Abstract
Sexually transmitted diseases are a major health problem worldwide, but there is still a lack of knowledge about how to induce an optimal immune response in the genital tract of humans. In this study we vaccinated 21 volunteers nasally or vaginally with the model mucosal antigen cholera toxin B subunit and determined the level of specific immunoglobulin A (IgA) and IgG antibodies in vaginal and cervical secretions as well as in serum. To assess the hormonal influence on the induction of antibody responses after vaginal vaccination, we administered the vaccine either independently of the stage in the menstrual cycle or on days 10 and 24 in the cycle in different groups of subjects. Vaginal and nasal vaccinations both resulted in significant IgA and IgG anti-cholera toxin B subunit responses in serum in the majority of the volunteers in the various vaccination groups. Only vaginal vaccination given on days 10 and 24 in the cycle induced strong specific antibody responses in the cervix with 58-fold IgA and 16-fold IgG increases. In contrast, modest responses were seen after nasal vaccination and in the other vaginally vaccinated group. Nasal vaccination was superior in inducing a specific IgA response in vaginal secretions, giving a 35-fold increase, while vaginal vaccination induced only a 5-fold IgA increase. We conclude that a combination of nasal and vaginal vaccination might be the best vaccination strategy for inducing protective antibody responses in both cervical and vaginal secretions, provided that the vaginal vaccination is given on optimal time points in the cycle.
To halt the spread of sexually transmitted diseases, attempts are made to induce locally secreted, pathogen-specific, neutralizing antibodies in the genital tract by vaccination. We and other groups have shown that it is possible to induce a specific antibody response in the human female genital tract by vaccination, by either the oral, the nasal, or the vaginal route (2, 15, 29). Direct application of an antigen at the target mucosa has proven to be the most efficient route for induction of a local antibody response in the intestine and upper respiratory tract (8, 20, 21). In line with this, vaginal administration has been shown to be superior to the oral route for the induction of specific immunoglobulin A (IgA) and IgG antibody responses in cervical secretions (15, 29). However, we have also demonstrated that the nasal mucosa can serve as an efficient site for the induction of specific IgA and IgG responses in vaginal secretions in humans (2), but it is not known if specific antibody responses can be induced in cervical secretions by nasal vaccination. Furthermore, a comparison of the nasal and vaginal vaccination routes for the induction of antibodies in genital secretions has never been performed in humans. Recent animal studies indicate that there is a compartmentalization of the immune response within the genital tract, i.e., the recruitment of CD4+ lymphocytes is differentially regulated in the upper and lower genital tract (14). We have also found in mice that nasal and vaginal immunizations result in differential distributions of specific antibodies within the genital tract (11). It is conceivable that this is the case also in humans.
Nasal vaccination is an interesting alternative for inducing specific antibody responses in the human female genital tract, both for convenience and because the outcome of vaginal vaccination might be dependent on the time point in the menstrual cycle for vaccine administration. It is known from experiments in rodents that hormone levels, e.g., progesterone, can influence the outcome of vaginal immunization (11). In rodents the estrous cycle has a major influence on both the antigen uptake in the vagina and the ability of antigen-presenting cells to present antigens for the T cells in the vagina and uterus (18, 30). Although there is strong variation in hormone levels during the menstrual cycle, it is not known whether this influences the outcome of vaginal vaccination in humans.
Relatively little is known about the presence of specific antibody-secreting cells (ASCs) in the genital tract. Results from studies in mice and monkeys have shown that vaginal but not oral vaccination induces cholera toxin B subunit (CTB)-specific ASCs in the female genital tract, and in mice specific ASCs in the genital tract have also been demonstrated after nasal vaccination (6, 11). There are no reports regarding the presence of antigen-specific ASCs in the human genital tract, which is particularly interesting since the lymphocyte homing mechanisms to the genital tract are elusive.
The first aim of our study was to compare the induction of specific antibodies in cervical and vaginal secretions after vaginal and nasal vaccinations. Recombinant CTB was used as a model antigen, since it is one of the best-characterized mucosal antigens, both regarding safety and immunogenicity in humans (2, 7, 9). The second aim was to evaluate the hormonal influence on the induction of local antibody responses after vaginal vaccination. Finally, we examined the presence of antigen-specific ASCs in the female genital tract after nasal and vaginal vaccination.
MATERIALS AND METHODS
Subjects.
Twenty-one Swedish female volunteers at reproductive ages gave informed consent to participate in the study, which was approved by the Swedish Medical Products Agency and the local Human Research Ethical Committee of the Medical Faculty, Göteborg University, Göteborg, Sweden. All volunteers were scheduled for hysterectomy because of bleeding disorders, except for five women (vaginalcorr) who were healthy premenopausal volunteers (Table 1). Exclusion criteria for participation included previous vaccination against cholera, endometrial cancer, and acute sexually transmitted disease. The volunteers were vaccinated either nasally or vaginally. The vaginally vaccinated women were divided into two groups. One group was vaccinated independently of the stage in the menstrual cycle (vaginalind), and the other group was vaccinated on days 10 and 24 in the cycle (vaginalcorr) (Table 1).
TABLE 1.
Characteristics of the vaccination groups
| Vaccination group | No. of individuals | Mean age in yr (range) | CTB dose | Vaccination point in relation to the menstrual cycle |
|---|---|---|---|---|
| Vaginalcorr | 5 | 39 (34–44) | 1 mg | Days 10 and 24 |
| Vaginalind | 8 | 41.5 (33–50) | 1 mg | Independent |
| Nasal | 8 | 42.4 (38–49) | 250 μg | Independent |
Vaccination.
The nasal vaccine consisted of purified recombinant CTB (23) diluted in phosphate-buffered saline (PBS) to a concentration of 0.625 mg/ml and was produced by Active Biotech (Stockholm, Sweden). The vaccine was administered as a 100-μl spray given twice in both nostrils by using an atomizer (Apoteksbolaget AB), i.e., a total volume of 400 μl, containing 250 μg of CTB, was given per dose. The vaginal vaccine consisted either of recombinantly produced CTB alone (vaginalind) or of a licensed oral cholera vaccine which, in addition to CTB, also contained inactivated cholera vibrios (Dukoral; Active Biotech) (vaginalcorr). Then, 2 ml of either form of vaginal vaccine, containing 0.5 mg of CTB per ml, was mixed with 340 μg of a biologically inert polysaccharide (Eldexomer, batch 015; Perstorp Pharma, Perstorp, Sweden). The freshly made preparation was deposited in the upper fornix of the vagina, and the women did not move for 10 min after vaccination. Irrespective of the vaccination route, all volunteers were given two doses of vaccine with a 2-week interval.
Collection of specimens.
Blood and vaginal and cervical secretions were collected immediately before the first vaccination and 10 days after the second vaccination. In addition, serum samples were collected 6 weeks after the last vaccination dose. Secretions were never collected during menstruation. The vaginal secretions were sampled by using a polywick tampon (10 by 30 mm; Polyfiltronics Group, Inc., Rockland, Mass.), which was inserted deeply into the vagina by the female volunteer herself. After 2 h, the tampon was taken out by the volunteer with the aid of a thread attached to the tampon and placed in 1 ml of PBS, resulting in a sample dilution of ca 1:10, and stored at −70°C. The tampons were thawed, and the fluid was squeezed out by centrifugation for 10 min at 3,500 × g in a pierced Eppendorf tube placed on top of another tube. Thereafter the samples were treated with bromelain (Sigma Chemical Co., St. Louis, Mo.) to solubilize the mucus. Then, 25 μg of bromelain per ml of sample was added, and the samples were incubated for 60 min at 37°C, followed by centrifugation for 10 min at 9,500 × g. Cervical secretion samples were collected with a syringe (Aspiglaire; Biotechnologies International, Aigle, France). The volume was recorded, and the samples were diluted 1:10 in PBS and treated with bromelain as described above.
Cervical and uterine tissues were obtained from six volunteers undergoing hysterectomy 10 days after the second vaccination. The samples were taken randomly from the different vaccination groups. Three of the volunteers were vaccinated vaginally, and three were vaccinated nasally. In addition, we also collected samples from two nonvaccinated individuals.
Determination of total immunoglobulin and specific antibodies.
The contents of total IgA and IgG in vaginal and cervical secretions were determined by enzyme-linked immunosorbent assay (ELISA) as described previously (2). Briefly, the plates were coated with goat anti-human IgA, α-chain-specific IgG (Jackson ImmunoResearch Laboratories, West Grove, Pa.), and goat anti-human IgG F(ab)2 (Jackson). Thereafter, the samples and standards (polyclonal human plasma IgA and IgG; Calbiochem Corp., La Jolla, Calif.) were added in duplicates and serially diluted. Bound total IgA and IgG antibodies were demonstrated by using horseradish peroxidase (HRP)-conjugated goat anti-human serum IgA (α-chain specific; Jackson) and HRP-conjugated goat anti-human IgG (Fcγ specific; Jackson), followed by treatment with o-phenylenediamine and H2O2 as the enzyme substrate. The endpoint titers were determined as the reciprocal dilution giving an absorbance at 450 nm of 0.4 above background (Labsystems Multiscan PLUS) after 20 min of enzyme reaction.
CTB-specific antibodies in serum and secretions were determined by a modified GM1-ELISA method as previously described (2). Briefly, plates were coated first with GM1 ganglioside (Sigma) and then with CTB. The samples and a positive serum reference (a serum pool from Swedish volunteers vaccinated orally with the whole-cell–CTB cholera vaccine) were added in duplicates and serially diluted. Bound CTB- specific IgA and IgG antibodies were demonstrated with HRP-conjugated rabbit anti-human serum IgA (α-chain specific; Jackson) and HRP-conjugated goat anti-human IgG (Fcγ specific; Jackson) and then developed as described above. Responders were defined as having a >2-fold increase in specific antibody titers between pre- and postvaccination specimens (10).
Determination of total and specific immunoglobulin-secreting cells in the cervix and uterus.
Cervical and vaginal tissues were obtained from eight volunteers undergoing hysterectomy. The mucosa was excised from the tissue, cut into small pieces, and treated with 10 mM dithiothreitol and thermolysin buffer (0.5 mg/ml; Boehringer-Mannheim, Mannheim, Germany). Thereafter, the tissue suspensions were filtered through a 150-μm (pore-size) mesh, and the separated lamina propria cells were incubated in a prewarmed collagenase-DNase solution (1 mg of collagenase [Sigma] per ml plus 2 mg of DNase [Sigma] per ml in 10% fetal calf serum-Iscove medium) for 45 min at 37°C and refiltered first through a 150-μm mesh and then through a 50-μm mesh. The intraepithelial cells were incubated with DNase (2 mg/ml) for 30 min at 37°C and then filtered through a 50-μm mesh. Both cell populations were then mixed and counted. Plasma cells were selected by separation with anti-CD38-coated magnetic beads according to the manufacturer's protocol (Dynal, Oslo, Norway). Total IgA and IgG, as well as CTB-specific spots, were analyzed by ELISPOT (21).
Statistical methods.
The specific antibody titers in vaginal and cervical secretions were adjusted for variations in total immunoglobulin content and are expressed as units per microgram of total IgA or IgG. Analyses of the significance of the differences between pre- and postvaccination titers were performed with a paired Student's t test. Before calculations, all values were log10 transformed. A difference was considered significant at P < 0.05.
RESULTS
We determined local immune responses in the genital tract as well as the antibody responses in serum in volunteers who had been given two nasal or vaginal vaccinations with recombinant CTB. The vaginally vaccinated women obtained the vaccine either independently of the stage in the menstrual cycle (vaginalind) or on days 10 and 24 in the cycle (vaginalcorr).
Antibody responses in serum.
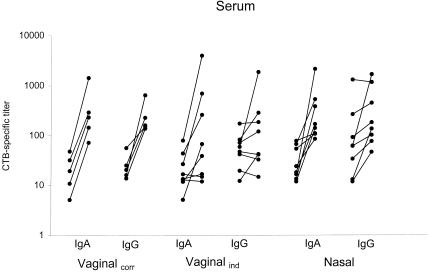
Vaccination of the vaginalcorr group resulted in a 14-fold anti-CTB IgA geometric mean increase and a 9.5-fold IgG geometric mean increase, with a significant response seen in all of the five vaccinated individuals (Fig. 1, Table 2). However, if the vaginal vaccinations were instead given independently of the stage in the cycle (vaginalind), only five of eight subjects responded in IgA and only three of eight subjects responded in IgG. Nasal vaccination resulted in an 8.7-fold increase in anti-CTB IgA with six of eight individuals responding, but there was only a 3.3-fold increase in IgG with half of the subjects responding (Fig. 1, Table 2). A difference in the kinetics of the antibody response could also be observed between the nasal and vaginal vaccinations. The IgA and IgG titers after nasal vaccination were higher after 6 weeks compared to 10 days after vaccination, i.e., 11-fold increases in specific IgA and 7.5-fold increases in IgG at 6 weeks postvaccination. In contrast, the titers in serum after vaginal vaccination remained at the same level 6 weeks after vaccination as they had been at 2 weeks after vaccination (not shown). We have previously reported such a delayed response after nasal vaccination compared with oral vaccination (21).
FIG. 1.
Anti-CTB IgA and IgG titer increases in serum are shown after vaginal vaccination at days 10 and 24 of the cycle (vaginalcorr), after vaginal vaccination independently of the stage in the cycle (vaginalind), and after nasal vaccination. Pre- and postvaccination titers are shown for each individual.
TABLE 2.
CTB-specific IgA and IgG responses in seruma
| Vaccination group | IgA
|
IgG
|
||||
|---|---|---|---|---|---|---|
| No. of responders/total no. | Fold increase in GMT (P)
|
No. of responders/total no. | Fold increase in GMT (P)
|
|||
| All | Responders | All | Responders | |||
| Vaginalcorr | 5/5 | 14.0 (0.0002) | 14.0 | 5/5 | 9.5 (0.006) | 9.5 |
| Vaginalind | 5/8 | 4.9 (0.02) | 12.8 | 3/8 | 2.1 (0.14) | 7.3 |
| Nasal | 6/8 | 8.7 (0.004) | 15.0 | 4/8 | 3.3 (0.02) | 6.0 |
For statistically significant increases, the P values are shown in boldface. GMT, geometric mean titer.
Antibody responses in vaginal secretions.
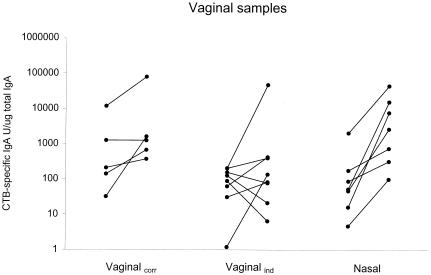
After vaccination of the vaginalcorr group, a 4.9-fold increase in the specific IgA titer was induced in the whole group, and three of five subjects responded (Fig. 2, Table 3). In the vaginalind group there was a 2.9-fold increase in specific IgA, with four of eight individuals responding. Nasal vaccination was superior to vaginal vaccination in inducing specific IgA responses in vaginal secretions. All individuals in the nasal group responded to the vaccination, with a significant 34.5-fold geometric mean IgA titer increase (Fig. 2, Table 3). In contrast to the IgA responses, no significant IgG increases were found in either of the vaccination groups (Table 3).
FIG. 2.
Anti-CTB IgA increases in vaginal secretions are shown after vaginal vaccination on days 10 and 24 of the menstrual cycle (vaginalcorr), after vaginal vaccination independently of the stage in the cycle (vaginalind), and after nasal vaccination. Data are presented as the CTB-specific IgA titer units/microgram of total IgA for each sample. Pre- and postvaccination values are shown for each individual.
TABLE 3.
CTB-specific IgA and IgG responses in vaginal secretionsa
| Vaccination group | IgA
|
IgG
|
||||
|---|---|---|---|---|---|---|
| No. of responders/total no. | Fold increase in GMT (P)
|
No. of responders/total no. | Fold increase in GMT (P)
|
|||
| All | Responders | All | Responders | |||
| Vaginalcorr | 3/5 | 4.9 (0.08) | 11.7 | 2/5 | 2.1 (0.45) | 12.4 |
| Vaginalind | 4/8 | 2.9 (0.33) | 26.2 | 3/8 | 1.4 (0.74) | 25.0 |
| Nasalb | 7/7 | 34.5 (0.003) | 34.5 | 2/5 | 2.2 (0.17) | 6.6 |
For statistically significant increases, the P values are shown in boldface. GMT, geometric mean titer.
In this group, some samples were not analyzed because the sample volume was too small.
Antibody responses in cervical secretions.
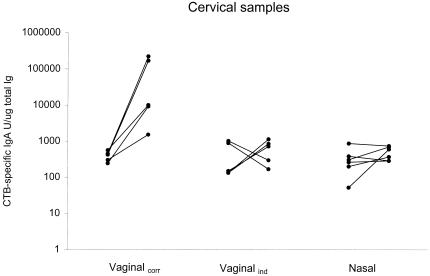
Vaccination of the vaginalcorr group resulted in a 58-fold increase in anti-CTB IgA levels with all five individuals responding (Fig. 3, Table 4). The IgG response increased 16-fold, with three of five subjects responding (Table 4). To obtain high cervical antibody responses, it proved to be essential to correlate the administration of the vaccine to the phase in the menstrual cycle, since only weak responses in IgA and IgG were found after vaccination of the vaginalind group. Nasal vaccination only induced moderate IgG antibody responses and no significant IgA (Fig. 3, Table 4). Thus, vaginal vaccination was superior to the nasal route in inducing antibody responses in cervical secretions, given that the vaccine was administered on days 10 and 24 in the cycle.
FIG. 3.
Anti-CTB IgA increases in cervical secretions are shown after vaginal vaccination on days 10 and 24 of the menstrual cycle (vaginalcorr), after vaginal vaccination independently of the stage in the cycle (vaginalind), and after nasal vaccination. Data are presented as CTB-specific IgA titer units/microgram of total IgA for each sample. Pre- and postvaccination values are shown for each individual.
TABLE 4.
CTB-specific IgA and IgG responses in cervical secretionsa
| Vaccination group | IgA
|
IgG
|
||||
|---|---|---|---|---|---|---|
| No. of responders/total no. | Fold increase in GMT (P)
|
No. of responders/total no. | Fold increase in GMT (P)
|
|||
| All | Responders | All | Responders | |||
| Vaginalcorrb | 5/5 | 58.3 (0.01) | 58.3 | 3/5 | 16 (0.064) | 68.6 |
| Vaginalind | 3/5 | 1.7 (0.55) | 6.4 | 2/5 | 1.5 (0.59) | 7.3 |
| Nasalb | 2/6 | 1.8 (0.21) | 5.1 | 3/6 | 1.9 (0.02) | 2.9 |
For statistically significant increases, the P values are shown in boldface. GMT, geometric mean titer.
In this group, some samples were not analyzed because the sample volume was too small.
ASCs in the female genital tract.
The cervical mucosa contained significantly more IgG than IgA ASCs, i.e., 19,430 ± 14,270 (mean ± the standard deviation) IgG compared to 5,290 ± 3,730 IgA ASCs per 106 mononuclear cells (MNCs) (P = 0.018). Also, in the uterine mucosa the majority of ASCs produced IgG antibodies, i.e., 12,770 ± 16,040 IgG ASCs compared to 3,430 ± 6,610 IgA ASCs per 106 MNCs, although this difference was not statistically significant. In the cervix, CTB-specific IgA ASCs were detected in two of three individuals after nasal vaccination (150 and 400 ASCs per 106 MNCs, respectively) and in one of three individuals after vaginal vaccination (125 ASCs per 106 MNCs), whereas no specific IgG ASCs were found. Neither the nasal nor the vaginal vaccination route induced any CTB-specific ASCs in the uterine mucosa. None of the individuals in the nonvaccinated group had any CTB-specific ASCs.
DISCUSSION
Studies in animals as well as in humans have shown that both nasal and vaginal vaccination can be efficient in inducing specific antibodies in the genital tract (2, 11, 22, 24, 29). This is, to our knowledge, the first study comparing the two vaccination routes in humans. We found that nasal vaccination elicited the strongest IgA antibody responses in vaginal secretions, while vaginal vaccination on days 10 and 24 of the cycle was superior in inducing a specific IgA or IgG antibody response in the cervix. Our results indicate that there is a compartmentalization within the genital tract and that the induction of specific antibodies in cervical secretions is differentially regulated from that in vaginal secretions. Also, in mice the antibody responses in the upper and lower female genital tract seem to be differentially regulated. However, in contrast to what we found in humans, vaginal vaccination in mice induced higher IgA levels in the vagina than in the upper genital tract, while nasal vaccination induced strong IgA responses in the vagina as well as in the uterus and fallopian tubes (11). One explanation for the difference in antibody distribution found in humans might be that vaginal vaccination induces the homing of ASCs mainly to the cervix, while nasal vaccination is more efficient in stimulating the homing of such cells to the vaginal mucosa.
The specific mechanisms guiding the lymphocytes to the genital mucosa are unknown. The mucosal addressin cell adhesion molecule 1 (MAdCAM-1) is thus far the only known mucosa-specific adhesion molecule that is expressed by the endothelium, and it is known to specifically recruit lymphocytes to the intestinal mucosa (25). We have recently investigated the expression of adhesion molecules on the vaginal and cervical endothelium in hysterectomized women (12). MAdCAM-1 was not, however, expressed in the genital mucosa, which indicates that other sets of adhesion molecules are important in the homing of lymphocytes to the female genital tract. In mice, studies have shown that MAdCAM-1 is not present in normal genital mucosa but could be expressed upon inflammation in the genital tract (13). These results are controversial, however, since another study found no MAdCAM-1 expression in a similar mouse model of chlamydia infection (19).
The subjects in the vaginalcorr group were vaccinated with the licensed oral cholera vaccine that, in addition to CTB, consists of killed bacteria (whole-cell component), whereas the subjects in the vaginalind group and the nasal group were vaccinated with CTB only. We do not think that the stronger antibody responses in the vaginalcorr group are due to an adjuvant effect of the whole-cell component. On the contrary, previous studies using oral administration showed that vaccination with B subunit induced a sixfold increase in the intestinal IgA compared to only a twofold increase in individuals vaccinated with the B-subunit–whole-cell vaccine (26, 27). Another difference between the groups is that women in the vaginalind group had bleeding disorders, while the women in the vaginalcorr group were healthy. However, since the serum responses in the vaginalind group did not differ from the responses found in one of our previous studies in which healthy women were vaccinated vaginally with the B-subunit–whole-cell vaccine, we do not believe that bleeding disorders affect the outcome of vaccination (29). For example, in the present study five of eight women in the group with bleeding disorders responded with specific IgA in serum compared to four of seven of the healthy women in the previous study.
Our results show that it is important to synchronize vaginal vaccinations to the menstrual cycle in order to obtain high and less-variable local antibody responses in genital secretions. Immunohistochemical studies in humans have shown that both the number of IgA-producing plasma cells and the level of immunoglobulins within the endometrial glands increase in the cervix during the late secretory phase, when the progesterone level is high (3, 16). Recently, it has also been demonstrated in mice that vaginal immunization can in fact induce tolerance if administered during estrus (high levels of estrogen), while tolerance induction was not found when the antigen was administered during diestrus (low estrogen levels) (4). We were successful in obtaining a strong antibody response with vaccinations on days 10 and 24 in the menstrual cycle. These time points were selected based on practical reasons, i.e., avoiding menstruation at the time of administration, while allowing for a 2-week interval between the vaccinations. The estrogen level is relatively high at both time points, but the progesterone level, on the other hand, is low on day 10 and high on day 24 in the cycle.
Organized inductive structures in the mucosa have not been found in the genital tract as opposed to the gut and the respiratory tract. Studies in mice have shown that dendritic cells can take up antigen from the vaginal lumen and migrate to the draining lymph nodes, where they are thought to present antigens to T and B cells (17, 18). Since vaginal vaccination of humans on days 10 and 24 of the cycle induced high systemic antibody responses, the alternative inductive pathway involving antigen uptake by dendritic cells present in the vaginal epithelium seems to be at least as efficient as the local uptake of antigen into organized lymphoid structures.
Antigen-specific antibody-producing cells have thus far never been demonstrated in the human genital tract. In accordance with an earlier study by Crowley-Nowick et al. (5), we found that in both the cervical and the uterine mucosa the majority of plasma cells were producing IgG. We were also able to isolate CTB-specific IgA ASCs in the cervical mucosa after both nasal and vaginal vaccinations. Unfortunately, the number of lymphocytes isolated by enzymatic digestion of the various tissues was too low to allow for an accurate determination of the absolute number of ASCs induced after vaccination. Thus, it was not possible to compare the difference in the number of ASCs in the female genital tract after nasal and vaginal vaccinations.
In the present study we have shown that vaginal administration of antigen on days 10 and 24 in the menstrual cycle is optimal for obtaining specific antibody responses in the cervix, while nasal administration is superior in inducing strong antibody levels in the vagina. In earlier studies, vaginal vaccination has been shown to be superior to both oral and rectal vaccination in inducing strong antibody responses in cervical secretions (15, 29). In contrast, oral and nasal vaccinations are equally efficient in inducing vaginal antibody responses (21). The advantage of nasal over oral vaccination is that the dose of antigen could be reduced fourfold and still induce strong CTB-specific IgA and IgG antibody responses both in serum and in vaginal secretions (21). A combination of efficient systemic and local immune responses is desirable in the protection against genital tract infections with chlamydia and human immunodeficiency virus (1, 28). Furthermore, for protection against most sexually transmitted diseases, it would probably be beneficial to have a high antibody response both in the vaginal and in the cervical secretions to hinder both the initiation and the spread of the infection. We therefore suggest that a combination of nasal and vaginal vaccinations might be the optimal vaccination strategy for inducing a protective antibody response in the female genital tract.
ACKNOWLEDGMENTS
This work was supported by Swedish Medical Research Council; The Swedish Physicians against AIDS Research Foundation; the Medical Faculty, Göteborg University; Magnus Bergwalls Stiftelse; and the Swedish Society of Medicine.
We gratefully acknowledge Margareta Fredriksson and Kerstin Andersson for excellent technical assistance and Active Biotech for providing us with the CTB vaccine preparations.
REFERENCES
- 1.Beagley K W, Timms P. Chlamydia trachomatis infection: incidence, health costs and prospects for vaccine development. J Reprod Immunol. 2000;48:47–68. doi: 10.1016/s0165-0378(00)00069-3. [DOI] [PubMed] [Google Scholar]
- 2.Bergquist C, Johansson E L, Lagergard T, Holmgren J, Rudin A. Intranasal vaccination of humans with recombinant cholera toxin B subunit induces systemic and local antibody responses in the upper respiratory tract and the vagina. Infect Immun. 1997;65:2676–2684. doi: 10.1128/iai.65.7.2676-2684.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bjercke S, Brandtzaeg P. Glandular distribution of immunoglobulins, J chain, secretory component, and HLA-DR in the human endometrium throughout the menstrual cycle. Hum Reprod. 1993;8:1420–1425. doi: 10.1093/oxfordjournals.humrep.a138271. [DOI] [PubMed] [Google Scholar]
- 4.Black C A, Rohan L C, Cost M, Watkins S C, Draviam R, Alber S, Edwards R P. Vaginal mucosa serves as an inductive site for tolerance. J Immunol. 2000;165:5077–5083. doi: 10.4049/jimmunol.165.9.5077. [DOI] [PubMed] [Google Scholar]
- 5.Crowley-Nowick P A, Bell M, Edwards R P, McCallister D, Gore H, Kanbour-Shakir A, Mestecky J, Partridge E E. Normal uterine cervix: characterization of isolated lymphocyte phenotypes and immunoglobulin secretion. Am J Reprod Immunol. 1995;34:241–247. doi: 10.1111/j.1600-0897.1995.tb00948.x. [DOI] [PubMed] [Google Scholar]
- 6.Eriksson K, Quiding-Jarbrink M, Osek J, Moller A, Bjork S, Holmgren J, Czerkinsky C. Specific-antibody-secreting cells in the rectums and genital tracts of nonhuman primates following vaccination. Infect Immun. 1998;66:5889–5896. doi: 10.1128/iai.66.12.5889-5896.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holmgren J. Actions of cholera toxin and the prevention and treatment of cholera. Nature. 1981;292:413–417. doi: 10.1038/292413a0. [DOI] [PubMed] [Google Scholar]
- 8.Hopkins S, Kraehenbuhl J P, Schodel F, Potts A, Peterson D, de Grandi P, Nardelli-Haefliger D. A recombinant Salmonella typhimurium vaccine induces local immunity by four different routes of immunization. Infect Immun. 1995;63:3279–3286. doi: 10.1128/iai.63.9.3279-3286.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jertborn M, Svennerholm A M, Holmgren J. Safety and immunogenicity of an oral recombinant cholera B subunit-whole cell vaccine in Swedish volunteers. Vaccine. 1992;10:130–132. doi: 10.1016/0264-410x(92)90030-n. [DOI] [PubMed] [Google Scholar]
- 10.Jertborn M, Svennerholm A M, Holmgren J. Saliva, breast milk, and serum antibody responses as indirect measures of intestinal immunity after oral cholera vaccination or natural disease. J Clin Microbiol. 1986;24:203–209. doi: 10.1128/jcm.24.2.203-209.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johansson E L, Rask C, Fredriksson M, Eriksson K, Czerkinsky C, Holmgren J. Antibodies and antibody-secreting cells in the female genital tract after vaginal or intranasal immunization with cholera toxin B subunit or conjugates. Infect Immun. 1998;66:514–520. doi: 10.1128/iai.66.2.514-520.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johansson E L, Rudin A, Wassen L, Holmgren J. Distribution of lymphocytes and adhesion molecules in human cervix and vagina. Immunology. 1999;96:272–277. doi: 10.1046/j.1365-2567.1999.00675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelly K A, Rank R G. Identification of homing receptors that mediate the recruitment of CD4 T cells to the genital tract following intravaginal infection with Chlamydia trachomatis. Infect Immun. 1997;65:5198–5208. doi: 10.1128/iai.65.12.5198-5208.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kelly K A, Walker J C, Jameel S H, Gray H L, Rank R G. Differential regulation of CD4 lymphocyte recruitment between the upper and lower regions of the genital tract during Chlamydia trachomatis infection. Infect Immun. 2000;68:1519–1528. doi: 10.1128/iai.68.3.1519-1528.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kozlowski P A, Cu-Uvin S, Neutra M R, Flanigan T P. Comparison of the oral, rectal, and vaginal immunization routes for induction of antibodies in rectal and genital tract secretions of women. Infect Immun. 1997;65:1387–1394. doi: 10.1128/iai.65.4.1387-1394.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murdoch A J, Buckley C H, Fox H. Hormonal control of the secretory immune system of the human uterine cervix. J Reprod Immunol. 1982;4:23–30. doi: 10.1016/0165-0378(82)90020-1. [DOI] [PubMed] [Google Scholar]
- 17.Parr M B, Kepple L, Parr E L. Antigen recognition in the female reproductive tract. II. Endocytosis of horseradish peroxidase by Langerhans cells in murine vaginal epithelium. Biol Reprod. 1991;45:261–265. doi: 10.1095/biolreprod45.2.261. [DOI] [PubMed] [Google Scholar]
- 18.Parr M B, Parr E L. Antigen recognition in the female reproductive tract. I. Uptake of intraluminal protein tracers in the mouse vagina. J Reprod Immunol. 1990;17:101–114. doi: 10.1016/0165-0378(90)90029-6. [DOI] [PubMed] [Google Scholar]
- 19.Perry L L, Feilzer K, Portis J L, Caldwell H D. Distinct homing pathways direct T lymphocytes to the genital and intestinal mucosae in chlamydia-infected mice. J Immunol. 1998;160:2905–2914. [PubMed] [Google Scholar]
- 20.Quiding-Jarbrink M, Granstrom G, Nordstrom I, Holmgren J, Czerkinsky C. Induction of compartmentalized B-cell responses in human tonsils. Infect Immun. 1995;63:853–857. doi: 10.1128/iai.63.3.853-857.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rudin A, Johansson E L, Bergquist C, Holmgren J. Differential kinetics and distribution of antibodies in serum and nasal and vaginal secretions after nasal and oral vaccination of humans. Infect Immun. 1998;66:3390–3396. doi: 10.1128/iai.66.7.3390-3396.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Russell M W, Moldoveanu Z, White P L, Sibert G J, Mestecky J, Michalek S M. Salivary, nasal, genital, and systemic antibody responses in monkeys immunized intranasally with a bacterial protein antigen and the cholera toxin B subunit. Infect Immun. 1996;64:1272–1283. doi: 10.1128/iai.64.4.1272-1283.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanchez J, Holmgren J. Recombinant system for overexpression of cholera toxin B subunit in Vibrio cholerae as a basis for vaccine development. Proc Natl Acad Sci USA. 1989;86:481–485. doi: 10.1073/pnas.86.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shen X, Lagergard T, Yang Y, Lindblad M, Fredriksson M, Holmgren J. Systemic and mucosal immune responses in mice after mucosal immunization with group B streptococcus type III capsular polysaccharide-cholera toxin B subunit conjugate vaccine. Infect Immun. 2000;68:5749–5755. doi: 10.1128/iai.68.10.5749-5755.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Streeter P R, Berg E L, Rouse B T, Bargatze R F, Butcher E C. A tissue-specific endothelial cell molecule involved in lymphocyte homing. Nature. 1988;331:41–46. doi: 10.1038/331041a0. [DOI] [PubMed] [Google Scholar]
- 26.Svennerholm A M, Jertborn M, Gothefors L, Karim A M, Sack D A, Holmgren J. Mucosal antitoxic and antibacterial immunity after cholera disease and after immunization with a combined B subunit-whole cell vaccine. J Infect Dis. 1984;149:884–893. doi: 10.1093/infdis/149.6.884. [DOI] [PubMed] [Google Scholar]
- 27.Svennerholm A M, Sack D A, Holmgren J, Bardhan P K. Intestinal antibody responses after immunisation with cholera B subunit. Lancet. 1982;i:305–308. doi: 10.1016/s0140-6736(82)91568-9. [DOI] [PubMed] [Google Scholar]
- 28.Velin D, Kraehenbuhl J P. Delivery systems and adjuvants for vaccination against HIV. Exper Suppl (Basel) 2000;89:227–237. doi: 10.1007/978-3-0348-8393-1_14. [DOI] [PubMed] [Google Scholar]
- 29.Wassen L, Schon K, Holmgren J, Jertborn M, Lycke N. Local intravaginal vaccination of the female genital tract. Scand J Immunol. 1996;44:408–414. doi: 10.1046/j.1365-3083.1996.d01-320.x. [DOI] [PubMed] [Google Scholar]
- 30.Wira C R, Rossoll R M. Antigen-presenting cells in the female reproductive tract: influence of the estrous cycle on antigen presentation by uterine epithelial and stromal cells. Endocrinology. 1995;136:4526–4534. doi: 10.1210/endo.136.10.7664673. [DOI] [PubMed] [Google Scholar]