitor prevention and eradication trials. To determine feasibility of trial design and strategic resources and partnerships to be leveraged, two clinical trial concepts were considered.
Results:
For the Inhibitor Prevention Trial, we considered adaptive design with early stopping rules, dynamic randomization and Master Protocol models to reduce sample size; and registries to provide concurrent controls and natural history data. For the Inhibitor Eradication Trial using gene therapy, an adaptive design was considered in a small number of subjects, and, if safe and meeting regulatory requirements, enrolment would be expanded. A Haemophilia Clinical Trials Group (HCTG) infrastructure was envisioned, with uniform procedures and standardized outcomes, data collection and assays, within which trial concepts would be developed, vetted and prioritized by a Steering Committee, and submitted to NIH and other research sponsors for review and funding. Mechanistic studies would be embedded within the trials, early stage investigators trained and mentored, and the research infrastructure established within the haemophilia centre (HTC) network and supported by partnerships with foundations, community, federal partners and industry.
Conclusion:
The success of inhibitor trials will depend on innovative trial design and an organized HCTG research infrastructure, leveraged through community partnerships.
Keywords: clinical trials, haemophilia, inherited bleeding disorders, inhibitor formation
1 ∣. INTRODUCTION
Inhibitor formation is the major burden and complication of haemophilia, with an incidence of 30% in severe haemophilia A,1 caused by a T cell-dependent antibody-mediated immune response to exogenous factor VIII (FVIII),2 that renders life-saving FVIII ineffective. However, to treat inhibitors is difficult, as bypass agents, recombinant factor VIIa or activated prothrombin complex concentrate are poorly effective in controlling bleeds, resulting in 2x the hospitalizations, 10x the cost and 3.5x the mortality of non-inhibitor patients.3-5 To eradicate inhibitors by high-dose daily, FVIII (immune tolerance induction, ITI) is inconvenient, costly and ineffective in ~20% of patients. Thus, the burden of disease remains high and better therapies are needed to both treat and prevent inhibitors. Given the increasing number of novel haemostatic non-factor and immune-modulatory therapies in clinical trials and coming to market, this is an exciting time to plan clinical trials for haemophilia.
The challenges of clinical trial design in rare diseases, however, are considerable (Table 1).6-9 There are limited subjects; a recent survey of haemophilia treatment centres (HTCs) indicated 257 children with severe haemophilia A are born annually and followed at ~140 HTCs or about 1.8 previously untreated patients (PUPs) per HTC. Among PUPs, an estimated 75% may not qualify to participate in an inhibitor prevention trial due to past bleeds, past treatment, refusal, travel barriers and competing trials.10 As only 30% develop inhibitors, even if minimally treated patients or those with past bleeds are included, the resulting sample size is insufficient to conduct a classic randomized controlled trial. Thus, to conduct small trials in orphan populations such as haemophilia, the use of innovative trial design with targeted and novel clinical endpoints may maximize statistical power and complement a coordinated national or international strategy in trial implementation.
TABLE 1.
Challenges to the conduct of rare disease clinical trials
| Limited subjects: | 1.82 PUPs per HTC per year or ~257 PUPs/y |
| Rare outcomes: | 30% develop anti-VIII inhibitor antibody |
| Trial design: | Lack of novel design to conduct randomized trials in haemophilia |
| Competing studies: | Competition for PUPs by registries, single-arm and pharma trials |
| Ineligibility: | Exclusion due to past factor, bleeds, cost and competing trials |
| HTC organization: | Lack of unified data collection, sampling and standard of care protocol |
| Partnership: | Lack of foundation, industry and community support for inhibitor trials |
| HTC support: | Lack of personnel or sample preparation support by 40% of HTCs |
| Data collection: | Lack of uniform data collection, blood processing and shipping at HTC |
| Mechanistic studies: | Need for collaborative MD-scientist, immunology network input |
HTC, haemophilia treatment centre; PUP, previously untreated patient.
2 ∣. METHODS
The charge to Working Group 1 was to establish scientific priorities and innovative implementation strategies for clinical trials in haemophilia A patients to prevent and eradicate inhibitors, including gene therapy, and to leverage the resources and partnerships to conduct such trials. The Working Group 1 membership was multidisciplinary, with broad representation across the national and international haemophilia community, complemented by broad expertise in clinical trials, biostatistical design, industry collaboration and international scientific perspective from outside the field of haemophilia (Table 2). The ideas presented in this paper arose from extensive Working Group deliberations and State of the Science (SOS) breakout and large group discussions.
TABLE 2.
Working group 1 members
| Expertise | Investigator | Affiliation |
|---|---|---|
| Co-Chairs | Margaret Ragni, MD, MPH | University of Pittsburgh |
| Lindsey George, MD | University of Pennsylvania | |
| Biostatistics | Alfonso Iorio, MD, PhD | McMaster University, CA |
| John Scott, PhD | Food & Drug Administration/Center Biologics Evaluation |
|
| Clinical Trials | James Casella, MD | Johns Hopkins University |
| Charles Hay, MD | Manchester Royal Infirmary, UK | |
| Alice Long, PhD | Benaroya Research Institute | |
| Marilyn Manco-Johnson, MD | University of Colorado | |
| Industry | Marcus Carr, MD, PhD | Spark Therapeutics |
| Haemophilia Community Perspective | Jorge de la Riva | National Haemophilia Foundation |
| Ilene Goldberg, RN | Weill Cornell Medical College | |
| Matthew Igelman | PWH, Haemophilia Federation of America | |
| Jay Konduros | PWH, Gene Trial Participant |
PWH, person with haemophilia.
3 ∣. RESULTS AND DISCUSSION
3.1 ∣. Scientific priorities
Given the burden of disease and the considerable challenges to conduct rare disease trials, the Working Group considered the major scientific priority to determine innovative approaches to the design and implementation of feasible clinical trials in the prevention and eradication of FVIII inhibitors, with special consideration for the integration of novel non-factor therapeutics and gene therapy into the standard of care for FVIII inhibitor patients. In order to determine the feasibility of trial design and the strategic resources and partnerships that would be required to conduct such trials, we considered two clinical trial concepts.
3.1.1 ∣. Trial concept I: inhibitor prevention by novel non-haemostatic agent trial
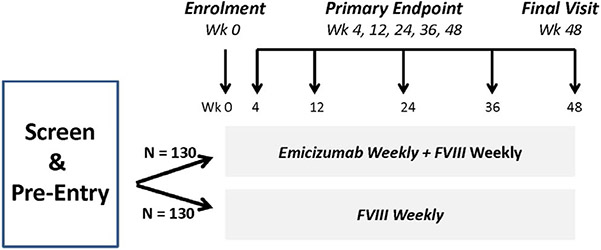
The first proposed trial concept was an Inhibitor Prevention Trial (Figure 1) to determine if weekly emicizumab, a bispecific monoclonal antibody FVIII mimetic,11,12 when combined with factor VIII concentrate (FVIII) is superior to weekly FVIII alone in reducing the rate of inhibitor formation in severe haemophilia A PUPs. The hypothesis is that as emicizumab reduces bleeds in haemophilia A patients to a greater degree than FVIII,12 it would be less likely to trigger the immune system to react against co-administered FVIII and thereby reduce the anti-FVIII inhibitor rate to a greater degree than FVIII alone. The regimens are started before the first bleed, which occurs at a median 7 months of age,10 and the primary outcome, inhibitor formation, is measured at prespecified time-points over a 48-week period. Secondary endpoints include bleeding frequency and mechanistic studies, including FVIII-specific T-cell ELISPOT assays, cytokine expression and microbiome assays addressed by Working Group 3.13 Breakthrough bleeds would be managed as in clinical practice by escalation to twice-daily dosing. To demonstrate a 50% reduction in inhibitor rate, using a traditional randomized trial design would require 130 subjects per arm and over 5 years to enroll (if conducted in the United States only). If a steeper reduction in inhibitor rate is chosen, that is 83%, only 39 subjects per arm would be required, but the reduction might be unrealistic or might miss an inferior, but clinically relevant, endpoint.
FIGURE 1.
Clinical Trial Concept 1: Inhibitor Prevention: Emicizumab plus FVIII vs FVIII Alone in PUPs Trial. Previously untreated patients (PUPs) with severe haemophilia A are randomized to receive weekly emizicumab plus FVIII vs weekly FVIII alone and are followed for inhibitor formation >5.0 BU at prespecified time-points for 48 wk
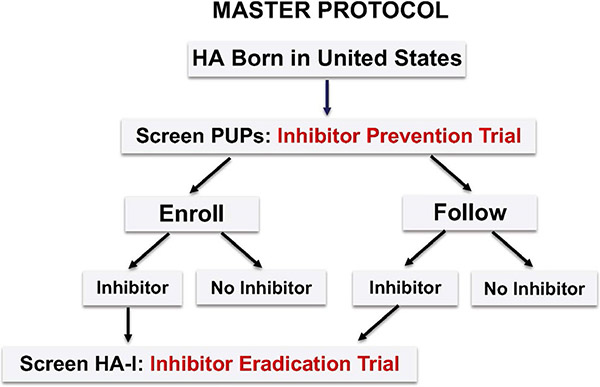
Alternatively, there are several potential statistical considerations to help maximize the information gathered from a rare disease cohort (Table 3). These include the adoption of stopping rules that would allow the study to run to completion only if there is no excess inhibitor development. For example, after 20 subjects complete the trial, if 10 of more develop inhibitors, the true rate is likely greater than the hypothetical background rate of 30%, and the trial may stop for safety concerns. Another strategy is dynamic randomization; in this approach, after a designated number of inhibitors develop, for example 4, the randomization would be changed in favour of the superior arm by 2:1 or 3:1. This strategy reduces the number of participants who receive the less effective arm, an ethical consideration.14,15 Other approaches to reduce sample size include the use of historic controls,16 concurrent controls such as in a Master Protocol 17 and prospective cohorts or registries, including international registries, within which trials can be embedded.18 Although international registries would greatly increase the number of potential subjects, there continue to be significant differences in regulatory and ethical review, which slow the implementation of international research collaborations. If a Master Protocol design is chosen to prevent inhibitors, all PUPs would be enrolled and undergo uniform data and specimen collection at predefined intervals before and after the intervention. The primary endpoint, inhibitor rate, is compared between those agreeing to receive the intervention (treatment group) and those agreeing only to be followed (control group)17 (Figure 2). The advantages of this strategy are that all enrolled subjects receive the intervention, and the control group provides concurrent natural history data for comparison with the intervention group. Once an inhibitor develops, whether in the treatment group or control group, subjects have the option to join an Inhibitor Eradication Trial. In this way, the protocol achieves maximum utilization of participants, critical in a rare disease for which new therapeutics are increasingly emerging. By using the untreated group as concurrent controls, a randomized trial design can be achieved with fewer controls, a greatly reduced sample size and greater proportion treated, while achieving a lower type 1 error rate and higher power.17 This approach results in lower cost and more rapid trial completion, and, importantly, new therapeutics can be tested in trial designs that are informed by prior clinical studies.
TABLE 3.
Statistical considerations to maximize information from rare disease cohorts
| Stopping rules | Run study to completion, if no excess inhibitor incidence Assess inhibitor rate after 20 subjects complete follow-up If ≥10 subjects develop inhibitors, halt trial, as>30% |
| Dynamic randomization | Base sample size on number of events After four inhibitors, randomization favours better arm 2:1, 3:1 Lessens inhibitor risk, preserves statistical integrity |
| Historic controls | Reduce sample size by baseline data from contemporary controls Use surveillance registries: CDC, international registry for controls Employ prospective cohorts, observational studies for controls |
| Master protocol | Follow all enrolled PUPs and screen for inhibitor development Establish baseline pre-inhibitor data and specimens Establish inhibitor natural history among screened subjects |
CDC, Centers for Disease Control; PUP, previously untreated patient.
FIGURE 2.
Master Protocol. All individuals with severe haemophilia A (HA) born in the United States are enrolled and screened for inhibitor formation at a uniform prespecified frequency. The inhibitor rate is compared between those who agree to enroll in the Inhibitor Prevention Trial, and those who agree to be followed only, thereby serving as concurrent controls and providing natural history data for inhibitor formation. Participants who develop inhibitors (HA-I), whether in the Enroll or Follow Groups may be screened to enroll in the Inhibitor Eradication Trial
3.1.2 ∣. Trial concept II: inhibitor eradication by gene therapy trial
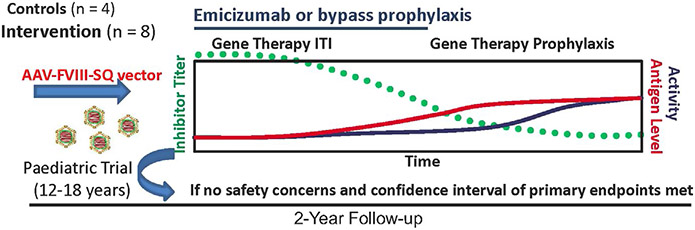
The second proposed trial concept was a gene therapy Inhibitor Eradication Trial in adults with severe haemophilia A and inhibitors refractory to immune tolerance induction (ITI) (Figure 3). Although there have been successful liver-directed AAV gene transfer trials in haemophilia A and B,19-21 current trials exclude patients with current or past inhibitors. Thus, there is little information on the capacity of gene therapy to induce FVIII tolerance or to break FVIII tolerance following gene transfer. In this inhibitor eradication trial, subjects would undergo liver-directed AAV-FVIII gene therapy to express FVIII, with the hypothesis that continuous hepatic expression of transgene-derived FVIII would induce FVIII tolerance, as has been demonstrated in murine and haemophilia A dog inhibitor models.22 The vector pharmacokinetic and safety profile would be determined in non-inhibitor patients with demonstrated ability to safely achieve sustained, steady-state FVIII activity, at least in the mild range. Until inhibitor resolution, tolerance induction or FVIII detection from transgene expression, subjects would remain on emicizumab prophylaxis or bypass therapy, at the discretion of the investigator. Subjects would be followed for two years on an initial vector dosing study and for a minimum of three years on a long-term follow-up study as required by the FDA. The primary outcome would be the presence or absence of an inhibitor at the end of study. The secondary outcomes would be end-of-study steady-state FVIII activity and mechanism of tolerance induction, as determined by basic science studies systematically addressed by Working Group 3.13 Preliminary efficacy data, including bleeding frequency, factor use, cessation of bypass therapy or emicizumab prophylaxis, would be collected as exploratory endpoints. Safety evaluation would be similar to current liver-directed AAV haemophilia gene therapy trials and include measures of liver toxicity, immunogenicity to AAV and FVIII, and vector shedding with at least five years of follow-up as required by the FDA. Due to theoretical risks associated with any gene therapy trial and costs of conducting a proof-of-principle trial, an adaptive design model is prudent, beginning with enrolment of a small number of subjects (~5). The trial would be expanded to enroll additional subjects and a control group to permit statistical analysis of preliminary endpoints, as long as (a) there are no safety concerns, that is clinically significant liver toxicity or vector or transgene immunogenicity and (b) at least half of the initial participants achieve FVIII tolerance induction. Should this gene therapy approach be proven safe and effective in inducing tolerance in adults, it could, with careful regulatory oversight, begin enrolment in adolescents.
FIGURE 3.
Clinical Trial Concept 2: Inhibitor Eradication Gene Therapy Immune Tolerance Induction Trial. Subjects with severe haemophilia A and inhibitors receive AAV-hFVIII gene therapy and emicizumab or bypass (rFVIIa or FEIBA), and are followed for inhibitor resolution, <0.6 BU, at prespecified time-points
Given the cost of AAV vector production, considerable regulatory requirements, and the intensity and duration of follow-up, significant resources outside the scope of traditional grant funding would be required to conduct the trial. While industry plays a predominant role in haemophilia gene therapy trials, there is opportunity for NIH-industry partnerships to allow shared resources for mutual benefit. This would include leveraging NIH resources in trial design and mechanistic studies and trial funding through parent R01 mechanisms, and leveraging industry's expertise in regulatory oversight of gene therapy trials and financial resources to support vector production and clinical trial administration. An NIH-industry partnership would maximize available scientific, financial and regulatory resources to assure trial feasibility, public disclosure and mechanistic studies of tolerance induction. By this approach, the therapeutic indications for liver-directed FVIII gene transfer would be advanced, specifically to induce FVIII tolerance 23 and to maintain or break tolerance in those with past inhibitors. Within collaborative NIH-industry partnerships, the goal of implementation of gene therapy into standard of care could be realized.
3.2 ∣. Strategic implementation
3.2.1 ∣. Scope of trials supported within the HTC network
Given the significant cost and paucity of subjects available to conduct inhibitor prevention and eradication trials, the Working Group developed an estimate of the number of trials that could be supported within the HTC network (Table 4). With approximately 1.82 severe haemophilia A PUPs born per year within the 141 US HTCs or approximately 257 per year,10 it is estimated only about 64 would qualify for a PUP prevention trial, with 75% disqualified for past factor, past bleed, refusal to participate in a trial, travel and time barriers, and competing trials.24 Thus, it is estimated up to 2-3 PUP Inhibitor Prevention Trials utilizing novel design or embedded within registries could be supported within the HTC network. Approximately 10% of the severe haemophilia A population, or 1000, have inhibitors refractory to immune tolerance induction.24,25 Because they have such a poor response to treatment and are ineligible for gene therapy, it is anticipated such patients would be likely to enroll in an inhibitor eradication protocol. Thus, it is estimated up to 2-3 Inhibitor Eradication Trials could be supported within the HTC network.
TABLE 4.
Inhibitor trials able to be supported within HTCs
| HA PUP Inhibitor Prevention Trial |
|---|
| PUPs (HA): Estimate approximately 257/y |
| HTCs: N = 141 |
| Newborn severe HA PUPs: 1.82/y/centre10 |
| Newborn severe HA PUPs in 141 HTC: N = 257 |
| Treatment with FVIII: Median age 1st bleed, start FVIII =7 mo |
| New inhibitors in severe HA PUPs: 30% (N = 77)1 |
| Proportion joining clinical trial: 25% (N = 64) |
| Proportion not joining clinical trial: 75% (N = 193) |
| For past factor; bleed; refusal; travel/time barriers; competing trials24 |
| HA-I Inhibitor Eradication Trial |
| Refractory Inhibitor (HA-I): Estimate approximately 1000 total |
| Estimated prevalence HA-I (12% severe HA)25 |
| Likely to enroll, as poor response to Rx, and ineligible for gene therapy |
| ESTIMATE: trials that could be supported in HTCs |
| 2-3 HA PUP inhibitor prevention trials |
| 2-3 HA-I PUP inhibitor eradication trials |
HA, haemophilia A; HA-I, haemophilia A with inhibitor; HTC, haemophilia treatment centre; PUP, previously untreated patient.
3.2.2 ∣. Haemophilia clinical trials group network
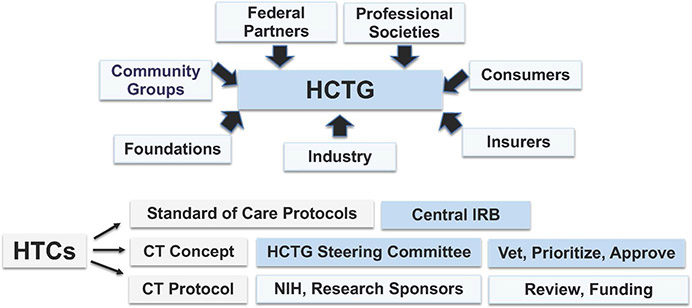
For haemophilia, a rare disease with the rapidly evolving drug discovery pipeline, it is critical to facilitate and develop clinical trials within a large multi-centre trial network (Figure 4), such as has been developed for the rare disease cystic fibrosis (CF).26 A haemophilia-specific network requires a multi-centre infrastructure with uniform procedures and standardized haemophilia-specific outcome parameters, protocol review and feasibility assessment for each proposed trial. The Working Group envisioned, similar to the successful CF network,26 the development of a haemophilia-specific clinical trials network to enable haemophilia investigators to develop new treatments and to implement new interventional trials, conducted within the successful HTC network and incorporating research staff and laboratory with support of government, consumers, industry, insurers, community groups, foundations and professional societies. The support would enable HTC clinicians to provide care to all patients at HTCs through standard of care protocols approved through single IRBs and with standardized collection of natural history data. HTC investigators would develop hypothesis-driven protocol concepts informed by national cohort studies, as addressed by Working Group 2,27 which would be vetted, prioritized and approved by a Steering Committee, developed into trial protocols and submitted to NIH and other research sponsors for review and funding recommendations.
FIGURE 4.
Haemophilia Clinical Trials Group Infrastructure. The haemophilia clinical trials group (HCTG) infrastructure was modelled after the Children's Oncology Group (COG), in which clinical trials are conducted within the organizational structure of the haemophilia treatment centre (HTC), supported by federal partners, foundations, industry, insurers, consumers and professional societies. Within HCTG, HTC MDs develop standard of care protocols and intervention trial concepts which are approved by a single IRB. Trial concepts are vetted and prioritized by the HCTG Steering Committee, and approved concepts are developed into protocols, which are submitted to NIH and other research sponsors for review and funding recommendations
3.2.3 ∣. Strategic partnerships, community engagement and future workforce development
Many opportunities and challenges to clinical trial implementation are recognized in the rare disease haemophilia. Successful clinical trial implementation will require collaboration and scientific partnerships to make meaningful progress towards the goal of preventing and eradicating inhibitors. It will require the engagement of stakeholders, including researchers, health care providers, community leaders, funding agencies, health policy experts, insurance experts, as well as patients and their families to formulate the research questions and assure the oversight and implementation of inhibitor prevention and eradication trials (Table 5). It will require leveraging the existing HTC infrastructure to incorporate research into routine practice. It will require leveraging trans-NIH resources for training, consultative support and engagement of cross-disciplinary scientists.28 It will require education and engagement of HTC staff, patients and families to understand the importance of clinical trials and their own personal participation in these trials to ultimately improve haemophilia care. The optimal haemophilia clinical trials network is within the HTC organizational network. This would permit rapid vetting, review and approval of protocols by a Steering Committee, including representatives of industry, government, health care, community, insurance and scientific agencies, to shorten the time to protocol development and submission for funding, and ultimate translation of the findings to patient care in the HTC clinic. Within the clinical trials management and infrastructure, mentorship and training of early stage investigators should be supported to assure development of the future workforce.
TABLE 5.
Scientific Priorities for Clinical Trials to Prevent and Eradicate Inhibitors
| Partner | Resource |
|---|---|
| I. Define resources and partnerships for clinical trials | |
| Industry | Contributes licensed drug, novel mechanistic assays and high-risk studies |
| Foundation | Supports HTCs to conduct trials; educate and engage community |
| Federal Agencies | Continue to promote surveillance registries, standardizes assays, procedures |
| NHLBI | Continues to develop mechanisms and resources, and review strategies to support small clinical trials |
| Community | Supports consumer education on research and promote trial participation |
| Haemophilia Centre | Trains staff to educate patients about inhibitors and participation in trials |
| HTC Physician | Engage in clinical trials, participate in writing protocols and enrolling patients |
| Partner | Resource |
| II. Leverage support for the haemophilia treatment centre research infrastructure | |
| Network | Provides for personnel, data collection, assays and statistical consultant |
| Capacity | Incorporates research within HTC, where patients receive medical care |
| CDC, ATHN | Establishes surveillance, uniform data collection and blood sampling/shipping |
| Partners | Community, industry and foundations support HTC research |
| HTC | Trains, educates staff, patients and families on importance of research |
| HTC Physician | Develops and proposes clinical trial concepts with community agreement |
| Steering Committee | Reviews, vets and prioritizes clinical trial concepts and new protocols |
| Trial Protocols | Developed from concept, submitted to and reviewed by NIH/other funding organizations |
| Goal | Specific mechanistic study |
| III. Embed mechanistic studies into clinical trials | |
| Feasibility | Anti-FVIII IgG subclasses ELISPOT assays on peripheral blood mononuclear cells FVIII-specific T-cell proliferation assays FVIII-specific T regulatory cell induction studies Intracellular cytokine expression assays Microbiome studies |
| Implementation | Fingerstick sampling for RNA sequencing or qPCR Validation of assays for small volumes in infants Epitope mapping and single cell assays |
| Network Utilization (ITN, CTSA) | Existing resources utilized to implement assays Alternate networks leveraged to help implement trial, serve as data centre |
| Partner | Resource |
| IV. Optimize public-private partnerships in clinical trials | |
| NHF/HFA | Serve as a philanthropic partner to promote research Educate providers, consumers on importance and commitment to research Set up a well-crafted Public Relations campaign on research Encourage providers, consumers to participate in registries, clinical trials Incorporate clinical trial importance into national, regional meetings Include clinical trial information on social media, publications and web portal |
| Industry/foundations | Engage in support of clinical trials and in HTC network to conduct trials Support novel agent assay development and mechanistic assays and studies |
| CDC | Promote surveillance registries to embed in trials Provide standardized assays and uniform data collection by HTCs |
| Research networks | Provide expertise, immune, mechanistic assays, models and resources |
| Foundations | Help support registries, trial network, HTC community to engage in trials |
| Resource/Group | Goal |
| V. Engage the patient community in clinical trials | |
| HTC MD/Staff | Discusses research with patients at each HTC visit Evaluates available studies for which patient may be eligible Reviews risks, benefits and subject protection Encourages participation in registries and clinical trials Supports “twinning” of new families with experienced families |
| Chapter/Community | Promote discussion, educate and engage the community Sponsor educational webinars on trials as part of “culture of care” Inform the community of importance of clinical trials Provide information/webinars on registries, novel therapies |
| Role | Training |
| VI. Embed training opportunities for early stage investigators in haemophilia trials | |
| Mentor (Grant recipient) | Incorporates training of young investigators in clinical trials Provides mentorship for trainees to learn about clinical trial operations |
| Mentee (Fellow, trainee) | Gains clinical trial research skills Learns how to plan, design and conduct a clinical trial Gains insight on the development of a clinical trial protocol Applies human subject protection principles to informed consent process Understands randomization techniques and intervention arm assignment Studies principles of analysis of study endpoints Gains knowledge of implementation of quality control standards Recognizes roles of safety monitoring and data safety monitoring boards Learns trial organization: clinical and data coordinating centres Furthers training through career-building opportunities provided by NIH, Foundations and professional societies |
CDC, Center for Disease Control; CTSA, Clinical and Translational Science Awards Program (NIH-sponsored); HFA is Haemophilia Federation of America; HTC, haemophilia Treatment Centre; ITN is immune tolerance network; NHF is National Haemophilia Foundation; NHLBI, National Institutes of Health Heart Lung Blood Institute; qPCR is quantitative polymerase chain reaction; RNA is ribonucleic acid.
4 ∣. SUMMARY
In conclusion, given the high burden of haemophilia inhibitor formation and the rapid development of novel non-factor haemostatic therapeutics, it is timely to assure the haemophilia community is poised to conduct clinical trials to prevent and eradicate inhibitors. The success of this effort will depend on novel statistical designs that address feasibility constraints, integration of clinical trials and standardized procedures within the HTC network, and establishment of partnerships with multiple stakeholders including industry, foundations, scientists, government agencies, and community groups and patients. By leveraging resources from these partners, the haemophilia community will meet the scientific, economic, organizational and educational challenges to develop scientifically valid and time-sensitive clinical trials to improve the lives of haemophilia patients with inhibitors.
ACKNOWLEDGEMENTS
We thank all members of Working Group 1 for their contributions.
Footnotes
DISCLOSURES
M. Ragni reported institutional research funding from Alnylam (Sanofi), Biomarin, Bioverativ, CSL Behring, Pfizer, Sangamo, Shire, Spark; and advisory board service to Alnylam (Sanofi), Biomarin and Spark Therapeutics; L. George reported employment at University of Pennsylvania which holds equity in Spark Therapeutics and consultancy with Pfizer; M. Manco-Johnson reported research support from Bayer HealthCare and advisory board service with Bioverativ, CSL Behring, Genentech, Novo Nordisk and Shire; M. Carr reported Consultancy with CSL Behring and Spark Therapeutics and former employment with Novo Nordisk, Pfizer, and Spark, and Stock in Pfizer and Spark; M. Igelman reported membership on the Board of Directors, Haemophilia of North Carolina; J. de la Riva reported membership on the Board of Directors, National Haemophilia Foundation; J. Scott, A. Iorio, J. Casella, A. Long, C. Hay, I. Goldberg and J. Konduros reported no conflict of interest.
REFERENCES
- 1.Gouw SC, van den Berg HM, Fischer K, et al. Intensity of factor VIII treatment and inhibitor development in children with severe hemophilia A: the RODIN study. Blood. 2013;121:4046–4055. [DOI] [PubMed] [Google Scholar]
- 2.Ragni MV, Bontempo FA, Lewis JH. Disappearance of inhibitor to factor VIII in HIV-infected hemophiliacs with progression to AIDS or severe ARC. Transfusion. 1989;29:447–449. [DOI] [PubMed] [Google Scholar]
- 3.Soucie JM, Symons J, Evatt B, Brettler D, Huszti H, Linden J. Homebased infusion therapy and hospitalization for bleeding complications among males with hemophilia. Haemophilia. 2001;7:198–206. [DOI] [PubMed] [Google Scholar]
- 4.Goudemand J. Pharmacoeconomic aspects of inhibitor treatment. Eur J Haematol. 1998;63:24–27. [DOI] [PubMed] [Google Scholar]
- 5.Walsh CE, Soucie JM, Miller CH. Impact of inhibitors on hemophilia A mortality in the United States. Am J Hematol. 2015;90:400–405. [DOI] [PubMed] [Google Scholar]
- 6.Ragni MV, Moore CG, Bias V, Key NS, Kouides PA, Francis CW. Challenges of rare disease research: limited patients and competing priorities. Haemophilia. 2012;18:192–194. [DOI] [PubMed] [Google Scholar]
- 7.Iorio A, Marcucci M. Clinical trials and haemophilia: does the Bayesian approach make the ideal and desirable good friend? Haemophilia. 2009;15:900–903. [DOI] [PubMed] [Google Scholar]
- 8.Peyvandi F, Farrugia A, Iorio A, Key NS, Srivastava A. Joint WFH-ISTH session: issues in clinical trial design. Haemophilia. 2014;20(Suppl 4):137–144. [DOI] [PubMed] [Google Scholar]
- 9.Cheng J, Iorio A, Marcucci M, et al. Bayesian approach to the assessment of the population-specific risk of inhibitors in hemophilia A patients: a case study. J Blood Med. 2016;7:239–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ragni MV, Kessler CM, Fogarty PJ, Josephson NC, Neff AT, Raffini LJ. Survey of current prophylaxis practice in U.S. Hemophilia treatment centers. Haemophilia. 2012;18:63–68. [DOI] [PubMed] [Google Scholar]
- 11.Oldenburg J, Mahlangu JN, Kim B, et al. Emicizumab prophylaxis in hemophilia A with inhibitors. New Engl J Med. 2017;377:809–818. [DOI] [PubMed] [Google Scholar]
- 12.Mahlangu J, Oldenburg J, Paz-Priel I, et al. Emizumab prophylaxis in hemophilia A without inhibitors. N Engl J Med. 2018;379:811–822. [DOI] [PubMed] [Google Scholar]
- 13.Meeks SL, Herzog RW; on behalf of the Members of Working Group 3. The national blueprint for future basic and translational research to understand factor VIII immunogenicity: NHLBI State of the Science (SOS) Workshop on factor VIII inhibitors. Haemophilia. 2019;25(4):595–602. [DOI] [PubMed] [Google Scholar]
- 14.DiMichele DM. Ethical considerations in clinical investigation: exploring relevance in haemophilia research. Haemophilia. 2008;14(Suppl 3): 122–129. [DOI] [PubMed] [Google Scholar]
- 15.Berry DA. Emerging innovations in clinical trial design. Clin Pharm Ther. 2016;99:82–91. [DOI] [PubMed] [Google Scholar]
- 16.Viele K, Berry S, Neuenschwander B, et al. Use of historical control data for assessing treatment effects in clinical trials. Pharm Stat. 2014;13:41–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Redman MW, Allegra CJ. The master protocol concept. Sem Oncol. 2015;42:724–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Newman AB, Avilés-Santa ML, Anderson G, et al. Embedding clinical interventions into observational studies. Contemp Clin Trials. 2016;46:100–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nathwani AC, Reiss UM, Tuddenham E, et al. Long-term safety and efficacy of factor IX gene therapy in hemophilia B. N Engl J Med. 2011;371:1994–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.George LA, Sullivan SK, Giermasz A, et al. Endogenous prophylaxis for hemophilia B following gene therapy with a high specific factor IX variant. N Engl J Med. 2017;377:2215–2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rangarajan S, Walsh L, Lester W, et al. AAV5-factor VIII gene transfer in severe hemophilia A. N Engl J Med. 2017;377:2519–2530. [DOI] [PubMed] [Google Scholar]
- 22.Finn JD, Ozelo MC, Sabatino DE, et al. Eradication of neutralizing antibodies to factor VIII in canine hemophilia A after liver gene therapy. Blood. 2010;116:5842–5848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.El-Enein M, Grainger DW, Kili S. Registry contributions to strengthen cell and gene therapeutic evidence. Mol Ther. 2018;26:1172–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ragni MV, Malec LM. Design of the INHIBIT trial: Preventing inhibitors by avoiding “danger”, prolonging half-life, and promoting tolerance. Expert Rev Hematol. 2014;7:747–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wight J, Paisley S. The epidemiology of inhibitors in haemophilia A: a systematic review. Haemophilia. 2003;9:418–435. [DOI] [PubMed] [Google Scholar]
- 26.De Boeck K, Bulteel V, Fajac I. Disease-specific clinical trials networks: the example of cystic fibrosis. Eur J Pediatr. 2016;175:817–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Konkle BA, Recht M; on behalf of the Members of Working Group 2. The national blueprint for 21st century data and specimen collection and observational cohort studies: NHLBI State of the Science (SOS) Workshop on factor VIII inhibitors. Haemophilia. 2019;25(4):590–594. [DOI] [PubMed] [Google Scholar]
- 28.Nexus Extramural.The roles of fellows and trainees in NIH-supported trials. https://nexus.od.nih.gov/all/2018/10/12/the-roles-of-fellows-and-trainees-in-nih-supportedclinical-trials/. Accessed October 10, 2019