Abstract
Background
Percutaneous mechanical circulatory support (pMCS) by an Impella™ device implies the initiation of systemic anticoagulation to prevent systemic thrombotic complications and a purge fluid to prevent device blockage. Traditionally, unfractionated heparin (UFH) was used for both. In April 2022, the use of bicarbonate-based purge solution (BBPS) as an alternative to UFH in dextrose solution was approved by the Food and Drug Administration in case of contraindications for UFH.
Case summary
We present the case of a 73-year-old female that was admitted to the cardiac intensive care unit with cardiogenic shock, requiring upgrade with pMCS by an axillary Impella CP™. When she developed a severe haemothorax, all UFH was stopped and the purge was switched to BBPS-dextrose solution without increase in purge pressures nor development of haemolysis. The bleeding stagnated and the patient could be weaned from the Impella™ after 2 days.
Discussion
Here, we present the first case report of the switch to BBPS in an Impella CP™ supported patient with major bleeding since the FDA approval in April 2022. The switch to BBSP in addition with the administration of platelets and protamine resulted in cessation of the bleeding in this case.
Keywords: Mechanical circulatory support, Bleeding, Purge solution, Bicarbonate, Case report
Learning points.
Despite the growing success and use of pMCS, bleeding complications remain frequent and jeopardize patients’ outcomes.
Proper bleeding management strategies are important during severe bleeding complications on pMCS.
Switch to BBPS can be considered in case of clinically significant bleedings during Impella™ support.
Introduction
Percutaneous mechanical circulatory support (pMCS) with transvalvular micro-axial flow pumps, such as the group of the Impella™ devices, has become a life-saving tool during the management of refractory cardiogenic shock (CS) and its success has greatly increased over the last decade.1 During Impella™-support, unfractionated heparin (UFH) is traditionally used for systemic anticoagulation to prevent device related thrombosis as well as added to the dextrose in water purge solution to prevent its blockage. Since some patients have contraindications for the use of UFH [e.g. heparin induced trombocytopenia (HIT)] or develop major bleeding problems, the manufacturer obtained recent Food and Drug Administration (FDA) approval to use bicarbonate-based purge solution (BBPS) as an UFH alternative in the purge. Here, we illustrate a case of a 73-year-old female patient that was successfully switched to BBPS when she developed a haemothorax under Impella CP™ support.
Timeline
| Day | Event |
|---|---|
| 1 | Presentation at the emergency department with inflammatory syndrome of unknown aetiology and anorexia. Start of IV antibiotics, IV fluids, and nasogastric tube feeding. |
| 6 | Acute respiratory failure resulting in ICU admission and intubation. Diagnosis of acute heart failure with reduced ejection fraction was made. Patient was transferred to our tertiary centre for upgrade with left-sided axillary Impella™. |
| 7 | Axillary Impella CP™ implantation with administration of UFH both systemically and via the purge. |
| 16 | Diagnostic thoracentesis was performed to exclude an empyema since ongoing high fever associated with a unilateral right-sided pleural effusion. Coagulant parameters are presented in Table 1. |
| 17 | Diagnosis of active haemothorax. Systemic UFH was stopped and purge was switched to BBPS. Coagulant parameters after administration of one pool of platelets and protamine are presented in Table 1. Bleeding stagnated. |
| 18 | Inotropes were weaned to stop. |
| 19 | Patient was weaned from the Impella™ and the device was removed. |
Case presentation
A 73-year-old female was transferred to the cardiac intensive care unit of our tertiary care centre for deteriorating CS despite high doses of inotropes, requiring escalation of therapy with pMCS (SCAI-D; no high oxygen requirements).2 She had no other medical history besides a diffuse large B-cell lymphoma (Stage IV), which had been diagnosed six months earlier and necessitating five cycles of oncological therapy with anthracyclines amongst others. She had been hospitalized for six days for an inflammatory syndrome of unknown aetiology and anorexia and had received empiric IV antibiotics, IV fluids and nasogastric tube feeding, when she was transferred to the intensive care unit because of acute respiratory failure with the need for intubation and positive pressure ventilation. Chest X-ray showed new bilateral infiltrates and transthoracic echocardiography revealed a strongly reduced left ventricular output (LVOT-VTI 6 cm (see Supplementary Figure S1); EF 20%) where LVEF prior to the start of the oncological treatment was reported normal (see Supplementary video S1 and S2). There were no manifest valvular problems and right ventricular contractility was preserved. n-terminal pro-brain natriuretic peptide was 27.745 ng/L (reference value <349 ng/L). An additional coronary angiography showed a normal coronary angiogram, but the ventriculography revealed some discrete apical ballooning. Therefore, a differential diagnosis of acute heart failure with reduced ejection fraction based on a toxic (anthracyclines) vs. Takotsubo cardiomyopathy was made. Given the progression into sliding CS on high doses of inotropes (milrinone 0.25 µg/kg/min and norepinephrine 0.4 µg/kg/min), the patient was considered for upgrade to short-term MCS as recommended by the European Society of Cardiology guidelines for acute heart failure,3,4 and considered suitable for MCS since her oncological diagnosis was not supposed to alter the short-term prognosis. She was transferred to our centre and an Impella CP™ was surgically inserted via the left subclavian artery, supported with a purge of dextrose 5% in water (D5W). Anticoagulation was provided by UFH via the purge (25 IU/mL) and systemically (calculated to reach a total of 12 IU/kg bodyweight) and monitored as previously described by our group, based on the anti-factor Xa assay (anti-Xa; target 0.3–0.5 IU/mL) with parallel measurements of activated thromboplastin time (APTT).5 On day 16, a diagnostic thoracentesis was performed to exclude an empyema since ongoing high fever associated with a unilateral right-sided pleural effusion. The result came back as chylous. Anticoagulant parameters at the time of the pleural drainage were normalized after cessation of peripheral UFH (Table 1). One night after the procedure and after peripheral UFH was restarted, the patient developed a haemothorax with a significant drop in haemoglobin (BARC 3 bleeding) and a prominent expansion of the right-sided pleural effusion on chest X-ray (Figure 1A) plus escalation of vasopressor doses. A percutaneous thoracic pigtail was inserted. Systemic UFH was immediately ceased and the purge was switched to BBPS (25 mEq sodium bicarbonate in 1L D5W solution, prepared by adding 12.5 mL of sodium bicarbonate 8.4% to a 500 mL solution of glucose 5%). One unit of both platelets and red blood cells as well as protamine sulphate were administered. After evacuation of two liters of haemorrhagic fluid, the bleeding stopped over the next few hours and the chest X-ray could not show any residual pleural effusion after drainage (Figure 1B). During the use of the BBPS (48 hours in total), there were no signs of pump thrombosis nor rising purge pressures or signs of haemolysis (Figure 2) and pH and natremia stayed within their normal range. Coagulant parameters on day 16 and 17 are presented in Table 1. On day 18, the haemodynamic state of the patient was markedly improved and inotropes/vasopressors could be weaned off. In total, UFH was stopped for 48 hours. The next day, the patient could be weaned from the Impella™. After removal, the device showed macroscopically no signs of thrombosis. Unfortunately, the patient developed several infectious problems afterwards and died on day 35.
Table 1.
Coagulant parameters on day 16 and 17 (day 10 and 11 after pMCS insertion)
| Parameter | Day 16 (UFH in purge, systemic UFH halted) | Day 17 (BPPS, no systemic UFH) | Reference values |
|---|---|---|---|
| Anti-Xa (IU/mL) | 0.25 | <0.15 | 0.30–0.50 |
| APTT (s) | 41.0 | 23.1 | 25.1–36.5 s |
| PT (INR) | 1.0 | 1.1 | |
| Platelets (0.103/µL) | 83 | 63 | 150–450 0.103/µL |
| Fibrinogen (g/L) | 3.19 | 3.95 | 2.00–3.93 g/L |
| pfHb | <5 | <5 | <10 mg/dL |
Anti-Xa, heparin anti-factor Xa assay; APTT, activated partial thromboplastin time; PT, prothrombin time; pfHb, plasma-free haemoglobin.
Figure 1.
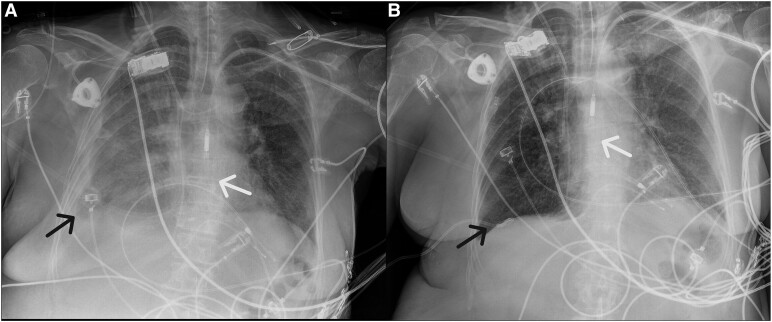
Left panel: Chest X-ray on day 16, showing a unilateral pleural effusion on the right side (black arrow), for which a diagnostic pleural punction was performed to exclude empyema. The Impella™-device is shown by the white arrow. Right panel: Chest X-ray on day 17 after pleural drainage and insertion of a pig tail catheter (black arrow), which resulted in full drainage of the pleural effusion.
Figure 2.
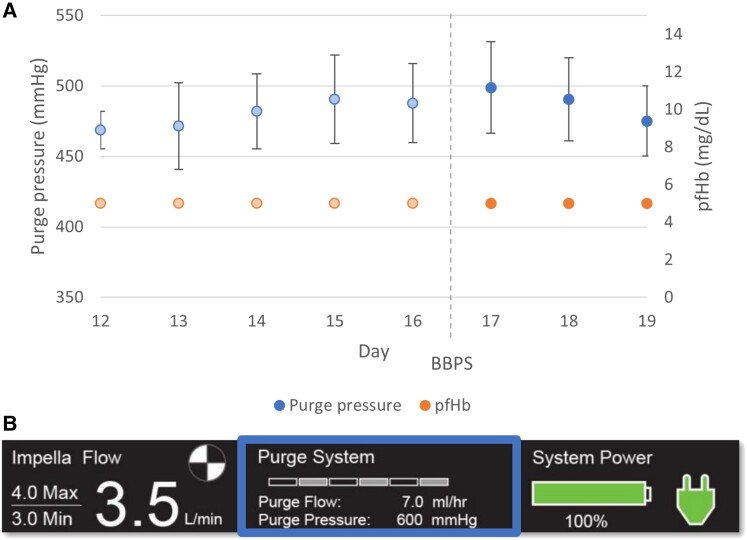
Upper panel: purge pressures (mean ± standard deviation) and evolution of pfHb (measurement once daily). Lower panel: Impella™ console indicating purge pressures within normal range (300–1000 mmHg) during support with BBPS.
Discussion
Despite the growing success and use of Impella™ devices, bleeding complications remain a major challenge and jeopardize patients’ outcome.6,7 The precarious balance between bleeding and thrombosis in this setting is a consequence of pre-existing coagulopathy (e.g. CS and multi-organ failure with systemic inflammatory response syndrome), access-site vascular complications, eventual concomitant antiplatelet therapy, device related coagulopathy and the need for systemic anticoagulation.5 Indeed, the use of systemic anticoagulation, most frequently with UFH, is necessary in Impella™ supported patients to counteract the activation of the coagulation system caused by shear forces and the pump surface.5 Alternatively to UFH, direct thrombin inhibitors can be used as well. The Impella™ device is equipped with a dextrose in water purge system, which traditionally contains UFH at a concentration of 25–50 IU/mL. The purge prevents blood from entering the motor compartment and leading to massive haemolysis.8,9 However, as the infusion rate of this purge solution is automatically controlled by the console to maintain purge pressures of 300–1100 mmHg, variable doses of UFH are administered by the pump. Until recently, the only way to influence the purge flow and thus the amount of purge UFH administration, was to alter the dextrose concentration in the purge, which determines the viscosity and therefore also the flow rate of the purge. Various dextrose concentrations have been evaluated (from 5% to 40%) and, for example, a dextrose purge solution of 20% will result in a 40% reduction in flow rates compared to a 5% solution.10 In April 2022, the manufacturer obtained FDA approval for the use of BBPS for patients intolerant to UFH (e.g. HIT) or in whom UFH is contraindicated due to bleeding complications. This resulted in a simplification of the anticoagulation management because there was no longer a need to account for fluctuating UFH-doses delivered through the device.11 In a recent retrospective study, sodium bicarbonate has been proven to be superior in preventing catheter related thrombosis in haemodialysis catheters compared to saline.12 The proposed working mechanism of BBPS is through bicarbonate chelation of calcium and neutralization of the acidic pH of dextrose solution,13 suppressing fibrin assembly, improving major blood protein stability and influencing bio-build-up lysis via buffering effect.14 In our case report, the major bleeding stabilized after switch to BBPS with parallel cessation of systemic UFH and administration of protamine and platelet concentrates. There were no overt signs of pump thrombosis nor purge pressure rise and pH as well as natremia remained within their normal range during the use of BBPS. Forty-eight hours after cessation of anticoagulant therapy, reintroduction of UFH can be considered although our patient could be successfully weaned from the device.
Conclusion
After recent FDA approval, this is the first case report describing the uneventful switch of the device purge system to BBPS in a left-sided Impella™-supported CS patient with major bleeding complications.
Supplementary Material
Acknowledgements
We sincerely thank the staff of the cardiac intensive care unit at UZ Leuven and the perfusionist team.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: Ethical approval as well as informed consent from the relatives in accordance with COPE guidelines was obtained for this case report.
Funding: None declared.
Contributor Information
Charlotte Van Edom, Department of Cardiovascular Sciences, University of Leuven, Herestraat 49, 3000 Leuven, Belgium.
Tim Van Puyvelde, Department of Cardiovascular Diseases, University Hospitals Leuven, Herestraat 49, 3000 Leuven, Belgium.
Steven Jacobs, Department of Cardiac Surgery, Unversity Hospitals Leuven, Herestraat 49, 3000 Leuven, Belgium; Department of Cardiovascular Sciences, University of Leuven, Herestraat 49, 3000 Leuven, Belgium.
Christophe Vandenbriele, Department of Cardiovascular Sciences, University of Leuven, Herestraat 49, 3000 Leuven, Belgium; Department of Cardiovascular Diseases, University Hospitals Leuven, Herestraat 49, 3000 Leuven, Belgium; Department of Adult Intensive Care, Royal Brompton and Harefield Hospitals, Guy's and St Thomas' NHS Foundation Trust, Sydney St, London SW3 6NP, UK.
Lead author biography
 Charlotte Van Edom graduated as MD at KU Leuven (Belgium) in 2019 and first spent two years in clinical training as a resident of Internal Medicine. In 2021, she started her PhD on haemocompatibility (haemolysis, thrombosis, haemostasis) on percutaneous mechanical circulatory support.
Charlotte Van Edom graduated as MD at KU Leuven (Belgium) in 2019 and first spent two years in clinical training as a resident of Internal Medicine. In 2021, she started her PhD on haemocompatibility (haemolysis, thrombosis, haemostasis) on percutaneous mechanical circulatory support.
Supplementary material
Supplementary material is available at European Heart Journal – Case Reports.
References
- 1. Balthazar T, Vandenbriele C, Verbrugge FH, Den Uil C, Engström A, Janssens S, et al. Managing patients with short-term mechanical circulatory support: JACC review topic of the week. J Am Coll Cardiol 2021;77:1243–1256. [DOI] [PubMed] [Google Scholar]
- 2. Naidu SS, Baran DA, Jentzer JC, Hollenberg SM, van Diepen S, Basir MB, et al. SCAI SHOCK stage classification expert consensus update: a review and incorporation of validation studies. J Am Coll Cardiol 2022;79:933–946. [DOI] [PubMed] [Google Scholar]
- 3. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021;42:3599–3726. [DOI] [PubMed] [Google Scholar]
- 4. Chieffo A, Dudek D, Hassager C, Combes A, Gramegna M, Halvorsen S, et al. Joint EAPCI/ACVC expert consensus document on percutaneous ventricular assist devices. Eur Heart J Acute Cardiovasc Care 2021;10:570–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vandenbriele C, Arachchillage DJ, Frederiks P, Guistino G, Gorog DA, Gramegna M, et al. Anticoagulation for percutaneous ventricular assist device-supported cardiogenic shock: JACC review topic of the week. J Am Coll Cardiol 2022;79:1949–1962. [DOI] [PubMed] [Google Scholar]
- 6. Amin AP, Spertus JA, Curtis JP, Desai N, Masoudi FA, Bach RG, et al. The evolving landscape of impella use in the United States among patients undergoing percutaneous coronary intervention with mechanical circulatory support. Circulation 2020;141:273–284. [DOI] [PubMed] [Google Scholar]
- 7. Dhruva SS, Ross JS, Mortazavi BJ, Hurley NC, Krumholz HM, Curtis JP, et al. Association of use of an intravascular microaxial left ventricular assist device vs intra-aortic balloon pump with in-hospital mortality and Major bleeding among patients with acute myocardial infarction complicated by cardiogenic shock. JAMA 2020;323:734–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dietrich JN, Kazmi H. Bleeding risks in patients on percutaneous ventricular assist devices receiving two different dextrose concentrations of heparinized purge solution: a case series. J Pharm Pract 2019;32:464–469. [DOI] [PubMed] [Google Scholar]
- 9. Beavers CJ, DiDomenico RJ, Dunn SP, Cox J, To L, Weeks P, et al. Optimizing anticoagulation for patients receiving impella support. Pharmacotherapy 2021;41:932–942. [DOI] [PubMed] [Google Scholar]
- 10. Succar L, Sulaica EM, Donahue KR, Wanat MA. Management of anticoagulation with impella® percutaneous ventricular assist devices and review of new literature. J Thromb Thrombolysis 2019;48:284–291. [DOI] [PubMed] [Google Scholar]
- 11. Beavers CJ, Dunn SP, DiDomenico RJ, Moretz J, Jennings DL. Bicarbonate-based purge solution during impella support: a growing alternative. J Am Coll Cardiol 2022;79:633. [Google Scholar]
- 12. El-Hennawy AS, Frolova E, Romney WA. Sodium bicarbonate catheter lock solution reduces hemodialysis catheter loss due to catheter-related thrombosis and blood stream infection: an open-label clinical trial. Nephrol Dial Transplant 2019;34:1739–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Simonsen KA, Gunn BL, Malhotra A, Beckles DL, Koerner MM, Tavilla G, et al. Use of a novel bicarbonate-based impella 5.5 purge solution in a coagulopathic patient. J Card Surg 2021;36:4773–4775. [DOI] [PubMed] [Google Scholar]
- 14. Gilman V, Popovsky M, McMinn S. Bicarbonate as an alternative to heparin in Impella purge fluid: understanding the biochemical basis. Paper presented at: ASAIO 66th Annual Conference; June 10–12, 2021; Washington DC. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.