Abstract
During pregnancy, Plasmodium falciparum-infected erythrocytes sequester in the placenta by adhering to chondroitin 4-sulfate, creating a risk factor for both the mother and the fetus. The primigravidae are at higher risk for placental malaria than the multigravidae. This difference in susceptibility has been attributed to the lack of antibodies that block the adhesion of infected erythrocytes to placental chondroitin 4-sulfate in primigravid women. However, recent results show that many primigravidae at term have antibody levels similar to those of multigravidae, and thus the significance of antiadhesion antibodies in providing protection against malaria during pregnancy remains unclear. In this study, we analyzed plasma samples from women of various gravidities at different gestational stages for antiadhesion antibodies. The majority of women, regardless of gravidity, had similar levels of antibodies at term. Most primigravidae had low levels of or no antiadhesion antibodies prior to ∼20 weeks of pregnancy and then produced antibodies. Multigravidae also lacked antibodies until ∼12 weeks of pregnancy, but thereafter they efficiently produced antibodies. In pregnant women who had placental infection at term, higher levels of antiadhesion antibodies correlated with lower levels of placental parasitemia. The difference in kinetics of antibody production between primigravidae and multigravidae correlated with the prevalence of malaria in these groups, suggesting that antibodies are produced during pregnancy in response to placental infection. The early onset of efficient antibody response in multigravidae and the delayed production to antibodies in primigravidae appear to account for the gravidity-dependent differential susceptibilities of pregnant women to placental malaria.
Plasmodium falciparum malaria is one of the major health problems in Africa and many other tropical countries around the world. Accumulated evidence indicates that the severity of falciparum malaria is due to the sequestration of P. falciparum-infected red blood cells (IRBC) in the microvascular capillaries of vital organs (15, 21, 23, 25, 26). During the past decade, a number of studies have shown that IRBC sequestration is mediated by the binding of P. falciparum erythrocyte membrane protein 1, an antigenic var gene family protein expressed on the surface of IRBC, to the host endothelial cell adhesion molecules (23, 25). It is thought that adherence of IRBC results in capillary obstruction and induction of proinflammatory cytokines locally in response to toxic parasite factors, resulting in tissue damage and clinical manifestations (10, 15, 21, 23, 25, 26). Adults in areas where malaria is endemic, however, have natural immunity against developing severe malaria, which is acquired in response to repeated infections with P. falciparum (3, 10, 30). A significant component of the protective immunity appears to be due to the anti-IRBC adhesion antibody response, which can limit IRBC sequestration to microvascular endothelia.
Despite previously acquired immunity, pregnant women are at risk of developing placental malaria, which is characterized by a poor pregnancy outcome (4, 19). In pregnant women, the placenta provides a new opportunity for selective accumulation of P. falciparum IRBC that express an antigenically distinct form of P. falciparum erythrocyte membrane protein 1 (5). Multiplication of this parasite phenotype and extensive accumulation of IRBC in the placenta could lead to massive infiltration of mononuclear cells and induction of proinflammatory cytokines, causing severe placental pathology (7, 11, 20, 22, 24). Primigravidae are at the highest risk for malaria, and their susceptibility decreases with subsequent pregnancies, suggesting gravidity-dependent acquisition of placental malaria-specific immunity.
Chondroitin 4-sulfate (C4S) mediates the adherence of IRBC in the human placenta (5, 6, 18, 27, 28, 31). In areas where malaria is endemic, pregnant women, but not adult males and nulligravid women, have been shown to have antibodies that inhibit adherence of IRBC to C4S, and the antibody level increases with increasing gravidity (8, 9, 17, 29). One study reported that multigravidae but not primigravidae produce antibodies that block the adhesion of IRBC to C4S and attributed the greater risk of malaria in primigravidae to the lack of antibodies (8). However, other studies have shown that both primigravidae and multigravidae have antibodies at term (9, 17, 29). The latter studies have also reported increased levels of antibodies with increasing gravidity, which has been attributed to the gravidity-dependent increased protection against developing severe placental malaria (9, 17, 29). A comparison of the reported data (8, 9, 17, 29), however, shows that a significant number of primigravidae have high levels of anti-C4S adhesion antibodies, and thus the reason why primigravidae are more susceptible to placental malaria remains unclear.
We have recently purified and characterized chondroitin sulfate proteoglycan (CSPG) receptors that mediate the adherence of IRBC to the intervillous spaces of human placenta; the C4S chains of the placental CSPG have unusually low sulfate content (<10% of disaccharide repeats are sulfated) (1). In the present study, using placental CSPG in an in vitro cytoadherence assay, we analyzed plasma from Cameroonian women of different gravidity and gestational ages for anti-C4S adhesion antibodies. The data showed that these women, irrespective of their gravidity, had low levels of antibodies or no antibodies prior to ∼12 weeks of gestation, and most pregnant women had antibodies at term. The data also indicated that most primigravidae produced antibodies beginning at 20 to 24 weeks of gestation, whereas multigravidae produced high levels of antibodies beginning at 12 to 16 weeks of gestation. The substantial delay in eliciting a primary antibody response in primigravidae as opposed to an early secondary response in multigravidae may contribute to the greater susceptibility of the former group to malaria.
MATERIALS AND METHODS
Participants and sample collection.
The samples were collected at the Biyem Assi Hospital in Yaounde, Cameroon. P. falciparum is transmitted throughout the year in Yaounde, with the transmission rate estimated to be ∼13 infectious bites per year in areas near the hospital (16). Over a 3-year period, pregnant women living near the hospital were recruited into the study. Prior to delivery, the nature of the project was explained to the women and verbal informed consent was obtained. Clinical histories, a sample of heparinized maternal venous blood, and a piece of the placenta were collected at the time of delivery. Ethical clearance for the research was obtained from the Ethical Committee, Ministry of Health, Cameroon, and the Institutional Review Board at Georgetown University. The project is covered by single project assurance number S-9601-01.
Plasma samples from 198 women were selected from the above-described panel to measure antibody levels at term. Equal numbers of women were selected based on gravidity statues 1 (primigravidae), 2, 3, 4, and ≥5, approximately 50% of whom had IRBC in the placenta at the time of delivery. To measure the kinetics of antibody formation, samples from another group of women were obtained at different time points during pregnancy. This group consisted of 45 primigravidae and 43 trigravidae (total, n = 129 samples). Samples were also obtained from 7 nulligravidae women and 11 nonpregnant multigravidae living in the same area and were used as controls.
Parasitological studies.
Thick and thin peripheral blood films, as well as impression smears of placental tissues, were prepared, stained with Dif-Quick (Baxter Scientific, New Providence, N.J.), and examined for the presence of parasitized erythrocytes using routine microscopy. To determine the level of parasitemia, 500 to 2,000 erythrocytes were examined and the number of infected erythrocytes was determined. In addition, sections of placental tissues were fixed in 10% buffered formalin, embedded, stained with hematoxylin-eosin, and examined for the presence of parasitized erythrocytes.
Parasites and parasite culturing.
P. falciparum parasites used for the C4S-IRBC adhesion inhibition assay were initially selected from the 3D7 clone for adherence to bovine trachea C4S and were subsequently selected for IRBC adherence to CSPG purified from human placenta (2). After continuous culturing for 6 to 8 weeks, the adherent IRBC were reselected on CSPG-coated plates. The parasites were cultured in RPMI-1640 medium supplemented with 10% O-positive human serum and O-positive human red blood cells at 37°C. Parasites were routinely synchronized at the early ring stage with 5% sorbitol as described previously (14). The cultures were harvested at the mid-trophozoite stage.
IRBC from cultures with >20% parasitemia were directly used for the adhesion assay (see below), and IRBC from cultures with lower parasitemia levels were enriched by gelatin flotation (12). For enriching IRBC, harvested cells were suspended in 0.65% gelatin in phosphate-buffered saline (PBS), pH 7.2, and incubated at 37°C for 15 to 20 min. When most erythrocytes had settled to the bottom, the supernatant containing the trophozoite-stage IRBC (50 to 60% parasitemia) was collected, centrifuged, and IRBC washed with sterile PBS.
Purification of placental intervillous CSPG.
The CSPG from placental intervillous spaces was isolated by DEAE-Sephacel chromatography as described previously (1). The CSPG was purified by successive DEAE-Sephacel chromatography using a salt gradient, CsBr density gradient centrifugation, and gel filtration using Sepharose CL-6B and Sepharose CL-4B (1).
Cytoadherence inhibition assays.
The CSPG was coated as circular spots on 15-by-150-mm-diameter plastic Petri dishes by spotting 10 to 15 μl of CSPG (200 ng/ml) in PBS, pH 7.2, and incubating it at 4°C overnight in a humidified chamber (2). The spots were blocked with 20 μl of 2% bovine serum albumin in PBS for 2 h at room temperature. Human plasma, diluted with PBS containing 2% bovine serum albumin, was mixed with equal volumes of 4% suspension of IRBC in PBS, in 96-well microtiter plates (final plasma dilutions were 1:10, 1:20, and 1:40). The suspension was incubated at room temperature for 30 min with intermittent mixing and then layered onto CSPG-coated spots. After 40 min at room temperature, the unbound cells were washed off with PBS. The bound cells were fixed with 2% glutaraldehyde and stained with Giemsa reagent. All assays were performed in duplicate. IRBC in several randomly selected low-power fields were counted under a light microscope, and the mean number of IRBC per mm2 was determined; values usually ranged from 5,000 to 6,000 for the PBS control. The percent inhibition of the IRBC binding was calculated by the formula (1 − [number of IRBC bound to the CSPG-coated spots in the presence of plasma/number of IRBC bound to CSPG coated spots in the absence of plasma]) × 100.
Statistical data analysis.
Statistical differences in the levels of antiadhesion antibodies between two groups were analyzed using Student's t test (13). Differences in the proportions of women with antiadhesion antibodies were analyzed using the Chi-square test. The Pearson's correlation analysis was used to test the correlation between the log-transformed placental parasitemia and level of antiadhesion antibody (13).
RESULTS
The study group.
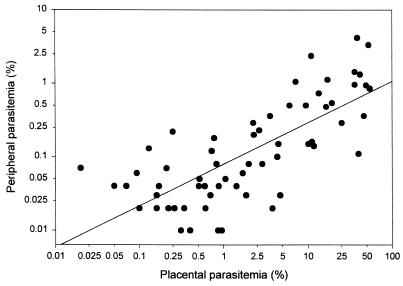
The study group for the assessment of anti-C4S adhesion antibodies at term consisted of 198 pregnant women. The numbers, ages, and the levels of peripheral and placental parasitemia of these women are shown in Table 1. For 69% of the malaria-positive women, parasites were detected in both peripheral and placental blood, with substantially higher levels of parasitemia in the placenta. In these women, as expected, there was a direct correlation between the levels of peripheral and placental parasitemias (r2 = 0.55) (Fig. 1). Among the remaining malaria-positive women, 29% had placental but not peripheral parasites while only 2% had peripheral but not placental parasitemias. These results are consistent with sequestration of P. falciparum IRBC in the placentas of pregnant women.
TABLE 1.
Description of pregnant women included in the studya
| Gravidity | No. of pregnant women
|
Mean age (yr) | % Parasitemia in M+ women (range)
|
|||
|---|---|---|---|---|---|---|
| Total | M− | M+ | Peripheral | Placental | ||
| 1 | 47 | 23 | 24 | 20 | 0–3.3 | 0.001–52.7 |
| 2 | 32 | 18 | 14 | 22 | 0–1.4 | 0.08–54.4 |
| 3 | 48 | 31 | 17 | 24 | 0–4.2 | 0.02–38.6 |
| 4 | 36 | 19 | 17 | 27 | 0–0.29 | 0.001–2.3 |
| ≥5 | 35 | 10 | 25 | 31 | 0–0.48 | 1.0–39.8 |
| Total | 198 | 101 | 97 | |||
M+, malaria positive. M−, malaria negative.
FIG. 1.
Relationship between peripheral and placental parasitemia. Each data point represents the mean percent parasitemias for individual malaria-positive women. Data from women who had placental but not peripheral parasites (n = 28) or who had peripheral but not placental parasites (n = 2) are not shown in the figure.
Assessing the levels of inhibitory antibodies in nonpregnant women.
We have previously shown that adhesion of IRBC to the placental CSPG is saturable at coating concentrations of 100 to 200 ng/ml (1). Therefore, in this study, all assays were performed at a coating concentration of 200 ng of CSPG/ml to ensure maximum adhesion of IRBC. The inhibition of IRBC adhesion was considered to be the measure of the level of antiadhesion antibodies in the plasma. Initially, plasma samples from nulligravidae (n = 7) and nonpregnant multigravidae (n = 11) were screened in the assay. The level of inhibition for nulligravidae and nonpregnant multigravidae was 4.5 ± 4.0%. Therefore, plasma samples producing greater than 12.5% inhibition (i.e., mean plus 2 standard deviations) were considered to be positive for antiadhesion antibodies. Using this criterion, none of the nulligravidae and 1 out of 11 nonpregnant multigravidae had inhibitory antibodies.
Prevalence and levels of antiadhesion antibodies at term.
Plasma samples collected at term from 198 pregnant women (see Table 1) were tested for the presence of inhibitory antibodies. Plasma from 175 women (88.4%) inhibited the binding of IRBC to C4S at a 1:10 dilution (Table 2). Similar results were obtained using 1:20 and 1:40 dilutions of plasma, but the percent inhibition was generally lower. The prevalence of antibodies was essentially the same in all gravidity groups including primigravidae (P = 0.11). These results agree with those reported in previous studies showing that the presence of inhibitory antibodies is pregnancy associated and that most pregnant women, regardless of gravidity, have adhesion-blocking antibodies at the end of the third trimester of pregnancy (9, 17, 29).
TABLE 2.
Proportion of pregnant women who were antiadhesion antibody positive and mean antibody levels at terma
| Gravidae | Total no. of women | % Antibody-positive women | Inhibition of IRBC adhesion to C4S by plasma from antibody-positive women (Mean % inhibition ± SD) |
|---|---|---|---|
| 1 | 47 | 92 | 43.3 ± 20.1 |
| 2 | 32 | 78 | 43.1 ± 22.5 |
| 3 | 48 | 90 | 51.3 ± 21.0 |
| 4 | 36 | 97 | 49.7 ± 26.6 |
| ≥5 | 35 | 86 | 53.9 ± 22.6 |
| Total | 198 | 88.4 | 48.3 ± 22.6 |
Note that women were considered to be antibody positive if their plasma inhibited adhesion of IRBC to C4S by > 12.5%.
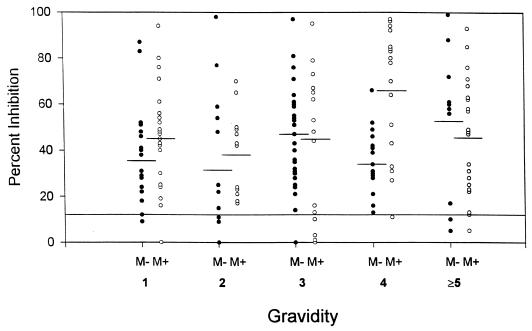
The levels of antiadhesion antibodies at term appeared to increase with gravidae (Table 2), but the increase was minimal. Comparison of levels of inhibitory activity between malaria-positive and malaria-negative women showed that both groups of women had similar levels at term (Fig. 2). Although malaria-positive women in their fourth pregnancies appeared to have higher levels (Fig. 2), this difference was not seen at plasma dilutions of 1:20 and 1:40 (data not shown). Thus, the levels of antiadhesion antibodies present at term did not explain gravidity-dependent protection against malaria (Table 2).
FIG. 2.
Inhibition of binding of IRBC to the placental CSPG by plasma samples from women without (M−) or with (M+) placental malaria at term. Each point represents the mean level of inhibition for individual primigravidae (1), secundigravidae (2), and multigravidae with three or more pregnancies; malaria-negative women (●), and malaria-positive women (○). Results for 1:10 plasma dilutions are shown. Based on results from nonpregnant women, the cut-off value for the presence of inhibitory antibodies was 12.5% (solid continuous horizontal line). Means for M− and M+ women with different gravidities are indicated by a short line.
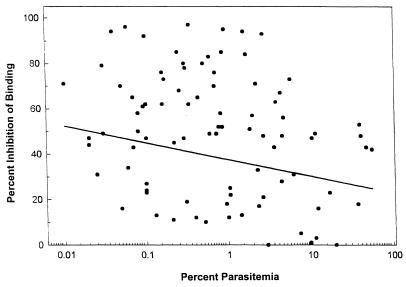
Data from malaria-positive women, however, showed that those with high levels of antiadhesion antibodies generally had low levels of placental parasitemias (Fig. 3), with correlation coefficient values (r2) of −0.37 (P = 0.02) at a 1:10 dilution and r2 = −0.22 (P = 0.04) at a 1:40 dilution. These data suggest that the presence of high titers of inhibitory antibodies helps in reducing placental parasitemias. Therefore, it is likely that women who had high placental parasitemias at the time of delivery were those who either poorly produced antibodies to the parasite adhesion ligand or developed placental infections late in their pregnancy.
FIG. 3.
Linear regression analysis of levels of antiadhesion antibodies and placental parasitemias. Each point represents the mean level of antiadhesion antibodies for individual women who had placental malaria at the time of delivery (n = 97). The cytoadherence assay was performed as described in Materials and Methods. Results using a 1:10 dilution of plasma are shown.
Kinetics of the production of inhibitory antibodies in primigravidae and multigravidae.
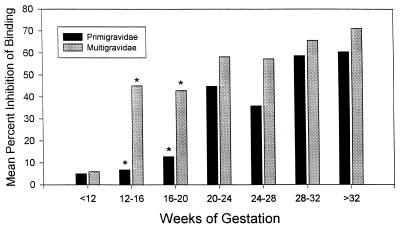
To determine when antibodies are produced during pregnancy, plasma collected longitudinally from pregnant primigravidae and multigravidae were analyzed (Fig. 4). All women lacked inhibitory antibodies during the first trimester of pregnancy (i.e., prior to 12 weeks of gestation). These data correlate with the observation that only 1 out of 18 nonpregnant women had antiadhesion antibodies. Multigravidae produced high levels of antibodies between 12 and 16 weeks of pregnancy. Thereafter, the level of inhibitory antibodies remained high throughout the pregnancy in multigravidae (Fig. 4). On the other hand, primigravidae lacked the antibodies early in pregnancy and did not begin producing significant amounts of antibodies until the fifth month (∼20 weeks) of pregnancy (Fig. 4). Antibody levels were significantly lower in primigravidae between weeks 12 and 20 (i.e., the third and fifth month of pregnancy) than in multigravidae (P < 0.0001) (Fig. 4). After 20 weeks of gestation, however, there was no significant difference in the levels of inhibitory antibodies between primi- and multigravidae (20 to 24 weeks, P = 0.39; 24 to 28 weeks, P = 0.11; 28 to 32 weeks, P = 0.56; and >32 weeks, P = 0.31). The presence of high levels of antibodies in multigravidae during the early stages of pregnancy (12 to 16 weeks) and the lack of antibodies in primigravidae until ∼20 weeks (Fig. 4) likely represent the induction of preexisting memory responses in the former and primary antibody responses in the latter.
FIG. 4.
Gravidity-dependent production of the adhesion inhibition antibodies during pregnancy. The inhibition of the adhesion of IRBC to placental CSPG-coated plates was measured using a 1:10 dilution of plasma as outlined in Materials and Methods. Asterisk, P = 0.0001 (Student's t test, comparing primigravidae and multigravidae).
DISCUSSION
In this study, we show for the first time that the majority of women, regardless of gravidity, lack antiadhesion antibodies during the early period of gestation and that 88% of pregnant women produce antiadhesion antibodies during the second trimester (Table 2; Fig. 4). We also show that the kinetics of the production of antibodies in primigravidae is substantially different from that in multigravidae. Based on these data and the previously observed differential prevalences of P. falciparum infection in primigravidae and multigravidae (4), we suggest that differences in the rate of production of antibodies in these women during the second trimester contribute significantly to their differential susceptibilities to placental malaria.
The presence of antiplacental IRBC adhesion antibodies in primigravidae has been controversial (8, 9, 17, 29). As noted in the introduction, while one study reported the absence of antibodies in primigravidae (8), other studies have shown that primigravidae do produce antiadhesion antibodies (9, 17, 29). All studies have found increased levels of antibodies with increasing gravidities. The results presented here, however, show that 92% of primigravidae have antiadhesion antibodies at term and that primigravidae have antibody levels almost similar to those found in multigravidae at term (Table 2 and Fig. 2), suggesting that antibody levels alone do not explain the phenomenon.
The antiadhesion antibodies are produced in pregnant women, mainly during the second trimester, presumably in response to the presence of placental infection by C4S-adherent parasites. This conclusion is based on the lack of or very low levels of antiadhesion antibodies in primigravidae and multigravidae until ∼20 and ∼12 weeks of gestation, respectively. These data, together with the observation that many pregnant women in the study population, regardless of gravidity, have significantly high levels of anti-placental IRBC adhesion antibodies at term, establish that antibodies are produced during pregnancy. Our data further show that by the time of the next pregnancy, which is usually about 2 years in this study group, the antibodies are cleared from the circulation. Although the kinetics of antibody decay after delivery remains to be established, the results of a recent study suggest that antibodies persist, presumably at low levels, for at least 6 months after delivery (17).
The anti-placental IRBC adhesion antibodies are differentially produced in Cameroonian primigravidae and multigravidae during gestation. As shown in Fig. 4, multigravidae developed rapid and high levels of antibodies beginning at ∼12 weeks of gestation, whereas the primigravidae did not produce significant levels of antibodies until the 20th week (Fig. 4). Interestingly, the period of onset of antibody response in both groups of women correlates with the peak prevalence of P. falciparum infection in these women (i.e., weeks 16 to 19). It is likely that toward the end of the first or the beginning of the second trimester (12 to 16 weeks), IRBC begin to sequester in the placenta and trigger the antiadhesion antibody response. Thus, our results offer an explanation for the gravidity-associated differential risks of women for placental malaria. Based on our data, it seems logical that because of preexisting memory acquired during previous pregnancies, multigravidae are able to efficiently produce antiadhesion antibodies in response to the sequestration of C4S-adherent IRBC. The rapid production of sufficient levels of antibodies during the early part of the second trimester in multigravidae prevents heavy accumulation of IRBC in the placenta, limiting the risk of severe malaria. In contrast, primigravidae produce antibodies beginning at ∼20 weeks, and the production of sufficient levels of antibodies for effective blocking of IRBC adherence is likely to require a period of ∼8 weeks. Thus, the presence of very low levels of antibodies in primigravidae during the early part of the second trimester, a period when the placenta undergoes rapid development, could lead to a heavy accumulation of IRBC in the placenta that affects the health of the fetus and the mother.
The results from this study help explain changes in the prevalence of P. falciparum infection during the course of pregnancy in western Kenyan women (4). In this area of holoendemicity, the peak prevalence of P. falciparum infection in both primigravidae and multigravidae is reported to occur at 13 to 16 weeks of pregnancy (4). Thereafter, however, the prevalence of parasites rapidly declines in multigravidae but persists until around 20 weeks in primigravidae. The rate of recovery during the third trimester is similar in the two groups (4). These results imply that pregnant women, regardless of gravidity, are at risk of placental infection during the early part of pregnancy. The data taken together, however, argue that the peak prevalence of parasite infections during the second trimester in primigravidae occurs when the women lack antiadhesion antibodies. Similarly, the rapid recovery from peak prevalence of infection during the second trimester in multigravidae occurs immediately after the initiation of an efficient antibody response due to preexisting memory. Thus, data from Cameroon support our conclusion that antiadhesion antibodies play a significant role in the reduction of placental parasitemia during pregnancy.
ACKNOWLEDGMENTS
This study was supported by grants AI45086 from NIAID, NIH, the New Initiative in Malaria Award from the Burroughs Wellcome Fund (D.C.G.), and AI35839 (sample collection) and AI43888 (sample analysis) (D.W.T.).
I.O.-D., R.N.A., and S.T.A-E. contributed equally to this study.
We thank the Yaounde Malaria Research Team, especially J. Fogako and S. Metenou for sample collection and parasite analysis; Manonmani Venkatesan and Vijaykumar Matam for culturing parasites; and all of the women in Yaounde who participated in the study.
REFERENCES
- 1.Achur R N, Valiyaveettil M, Alkhalil A, Ockenhouse C F, Gowda D C. Characterization of proteoglycans of human placenta and identification of unique chondroitin sulfate proteoglycans of the intervillous spaces that mediate the adherence of Plasmodium falciparum-infected erythrocytes to the placenta. J Biol Chem. 2000;275:40344–40356. doi: 10.1074/jbc.M006398200. [DOI] [PubMed] [Google Scholar]
- 2.Alkhalil A, Achur R N, Valiyaveettil M, Ockenhouse C F, Gowda D C. Structural requirements for the adherence of Plasmodium falciparum-infected erythrocytes to chondroitin sulfate proteoglycans of human placenta. J Biol Chem. 2000;275:40357–40364. doi: 10.1074/jbc.M006399200. [DOI] [PubMed] [Google Scholar]
- 3.Baird J K. Host age as a determinant of naturally acquired immunity to Plasmodium falciparum malaria. Parasitol Today. 1995;11:105–111. doi: 10.1016/0169-4758(95)80167-7. [DOI] [PubMed] [Google Scholar]
- 4.Brabin B J. An analysis of malaria in pregnancy in Africa. Bull W H O. 1983;61:1005–1016. [PMC free article] [PubMed] [Google Scholar]
- 5.Fried M, Duffy P E. Adherence of Plasmodium falciparum to chondroitin sulfate A in the human placenta. Science. 1996;272:1502–1504. doi: 10.1126/science.272.5267.1502. [DOI] [PubMed] [Google Scholar]
- 6.Fried M, Duffy P E. Maternal malaria and parasite adhesion. J Mol Med. 1998;76:162–171. doi: 10.1007/s001090050205. [DOI] [PubMed] [Google Scholar]
- 7.Fried M, Muga R O, Misore A O, Duffy P E. Malaria elicits type 1 cytokines in the human placenta: IFN-gamma and TNF-alpha associated with pregnancy outcomes. J Immunol. 1998;160:2523–2530. [PubMed] [Google Scholar]
- 8.Fried M, Nosten F, Brockman A, Brabin B J, Duffy P E. Maternal antibodies block malaria. Nature. 1998;395:851–852. doi: 10.1038/27570. [DOI] [PubMed] [Google Scholar]
- 9.Gysin J, Pouvelle B, Fievet N, Scherf A, Lepolard C. Ex vivo desequestration of Plasmodium falciparum-infected erythrocytes from human placenta by chondroitin sulfate A. Infect Immun. 1999;67:6596–6602. doi: 10.1128/iai.67.12.6596-6602.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hommel M. Amplification of cytoadherence in cerebral malaria: towards a more rational explanation of disease. Ann Trop Med Parasitol. 1993;87:627–635. doi: 10.1080/00034983.1993.11812821. [DOI] [PubMed] [Google Scholar]
- 11.Ismail M R, Ordi J, Menendez C, Ventura P J, Aponte J J, Kahigwa E, Hirt R, Cardesa A, Alonso P L. Placental pathology in malaria: a histological, immunohistochemical, and quantitative study. Hum Pathol. 2000;31:85–93. doi: 10.1016/s0046-8177(00)80203-8. [DOI] [PubMed] [Google Scholar]
- 12.Jensen J B. Concentration from continuous culture of erythrocytes infected with trophozoites and schizonts of Plasmodium falciparum. Am J Trop Med Hyg. 1978;27:1274–1276. doi: 10.4269/ajtmh.1978.27.1274. [DOI] [PubMed] [Google Scholar]
- 13.Kirkwood B R. Essentials of medical statistics. Cambridge, Mass: Blackwell Science Ltd.; 1996. pp. 1–234. [Google Scholar]
- 14.Lambros C, Vanderberg J P. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol. 1979;65:418–420. [PubMed] [Google Scholar]
- 15.MacPherson G G, Warrell M J, White N J, Looreesuwan S, Warrell D A. Human cerebral malaria. A quantitative ultrastructural analysis of parasitized erythrocyte sequestration. Am J Pathol. 1985;119:385–401. [PMC free article] [PubMed] [Google Scholar]
- 16.Manga L, Robert V, Messi J, Despontaine M, Carnevalle P. Le plaudisme urbain à Yaoundé, Cameroun 1. Etude entomologigue dans deux quartiers centraux. Mém Soc R Belge Entomol. 1992;35:155–162. [Google Scholar]
- 17.Maubert B, Fievet N, Tami G, Cot M, Boudin C, Deloron P. Development of antibodies against chondroitin sulfate A-adherent Plasmodium falciparum in pregnant women. Infect Immun. 1999;67:5367–5371. doi: 10.1128/iai.67.10.5367-5371.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maubert B, Fievet N, Tami G, Boudin C, Deloron P. Cytoadherence of Plasmodium falciparum-infected erythrocytes in the human placenta. Parasite Immunol. 2000;22:191–199. doi: 10.1046/j.1365-3024.2000.00292.x. [DOI] [PubMed] [Google Scholar]
- 19.Menendez C. Malaria during pregnancy: a priority area of malaria research and control. Parasitol Today. 1995;11:178–183. doi: 10.1016/0169-4758(95)80151-0. [DOI] [PubMed] [Google Scholar]
- 20.Menendez C, Ordi J, Ismail M R, Ventura P J, Aponte J J, Kahigwa E, Font F, Alonso P L. The impact of placental malaria on gestational age and birth weight. J Infect Dis. 2000;181:1740–1745. doi: 10.1086/315449. [DOI] [PubMed] [Google Scholar]
- 21.Miller L H, Good M F, Milon G. Malaria pathogenesis. Science. 1994;264:1878–1883. doi: 10.1126/science.8009217. [DOI] [PubMed] [Google Scholar]
- 22.Miller L H, Smith J D. Motherhood and malaria. Nat Med. 1998;4:1244–1245. doi: 10.1038/3223. [DOI] [PubMed] [Google Scholar]
- 23.Newbold C I, Craig A G, Kyes S, Berendt A R, Snow R W, Peshu N, Marsh K. PfEMP-1, polymorphism and pathogenesis. Ann Trop Med Parasitol. 1997;91:551–557. doi: 10.1080/00034989760923. [DOI] [PubMed] [Google Scholar]
- 24.Ordi J, Menendez C, Ismail M R, Ventura P J, Palacin A, Kahigwa E, Ferrer B, Cardesa A, Alonso P L. Placental malaria is associated with cell-mediated inflammatory responses with selective absence of natural killer cells. J Infect Dis. 2001;183:1100–1107. doi: 10.1086/319295. [DOI] [PubMed] [Google Scholar]
- 25.Pasloske B L, Howard R J. Malaria, the red cell, and the endothelium. Annu Rev Med. 1994;45:283–295. doi: 10.1146/annurev.med.45.1.283. [DOI] [PubMed] [Google Scholar]
- 26.Pongponratn E, Riganti M, Punpoowong B, Aikawa M. Microvascular sequestration of parasitized erythrocytes in human falciparum malaria: a pathological study. Am J Trop Med Hyg. 1991;44:168–175. doi: 10.4269/ajtmh.1991.44.168. [DOI] [PubMed] [Google Scholar]
- 27.Pouvelle B, Meyer P, Robert C, Bardel L, Gysin J. Chondroitin-4-sulfate impairs in vitro and in vivo cytoadherence of Plasmodium falciparum-infected erythrocytes. Mol Med. 1997;3:508–518. [PMC free article] [PubMed] [Google Scholar]
- 28.Pouvelle B T, Lepolard C, Gysin J. Biological and biochemical characteristics of cytoadhesion of Plasmodium falciparum-infected erythrocytes to chondroitin-4-sulfate. Infect Immun. 1998;66:4950–4956. doi: 10.1128/iai.66.10.4950-4956.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ricke C H, Staalsoe T, Koram K, Akanmori B D, Riley E M, Theander T G, Hviid L. Plasma antibodies from malaria-exposed pregnant women recognize variant surface antigens on Plasmodium falciparum-infected erythrocytes in a parity-dependent manner and block parasite adhesion to chondroitin sulfate A. J Immunol. 2000;165:3309–3316. doi: 10.4049/jimmunol.165.6.3309. [DOI] [PubMed] [Google Scholar]
- 30.Riley E M, Hviid L, Theander T G. Malaria. In: Kierszenbaum F, editor. Parasite infections and the immune system. New York, N.Y: Academic Press; 1994. pp. 119–143. [Google Scholar]
- 31.Rogerson S J, Brown G V. Chondroitin sulfate A as an adherence receptor for P. falciparum-infected erythrocytes. Parasitol Today. 1997;134:70–75. doi: 10.1016/s0169-4758(96)10081-8. [DOI] [PubMed] [Google Scholar]