Abstract
The receptor activator of nuclear factor-kappa B ligand (RANKL) induces osteoclastogenesis by induction of Ca2+ oscillation, calcineurin activation and translocation into the nucleus of nuclear factor of activated T cells type c1 (NFATc1). Homer proteins are scaffold proteins. They regulate Ca2+ signaling by modulating the activity of multiple Ca2+ signaling proteins. Homers 2 and 3, but not Homer1, also independently affect the interaction between NFATc1 and calcineurin. However, to date, whether and how the Homers are involved in osteoclastogenesis remains unknown. In the present study, we investigated Homer2 and Homer3 roles in Ca2+ signaling and NFATc1 function during osteoclast differentiation. Deletion of Homer2/Homer3 (Homer2/3) markedly decreased the bone density of the tibia, resulting in bone erosion. RANKL-induced osteoclast differentiation is greatly facilitated in Homer2/3 DKO bone marrow-derived monocytes/macrophages (BMMs) due to increased NFATc1 expression and nuclear translocation. However, these findings did not alter RANKL-induced Ca2+ oscillations. Of note, RANKL treatment inhibited Homer proteins interaction with NFATc1, but it was restored by cyclosporine A treatment to inhibit calcineurin. Finally, RANKL treatment of Homer2/3 DKO BMMs significantly increased the formation of multinucleated cells. These findings suggest a novel potent mode of bone homeostasis regulation through osteoclasts differentiation. Specifically, we found that Homer2 and Homer3 regulate NFATc1 function through its interaction with calcineurin to regulate RANKL-induced osteoclastogenesis and bone metabolism.
Keywords: RANKL, osteoclast differentiation, scaffold protein, osteoporosis, NFATc1
Introduction
Bone is a dynamic organ. It is constantly renewing through a bone remodeling process, mediated by osteoblastic bone formation and osteoclastic bone resorption. In numerous bone diseases, an imbalance in this process can be found. Examples are autoimmune arthritis, osteoporosis, periodontitis, osteopetrosis and bone tumors (Takayanagi 2007, Zaidi 2007). Multinucleated mature osteoclasts originate from bone marrow-derived monocytes/macrophages (BMMs) through two essential factors. One is receptor activator of nuclear factor-kappa B ligand (RANKL) secreted by osteoblasts. The other is macrophage-colony-stimulating factor (M-CSF) (Takayanagi 2007). RANK receptor’s activation by RANKL leads to oscillations in intracellular Ca2+ concentration ([Ca2+]i). The latter is linked to the activation of Gq. In turn, Gq generates inositol 1,4,5-triphosphate (IP3) to activate the IP3 receptors (IP3Rs), Ca2+ release from the ER and activation of Ca2+ influx from the extracellular space by the Orai channels and transient receptor potential (TRP) channels. Ultimately, as previously reported, this generates an increase in intracellular reactive oxygen species (Yang et al. 2009, 2013, Kim et al. 2010, Son et al. 2012, Park et al. 2013). Subsequently, the RANKL-mediated [Ca2+]i oscillations increase NFATc1’s nuclear translocation, multinucleated cells’ formation and bone resorption (Takayanagi et al. 2002, Yang et al. 2009, Kim et al. 2010).
Members of the NFAT family of transcription factors play a central role in immune responses and in the development of cardiac and skeletal muscles, bone and the nervous system. In these tissues, the NFAT family members are expressed in most cells. The NFAT family consists of five members (NFATc1-5). NFATc1 through NFATc4 are regulated by Ca2+ signaling. Specifically, Ca2+ activates calcineurin by calmodulin. This dephosphorylates NFAT to expose its nuclear import signal (Olson & Williams 2000, Crabtree & Olson 2002, Hogan et al. 2003, Takayanagi 2007).
Ca2+ is a universal intracellular messenger that is involved in various cellular functions and processes, such as cell proliferation, differentiation and apoptosis (Bootman et al. 2001, Berridge et al. 2003). Disruption of Ca2+ signaling is one mechanism by which RANKL function, NFATc1 activation and the associated bone metabolism can be altered. An additional less explored mechanism NFAT function’s regulation is through their interaction with adapter proteins. As for example SH3 domain-binding protein 2 (3BP2), NFAT-interacting protein (NIP45) and most prominently the Homer proteins (Takayanagi et al. 2002, Foucault et al. 2005, Huang et al. 2008, GuezGuez et al. 2010, Shanmugarajan et al. 2012). The Homer family of scaffolding proteins consists of Homer1, Homer2 and Homer3 and several splice variants. The short Homer1a’s gene coding was identified as an immediate early gene transcriptionally upregulated by synaptic activity in neuronal cells (Brakeman et al. 1997, Xiao et al. 1998, Bottai et al. 2002). Homer1a predominantly consists of the N-terminal Ena/VASP homology 1 (EVH) protein-binding domain. The remaining Homer proteins (i.e., Homer1b/c, Homer2 and Homer3, referred to as ‘long Homers’) are composed of an EVH domain and a C-terminus. The latter includes a coiled-coil multimerization domain and leucine zipper (Tu et al. 1998, Worley et al. 2007). Of note, the EVH domain binds to several G protein coupled-receptors (GPCRs). Specifically, these are as follows: canonical TRP (TRPC) channels, IP3Rs, ryanodine receptors (RyRs) and the Shank family of scaffold proteins (Tu et al. 1998, 1999, Xiao et al. 1998, Feng et al. 2002, Shin et al. 2003, Yuan et al. 2003). Homer proteins were previously identified as synaptic proteins regulating synaptic activity in neurons. However, earlier studies have reported on the importance of the Homer family in other tissues (Brakeman et al. 1997, Tu et al. 1998, 1999, Xiao et al. 1998). In previous studies, it has been shown that Homer2 modulates the intensity of the GPCRs stimulus. Of note, this is done by regulating the GAP activity of RGS proteins and of PLCβ in pancreas acinar cells (Shin et al. 2003). In the skeletal muscle, Homer2 enhances the NFATc1-dependent signaling pathway by increasing the release of RyR-dependent Ca2+ during myogenic differentiation (Stiber et al. 2005). It has been demonstrated that Homer3’s deletion induces severe autoimmune phenotypes in the lung and liver. Additionally, Homer3’s phosphorylation by the Ca2+/calmodulin-dependent protein kinase (CaMK) II regulates Purkinje cells synaptic activity (Huang et al. 2008, Mizutani et al. 2008). Of note, Homer2 and Homer3 have been shown to regulate T cell activation through the interaction with NFATc2. Specifically, this is done by competing with calcineurin to bind with NFATc2 (Huang et al. 2008). Additionally, Homer2 identified a co-localization with NFATc1 at the neuromuscular junction. The latter is part of the calcineurin-NFATc1 signaling pathway (Salanova et al. 2011). However, to date, there is a poor understanding of the characteristics of the RANKL-induced NFATc1 signaling pathway during osteoclastogenesis and whether and how it is regulated by Homer proteins.
In the present study, we investigated the role of Homer proteins in the RANKL-induced NFATc1 signaling pathway, osteoclast differentiation and bone metabolism with the use of Homer2 and Homer3 (Homer2/3) double-knockout (DKO) mice.
Materials and methods
Cell culture and reagents
The Homer2/3 DKO mice were generously provided by Dr Paul F Worley (Johns Hopkins University School of Medicine, Baltimore, MD) (Huang et al. 2008) and all animal care and experimental procedures complied with institutional guidelines and were approved by the Institutional Animal Care and Use Committee (IACUC) in Yonsei University (IACUC approval no. 2014-0067). Primary cultured bone marrow-derived monocytes (BMMs) were maintained in Dulbecco’s modified Eagle’s medium (DMEM, Invitrogen) and α-minimum essential medium (α-MEM) supplemented with 10% fetal bovine serum (FBS, Invitrogen) and incubated in 5% CO2. To maintain BMMs, α-MEM was supplemented with 30 ng/mL M-CSF. Receptor activator of RANKL and M-CSF were purchased from KOMA Biotech (Seoul, Korea). Fura-2/AM was purchased from Teflabs (Austin, TX, USA). Pluronic F-127 was obtained from Molecular Probes. Anti-Homer2, anti-Homer3 and anti-NFATc1 antibodies were obtained from Santa Cruz Biotechnology. Anti-actin antibodies were purchased from Sigma-Aldrich.
Preparation of BMMs
The femur and tibia were isolated from 4~6-week-old mice as described previously (Kim et al. 2010). All cells derived from bone marrow of femur and tibia were collected and cultured in α-MEM medium containing 10% FBS and 30 ng/mL M-CSF. The following day, non-adherent cells in media were collected and seeded on adequate plates and treated with M-CSF (30 ng/mL). After 2 days nonadherent cells were washed out and adherent cells were used as BMMs.
Analysis of bone density and skeletal morphology
Tibias and femora were isolated from 6-week-old WT and Homer2/3 DKO mice. To exclude differences caused by threshold resolution set-up, samples from WT and Homer2/3 DKO were set in one soft X-ray apparatus. Bone density was measured with a 3DμCT (SkyScan-1076 high resolution in vivo μCT system; SkyScan, Aartselaar, Belgium) and analyzed with CTAn and Cone beam reconstruction software. Histological examination was performed as described elsewhere (Mayingi et al. 2007). Dynamic histomorphometric method was as described before (Kim et al. 2010). Briefly, 4-week-old male mice were injected twice with calcein (15 mg/kg intraperitoneally) 2 days apart. The animals were killed on day 4, and undecalcified bones were embedded in methylmethacrylate. Longitudinal sections (10 μm thickness) of the tibias were prepared, and new bone formation was assessed by confocal microscopy (model LSM 510; Carl Zeiss) by capturing images of calcein green.
[Ca2+]i measurement
The cells were seeded on cover glass in a 35 mm dishes (5 × 104 cells/coverslip) and stimulated with RANKL (50 ng/mL) for the indicated times. The cells were loaded with 5 μM Fura-2/AM and 0.05% Pluronic F-127 for 30 min in physiological salt solution (140 mM NaCl, 5 mM KCl, 1 mM MgCl2, 1 mM CaCl2, 10 mM HEPES, 10 mM glucose, 310 mosmol, pH 7.4). Fura-2 fluorescence intensity was measured using excitation wavelengths of 340 and 380 nm, and emitted fluorescence 510 nm (ratio = F340/F380) was collected and monitored at 2 s intervals using a CCD camera (Universal Imaging Co., Downingtown, PA, USA) as described previously (Yang et al. 2009). Images were digitized and analyzed by MetaFlour software (Universal Imaging).
Western blot
Whole-cell lysates were prepared using RIPA lysis buffer (20 mM Tris, pH 7.4, 250 mM NaCl, 2 mM EDTA, pH 8.0, 0.1% Triton X-100, 0.01 mg/mL aprotinin, 5 μg/mL leupeptin, 0.4 mM PMSF and 4 mM NaVO4) and spun at 13,000 g for 10 min to remove insoluble material. Proteins (50~100 μg/well) were subjected to 6~8% SDS-PAGE and were separated by size. Proteins were electro-transferred to a nitrocellulose membrane, blocked with 6% skimmed milk and probed with Abs against NFATc1 (1:1000) and actin (1:2000). Thereafter, blots were washed, exposed to horseradish peroxidase-conjugated secondary antibodies for 1 h and finally detected by chemiluminescence (Amersham Pharmacia Biotech).
Immunoprecipitation
The immunoprecipitation was as before (Shin et al. 2003, Yang et al. 2014) with several modifications. In brief, microsomes were prepared from BMMs harvested from WT mice in a buffer containing 20 mM Mops (pH 6.7 with KOH), 250 mM sucrose, 1 mM EDTA, 1 mM MgCl2, 10 mM benzamidine and 0.2 mM PMSF. The homogenates were centrifuged at 400 g for 10 min and the supernatants were collected and re-centrifuged at 900 g for 10 min at 4°C. Microsomes were lysed in a buffer containing 50 mM Tris (pH 6.8 with HCl), 150 mM NaCl, 3 mM EDTA, 2 mM EGTA and 0.5% Triton X-100 supplemented with protease inhibitors. The lysates were cleared by centrifugation at 14,000 g for 15 min. About 150 μL of the extract (300 μg of protein/sample) was incubated for 10 days in a buffer containing 50 mM Tris (pH 6.8 with HCl), 150 mM NaCl, 3 mM EDTA, 2 mM EGTA. Protein A/G-agarose (Thermo Fisher Scientific) was added to each mixture, and rocking was continued overnight at 4°C. Protein A/G-agarose was pelleted at 1000 g for 10 s and the beads were quickly washed with cold phosphate buffered saline (PBS). The immunoprecipitated proteins were separated by SDS-PAGE and probed with anti-Homer3 (1:1000) by overnight incubation at 4°C. To avoid protein degradation by digestive enzymes, immunoprecipitation was initiated immediately after completion of microsomal preparation.
Immunocytochemistry
Cells were seeded on coverslips (12 mm) and treated for 4 days with 50 ng/mL RANKL. After fixation in 4% paraformaldehyde (PFA) for 5 min, cells were sequentially incubated in blocking solution (0.1% gelatin, 1% BSA, 0.01% sodium azide, 5% goat serum) for 1 h, overnight in blocking solution containing Abs against NFATc1 (1:100), and finally were treated with Alexa 488-labeled anti-mouse IgG antibody (Molecular Probes) in blocking solution for 1 h. Nuclei was separately stained with TO-PRO3. F-actin was visualized with Alexa Fluor 488-Phalloidin.
TRAP stain assay
BMMs were seeded in 96-well plates at a density of 3 × 105 cells per well and pretreated with the indicated compounds. Cells were then stimulated with 50 ng/mL RANKL. After 4 days, a TRAP stain assay was performed to evaluate the cell differentiation rate. TRAP staining was performed as described previously (Yang et al. 2009). Cells were fixed with 10% PFA and with ethanol/acetone (1:1) solution. 100 μL of TRAP staining solution were added to each well and stained for 5 min. TRAP-positive multinucleated cells (containing ≥3 nuclei) were then counted.
Measurement of bone resorption rate (pit assay)
Collected BMMs were seeded on Osteo assay plates (Corning) coated with a proprietary hydroxyapatite mineral surface. Following stimulation (50 ng/mL M-CSF and sRANKL) of the BMMs for 6 days, the plates were washed with sodium hypochlorite solution for 1h at room temperature. Pits formed on the surface of the hydroxyapatite mineral were imaged and calculated using MetaMorph software (Molecular Devices).
Alkaline phosphatase activity and Alizarin red S staining
After bone marrow stromal cells (BMSCs) were transduced, they were seeded into 48-well plates at a density of 5 × 105 cells per well. After osteogenic induction for indicated days, cells were rinsed two times with PBS. ALP and ARS (both from Sigma-Aldrich) were added into cells for the staining during 30 min. Staining agent was discarded and cells were rinsed 4–5 times with water and air-dried. Images of each sample were acquired using a CCD camera. To quantify matrix mineralization, cells were incubated in 100 mM cetylpyridinium chloride for ARS and alkaline phosphatase yellow for ALP. The concentration of ARS was measured using a microplate spectrophotometer at a 562 nm wavelength and ALP activity was measured at the wavelength of 405 nm.
Statistical analysis
All data were expressed as the mean±s.e.m. Statistical significance was determined by using a paired Student’s t-test. Statistical significance was set at P < 0.05 level.
Results
Decreased bone density in Homer2/3 DKO mice
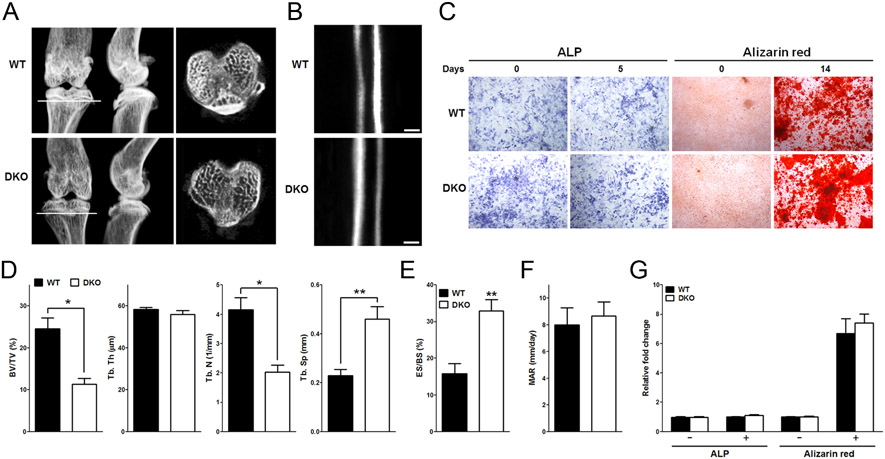
Homer proteins have been shown to be regulators of Ca2+ signaling pathways and direct regulators of NFAT activation in T cells (Huang et al. 2008). In the present study, we examined whether the deletion of Homer proteins affects bone metabolism through bone remodeling. The tibias of WT and Homer2/3 DKO mice were dissected. Subsequently, we evaluated bone density by microradiographic and histological analyses. The tibias’ bone density (bone volume/total volume, BV/TV) and the trabecular bone’s thickness from Homer2/3 DKO mice were significantly reduced (~44.5 and ~38.5%, respectively) vs WT (Fig. 1A). Histologically, Homer2/3 DKO mice exhibited an extensive resorption of trabecular bone below the growth plate and epiphysis of the tibia. Additionally, Homer2/3 DKO mice had a significantly increased erosion surface on bone by ~1.08-fold (eroded surface/bone surface, ES/BS) (Fig. 1D).
Figure 1.
Decreased bone density in Homer2/3 DKO mice. (A) Microradiographic analyses of tibias in WT and Homer2/3 DKO mice. Note that in Homer2/3 DKO tibias, trabecular and cortical bone mass decreased (6 weeks). (B) Representative calcein-labeled sections of proximal tibias in WT and Homer2/3 DKO mice (scale bar, 20 μm). (C) Alkaline phosphatase (ALP) Alizarin red S (ARS) staining (n = 4). (D) μCT data of proximal femur obtained from WT and Homer2/3 DKO mice. Each parameter, including BV/TV, Tb. Th, Tb. N and Tb. Sp were calculated and presented. (E) Histomorphometric parameters. The percentage of eroded surface (ES) on bone surface (BS) increased in Homer2/3 DKO mice (left, n = 3). (F) We observed no clear difference in mineral apposition rate (MAR) between Homer2/3 DKO and WT mice (middle, n = 7). (G) Following ALP activity and ARS staining, we found no difference in osteoblastogenesis between WT and DKO mice (right, n = 4). Data were normalized to bone volume in WT mice and expressed as means ± s.e.m. *P < 0.05, **P < 0.01 compared with WT.
Next, we aimed at analyzing the impact of Homer2/3 deletion on bone formation rates. To this end, we performed dynamic histomorphometric measurements on 4-week-old mice double-labeled with sequential doses of calcein. We did not observe a clear difference in bone-formation rate and mineral apposition rate (MAR) between Homer2/3 DKO and WT mice (Fig. 1B and E). Of note, the results for osteoblastogenesis by alkaline phosphatase (ALP) activity and alizarin red S (ARS) staining showed no difference between DKO and WT mice (Fig. 1C and F).
Homer2/3 deletion does not affect RANKL-induced [Ca2+]i oscillation
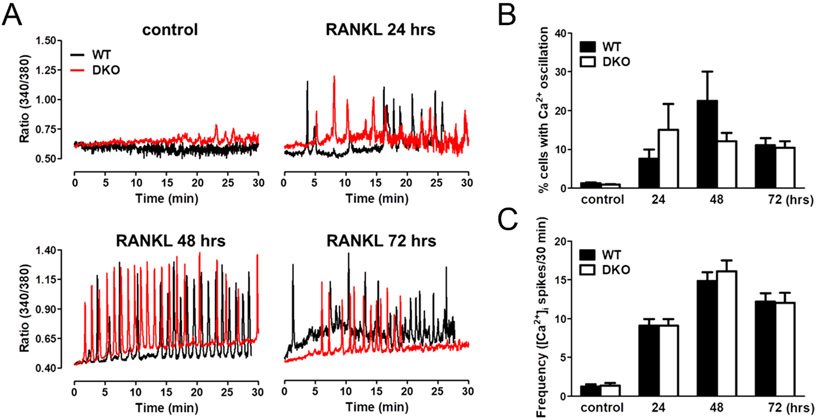
It has been previously shown that RANKL-induced [Ca2+]i oscillations are essential for osteoclast differentiation (Takayanagi et al. 2002). As a consequence, we analyzed Ca2+ signaling in RANKL-treated BMMs obtained from WT and Homer2/3 DKO mice. We initiated [Ca2+]i oscillations after 24 h of RANKL treatment in both WT and Homer2/3 DKO BMMs (Fig. 2A and B). We observed that the number of responding cells and the frequency of [Ca2+]i oscillations were not altered by the deletion of Homer2/3 (Fig. 2B and C).
Figure 2.
RANKL-induced [Ca2+]i oscillations in WT and Homer2/3 DKO BMMs. (A) Oscillatory change in [Ca2+]i in WT and Homer2/3 DKO BMMs before and after RANKL treatment. (B) Quantitative analysis of the number of cells with [Ca2+]i oscillation induced by RANKL in WT and Homer2/3 DKO BMMs (n = 3). (C) Oscillation frequency at 24, 48 and 72 h. Data are expressed as mean ± s.e.m.
Key role of Homer2/3 proteins in RANKL-induced NFATc1 expression and osteoclastogenesis
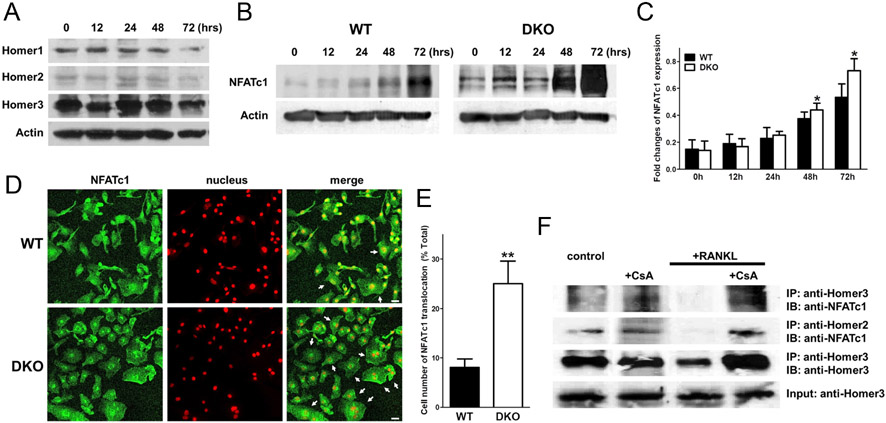
It has been previously shown that Homer2 and Homer3 interfere with the interaction of NFAT with calcineurin (Huang et al. 2008, Salanova et al. 2011). Therefore, we examined the effects of Homer2/3 proteins on NFATc1 expression and translocation into the nucleus in BMMs after RANKL treatment. We observed that the expression of Homer proteins was not affected by RANKL’s treatment of WT BMMs (Fig. 3A). RANKL induced a transient and moderate increase in NFATc1 protein levels after 12 h of treatment. Subsequently, it caused a sustained upregulation, which persisted for several days in WT and Homer2/3 DKO BMMs (Fig. 3B). The RANKL-induced upregulation of NFATc1 in Homer2/3 DKO cells was significantly higher after 48 and 72 h vs WT BMMs (Fig. 3C). Of note, we found that NFATc1 translocation into the nucleus was markedly increased in Homer2/3 DKO BMMs (Fig. 3D and E).
Figure 3.
RANKL-induced NFATc1 expression and translocation depend on the interaction of Homer proteins with NFATc1. (A) Expression levels of Homer proteins (45 kDa) and β-actin (42 kDa) in WT BMMs after RANKL stimulation. (B) Expression level of NFATc1 (approximately 90~110 kDa) and β-actin in WT and Homer2/3 DKO BMMs following RANKL stimulation. (C) Mean NFATc1 expression levels in WT and Homer2/3 DKO BMMs. We analyzed by densitometry the expression levels in experiments similar to those in B. Specifically, we observed that NFATc1’s expression levels between 48 and 72 h were significantly increased in Homer2/3 DKO cells (n = 6). Data were normalized to expression level in cells from WT mice and expressed as the mean ± s.e.m. (D) NFATc1 protein’s immunostaining in WT and Homer2/3 DKO BMMs post 72 h RANKL stimulation. Of note, NFATc1’s nuclear translocation was significantly higher in Homer2/3 DKO vs WT BMMs. Arrows indicate nuclear localized NFATc1. TO-PRO3 counterstaining was performed on the nuclei (scale bar, 20 μm). (E) Quantification of cell number with nuclear NFATc1. Homer2/3 DKO cells had a significant increase in NFATc1 translocation (n = 7). (F) Homer proteins and NFATc1 co-immunoprecipitation in cell lysates prepared from WT BMMs before and after 24-h RANKL stimulation. A reduced interaction of NFATc1 with Homer3 and Homer2 was found following RANKL stimulation. As a control, inhibition of calcineurin with cyclosporine A (CsA, 0.01 μg/mL) to inhibit NFATc1 dephosphorylation increased NFATc1’s interaction with Homer3 and Homer2 and reversed RANKL stimulation’s effect. *P < 0.05 and **P < 0.01 compared with WT.
Earlier studies have reported that T cells and skeletal muscle Homer proteins interact with and sequester NFAT in the cytosol (Huang et al. 2008, Salanova et al. 2011). We aimed at examining the interaction of Homer proteins with NFATc1 in BMMs and determining if it is affected by the induction of osteoclastogenesis. As a consequence, we examined both whether Homer proteins selectively bind NFATc1 and RANKL stimulation affects the interaction in WT and Homer2/3 DKO BMMs. Figure 3F shows the co-immunoprecipitation with NFATc1 of Homer2 and Homer3 prior to RANKL stimulation. Importantly, RANKL stimulation significantly reduced the interaction aimed at dissociating the NFATc1/Homers complexes. Of note, following treatment with the specific calcineurin inhibitor cyclosporine A, Homer proteins and NFATc1’s interaction were restored and complex formation was enhanced.
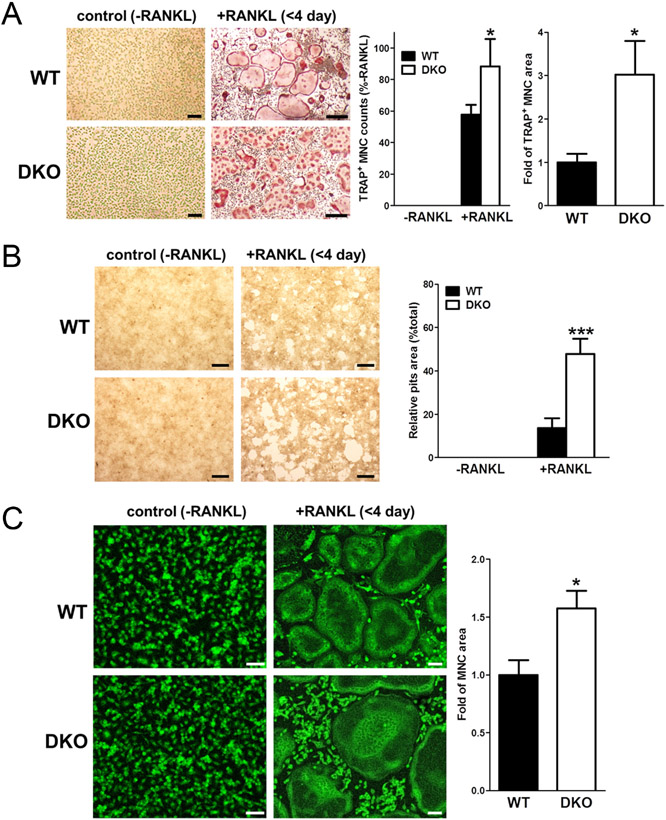
Next, we aimed at confirming the role of Homer proteins in RANKL-mediated osteoclasts differentiation. To this end, we used TRAP staining with the goal of determining the difference between the morphology of differentiated Homer2/3 DKO osteoclasts and that of WT cells. We found that a 4-day RANKL stimulation increased the formation of TRAP+ MNCs such that the increase was higher in cells from Homer2/3 DKO. Additionally, the morphological features characteristic of osteoclast differentiation (i.e., the number of nuclei and area of MNCs) were enhanced in the RANKL-induced Homer2/3 DKO BMMs vs WT BMMs (Fig. 4A). We then examined Homer2/3’s effect on osteoclast function by performing pit formation and actin ring formation assays. Osteoclasts were generated on a bone resorption assay plate. We observed that the percentage of bone resorption area increased in the RANKL-induced Homer2/3 DKO cells. Additionally, the actin staining results demonstrated that area of MNCs is higher in Homer2/3 DKO cells (Fig. 4B and C).
Figure 4.
RANKL-induced osteoclastogenesis is enhanced in Homer2/3 DKO BMMs. (A) RANKL-induced osteoclast differentiation in vitro in WT and Homer2/3 DKO BMMs (TRAP staining; scale bar, 50 μm). Columns show quantitative analysis of the number (middle) and the area (right) of TRAP-positive (TRAP+) multinucleated cells (MNCs; ≥3 nuclei) in WT and Homer2/3 DKO mice. The number and area of TRAP+ MNCs are significantly higher in Homer2/3 DKO mice (n = 4). (B) Pits area during osteoclastogenesis is higher in Homer2/3 DKO cells (n = 5). (C) Actin staining of TRAP+ MNCs in WT and Homer2/3 DKO cells (scale bar, 20 μm). The area of actin ring formation in TRAP+ MNCs during osteoclastogenesis is significantly higher in Homer2/3 DKO cells (n = 6). We normalized the data to the number and area of MNCs in RANKL-treated BMMs of WT mice. Results were expressed as the mean ± s.e.m. *P < 0.05 and ***P < 0.001 compared with RANKL-treated WT.
Discussion
In the present study, we report a novel interaction between Homer2/3 and NFATc1 for the regulation of osteoclastogenesis and bone metabolism. Our study demonstrates that inhibition of Homer2/3 expression strongly facilitates osteoclastogenesis. Homer2/3 DKO mice had an osteoporotic phenotype. The latter was caused by increased osteoclast differentiation with a wider bone erosion surface, independent of the rate of osteoblastic bone formation. We traced the aberrant bone metabolism to reduced Homer2/3 expression responsible for inducing NFATc1 with a sustained increase in its levels several days from RANKL treatment and a marked increase in its nuclear localization. These findings suggest that Homer2 and Homer3 are key regulators of RANKL-mediated osteoclastogenesis by linking RANKL-induced NFATc1 activation to the last stages of osteoclast differentiation.
It has been shown that Ca2+ oscillations have a prominent role in osteoclastogenesis (Takayanagi et al. 2002, Yang et al. 2009, Kim et al. 2010). Furthermore, Homer proteins were reported to interact with several Ca2+ signaling proteins (Shin et al. 2003, Worley et al. 2007, Yang et al. 2014). However, we found that the increase in long-lasting [Ca2+]i oscillations occurring between 24 and 72 h from RANKL stimulation (Fig. 2) occurred independently in Homer2/3 expressions. Therefore, it appears that Homer2 and Homer3 have another role in BMMs. Homer1 is the major regulator of Ca2+ signaling (Yuan et al. 2003), while Homer2 modulates only GPCR by affecting Gq-related signaling through RGS2 (Shin et al. 2003) and Homer3 has no known role in Ca2+ signaling. However, Homer2/3 competes with calcineurin for interaction with NFAT and may, therefore, regulate NFAT-dependent transcription (Huang et al. 2008). This accounts for the lack of effect of the DKO on RANKL-mediated Ca2+ oscillations. Specifically, we believe that they regulate the NFATc1 system’s function. As a consequence, the expression and translocation of NFATc1 appeared 12 h post RANKL treatment. Of note, these effects were enhanced in the RANKL-induced Homer2/3 DKO BMMs.
Previous reports showed that NFAT activation driving osteoclast differentiation requires NFAT expression’s induction via NF-κB and AP-1 and posttranslational activation induced by ITAM-associated immunoreceptors (Koga et al. 2004, Mocsai et al. 2004, Kuroda et al. 2008). Several studies suggest that NFAT binds Homer proteins participating in skeletal muscle differentiation and T cell activation (Stiber et al. 2005, Huang et al. 2008, Salanova et al. 2011). Huang and colleagues also reported that Homer2 and Homer3 play the action as a selective inhibitor to calcineurin-NFAT activation which the action was not related with AP1 and NF-kB pathway (Huang et al. 2008). Such mode of NFATc1 regulation appears to operate in both BMMs and osteoclasts. This is based on Homer proteins and NFATc1’s interaction, which is dependent on calcineurin and markedly inhibited by RANKL stimulation during osteoclastogenesis (Fig. 3F). Therefore, our study shows that Homer proteins are necessary for the control of RANKL-mediated NFATc1 induction and translocation. Additionally, they represent key molecules of the NFATc1 signaling pathway and autoamplification during osteoclastogenesis. Accordingly, the deletion of Homer2/3 facilitated the formation of MNCs and bone resorption in late-stage osteoclastogenesis to reduced bone density.
In summary, these results provide strong evidence for the regulation of NFATc1 availability for transcriptional regulation by the Homers in osteoclasts and that the Homers are major regulators of osteoclasts differentiation and bone metabolism. Specifically, our findings indicate that Homer proteins constitute a node at which NFATc1 activity, formation of MNCs, and physiological activity of osteoclasts are sequentially regulated. We believe that our findings may have a clinical relevance in pathological conditions of the skeletal system. We demonstrated essential roles of Homer proteins in the RANKL-induced NFATc1 signaling pathway during osteoclasts differentiation. Such observations suggest a specific and potent therapeutic target for various bone diseases caused by the abnormal formation of osteoclasts.
Acknowledgement
The authors thank Dr Paul F Worley for sharing the Homer2/3 DKO mice.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government MSIP (2016R1A5A2008630) and MOE (2012R1A1A2007673, 2015R1D1A1A01057277).
Footnotes
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- Berridge MJ, Bootman MD & Roderick HL 2003. Calcium signalling: dynamics, homeostasis and remodelling. Nature Reviews: Molecular Cell Biology 4 517–529. ( 10.1038/nrm1155) [DOI] [PubMed] [Google Scholar]
- Bootman MD , Lipp P & Berridge MJ 2001. The organisation and functions of local Ca2+ signals. Journal of Cell Science 114 2213–2222. [DOI] [PubMed] [Google Scholar]
- Bottai D, Guzowski JF, Schwarz MK, Kang SH, Xiao B, Lanahan A, Worley PF & Seeburg PH 2002. Synaptic activity-induced conversion of intronic to exonic sequence in Homer 1 immediate early gene expression. Journal of Neuroscience 22 167–175. ( 10.1523/JNEUROSCI.22-01-00167.2002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brakeman PR, Lanahan AA, O’Brien R, Roche K, Barnes CA, Huganir RL & Worley PF 1997. Homer: a protein that selectively binds metabotropic glutamate receptors. Nature 386 284–288. ( 10.1038/386284a0) [DOI] [PubMed] [Google Scholar]
- Crabtree GR & Olson EN 2002. NFAT signaling: choreographing the social lives of cells. Cell 109 (Supplement) S67–S79. ( 10.1016/s0092-8674(02)00699-2) [DOI] [PubMed] [Google Scholar]
- Feng W, Tu J, Yang T, Vernon PS, Allen PD, Worley PF & Pessah IN 2002. Homer regulates gain of ryanodine receptor type 1 channel complex. Journal of Biological Chemistry 277 44722–44730. ( 10.1074/jbc.M207675200) [DOI] [PubMed] [Google Scholar]
- Foucault I, Le Bras S, Charvet C, Moon C, Altman A & Deckert M 2005. The adaptor protein 3BP2 associates with VAV guanine nucleotide exchange factors to regulate NFAT activation by the B-cell antigen receptor. Blood 105 1106–1113. ( 10.1182/blood-2003-08-2965) [DOI] [PubMed] [Google Scholar]
- GuezGuez A, Prod’homme V, Mouska X, Baudot A, Blin-Wakkach C, Rottapel R & Deckert M 2010. 3BP2 adapter protein is required for receptor activator of NFkappaB ligand (RANKL)-induced osteoclast differentiation of RAW264.7 cells. Journal of Biological Chemistry 285 20952–20963. ( 10.1074/jbc.M109.091124) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan PG, Chen L, Nardone J & Rao A 2003. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes and Development 17 2205–2232. ( 10.1101/gad.1102703) [DOI] [PubMed] [Google Scholar]
- Huang GN, Huso DL, Bouyain S, Tu J, McCorkell KA, May MJ, Zhu Y, Lutz M, Collins S, Dehoff M, et al. 2008. NFAT binding and regulation of T cell activation by the cytoplasmic scaffolding Homer proteins. Science 319 476–481. ( 10.1126/science.1151227) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MS, Yang YM, Son A, Tian YS, Lee SI, Kang SW, Muallem S & Shin DM 2010. RANKL-mediated reactive oxygen species pathway that induces long lasting Ca2+ oscillations essential for osteoclastogenesis. Journal of Biological Chemistry 285 6913–6921. ( 10.1074/jbc.M109.051557) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga T, Inui M, Inoue K, Kim S, Suematsu A, Kobayashi E, Iwata T, Ohnishi H, Matozaki T, Kodama T, et al. 2004. Costimulatory signals mediated by the ITAM motif cooperate with RANKL for bone homeostasis. Nature 428 758–763. ( 10.1038/nature02444) [DOI] [PubMed] [Google Scholar]
- Kuroda Y, Hisatsune C, Nakamura T, Matsuo K & Mikoshiba K 2008. Osteoblasts induce Ca2+ oscillation-independent NFATc1 activation during osteoclastogenesis. PNAS 105 8643–8648. ( 10.1073/pnas.0800642105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayingi J, Helary G, Noirclere F, Bacroix B & Migonney V 2007. Grafting of bioactive polymers onto titanium surfaces and human osteoblasts response. Conference Proceedings Annual International Conference of the IEEE Engineering in Medicine and Biology Society 2007 5119–5122. ( 10.1109/IEMBS.2007.4353492) [DOI] [PubMed] [Google Scholar]
- Mizutani A, Kuroda Y, Futatsugi A, Furuichi T & Mikoshiba K 2008. Phosphorylation of Homer3 by calcium/calmodulin-dependent kinase II regulates a coupling state of its target molecules in Purkinje cells. Journal of Neuroscience 28 5369–5382. ( 10.1523/JNEUROSCI.4738-07.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocsai A, Humphrey MB, Van Ziffle JA, Hu Y, Burghardt A, Spusta SC, Majumdar S, Lanier LL, Lowell CA & Nakamura MC 2004. The immunomodulatory adapter proteins DAP12 and Fc receptor gamma-chain (FcRgamma) regulate development of functional osteoclasts through the Syk tyrosine kinase. PNAS 101 6158–6163. ( 10.1073/pnas.0401602101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson EN & Williams RS 2000. Calcineurin signaling and muscle remodeling. Cell 101 689–692. ( 10.1016/s0092-8674(00)80880-6) [DOI] [PubMed] [Google Scholar]
- Park B, Yang YM, Choi BJ, Kim MS & Shin DM 2013. Activation of G proteins by aluminum fluoride enhances RANKL-mediated osteoclastogenesis. Korean Journal of Physiology and Pharmacology 17 427–433. ( 10.4196/kjpp.2013.17.5.427) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salanova M, Bortoloso E, Schiffl G, Gutsmann M, Belavy DL, Felsenberg D, Furlan S, Volpe P & Blottner D 2011. Expression and regulation of Homer in human skeletal muscle during neuromuscular junction adaptation to disuse and exercise. FASEB Journal 25 4312–4325. ( 10.1096/fj.11-186049) [DOI] [PubMed] [Google Scholar]
- Shanmugarajan S, Haycraft CJ, Reddy SV & Ries WL 2012. NIP45 negatively regulates RANK ligand induced osteoclast differentiation. Journal of Cellular Biochemistry 113 1274–1281. ( 10.1002/jcb.23460) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin DM, Dehoff M, Luo X, Kang SH, Tu J, Nayak SK, Ross EM, Worley PF & Muallem S 2003. Homer 2 tunes G protein-coupled receptors stimulus intensity by regulating RGS proteins and PLCbeta GAP activities. Journal of Cell Biology 162 293–303. ( 10.1083/jcb.200210109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son A, Kim MS, Jo H, Byun HM & Shin DM 2012. Effects of inositol 1,4,5-triphosphate on osteoclast differentiation in RANKL-induced osteoclastogenesis. Korean Journal of Physiology and Pharmacology 16 31–36. ( 10.4196/kjpp.2012.16.1.31) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiber JA, Tabatabaei N, Hawkins AF, Hawke T, Worley PF, Williams RS & Rosenberg P 2005. Homer modulates NFAT-dependent signaling during muscle differentiation. Developmental Biology 287 213–224. ( 10.1016/j.ydbio.2005.06.030) [DOI] [PubMed] [Google Scholar]
- Takayanagi H 2007. The role of NFAT in osteoclast formation. Annals of the New York Academy of Sciences 1116 227–237. ( 10.1196/annals.1402.071) [DOI] [PubMed] [Google Scholar]
- Takayanagi H, Kim S, Koga T, Nishina H, Isshiki M, Yoshida H, Saiura A, Isobe M, Yokochi T, Inoue J, et al. 2002. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Developmental Cell 3 889–901. ( 10.1016/S1534-5807(02)00369-6) [DOI] [PubMed] [Google Scholar]
- Tu JC, Xiao B, Yuan JP, Lanahan AA, Leoffert K, Li M, Linden DJ & Worley PF 1998. Homer binds a novel proline-rich motif and links group 1 metabotropic glutamate receptors with IP3 receptors. Neuron 21 717–726. ( 10.1016/S0896-6273(00)80589-9) [DOI] [PubMed] [Google Scholar]
- Tu JC, Xiao B, Naisbitt S, Yuan JP, Petralia RS, Brakeman P, Doan A, Aakalu VK, Lanahan AA, Sheng M, et al. 1999. Coupling of mGluR/Homer and PSD-95 complexes by the Shank family of postsynaptic density proteins. Neuron 23 583–592. ( 10.1016/S0896-6273(00)80810-7) [DOI] [PubMed] [Google Scholar]
- Worley PF, Zeng W, Huang G, Kim JY, Shin DM, Kim MS, Yuan JP, Kiselyov K & Muallem S 2007. Homer proteins in Ca2+ signaling by excitable and non-excitable cells. Cell Calcium 42 363–371. ( 10.1016/j.ceca.2007.05.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao B, Tu JC, Petralia RS, Yuan JP, Doan A, Breder CD, Ruggiero A, Lanahan AA, Wenthold RJ & Worley PF 1998. Homer regulates the association of group 1 metabotropic glutamate receptors with multivalent complexes of homer-related, synaptic proteins. Neuron 21 707–716. ( 10.1016/S0896-6273(00)80588-7) [DOI] [PubMed] [Google Scholar]
- Yang YM, Kim MS, Son A, Hong JH, Kim KH, Seo JT, Lee SI & Shin DM 2009. Alteration of RANKL-induced osteoclastogenesis in primary cultured osteoclasts from SERCA2+/− mice. Journal of Bone and Mineral Research 24 1763–1769. ( 10.1359/jbmr.090420) [DOI] [PubMed] [Google Scholar]
- Yang YM, Jung HH, Lee SJ, Choi HJ, Kim MS & Shin DM 2013. TRPM7 is essential for RANKL-induced osteoclastogenesis. Korean Journal of Physiology and Pharmacology 17 65–71. ( 10.4196/kjpp.2013.17.1.65) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YM, Lee J, Jo H, Park S, Chang I, Muallem S & Shin DM 2014. Homer2 protein regulates plasma membrane Ca2+-ATPase-mediated Ca2+ signaling in mouse parotid gland acinar cells. Journal of Biological Chemistry 289 24971–24979. ( 10.1074/jbc.M114.577221) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan JP, Kiselyov K, Shin DM, Chen J, Shcheynikov N, Kang SH, Dehoff MH, Schwarz MK, Seeburg PH, Muallem S, et al. 2003. Homer binds TRPC family channels and is required for gating of TRPC1 by IP3 receptors. Cell 114 777–789. ( 10.1016/s0092-8674(03)00716-5) [DOI] [PubMed] [Google Scholar]
- Zaidi M 2007. Skeletal remodeling in health and disease. Nature Medicine 13 791–801. ( 10.1038/nm1593) [DOI] [PubMed] [Google Scholar]